Abstract
Postweaning mortality is a complex causal matrix involving animal, environment, and infectious etiologic factors. Despite advances in swine productivity such as total pigs born, growth rate, feed intake, and efficiency, there have been modest to no improvements in postweaning mortality rates over the last several years. Industry averages for postweaning mortality range from four to eight percent for each the nursery, grow-finish, or wean-finish stages. Retrospective mortality causal analyses of individual databases have been performed. However, little information derived from meta-analysis, systematic review, or comprehensive literature reviews are available. In order to develop and evaluate strategies to comprehensively manage and reduce postweaning mortality, addressing the complexity and range of impact that factors have on mortality is necessary to identify and prioritize such contributing factors. Our objective is to describe the current state of knowledge regarding non-infectious causes of postweaning mortality, focusing on estimates of frequency and magnitude of effect where available. Postweaning mortality can be generalized into non-infectious and infectious causes, with non-infectious factors further classified into anatomic abnormalities, toxicity, animal factors, facility factors, nutritional inadequacies, season, and management factors. Important non-infectious factors that have been identified through review of literature include birth weight, pre-weaning management, weaning age and weight, and season. Additionally, reasons for mortality with a low incidence but a high magnitude include abdominal organ torsion/volvulus, sodium ion or ionophore toxicosis, or dietary imbalance due to feed formulation or manufacture error. Many interactive effects are present between and among infectious and non-infectious factors, but an important trend is the impact that non-infectious factors have on the incidence, severity, and resolution of infectious disease. Strategies to reduce postweaning mortality must consider the dynamic, complex state that forms the causal web. Control of postweaning mortality through understanding of the complexity, evaluation of mortality reduction strategies through rigorous scientific evaluation, and implementation remains an area of opportunity for continued growth and development in the global swine industry.
Keywords: death loss, mortality, non-infectious, postweaning, swine
INTRODUCTION
Of the total pigs born per litter, over one-third succumb to birth, pre-weaning, and postweaning death loss (Knauer and Hostetler, 2013). Numerous studies have identified causes of birth and pre-weaning mortality (Alonso-Spilsbury et al., 2007; Kirkden et al., 2013; Muns et al., 2016). Less effort has been directed toward summarizing postweaning mortality. Retrospective analyses of individual databases have been performed (Losinger et al., 1998; Maes et al., 2001, 2004; Larriestra et al., 2005a; Oliveira et al., 2007, 2009; Agostini et al., 2013, 2014, 2015; Serrano et al., 2014). Meta-analysis, systematic review, or comprehensive literature review of causes and contributors to mortality from weaning to market is limited. A review addressing the complexity and the range of impact that factors have on mortality is necessary. In doing so, factors can be identified and prioritized for which strategies can be implemented to comprehensively manage and reduce postweaning mortality. Factors contributing to mortality are often not clearly defined. Determining a causal relationship resulting in mortality is particularly challenging, especially with the multitude of component causes that in various combinations can result in a sufficient causal mechanism of mortality (Rothman and Greenland, 2005). Further complicating this issue is the fact that many of the component causes of mortality often do not need to occur simultaneously, that is a particular event can increase the severity of a later event. When deciding to what cause(s) the mortality should be attributed, it is often the cause most closely related to the fatal outcome or proximate cause that is assigned responsibility (Rothman and Greenland, 2005). Causation of mortality is often very challenging to understand, but an important step is identification of potential contributing component causes and attempt to identify and quantify interactive effects. Our objective is to summarize the current state of knowledge regarding non-infectious factors contributing to postweaning mortality, focusing on estimates of frequency of mortal occurrences and magnitude of effect on mortality within a population when the factor is present.
MATERIALS AND METHODS
The search was performed using Web of Science (https://login.webofknowledge.com/) which incorporated the Web of Science Core Collection, CAB Abstracts, and Medline databases, PubMed (www.ncbi.nlm.nih.gov/pubmed/), as well as the Scopus (www.scopus.com). Search terms including (SWINE OR PIG) AND (MORTALITY OR MORBIDITY OR DEAD OR REMOVAL) AND the respective search item of interest (e.g., feed, mycotoxin, lameness, weaning age, and sex). Additionally, assessment of bibliographical items resulted in further identification of relevant sources. Articles were restricted to primarily English language; however, non-English articles that contained an English abstract where relevant information could be extracted were included. Once relevant articles were identified, they were filed and categorized according to topic for further evaluation. This review only includes peer-reviewed literature; therefore, conference proceedings and other non-peer reviewed literature is not included.
RESULTS AND DISCUSSION
Overview of Postweaning Mortality
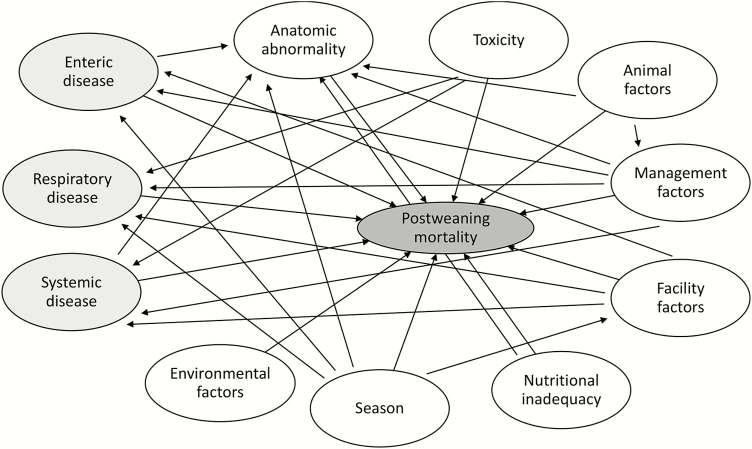
Postweaning mortality is a complex causal matrix involving animal, environment, and infectious etiologic factors. While all three can individually result in a cascade that leads to mortality, oftentimes mortality is due to interactive effects. Postweaning mortality can be generalized into non-infectious and infectious factors (Figure 1), with non-infectious factors further classified into anatomic abnormalities, toxicity, animal factors, facility factors, nutritional inadequacies, season, and management factors. Defining causation of mortality is extremely difficult. Multiple factors contribute, and the relative contribution is extremely difficult to differentiate. Hereafter, all discussion regarding the contribution of various factors on postweaning mortality is done with clear acknowledgement that proximate and ultimate cause of mortality is often not clear. Nonetheless, we attempt to provide context regarding the scope of impact these factors contribute to postweaning mortality.
Figure 1.
Causal web of factors contributing to postweaning mortality including non-infectious (white) and infectious (gray) causes. Arrows represent pathway by which one cause influences another cause.
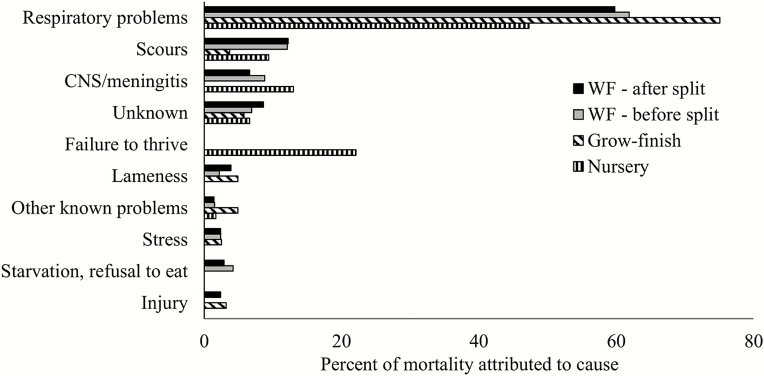
Knauer and Hostetler (2013) reported mortality for nursery, finishing, and wean–finish in the United States between 2005 and 2010 was 3.6%, 5.0%, and 7.6%, respectively. The National Animal Health Monitoring System Swine 2012 report (USDA, 2015) reported that across all site sizes, 3.6% of pigs entering the nursery phase died, 4.1% of pigs entering the grower/finisher phase died, and 5.6% of pigs entering the wean–finish phase died. The most common cause of mortality as reported by USDA (2015) for all phases of postweaning production was respiratory disease as reported by producers (Figure 2). Straw et al. (1983) observed that finishing pig mortality was caused by pneumonia (24.7%), gastric ulceration (14.0%), enteritis (6.4%), trauma (4.3%), gastrointestinal displacement (4.3%), followed by miscellaneous causes with lower incidence (15.1%) or undiagnosed (31.2%). A total of 11 retrospective analyses of production system records are summarized in Table 1 with 19 unique factors identified as significant contributors to postweaning mortality. Factors contributing to postweaning mortality are summarized by estimate of incidence when appropriate, and qualitative magnitude of effect for morbidity and mortality attributed to the factor or when it does occur (Table 2). Many factors are always present such as season, sex, and genetics, while other factors occur sporadically such as ionophore toxicosis.
Figure 2.
Percentage of mortality by producer-identified cause [adapted from USDA (2015)]. Listed in the order of sum of mortality by production phase. CNS = central nervous system.
Table 1.
Summary of explanatory variables associated with postweaning mortality as identified in retrospective analysis of commercial production records
| Weight, kg | ||||||
|---|---|---|---|---|---|---|
| Article | Country | Year | Number of pigs | Start | End | Significant factors (P ≤ 0.05) |
| Losinger et al. (1998) | USA | 1995–1996 | N/A | N/A | N/A | Weaning age |
| Source | ||||||
| Maes et al. (2001) | USA | 1996–2000 | 1,345,127 | N/A | N/A | Year |
| Timing within finishing | ||||||
| Maes et al. (2004) | Belgium | 1999–2002 | 828,385 | 25 | 113 | Season |
| Source | ||||||
| Feeding duration | ||||||
| Larriestra et al. (2005a) | USA | 1996–2000 | 1,720,040 | 23 | N/A | Entry weight |
| Days on feed | ||||||
| Season | ||||||
| Oliveira et al. (2007) | Spain | 1996–1997 | 120,751 | 18–20 | N/A | Farm type |
| Herd size | ||||||
| Season | ||||||
| Feeding duration | ||||||
| Oliveira et al. (2009) | Spain | 1999–2002 | 158 batches | N/A | N/A | Quality of care |
| Source | ||||||
| Season | ||||||
| Year | ||||||
| Agostini et al. (2013) | Spain | 2008–2010 | 1,157,212 | 19 | 108 | Season |
| Number of pigs placed | ||||||
| Number of sources | ||||||
| Circovirus vaccine | ||||||
| Antibiotic route | ||||||
| Water source | ||||||
| Serrano et al. (2014) | Spain | 2003–2005 | 42 farms | N/A | N/A | Presence of viral antibodies |
| Farm type | ||||||
| Agostini et al. (2014) | Spain | 2008–2009 | 454,855 | 20 | 104 | Season |
| Number of sources | ||||||
| Ventilation type | ||||||
| Initial bodyweight (IBW) | ||||||
| IBW × ventilation type | ||||||
| IBW × number of sources | ||||||
| Agostini et al. (2015) | Spain | 2008–2010 | 1,040,116 | 19 | 106 | Season |
| Number of sources | ||||||
| Ventilation type | ||||||
| Number of pigs placed | ||||||
| Mehling et al. (2019) | USA | 2015 | 115,213 | 7 | 115 | Stocking density |
N/A = information not provided.
Table 2.
Summary of non-infectious factors contributing to postweaning mortality based on incidence and magnitude of potential mortality1
| Item | Sub-classification | Incidence2 | Magnitude3 |
|---|---|---|---|
| Anatomic abnormality | |||
| Lameness/physical injury | ++ | ++ | |
| Gastric ulceration | +++ | ++ | |
| Acute abdominal incidents | + | +++ | |
| Umbilical herniation | + | + | |
| Rectal prolapse | ++ | ++ | |
| Scrotal/inguinal herniation | + | ++ | |
| Toxicity | |||
| Mycotoxins | +++ | + | |
| Sodium ion toxicosis | + | +++ | |
| Ionophore toxicosis | + | +++ | |
| Toxins of lower incidence | + | ++ | |
| Animal factors | |||
| Sex | N/A | + | |
| Genetics | N/A | ++ | |
| Birth weight | N/A | +++ | |
| Dam parity | N/A | ++ | |
| Facility factors | N/A | + | |
| Environmental factors | N/A | ++ | |
| Season | N/A | +++ | |
| Nutritional inadequacy | + | +++ | |
| Management factors | |||
| Preweaning management | N/A | +++ | |
| Weaning age and weight | N/A | +++ | |
| Barn fill length and sources of pigs | N/A | ++ | |
| Resource availability | N/A | ++ | |
| Group size | N/A | + | |
| Transportation | N/A | + | |
| Sanitation | N/A | ++ |
1 Qualitative assignment of relative incidence and magnitude of mortality was performed by primary author (J.T.G.) based on summarization of published literature.
2 Where appropriate, relative incidence of mortality attributed to the factor was denoted using a system ranging from + to +++. Where estimates of incidence were not appropriate, N/A was indicated.
3 Relative magnitude of mortality in a population attributed to the presence of factor was described as + (low potential), ++ (moderate potential), and +++ (significant potential).
Anatomic Abnormality
Anatomic abnormalities can both directly cause mortality (e.g., abdominal organ torsion or volvulus) and reduce competitiveness that leads to gradual decline with euthanasia often the outcome (e.g., lameness, umbilical herniation, and rectal prolapse). While incidence of anatomic abnormalities can vary greatly, the case fatality rate can be very high.
Acute abdominal incidents.
Distention of abdominal organs can be classified into two primary causes including rotation around the mesenteric axis (mesenteric torsion or twisted gut) or simple gas distention leading to increased abdominal pressure with or without secondary torsion. Factors thought to lead to mesenteric torsion include rapid pig movement or directional changes, inconsistent feed intake pattern characterized as periodic fasting and engorgement, and distended viscera due to fermentative digestion (Thomson and Friendship, 2019). Gas distention of the colon can be due to a number of factors including diet and colonic microflora imbalance. This can result in subsequent intestinal distention and possibly torsion, vascular occlusion, and death (Martineau et al., 2008). A significant amount of discussion has been focused on hemorrhagic bowel syndrome as a cause of acute death in finishing pigs. Hemorrhagic bowel syndrome as an independent disease process from volvulus/torsion remains speculative. The reported incidence of organ torsion or volvulus is not widely understood, but overall is relatively low. Once abdominal organs have been fully occluded, mortality rapidly occurs resulting in a high case fatality rate. Additional research is necessary to understand the cause of acute abdominal incidents and how the incidence can be reduced.
Lameness/physical injury.
Lameness can be caused by a multitude of factors including trauma, infectious causes resulting in arthritis or osteomyelitis (Hill et al., 1996; Gomes-Neto et al., 2016), developmental orthopedic diseases such as osteochondritis dissecans, nutritional imbalance resulting in abnormal structural integrity of the skeletal system, or combination of these factors (Madson et al., 2019). Canning et al. (2019) reviewed lameness diagnostic laboratory case submissions in grow–finish pigs and found that 31% of cases did not result in conclusive cause or no abnormal findings, 42% had a diagnosis of infectious etiology, whereas the remaining diagnoses included metabolic bone disease, or other/unknown causes. It is important to note that the before mentioned estimates were based on case submissions, likely underrepresenting cases that can be easily field diagnosed. Lameness is estimated to affect between 0.6% and 2.2% of finishing pigs (Krieter et al., 2004; Cagienard et al., 2005; Petersen et al., 2008; Neumann et al., 2014). Breaches in integument may be portals of entry for bacteria and include injuries from tail bites, flank wounds, among others, which are estimated to affect 0.5% to 2% of finishing pigs (Cagienard et al., 2005; Petersen et al., 2008; Neumann et al., 2014). Lameness and physical injury is a broad categorization, oftentimes not leading directly to mortality but rather morbidity that results in reduced competitiveness and eventual culling or euthanasia.
Umbilical herniation.
Umbilical herniation is a lack of appropriate fusion of the body wall at the umbilicus, with subsequent protrusion of abdominal organs. Umbilical infections with pyogenic bacteria may be a risk factor for umbilical hernia, both as causal and contributory, with abscesses often present (Anderson and Mulon, 2019). A genetic association has been reported for umbilical herniation (Searcy-Bernal et al., 1994; Rutten-Ramos and Deen, 2006; Ding et al., 2009; Liao et al., 2015; Long et al., 2016). Additionally, as weaning age is increased from 12 to 15 days of age, belly-nosing behavior and subsequent umbilical lesions were observed to be less frequent (Main et al., 2005b). The prevalence of umbilical herniation has been reported to be 0.7% to 1.5% in postweaning pigs (Searcy-Bernal et al., 1994; Petersen et al., 2008; Straw et al., 2009; Yun et al., 2017; Ramirez, 2019) with case fatality rate ranging from 0% to 7.2% (Searcy-Bernal et al., 1994; Straw et al., 2009). Umbilical hernias often do not lead to mortality in a commercial setting due to the common practice of marketing affected animals prematurely to capture value. Overall, this condition can result in mild levels of morbidity and mortality.
Scrotal/inguinal herniation.
Scrotal or inguinal herniation is a condition where abdominal organs extend beyond the inguinal ring and protrude into the inguinal canal (Anderson and Mulon, 2019). With chronic herniation including displacement of intestine, strangulation can occur and lead to extreme pain, necrosis, and death. While exact pathogenesis is unknown, it has been reported that scrotal/inguinal herniation is associated with specific genetic lines (Magee, 1951; Grindflek et al., 2006; Ding et al., 2009; Lago et al., 2018). Surgical correction has been described by Anderson and Mulon (2019); however, it is not commonly performed in a commercial setting. Prevalence of inguinal herniation has been reported to be 0.1% to 0.7% (Straw et al., 2009; Yun et al., 2017; Ramirez, 2019) with a case fatality rate of 25.0% (Straw et al., 2009).
Rectal prolapse.
Animals experiencing rectal prolapse often are euthanized for welfare concerns. Factors reported to lead to greater incidence of rectal prolapse include piling due to cold temperatures, abdominal straining, mycotoxins, having a short tail, being a barrow, offspring from a low parity dam, low birth weight, genetic predisposition, chronic water shortage, and excess dietary lysine (Gardner et al., 1988; Amass et al., 1995; Anderson and Mulon, 2019). Multiple reports also have challenged the theory of seasonality and sex differences in prolapse rate (Garden et al., 1988) and association with coughing, pneumonia, constipation, or diarrhea (Garden et al., 1988; Gardner et al., 1988). It has been reported that the incidence rate is ~9.1 cases per 100,000 days at risk (Gardner et al., 1988), and prevalence rates at market have been reported as high as 5.8% (Neumann et al., 2014). Many of the pigs will heal if properly segregated prior to onset of significant bleeding; however, early culling and euthanasia are common outcomes.
Gastric ulceration.
Gastric ulceration is thought to be multifactorial in nature. An important factor that has been associated with gastric ulcers include feed, both the particle size of ground grain and diet form. Feeding pelleted diets has been associated with gastric ulcers at harvest (Robertson et al., 2002). The most severe morphologic changes occur in pigs fed pelleted diets immediately before marketing for periods of longer than 2 weeks (De Jong et al., 2016). Additionally, feeding pelleted diets has been associated with a greater number of pigs removed per pen (De Jong et al., 2016). Additional studies have reported pigs fed pelleted diets had greater presence of stomach lesions compared with pigs fed mash diets (Grosse Liesner et al., 2009; Cappai et al., 2013; Mosseler et al., 2014). In many of these experiments, mash diets had a coarser particle size thus confounding diet form and particle size. This results in lack of clarity regarding the underlying cause of the measured association between diet form and increased risk of gastric ulceration. In the absence of additional published data, swine production systems and technical support companies have demonstrated the association between feeding pellets and gastric ulcers. Reports on such investigations are not found in published literature.
Multiple studies have evaluated particle size in multiple grain types including corn, sorghum, and wheat and observed morphologic changes to the stomach including keratinization and ulcer scores (Hedde et al., 1985; Healy et al., 1994; Wondra et al., 1995; Mavromichalis et al., 2000; Millet et al., 2012; Rojas et al., 2016). The resulting impact on mortality is not clearly defined in the literature. Additional investigation in a commercial setting using a large sample size is necessary to determine whether morphologic changes associated with feeding fine particle size diets results in morbidity and mortality. Once the impact on mortality is clearly defined, the findings can be incorporated with economic value of expected feed efficiency improvements to determine the best grinding strategy.
Additional factors thought to increase risk of gastric ulceration include recurrent interruption of feed intake such as feed outages, increased fluidity of digesta, Mycoplasma spp. vaccination, season (Ramis et al., 2006; Gottardo et al., 2017; Thomson and Friendship, 2019), and possibly infectious etiologies (De Witte et al., 2018). Additionally, treatment of finishing pigs with an anthelmintic has been associated with reduced risk of gastric ulceration (Gottardo et al., 2017). While gross and histologic changes in the esophageal region of the stomach are very common with 12% to 28% of sites reporting ulcers and 30% to 90% of market pigs having visible pathology (Robertson et al., 2002; van den Berg et al., 2005; Rodriguez et al., 2008; de Oliveira et al., 2010; Swaby and Gregory, 2012; USDA, 2016), mortality is commonly much less at an estimated 2.5% (Doster, 2000). In extreme cases, mortality as high as 27% within a group of finishing pigs during a single week has been reported (Melnichouk, 2002). Formation of gastric ulcers is multi-factorial and is discussed further in dietary factors.
Toxicity
Toxicity can occur due to a nearly infinite list of compounds (natural and synthetic) that can be introduced to swine via a number of routes including aerosol, feed, and water. Perhaps the most common sources of toxicity leading to death include the sustained lack of water access followed by a period of sudden availability leading to swelling of the brain known as sodium ion toxicity, accidental ionophore intoxication, followed by mycotoxins. Although many of these toxicities will not be discussed in further detail due to rare incidence, the magnitude of effect on mortality or necessity to depopulate to prevent human harm via ingestion of products may be great.
Mycotoxins.
The dose and duration of mycotoxin exposure resulting in mortality is not well defined for all mycotoxins affecting swine. A summary of available information is provided in Table 3. Reports vary regarding the dose and feeding duration of aflatoxin required to cause mortality under commercial conditions (Hayes et al., 1978, Harvey et al., 1989; Marin et al., 2002; Ensley et al., 2019), but mortality has been reported at levels as low as 0.3 mg/kg bodyweight when fed for 42 days (Cook et al., 1989). In very high, acute situations, the aflatoxin concentration required to kill 50% of exposed animals was 0.62 mg/kg bodyweight (equivalent to dietary concentration of ~20 mg/kg) for 1 day (Ensley and Radke, 2019). A dose of 1 mg/kg bodyweight ochratoxin has been reported to result in 38% mortality in nursery pigs when fed for 5 days (Szczech et al., 1973). In contrast, a higher dose of 2 mg/kg ochratoxin fed to pigs for 28 day has been observed to have no lethal effects (Harvey et al., 1989). Fumonisin has a wide range of attack and case fatality risks (Harrison et al., 1990; Haschek et al., 1992; Osweiler et al., 1992; Colvin et al., 1993; Zomborszky et al., 2000), but dietary concentrations >120 mg/kg for a period of 4 days or more is most commonly associated with mortality (Ensley and Radke, 2019). Mycotoxins are a problem across many regions of the United States, and USDA (2016) reported that 73% of all sites were currently using or have used mycotoxin binders. Significant questions remain regarding the interactive impacts of long-term feeding of mycotoxins on disease, management, dietary, or other causes of mortality. Mycotoxins are often associated with morbidity and reduced growth performance. Due to the variable and often aged information, more research is needed to further understand the impact on mortality.
Table 3.
Dose and duration of various mycotoxins required to cause death
| Item | Attack rate1 | Case fatality risk2 | Dose, dietary mg/kg | Duration of experiment | Onset of mortality | Source |
|---|---|---|---|---|---|---|
| Aflatoxin | — | LD503 | 0.62 mg/kg bodyweight | — | 1 d | Ensley and Radke (2019) |
| — | — | 2–4 | — | Extended time | Ensley and Radke (2019) | |
| 0% | — | 0.28 | 28 d | — | Marin et al. (2002) | |
| 33 to 100% | 0 to 15% mortality | 0.30 to 1.07 B1, 0.01 to 0.13 B24 | 17 to 84 d | — | Cook et al. (1989) | |
| — | 0% mortality | 2 | 28 d | — | Harvey et al. (1989) | |
| — | 20% mortality | 0.8 B1, 0.2 B2 | — | — | Hayes et al. (1978) | |
| Citrinin | Not established | — | — | — | — | — |
| Ergot | Not established | — | — | — | — | — |
| Fumonisin (B1 and B2) | 66% | 100% | 92 B1, 28 B2 | 21 d | 5 d | Osweiler et al. (1992) experiment |
| 6% | 100% | 24 to 120 B1 | — | 4 d | Osweiler et al. (1992) field cases | |
| 100%5 | 0% | 40 B1 | 28 d | 28 d | Zomborszky et al. (2000) | |
| 100% | 66% | 166 B1, 48 B26 | 15 d | 5 d | Haschek et al. (1992) | |
| 66% | 100% | 155 | 28 d | 7 d | Harrison et al. (1990) | |
| 0%7 | — | 105 | 28 d | — | Harrison et al. (1990) | |
| 100% | 50%8 | 200 B1 | 21 d | 21 d | Colvin et al. (1993) | |
| Ochratoxin | — | 0% mortality | 2 | 28 d | — | Harvey et al. (1989) |
| — | 38% mortality | 1 mg/kg bodyweight | 6 d | 5 d | Szczech et al. (1973) | |
| Trichothecenes | ||||||
| T-2 toxin | Not established | — | — | — | — | — |
| Deoxynivalenol | Not established | — | — | — | — | — |
| Zearalenone (f-2 toxin) | Not established | — | — | — | — | — |
1Attack rate = number of cases in population at risk divided by number of animals in the population exposed (Dohoo et al., 2003).
2Case fatality risk = proportion of animals with a specific disease that result in death (Dohoo et al., 2003).
3Lethal dose 50%, dose required to cause mortality in 50% of test subjects.
4 Cook et al. (1989) reported findings from an investigation of eight farms diagnosed with aflatoxicosis from 1983 to 1985. Dietary aflatoxin levels ranged from 0.30 to 1.07 mg/kg aflatoxin B1 and 0.01 to 0.13 mg/kg aflatoxin B2. Complete diet samples were not available for all farms, so shelled corn was reported as well ranging from 0.23 to 1.90 mg/kg aflatoxin B1 and 0.01 to 0.12 mg/kg aflatoxin B2.
5All five animals fed 40 mg/kg fumonisin B1 demonstrated histological changes associated with pulmonary edema at necropsy on day 28.
6Corn screenings also contained 0.3 mg/kg deoxynivalenol and 20 µg/kg aflatoxin.
7 One out of three animals was euthanized on d 14 of study due to severe anorexia, but did not shown evidence of pulmonary edema/hydrothorax.
8 Two out of four animals euthanized and necropsied on day 21 of study. Remaining animals were switched to fumonisin-free diet and were continued to be monitored.
Sodium ion toxicosis.
Sodium ion toxicosis, salt poisoning, or water deprivation is most commonly associated with interrupted water supply to pigs for periods of 24 h or greater due to mechanical failure, frozen water supply, oversight to turn on water supply upon animal placement in the facility, or improper control valve placement around the time of instituting a water medication regimen (Brito et al., 2001; Ensley and Radke, 2019). The prevalence of sodium ion toxicosis is low, but morbidity and mortality can be high with extreme cases.
Ionophore toxicosis.
Narasin is an ionophore and is approved for increased rate of gain (15 to 30 g/tonne) or increased rate of gain and improved feed efficiency (20 to 30 g/tonne; FDA, 2018). When accidently fed at higher doses, ionophore toxicity can occur leading to altered cardiac rhythm, myocyte damage, and necrosis (McKellar and Lawrence, 1996) with significant mortality when fed at levels of 139 g/tonne or greater (Armien et al., 1997; Sturos et al., 2016). Pleuromutilin antibiotics, such as tiamulin, potentiate ionophore toxicity due to inhibition of cytochrome P450, thereby reducing the metabolism and clearance of ionophores (McKellar and Burch, 1996; Carpenter et al., 2005). Carpenter et al. (2005) reported that 100% of pigs inadvertently fed narasin at 83 g/tonne and tiamulin at 31 g/tonne exposed were euthanized following development of clinical signs. Monensin is another commonly used ionophore fed to ruminants and has been shown to be lethal to swine at 300 mg/kg (Dilov et al., 1981; Iotsev, 1985). Ionophore intoxication due to feed manufacture error does occur in swine and can result in significant morbidity and mortality.
Toxins of lower incidence.
A multitude of additional toxins have the potential in unique situations to cause mortality in postweaning swine. Toxin sources include water contaminants such as nitrates, nitrites, and toxins produced by microorganisms living in water such as blue-green algae (Classen et al., 2017). Gas intoxication including hydrogen sulfide, carbon monoxide, ammonia, and carbon dioxide as well as hyperthermia in the event of power loss to enclosed facilities and/or fire can also lead to high mortality. Additional information is discussed by Ensley and Radke (2019).
Animal Factors
Pig factors are causes of mortality that are attributed to certain characteristics of the animal such as sex, genetics, and birth weight. Information is relatively scarce regarding genetic associations with postweaning mortality, and improvements in genetic selection is largely done internally and does not generate peer reviewed publications
Sex.
Research investigating the impact of sex on mortality has largely shown no effect (van Veen et al., 1985; Brumm, 2004; Serrano et al., 2013) with the exception of Larriestra et al. (2006) who observed that barrows were 1.75 times more likely to die during the nursery phase compared with gilts. Based on the body of evidence, it is evident that sex is not commonly associated with substantial differences in mortality. Castration is a common practice performed within the first 2 to 5 days of age in male pigs to reduce aggressive behavior and non-desirable meat characteristics. Immunocastration has been developed and does not necessitate physical castration; however, this practice has not been widely implemented in the United States. No evidence of a difference in mortality has been observed between intact, castrated, and immunocastrated males from birth through harvest (Aluwe et al., 2015) or between intact males and immunocastrates (Guay et al., 2013). Additionally, no evidence of a difference in mortality was observed comparing barrows castrated using conventional castration with no analgesia or anesthesia compared with the use of lidocaine, meloxicam, the combination of lidocaine and meloxicam, or a sham castration (Kluivers-Poodt et al., 2012). Based on published literature, sex and castration does not significantly contribute to postweaning mortality.
Genetics.
Genetic evaluation has been performed using parameters for preweaning mortality (Varona et al., 2010; Dufrasne et al., 2013; Strange et al., 2013) and farrowing mortality (Ibanez-Escriche et al., 2009). Dufrasne et al. (2014) found that the correlation between pre-weaning and postweaning mortality was weak, indicating that genetic selection for one would not guarantee improvements in the other. Differences in mortality have been observed in field settings between various commercially available genetic lines. This information is not available in peer-reviewed publications. The use of genomic technologies to improve resistance to diseases was summarized by Mellencamp et al. (2008), and provides a useful strategy to reduce clinical disease and subsequent mortality. In additional to traditional genetic selection techniques, technology is currently in development where susceptibility to various pathogens has been reduced or eliminated through use of genetic modification technologies. These technologies have been shown to be successful at preventing infection of porcine reproductive and respiratory syndrome virus (PRRSV; Whitworth et al., 2016), porcine epidemic diarrhea virus (PEDV), and transmissible gastroenteritis virus (TGEV; Whitworth et at., 2019). While these technologies provide a huge opportunity for improved pig health and welfare, they are currently undergoing regulatory review within the United States and further steps are required prior to implementation in the industry. Genetics clearly play an important role in morbidity and mortality and is an opportunity for future development and implementation.
Birth weight.
Low birth weight has been reported to be positively associated with preweaning as well as postweaning mortality (Smith et al., 2007; Pardo et al., 2013; Declerck et al., 2016; Zotti et al., 2017; Skorput et al., 2018). It has been reported that pigs with a birth weight of ≤0.7 kg at birth have <40% chance of survival through the nursery, compared with nearly 90% chance of survival if birth weight is 1.7 kg and nearly 100% if 2.7 kg at birth (Fix et al., 2010). Each additional kilogram of bodyweight at birth results in pigs that are 20 times less likely to result in nursery mortality (Declerck et al., 2016). When assessing lifetime mortality, only 28.4% of pigs with a birth weight of <0.95 kg were found to survive from birth to market compared with 87.1% of pigs ≥0.95 kg at birth (Calderon Diaz et al., 2017). Not all investigations have observed an association between birth weight and postweaning mortality (de Almeida et al., 2014); however, across the body of literature low birth weight has been shown to be highly correlated with both pre- and postweaning mortality.
Dam parity.
Offspring from gilts have been associated with greater preweaning and postweaning mortality (Craig et al., 2017; Pineiro et al., 2019). In contrast, no evidence of a difference in preweaning mortality was observed between first and fourth parity dams using a small sample size (Carney-Hinkle et al., 2013). Additionally, Miller et al. (2013) observed greater preweaning mortality in offspring born to gilts compared with offspring born to sows with no evidence of a difference in postweaning mortality. Multiple studies have evaluated differences in yield and composition of colostrum and milk from gilts and sows (Devillers et al., 2007; Quesnel, 2011; Declerck et al., 2015; Craig et al., 2019) with no clear consensus. Another consideration which may contribute to the observed differences in mortality by parity beyond the differences in maternal growth and development and remains poorly defined includes differences in maternal immune status through natural exposures to infectious agents and controlled acclimation procedures of females entering the breeding herd. Offspring from gilts have been shown to have increased postweaning mortality, although the exact mechanism remains unclear.
Facility Factors
A great deal of variability exists in facility design and utilization. USDA (2015) found that the largest percentage of finishing facilities is total confinement (71.6%), with over 99% of all grower–finisher pigs having no outdoor access. Differences in postweaning mortality have been described based on facility type (wean–finish compared with nursery and finish), providing a realistic illustration that facility type and associated health and management factors can result in differences in mortality (Fangman et al., 2001).
Modern production in the United States uses primarily complete slats, partial slats, or mesh depending on the phase of production with the most common construction materials including concrete and plastic (USDA, 2015). Multiple studies have shown differences in feet lesions between flooring types. Currently, the literature does not indicate that flooring type has a substantial impact on postweaning mortality (Mouttotou et al., 1999; Kilbride et al., 2008; Gillman et al., 2009). Damage and improper maintenance of facilities, such as broken slats, could obviously increase the likelihood of injury and progressive decline leading to culling or euthanasia; however, specific data are unavailable. Little evidence is currently available that flooring type within the scope of commercial production in the United States has a significant impact on postweaning mortality.
Environmental Factors
Regulation of environmental factors involves temperature, humidity, and facility ventilation. Temperature has a significant impact on growth performance, with optimum performance occurring within the animal’s thermoneutral zone. Temperature and humidity have a significant economic impact to postweaning production (St-Pierre et al., 2003). Limited published literature is available providing specific estimates of magnitude on mortality attributable to environment. Appropriate ventilation is necessary to control humidity and temperature and remove noxious gases such as ammonia. The majority of barns are either naturally or mechanically ventilated. In addition to ventilation technique, multiple control methods have historically been used including manual controls and automatic controls. As would be expected, automatic ventilation controls have been associated with decreased mortality compared with manual control (Agostini et al., 2014, 2015), with the greatest magnitude of effect with recently weaned pigs. Additionally, aerosolized particulate matter and ammonia have been shown to impact finishing pig morbidity (Michiels et al., 2015). When ventilation is not properly controlled, morbidity and subsequently mortality increase primarily due to interactions with infectious agents including increased incidence of respiratory disease.
Season
Season has been shown to be strongly correlated with postweaning mortality, with mortality generally decreased for pigs entering growing facilities during the summer months compared with other times of the year (Maes et al., 2001, 2004; Larriestra et al., 2005a; Ramis et al., 2006; Oliveira et al, 2007; 2009; Agostini et al., 2013; 2014; 2015; Hopkins et al., 2018). The primary reason for this is the barn microenvironment that can be difficult to manage with large daily ambient temperature variation (Haeussermann et al., 2006; Van Huffel et al., 2019). Maintaining appropriate microenvironment is important to maintain low morbidity and mortality, thus proper design and utilization of ventilation systems is critical to reduce the magnitude of seasonal mortality differences. Infectious disease incidence also follows a seasonal pattern with increased incidence in cold months of the year as described by Tousignant et al. (2015) with PRRSV, Chen et al. (2019) for TGEV, and Poljak et al. (2014) for swine influenza virus. Photoperiod has been shown not to be a contributing factor to nursery mortality (McGlone et al., 1988). Seasonal variation has been shown to have a significant impact on morbidity and mortality, with possible underlying mechanisms being challenges with maintaining appropriate microenvironment and/or seasonality trends of infectious disease incidence.
Nutrient Inadequacies
Nutritional inadequacies can include inappropriate regulation of antioxidant status is often associated with inadequate levels of vitamin E and/or selenium. Mulberry heart disease and hepatosis dietetica have historically been associated with deficiencies of vitamin E and/or selenium (Van Vleet et al., 1970; Sharp et al., 1972a, b; Mahan et al., 1973; Moir and Masters, 1979; Rice and Kennedy, 1989). Recent investigations have shown the presence of the disorder in the absence of vitamin E/selenium deficiencies indicating a more complex pathogenesis (Pallares et al., 2002; Shen et al., 2011). Interactions between heavy metals (Van Vleet, 1976, 1981) and other pro-oxidant substances including iron, oxygen, ozone, and various drugs (Van Vleet and Ferrans, 1992) potentially contribute to loss of biological antioxidant capacity. Mulberry heart disease and associated diseases can be induced by low levels of dietary vitamin E and/or selenium. In clinical cases, however, there appears to be a complicated interaction with multiple other factors influencing the physiological antioxidant status.
Regulation of Ca and P metabolism is closely controlled by dietary Ca, P, and vitamin D. Imbalance of these nutrients has been associated with fractures (Hejazi and Danyluk, 2009), kyphosis (Rortvedt and Crenshaw, 2012), rickets (Madson et al., 2012), and acute seizures and death in association with hypovitaminosis A (Lakritz et al., 1993). Additionally, vitamin D toxicosis has been reported (Long, 1984; Hulsmann et al., 1991) indicating that oversupply can be detrimental as well. When veterinarians suspect abnormalities in the homeostasis of Ca, P, and vitamin D, dietary and biological samples are often collected and analyzed (Hejazi and Danyluk, 2009; Madson et al., 2012). Diet sampling and analysis is quite variable (Dritz et al., 2019), and animal-level testing has been shown to have limited value (Amundson et al., 2017) making diagnostic workups challenging. Many cases of dietary imbalance leading to physiological changes resulting in mortality are not caused by minor formulation error, but significant oversight in diet formulation or diet manufacturing and in many cases, issues are more likely to manifest as the duration of the imbalance increases. Greater discussion regarding additional nutrients balanced in swine diets and the consequences of inappropriate formulation and intake is described by Dritz et al. (2019). The impact on morbidity and mortality is relatively uncommon, but can have a large magnitude of effect.
Management Factors
Management factors are a broad category that encompass the production decisions that often can be manipulated. One of the largest factors where management contributes to postweaning mortality is through variation within a group of pigs. Variation in preweaning management, such as colostrum intake, weaning age, weaned facility fill length, and source(s) of pigs within the population, increases the likelihood of mortality due to infectious causes. Additionally, the lightest pigs in a group oftentimes lose competitiveness within the population for limited resources, which begins a slow process of decline often ending in culling or euthanasia.
Preweaning management.
Preweaning management factors that can carry over into the postweaning period include cross fostering and colostrum intake. Cross fostering has been associated with increased risk of postweaning mortality compared with nonfostered pigs (Calderon Diaz et al., 2017, 2018). Reasons for this response are likely the fundamental differences in the population of cross-fostered pigs, such as being small in size, as well as abrupt changes in social hierarchy within litter leading to competition for resources. Sufficient colostrum intake has been associated with low postweaning mortality, with the greatest impact on pigs with low birth weights (Decaluwe et al., 2014; Ferrari et al., 2014; Declerck et al., 2016). Management practices including cross-fostering and low levels of colostrum intake in the preweaning period can have a significant impact on postweaning mortality.
Weaning age and weight.
Understanding the variability associated with weaning age within a cohort of weaned pigs is important when considering the impact on postweaning mortality. USDA (2015) reported the average weaning age across all operation sizes was 20.8 days. Multiple investigations have shown that increasing weaning age is associated with reduced mortality (Losinger et al., 1998; Main et al., 2004; Davis et al., 2006; Leliveld et al., 2013; Lopez-Verge, 2019) with every additional day of age from 12 to 21.5 days at weaning was associated with a reduction in wean–finish mortality of 0.47% with improvements in economic outcomes (Main et al., 2005a). Not all investigations have shown statistically significant impact of weaning age on postweaning mortality including Smith et al. (2008). Body weight when entering the grow–finish period has been shown to be inversely related to postweaning mortality (de Grau et al., 2005; Larriestra et al., 2005b; Dewey et al., 2006; Larriestra et al., 2006; Agostini et al., 2014; Declerck et al., 2016; Collins et al., 2017). Weaning age and weight are correlated, and both serve as relative (not absolute) indicators of physiologic maturity. Additional research is needed evaluating the impact of weaning age in both low and high health production systems. Changes in management including older weaning age and implementation of supplemental litter nutrition in late lactation may be considered depending on the health status of the herd with the entirety of the production system in mind.
Barn fill length and sources of pigs.
Fill length can be defined as the duration of time required to bring in all animals intended to be raised in a given airspace such as room within barn or whole barn. All in/all out commercial production has become the predominant production practice with 80 to 90% of nursery, wean–finish, and grow–finish sites using all in/all out practices either by room, building, or site (USDA, 2015). Wu et al. (2019) reported that average fill length was much shorter for finisher sites, with the majority of facilities filling in ≤2 days, whereas nursery fill length was much longer with approximately 80% of batches filled within 20 days. Variation in age and weight have been shown to result in high or variable nursery mortality (Dewey et al., 2006). In addition to all-in/all-out, reducing commingling of pigs from multiple farms or sources with varying health statuses can help reduce disease incidence. Of the sites surveyed by USDA (2015), it is estimated that 81% or greater of all postweaning sites used a single source of pigs when filling. Multiple sources of pigs within a production batch have been shown to be associated with increased mortality (Maes et al., 2004; Agostini et al., 2013, 2014, 2015). Additionally, increases in postweaning mortality have been reported due to pig source characteristics, such as origin farm or source (Tuovinen et al., 1994; Losinger et al., 1998; Maes et al., 2004; Larriestra et al., 2006; Oliveira et al., 2007, 2009; Serrano et al., 2014), larger farm sizes (Oliveira et al., 2007), or the larger number of pigs placed at any given time (Agostini et al., 2013, 2015). Variability associated with barn fill length and pig source can lead to mild-to-moderate morbidity and mortality.
Resource availability.
Space and feed/water access per pig are resources that are limited within groups of pigs. Data generated by USDA (2015) indicate that 44.2% of wean-to-finish sites always or sometimes overstock with the majority at 150% to 200% over stocked. Stocking density was positively associated with mortality in retrospective analyses of wean-to-finish records from a commercial production system (Mehling et al., 2019). A positive correlation in nursery mortality attributable to stocking density was reported by several authors (Table 4; Brumm et al., 1996; Wolter et al., 2003a; b; DeDecker et al., 2005; Stojanac et al., 2014). However, multiple studies have not observed a difference in death loss related to stocking density (Brumm et al., 1996, 2001, 2004; Turner et al, 2000; Wolter et al., 2002; Morrison et al., 2003; Street and Gonyou, 2008; Jensen et al., 2012; Serrano et al., 2013; Callahan et al., 2017; Jang et al., 2017; Wastell et al., 2018). Stocking density has been described to be positively correlated with tail and skin lesions with no evidence of a difference in mortality (Turner et al., 2000). Stocking density has been extensively evaluated regarding production and health outcomes, and results indicate that stocking density may influence postweaning mortality. There is significant variability for this association and it is not uniformly observed.
Table 4.
Effect of stocking density on postweaning mortality
| Weight, kg | |||||||
|---|---|---|---|---|---|---|---|
| Article | Outcome measured | Start | End | No. of stocking densities | High stocking density, m2 | Low stocking density, m2 | Statistical interpretation1 |
| Brumm et al. (1996) experiment 1 | Dead/ removed | 21 | 111 | 2 | 0.56 | 0.78 | + |
| Brumm et al. (1996) experiment 2 | Dead/ removed | 23 | 107 | 2 | 0.56 | 0.78 | NS |
| Brumm et al. (1996) experiment 3 | Dead/ removed | 21 | 106 | 2 | 0.56 | 0.78 | NS |
| Turner et al. (2000) | Removed | 30 | 60 | 2 | 50 kg/m2 | 32 kg/m2 | NS |
| Brumm et al. (2001) 2 | Dead/ removed | 7 | 20 | 2 | 0.16 | 0.25 | NS |
| Brumm et al. (2001) 3 | Dead/ removed | 20 | 110 | 2 | 0.56 | 0.78 | NS |
| Brumm et al. (2001) 4 | Dead/ removed | 5 | 22 | 2 | 0.16 | 0.23 | NS |
| Brumm et al. (2001) 5 | Dead/ removed | 22 | 110 | 2 | 0.60 | 0.74 | NS |
| Wolter et al. (2002) Study 1 | Removed | 6 | 41 | 2 | 0.33 | 0.65 | NS |
| Wolter et al. (2002) Study 2 | Removed | 5 | 43 | 2 | 0.33 | 0.65 | NS |
| Hamilton et al. (2003) | Mortality, morbidity | 41 | 120 | 2 | 0.37 m2 to 80 kg, 0.56 m2 after | 0.93 | ~ |
| Morrison et al. (2003) | Mortality | 24 | 104 | 2 | 0.45 m2 wk. 10 to 13, 0.55 m2 wk. 14 to 16, 0.74 m2 wk. 17 to 23 | 0.88 | NS |
| Wolter et al. (2003a) | Removed | 6 | 57 | 2 | 0.32 | 0.63 | + |
| Wolter et al. (2003b) 6 | Removed | 5 | 28 | 2 | 0.21 | 0.63 | + |
| Brumm (2004) experiment 1 | Dead/ removed | 23 | 115 | 2 | 0.58 barrows, 0.65 gilts | 0.65 barrows, 0.74 gilts | NS |
| Brumm (2004) experiment 2 | Dead/ removed | 5 | 124 | 2 | 0.58 | 0.74 | NS |
| DeDecker et al. (2005) | Combined morbidity/ mortality | 5 | 117 | 3 | 0.55 | 0.80 | + |
| Street and Gonyou (2008) | Injury scores, Removed, medicated | 37 | 94 | 2 | 0.52 | 0.78 | NS |
| Jensen et al. (2012) | Mortality | 31 | 91 | 3 | 0.67 | 0.79 | NS |
| Serrano et al. (2013) | Mortality | 19 | 110 | 2 | 0.76 | 0.84 | NS |
| Stojanac et al. (2014) | Mortality | 7 | 22 | 3 | 0.16 | 0.32 | + |
| Jang et al. (2017) | Mortality | 30 | 98 | 3 | 0.69 | 0.96 | N/A |
| Johnston et al. (2017) | Dead, removed, treated, lameness, lesions | 27 | 139 | 5 | 0.71 | 1.07 | ~ |
| Callahan et al. (2017) | Mortality | 6 | 26 | 2 | 0.15 | 0.27 | NS |
| Wastell et al. (2018) | Dead/ removed | 28 | 129 | 2 | 0.65 | 0.78 | NS |
| Mehling et al. (2019) | Mortality | 7 | 115 | 3 | 0.62 | 0.69 | + |
1 P < 0.05 represented by +; 0.05 < P ≤ 0.10 represented by ~; P > 0.10 represented by NS; N/A indicates statistical analysis was not provided in publication.
2 Nursery portion of experiment 1 as described by Brumm et al. (2001).
3 Grow-finish portion of experiment 1 as described by Brumm et al. (2001).
4 Nursery portion of experiment 2 as described by Brumm et al. (2001).
5 Grow-finish portion of experiment 2 as described by Brumm et al. (2001).
6 Study was designed with an early wean-finish (5 to 28 kg BW) and late wean-finish (28 to 114 kg BW) periods. Space restriction (0.21 m2) was applied during the first 8 weeks to one group, and both groups had 0.63 m2 from week 9 until marketing.
Feeder access (represented as feeder spaces or linear feeder space per pig) is fundamentally utilization of restricted resources similar to stocking density. Feeder access or location has shown no impact on postweaning mortality (Wolter et al., 2000; 2003b; Morrison et al., 2003; Weber et al., 2015; Wastell et al., 2018; Laskoski et al., 2019). Additionally, number of pigs per water drinker has not been shown to influence mortality (Turner et al., 1999). Design of feeder and waterer used can contribute to overall cleanliness. If feeders and water bowls are not managed properly, the buildup of feces, urine, and other debris may result in reduced feed intake as well as result in increased morbidity. Experimentally, no evidence of a difference in number of pigs dead or removed has been observed between dry and wet/dry feeders, between swing and nipple-style drinkers, or between swing and bowl drinkers (Brumm et al., 2000). Little evidence indicating interactions between feeder/waterer access and stocking density is available in published literature. Neither feeder/waterer access nor design has been shown to be a significant factor contributing to postweaning mortality.
Number of animals per pen.
The number of pigs per pen is important for social hierarchy structure and independent of resource access. Numerous investigations have been performed assessing the impact of postweaning group size on mortality. Olsen et al. (2018) reported that pigs housed in groups of 31 pigs/pen had a greater proportion removed from the experiment compared with 11 pigs/pen with similar stocking densities and feeder access per pig. The majority of these studies, however, did not observe any differences in mortality among different number of pigs per pen (McGlone and Newby, 1994; Spoolder et al., 1999; Turner et al., 1999, 2000; Wolter et al., 2001; Schmolke et al., 2003; Samarakone and Gonyou, 2008; Callahan et al., 2017; Laskoski et al., 2019). In general, it appears that group size has not been shown to impact postweaning mortality.
Transportation.
Ritter et al. (2009) summarized transport losses in market pigs, and estimated that the yearly incidence of pigs dead on arrival (DOA) at USDA-inspected packing facilities in 2006 was 0.22%. In the current review, DOA ranged from 0.01 to 0.25% in reports investigating transportation factors associated with increased DOA rates of both wean/feeder pigs and market pigs (Gosalvez et al., 2006, Gade et al., 2007; Werner et al., 2007; Averos et al., 2008; Fitzgerald et al., 2009; Sutherland et al., 2009; Averos et al., 2010; Haley et al., 2010; Kephart et al., 2010; Vitali et al., 2014; Zhao et al., 2016; Harmon et al., 2017; Voslarova et al., 2017; Dalla Costa et al., 2019; Table 5). Increased animal density during transportation and transport duration has been shown to increase transport-related death loss (Ritter et al., 2006, 2007). Similar observations were not seen by Pilcher et al. (2011). Ritter et al. (2008) observed no evidence of a difference in DOA at the packing plant when market pigs were transported at different times of the year or in different trailer designs. Additionally, no evidence of a difference in market pig DOA was observed when pigs were walked a short distance during the loading process compared with long distance (Ritter et al., 2007). Transportation mortality is a contributor to postweaning mortality with a relatively low magnitude of effect.
Table 5.
Summary of explanatory variables associated with transportation mortality (dead on arrival; DOA) as identified in retrospective analysis of transportation records
| Article | Country | Number of pigs | Weight, kg | Mortality, % | Significant factors (P ≤ 0.05) |
|---|---|---|---|---|---|
| Wean/feeder pig transport | |||||
| Averos et al. (2010) | EU | 58,682 | N/A | 0.07 | Temperature × transport time |
| Fasting time | |||||
| Water availability | |||||
| Ventilation type | |||||
| Zhao et al. (2016) | USA | 13,344 records | N/A | 0.033 for weaned pigs, 0.024 for feeder pigs | Pig type (weaned or feeder pig) |
| Temperature | |||||
| Travel distance | |||||
| Type × temperature | |||||
| Temperature × distance | |||||
| Type × distance | |||||
| Type × temperature × distance | |||||
| Harmon et al. (2017) | USA | 79,715 | 7 | 0.035 | Temperature, quadratic form |
| Temperature × density × compartment | |||||
| Density × compartment | |||||
| Temperature × compartment | |||||
| Compartment | |||||
| Market pig transport | |||||
| Gosalvez et al. (2006) | Spain | 90,366 | 100 | N/A | Distance × number of farms |
| Distance | |||||
| Number of farms | |||||
| Gade et al. (2007) | Denmark | 17,882,622 | N/A | 0.012 | Season |
| Abattoir | |||||
| Producer | |||||
| Hauler | |||||
| Transport distance | |||||
| Werner et al. (2007) | Germany | 7,194,120 | 100 to 110 | 0.187 | Year |
| Season | |||||
| Transport duration | |||||
| Averos et al. (2008) | EU | 112,842 | N/A | 0.107 | Injuries recorded prior to transport |
| Transport time | |||||
| Temperature | |||||
| Barn fill length | |||||
| Fasting × transport time | |||||
| Fitzgerald et al. (2009) 1 | USA | 2,053,945 | 118 | 0.25 | Week |
| Driver | |||||
| Farm | |||||
| Load crew | |||||
| Sort of barn | |||||
| Load type | |||||
| Receiving crew | |||||
| Trailer density | |||||
| Temperature-humidity index, linear | |||||
| Temperature-humidity index, quadratic | |||||
| Wind speed | |||||
| Barn fill length | |||||
| Wait time | |||||
| Sutherland et al. (2009) | USA | 2,730,754 | 115 to 135 | 0.19 | Waiting time |
| Bedding type | |||||
| Sex | |||||
| Haley et al. (2010) | Canada | 728,087 | N/A | 0.219 | Temperature |
| Humidity | |||||
| Trailer density | |||||
| Kephart et al. (2010) | USA | 41,744 | N/A | 0.06 | No factors identified |
| Vitali et al. (2014) | Italy | 3,676,153 | 160 | 0.032 | Season |
| Transport time | |||||
| Voslarova et al. (2017) | Czech Republic | 16,470,730 | N/A | 0.069 | Year |
| Transport distance | |||||
| Season | |||||
| Ambient temperature | |||||
| Dalla Costa et al. (2019) | Brazil | 37,962 | 119 | 0.08 | Previous health problems |
| Loading ramp slope | |||||
| Transport time |
N/A = information not provided.
1 Model based on outcome of total losses including fatigued, injured, and dead pigs during transport.
Sanitation.
Sanitation practices, such as cleaning and disinfection, is an effective method to reduce environmental pathogen load of both animal housing facilities and other fomites including trailers used for animal transportation. Sanitation also is a critical component of biosecurity: both internal biosecurity aimed to prevent pathogen spread within a farm and external biosecurity which prevents entry of pathogens onto a farm. Certain areas have been shown to remain contaminated following cleaning and disinfection including feeders and drinkers (Mannion et al., 2007) and rough surfaces (Madec et al., 1999). Although time between groups of animals in addition to cleaning and disinfection is a common sanitation approach, Luyckx et al. (2016) observed that a period of 10 days following cleaning and disinfection of a nursery facility does not reduce bacterial loads. Sanitation is also critical for fomites involved with animal transportation such as trailers. Animal transportation has been associated with spread of Brachyspira hyodysenteriae and porcine epidemic diarrhea virus through ineffective trailer cleaning and disinfection procedures (Lowe et al., 2014; Boniotti et al., 2018; Giacomini et al., 2018). Thus, proper removal of organic material and disinfection is critical to reduce environmental pathogen load of both animal housing facilities but also transportation equipment. It is known that poor sanitary conditions can lead to increased morbidity through interactions with other factors. A standalone estimate of magnitude of effect is not currently available.
CONCLUSION
Postweaning mortality is extremely complex in nature and affected by numerous non-infectious and infectious causes. Important non-infectious causes that have been identified through review of literature and can be influenced with intervention strategies include preweaning management, weaning age and weight, effective control of microenvironment, and reducing likelihood of feed formulation and manufacturing errors. Many interactive effects are present between and among infectious and non-infectious causes, but an important trend is the recognition of the impact that non-infectious causes have on the incidence, severity, and resolution of disease. Thus strategies to reduce postweaning mortality must consider the dynamic, complex state that forms the causal web. Reduction of postweaning mortality can be accomplished through identification of contributing factors and rigorous scientific evaluation of reduction strategies. Understanding the factors influencing postweaning mortality and intervention strategies remains an area of opportunity for growth in the swine industry.
ACKNOWLEDGMENTS
This article is a contribution no. 20-213-J from the Kansas Agric. Exp. Stn., Manhattan, KS 66506. Appreciation is expressed to Dr Kent Schwartz for thoughtful input and content expertise. Funding, wholly or in part, was provided by The National Pork Board as project no. 18-147 and The Foundation for Food & Agriculture Research.
Conflict of interest statement. None declared.
LITERATURE CITED
- Agostini P. S., Fahey A. G., Manzanilla E. G., O’Doherty J. V., de Blas C., and Gasa J... 2014. Management factors affecting mortality, feed intake and feed conversion ratio of grow-finishing pigs. Animal. 8:1312–1318. doi: 10.1017/S1751731113001912 [DOI] [PubMed] [Google Scholar]
- Agostini P. S., Gasa J., Manzanilla E. G., Da Silva C. A., and de Blas C... 2013. Descriptive study of production factors affecting performance traits in growing-finishing pigs in Spain. Span. J. Agric. Res. 11:371–381. doi: 10.5424/sjar/2013112-3011 [DOI] [Google Scholar]
- Agostini P. S., Manzanilla E. G., Blas C., Fahey A. G., Silva C. A., and Gasa J... 2015. Managing variability indecision making in swine growing-finishing units. Ir. Vet. J. 68:20. doi: 10.1186/s13620-015-0048-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Almeida M., Bernardi M. L., Motta A. P., Bortolozzo F. P., and Wentz I... 2014. Effect of birth weight and litter size on the performance of landrace gilts until puberty. Acta. Sci. Vet. 42:1–8. [Google Scholar]
- Alonso-Spilsbury M., Ramirez-Necoechea R., Gonzalez-Lozano M., Mota-Rojas D., and Trujillo-Ortega M. E... 2007. Piglet survival in early lactation: A review. J. Anim. Vet. Adv. 6:76–86. [Google Scholar]
- Amundson L. A., Hernandez L. L., and Crenshaw T. D... 2017. Serum and tissue 25-OH vitamin D3 concentrations do not predict bone abnormalities and molecular markers of vitamin D metabolism in the hypovitaminosis D kyphotic pig model. Br. J. Nutr. 118:30–40. doi: 10.1017/S0007114517001751 [DOI] [PubMed] [Google Scholar]
- Anderson D. E., and Mulon P. Y... 2019. Anesthesia and surgical procedures in swine. In: Zimmerman J. J., Karriker L. A., Ramirez A., Schwartz K. J., and Stevenson G. W., and Zhang J.. Diseases of swine, 11th ed. Ames, IA: John Wiley & Sons. [Google Scholar]
- Armien A. G., Peixoto P. V., Dobereiner J., and Tokarnia C. H... 1997. Outbreak of narasin toxicity in swine. Pesq. Vet. Bras. 17:63–68. doi: 10.1590/S0100-736X1997000200004 [DOI] [Google Scholar]
- Averós X., Knowles T. G., Brown S. N., Warriss P. D., and Gosálvez L. F... 2010. Factors affecting the mortality of weaned piglets during commercial transport between farms. Vet. Rec. 167:815–819. doi: 10.1136/vr.c6226 [DOI] [PubMed] [Google Scholar]
- Averós X., Knowles T. G., Brown S. N., Warriss P. D., and Gosálvez L. F... 2008. Factors affecting the mortality of pigs being transported to slaughter. Vet. Rec. 163:386–390. doi: 10.1136/vr.163.13.386 [DOI] [PubMed] [Google Scholar]
- van den Berg A., Brülisauer F., and Regula G... 2005. [Prevalence of gastric lesions in the pars proventricularis in finishing pigs at slaughter in Switzerland]. Schweiz. Arch. Tierheilkd. 147:297–303. doi: 10.1024/0036-7281.147.7.297 [DOI] [PubMed] [Google Scholar]
- Boniotti M. B., Papetti A., Bertasio C., Giacomini E., Lazzaro M., Cerioli M., Faccini S., Bonilauri P., Vezzoli F., Lavazza A., and Alborali G. L... 2018. Porcine epidemic diarrhea virus in Italy: disease spread and the role of transportation. Transbound. Emerg. Dis. 65:1935–1942. doi: 10.1111/tbed.12974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brito L. A., Matos M. P., Sobestiansky J., Sucupira M. C., and Ortolani E. L... 2001. Accumulative sodium poisoning in Brazilian swine fed whey. Vet. Hum. Toxicol. 43:88–90. [PubMed] [Google Scholar]
- Brumm M. C. 2004. The effect of space allocation on barrow and gilt performance. J. Anim. Sci. 82:2460–2466. doi: 10.2527/2004.8282460x [DOI] [PubMed] [Google Scholar]
- Brumm M. C., Dahlquist J. M., and Heemstra J. M... 2000. Impact of feeders and drinker devices on pig performance, water use, and manure volume. J. Swine Health Prod. 8:51–57. [Google Scholar]
- Brumm M. C., Ellis M., Johnston L. J., Rozeboom D. W., and Zimmerman D. R.; NCR-89 Committee on Swine Management 2001. Interaction of swine nursery and grow-finish space allocations on performance. J. Anim. Sci. 79:1967–1972. doi: 10.2527/2001.7981967x [DOI] [PubMed] [Google Scholar]
- Brumm M. C., and Miller P. S... 1996. Response of pigs to space allocation and diets varying in nutrient density. J. Anim. Sci. 74:2730–2737. doi: 10.2527/1996.74112730x [DOI] [PubMed] [Google Scholar]
- Cagienard A., Regula G., and Danuser J... 2005. The impact of different housing systems on health and welfare of grower and finisher pigs in Switzerland. Prev. Vet. Med. 68:49–61. doi: 10.1016/j.prevetmed.2005.01.004 [DOI] [PubMed] [Google Scholar]
- Calderón Díaz J. A., Boyle L. A., Diana A., Leonard F. C., Moriarty J. P., McElroy M. C., McGettrick S., Kelliher D., and García Manzanilla E... 2017. Early life indicators predict mortality, illness, reduced welfare and carcass characteristics in finisher pigs. Prev. Vet. Med. 146:94–102. doi: 10.1016/j.prevetmed.2017.07.018 [DOI] [PubMed] [Google Scholar]
- Calderon Diaz J. A., Manzanilla E. G., Diana A., and Boyle L. A... 2018. Cross-fostering implications for pig mortality, welfare, and performance. Front. Vet. Sci. 5:123. doi: 10.3389/fvets.2018.00123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan S. R., Cross A. J., DeDecker A. E., Lindemann M. D., and Estienne M. J... 2017. Effects of group-size-floor space allowance during the nursery phase of production on growth, physiology, and hematology in replacement gilts. J. Anim. Sci. 95:201–211. doi: 10.2527/jas.2016.0842 [DOI] [PubMed] [Google Scholar]
- Canning P., Costello N., Mahan-Riggs E., Schwartz K. J., Skoland K., Crim B., Ramirez A., Linhares D., Gauger P., and Karriker L... 2019. Retrospective study of lameness cases in growing pigs associated with joint and leg submissions to a veterinary diagnostic laboratory. J. Swine Health Prod. 27:118–124. [Google Scholar]
- Cappai M. G., Picciau M., and Pinna W... 2013. Ulcerogenic risk assessment of diets for pigs in relation to gastric lesion prevalence. BMC Vet. Res. 9:36. doi: 10.1186/1746-6148-9-36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F., Knuston T. P., Rossow S., Saif L. J., and Marthaler D. G... 2019. Decline of transmissible gastroenteritis virus and its complex evolutionary relationship with porcine respiratory coronavirus in the United States. Sci. Rep. 9:3953. doi: 10.1038/s41598-019-40564-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney-Hinkle E. E., Tran H., Bundy J. W., Moreno R., Miller P. S., and Burkey T. E... 2013. Effect of dam parity on litter performance, transfer of passive immunity, and progeny microbial ecology. J. Anim. Sci. 91:2885–2893. doi: 10.2527/jas.2011-4874 [DOI] [PubMed] [Google Scholar]
- Carpenter J. A., Charbonneau G., and Josephson G... 2005. Tiamulin and narasin toxicosis in nursery pigs. J. Swine Health Prod. 13:333–336. [Google Scholar]
- Classen D. M., Schwartz K. J., Madson D., and Ensley S. M... 2017. Microcystin toxicosis in nursery pigs. J. Swine Health Prod. 25:198–205. [Google Scholar]
- Colvin B. M., Cooley A. J., and Beaver R. W... 1993. Fumonisin toxicosis in swine: clinical and pathologic findings. J. Vet. Diagn. Invest. 5:232–241. doi: 10.1177/104063879300500215 [DOI] [PubMed] [Google Scholar]
- Cook W. O., Van Alstine W. G., and Osweiler G. D... 1989. Aflatoxicosis in Iowa swine: eight cases (1983-1985). J. Am. Vet. Med. Assoc. 194:554–558. [PubMed] [Google Scholar]
- Craig J. R., Collins C. L., Bunter K. L., Cottrell J. J., Dunshea F. R., and Pluske J. R... 2017. Poorer lifetime growth performance of gilt progeny compared with sow progeny is largely due to weight differences at birth and reduced growth in the preweaning period, and is not improved by progeny segregation after weaning. J. Anim. Sci. 95:4904–4916. doi: 10.2527/jas2017.1868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig J. R., Dunshea F. R., Cottrell J. J., Wijesiriwardana U. A., and Pluske J. R... 2019. Primiparous and multiparous sows have largely similar colostrum and milk composition profiles throughout lactation. Animals. 9:35. doi: 10.3390/ani9020035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalla Costa O. A., Dalla Costa F. A., Feddern V., Lopes L. D. S., Coldebella A., Gregory N. G., and de Lima G. J. M. M... 2019. Risk factors associated with pig pre-slaughtering losses. Meat Sci. 155:61–68. doi: 10.1016/j.meatsci.2019.04.020 [DOI] [PubMed] [Google Scholar]
- Davis M. E., Sears S. C., Apple J. K., Maxwell C. V., and Johnson Z. B... 2006. Effect of weaning age and commingling after the nursery phase of pigs in a wean-to-finish facility on growth, and humoral and behavioral indicators of well-being. J. Anim. Sci. 84:743–756. doi: 10.2527/2006.843743x [DOI] [PubMed] [Google Scholar]
- Decaluwe R., Maes D., Wuyts B., Cools A., Piepers S., and Janssens G. P. J... 2014. Piglets’ colostrum intake associates with daily weight gain and survival until weaning. Livest. Sci. 162:185–192. doi: 10.1016/j.livsci.2014.01.024 [DOI] [Google Scholar]
- Declerck I., Dewulf J., Piepers S., Decaluwé R., and Maes D... 2015. Sow and litter factors influencing colostrum yield and nutritional composition. J. Anim. Sci. 93:1309–1317. doi: 10.2527/jas.2014-8282 [DOI] [PubMed] [Google Scholar]
- Declerck I., Dewulf J., Sarrazin S., and Maes D... 2016. Long-term effects of colostrum intake in piglet mortality and performance. J. Anim. Sci. 94:1633–1643. doi: 10.2527/jas2015-9564 [DOI] [PubMed] [Google Scholar]
- DeDecker J. M., Ellis M., Wolter B. F., Corrigan B. P., Curtis S. E., and Hollis G. R... 2005. Effect of stocking rate on pig performance in a wean-to-finish production system. Can. J. Anim. Sci. 85:1–5. doi: 10.4141/A04-042 [DOI] [Google Scholar]
- De Jong J. A., DeRouchey J. M., Tokach M. D., Dritz S. S., Goodband R. D., Woodworth J. C., and Allerson M. W... 2016. Evaluating pellet and meal feeding regimens on finishing pig performance, stomach morphology, and carcass characteristics. J. Anim. Sci. 94:4781–4788. doi: 10.2527/jas.2016-0461 [DOI] [PubMed] [Google Scholar]
- Devillers N., Farmer C., Le Dividich J., and Prunier A... 2007. Variability of colostrum yield and colostrum intake in pigs. Animal. 1:1033–1041. doi: 10.1017/S175173110700016X [DOI] [PubMed] [Google Scholar]
- Dewey C. E., Johnston W. T., Gould L., and Whiting T. L... 2006. Postweaning mortality in Manitoba swine. Can. J. Vet. Res. 70:161–167. [PMC free article] [PubMed] [Google Scholar]
- De Witte C., Ducatelle R., and Haesebrouck F... 2018. The role of infectious agents in the development of porcine gastric ulceration. Vet. J. 236:56–61. doi: 10.1016/j.tvjl.2018.04.015 [DOI] [PubMed] [Google Scholar]
- Dilov P., Dimitrov S., Dzhurov A., Nikolov A., and Panchev I... 1981. Toxicity of sodium monensin for pigs. Vet. Med. Nauki. 18:55–63. [PubMed] [Google Scholar]
- Ding N. S., Mao H. R., Guo Y. M., Ren J., Xiao S. J., Wu G. Z., Shen H. Q., Wu L. H., Ruan G. F., Brenig B.,. et al. 2009. A genome-wide scan reveals candidate susceptibility loci for pig hernias in an intercross between White Duroc and Erhualian. J. Anim. Sci. 87:2469–2474. doi: 10.2527/jas.2008-1601 [DOI] [PubMed] [Google Scholar]
- Dohoo I., Martin W., and Stryhn H... 2003. Veterinary epidemiologic research. P.E.I., Canada: AVC Inc. Charlottetown. [Google Scholar]
- Doster A. R. 2000. Porcine gastric ulcer. Vet. Clin. North Am. Food Anim. Pract. 16:163–174. doi: 10.1016/s0749-0720(15)30141-9 [DOI] [PubMed] [Google Scholar]
- Dritz S. S., Goodband R. D., DeRouchey J. M., Tokach M. D., and Woodworth J. C... 2019. Nutrient deficiencies and excesses. In: Zimmerman J. J., Karriker L. A., Ramirez A., Schwartz K. J., and Stevenson G. W., and Zhang J.. Diseases of swine, 11th ed. Ames, IA: John Wiley & Sons. [Google Scholar]
- Dufrasne M., Misztal I., Tsuruta S., Gengler N., and Gray K. A... 2014. Genetic analysis of pig survival up to commercial weight in a crossbred population. Livest. Sci. 167:19–24. doi: 10.1016/j.livsci.2014.05.001 [DOI] [Google Scholar]
- Dufrasne M., Misztal I., Tsuruta S., Holl J., Gray K. A., and Gengler N... 2013. Estimation of genetic parameters for birth weight, preweaning mortality, and hot carcass weight of crossbred pigs. J. Anim. Sci. 91:5565–5571. doi: 10.2527/jas.2013-6684 [DOI] [PubMed] [Google Scholar]
- Ensley S. M., and Radke S. L... 2019. Toxic minerals, chemicals, plants, and gases. In: Zimmerman J. J., Karriker L. A., Ramirez A., Schwartz K. J., and Stevenson G. W., and Zhang J.. Diseases of swine, 11th ed. Ames, IA: John Wiley & Sons. [Google Scholar]
- Fangman T. J., Hardin L. E., Grellner G., Carlson M. S., Zulovich J. M., and Coleman J. L... 2001. Performance and disease status of pigs grown in a wean-to-finish facility compared to pigs grown in a conventional nursery and grower-finisher facility. J. Swine Health Prod. 9:71–76. [Google Scholar]
- FDA, Department of Health and Human Services 2018. Specific new animal drugs for use in animal feeds. 21 C.F.R. Sec. 558.363. Fed. Regist. 51:29098. [Google Scholar]
- Ferrari C. V., Sbardella P. E., Bernardi M. L., Coutinho M. L., Vaz I. S. Jr, Wentz I., and Bortolozzo F. P... 2014. Effect of birth weight and colostrum intake on mortality and performance of piglets after cross-fostering in sows of different parities. Prev. Vet. Med. 114:259–266. doi: 10.1016/j.prevetmed.2014.02.013 [DOI] [PubMed] [Google Scholar]
- Fitzgerald R. F., Stalder K. J., Matthews J. O., Schultz Kaster C. M., and Johnson A. K... 2009. Factors associated with fatigued, injured, and dead pig frequency during transport and lairage at a commercial abattoir. J. Anim. Sci. 87:1156–1166. doi: 10.2527/jas.2008-1270 [DOI] [PubMed] [Google Scholar]
- Fix J. S., Cassady J. P., Holl J. W., Herring W. O., Culbertson M. S., and See M. T... 2010. Effect of piglet birth weight on survival and quality of commercial market swine. Livest. Sci. 132:98–106. doi: 10.1016/j.livsci.2010.05.007 [DOI] [Google Scholar]
- Gade B. P., Christensen L., Baltzer M., and Petersen J. V... 2007. Causes of pre-slaughter mortality in Danish slaughter pigs with special emphasis on transport. Anim. Welf. 16:459–470. [Google Scholar]
- Garden S. 1988. Rectal prolapse in pigs. Vet. Rec. 123:654. [PubMed] [Google Scholar]
- Gardner I. A., Hird D. W., Franti C. E., and Glenn J... 1988. Patterns and determinants of rectal prolapse in a herd of pigs. Vet. Rec. 123:222–225. doi: 10.1136/vr.123.9.222 [DOI] [PubMed] [Google Scholar]
- Giacomini E., Gasparrini S., Lazzaro M., Scali F., Boniotti M. B., Corradi A., Pasquali P., and Alborali G. L... 2018. The role of transportation in the spread of Brachyspira hyodysenteriae in fattening farms. BMC Vet. Res. 14:10. doi: 10.1186/s12917-017-1328-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillman C. E., KilBride A. L., Ossent P., and Green L. E... 2009. A cross-sectional study of the prevalence of foot lesions in post-weaning pigs and risks associated with floor type on commercial farms in England. Prev. Vet. Med. 91:146–152. doi: 10.1016/j.prevetmed.2009.05.023 [DOI] [PubMed] [Google Scholar]
- Gomes-Neto J. C., Raymond M., Bower L., Ramirez A., Madson D. M., Strait E. L., Rosey E. L., and Rapp-Gabrielson V. J... 2016. Two clinical isolates of Mycoplasma hyosynoviae showed differing pattern of lameness and pathogen detection in experimentally challenged pigs. J. Vet. Sci. 17:489–496. doi: 10.4142/jvs.2016.17.4.489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosálvez L. F., Averós X., Valdelvira J. J., and Herranz A... 2006. Influence of season, distance and mixed loads on the physical and carcass integrity of pigs transported to slaughter. Meat Sci. 73:553–558. doi: 10.1016/j.meatsci.2006.02.007 [DOI] [PubMed] [Google Scholar]
- Gottardo F., Scollo A., Contiero B., Bottacini M., Mazzoni C., and Edwards S. A... 2017. Prevalence and risk factors for gastric ulceration in pigs slaughtered at 170 kg. Animal. 11:2010–2018. doi: 10.1017/S1751731117000799 [DOI] [PubMed] [Google Scholar]
- de Grau A., Dewey C., Friendship R., and de Lange K... 2005. Observational study of factors associated with nursery pig performance. Can. J. Vet. Res. 69:241–245. [PMC free article] [PubMed] [Google Scholar]
- Grindflek E. M. M., Taubert H., Simianer H., Lien S., and Moen T... 2006. Genome-wide linkage analysis of inguinal hernia in pigs using affected sib pairs. BMC Genet. 7:25. doi: 10.1186/1471-2156-7-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosse Liesner V., Taube V., Leonhard-Marek S., Beineke A., and Kamphues J... 2009. Integrity of gastric mucosa in reared piglets-effects of physical form of diets (meal/pellets), pre-processing grinding (coarse/fine) and addition of lignocellulose (0/2.5%). J. Anim. Physiol. Anim. Nutr. (Berl.). 93:373–380. doi: 10.1111/j.1439-0396.2008.00871.x [DOI] [PubMed] [Google Scholar]
- Guay K., Salgado G., Thompson G., Backus B., Sapkota A., Chaya W., and McGlone J. J... 2013. Behavior and handling of physically and immunologically castrated market pigs on farm and going to market. J. Anim. Sci. 91:5410–5417. doi: 10.2527/jas.2012-5726 [DOI] [PubMed] [Google Scholar]
- Haeussermann A., Hartung E., Gallmann E., and Jungbluth T... 2006. Influence of season, ventilation strategy, and slurry removal on methane emissions from pig houses. Agric. Ecosyst. Environ. 112:115–121. doi: 10.1016/j.agee.2005.08.011 [DOI] [Google Scholar]
- Haley C., Dewey C. E., Widowski T., and Friendship R... 2010. Relationship between estimated finishing-pig space allowance and in-transit loss in a retrospective survey of 3 packing plants in Ontario in 2003. Can. J. Vet. Res. 74:178–184. [PMC free article] [PubMed] [Google Scholar]
- Hamilton D. N., Ellis M., Wolter B. F., Schinckel A. P., and Wilson E. R... 2003. The growth performance of the progeny of two swine sire lines reared under different floor space allowances. J. Anim. Sci. 81:1126–1135. doi: 10.2527/2003.8151126x [DOI] [PubMed] [Google Scholar]
- Harmon J. D., Hoff S. J., Baas T. J., Zhao Y., Xin H., and Follett L. R... 2017. Evaluation of conditions during weaned pig transport. Appl. Eng. Agric. 33:901–912. doi: 10.13031/aea.12367 [DOI] [Google Scholar]
- Harrison L. R., Colvin B. M., Greene J. T., Newman L. E., and Cole J. R. Jr. 1990. Pulmonary edema and hydrothorax in swine produced by fumonisin B1, a toxic metabolite of Fusarium moniliforme. J. Vet. Diagn. Invest. 2:217–221. doi: 10.1177/104063879000200312 [DOI] [PubMed] [Google Scholar]
- Harvey R. B., Huff W. E., Kubena L. F., and Phillips T. D... 1989. Evaluation of diets contaminated with aflatoxin and ochratoxin fed to growing pigs. Am. J. Vet. Res. 50:1400–1405. [PubMed] [Google Scholar]
- Haschek W. M., Motelin G., Ness D. K., Harlin K. S., Hall W. F., Vesonder R. F., Peterson R. E., and Beasley V. R... 1992. Characterization of fumonisin toxicity in orally and intravenously dosed swine. Mycopathologia 117:83–96. doi: 10.1007/BF00497283 [DOI] [PubMed] [Google Scholar]
- Hayes A. W., King R. E., Unger P. D., Phillips T. D., Hatkin J., and Bowen J. H... 1978. Aflatoxicosis in swine. J. Am. Vet. Med. Assoc. 172:1295–1297. [PubMed] [Google Scholar]
- Healy B. J., Hancock J. D., Kennedy G. A., Bramel-Cox P. J., Behnke K. C., and Hines R. H... 1994. Optimum particle size of corn and hard and soft sorghum for nursery pigs. J. Anim. Sci. 72:2227–2236. doi: 10.2527/1994.7292227x [DOI] [PubMed] [Google Scholar]
- Hedde R. D., Lindsey T. O., Parish R. C., Daniels H. D., Morgenthien E. A., and Lewis H. B... 1985. Effect of diet particle size and feeding of H2-receptor antagonists on gastric ulcers in swine. J. Anim. Sci. 61:179–186. doi: 10.2527/jas1985.611179x [DOI] [PubMed] [Google Scholar]
- Hejazi R., and Danyluk A. J... 2009. Epidemiological investigation of femoral fractures in market pigs and the associated economic implications. Can. Vet. J. 50:516–518. [PMC free article] [PubMed] [Google Scholar]
- Hill B. D., Corney B. G., and Wagner T. M... 1996. Importance of Staphylococcus hyicus ssp. hyicus as a cause of arthritis in pigs up to 12 weeks of age. Aust. Vet. J. 73:179–181. doi: 10.1111/j.1751-0813.1996.tb10022.x [DOI] [PubMed] [Google Scholar]
- Hopkins D., Poljak Z., Farzan A., and Friendship R... 2018. Factors contributing to mortality during a Streptoccocus suis outbreak in nursery pigs. Can. Vet. J. 59:623–630. [PMC free article] [PubMed] [Google Scholar]
- Hulsmann H. G., Stockhofe-Zurwieden N., Ganter M., and Muller E... 1991. Clinical findings in vitamin D poisoning in swine. Tierarztl. Prax. 19:488–492. [PubMed] [Google Scholar]
- Ibáñez-Escriche N., Varona L., Casellas J., Quintanilla R., and Noguera J. L... 2009. Bayesian threshold analysis of direct and maternal genetic parameters for piglet mortality at farrowing in Large White, Landrace, and Pietrain populations. J. Anim. Sci. 87:80–87. doi: 10.2527/jas.2007-0670 [DOI] [PubMed] [Google Scholar]
- Iotsev M. 1985. Tolerance, toxicity and residues of Pharmachim’s sodium monensin in pigs. Vet. Med. Nauki. 22:98–104. [PubMed] [Google Scholar]
- Jang J. C., Jin X. H., Hong J. S., and Kim Y. Y... 2017. Effects of different space allowances on growth performance, blood profile and pork quality in a grow-to-finish production system. Asian-Australas. J. Anim. Sci. 30:1796–1802. doi: 10.5713/ajas.17.0076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen T., Nielsen C. K., Vinther J., D’Eath R. B... 2012. The effect of space allowance for finishing pigs on productivity and pen hygiene. Livest. Sci. 149:33–40. doi: 10.1016/j.livsci.2012.06.018 [DOI] [Google Scholar]
- Johnston L. J., Rozeboom D. W., Goodband R. D., Moeller S. J., Shannon M. C., and Schieck S. J... 2017. Effect of floor space allowances on growth performance of finishing pigs marketed at 138 kilograms. J. Anim. Sci. 95:4917–4925. doi: 10.2527/jas2017.1870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kephart K. B., Harper M. T., and Raines C. R... 2010. Observations of market pigs following transport to a packing plant. J. Anim. Sci. 88:2199–2203. doi: 10.2527/jas.2009-2440 [DOI] [PubMed] [Google Scholar]
- KilBride A. L., Gillman C. E., Ossent P., and Green L. E... 2008. A cross-sectional study of the prevalence and associated risk factors for capped hock and the associations with bursitis in weaner, grower and finisher pigs from 93 commercial farms in England. Prev. Vet. Med. 83:272–284. doi: 10.1016/j.prevetmed.2007.08.004 [DOI] [PubMed] [Google Scholar]
- Kirkden R. D., Broom D. M., and Andersen I. L... 2013. Invited review: piglet mortality: management solutions. J. Anim. Sci. 91:3361–3389. doi: 10.2527/jas.2012-5637 [DOI] [PubMed] [Google Scholar]
- Kluivers-Poodt M., Houx B. B., Robben S. R., Koop G., Lambooij E., and Hellebrekers L. J... 2012. Effects of a local anaesthetic and NSAID in castration of piglets, on the acute pain responses, growth and mortality. Animal. 6:1469–1475. doi: 10.1017/S1751731112000547 [DOI] [PubMed] [Google Scholar]
- Knauer M. T., and Hostetler C. E... 2013. US swine industry productivity analysis, 2005 to 2010. J. Swine Health Prod. 21:248–252. [Google Scholar]
- Krieter J., Schnider R., and Tölle K. H... 2004. Health conditions of growing-finishing pigs in fully-slatted pens and multi-surface systems. Dtsch. Tierarztl. Wochenschr. 111:462–466. [PubMed] [Google Scholar]
- Lago L. V., da Silva A. N., Zanella E. L., Marques M. G., Peixoto J. O., da Silva M. V. G. B., Ledur M. C., and Zanella R... 2018. Identification of genetic regions associated with scrotal hernias in a commercial swine herd. Vet. Sci. 5:15. doi: 10.3390/vetsci5010015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakritz J., George L. W., and Moore J... 1993. Seizures and acute death attributable to hypovitaminosis A and suspected hypovitaminosis D in feeder pigs. J. Am. Vet. Med. Assoc. 202:1276–1278. [PubMed] [Google Scholar]
- Larriestra A. J., Maes D. G., Deen J., and Morrison R. B... 2005a. Mixed models applied to the study of variation of grower-finisher mortality and culling rates of a large swine production system. Can. J. Vet. Res. 69:26–31. [PMC free article] [PubMed] [Google Scholar]
- Larriestra A. J., Morrison R. B., and Deen J... 2005b. A decision-making framework for evaluating interventions used at weaning to reduce mortality in lightweight pigs and improve weight gains in the nursery. J. Swine Health Prod. 13:143–149. [Google Scholar]
- Larriestra A. J., Wattanaphansak S., Neumann E. J., Bradford J., Morrison R. B., and Deen J... 2006. Pig characteristics associated with mortality and light exit weight for the nursery phase. Can. Vet. J. 47:560–566. [PMC free article] [PubMed] [Google Scholar]
- Laskoski F., Faccin J. E. G., Vier C. M., Goncalves M. A. D., Orlando U. A. D., Kummer R., Mellagi A. P. G., Bernardi M. L., Wentz I., and Bortolozzo F. P... 2019. Effects of pigs per feeder hole and group size on feed intake onset, growth performance, and ear and tail lesions in nursery pigs with consistent space allowance. J. Swine Health Prod. 27:12–18. [Google Scholar]
- Leliveld L. M. C., Riemensperger A. V., Gardiner G. E., O’Doherty J. V., Lynch P. B., and Lawlor P. G... 2013. Effect of weaning age and postweaning feeding programme on the growth performance of pigs to 10 weeks of age. Livest. Sci. 157:225–233. doi: 10.1016/j.livsci.2013.06.030 [DOI] [Google Scholar]
- Liao X. J., Lia L., Zhang Z. Y., Long Y., Yang B., Ruan G. R., Su Y., Ai H. S., Zhang W. C., Deng W. Y.,. et al. 2015. Susceptibility loci for umbilical hernia in swine detected by genome-wide association. Genetika. 51:1163–1170. doi: 10.7868/s0016675815100100 [DOI] [PubMed] [Google Scholar]
- Long G. G. 1984. Acute toxicosis in swine associated with excessive dietary intake of vitamin D. J. Am. Vet. Med. Assoc. 184:164–170. [PubMed] [Google Scholar]
- Long Y., Su Y., Ai H., Zhang Z., Yang B., Ruan G., Xiao S., Liao X., Ren J., Huang L.,. et al. 2016. A genome-wide association study of copy number variations with umbilical hernia in swine. Anim. Genet. 47:298–305. doi: 10.1111/age.12402 [DOI] [PubMed] [Google Scholar]
- Lopez-Verge S., Gasa J., Coma J., Bonet J., and Sola-Oriol D... 2019. Effect of lactation length caused by the management production system on piglet performance until slaughter. Livest. Sci. 224:26–30. doi: 10.1016/j.livsci.2019.04.003 [DOI] [Google Scholar]
- Losinger W. C., Bush E. J., Smith M. A., and Corso B. A... 1998. An analysis of mortality in the grower/finisher phase of swine production in the United States. Prev. Vet. Med. 33:121–145. doi: 10.1016/s0167-5877(97)00052-4 [DOI] [PubMed] [Google Scholar]
- Lowe J., Gauger P., Harmon K., Zhang J., Connor J., Yeske P., Loula T., Levis I., Dufresne L., and Main R... 2014. Role of transportation in spread of porcine epidemic diarrhea virus infection, United States. Emerg. Infect. Dis. 20:872–874. doi: 10.3201/eid2005.131628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luyckx K., Millet S., Van Weyenberg S., Herman L., Heyndrickx M., Dewulf J., and De Reu K... 2016. A 10-day vacancy period after cleaning and disinfection has no effect on the bacterial load in pig nursery units. BMC Vet. Res. 12:236. doi: 10.1186/s12917-016-0850-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madec F., Humbert F., Salvat G., and Maris P... 1999. Measurement of the residual contamination of post-weaning facilities for pigs and related risk factors. Zentralbl. Veterinarmed. B. 46:37–45. doi: 10.1046/j.1439-0450.1999.00207.x [DOI] [PubMed] [Google Scholar]
- Madson D. M., Arruda P. H. E., and Arruda B. L... 2019. Nervous and locomotor system. In: Diseases of Swine, 11th ed. Zimmerman J. J., Karriker L. A., Ramirez A., Schwartz K. J., and Stevenson G. W., and Zhang J.. Ames, IA: John Wiley & Sons. [Google Scholar]
- Madson D. M., Ensley S. M., Gauger P. C., Schwartz K. J., Stevenson G. W., Cooper V. L., Janke B. H., Burrough E. R., Goff J. P., and Horst R. L... 2012. Rickets: case series and diagnostic review of hypovitaminosis D in swine. J. Vet. Diagn. Invest. 24:1137–1144. doi: 10.1177/1040638712461487 [DOI] [PubMed] [Google Scholar]
- Maes D. G., Duchateau L., Larriestra A., Deen J., Morrison R. B., and de Kruif A... 2004. Risk factors for mortality in grow-finishing pigs in Belgium. J. Vet. Med. B. Infect. Dis. Vet. Public Health 51:321–326. doi: 10.1111/j.1439-0450.2004.00780.x [DOI] [PubMed] [Google Scholar]
- Maes D., Larriestra A., Deen J., and Morrision R... 2001. A retrospective study of mortality in grow-finish pigs in a multi-site production system. J. Swine Health Prod. 9:267–273. [Google Scholar]
- Magee W. T. 1951. Inheritance of scrotal hernia in swine. J. Anim. Sci. 10:516–522. doi: 10.2527/jas1951.102516x [DOI] [PubMed] [Google Scholar]
- Main R. G., Dritz S. S., Tokach M. D., Goodband R. D., and Nelssen J. L... 2004. Increasing weaning age improves pig performance in a multisite production system. J. Anim. Sci. 82:1499–1507. doi: 10.2527/2004.8251499x [DOI] [PubMed] [Google Scholar]
- Main R. G., Dritz S. S., Tokach M. D., Goodband R. D., and Nelssen J. L... 2005a. Effects of weaning age on growing-pig costs and revenue in a multi-site production system. J. Swine Health Prod. 13:189–197. [Google Scholar]
- Main R. G., Dritz S. S., Tokach M. D., Goodband R. D., Nelssen J. L., and Loughin T. M... 2005b. Effects of weaning age on postweaning belly-nosing behavior and umbilical lesions in a multi-site production system. J. Swine Health Prod. 13:259–264. [Google Scholar]
- Mannion C., Leonard F. C., Lynch P. B., and Egan J... 2007. Efficacy of cleaning and disinfection on pig farms in Ireland. Vet. Rec. 161:371–375. doi: 10.1136/vr.161.11.371 [DOI] [PubMed] [Google Scholar]
- Marin D. E., Taranu I., Bunaciu R. P., Pascale F., Tudor D. S., Avram N., Sarca M., Cureu I., Criste R. D., Suta V.,. et al. 2002. Changes in performance, blood parameters, humoral and cellular immune responses in weanling piglets exposed to low doses of aflatoxin. J. Anim. Sci. 80:1250–1257. doi: 10.2527/2002.8051250x [DOI] [PubMed] [Google Scholar]
- Martineau G. P., Morvan H., Decoux M... 2008. Porcine intestinal distention syndrome (PIDS). J. Rech. Porc. 40:33–42. [Google Scholar]
- Mavromichalis I., Hancock J. D., Senne B. W., Gugle T. L., Kennedy G. A., Hines R. H., and Wyatt C. L... 2000. Enzyme supplementation and particle size of wheat in diets for nursery and finishing pigs. J. Anim. Sci. 78:3086–3095. doi: 10.2527/2000.78123086x [DOI] [PubMed] [Google Scholar]
- McGlone J. J., and Newby B. E... 1994. Space requirements for finishing pigs in confinement: behavior and performance while group size and space vary. Appl. Anim. Beh. Sci. 39:331–338. doi: 10.1016/0168-1591(94)90166-X [DOI] [Google Scholar]
- McGlone J. J., Stansbury W. F., Tribble L. F., and Morrow J. L... 1988. Photoperiod and heat stress influence on lactating sow performance and photoperiod effects on nursery pig performance. J. Anim. Sci. 66:1915–1919. doi: 10.2527/jas1988.6681915x [DOI] [PubMed] [Google Scholar]
- Mehling S., Henao-Diaz A., Maurer J., Kluber E., Stika R., Rademacher C., Zimmerman J., Gimenez-Lirola L., Wang C., Holtkamp D. J., Main R., Karriker L., Linhares D. C. L., Breuer M., Goodell C., and Baum D... 2019. Mortality patterns in a commercial wean-to finish swine production system. Vet. Sci. 6:49. doi: 10.3390/vetsci6020049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKellar Q., and Lawrence K... 1996. Ionophores. In Pract. 18:385–386. doi: 10.1136/inpract.18.8.385 [DOI] [Google Scholar]
- Mellencamp M. A., Galina-Pantoja L., Gladney C. D., and Torremorell M... 2008. Improving pig health through genomics: a view from the industry. Dev. Biol. (Basel). 132:35–41. doi: 10.1159/000317142 [DOI] [PubMed] [Google Scholar]
- Melnichouk S. I. 2002. Mortality associated with gastric ulceration in swine. Can. Vet. J. 43:223–225. [PMC free article] [PubMed] [Google Scholar]
- Michiels A., Piepers S., Ulens T., Van Ransbeeck N., Del Pozo Sacristán R., Sierens A., Haesebrouck F., Demeyer P., and Maes D... 2015. Impact of particulate matter and ammonia on average daily weight gain, mortality and lung lesions in pigs. Prev. Vet. Med. 121:99–107. doi: 10.1016/j.prevetmed.2015.06.011 [DOI] [PubMed] [Google Scholar]
- Miller Y. J., Collins A. M., Emery D., Begg D. J., Smits R. J., and Holyoake P. K... 2013. Piglet performance and immunity is determined by the parity of both the birth dam and rearing dam. Anim. Prod. Sci. 53:46–51. doi: 10.1071/AN12063 [DOI] [Google Scholar]
- Millet S., Kumar S., De Boever J., Meyns T., Aluwé M., De Brabander D., and Ducatelle R... 2012. Effect of particle size distribution and dietary crude fibre content on growth performance and gastric mucosa integrity of growing-finishing pigs. Vet. J. 192:316–321. doi: 10.1016/j.tvjl.2011.06.037 [DOI] [PubMed] [Google Scholar]
- Moir D. C., and Masters H. G... 1979. Hepatosis dietetica, nutritional myopathy, mulberry heart disease and associated hepatic selenium level in pigs. Aust. Vet. J. 55:360–364. doi: 10.1111/j.1751-0813.1979.tb15889.x [DOI] [PubMed] [Google Scholar]
- Morrison R. S., Hemsworth P. H., Cronin G. M., and Campbell R. G... 2003. The effect of restricting pen space and feeder availability on the behavior and growth performance of entire male growing pigs in a deep-litter, large group housing system. Appl. Anim. Behav. Sci. 83:163–176. doi: 10.1016/S0168-1591(03)00117-5 [DOI] [Google Scholar]
- Mosseler A. K., Wintermann M. F., Beyerbach M., and Kamphues J... 2014. Effects of grinding intensity and pelleting of the diet – fed either dry or liquid – on intragastric milieu, gastric lesions and performance of swine. Anim. Feed Sci. Technol. 194:113–120. doi: 10.1016/j.anifeedsci.2014.05.005 [DOI] [Google Scholar]
- Mouttotou N., Hatchell F. M., and Green L. E... 1999. Foot lesions in finishing pigs and their associations with the type of floor. Vet. Rec. 144:629–632. doi: 10.1136/vr.144.23.629 [DOI] [PubMed] [Google Scholar]
- Muns R., Nuntapaitoon M., and Tummaruk P... 2016. Non-infectious causes of pre-weaning mortality in piglets. Livest. Sci. 184:46–57. doi: 10.1016/j.livsci.2015.11.025 [DOI] [Google Scholar]
- Neumann E., Hall W., Stevenson M., Morris R., and Ling Min Than J... 2014. Descriptive and temporal analysis of post-mortem lesions recorded in slaughtered pigs in New Zealand from 2000 to 2010. N. Z. Vet. J. 62:110–116. doi: 10.1080/00480169.2013.853278 [DOI] [PubMed] [Google Scholar]
- de Oliveira S. J., Bernardi R. T., Vogt F. I., Scartezzini M., Hepp D., and Lunge V. R... 2010. Gastric ulcers in fattening pigs: Isolation of Arcobacter spp. from stomachs with different severity of lesions. Acta. Sci. Vet. 38:351–356. [Google Scholar]
- Oliveira J., Guitián F. J., and Yus E... 2007. Effect of introducing piglets from farrow-to-finish breeding farms into all-in all-out fattening batches in Spain on productive parameters and economic profit. Prev. Vet. Med. 80:243–256. doi: 10.1016/j.prevetmed.2007.02.003 [DOI] [PubMed] [Google Scholar]
- Oliveira J., Yus E., and Guitian F. J... 2009. Effects of management, environmental and temporal factors on mortality and feed consumption in integrated swine fattening farms. Livest. Sci. 123:221–229. doi: 10.1016/j.livsci.2008.11.016 [DOI] [Google Scholar]
- Olsen K. M., Gabler N. K., Rademacher C. J., Schwartz K. J., Schweer W. P., Gourley G. G., and Patience J. F... 2018. The effects of group size and subtherapeutic antibiotic alternatives on growth performance and morbidity of nursery pigs: a model for feed additive evaluation. Transl. Anim. Sci. 2:298–310. doi: 10.1093/tas/txy068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osweiler G. D., Ross P. F., Wilson T. M., Nelson P. E., Witte S. T., Carson T. L., Rice L. G., and Nelson H. A... 1992. Characterization of an epizootic of pulmonary edema in swine associated with fumonisin in corn screenings. J. Vet. Diagn. Invest. 4:53–59. doi: 10.1177/104063879200400112 [DOI] [PubMed] [Google Scholar]
- Pallares F. J., Yaeger M. J., Janke B. H., Fernandez G., and Halbur P. G... 2002. Vitamin E and selenium concentration in livers of pigs diagnosed with mulberry heart disease. J. Vet. Diagn. Invest. 14:412–414. doi: 10.1177/104063870201400509 [DOI] [PubMed] [Google Scholar]
- Pardo C. E., Kreuzer M., and Bee G... 2013. Effect of average litter weight in pigs on growth performance, carcass characteristics and meat quality of the offspring as depending on birth weight. Animal. 7:1884–1892. doi: 10.1017/S1751731113001419 [DOI] [PubMed] [Google Scholar]
- Petersen H. H., Nielsen E. O., Hassing A. G., Ersbøll A. K., and Nielsen J. P... 2008. Prevalence of clinical signs of disease in Danish finisher pigs. Vet. Rec. 162:377–382. doi: 10.1136/vr.162.12.377 [DOI] [PubMed] [Google Scholar]
- Pilcher C. M., Ellis M., Rojo-Gómez A., Curtis S. E., Wolter B. F., Peterson C. M., Peterson B. A., Ritter M. J., and Brinkmann J... 2011. Effects of floor space during transport and journey time on indicators of stress and transport losses of market-weight pigs. J. Anim. Sci. 89:3809–3818. doi: 10.2527/jas.2010-3143 [DOI] [PubMed] [Google Scholar]
- Piñeiro C., Manso A., Manzanilla E. G., and Morales J... 2019. Influence of sows’ parity on performance and humoral immune response of the offspring. Porcine Health Manag. 5:1. doi: 10.1186/s40813-018-0111-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poljak Z., Carman S., and McEwen B... 2014. Assessment of seasonality of influenza in swine using field submissions to a diagnostic laboratory in Ontario between 2007 and 2012. Influenza Other Respir. Viruses. 8:482–492. doi: 10.1111/irv.12248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quesnel H. 2011. Colostrum production by sows: variability of colostrum yield and immunoglobulin G concentrations. Animal. 5:1546–1553. doi: 10.1017/S175173111100070X [DOI] [PubMed] [Google Scholar]
- Ramirez A. 2019. Differential diagnosis of diseases. In: Zimmerman J. J., Karriker L. A., Ramirez A., Schwartz K. J., and Stevenson G. W., and Zhang J.. Diseases of swine, 11th ed. Ames, IA: John Wiley & Sons. [Google Scholar]
- Ramis G., Gomez S., Ballesta M., and Munoz A... 2006. Esophagogastric ulcer in finishing pigs from twelve large multi-site herds in southeastern Spain, 1995–2000: Descriptive epidemiology. J. Swine Health Prod. 14:18–24. [Google Scholar]
- Rice D. A., and Kennedy S... 1989. Vitamin E, selenium, and polyunsaturated fatty acid concentrations and glutathione peroxidase activity in tissues from pigs with dietetic microangiopathy (mulberry heart disease). Am. J. Vet. Res. 50:2101–2104. [PubMed] [Google Scholar]
- Ritter M. J., Ellis M., Bertelsen C. R., Bowman R., Brinkmann J., Dedecker J. M., Keffaber K. K., Murphy C. M., Peterson B. A., Schlipf J. M.,. et al. 2007. Effects of distance moved during loading and floor space on the trailer during transport on losses of market weight pigs on arrival at the packing plant. J. Anim. Sci. 85:3454–3461. doi: 10.2527/jas.2007-0232 [DOI] [PubMed] [Google Scholar]
- Ritter M. J., Ellis M., Berry N. L., Curtis S. E., Anil L., Berg E., Benjamin M., Butler D., Dewey C., Driessen B., DuBois P., Hill J. D., Marchant-Forde J. N., Matzat P., McGlone J., Mormede P., Moyer T., Pfalzgraf K., Salak-Johnson J., Siemens M., Sterle J., Stull C., Whiting T., Wolter B., Niekamp S. R., and Johnson A. K... 2009. Review: transport losses in market weight pigs: I. A review of definitions, incidence, and economic impact. Prof. Anim. Sci. 25:404–414. doi: 10.15232/S1080-7446(15)30735-X [DOI] [Google Scholar]
- Ritter M. J., Ellis M., Bowman R., Brinkmann J., Curtis S. E., DeDecker J. M., Mendoza O., Murphy C. M., Orellana D. G., Peterson B. A.,. et al. 2008. Effects of season and distance moved during loading on transport losses of market-weight pigs in two commercially available types of trailer. J. Anim. Sci. 86:3137–3145. doi: 10.2527/jas.2008-0873 [DOI] [PubMed] [Google Scholar]
- Ritter M. J., Ellis M., Brinkmann J., DeDecker J. M., Keffaber K. K., Kocher M. E., Peterson B. A., Schlipf J. M., and Wolter B. F... 2006. Effect of floor space during transport of market-weight pigs on the incidence of transport losses at the packing plant and the relationships between transport conditions and losses. J. Anim. Sci. 84:2856–2864. doi: 10.2527/jas.2005-577 [DOI] [PubMed] [Google Scholar]
- Robertson I. D., Accioly J. M., Moore K. M., Driesen S. J., Pethick D. W., and Hampson D. J... 2002. Risk factors for gastric ulcers in Australian pigs at slaughter. Prev. Vet. Med. 53:293–303. doi: 10.1016/s0167-5877(01)00286-0 [DOI] [PubMed] [Google Scholar]
- Rodriguez B. de J., Taborda D. A., Franco O. I., Ortiz L. C... 2008. Frequency of gastric lesions found post mortem in pigs in the city of Medellin (Colombia). Rev. Colomb. Cienc. Pecu. 21:219–227. [Google Scholar]
- Rojas O. J., Liu Y., and Stein H. H... 2016. Effects of particle size of yellow dent corn on physical characteristics of diets and growth performance and carcass characteristics of growing-finishing pigs. J. Anim. Sci. 94:619–628. doi: 10.2527/jas2015-9054 [DOI] [PubMed] [Google Scholar]
- Rortvedt L. A., and Crenshaw T. D... 2012. Expression of kyphosis in young pigs is induced by a reduction of supplemental vitamin D in maternal diets and vitamin D, Ca, and P concentrations in nursery diets. J. Anim. Sci. 90:4905–4915. doi: 10.2527/jas.2012-5173 [DOI] [PubMed] [Google Scholar]
- Rothman K. J., and Greenland S... 2005. Causation and causal inference in epidemiology. Am. J. Public Health. 95(Suppl. 1):S144–150. doi: 10.2105/AJPH.2004.059204 [DOI] [PubMed] [Google Scholar]
- Rutten-Ramos S. C., and Deen J... 2006. Association between umbilical hernias and genetic line in a swine multiplication herd and methods to differentiate the role of sire in the incidence of umbilical hernias in offspring. J. Swine Health Prod. 14:317–322. [Google Scholar]
- Samarakone T. S., and Gonyou H. W... 2008. Productivity and aggression at grouping of grower-finisher pigs in large groups. Can. J. Anim. Sci. 88:9–17. doi: 10.4141/CJAS07106 [DOI] [Google Scholar]
- Schmolke S. A., Li Y. Z., and Gonyou H. W... 2003. Effect of group size on performance of growing-finishing pigs. J. Anim. Sci. 81:874–878. doi: 10.2527/2003.814874x [DOI] [PubMed] [Google Scholar]
- Searcy-Bernal R., Gardner I. A., and Hird D. W... 1994. Effects of and factors associated with umbilical hernias in a swine herd. J. Am. Vet. Med. Assoc. 204:1660–1664. [PubMed] [Google Scholar]
- Serrano M. P., Camara L., Morales J. I., Berrocoso J. D., Lopez Bote C. J., and Mateos G. G... 2013. Effect of gender, housing density and the interaction on growth performance and carcass and meat quality of pigs slaughtered at 110 kg body weight. Span. J. Agric. Res. 11:89–99. doi: 10.5424/sjar/2013111-2869 [DOI] [Google Scholar]
- Serrano E., Lopez-Soria S., Trinchera L., and Segales J... 2014. The use of null models and partial least squares approach path modelling (PLS-PM) for investigating risk factors influencing post-weaning mortality in indoor pig farms. Epidemiol. Infect. 142:530–539. doi: 10.1017/S0950268813001295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp B. A., Young L. G., and Van Dreumel A. A... 1972a. Dietary induction of mulberry heart disease and hepatosis dietetica in pigs. I. Nutritional aspects. Can. J. Comp. Med. 36:371–376. [PMC free article] [PubMed] [Google Scholar]
- Sharp B. A., Young L. G., and Van Dreumel A. A... 1972b. Effect of supplemental vitamin E and selenium in high moisture corn diets on the incidence of mulberry heart disease and hepatosis dietetica in pigs. Can. J. Comp. Med. 36:393–397. [PMC free article] [PubMed] [Google Scholar]
- Shen H., Thomas P. R., Ensley S. M., Kim W. I., Loynachan A. T., Halbur P. G., and Opriessnig T... 2011. Vitamin E and selenium levels are within normal range in pigs diagnosed with mulberry heart disease and evidence for viral involvement in the syndrome is lacking. Transbound. Emerg. Dis. 58:483–491. doi: 10.1111/j.1865-1682.2011.01224.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skorput D., Dujmovic Z., Karolyi D., and Lukovic Z... 2018. Variability of birth weight and growth of piglets in highly prolific sows. J. Cent. Eur. Agric. 19:823–828. doi: 10.5513/JCEA01/19.4.2355 [DOI] [Google Scholar]
- Smith A. L., Stalder K. J., Serenius T. V., Baas T. J., and Mabry J. W... 2007. Effect of piglet birth weight on weights at weaning and 42 days post weaning. J. Swine Health Prod. 15:213–218. [Google Scholar]
- Smith A. L., Stalder K. J., Serenius T. V., Baas T. J., and Mabry J. W... 2008. Effect of weaning age on nursery pig and sow reproductive performance. J. Swine Health Prod. 16:131–137. [Google Scholar]
- Spoolder H. A. M., Edwards S. A., and Corning S... 1999. Effects of group size and feeder space allowance on welfare in finishing pigs. Animal Sci. 69:481–489. doi: 10.1017/S135772980005133X [DOI] [Google Scholar]
- Stojanac N., Stevancevic O., Potkonjak A., Savic B., Stancic I., and Vracar V... 2014. The impact of space allowance on productivity performance and Salmonella spp. shedding in nursery pigs. Livest. Sci. 164:149–153. doi: 10.1016/j.livsci.2014.03.027 [DOI] [Google Scholar]
- St-Pierre N. R., Cobanov B., and Schnitkey G... 2003. Economic losses from heat stress by US livestock industries. J. Dairy. Sci. 86:(E. Suppl.):E52–E77. doi: 10.3168/jds.S0022-0302(03)74040-5 [DOI] [Google Scholar]
- Strange T., Ask B., and Nielsen B... 2013. Genetic parameters of the piglet mortality traits stillborn, weak at birth, starvation, crushing, and miscellaneous in crossbred pigs. J. Anim. Sci. 91:1562–1569. doi: 10.2527/jas.2012-5584 [DOI] [PubMed] [Google Scholar]
- Straw B., Bates R., and May G... 2009. Anatomical abnormalities in a group of finishing pigs: prevalence and pig performance. J. Swine Health Prod. 17:28–31. [Google Scholar]
- Straw B. E., Neubauer G. D., and Leman A. D... 1983. Factors affecting mortality in finishing pigs. J. Am. Vet. Med. Assoc. 183:452–455. [PubMed] [Google Scholar]
- Street B. R., and Gonyou H. W... 2008. Effects of housing finishing pigs in two group sizes and at two floor space allocations on production, health, behavior, and physiological variables. J. Anim. Sci. 86:982–991. doi: 10.2527/jas.2007-0449 [DOI] [PubMed] [Google Scholar]
- Sturos M. J., Robbins R. C., Moreno R., McLamb B. L., Rossow S. A... 2016. Narasin toxicosis in finishing pigs. J. Swine Health Prod. 24:205–211. [Google Scholar]
- Sutherland M. A., McDonald A., and McGlone J. J... 2009. Effects of variations in the environment, length of journey and type of trailer on the mortality and morbidity of pigs being transported to slaughter. Vet. Rec. 165:13–18. doi: 10.1136/vetrec.165.1.13 [DOI] [PubMed] [Google Scholar]
- Swaby H., and Gregory N. G... 2012. A note on the frequency of gastric ulcers detected during post-mortem examination at a pig abattoir. Meat Sci. 90:269–271. doi: 10.1016/j.meatsci.2011.06.015 [DOI] [PubMed] [Google Scholar]
- Szczech G. M., Carlton W. W., Tuite J., and Caldwell R... 1973. Ochratoxin A toxicosis in swine. Vet. Pathol. 10:347–364. doi: 10.1177/030098587301000408 [DOI] [PubMed] [Google Scholar]
- Thomson J. R., and Friendship R. M... 2019. Digestive system. In: Zimmerman J. J., Karriker L. A., Ramirez A., Schwartz K. J., and Stevenson G. W., and Zhang J.. Diseases of swine, 11th ed. Ames, IA: John Wiley & Sons. [Google Scholar]
- Tousignant S. J., Perez A. M., Lowe J. F., Yeske P. E., and Morrison R. B... 2015. Temporal and spatial dynamics of porcine reproductive and respiratory syndrome virus infection in the United States. Am. J. Vet. Res. 76:70–76. doi: 10.2460/ajvr.76.1.70 [DOI] [PubMed] [Google Scholar]
- Tuovinen V. K., Grohn Y. T., and Straw B. E... 1994. Health classification of multisource feeder pigs – a field trial. Prev. Vet. Med. 20:11–22. doi: 10.1016/0167-5877(94)90104-X [DOI] [PubMed] [Google Scholar]
- Turner S. P., Edwards S. A., and Bland V. C... 1999. The influence of drinker allocation and group size on the drinking behavior, welfare and production of growing pigs. Animal Sci. 68:617–624. doi: 10.1017/S1357729800050645 [DOI] [Google Scholar]
- Turner S. P., Ewen M., Rooke J. A., and Edwards S. A... 2000. The effect of space allowance on performance, aggression, and immune competence of growing pigs housed on straw deep-litter at different group sized. Livest. Prod. Sci. 66:47–55. doi: 10.1016/S0301-6226(00)00159-7 [DOI] [Google Scholar]
- USDA 2015. Swine 2012: Part I: Baseline reference of swine health and management in the United States, 2012. Fort Collins, CO: USDA-APHIS-VS, CEAH. #663.0814. [Google Scholar]
- USDA 2016. Swine 2012: Part II: Reference of swine health and health management in the United States, 2012. Fort Collins, CO: USDA-APHIS-VS-CEAH-NAHMS. #676.0216. [Google Scholar]
- Van Huffel K., Hansen M. J., Bruneel J., Van Langenhove H., and Feilberg A... 2019. Extraction efficiency of odorous compounds during a winter and summer period for partial pit ventilation in pig houses with diffuse ceiling inlet and wall inlets. Biosyst. Eng. 179:71–79. doi: 10.1016/j.biosystemseng.2018.12.006 [DOI] [Google Scholar]
- van Veen H. M., Vcllenga L., and Hoogerbrugge A... 1985. Mortality, morbidity, and external injuries in piglets housed in two different housing systems. Vet. Q. 7:127–132. doi: 10.1080/01652176.1985.9693968 [DOI] [PubMed] [Google Scholar]
- Van Vleet J. F. 1976. Induction of lesions of selenium-vitamin E deficiency in pigs fed silver. Am. J. Vet. Res. 37:1415–1420. [PubMed] [Google Scholar]
- Van Vleet J. F., Boon G. D., and Ferrans V. J... 1981. Induction of lesions of selenium-vitamin E deficiency in weanling swine fed silver, cobalt, tellurium, zinc, cadmium, and vanadium. Am. J. Vet. Res. 42:789–799. [PubMed] [Google Scholar]
- Van Vleet J. F., Carlton W., and Olander H. J... 1970. Hepatosis dietetica and mulberry heart disease associated with selenium deficiency in Indiana swine. J. Am. Vet. Med. Assoc. 157:1208–1219. [PubMed] [Google Scholar]
- Van Vleet J. F., and Ferrans V. J... 1992. Etiologic factors and pathologic alterations in selenium-vitamin E deficiency and excess in animals and humans. Biol. Trace Elem. Res. 33:1–21. doi: 10.1007/BF02783988 [DOI] [PubMed] [Google Scholar]
- Varona L., and Sorensen D... 2010. A genetic analysis of mortality in pigs. Genetics. 184:277–284. doi: 10.1534/genetics.109.110759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitali A., Lana E., Amadori M., Bernabucci U., Nardone A., and Lacetera N... 2014. Analysis of factors associated with mortality of heavy slaughter pigs during transport and lairage. J. Anim. Sci. 92:5134–5141. doi: 10.2527/jas.2014-7670 [DOI] [PubMed] [Google Scholar]
- Voslarova E., Vecerek V., Passantino A., Chloupek P., and Bedanova I... 2017. Transport losses in finisher pigs: impact of transport distance and season of the year. Asian-Australas. J. Anim. Sci. 30:119–124. doi: 10.5713/ajas.16.0265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wastell M. E., Garbossa C. A. P., and Schinckel A. P... 2018. Effects of wet/dry feeder and pen stocking density on grow-finish pig performance. Transl. Anim. Sci. 2:358–364. doi: 10.1093/tas/txy073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber E. K., Stalder K. J., and Patience J. F... 2015. Wean-to-finish feeder space availability effects on nursery and finishing pig performance and total tract digestibility in a commercial setting when feeding dried distillers grains with solubles. J. Anim. Sci. 93:1905–1915. doi: 10.2527/jas.2014-8136 [DOI] [PubMed] [Google Scholar]
- Werner C., Reiners K., and Wicke M... 2007. Short as well as long transport duration can affect the welfare of slaughter pigs. Anim. Welf. 16:385–389. [Google Scholar]
- Whitworth K. M., Rowland R. R., Ewen C. L., Trible B. R., Kerrigan M. A., Cino-Ozuna A. G., Samuel M. S., Lightner J. E., McLaren D. G., Mileham A. J.,. et al. 2016. Gene-edited pigs are protected from porcine reproductive and respiratory syndrome virus. Nat. Biotechnol. 34:20–22. doi: 10.1038/nbt.3434 [DOI] [PubMed] [Google Scholar]
- Whitworth K. M., Rowland R. R. R., Petrovan V., Sheahan M., Cino-Ozuna A. G., Fang Y., Hesse R., Mileham A., Samuel M. S., Wells K. D.,. et al. 2019. Resistance to coronavirus infection in amino peptidase N-deficient pigs. Transgenic Res. 28:21–32. doi: 10.1007/s11248-018-0100-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolter B. F., Ellis M., Corrigan B. P., DeDecker J. M., Curtis S. E., Parr E. N., and Webel D. M... 2003a. Effect of restricted postweaning growth resulting from reduced floor and feeder-trough space on pig growth performance to slaughter weight in a wean-to-finish production system. J. Anim. Sci. 81:836–842. doi: 10.2527/2003.814836x [DOI] [PubMed] [Google Scholar]
- Wolter B. F., Ellis M., Corrigan B. P., DeDecker J. M., Curtis S. E., Parr E. N., and Webel D. M... 2003b. Impact of early postweaning growth rate as affected by diet complexity and space allocation on subsequent growth performance of pigs in a wean-to-finish production system. J. Anim. Sci. 81:353–359. doi: 10.2527/2003.812353x [DOI] [PubMed] [Google Scholar]
- Wolter B. F., Ellis M., Curtis S. E., Augspurger N. R., Hamilton D. N., Parr E. N., and Webel D. M... 2001. Effect of group size on pig performance in a wean-to-finish production system. J. Anim. Sci. 79:1067–1073. doi: 10.2527/2001.7951067x [DOI] [PubMed] [Google Scholar]
- Wolter B. F., Ellis M., Curtis S. E., Parr E. N., and Webel D. M... 2000. Feeder location did not affect performance of weanling pigs in large groups. J. Anim. Sci. 78:2784–2789. doi: 10.2527/2000.78112784x [DOI] [PubMed] [Google Scholar]
- Wolter B. F., Ellis M., DeDecker J. M., Curtis S. E., Hollis G. R., Shanks R. D., Parr E. N., and Webel D. M... 2002. Effects of double stocking and weighing frequency on pig performance in wean-to-finish production systems. J. Anim. Sci. 80:1442–1450. doi: 10.2527/2002.8061442x [DOI] [PubMed] [Google Scholar]
- Wondra K. J., Hancock J. D., Behnke K. C., Hines R. H., and Stark C. R... 1995. Effects of particle size and pelleting on growth performance, nutrient digestibility, and stomach morphology in finishing pigs. J. Anim. Sci. 73:757–763. doi: 10.2527/1995.733757x [DOI] [PubMed] [Google Scholar]
- Wu F., Liao J., Tokach M. D., Dritz S. S., Woodworth J. C., Goodband R. D., DeRouchey J. M., Vahl C. I., Calderon-Cartagena H. I., and Van De Stroet D. L... 2019. A retrospective analysis of seasonal growth patterns of nursery and finishing pigs in commercial production. J. Swine Health Prod. 27:19–33. [Google Scholar]
- Yun J., Olkkola S., Hanninen J., Oliviero C., and Heinonen M... 2017. The effects of amoxicillin treatment of newborn piglets on the prevalence of hernias and abscesses, growth and ampicillin resistance of intestinal coliform bacteria in weaned pigs. PLoS ONE. 12:e0172150. doi: 10.1371/journal.pone.0172150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Xin H., Harmon J. D., and Baas T. J... 2016. Mortality rate of weaned and feeder pigs as affected by ground transport conditions. Trans. ASABE. 59:943–948. doi: 10.13031/trans.59.11671 [DOI] [Google Scholar]
- Zomborszky M. K., Vetesi F., Repa I., Kovacs F., Bata A., Horn P., Toth A., and Romvari R... 2000. Experiment to determine the limits of tolerance for Fumonisin B1 in weaned piglets. J. Vet. Med. B. Infect. Dis. Vet. Public Health. 47:277–286. [DOI] [PubMed] [Google Scholar]
- Zotti E., Resmini F. A., Schutz L. G., Volz N., Milani R. P., Bridi A. M., Alfieri A. A., and da Silva C. A... 2017. Impact of piglet birthweight and sow parity on mortality rates, growth performance, and carcass traits in pigs. R. Bras. Sootec. 46:856–862. doi: 10.1590/S1806-92902017001100004 [DOI] [Google Scholar]