Abstract
Indolamine-2,3-dioxygenase (IDO) is an intracellular enzyme that catalyzes amino acid tryptophan to L-kynurenine. IDO is overexpressed in various cancers and several IDO inhibitors have been assessed in multiple clinical trials. If an IDO inhibitor is to be commercialized, IDO immunohistochemistry will be an important method. In this study, 80% (28/35) of mature T- and natural killer (NK)-cell neoplasms showed positivity for IDO protein (score 1: five, score 2: one, score 3: seven, score 4: fifteen). In addition, 29.9% (23/77) of mature B-cell lymphomas showed positivity for IDO protein (score 1: three, score 2: tewelve, score 3: four, score 4: four). In mature B-cell lymphomas, 95.7% (22/23) of IDO positive cases were diffuse B-cell lymphomas. Our study includes various types of lymphoma that were previously unreported and shows various patterns of IDO stain according to the type. When the results are accumulated, IDO immunohistochemistry will be a useful tool to diagnose lymphomas and to predict their prognosis.
Keywords: Indolamine-2,3-dioxygenase (IDO); lymphoma; immunohistochemistry (IHC)
1. Introduction
Tumors express the antigens that induce the host immune response. Progression of tumors requires avoidance of host immune surveillance [1,2]. Recent studies have shown that tryptophan catabolism is one means of avoiding immune surveillance [3,4]. Indolamine-2,3-dioxygenase (IDO) is a cytosolic enzyme that catalyzes tryptophan. IDO converts the amino acid tryptophan to L-kynurenine [5]. The depletion of tryptophan and the production of L-kynurenine induces the apoptosis of T-cells and natural killer (NK)-cells [6,7,8]. In addition, the IDO-expressing macrophages, dendritic cells, and tumor cells suppress T-cell proliferation [7,8,9,10]. In previous reports, IDO expression and the serum concentration of L-kynurenine were negative prognostic factors in diffuse large B-cell lymphomas and adult T-cell leukemia/lymphomas [11,12,13]. In a previous immunohistochemical analysis for IDO expression in diffuse large B-cell lymphomas treated with R-CHOP chemotherapy, the IDO-positive group showed resistance to the treatment and a poorer prognosis than the IDO-negative group [14]. Immunohistochemistry is a relatively fast and inexpensive utility in diagnostic surgical pathology. Immunohistochemistry is widely used for subtyping of lymphomas and plays an important role in hematopathology. There are very few recent immunohistochemical assays of IDO in lymphomas [14,15,16]. To address different immunohistochemical features in various lymphomas, we performed immunohistochemistry of IDO in a Korean lymphoma cohort of a single center.
2. Materials and Methods
2.1. Study Population
This study was approved by the Institutional Review Board (IRB) of Samsung Changwon Hospital, Changwon, Korea (IRB FILE No. 2020-01-003, 23 January 2020). The study was retrospective, therefore the IRB waived the need for written informed consent. The medical records of Samsung Changwon Hospital between January 2014 and December 2019 were gathered. All slides of diagnosed lymphomas during the period were independently reviewed by two authors (H.Y.L and T.I.P) according to the World Health Organization (WHO) classification of tumors of hematopoietic and lymphoid tissues, 4th Edition. Of a total of 171 cases obtained by biopsy or excision, those with an insufficient amount of specimen (cut off: 0.25 cm²) and cases of controversial diagnosis were excluded from the study. The remaining 120 cases were enrolled in this study (Male:Female = 5:3; aged 10–86, mean = 59.4 years, median = 62 years). Of the 120 cases of lymphoma, 103 cases were Ann Arbor stage I, 12 cases were stage II, and five cases were stage III. In situ hybridization (ISH) with the Epstein-Barr virus (EBV)-encoded small RNA (EBER) were performed in 91 cases of lymphoma. A total of 26.4% (24/91) of cases showed positivity for ISH with EBER (Hodgkin Lymphoma: five, EBV-Positive diffuse large B-cell lymphoma (DLBCL), not otherwise specified (NOS): two, Extranodal NK-/T-cell Lymphoma: twelve, Peripheral T-cell Lymphoma, NOS: three, Angioimmunoblastic T-cell Lymphoma: one, Enteropathy-associated T-cell Lymphoma, Type II: one). All cases were negative for HIV infection. All specimens were obtained at the time of pathologic diagnosis before initiation of treatment. A total of seven cases of Hodgkin lymphoma, 77 cases of mature B-cell lymphoma, one B-Lymphoblastic lymphoma, and 35 cases of mature T- and NK-cell neoplasm were enrolled the study.
2.2. Immunohistochemistry for Indoleamine 2, 3-Dioxygenase
We reviewed all slides of the cases and selected one representative formalin-fixed, paraffin-embedded (FFPE) block from each case for immunohistochemistry. The representative blocks were cut on 4 μm thick sections and immunohistochemical staining was performed for Indoleamine 2, 3-dioxygenase (rabbit recombinant monoclonal, clone EPR20374, Abcam, 1:2000 dilution). All immunohistochemical staining was performed using the BenchMark XT autostainer (Ventana Medical Systems, Tucson, Arizona), according to the manufacturer’s protocol. The results were evaluated by two authors (H.Y.L and M.S.K) independently, and any discrepancies were reviewed to achieve a consensus. In previous studies, the reactive immune cells around the tumors were stained by IDO protein [17,18]. It was difficult or impossible to distinguish reactive immune cells from tumor cells. Considering the possibility of containing reactive cells in the tumors, percentage of stained cells equal to or higher than 5% were regards as positive. All lymphomas were scored based on percentage of tumor cell staining: 0 = <5%, 1 = 5–25%, 2 = 26–50%, 3 = 51–75%, and 4 = >75%. The moderate-to-strong cytoplasmic staining was regards as positive. In Hodgkin lymphomas, due to the scant cellularity of tumor cells, the expressions were evaluated to be positive or negative. The positivity for IDO in the vessels was judged in comparison with hematoxylin and eosin (H&E) stain. The vessels were lined by endothelium and covered by pericytes in H&E stain.
3. Results
3.1. Hodgkin Lymphoma
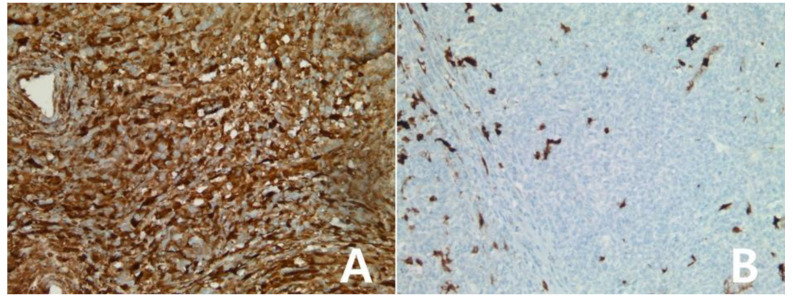
The clinicopathologic characteristics and the immunohistochemical results of IDO are summarized in Table 1. The male:female ratio was 1.3:1, and the age distribution was 35–79 years (mean = 61 years, median = 70 years). Four cases of classic Hodgkin lymphoma (three mixed cellularity and one nodular sclerosis), and three cases of nodular lymphocyte predominant Hodgkin lymphoma were included in the study. There was no difference of IDO staining pattern between classic type and nodular lymphocyte predominant type. In the positive cases, the tumor cells and vessels showed positivity for IDO protein (Figure 1). In the negative cases, only dendritic cells and macrophages showed positivity for IDO (Figure 2). Three cases were positive, and four cases were negative. In past studies [15,16], IDO had been expressed in the dendritic cells and the macrophages only. However, our results showed tumor cell positivity for IDO in several cases.
Table 1.
The clinicopathologic characteristics and the Indolamine-2,3-dioxygenase (IDO) expressions of Hodgkin lymphomas.
| Histologic Type | Age | Sex | Site of Involvement | IDO | |
|---|---|---|---|---|---|
| Classic Hodgkin Lymphoma | Tumor Cell | Vessel | |||
| Mixed Cellularity | 70 | M | Cervical lymph node | Positive | Positive |
| 35 | F | Cervical lymph node | Negative | Negative | |
| 79 | F | Palatine tonsil | Positive | Positive | |
| Nodular Sclerosis | 72 | M | Axillary lymph node | Negative | Negative |
| Nodular Lymphocyte Predominant | 33 | M | Cervical lymph node | Negative | Negative |
| 77 | M | Cervical lymph node | Positive | Positive | |
| 61 | F | Cervical lymph node | Negative | Negative | |
Figure 1.
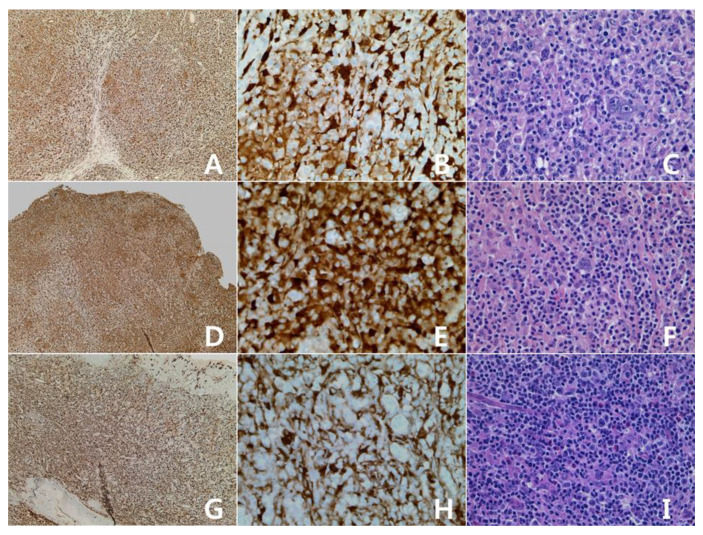
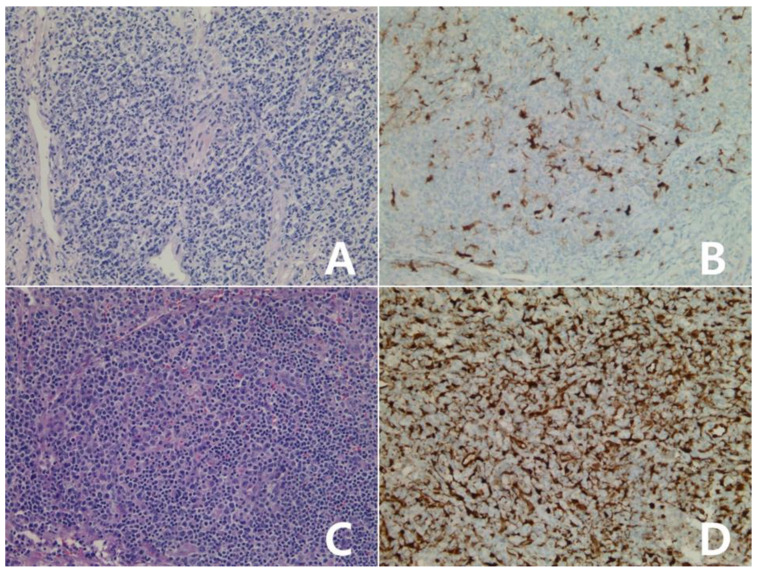
Hodgkin lymphomas with expression of Indolamine-2,3-dioxygenase (IDO): classic Hodgkin lymphoma, mixed cellularity. (A–C): cervical lymph node; (G–I): palatine tonsil. Some tumor cells and vascular structures show reactivity for IDO protein (A,G): IDO stain, ×40; (B,H): IDO stain, ×400; (C,I): H&E stain, ×400. Nodular lymphocyte predominant Hodgkin lymphoma (D–F): cervical lymph node. Some tumor cells and vascular structures show reactivity for IDO protein (D): IDO stain, ×40; E: IDO stain, ×400; F: H&E stain, ×400).
Figure 2.
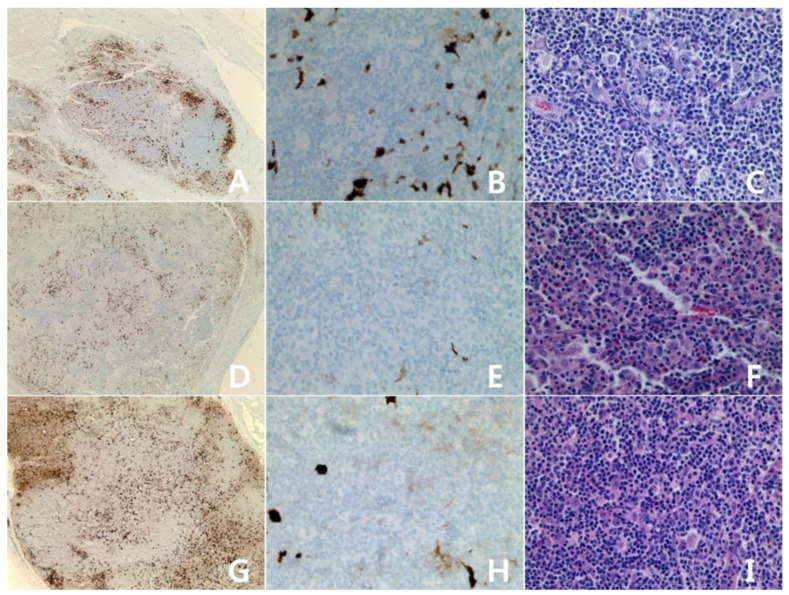
Hodgkin lymphomas, negative for IDO: Classic Hodgkin lymphoma, nodular sclerosis type (A–C): axillary lymph node. Classic Hodgkin lymphoma, mixed cellularity (D–F): cervical lymph node. Nodular lymphocyte predominant Hodgkin lymphoma (G–I): cervical lymph node. Some dendritic cells and macrophages show reactivity for IDO protein (A,D,G): IDO stain, ×40; (B,E,H): IDO stain, ×400: (C,F,I): H&E stain, ×400.
3.2. Diffuse Large B-Cell Lymphoma (DLBCL)
3.2.1. Diffuse Large B-Cell Lymphoma (DLBCL), NOS
A total of 26 cases of diffuse large B-cell lymphoma, NOS were enrolled the study. The clinicopathologic characteristics and the immunohistochemical results of IDO are summarized in Table 2. The male:female ratio was 2.7:1, and the age distribution was 10–84 years (mean = 61 years, median = 65.5 years). Six cases were germinal center type and 20 cases were activated B-cell type. There was no difference in the IDO staining pattern between germinal center type and activated B-cell type. The lymphomas were scored based on percentage of tumor cell staining: 0 = <5%, 1 = 5–25%, 2 = 26–50%, 3 = 51–75%, and 4 = >75% (Figure 3A: score 0; 3B: 1; 3C: 2; 3D: 3; 3E: 4). The staining distribution was scored from 0 to 4. Eight cases were scored 0, three cases were 1, 10 cases were 2, three cases were 3, and two cases were 4.
Table 2.
The clinicopathologic characteristics and the IDO expressions in diffuse large B-cell lymphomas.
| Histologic Type | Age | Sex | Site of Involvement | IDO |
|---|---|---|---|---|
| Diffuse Large B-cell Lymphoma, NOS | ||||
| Germinal Center B-cell | 42 | M | Nasal cavity | score 3 |
| 65 | M | Nasal cavity | score 1 | |
| 10 | M | Uvula, palatine tonsil | score 2 | |
| 81 | F | Femur, brain | score 0 | |
| 59 | F | Nasal cavity | score 0 | |
| 80 | M | Testis | score 0 | |
| Activated B-cell | 56 | M | Inguinal lymph node | score 3 |
| 42 | F | Palatine tonsil | score 2 | |
| 66 | M | Testis | score 2 | |
| 47 | M | Palatine tonsil | score 0 | |
| 66 | F | Axillary LN | score 2 | |
| 55 | M | Palatine tonsil | score 2 | |
| 62 | M | Testis | score 2 | |
| 54 | M | Inguinal LN | score 2 | |
| 67 | M | Palatine tonsil | score 4 | |
| 74 | M | Heart | score 0 | |
| 67 | M | Supraclavicular LN | score 0 | |
| 50 | M | Palatine tonsil | score 1 | |
| 70 | M | Nasal cavity | score 2 | |
| 54 | F | Intestine, appendix, omentum, LN | score 0 | |
| 72 | M | Stomach, large intestine, LN | score 2 | |
| 48 | M | Cervical LN | score 0 | |
| 75 | F | Small intestine | score 3 | |
| 70 | M | Small intestine, appendix | score 4 | |
| 84 | F | Nasal cavity | score 1 | |
| 70 | M | Testis | score 2 | |
| T-cell/histiocyte–Rich Large B-cell Lymphoma | 72 | M | Neck, axillary LN | score 2 |
| Primary DLBCL of the CNS | 79 | F | Brain | score 0 |
| 78 | F | Brain | score 0 | |
| 60 | F | Brain | score 0 | |
| 78 | M | Brain | score 0 | |
| Primary Cutaneous DLBCL, Leg type | 77 | F | Skin, upper arm and low leg | score 4 |
| EBV-Positive DLBCL, NOS | 62 | M | Cervical LN | score 4 |
| 86 | F | Axillary LN | score 2 | |
* LN: lymph node, DLBCL: diffuse large B-cell lymphoma, CNS: central nervous system, EBV: Epstein-Barr virus, NOS: not otherwise specified.
Figure 3.
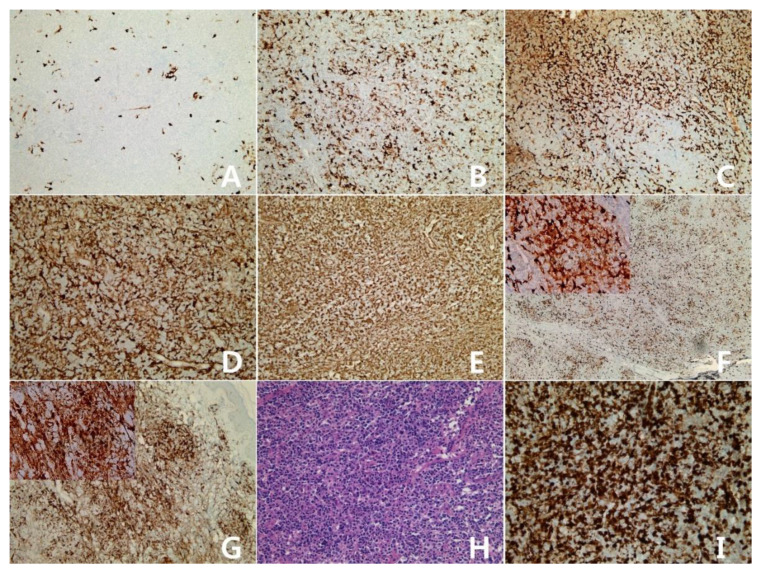
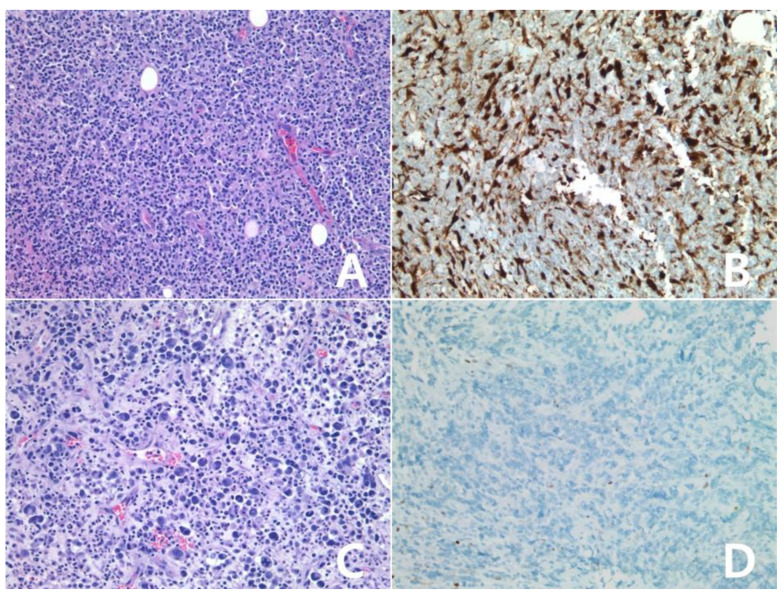
Diffuse large B-cell lymphoma with expression of IDO: Diffuse large B-cell lymphoma, not otherwise specified(A–E), ×100; (A): score 0 (<5%), (B): score 1 (5-25%), (C): score 2 (26–50%), (D): score 3 (51-75%), (E): score 4 (>75%)). T-cell/histiocyte–rich large B-cell lymphoma (F): score 2, ×40; Insert: ×400. Primary cutaneous DLBCL, leg type (G): score 4, ×100; Insert: ×400. Epstein-Barr virus (EBV) -positive DLBCL, NOS (H): H&E stain, (I): IDO stain, score 4, (H,I): ×200.
3.2.2. Diffuse Large B-Cell Lymphoma (DLBCL), Subtypes
The clinicopathologic characteristics and the immunohistochemical results of IDO are summarized in Table 2. A total of 30.8% (8/26) of DLBCL, NOS were scored 0 for IDO immunohistochemistry, but four cases of primary DLBCL of the central nervous system were scored 0 (Figure 4). A T-cell/histiocyte–rich large B-cell lymphoma was scored 2 (Figure 3F), a primary cutaneous DLBCL, leg type was scored 4 (Figure 3G), and one case of EBV-positive DLBCL, NOS was scored 2 and another was scored 4 (Figure 3H,I).
Figure 4.
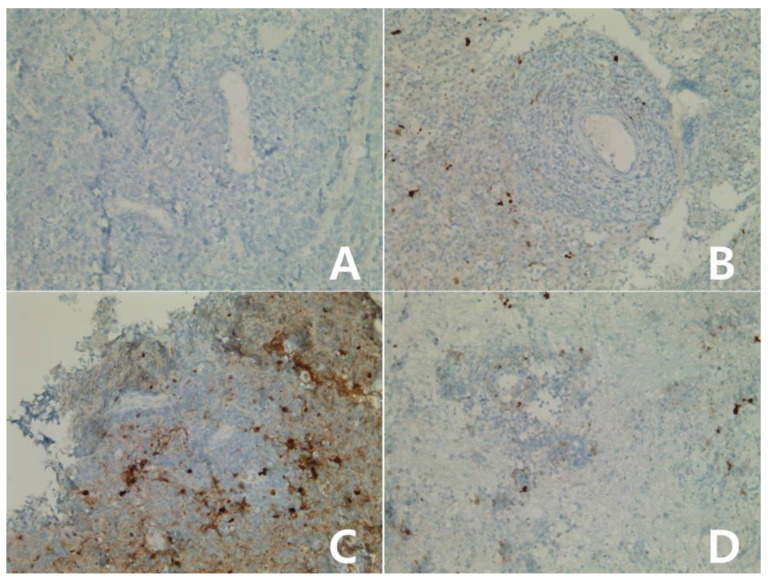
Immunohistochemical stain for IDO in primary DLBCL of the CNS (central nervous system): Atypical lymphocytic infiltration mainly in the perivascular area without IDO expression (A–D): score 0, ×400).
3.3. B-Cell Lymphomas with Low IDO Expression
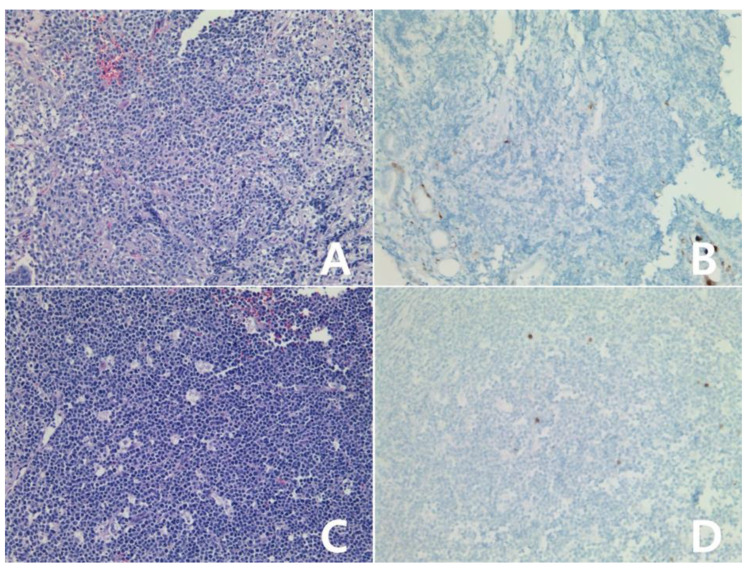
A total of 15 cases of follicular lymphoma (grade 1–2: 11, 3A: three, 3B: one) and 16 cases of marginal zone B-cell lymphoma of the mucosa-associated lymphoid tissue (MALT lymphoma) were enrolled in the study. The clinicopathologic characteristics and the immunohistochemical results of IDO are summarized in Table 3. The male:female ratio was 1.14:1, and the age distribution was 36–79 years (mean = 52.3 years, median = 50 years) in follicular lymphomas. The male:female ratio was 1:1, and the age distribution was 42–84 years (mean = 64.1 years, median = 64 years) in MALT lymphomas. A total of 93.3% (14/15) of follicular lymphomas were scored 0 (Figure 5A). One 3B case showed positivity for IDO in the centroblasts of follicles and the diffuse patterned areas (Figure 5B, submandibular lymph node and, 16 months later, the lymphoma transformed to DLBCL of the brain (IDO score 4). One pediatric follicular lymphoma was scored 0 for IDO expression. A total of 16 cases of MALT lymphoma (Figure 5C) and four cases of nodal marginal zone lymphoma were scored 0 for IDO. Three mantle lymphomas (Figure 5D), two chronic lymphocytic leukemia/small lymphocytic lymphomas (Figure 5E), and one B-lymphoblastic lymphoma/leukemia, NOS (Figure 5F) were scored 0 for IDO expression. Two Burkitt lymphomas were scored 0 for IDO (Figure 6).
Table 3.
The clinicopathologic characteristics of low IDO expression B-cell lymphomas.
| Histologic Type | Age | Sex | Site of Involvement | IDO |
|---|---|---|---|---|
| Follicular Lymphoma | ||||
| Grade 1–2 | 44 | M | Axillary LN | score 0 |
| 79 | M | Inguinal LN | score 0 | |
| 61 | F | Cervical LN | score 0 | |
| 40 | F | Inguinal LN | score 0 | |
| 51 | F | Inguinal LN | score 0 | |
| 36 | M | Abdominal LN | score 0 | |
| 62 | F | Stomach | score 0 | |
| 50 | M | Thoracic LN | score 0 | |
| 59 | M | Cervical LN | score 0 | |
| 36 | F | Cervical LN | score 0 | |
| 49 | F | Buccal mucosa | score 0 | |
| Grade 3A | 40 | M | Cervical LN | score 0 |
| 49 | F | Epitrochlear LN | score 0 | |
| 77 | M | Inguinal LN | score 0 | |
| Grade 3B | 51 | M | Submandibular LN | Diffuse area: score 3 |
| Pediatric Follicular Lymphoma | 32 | M | Cervical LN | score 0 |
| MALT (mucosa-associated lymphoid tissue)- Lymphoma | 80 | M | Eyelid | score 0 |
| 56 | F | Urinary bladder | score 0 | |
| 66 | F | Stomach | score 0 | |
| 44 | F | Stomach | score 0 | |
| 53 | M | Eyelid | score 0 | |
| 65 | M | Stomach | score 0 | |
| 71 | M | Small intestine, omentum, LN | score 0 | |
| 57 | F | Stomach | score 0 | |
| 81 | M | Stomach | score 0 | |
| 63 | M | Stomach | score 0 | |
| 42 | F | Stomach | score 0 | |
| 66 | M | Eyelid | score 0 | |
| 84 | F | Stomach | score 0 | |
| 82 | M | Stomach | score 0 | |
| 56 | F | Stomach | score 0 | |
| 60 | F | Rectum | score 0 | |
| Nodal Marginal Zone Lymphoma | 66 | F | Axillary LN | score 0 |
| 66 | F | Cervical LN | score 0 | |
| 67 | F | Inguinal LN | score 0 | |
| 17 | F | Palatine tonsil | score 0 | |
| Mantle Cell Lymphoma | 71 | M | Axillary LN | score 0 |
| 53 | M | Palatine tonsil | score 0 | |
| 66 | F | Palatine tonsil | score 0 | |
| Burkitt Lymphoma | 75 | M | Stomach, pancreas | score 0 |
| 47 | M | Small intestine, appendix, omentum | score 0 | |
| Chronic Lymphocytic Leukemia/Small Lymphocytic lymphoma | 62 | M | Cervical LN | score 0 |
| 71 | M | Inguinal LN | score 0 | |
| B-Lymphoblastic Lymphoma/Leukemia, NOS | 45 | M | Soft tissue, paravertebral area | score 0 |
Figure 5.
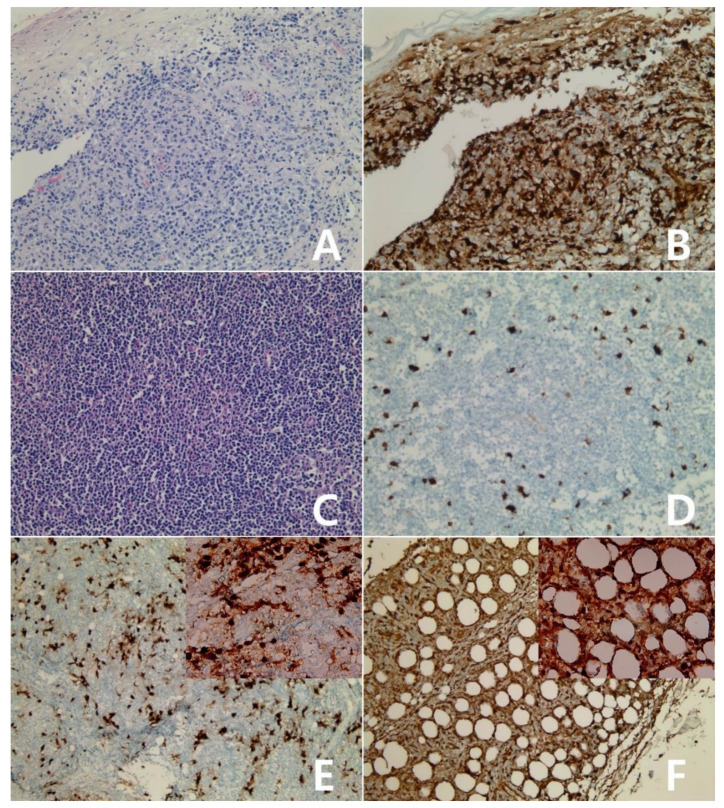
B-cell lymphomas with low IDO expression: Follicular lymphoma, grade 1–2 (A), ×40. Follicular lymphoma, grade 3B showed positivity in the centroblasts of follicles and the diffuse patterned areas (B), ×40; Insert: ×400. MALT lymphoma (C), ×100. Mantle cell lymphoma (D), ×40. Chronic lymphocytic leukemia/small lymphocytic lymphoma (E), ×40. B-lymphoblastic lymphoma/leukemia (F), ×40.
Figure 6.
Immunohistochemical stain for IDO in Burkitt lymphoma: Atypical lymphocytes showed negativity for IDO protein (A,B): case 1, (C,D): case 2, (B,D): IDO stain, (A,C): H&E, all ×200.
3.4. Mature T-and NK-Cell Neoplasms
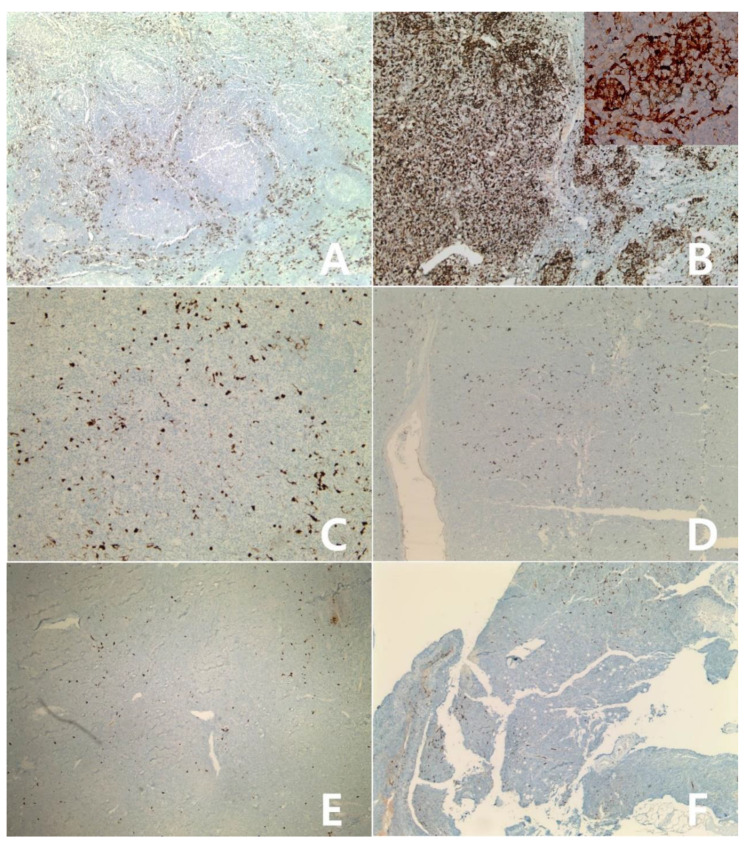
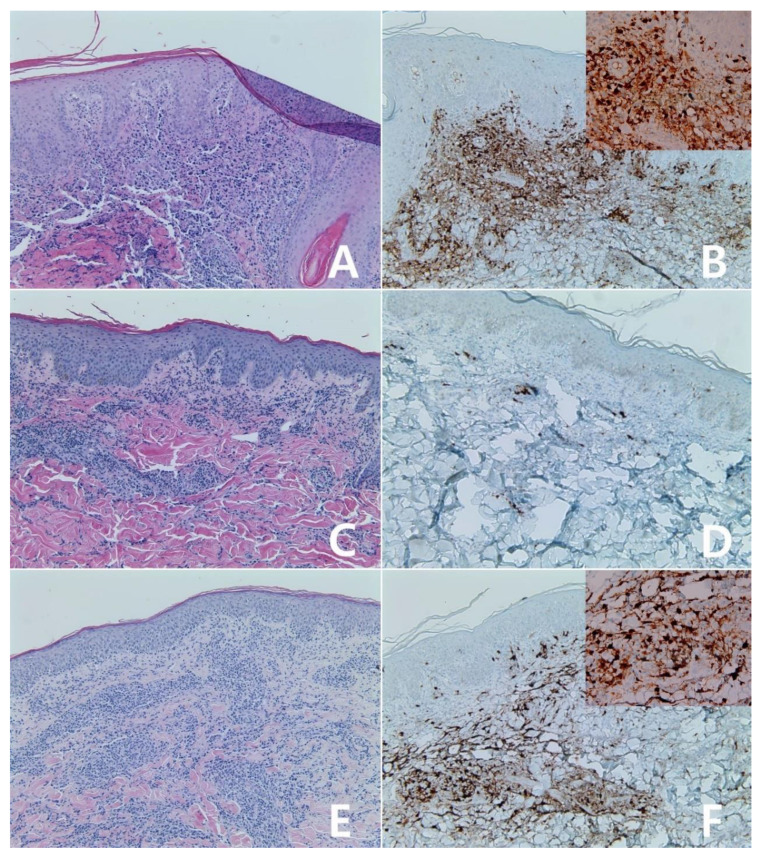
Twelve cases of extranodal NK-/T-cell lymphoma (aged 19–83 (mean = 54.2, median = 57), Male:Female = 1.4:1), eight cases of primary cutaneous CD30 positive T-cell proliferative disorder (six lymphomatoid papulosis, two primary cutaneous anaplastic large cell lymphoma), six cases of peripheral T-cell lymphoma, NOS, five cases of anaplastic large cell lymphoma (three ALK-positive and two ALK-negative), a primary cutaneous CD8-positive aggressive epidermotrophic cytotoxic T-cell lymphoma, a enteropathy-associated T-cell lymphoma, a angioimmunoblastic T-cell lymphoma, and a subcutaneous panniculitis-like T-cell lymphoma were enrolled in the study. The clinicopathologic characteristics and the immunohistochemical results of IDO are summarized in Table 4. Of the 12 cases of extranodal NK-/T-cell lymphoma, 10 cases showed diffuse positivity for IDO protein (Figure 7A) and only two cases were scored 0 (Figure 7B). The IDO negative cases were younger (19 and 26 years) than IDO positive cases (aged 43–83). The staining distribution of IDO in lymphomatoid papulosis was scored 0–4 (scored 0: one, scored 1: three, scored 4: two; Figure 8A–D). One case of primary cutaneous anaplastic large cell lymphoma was scored 3 (Figure 8E,F) and another was scored 0. The staining distribution of IDO in peripheral T-cell lymphoma, NOS was scored 1–3 (score 1: one, Figure 9A,B; score 3: 5, Figure 9C,D). The staining distribution of IDO in anaplastic large cell lymphoma, ALK(anaplastic lymphoma kinase)-positive was scored 2–4 (score 2: one, score 3: one, score 4: one, Figure 10A,B). All cases of anaplastic large cell lymphoma, ALK-negative were scored 0 (Figure 10C,D). Primary cutaneous CD8-positive aggressive epidermotrophic cytotoxic T-cell lymphoma was scored 4 (Figure 11A,B). Enteropathy-associated T-cell lymphoma, type II was scored 0 (Figure 11C and D). Angioimmunoblastic T-cell lymphoma was scored 1 (Figure 11E), and subcutaneous panniculitis-like T-cell lymphoma was scored 4 (Figure 11F).
Table 4.
The clinicopathologic characteristics and IDO expression in mature T- and NK-cell neoplasms.
| Histologic Type | Age | Sex | Site of Involvement | IDO |
|---|---|---|---|---|
| Extranodal NK-/T-cell Lymphoma | 65 | F | Nasal cavity | score 4 |
| 44 | M | Nasal cavity | score 4 | |
| 26 | F | Nasal cavity | score 0 | |
| 19 | M | Scrotum | score 0 | |
| 83 | M | Pharynx | score 4 | |
| 63 | F | Nasal cavity | score 4 | |
| 64 | M | Soft tissue, hip | score 4 | |
| 56 | F | Nasal cavity | score 4 | |
| 58 | M | Nasal cavity | score 4 | |
| 76 | F | Nasal cavity | score 4 | |
| 43 | M | Nasal cavity | score 4 | |
| 53 | M | Nasal cavity | score 4 | |
| Primary Cutaneous CD30 positive T-cell Proliferative Disorder | ||||
| Lymphomatoid Papulosis | 42 | M | Skin, forearm and thigh | score 4 |
| 57 | M | Skin, occipital, and abdomen | score 0 | |
| 62 | M | Eyelid | score 4 | |
| 40 | M | Skin, shoulder, and flank | score 1 | |
| 62 | M | Skin, thigh | score 1 | |
| 64 | M | Skin, chest | score 1 | |
| Primary Cutaneous Anaplastic Large Cell Lymphoma | 63 | M | Skin, shoulder | score 3 |
| 53 | F | Skin, lower leg | score 0 | |
| Peripheral T-cell Lymphoma, NOS | 78 | F | Skin, thigh, and inguinal LN | score 3 |
| 72 | M | Axillary LN | score 3 | |
| 61 | M | Skin, nose | score 1 | |
| 74 | M | Axillary LN | score 3 | |
| 70 | F | Cervical LN | score 3 | |
| 58 | M | Cervical LN | score 3 | |
| Anaplastic Large Cell Lymphoma, ALK-positive | 14 | M | Skin and soft tissue, face | score 3 |
| 21 | F | Inguinal LN | score 4 | |
| 36 | M | Soft tissue and bone, sternum | score 2 | |
| Anaplastic Large Cell Lymphoma, ALK-negative | 71 | M | Sphenoid sinus | score 0 |
| 86 | M | Vertebra, lumbar | score 0 | |
| Primary Cutaneous CD8-positive Aggressive | ||||
| Epidermotrophic Cytotoxic T-cell Lymphoma | 82 | M | Skin, abdomen | score 4 |
| Enteropathy-associated T-cell Lymphoma, Type II | 81 | F | Small intestine | score 0 |
| Angioimmunoblastic T-cell Lymphoma | 55 | M | Cervical LN | score 1 |
| Subcutaneous Panniculitis-like T-cell Lymphoma | 21 | M | Skin, flank | score 4 |
* ALK: anaplastic lymphoma kinase.
Figure 7.
Immunohistochemical stain for IDO in extranodal NK-/T-cell lymphoma: The tumor cells showed diffuse positivity for IDO protein (A), ×200. The tumor was scored 0 for IDO protein (B), ×200.
Figure 8.
Immunohistochemistry for IDO in primary cutaneous CD30 positive T-cell proliferative disorder: Lymphomatoid papulosis (A,B): case 1, (C,D): case 2, (A–D) ×100, (A,C): H&E stain. Atypical lymphocytes showed diffuse positivity for IDO protein (B), score 4; Insert: ×400, negative for IDO (D), score 0. One primary cutaneous anaplastic large cell lymphoma was scored 3 (E,F), ×100; Insert: ×400, (E): H&E stain.
Figure 9.
Immunohistochemistry for IDO in peripheral T-cell lymphoma, NOS: (A,B): scored 1, (C,D): scored 3, (A–D) ×200, (A,C): H&E stain. The tumor was scored 1 for IDO (B). The tumor cells showed positivity for IDO, score 3 (D).
Figure 10.
Immunohistochemistry for IDO in anaplastic large cell lymphoma: Anaplastic large cell lymphoma, ALK-positive (A,B), ×200, A: H&E stain. The tumor showed score 2 for IDO protein (B). Anaplastic large cell lymphoma, ALK-negative (C,D), ×200, C: H&E stain. The tumor showed no reactivity for IDO protein, score 0 (D).
Figure 11.
Immunohistochemistry for IDO in mature T- and NK-cell neoplasms: Primary cutaneous CD8-positive aggressive epidermotrophic cytotoxic T-cell lymphoma (A,B), ×200, A: H&E stain. The tumor showed diffuse reactivity for IDO protein, score 4 (B). Enteropathy-associated T-cell lymphoma, type II (C,D), ×200, C: H&E stain. The tumor showed weak reactivity for IDO protein, score 0 (D). Angioimmunoblastic T-cell lymphoma(E), ×100; Insert: ×400. The tumor was scored 1 for IDO protein (E). Subcutaneous panniculitis-like T-cell lymphoma (F), ×100; Insert: ×400. The tumor showed diffuse reactivity for IDO protein, score 4 (F).
4. Discussion
Indolamine-2,3-dioxygenase (IDO) is an intracellular enzyme that catalyzes amino acid tryptophan to L-kynurenine [19]. IDO is reportedly expressed by dendritic cells in tumor-draining lymph nodes [10]. In our study, dendritic cells and macrophages of lymph nodes or tonsils showed positivity for IDO protein. All lymphomas were scored based on percentage of tumor cell staining: score 0 = <5%, 1 = 5%–25%, 2 = 26%–50%, 3 = 51%–75%, and 4 = >75%. It was difficult or impossible to distinguish reactive immune cells from tumor cells. To clarify the distinction between the tumor cells and reactive cells, multiple immunofluorescence stainings against IDO and other antibodies can be helpful as has been done in previous research [17]. The staining distribution of IDO was scored 0–4. A total of 80% (28/35) of mature T- and NK-cell neoplasms showed positivity for IDO protein (score 1: five, score 2: one, score 3: seven, score 4: 15). There was no different staining pattern or positive rate between the histopathological types of mature T- and NK-cell neoplasms. A total of 78.6% (22/28) of positive cases were scored 3 or 4; 83.3% (10/12) of extranodal NK-/T-cell lymphomas showed diffuse positivity for IDO protein. The remaining two negative cases were of a relatively young age (19 and 26 years) than the positive extranodal NK-/T-cell lymphomas (aged 43–83). In anaplastic large cell lymphomas, all three ALK-positive cases were positive (scoring 2, 3, or 4) for IDO and both ALK-negative cases were negative for IDO. A total of six peripheral T-cell lymphomas, NOS showed positivity for IDO (five scoring 3 and one scoring 1). In contrast to mature T- and NK-cell neoplasms, 29.9% (23/77) of mature B-cell lymphomas showed positivity for IDO protein (score 1: three, score 2: 12, score 3: four, score 4: four). In mature B-cell lymphomas, 95.7% (22/23) of IDO positive cases were DLBCL, NOS, or DLBCL subtypes. There was no different staining pattern or positive rate between DLBCL, NOS, and DLBCL subtypes. All small B-cell neoplasms except one were negative for IDO protein. The remaining positive case was follicular lymphoma, grade 3B. The positive follicular lymphoma was scored 3 at centroblasts in follicle and diffuse areas. In contrast to other subtypes of DLBCL, all four primary DLBCLs of the CNS were negative for IDO protein. IDO proteins have been overexpressed in various cancers [20,21,22,23] and several IDO inhibitors have been assessed in multiple clinical trials. If an IDO inhibitor is to be commercialized, IDO immunohistochemistry will be important method. However, there are insufficient studies of immunohistochemistry for IDO in lymphomas. Previous studies have demonstrated only Hodgkin lymphoma and diffuse large B-cell lymphoma [14,15,16]. There was no immunohistochemical study of IDO protein for mature T- and NK-cell neoplasms. However, our study included various types of mature T- and NK-cell lymphoma (that have previously been unreported) and showed various patterns of IDO staining according to the type. Our study used scant sample size to determine the IDO pattern of various lymphomas and the relationship between IDO expression and prognosis. When the results are accumulated, IDO immunohistochemistry will be a useful tool to diagnose lymphomas and predict their prognosis.
Author Contributions
Conceptualization, M.-s.K. and H.W.L.; methodology, M.-s.K.; software, S.-A.S.; validation, H.W.L., T.I.P.; formal analysis, M.-s.K.; investigation, M.-s.K.; resources, H.W.L.; data curation, M.-s.K.; Writing—Original draft preparation, M.-s.K.; Writing—Review and editing, H.W.L.; visualization, S.-A.S.; supervision, H.W.L.; project administration, H.W.L.; funding acquisition, T.I.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Gajewski T.F. Identifying and Overcoming Immune Resistance Mechanisms in the Melanoma Tumor Microenvironment. Clin. Cancer Res. 2006;12:2326s–2330s. doi: 10.1158/1078-0432.CCR-05-2517. [DOI] [PubMed] [Google Scholar]
- 2.Whiteside T.L. Immune Suppression in Cancer: Effects on Immune Cells, Mechanisms and Future Therapeutic Intervention. Semin. Cancer Biol. 2006;16:3–15. doi: 10.1016/j.semcancer.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 3.Munn D.H., Mellor A.L. Indoleamine 2,3-Dioxygenase and Tumor-Induced Tolerance. J. Clin. Investig. 2007;117:1147–1154. doi: 10.1172/JCI31178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mellor A.L., Munn D.H. Tryptophan Catabolism and T-Cell Tolerance: Immunosuppression by Starvation? Immunol. Today. 1999;20:469–473. doi: 10.1016/S0167-5699(99)01520-0. [DOI] [PubMed] [Google Scholar]
- 5.Zhai L., Spranger S., Binder D.C., Gritsina G., Lauing K.L., Giles F.J., Wainwright D.A. Molecular Pathways: Targeting IDO1 and Other Tryptophan Dioxygenases for Cancer Immunotherapy. Clin. Cancer Res. 2015;21:5427–5433. doi: 10.1158/1078-0432.CCR-15-0420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nakamura N., Hara T., Shimizu M., Mabuchi R., Nagano J., Ohno T., Kochi T., Kubota M., Shirakami Y., Goto N., et al. Effects of Indoleamine 2,3-Dioxygenase Inhibitor in Non-Hodgkin Lymphoma Model Mice. Int. J. Hematol. 2015;102:327–334. doi: 10.1007/s12185-015-1835-8. [DOI] [PubMed] [Google Scholar]
- 7.Frumento G., Rotondo R., Tonetti M., Damonte G., Benatti U., Ferrara G.B. Tryptophan-Derived Catabolites are Responsible for Inhibition of T and Natural Killer Cell Proliferation Induced by Indoleamine 2,3-Dioxygenase. J. Exp. Med. 2002;196:459–468. doi: 10.1084/jem.20020121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Munn D.H., Shafizadeh E., Attwood J.T., Bondarev I., Pashine A., Mellor A.L. Inhibition of T Cell Proliferation by Macrophage Tryptophan Catabolism. J. Exp. Med. 1999;189:1363–1372. doi: 10.1084/jem.189.9.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hwu P., Du M.X., Lapointe R., Do M., Taylor M.W., Young H.A. Indoleamine 2,3-Dioxygenase Production by Human Dendritic Cells Results in the Inhibition of T Cell Proliferation. J. Immunol. 2000;164:3596–3599. doi: 10.4049/jimmunol.164.7.3596. [DOI] [PubMed] [Google Scholar]
- 10.Munn D.H., Sharma M.D., Lee J.R., Jhaver K.G., Johnson T.S., Keskin D.B., Marshall B., Chandler P., Antonia S.J., Burgess R., et al. Potential Regulatory Function of Human Dendritic Cells Expressing Indoleamine 2,3-Dioxygenase. Science. 2002;297:1867–1870. doi: 10.1126/science.1073514. [DOI] [PubMed] [Google Scholar]
- 11.Yoshikawa T., Hara T., Tsurumi H., Goto N., Hoshi M., Kitagawa J., Kanemura N., Kasahara S., Ito H., Takemura M., et al. Serum Concentration of L-Kynurenine Predicts the Clinical Outcome of Patients with Diffuse Large B-Cell Lymphoma Treated with R-CHOP. Eur. J. Haematol. 2010;84:304–309. doi: 10.1111/j.1600-0609.2009.01393.x. [DOI] [PubMed] [Google Scholar]
- 12.Ninomiya S., Hara T., Tsurumi H., Goto N., Saito K., Seishima M., Takami T., Moriwaki H. Indoleamine 2,3-Dioxygenase Expression and Serum Kynurenine Concentrations in Patients with Diffuse Large B-Cell Lymphoma. Leuk. Lymphoma. 2012;53:1143–1145. doi: 10.3109/10428194.2011.643472. [DOI] [PubMed] [Google Scholar]
- 13.Hoshi M., Ito H., Fujigaki H., Takemura M., Takahashi T., Tomita E., Ohyama M., Tanaka R., Saito K., Seishima M. Indoleamine 2,3-Dioxygenase is Highly Expressed in Human Adult T-Cell Leukemia/Lymphoma and Chemotherapy Changes Tryptophan Catabolism in Serum and Reduced Activity. Leuk. Res. 2009;33:39–45. doi: 10.1016/j.leukres.2008.05.023. [DOI] [PubMed] [Google Scholar]
- 14.Ninomiya S., Hara T., Tsurumi H., Hoshi M., Kanemura N., Goto N., Kasahara S., Shimizu M., Ito H., Saito K., et al. Indoleamine 2,3-Dioxygenase in Tumor Tissue Indicates Prognosis in Patients with Diffuse Large B-Cell Lymphoma Treated with R-CHOP. Ann. Hematol. 2011;90:409–416. doi: 10.1007/s00277-010-1093-z. [DOI] [PubMed] [Google Scholar]
- 15.Choe J.Y., Yun J.Y., Jeon Y.K., Kim S.H., Park G., Huh J.R., Oh S., Kim J.E. Indoleamine 2,3-Dioxygenase (IDO) is Frequently Expressed in Stromal Cells of Hodgkin Lymphoma and is Associated with Adverse Clinical Features: A Retrospective Cohort Study. BMC Cancer. 2014;14:335. doi: 10.1186/1471-2407-14-335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Masaki A., Ishida T., Maeda Y., Ito A., Suzuki S., Narita T., Kinoshita S., Takino H., Yoshida T., Ri M., et al. Clinical Significance of Tryptophan Catabolism in Hodgkin Lymphoma. Cancer. Sci. 2018;109:74–83. doi: 10.1111/cas.13432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heeren A.M., van Dijk I., Berry D.R.A.I., Khelil M., Ferns D., Kole J., Musters R.J.P., Thijssen V.L., Mom C.H., Kenter G.G., et al. Indoleamine 2,3-Dioxygenase Expression Pattern in the Tumor Microenvironment Predicts Clinical Outcome in Early Stage Cervical Cancer. Front. Immunol. 2018;9:1598. doi: 10.3389/fimmu.2018.01598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li F., Zhao Y., Wei L., Li S., Liu J. Tumor-Infiltrating Treg, MDSC, and IDO Expression Associated with Outcomes of Neoadjuvant Chemotherapy of Breast Cancer. Cancer. Biol. Ther. 2018;19:695–705. doi: 10.1080/15384047.2018.1450116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takikawa O. Biochemical and Medical Aspects of the Indoleamine 2,3-Dioxygenase-Initiated L-Tryptophan Metabolism. Biochem. Biophys. Res. Commun. 2005;338:12–19. doi: 10.1016/j.bbrc.2005.09.032. [DOI] [PubMed] [Google Scholar]
- 20.Brown Z.J., Yu S.J., Heinrich B., Ma C., Fu Q., Sandhu M., Agdashian D., Zhang Q., Korangy F., Greten T.F. Indoleamine 2,3-Dioxygenase Provides Adaptive Resistance to Immune Checkpoint Inhibitors in Hepatocellular Carcinoma. Cancer Immunol. Immunother. 2018;67:1305–1315. doi: 10.1007/s00262-018-2190-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gide T.N., Allanson B.M., Menzies A.M., Ferguson P.M., Madore J., Saw R.P.M., Thompson J.F., Long G.V., Wilmott J.S., Scolyer R.A. Inter- and Intrapatient Heterogeneity of Indoleamine 2,3-Dioxygenase Expression in Primary and Metastatic Melanoma Cells and the Tumour Microenvironment. Histopathology. 2019;74:817–828. doi: 10.1111/his.13814. [DOI] [PubMed] [Google Scholar]
- 22.Hornyak L., Dobos N., Koncz G., Karanyi Z., Pall D., Szabo Z., Halmos G., Szekvolgyi L. The Role of Indoleamine-2,3-Dioxygenase in Cancer Development, Diagnostics, and Therapy. Front. Immunol. 2018;9:151. doi: 10.3389/fimmu.2018.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Inaba T., Ino K., Kajiyama H., Yamamoto E., Shibata K., Nawa A., Nagasaka T., Akimoto H., Takikawa O., Kikkawa F. Role of the Immunosuppressive Enzyme Indoleamine 2,3-Dioxygenase in the Progression of Ovarian Carcinoma. Gynecol. Oncol. 2009;115:185–192. doi: 10.1016/j.ygyno.2009.07.015. [DOI] [PubMed] [Google Scholar]