Abstract
Aims/hypothesis
This study aimed to determine, in women with gestational diabetes (GDM), the changes in insulin sensitivity (Matsuda Insulin Sensitivity Index; ISOGTT), insulin response and disposition index (DI) from late pregnancy (34–37 weeks gestation, T1), to early postpartum (1–5 days, T2) and late postpartum (6–12 weeks, T3). A secondary aim was to correlate the longitudinal changes in maternal lipids, adipokines, cytokines and weight in relation to the changes in ISOGTT, insulin response and DI.
Methods
ISOGTT, insulin response and DI were calculated at the three time points (T1, T2 and T3) using the results of a 75 g OGTT. Adipokines, cytokines and lipids were measured prior to each OGTT. Linear mixed-effects models were used to compare changes across each time point. Changes in ISOGTT, insulin response and DI were correlated with changes in maternal adipokines, cytokines and lipids at each time point.
Results
A total of 27 women completed all assessments. Compared with T1, ISOGTT was 11.20 (95% CI 8.09, 14.31) units higher at 1–5 days postpartum (p < 0.001) and was 5.49 (95% CI 2.38, 8.60) units higher at 6–12 weeks postpartum (p < 0.001). Compared with T1, insulin response values were 699.6 (95% CI 957.5, 441.6) units lower at T2 (p < 0.001) and were 356.3 (95% CI 614.3, 98.3) units lower at T3 (p = 0.004). Compared with T1, the DI was 6434.1 (95% CI 2486.2, 10,381.0) units higher at T2 (p = 0.001) and was 4262.0 (95% CI 314.6, 8209.3) units higher at T3 (p = 0.03). There was a decrease in mean cholesterol, triacylglycerol, LDL-cholesterol and VLDL-cholesterol from T1 to T2 (all p < 0.001), and an increase in mean C-reactive protein, IL-6 and IL-8 from T1 to T2 (all p < 0.001). Mean leptin decreased from T1 to T2 (p = 0.001). There was no significant change in mean adiponectin (p = 0.99) or TNF-α (p = 0.81) from T1 to T2. The mean maternal BMI decreased from T1 to T2 (p = 0.001) and T3 (p < 0.001). There were no significant correlations between any measure of change in ISOGTT, insulin response and DI and change in maternal cytokines, adipokines, lipids or weight from T1 to T2.
Conclusions/interpretation
In women with GDM, delivery was associated with improvement in both insulin sensitivity and insulin production within the first few days. Improvement in insulin production persisted for 6–12 weeks, but insulin sensitivity deteriorated slightly. These changes in glucose metabolism were not associated to changes in lipids, leptin, inflammation markers or body weight.
Trial registration
Keywords: Disposition index, Gestational diabetes, Insulin response, Insulin sensitivity
Introduction
Gestational diabetes (GDM) is a common metabolic complication of pregnancy, affecting between 6% and 18% of all pregnancies in the USA depending on the criteria used for diagnosis [1, 2]. Among women with a GDM-affected pregnancy, the postpartum prevalence (4–20 weeks after delivery) of impaired glucose tolerance is 17–23% and of diabetes is 5–14% [3–5]. The American College of Obstetricians and Gynecologists (ACOG) recommends that women with a history of GDM have screening for diabetes at 6–12 weeks postpartum [6]. Owing to the difficulties in completing postpartum screening for women who developed GDM [7, 8], novel approaches, including screening prior to discharge during the delivery hospitalisation, have been proposed [9–11]. However, before a change in current practice is considered, a more robust evaluation of postpartum glucose metabolism and physiology is needed for women affected by GDM. Historically, waiting until 6 weeks postpartum to perform screening for diabetes was based on assumptions that pregnancy specific factors (such as human placental lactogen, postpartum weight loss and other potential placental mediated factors) that contribute to physiological insulin resistance during pregnancy will resolve by 6–8 weeks postpartum [12–14]. However, there are limited data in the literature to support this clinical practice.
Therefore, the purpose of this research was to prospectively assess glucose metabolism in women diagnosed with GDM and examine the longitudinal changes in late pregnancy, 1–5 days postpartum and 6–12 weeks postpartum. We hypothesised that maternal Matsuda Insulin Sensitivity Index (ISOGTT), insulin response and disposition index (DI) would improve immediately after delivery with a persistent improvement during the period 6–12 weeks postpartum. As a secondary aim we also assessed the longitudinal changes in maternal lipids, adipokines, cytokines and body weight in relation to the changes in glucose metabolism. We hypothesised that variations in postpartum glucose metabolism are associated with changes in maternal adipokines, cytokines, lipids and weight, as many of these factors are related to various aspects of glucose metabolism in non-pregnant individuals [15, 16].
Methods
We conducted a prospective observational study in which study-eligible women diagnosed with GDM completed a 75 g OGTT at three time points: at 34–37 weeks’ gestation (Time 1: T1), 1– 5 days postpartum, during the post-delivery hospitalisation (Time 2: T2), and again at 6–12 weeks postpartum (Time 3: T3). Nurse coordinators identified women diagnosed with GDM after 19 weeks of gestation based on a 100 g OGTT using the Carpenter–Coustan criteria [17]. Women identified as meeting the following eligibility criteria were then recruited with written informed consent: singleton pregnancy, ≥18 years of age, proficient in English, not currently incarcerated, and no evidence of overt diabetes in the last 12 months (defined as at least one of the following: an HbA1c value ≥48 mmol/mol (6.5%); a fasting blood sugar ≥7 mmol/l; or a random blood sugar >11.1 mmol/l, if confirmed with either a fasting blood sugar ≥7 mmol/l, 2 h post-OGTT glucose >11.1 mmol/l or an HbA1c ≥48 mmol/mol (6.5%) [18].
Eligible women who were willing to have a 75 g OGTT at all three time points (T1, T2 and T3) to assess the changes in ISOGTT, insulin response and DI in addition to fasting lipids, adipokines and cytokines, were recruited at MetroHealth Medical Center, a tertiary academic medical centre. Enrolled women became ineligible for participation if any of the following occurred: stillbirth, delivery <34 weeks of gestation, clinical evidence of infection, documented use of steroids within 7 days of the study visit, or documented use of glucose control medication at any time post delivery. Study participants signed a written consent form and were compensated for their time and travel. The study protocol and all data collection documents were approved by institutional review boards at Battelle Memorial Institute and MetroHealth Medical Center, Case Western Reserve University. The study was registered under ClinicalTrials.gov identifier NCT02082301.
Study OGTTs required an overnight fast with six blood samples drawn over the course of 2 h: −10 min, 0 min, 30 min, 60 min, 90 min and 120 min. All samples were assessed in duplicate. Glucose concentrations were analysed using the glucose oxidase method (YSI, Yellow Springs, OH, USA). Insulin concentration was assessed with ELISA (kit EZHI-14K, EMD Millipore, Billerica, MA, USA) with the following CV: 0.1–17.1% at each time point (−10, 0, 30, 60, 90 and 120 min). A fasting lipid profile (cholesterol, LDL-cholesterol, VLDL-cholesterol, HDL-cholesterol and triacylglycerol) was measured in the hospital laboratory before administration of the OGTT. Basal concentrations of IL-8, IL-6 and TNF-α in maternal plasma were measured using Quantikine ELISA kits according to the manufacturer’s instructions (R&D Systems, Minneapolis, MN, USA) with the following CVs: 0.2–11.9%, 0.3–12.3% and 0.1–14.8%, respectively. C-reactive protein (CRP) concentration in maternal plasma was determined by using ELISA (Alpha Diagnostics, San Antonio, TX, USA) according to the manufacturer’s instructions with CV 0.7–13.9%. Basal adiponectin and leptin in maternal plasma were determined using ELISA kits (EMD Millipore, Burlington, MA, USA) according to the manufacturer’s directions with CVs of 0.08–11.2% and 0.18–15.2%, respectively. The insulin, CRP and adipokines were batched to avoid interassay variation. All study visits were completed through the MetroHealth Medical Center clinical research units of the Clinical and Translational Science Collaborative at Case Western Reserve University. At the completion of the study, all participants and their obstetricians were provided with a letter including their 6 week results and information on appropriate follow-up, as needed.
The results of the laboratory tests at T1 and T2 were used to test the hypotheses as described previously. This protocol was not powered to assess the clinical utility of early postpartum OGTTs in women diagnosed with GDM. Immediate postpartum glucose testing for determining glucose intolerance will combine results from the T1 and T2 postpartum OGTTs from this protocol with results from a much larger research protocol where only immediate and 6–12 weeks postpartum OGTTs will be used. This manuscript is currently in preparation.
Research nurse coordinators abstracted medical record data on enrolled participants. Data abstracted included maternal demographic information, medical history, height, pre-pregnancy weight (self-reported), medications, delivery date, maternal weight at delivery, maternal and infant health at delivery (including newborn weight, Apgar score and mode of delivery), and breastfeeding status at discharge. If there was not adequate control of glucose with nutrition counselling and increased physical activity, the need for medication for glucose control during pregnancy was also abstracted. At the study institution, only insulin was used for glycaemic control during pregnancy, if diet therapy and increased physical activity were deemed insufficient to maintain glucose homeostasis. For analysis, we calculated a pre-pregnancy BMI, and relative gestational weight gain as [(last prenatal care visit weight) − (pre-pregnancy weight)]/(pre-pregnancy weight). Descriptive statistics were compiled from all study participants.
Insulin sensitivity was estimated using the ISOGTT, which has been validated in pregnancy [19]. ISOGTT was calculated as: 10,000/√[(glucose0 × insulin0) × (glucosemean × insulinmean)], using glucose and insulin measurements as mmol/l and pmol/l, respectively. First phase insulin response was calculated using the Stumvoll method as: [1283 + (1.829 × insulin30) − (138.7 × glucose30) + (3.772 × I0)] [20], using glucose and insulin measurements as mmol/l and pmol/l, respectively. The DI was calculated as the product of ISOGTT and first phase insulin response [21]. Glucose AUC was calculated using the trapezoidal method. Changes in ISOGTT, insulin response, DI and glucose AUC were compared for T1 vs T2, T1 vs T3 and T2 vs T3 with a p value of <0.05 considered significant. We also assessed changes in maternal lipids and cytokines over the same time intervals (p < 0.05 considered significant). Finally, we estimated the correlations of changes in ISOGTT, insulin response and DI with changes in maternal lipid adipokine and cytokine measurements. This was performed comparing the delta of ISOGTT, insulin response or DI with the delta of each maternal lipid and cytokine (T2 − T1, T3 − T1 and T3 − T2). For these analyses p ≤ 0.01 was considered significant owing to the number of comparisons.
An a priori sample size calculation showed that 26 women provided sufficient power (90%) to demonstrate a 10% improvement in ISOGTT from late pregnancy (7.5) to immediate postpartum (8.25) assuming an α level of 0.05 and an estimated SD of the paired difference of 1.09. In this study, participant characteristics were described using valid counts and proportions for all nominal and ordinal distributions, while median with interquartile range (IQR) was used to describe the distributions of age, pre-pregnancy BMI (kg/m2), birthweight (g), and gestational age (weeks).
For the primary outcome, a linear mixed-effects model was used to assess for change in participants’ mean ISOGTT from T1 to T2 and T3. Because participants could contribute multiple measures of ISOGTT to the analysis (i.e., one for each time point), random intercepts were allowed for each participant while specifying a completely general (unstructured) covariance matrix to account for their paired (dependent) observations. Further, because the overall type 3 test of the fixed effect was statistically significant, all possible pairwise comparisons of ISOGTT (i.e. from T1 to T2 and T3) were conducted using a Sidak correction to control the type 1 error rate. In these comparisons, a Kenward–Roger correction was used to adjust the denominator degrees of freedom for small sample bias [22]. The same approach was used to assess for change in insulin response, DI, cholesterol (mmol/l), triacylglycerol (mmol/l), HDL-cholesterol (mmol/l), LDL-cholesterol (mmol/l), VLDL-cholesterol (mmol/l), CRP (nmol/l), IL-6 (pg/ml), IL-8 (pg/ml), adiponectin (ng/ml), leptin (ng/ml), TNF-α (pg/ml), and BMI (kg/m2). For all models, the fundamental assumptions of normality and homogeneity were assessed using QQ plots and residual plots, respectively, while outliers were assessed using boxplots. Further, Friedman’s non-parametric rank test was used as sensitivity analysis to confirm all model conclusions. Regarding missing data, it is important to note that all available data were included in the analysis. However, all data were missing for ten women who did not complete all study visits. For this reason, these women were excluded from the analysis. Baseline characteristics were compared between participants who completed the study and those that were lost to follow up. Continuous variables were compared with the Mann–Whitney U test and categorical variables were compared with the Fisher’s exact test.
Finally, Spearman correlations were used to describe the association between change in participants’ laboratory values with their change in ISOGTT, insulin response and DI from late pregnancy to early and late postpartum. All analyses were completed using SAS version 9.4 (Cary, NC, USA).
Results
A total of 37 women were enrolled, with 27 women completing all three study visits. For women who did not complete the study (n = 10), indications for not completing the protocol included: being ineligible at follow-up visits (n = 6), participant refusal to complete the T2 or T3 study visit (n = 3) and lost to follow-up (n = 1). The laboratory specimens were not processed for the women who did not complete, and were discarded. For participants who completed all study visits, the median gestational age at enrolment was 31 (IQR 19–37) weeks with completion of the T1 OGTT at 34–37 weeks gestational age, completion of the T2 OGTT at a median of 2 days after delivery (range 1–5 days) and completion of the T3 OGTT at a median of 7 weeks after delivery (range 6–13 weeks). Table 1 includes the demographic characteristics and pregnancy histories for the 27 women who completed all three study visits. The median age was 31 years (IQR: 22.55–36.17) and a majority had less than a high school education. We observed no significant difference in maternal age at enrolment, race or ethnicity, gestational age at enrolment, or results of the 1 h 50 g glucose screening test between women who completed the study when compared with the ten women lost to follow-up.
Table 1.
Demographic characteristics, pregnancy histories, and glucose results of the OGTT of women who completed an OGTT at T1, T2 and T3
| Characteristic | Summary |
|---|---|
| N | 27 |
| Age (years) | 31.04 (22.55–36.17) |
| Race | |
| Non-Hispanic White | 13 (48) |
| Non-Hispanic Black | 10 (37) |
| Hispanic | 3 (11) |
| Asian | 1 (3.7) |
| Education ≥12 years | 5 (19) |
| Gestational age at enrolment (weeks) | 31 (19–37) |
| Nulliparous | 11 (41) |
| Pre-pregnancy BMI (kg/m2) (n = 26) | 36.25 (27.37–43.08) |
| Ordinal pre-pregnancy BMI (n = 26) | |
| Normal (18.5–24.9 kg/m2) | 2 (7.7) |
| Overweight (25.0–29.9 kg/m2) | 8 (31) |
| Obese (≥30 kg/m2) | 16 (62) |
| Tobacco use during pregnancy | 6 (22) |
| Insulin use during pregnancy | 17 (63) |
| Caesarean section delivery | 13 (48) |
| Birthweight (g) | 3490 (3150–3910) |
| Gestational age at delivery (weeks) | 39 (37–39) |
| Breastfeeding | |
| T2 | 17 (63) |
| T3 | 9 (33) |
| Fasting glucose (mmol/l) | |
| T1 | 4.94 (3.83–7.49) |
| T2 | 4.33 (3.61–5.55) |
| T3 | 5.38 (4.16–6.77) |
| 120 min OGTT glucose (mmol/l) | |
| T1 | 9.21 (6.77–13.38) |
| T2 | 8.32 (5.49–11.27) |
| T3 | 7.10 (3.66–10.99) |
Data are median (IQR) or n (%)
Unless otherwise stated, N = 27 for all summaries
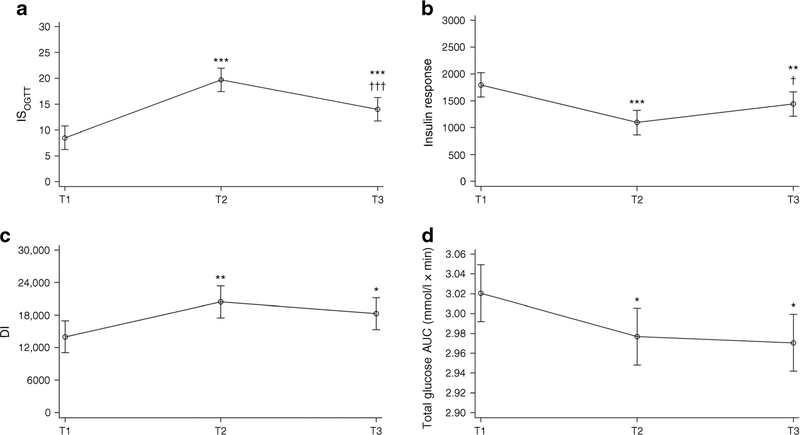
Figure 1 presents the results of the ISOGTT, insulin response, DI and glucose AUC at all three time points. Compared with T1, ISOGTT was 11.20 (95% CI 8.09, 14.31) units higher at 1–5 days postpartum (p < 0.001) and was 5.49 (95% CI 2.38, 8.60) units higher at 6–12 weeks postpartum (p < 0.001). Conversely, compared with T2, ISOGTT was 5.71 (95% CI 8.82, 2.60) units lower at T2 (p < 0.001) (Fig. 1a). As expected, there were also significant changes in insulin response (Fig. 1b). Compared with T1, insulin response values were 699.6 (95% CI 957.5, 441.6) units lower at T2 (p < 0.001) and were 356.3 (95% CI 614.3, 98.3) units lower at T3 (p = 0.004). Conversely, compared with T2, insulin response values were 343.3 (95% CI 85.3, 601.3) units higher at T3 (p = 0.01). We also observed improvement in the DI (Fig. 1c). Compared with T1, the DI was 6434.1 (95% CI 2486.2, 10,381.0) units higher at T2 (p = 0.001) and was 4262.0 (95% CI 314.6, 8209.3) units higher at T3 (p = 0.03). Results were similar for glucose AUC (Fig. 1d). Compared with T1, the glucose AUC was 0.04 (95% CI 0.08, 0.008) mmol/l × min lower at T2 (p = 0.049) and was 0.05 (95% CI 0.09, 0.01) mmol/l × min lower at T3 (p = 0.02).
Fig. 1.
ISOGTT (a), first phase insulin response (b), DI (c) and glucose AUC (d) at T1, T2 and T3, measured using a linear mixed-effects model. *p<0.05, **p<0.01, ***p<0.001 vs T1; †p<0.05, †††p<0.001 vs T2
Table 2 shows the lipid profile, cytokines, adipokines and maternal BMI across the three time points with the mean and standard deviation of each measured variable listed in Table 3. There was a significant decrease in mean cholesterol, triacylglycerol, LDL-cholesterol and VLDL-cholesterol from T1 to T2 (all p < 0.001) with no significant change in HDL-cholesterol over the same interval (p = 0.76). The majority of lipids, specifically triacylglycerol, HDL-cholesterol and VLDL-cholesterol, were also lower at T3 compared with T2 (all p < 0.001). All maternal lipids except LDL-cholesterol were significantly lower at T3 when compared with T1. No differences were noted for TNF-α or adiponectin over any interval. There was a significant increase in IL-6, IL-8 and CRP from T1 to T2 (all p < 0.001), followed by a decrease between early postpartum to late postpartum for IL-6 (p < 0.001) and CRP (p < 0.001). As expected, maternal BMI was significantly lower at each follow-up visit (overall p < 0.001).
Table 2.
Mean difference with 95% CI of maternal plasma metabolites at T1, T2 and T3
| Mean difference | 95% CI |
p value | ||
|---|---|---|---|---|
| Lower | Upper | |||
| Cholesterol (mmol/l) | <0.001a | |||
| T2 vs T1 | −0.70 | −1.02 | −0.37 | <0.001 |
| T3 vs T1 | −0.88 | −1.21 | −0.55 | <0.001 |
| T3 vs T2 | −0.18 | −0.51 | 0.15 | 0.45 |
| Triacylglycerol (mmol/l) | <0.001a | |||
| T2 vs T1 | −0.60 | −0.92 | −0.27 | <0.001 |
| T3 vs T1 | −1.19 | −1.52 | −0.86 | <0.001 |
| T3 vs T2 | −0.60 | −0.93 | −0.27 | <0.001 |
| HDL-cholesterol (mmol/l) | <0.001a | |||
| T2 vs T1 | −0.05 | −0.18 | 0.09 | 0.76 |
| T3 vs T1 | −0.48 | −0.62 | −0.35 | <0.001 |
| T3 vs T2 | −0.44 | −0.57 | −0.30 | <0.001 |
| LDL-cholesterol (mmol/l) | <0.001a | |||
| T2 vs T1 | −0.50 | −0.79 | −0.22 | <0.001 |
| T3 vs T1 | −0.11 | −0.39 | 0.18 | 0.74 |
| T3 vs T2 | 0.40 | 0.11 | 0.68 | 0.004 |
| VLDL-cholesterol (mmol/l) | <0.001a | |||
| T2 vs T1 | −0.22 | −0.34 | −0.10 | <0.001 |
| T3 vs T1 | −0.44 | −0.56 | −0.31 | <0.001 |
| T3 vs T2 | −0.22 | −0.34 | −0.09 | <0.001 |
| CRP (nmol/l) | <0.001a | |||
| T2 vs T1 | 653.73 | 488.49 | 818.97 | <0.001 |
| T3 vs T1 | −22.76 | −188.10 | 142.48 | 0.98 |
| T3 vs T2 | −676.49 | −841.73 | −511.25 | <0.001 |
| IL-6 (pg/ml) | <0.001a | |||
| T2 vs T1 | 8.18 | 6.34 | 10.01 | <0.001 |
| T3 vs T1 | −0.10 | −1.92 | 1.72 | 0.99 |
| T3 vs T2 | −8.28 | −10.11 | −6.44 | <0.001 |
| IL-8 (pg/ml) | <0.001a | |||
| T2 vs T1 | 2.37 | 1.12 | 3.61 | <0.001 |
| T3 vs T1 | 1.36 | 0.11 | 2.60 | 0.03 |
| T3 vs T2 | −1.01 | −2.28 | 0.26 | 0.16 |
| Adiponectin (ng/ml) | 0.96a | |||
| T2 vs T1 | −80.95 | −1273.61 | 1111.72 | 0.99 |
| T3 vs T1 | −138.15 | −1314.33 | 1038.03 | 0.99 |
| T3 vs T2 | −57.20 | −1249.87 | 1135.47 | 0.99 |
| Leptin (ng/ml) | 0.002a | |||
| T2 vs T1 | −20.51 | −33.93 | −7.09 | 0.001 |
| T3 vs T1 | −10.59 | −24.01 | 2.84 | 0.16 |
| T3 vs T2 | 9.92 | −3.50 | 23.34 | 0.21 |
| TNF-α (pg/ml) | 0.72a | |||
| T2 vs T1 | 0.07 | −0.14 | 0.27 | 0.81 |
| T3 vs T1 | 0.03 | −0.17 | 0.23 | 0.98 |
| T3 vs T2 | −0.04 | −0.24 | 0.17 | 0.96 |
| BMI (kg/m2) | <0.001a | |||
| T2 vs T1 | −0.93 | −1.50 | −0.36 | 0.001 |
| T3 vs T1 | −2.67 | −3.22 | −2.13 | <0.001 |
| T3 vs T2 | −1.74 | −2.31 | −1.17 | <0.001 |
Confidence intervals and significance values have been adjusted for multiple comparisons using a Sidak correction
Type 3 test of the fixed effect
Table 3.
Summary of mean values and standard deviations of maternal plasma metabolites at T1, T2 and T3
| Visit |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| T1 |
T2 |
T3 |
|||||||
| N | Mean | SD | N | Mean | SD | N | Mean | SD | |
| Cholesterol (mmol/l) | 27 | 5.71 | 1.08 | 27 | 5.02 | 0.98 | 27 | 4.84 | 1.00 |
| Triacylglycerol (mmol/l) | 27 | 2.66 | 0.92 | 27 | 2.07 | 0.88 | 26 | 1.41 | 0.64 |
| HDL-cholesterol (mmol/l) | 27 | 1.54 | 0.32 | 27 | 1.49 | 0.35 | 27 | 1.06 | 0.24 |
| LDL-cholesterol (mmol/l) | 27 | 3.39 | 1.00 | 27 | 2.89 | 0.88 | 27 | 3.28 | 0.72 |
| VLDL-cholesterol (mmol/l) | 27 | 0.98 | 0.34 | 27 | 0.76 | 0.32 | 25 | 0.53 | 0.23 |
| CRP (nmol/l) | 27 | 128.57 | 120.57 | 27 | 782.30 | 457.72 | 27 | 105.81 | 90.95 |
| IL-6 (pg/ml) | 27 | 4.65 | 1.77 | 26 | 12.83 | 4.18 | 27 | 4.55 | 1.78 |
| IL-8 (pg/ml) | 27 | 5.00 | 2.31 | 25 | 7.32 | 2.42 | 25 | 6.40 | 2.25 |
| Adiponectin (ng/ml) | 27 | 10,166.04 | 5299.78 | 26 | 9966.15 | 5170.54 | 27 | 10,027.89 | 4321.86 |
| Leptin (ng/ml) | 27 | 69.24 | 49.82 | 27 | 48.74 | 45.23 | 27 | 58.66 | 47.46 |
| TNF-α (pg/ml) | 26 | 1.39 | .42 | 25 | 1.46 | .38 | 26 | 1.42 | .31 |
| BMI (kg/m2) | 27 | 37.77 | 8.50 | 24 | 37.31 | 8.43 | 27 | 35.10 | 8.87 |
From T1 to T2 (electronic supplementary material [ESM] Table 1), there was a nominal negative association between change in leptin values and change in insulin response (Spearman r = −0.40, p = 0.04). Similarly, from early postpartum to late postpartum (ESM Table 2), there was a nominal negative association between change in TNF-α and change in insulin response (Spearman r = −0.40, p = 0.0499). There were no significant correlations between measure of change in glucose metabolism and change in maternal adipokines, cytokines, lipids or BMI. No significant correlations were noted from late pregnancy to late postpartum (ESM Table 3).
Discussion
The results of this study provide important physiological information, as we observed a significant improvement in ISOGTT and DI together with a decrease in insulin response from the third trimester of pregnancy (T1) to immediately after delivery (median 2 days postpartum; T2). Additionally, we observed similar improvements in ISOGTT, DI and insulin response at 6–12 week postpartum (T3) when compared with late pregnancy. We did not observe any significant associations among changes in circulating maternal lipids, adipokines or cytokines with changes in ISOGTT, insulin response or DI from late pregnancy to 1–5 days postpartum (T1 to T2) or from late pregnancy to 6–12 weeks postpartum (T1 to T3). These observations suggest that a majority of improvements in maternal glucose metabolism and insulin action occur in the immediate postpartum period. Further, we did not observe a significant correlation between changes in maternal ISOGTT, insulin response and DI with maternal weight changes over any time interval, despite a mean decrease of 5 kg from T2 to T3. Hence, the majority of the observed immediate postpartum changes in glucose metabolism are not related to the changes in lipids, adipokines, cytokines or maternal weight changes measured in this study. We hypothesise that this may be largely related to the delivery of the placenta.
The strengths of our study include the strict inclusion criteria (only women who had a diagnosis of GDM in the second trimester) and the prospective longitudinal nature of our study design. The diagnosis of GDM and implications for treatment prior to the second trimester remain controversial using current criteria for the diagnosis of GDM. Our study also has limitations. First, the sample size was small, and this limits potential subgroup analyses, such as women who were treated with diet alone or with insulin during pregnancy for glycaemic control. Further, there were limited data regarding degree of exclusive lactation and duration of breastfeeding among study participants. As a significant proportion of enrolled women (n = 37) did not complete all three study visits (n = 10 or 27.0%), owing to a variety of factors, there is the potential for selection bias. Finally, while we did not identify significant correlation between changes in glucose metabolism and measured lipids, adipokines or cytokines, other factors (such as human placental lactogen) made by the placenta, not assessed in this study, may have resulted in improvement in immediate postpartum glucose homeostasis.
There are limited data regarding the physiological changes in glucose metabolism in the immediate postpartum time period in women diagnosed with GDM [12]. This report, by Ryan et al, examining insulin sensitivity in late pregnancy and the immediate postpartum period in women with normal glucose tolerance and GDM, reported a significant improvement in insulin sensitivity 3 days postpartum. The strength of the Ryan et al study was that the investigators estimated insulin sensitivity using the euglycaemic clamp whereas we used the ISOGTT/Matsuda Insulin Sensitivity Index. While the euglycaemic clamp is the gold standard for estimating insulin sensitivity, the ISOGTT has been validated during pregnancy in women with normal glucose tolerance and GDM [19]. A relative weakness of the Ryan et al study is that there were only four women (2 women with normal glucose tolerance and two women diagnosed with GDM) examined in late pregnancy and immediately postpartum. Consistent with Ryan et al, we observed a greater than 132% improvement in insulin sensitivity immediately postpartum (T2) with a persistent improvement noted at 6–12 weeks postpartum (T3).
Mazaki-Tovi et al examined 27 women with normal glucose tolerance the day prior to a planned caesarean delivery and 4 days postpartum [23], and found a significant increase in estimates of insulin sensitivity postpartum using HOMA. HOMA estimates of insulin sensitivity, while correlated with clamp measures of insulin sensitivity, are not as robust as using the ISOGTT in pregnancy [19]. Similar to our findings, there were no differences in adiponectin before and after delivery but there was a decrease in leptin concentrations immediately postpartum. In summary, both the Ryan and Mazaki-Tovi studies report that there is a significant increase in insulin sensitivity in the first few days after delivery in women with normal glucose tolerance. Based on the Ryan study and our data, the significant improvement in insulin sensitivity extends to women with GDM. None of the studies found a correlation with any frequently measured hormones or cytokines frequently related to changes in insulin sensitivity. These data highlight that the improvements in insulin sensitivity immediately after delivery are not explained by changes in maternal weight, lipids, adipokines or cytokines and therefore may be related to other factors, such as those produced by the placenta.
Based on clamp studies, decreased insulin sensitivity in pregnancy has been speculated to be related to decreases in post-receptor insulin signalling [12]. Clamps and skeletal muscle biopsy studies have been performed in late pregnancy and 1 year postpartum in women with normal glucose tolerance and GDM. In women with normal glucose tolerance, improvements in insulin sensitivity were related to significant postpartum weight loss and increases in skeletal muscle IRS-1 [24]. By contrast, in women with GDM, who did not have significant weight loss or improvement in insulin sensitivity postpartum, there was evidence of increased skeletal muscle inflammation and persistent dysfunction of post-receptor insulin signalling [25]. In summary, as yet uncharacterised placental factors are likely to be related to the significant improvements in insulin sensitivity immediately postpartum. Longer term improvements in insulin sensitivity are probably related to other factors such as decreases in chronic inflammation and weight loss. Hence, avoiding excess gestational weight gain and postpartum weight retention is a reasonable first step to restoring insulin sensitivity in women with normal glucose tolerance and GDM [26].
Postpartum screening for both diabetes mellitus and impaired glucose tolerance in women diagnosed with GDM continues to be a relevant clinical concern. Efforts such as the Diabetes Prevention Program [27] have reported significant decreases in progression to type 2 diabetes using either intensive lifestyle intervention or metformin in women diagnosed with GDM. ACOG continues to recommend that women with a GDM-affected pregnancy have a 2 h 75 g OGTT performed 6 weeks postpartum to screen for diabetes [6]. However, given the poor follow-up of women with GDM and the limitations of screening for diabetes at 6 weeks postpartum, new approaches are needed. To that end, we report a significant improvement in ISOGTT, insulin response and DI within days of delivery in women diagnosed with GDM. These observed physiological changes in maternal metabolism support further clinical studies to evaluate the utility of early postpartum screening for glucose intolerance in women diagnosed with GDM.
The significant improvement in insulin sensitivity in the immediate postpartum period in women with normal glucose tolerance and those with GDM emphasise the importance of the placenta in affecting maternal glucose metabolism during pregnancy. Although the decrease in human insulin sensitivity during pregnancy has been well described for many years there is still no unifying concept or agreement as to the underlying mechanism(s) [28]. Suggested mediators responsible for the decrease in insulin sensitivity include, but are not limited to, human placental lactogen, cytokines such as TNF-α, placental growth hormone and cortisol, and ‘other factors’ produced by the placenta. However, the physiological mechanisms responsible for the significant changes in glucose metabolism remain poorly defined. [29, 30] We were unable to find any significant correlations for the changes in ISOGTT, insulin response and DI with lipids or cytokines, from late pregnancy to immediately postpartum. Further, there was neither a significant increase in adiponectin during this same time period nor a relationship between decrease in weight and improvement in insulin sensitivity. As postpartum changes in weight from immediately after birth to 6–12 weeks post-delivery are most likely to represent decreases in maternal plasma volume (or water), changes in weight would therefore not be anticipated to affect glucose metabolism. Our findings provide a possible physiological mechanism for clinical observations of decreased insulin requirements among women with GDM treated with insulin or oral agents immediately postpartum. Identifying mediators for the physiological improvement in insulin sensitivity postpartum in women with GDM has the potential for improving our understanding and the therapeutic implications for treatment of GDM during pregnancy and prevention of type 2 diabetes after pregnancy.
Supplementary Material
Research in context.
What is already known about this subject?
There is significant improvement in glucose metabolism among women with normal glucose tolerance in the early postpartum period
There is scant information regarding the changes in glucose metabolism in women affected by gestational diabetes in the immediate postpartum period
There is no consensus as to the factor(s) relating to the significant changes in postpartum insulin resistance
What is the key question?
Is there a significant improvement in insulin sensitivity, in the days after delivery, in women affected by gestational diabetes?
What are the new fingings?
There is a significant increase in insulin sensitivity and disposition index in the early and late postpartum period in women whose pregnancies were complicated by gestational diabetes
There is a significant decrease in insulin response in the early and late postpartum period in women who had gestational diabetes
There were no significant correlations of the longitudinal changes in maternal lipids, adipokines, cytokines and body weight with changes in insulin sensitivity, insulin response and disposition index from late pregnancy and during the early postpartum period
How might this impact on clinical practice in the foreseeable future?
Owing to difficulties in completing OGTT in the late postpartum period (6–12 weeks postpartum), screening for glucose intolerance prior to hospital discharge (1–5 days postpartum) may be a viable option for future research
Acknowledgements
We would like to thank the research staff of the Clinical Research Center at MetroHealth Medical Center, Case Western Reserve University, for their excellent contribution to the completion of the study protocol.
Funding The Clinical and Translational Science Collaborative (CTSC) Cleveland at Case Western Reserve University is supported by the National Institutes of Health (NIH) Clinical and Translational Science Award (CTSA) programme, grant number UL1TR000439.The CTSA programme is led by the NIH’s National Center for Advancing Translational Sciences (NCATS).
This manuscript was written in the course of employment by the United States Government with support from services provided by a contract with Battelle Memorial Institute (200-2008-27956, Task Order 23) and is not subject to copyright in the United States.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC, who were involved in the project but did not provide funding.
Abbreviations
- ACOG
American College of Obstetricians and Gynecologists
- CRP
C-reactive protein
- DI
The disposition index
- GDM
Gestational diabetes
- IQR
Interquartile range
- ISOGTT
Matsuda Insulin Sensitivity Index
- T1
Time 1: 34–37 weeks’ gestation
- T2
Time 2: 1–5 days postpartum
- T3
Time 3: 6–12 weeks postpartum
Footnotes
Duality of interest The study sponsor (Batetelle Memorial Institute) was involved in the design of the study, the collection, analysis, and interpretation of data, and drafting of the manuscript. The authors declare no other duality of interest associated with this manuscript.
Data availability Please contact the primary author for the availability of the data presented within.
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s00125-019-05051-0) contains peer-reviewed but unedited supplementary material, which is available to authorised users.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Correa A, Bardenheier B, Elixhauser A, Geiss LS, Gregg E (2015) Trends in prevalence of diabetes among delivery hospitalizations, United States, 1993–2009. Matern Child Health J 19(3):635–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Waters TP, Dyer AR, Scholtens DM et al. (2016) Maternal and neonatal morbidity for women who would be added to the diagnosis of GDM using IADPSG Criteria: a secondary analysis of the hyperglycemia and adverse pregnancy outcome study. Diabetes Care 39(12):2204–2210. 10.2337/dc16-1194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schaefer-Graf UM, Klavehn S, Hartmann R et al. (2009) How do we reduce the number of cases of missed postpartum diabetes in women with recent gestational diabetes mellitus? Diabetes Care 32(11):1960–1964. 10.2337/dc09-0627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kitzmiller JL, Dang-Kilduff L, Taslimi MM (2007) Gestational diabetes after delivery. Short-term management and long-term risks. Diabetes Care 30 Suppl 2:S225–S235. 10.2337/dc07-s221 [DOI] [PubMed] [Google Scholar]
- 5.Catalano PM, Vargo KM, Bernstein IM, Amini SB (1991) Incidence and risk factors associated with abnormal postpartum glucose tolerance in women with gestational diabetes. Am J Obstet Gynecol 165(4 Pt 1):914–919. 10.1016/0002-9378(91)90438-w [DOI] [PubMed] [Google Scholar]
- 6.American College of Obstetricians and Gynecologists Committee on Practice Bulletins—Obstetrics (2017) ACOG Practice Bulletin No. 180: Gestational Diabetes Mellitus. Obstet Gynecol 130(1): e17–e37. 10.1097/AOG.0000000000002159 [DOI] [PubMed] [Google Scholar]
- 7.Hale NL, Probst JC, Liu J, Martin AB, Bennett KJ, Glover S (2012) Postpartum screening for diabetes among Medicaid-eligible South Carolina women with gestational diabetes. Womens Health Issues 22(2):e163–e169. 10.1016/j.whi.2011.08.003 [DOI] [PubMed] [Google Scholar]
- 8.Hunt KJ, Logan SL, Conway DL, Korte JE (2010) Postpartum screening following GDM: how well are we doing? Curr Diab Rep 10(3):235–241. 10.1007/s11892-010-0110-x [DOI] [PubMed] [Google Scholar]
- 9.Werner EF, Has P, Tarabulsi G, Lee J, Satin A (2016) Early postpartum glucose testing in women with gestational diabetes mellitus. Am J Perinatol 33(10):966–971. 10.1055/s-0036-1583193 [DOI] [PubMed] [Google Scholar]
- 10.Dinglas C, Muscat J, Heo H, Islam S, Vintzileos A (2017) Immediate postpartum glucose tolerance testing in women with gestational diabetes: a pilot study. Am J Perinatol 34(12):1264–1270. 10.1055/s-0037-1606620 [DOI] [PubMed] [Google Scholar]
- 11.Carter EB, Martin S, Temming LA, Colditz GA, Macones GA, Tuuli MG (2018) Early versus 6–12 week postpartum glucose tolerance testing for women with gestational diabetes. J Perinatol 38(2): 118–121. 10.1038/jp.2017.159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ryan EA, O’Sullivan MJ, Skyler JS (1985) Insulin action during pregnancy. Studies with the euglycemic clamp technique. Diabetes 34(4):380–389. 10.2337/diab.34.4.380 [DOI] [PubMed] [Google Scholar]
- 13.Kirwan JP, Hauguel-De Mouzon S, Lepercq J et al. (2002) TNF-α is a predictor of insulin resistance in human pregnancy. Diabetes 51(7):2207–2213. 10.2337/diabetes.51.7.2207 [DOI] [PubMed] [Google Scholar]
- 14.Xiang AH, Peters RK, Trigo E, Kjos SL, Lee WP, Buchanan TA (1999) Multiple metabolic defects during late pregnancy in women at high risk for type 2 diabetes. Diabetes 48(4):848–854. 10.2337/diabetes.48.4.848 [DOI] [PubMed] [Google Scholar]
- 15.Plomgaard P, Nielsen AR, Fischer CP et al. (2007) Associations between insulin resistance and TNF-α in plasma, skeletal muscle and adipose tissue in humans with and without type 2 diabetes. Diabetologia 50(12):2562–2571. 10.1007/s00125-007-0834-6 [DOI] [PubMed] [Google Scholar]
- 16.Pirola L, Ferraz JC (2017) Role of pro- and anti-inflammatory phenomena in the physiopathology of type 2 diabetes and obesity. World J Biol Chem 8(2):120–128. 10.4331/wjbc.v8.i2.120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carpenter MW, Coustan DR (1982) Criteria for screening tests for gestational diabetes. Am J Obstet Gynecol 144(7):768–773. 10.1016/0002-9378(82)90349-0 [DOI] [PubMed] [Google Scholar]
- 18.American Diabetes Association (2015) Classification and Diagnosis of Diabetes. Diabetes Care 38 Suppl 1:S8–S16 [DOI] [PubMed] [Google Scholar]
- 19.Kirwan JP, Huston-Presley L, Kalhan SC, Catalano PM (2001) Clinically useful estimates of insulin sensitivity during pregnancy: validation studies in women with normal glucose tolerance and gestational diabetes mellitus. Diabetes Care 24(9):1602–1607. 10.2337/diacare.24.9.1602 [DOI] [PubMed] [Google Scholar]
- 20.Stumvoll M, Mitrakou A, Pimenta W et al. (2000) Use of the oral glucose tolerance test to assess insulin release and insulin sensitivity. Diabetes Care 23(3):295–301. 10.2337/diacare.23.3.295 [DOI] [PubMed] [Google Scholar]
- 21.Bergman RN, Phillips LS, Cobelli C (1981) Physiologic evaluation of factors controlling glucose tolerance in man: measurement of insulin sensitivity and beta-cell glucose sensitivity from the response to intravenous glucose. J Clin Invest 68(6):1456–1467. 10.1172/jci110398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kenward MG, Roger JH (1997) Small sample inference for fixed effects from restricted maximum likelihood. Biometrics 53:983–997 [PubMed] [Google Scholar]
- 23.Mazaki-Tovi S, Kanety H, Pariente C et al. (2011) Insulin sensitivity in late gestation and early postpartum period: the role of circulating maternal adipokines. Gynecol Endocrinol 27(9):725–731. 10.3109/09513590.2010.500426 [DOI] [PubMed] [Google Scholar]
- 24.Kirwan JP, Varastehpour A, Jing M et al. (2004) Reversal of insulin resistance postpartum is linked to enhanced skeletal muscle insulin signaling. J Clin Endocrinol Metab 89(9):4678–4684. 10.1210/jc.2004-0749 [DOI] [PubMed] [Google Scholar]
- 25.Friedman JE, Kirwan JP, Jing M, Presley L, Catalano PM (2008) Increased skeletal muscle tumor necrosis factor-α and impaired insulin signaling persist in obese women with gestational diabetes mellitus 1 year postpartum. Diabetes 57(3):606–613. 10.2337/db07-1356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berggren EK, Presley L, Amini SB et al. (2015) Are the metabolic changes of pregnancy reversible in the first year postpartum? Diabetologia. 58(7):1561–1568. 10.1007/s00125-015-3604-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Orchard TJ, Temprosa M, Goldberg R et al. (2005) The effect of metformin and intensive lifestyle intervention on the metabolic syndrome: the Diabetes Prevention Program randomized trial. Ann Intern Med 142(8):611–619. 10.7326/0003-4819-142-8-200504190-00009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kalkhoff RK, Jacobson M, Lemper D (1970) Progesterone, pregnancy and the augmented plasma insulin response. J Clin Endocrinol Metab 31(1):24–28. 10.1210/jcem-31-1-24 [DOI] [PubMed] [Google Scholar]
- 29.Catalano PM, Huston L, Amini SB, Kalhan SC (1999) Longitudinal changes in glucose metabolism during pregnancy in obese women with normal glucose tolerance and gestational diabetes mellitus. Am J Obstet Gynecol 180(4):903–916. 10.1016/s0002-9378(99)70662-9 [DOI] [PubMed] [Google Scholar]
- 30.Catalano PM, Tyzbir ED, Wolfe RR et al. (1993) Carbohydrate metabolism during pregnancy in control subjects and women with gestational diabetes. Am J Phys 264(1 Pt 1):E60–E67 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.