Abstract
Coronavirus disease 2019 (COVID-19) predominantly presents with symptoms of fever, fatigue, cough and respiratory failure. However, it appears to have a unique interplay with cardiovascular disease (CVD); patients with pre-existing CVD are at highest risk for mortality from COVID-19, along with the elderly. COVID-19 contributes to cardiovascular complications including arrhythmias, myocardial dysfunction and myocardial inflammation. Although the exact mechanism of myocardial inflammation in patients with COVID-19 is not known, several plausible mechanisms have been proposed based on early observational reports. In this article, the authors summarise the available literature on mechanisms of myocardial injury in COVID-19.
Keywords: Myocardial injury, myocarditis, cardiac failure, COVID-19, ACE-2 receptors
Coronavirus disease 2019 (COVID-19) has evolved into a global pandemic, having affected more than 2.3 million people and claiming more than 160,000 lives. Infection with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus predominantly causes fever (77–98% of cases), fatigue (52–75%) and cough (60–81%).[1,2] While it primarily affects the respiratory system, it also appears to have a unique interplay with the cardiovascular system.
Patients with pre-existing cardiovascular comorbidities appear to be at the highest risk for mortality from COVID-19, along with the elderly. The disease also contributes to cardiovascular complications, including acute coronary syndromes, arrhythmias, myocarditis, acute heart failure and, in the most severe cases, cardiogenic shock and death.[3]
Although only a few population studies have detailed the spectrum of cardiovascular complications, the high prevalence of myocardial injury in patients with COVID-19 is suggested by frequently elevated cardiac biomarkers. Elevated troponin levels are noted in 7–28% of COVID-19 patients on presentation, some associated with depressed left ventricular function and haemodynamic shock.[4–7] Although an elevation in cardiac troponin is a sensitive marker for myocardial injury, it does not distinguish between the various aetiologies of injury. Multiple potential mechanisms of acute myocardial injury from the viral infection have been proposed.[8]
The purpose of this article is primarily to summarise the available literature (Tables 1 and 2) on various proposed mechanisms of myocardial injury related to COVID-19 (Figure 1).
Table 1: Pooled Baseline Demographics and Comorbidities in Published Studies.
| Author | Study period | Cases | Died, Number (%) | Region, Country | Demographics and Baseline Cardiovascular Comorbidities | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean Age (Range) | Female | Hypertension | Type 2 Diabetes | Smoker | Cardiovascular Diseases | Chronic Kidney Disease | |||||
| Zhou et al. 2000[11] | 29 December 2019–31 January 2020 | 191 | 54 (28.2%) | Jinyintan/Wuhan, China | 56 (18–87) | 72 (38%) | 58 (30%) | 36 (19%) | 11 (6%) | CAD: 15 (8%); HF 44 (23%) | 2 (1%) |
| Bhatraju et al. 2000[5] | 24 February–9 March 2020 | 24 | 12 (50%) | Washington, US | 64 (±18) | 9 (38%) | – | 14 (58%) | 5 (22%) | – | 5 (21%) |
| Yang et al. 2000[54] | 24 December 2019–29 January 2020 | 52 | 32 (61.5%) | Wuhan, China | 59.7 (±13.3) | 17 (33%) | – | 9 (17%) | 2 (4%) | 5 (10%) | – |
| Phua, 2000[6] | 20 January 2020–10 February 2020 | 416 | 57 (13.7%) | Wuhan, China | 64 (21–95) | 211 (50.7%) | 127 (30.5%) | 60 (14.4%) | – | 44 (10.6%) | 14 (3.4% |
| Huang et al. 2000[29,55] | 31 December 2019–2 January 2020 | 41 | 6 (15%) | Wuhan, China | 49 (41–58) | 11 (27%) | 6 (15%) | 8 (20%) | 3 (7%) | 6 (15%) | – |
| Wu et al. 2000[55] | 201 | 44 (21.9%) | Wuhan, China | 52 (43–60) | 75 (36.3%) | 39 (19.4%) | 22 (10.9%) | – | 9 (4.0%) | 2 (1.0%) | |
| Chen et al. 2000[56] | 1–20 January 2020 | 99 | 11 (11%) | Wuhan, China | 55.5 (13.1) | 32 ( 32%) | – | – | – | 40 (40%) had both cardiovascular and cerebrovascular illness | – |
| Guan et al. 2000[57] | 11 December 2019–29 January 2020 | 1,099 | 15 (1.4%) | Entire China | 47 (35–58) | 459/1,096 (41.9%) | 165(15%) | 81 (7.4%) | 158 (14.5%) | CAD 27 (2.5%) | 8 (0.7%) |
| Guo et al. 2000[7] | 23 January–23 February 2020 | 187 | 43 (23.0%) | Wuhan, China | 58.5 (14.6) | 96 (51.3%) | 61(32.6%) | 28 (15.0%) | 18 (9.6%) | CAD 21 (11.2%); HF 8 (4.3%) | 6 (3.2%) |
| Petrilli et al. 2000[58] * (only hospitalised cohort) | 1 March–2 April 2020 | 1,999 | 292 (14.6%) | New York, US | 62 (50–74) | 1,052 (52.6%) | 742 (37.1%) | 503 (25.2%) | 520 (26%) | CAD 197 (9.9%); HF 124 (6.2%) | 195 (9.8%) |
| Richardson et al. 2000[59] | 1 March–1 April 2020 | 5,700 | 553/2,634 (21%) | New York, US | 63 (0–107) | 2,263 (39.7%) | 2036 (56.6%) | 1,808 (33.8%) | 558/3567 (15.6%) | CAD 595 (11.1%) | 268 (5%); ESRD 186 (3.5% |
| Arentz et al. 2000[60] | 20 February–5 March 2020 | 21 | 11 (52.4%) | Washington, US | 70 (43–92) | 9 (48%) | – | 7 (33.3%) | COPD 7 (33.3%) | HF 9 (42.9%) | 10 (47.6%) ESRD 2 (9.5%) |
CAD = coronary artery disease; COPD = chronic obstructive pulmonary disease; ESRD = end-stage renal disease; HF = heart failure. * = available on preprint server.
Table 2: Biomarkers, Clinical Parameters and Interventions in Published Studies.
| Author | Elevated Cardiac Biomarker | Natriuretic Peptide (NT pro-BNP) | Respiratory Involvement (Chest X-ray/CT) | Echocardiography Findings | Invasive Mechanical Ventilation | Glucocorticoids | ECMO Utilisation | Renal Replacement Therapy | Comments/Sentinel Findings |
|---|---|---|---|---|---|---|---|---|---|
| All patients: 24/145 (17%); non-survivor 23/50 (46%); survivor 1/95 (1%) (TnI) | – | Consolidation 112 (59%); GGO 136 (71%); BL infiltration 143 (75%) | – | 32 (17%) | 57 (30%) | 3 (2%) | 10 (5%) | Compared survivor to non-survivors and found older age, higher SOFA score, and D-dimer were associated with mortality | |
| Zhou et al. 2000[11] | 2/13 (15%) | – | BL infiltrates 23/23 (100%); GGO 4/5 (80%) | 0/9 (0%) | 18/24 (75%) | 0 | 0 | – | First published COVID-19 study in US; hypoxaemic respiratory failure was commonest reason for ICU admission |
| Bhatraju et al. 2000[5] | All patients: 12 (23%); non-survivor 9 (28%); survivor 3 (15%) Median TnI =161.0 pg/mL | – | – | – | 22 (42%) | 30 (58%) | 6 (11.5%) | 9 (17%) | Critically ill patients included only; ARDS (67%), AKI (29%), liver dysfunction (29%) |
| Yang et al. 2000[54] | 82 (19.7%); cardiac injury patients Median TnI = 0.19 µg/l | All patients: median = 219 pg/ml Cardiac injury patients: median = 1,689 pg/ml | UL infiltrate 105 (25.2%); BL 311 (74.8%); GGO 68 (16.3%) | – | 32 (7.7%) | 204 (73.1%) | – | 2 (0.5%) | HR 4.26 (95% CI [1.92–9.49]) for mortality from cardiac injury; only 22 (26.8%) underwent ECG. ARDS, AKI, coagulopathy, dyselectrolytemia more common in cardiac injury patients |
| Phua, 2000[6] | All patients: 5/41 (12%); ICU patients 4/13 (31%); non-ICU patients 1/28 (4%) Median TnI = 3.4 pg/mL | – | Bilateral involvement 40 (98%) | – | 4 (10%) | 9 (22%) | 2 (5%) | 3 (7%) | Earliest Chinese study reporting outcomes |
| Huang et al. 2000[29] | 9/198 (4.5%) (CK-MB) | – | UL infiltrate 10 (5.0%); BL infiltrate 191 (95%) | – | 5 (2.5%) | 62 (30.8%) | 1 (0.5%) | – | 10 patients overlap with Huang et al.[29] and Wu et al.[55] ARDS in 41.8% of patients. Methylprednisolone reduced death (HR 0.38; 95% CI [0.20–0.72]) |
| Wu et al. 2000[55] | 13 (13%) (CK) | – | UL PNA 25 (25%); BL PNA 74 (75%); GGO 14 (14%) | – | 4 (4%) | 19 (19%) | 3 (3%) | 9 (9%) | Symptoms: chest pain (2%); shortness of breath (31%); fever (83%); nausea/vomiting (1%) |
| Chen et al. 2000[56] | – | – | GGO 55/274 (20.1%); UL PNA 77/274 (28.1%); BL PNA 100/274 (36.5%) | – | 25 (2.3%) | 204 (18.6%) | 5 (0.5%) | 9 (0.8%) | From National Health Commission in China. Lymphocytopenia in 83.2% of patients |
| Guan et al. 2000[57] | All patients: 52 (27.8%) (TnT) | All patients: median 268.4 pg/ml Cardiac injury patients: median 817.4 pg/ml | – | – | 45 (24.1%) | 106 (56.7%) | – | – | ACEi/ARB use: 10.1%; VT/VF incidence: 5.9%); mortality in elevated TnT group was 31/52 (59.6%) versus 14/135 (10.4%) in normal TnT group |
| Petrilli et al. 2000[58] (only hospitalised cohort)* | 185/1,327 (13.9%) | – | – | – | 445 (22.2%) | – | – | – | No treatment characteristics reported |
| Richardson et al. 2000[59] | 801/3,533 (22.6%) | All patients: median 385.5 pg/ml | – | 320/2,634 (12.2%) | – | – | 81/2,634 (3.2%) | Obesity 1737/4170 (41.7%); morbid obesity (BMI >35) 791/4170 (19.0%); liver dysfunction 56 (2.1%). Did not report medications Mortality in patients not taking ACEi/ARB 26.7% and taking ACEi 32.7% or ARB 30.6%. ACEi/ARB use: 48.1%/50.1% continued during hospitalisation | |
| Arentz et al. 2000[60] | BL reticular opacities 18 (86%); GGO: 14 (67%) | New reduced left ventricular systolic function 7 (33.3%) | 15 (71%) | Included only critical patients. Liver dysfunction 3 (14.3%) |
ACEi = angiotensin converting enzyme inhibitor; AKI = acute kidney injury; ARB = angiotensin receptor blocker; ARDS = acute respiratory distress syndrome; BL = bilateral; CK = creatinine kinase; CK-MB = creatinine kinase-MB; COVID-19 = coronavirus disease 2019; ECMO = extracorporeal membrane oxygenation; ESRD = end-stage renal disease; HF=heart failure; ICU = intensive care unit; NT pro-BNP = N-terminal brain natriuretic peptide; GGO = ground-glass opacities; PNA = pneumonia; SOFA= sequential organ failure assessment; TnI = troponin I; TnT = troponin T; UL = unilateral. * = available on preprint server. Standard abbreviations for measurement values are used. Data extracted on 23 April 2020.
Figure 1: Mechanisms of Myocardial Injury in COVID-19.
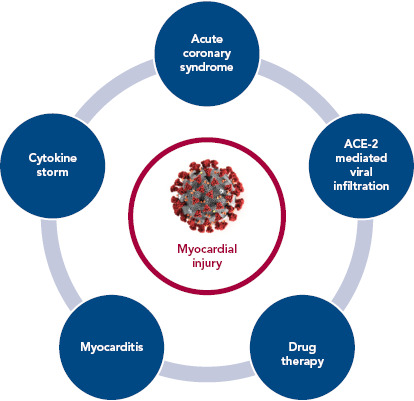
ACE-2 = angiotensin converting enzyme 2
Acute Coronary Syndromes
Although the reported incidence of acute coronary syndrome (ACS) has gone down during the COVID-19 pandemic, it is possible this is because patients are being hesitant in visiting hospitals for their medical care.[9] However, a variety of syndromes mimicking ACS have been reported in people with COVID-19 infection.
In a case series of 18 patients with COVID-19, 56% were found to have ST-elevation at time of presentation, with the rest developing it at some point during hospitalisation.[10] Focal ST elevations were present in 78% of patients. A total of nine patients (50%) underwent coronary angiography but only six patients (33%) were eventually found to have obstructive coronary artery disease (CAD). Inferior and lateral ST segment elevations were most common, and ECG findings were more likely to be focal in patients with obstructive CAD. Of note, all 18 patients had elevated D-dimer levels.
In a recently published retrospective study of 191 COVID-19 patients from two separate hospitals in China, the incidence of elevation in high-sensitivity cardiac troponin I (cTnI; >28 pg/ml) was 17%, and it was significantly higher among non-survivors (46% versus 1%, p<0.001).[11] Furthermore, elevation of this biomarker was noted to be a predictor of in-hospital death (univariable OR 80.07, 95% CI [10.34–620.36], p<0.0001). The most abrupt increase in cardiac troponin I in non-survivors was noted beyond day 16 after the onset of disease.
In the same study, the incidence of acute cardiac injury was 17% among all patients but significantly higher in non-survivors (59% versus 1%, p<0.0001). Another study reported the incidence of acute cardiac injury to be 7% (10 out of 138 patients), but significantly higher among patients requiring intensive care unit (ICU) admission (22.2% versus 2%, p<0.001).[1] A meta-analysis of cardiac biomarkers in patients with COVID-19 showed that the values of cardiac troponin I were significantly higher in those with severe disease than in those without (SMD 25.6 ng/l; 95% CI [6.8–44.5 ng/l]).[4]
Hypercoagulability has also been reported among COVID-19 patients, which makes them more prone to arterial thrombi and ACS.[12] In a recent review of COVID-19 patients, Violi et al. suggested that patients with severe disease (Pneumonia Severity Index >90, CURB-65 ≥2, acute respiratory distress syndrome, sepsis or requiring ICU admission) and those with elevated D-dimer levels have a hypercoagulable milieu and are likely to benefit from antithrombotic therapy with low molecular weight heparin or aspirin.[13]
It has been postulated that COVID-19 may cause direct vascular injury, as reported in a case report of right coronary artery subintimal haematoma associated with spontaneous coronary dissection.[14] In a recently published case series of COVID-19 patients, Varga et al. found evidence of direct viral infection of the endothelial cells and resultant endotheliitis.[15] Such endothelial inflammation would certainly predispose COVID-19 patients to ACS, especially given their hypercoagulable state. Although no studies have reported the precise incidence of ACS and acute MI with COVID-19, these conditions have been known to occur after severe acute respiratory syndrome coronavirus 1 (SARS-CoV-1) and influenza infections.[16–18]
Myocarditis
Myocarditis is another potential aetiology of acute myocardial injury in COVID-19 patients, although there remains a paucity of literature on cases confirmed with imaging or histopathology.
SARS-CoV-2 can directly infect the cardiac tissue via angiotensin-converting enzyme 2 (ACE-2) receptors, which may cause myocardial inflammation and damage. Such a mechanism of myocardial injury through SARS-CoV has been well-established in animal models and cardiac autopsies.[19,20] Some autopsy reports of COVID-19 patients have likewise reported myocardial interstitial infiltration by mononuclear cells and lymphocytic infiltration.[21,22] Endomyocardial biopsy showing low-grade myocardial inflammation and viral infiltration directly into the myocardial tissue has also been reported.[23]
Isolated cardiac involvement with no respiratory symptoms or infiltrate has also been reported with cardiac MRI demonstrating marked biventricular myocardial interstitial oedema with late gadolinium enhancement.[24,25] COVID-19-associated fulminant myocarditis has also been reported.[26] As most patients do not undergo advanced cardiac imaging or cardiac biopsies (because of concerns of field contamination and increased viral dissemination), the true incidence of myocarditis remains unknown.
In addition to myocardial inflammation, pericardial involvement in the form of pericardial effusion with tamponade has also been reported in COVID-19.[27] A mimicker of COVID-19-associated myocarditis is stress-induced cardiomyopathy, which is another known cause of acute heart failure in these patients.[28]
The exact aetiology of myocarditis in COVID-19 remains unknown. Possible mechanisms include direct viral infiltration and resultant inflammation, or a cytokine storm with myocardial inflammation.
Role of Cytokine Storm
An excessive immune response to SARS-CoV-2 infection has been demonstrated in certain subgroups of infected patients and is referred to as a cytokine storm.[29,30] This phenomenon has been demonstrated in previous studies where SARS-CoV-1 caused severe lung injury.[31]
A cytokine storm is triggered by an imbalanced response of type 1 and type 2 T helper cells, resulting in an excessive production of cytokines, particularly interleukin 6 (IL-6).[32,33] Yang et al. showed that in 53 clinically moderately to severely affected COVID-19 patients, 14 cytokines were elevated, with three of them – interferon gamma induced protein 10 (IP-10), monocyte chemotactic protein 3 (MCP-3) and interleukin-1 receptor antagonist (IL-1ra) – being independently associated with hypoxaemia, disease progression and death.[34]
The cytokine profile in COVID-19 is similar to that in secondary haemophagocytic hymphohistiocytosis (SHH), a hyper-inflammatory syndrome seen in other viral infections.[35,36] The inflammatory response may impact myocardial contractility and function by direct myocardial damage or via hypoxia-mediated myocardial damage.[37] Certain anti-cytokine treatments, such as tocilizumab, have been proposed to treat this cytokine storm.[38]
Role of ACE Receptor in Pathogenesis
ACE-2 is a membrane-bound aminopeptidate receptor expressed on the epithelial cells of the lungs, intestines, kidneys and blood vessels.[39] It has important immune and cardiovascular roles. Angiotensin-converting enzyme (ACE) cleaves angiotensin I to generate angiotensin II (Ang II), which binds to and activates AT1R, thus promoting vasoconstriction. ACE-2 cleaves angiotensin II and generates angiotensin 1–7, a powerful vasodilator acting through Mas receptors.
SARS-CoV-2 has a spike protein receptor-binding domain, similar to SARS-CoV-1, which interacts with the ACE-2 receptor and acts as the primary functional receptor for pathogenicity and human-to-human transmission.[40] Furthermore, SARS-CoV-2 binding to ACE-2 leads to its downregulation and increases angiotensin II. This subsequently leads to lower amount of angiotensin 1–7. This causes AT1R-mediated pulmonary vascular permeability.[41]
The ACE-2 receptor is upregulated in patients with cardiovascular disease, especially with the use of renin-angiotensin-aldosterone system inhibitors.[32] SARS-CoV-2 mainly affects the alveolar epithelial cells, resulting in respiratory symptoms, which are more severe in patients with cardiovascular disease.
Some animal studies have reported the protective role of ACE-2 in the development of severe acute lung injury.[42] This has led to speculation about the potential effects of antihypertensive medications with ACE-inhibitors or angiotensin receptor blockers on COVID-19 positive patients.
Guo et al. demonstrated that angiotensin-converting enzyme inhibitors/angiotensin receptor blockers had no effect of on mortality in 187 hospitalised patients with SARS-CoV-2.[7] Furthermore, a retrospective analysis of 1,128 adult COVID-19 patients with a previous history of hypertension, inpatient use of ACE inhibitors or angiotensin receptor blockers (ARBs) was associated with lower risk of all-cause mortality compared to non-users.[21] There is a lack of clear experimental or clinical data showing ACE receptor-mediated antihypertensive therapy has adverse effects in patients with COVID-19.[43] Therefore, modification of pre-existing therapy is not recommended as per guidelines from both American and European cardiovascular societies.[44,45]
On the other hand, sacubitril/valsartan reduces the concentration of pro-inflammatory cytokines and neutrophil count, while increasing lymphocyte count more than valsartan alone or placebo. This finding might be related to the increase in plasma levels of atrial/brain/C-type natriuretic peptide, Ang I/II, substance P, bradykinin and endothelin secondary to neprilisin inhibition by sacubitril.[46] Therefore, administration of an ACE, an ARB or sacubitril/valsartan may even be beneficial through inhibition of AT1R.[47]
Impact of Drug Therapy
Currently, there is no known no curative therapy for SARS-CoV-2. Many drugs are being used as prophylaxis or for treatment of COVID-19 patients in an expedited manner.[48] Hydroxychloroquine, proposed as an effective strategy for COVID-19 patients, has known cardiovascular toxicity, causing arrhythmias and biventricular failure.[5,49]
Hydroxychloroquine-related cardiac adverse events are rare but can be severe and, occasionally, life-threatening. A recent review of cardiac complications attributed to this medication found that most patients who developed cardiac symptoms had been on treatment for a long period of time (median 7 years), and had been exposed to large cumulative doses (median 1,235 g.[50] Conduction abnormalities (prolonged QT and PR intervals) were the most common adverse events, affecting 85% of these patients. Although it appears that conduction abnormalities are a long-term consequence of high-dose and prolonged use of hydroxychloroquine, we recommend monitoring all patients with COVID-19 treated with this drug for cardiac arrhythmias.
Azithromycin, which has been proposed for the treatment of COVID-19 pneumonia, is also known to increase the risk of adverse cardiovascular events. This risk is highest among patients with baseline cardiovascular comorbidities, conduction abnormalities and on concomitant QT-prolonging medications.[51] Indeed, concomitant use of hydroxychloroquine and azithromycin is associated with higher risk of QTc prolongation in COVID-19 patients than either medication alone.[52]
The potential of experimental treatment to cause myocardial damage remains a concern and patients receiving such therapies require close monitoring.
Long-term Cardiovascular Implications
In addition to acute myocardial damage, COVID-19-related long-term cardiovascular morbidity is also a concern. SARS-CoV-2 is structurally and genetically very similar to its predecessor SARS-CoV-1.
A small study of 25 patients who recovered from SARS-CoV-1 infection had abnormal lipid profiles and glucose metabolism, and a higher burden of cardiovascular abnormalities at 12 years’ follow-up.[53] As serological antibody testing becomes readily available to identify patients who have recovered from COVID-19, it will be important to observe their long-term cardiovascular health after infection.
Conclusion
Multiple mechanisms appear to contribute to myocardial injury in COVID-19. Individually or in conjunction with one another, they include respiratory failure induced hypoxia, inflammatory cytokine storms, direct viral infiltration and subsequent myocyte death, and myocardial dysfunction from acute illness.
Acknowledgments
ASR and SR contributed equally.
References
- 1.Wang D, Hu B, Hu C Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. Jama 2020. epub ahead of press. [DOI] [PMC free article] [PubMed]
- 2.Ruan Q, Yang K, Wang W Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020. epub ahead of press. [DOI] [PMC free article] [PubMed]
- 3.Rali AS, Sauer Andrew J. COVID-19 pandemic and cardiovascular disease. US Cardiology Review. 2020;14:e01. doi: 10.15420/usc.2020.14. [DOI] [Google Scholar]
- 4.Lippi G, Lavie CJ, Sanchis-Gomar F. Cardiac troponin I in patients with coronavirus disease 2019 (COVID-19): evidence from a meta-analysis. Prog Cardiovasc Dis. 2020. epub ahead of press. [DOI] [PMC free article] [PubMed]
- 5.Bhatraju PK, Ghassemieh BJ, Nichols M Covid-19 in critically ill patients in the Seattle region – case series. N Engl J Med. 2020. epub ahead of press. [DOI] [PMC free article] [PubMed]
- 6.Phua J, Weng L, Ling L Intensive care management of coronavirus disease 2019 (COVID-19): challenges and recommendations. Lancet Respir Med. 2020. epub ahead of press. [DOI] [PMC free article] [PubMed]
- 7.Guo T, Fan Y, Chen M Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020. epub ahead of press. [DOI] [PMC free article] [PubMed]
- 8.Madjid M, Safavi-Naeini P, Solomon SD, Vardeny O. Potential effects of coronaviruses on the cardiovascular system: a review. JAMA Cardiology. 2020. epub ahead of press. [DOI] [PubMed]
- 9.Metzler B, Siostrzonek P, Binder RK et al. Decline of acute coronary syndrome admissions in Austria since the outbreak of COVID-19: the pandemic response causes cardiac collateral damage. Eur Heart J. 2020;41:3. doi: 10.1093/eurheartj/ehaa314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bangalore S, Sharman A, Yatskar L ST-segment elevation in patients with Covid-19 – a case series. N Engl J Med. 2020. epub ahead of press. [DOI] [PMC free article] [PubMed]
- 11.Zhou F, Yu T, Du R et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–62. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Danzi GB, Loffi M, Galeazzi G, Gherbesi E. Acute pulmonary embolism and COVID-19 pneumonia: a random association? Eur Heart J. 2020;41:1858. doi: 10.1093/eurheartj/ehaa254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Violi F, Pastori D, Cangemi R Hypercoagulation and antithrombotic treatment in coronavirus 2019: a new challenge. Thromb Haemost. 2020. epub ahead of press. [DOI] [PMC free article] [PubMed]
- 14.Courand P-Y, Harbaoui B, Bonnet M, Lantelme P. Spontaneous coronary artery dissection in a patient with COVID-19. JACC: Cardiovasc Interv. 2020. in press. [DOI] [PMC free article] [PubMed]
- 15.Varga Z, Flammer AJ, Steiger P et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–8. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peiris JS, Chu CM, Cheng VC et al. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet. 2003;361:1767–72. doi: 10.1016/s0140-6736(03)13412-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chong PY, Chui P, Ling AE et al. Analysis of deaths during the severe acute respiratory syndrome (SARS) epidemic in Singapore: challenges in determining a SARS diagnosis. Arch Pathol Lab Med. 2004;128:195–204. doi: 10.1043/1543-2165(2004)1282.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 18.Kwong JC, Schwartz KL, Campitelli MA et al. Acute myocardial infarction after laboratory-confirmed influenza infection. N Engl J Med. 2018;378:345–53. doi: 10.1056/NEJMoa1702090. [DOI] [PubMed] [Google Scholar]
- 19.Oudit GY, Kassiri Z, Jiang C et al. SARS-coronavirus modulation of myocardial ACE2 expression and inflammation in patients with SARS. Eur J Clin Invest. 2009;39:618–25. doi: 10.1111/j.1365-2362.2009.02153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Booth C.M., Matukas LM, Tomlinson GA et al. Clinical features and short-term outcomes of 144 patients with SARS in the greater Toronto area. JAMA. 2003;289:2801–9. doi: 10.1001/jama.289.21.JOC30885. [DOI] [PubMed] [Google Scholar]
- 21.Zhang P, Zhu L, Cai J Association of inpatient use of angiotensin converting enzyme inhibitors and angiotensin II receptor blockers with mortality among patients with hypertension hospitalized with COVID-19. Circ Res. 2020. epub ahead of press. [DOI] [PMC free article] [PubMed]
- 22.Sala S, Peretto G, Gramegna M et al. Acute myocarditis presenting as a reverse Tako-Tsubo syndrome in a patient with SARS-CoV-2 respiratory infection. Eur Heart J. 2020;41:1861–2. doi: 10.1093/eurheartj/ehaa286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tavazzi G, Pellegrini C, Maurelli M Myocardial localization of coronavirus in COVID-19 cardiogenic shock. Eur J Heart Fail. 2020. epub ahead of press. [DOI] [PMC free article] [PubMed]
- 24.Inciardi RM, Lupi L, Zaccone G Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020. epub ahead of press. [DOI] [PMC free article] [PubMed]
- 25.Kim IC, Kim JY, Kim HA, Han S. COVID-19-related myocarditis in a 21-year-old female patient. Eur Heart J. 2020;41:1859. doi: 10.1093/eurheartj/ehaa288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hu H a F, Wei X, Fang Y. Coronavirus fulminant myocarditis saved with glucocorticoid and human immunoglobulin. Eur Heart J. 2020. epub ahead of press. [DOI] [PMC free article] [PubMed]
- 27.Hua A, O’Gallagher K, Sado D, Byrne J. Life-threatening cardiac tamponade complicating myo-pericarditis in COVID-19. Eur Heart J. 2020. epub ahead of press. [DOI] [PMC free article] [PubMed]
- 28.Meyer P, Degrauwe S, Van Delden C et al. Typical takotsubo syndrome triggered by SARS-CoV-2 infection. Eur Heart J. 2020;41:1860. doi: 10.1093/eurheartj/ehaa306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang C, Wang Y, Li X et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Siddiqi HK, Mehra MR. COVID-19 Illness in native and immunosuppressed states: a clinical-therapeutic staging proposal. J Heart Lung Transplant. 2020;39:405–7. doi: 10.1016/j.healun.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wong CK, Lam CW, Wu AK et al. Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clin Exp Immunol. 2004;136:95–103. doi: 10.1111/j.1365-2249.2004.02415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zheng YY, Ma YT, Zhang JY, Xie X. COVID-19 and the cardiovascular system. Nat Rev Cardiol. 2020;17:259–60. doi: 10.1038/s41569-020-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen C, Zhang XR, Ju ZY2 He WF. Advances in the research of cytokine storm mechanism induced by corona virus disease 2019 and the corresponding immunotherapies. [in Chinese] Zhonghua Shao Shang Za Zhi. 2020;36:E005. doi: 10.3760/cma.j.cn501120-20200224-00088. epub ahead of press. [DOI] [PubMed] [Google Scholar]
- 34.Vaninov N. In the eye of the COVID-19 cytokine storm. Nat Rev Immunol. 2020;20:277. doi: 10.1038/s41577-020-0305-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mehta P, McAuley DF, Brown M et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–4. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ramos-Casals M, Brito-Zerón P, López-Guillermo A et al. Adult haemophagocytic syndrome. Lancet. 2014;383:1503–16. doi: 10.1016/S0140-6736(13)61048-X. [DOI] [PubMed] [Google Scholar]
- 37.Chau VQ, Oliveros E, Mahmood K The imperfect cytokine storm: severe COVID-19 with ARDS in patient on durable LVAD Support. JACC Case Rep. 2020. epub ahead of press. [DOI] [PMC free article] [PubMed]
- 38.Zhang W, Zhao Y, Zhang F et al. The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): the perspectives of clinical immunologists from China. Clin Immunol. 2020;214:108393. doi: 10.1016/j.clim.2020.108393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Turner AJ, Hiscox JA, Hooper NM. ACE2: from vasopeptidase to SARS virus receptor. Trends Pharmacol Sci. 2004;25:291–4. doi: 10.1016/j.tips.2004.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wan Y, Shang J, Graham R et al. Receptor recognition by the novel coronavirus from wuhan: an analysis based on decade-long structural studies of SARS coronavirus. J Virol. 2020;94((7)):e00127–20. doi: 10.1128/JVI.00127-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kuba K, Imai Y, Rao S et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med. 2005;11:875–9. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Imai Y, Kuba K, Rao S et al. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436:112–6. doi: 10.1038/nature03712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vaduganathan M, Vardeny O, Michel T et al. Renin-angiotensin-aldosterone system inhibitors in patients with Covid-19. N Engl J Med. 2020;382:1653–9. doi: 10.1056/NEJMsr2005760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ñamendys-Silva SA, Respiratory support for patients with COVID-19 infection. Lancet Respir Med. 2020;8:e18. doi: 10.1016/S2213-2600(20)30110-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Giovanni S. Position Statement of the ESC Council on Hypertension on ACE-Inhibitors and Angiotension Receptor Blockers. 2020. http://tinyurl.com/tqd8uos (accessed 16 May)
- 46.Zhang H, Liu G, Zhou W et al. Neprilysin inhibitor-angiotensin II receptor blocker combination therapy (sacubitril/valsartan) suppresses atherosclerotic plaque formation and inhibits inflammation in apolipoprotein E-deficient mice. Sci Rep. 2019;9:6509. doi: 10.1038/s41598-019-42994-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gurwitz D. Angiotensin receptor blockers as tentative SARS-CoV-2 therapeutics. Drug Dev Res. 2020. epub ahead of press. [DOI] [PMC free article] [PubMed]
- 48.Kupferschmidt K, Cohen J. Race to find COVID-19 treatments accelerates. Science. 2020;367:1412. doi: 10.1126/science.367.6485.1412. [DOI] [PubMed] [Google Scholar]
- 49.Cortegiani A, Ingoglia G, Ippolito M, Giarratano A, Einav S. A systematic review on the efficacy and safety of chloroquine for the treatment of COVID-19. J Crit Care. 2020. pp. S0883–9441.pp. 30390–7. epub ahead of press. [DOI] [PMC free article] [PubMed]
- 50.Chatre C, Roubille F, Vernhet H et al. Cardiac complications attributed to chloroquine and hydroxychloroquine: a systematic review of the literature. Drug Saf. 2018;41:919–31. doi: 10.1007/s40264-018-0689-4. [DOI] [PubMed] [Google Scholar]
- 51.Maisch N.M., Kochupurackal JG, Sin J. Azithromycin and the risk of cardiovascular complications. J Pharm Pract. 2014;27:496–500. doi: 10.1177/0897190013516503. [DOI] [PubMed] [Google Scholar]
- 52.Mercuro NJ, Yen CF, Shim DJ Risk of QT interval prolongation associated with use of hydroxychloroquine with or without concomitant azithromycin among hospitalized patients testing positive for coronavirus disease 2019 (COVID- 19) JAMA Cardiol. 2020. epub ahead of press. [DOI] [PMC free article] [PubMed]
- 53.Wu Q, Zhou L, Sun X et al. Altered lipid metabolism in recovered SARS patients twelve years after infection. Sci Rep. 2017;7:9110. doi: 10.1038/s41598-017-09536-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yang X, Yu Y, Xu J et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–81. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wu C, Chen X, Cai Y. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 Pneumonia in Wuhan, China. JAMA Intern Med. 2020. epub ahead of press. [DOI] [PMC free article] [PubMed]
- 56.Chen N, Zhou M, Dong X et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–13. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Guan WJ, Ni ZY, Hu Y et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–20. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Petrilli CM, Jones SA, Yang J Factors associated with hospitalization and critical illness among 4,103 patients with COVID-19 disease in New York City. medRxiv. 2020. Preprint. [DOI]
- 59.Richardson S, Hirsch JS, Narasimhan M Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020. epub ahead of press. [DOI] [PMC free article] [PubMed]
- 60.Arentz M, Yim E, Klaff L et al. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State. JAMA. 2020;323:1612–4. doi: 10.1001/jama.2020.4326. [DOI] [PMC free article] [PubMed] [Google Scholar]


