Abstract
Objective
We aimed to investigate the relationship of Helicobacter pylori infection with alcohol and smoking.
Methods
We conducted a cross-sectional study among participants who underwent health check-ups for H. pylori infection between January 2013 and March 2017. We subsequently investigated the relationship of H. pylori infection with alcohol and smoking.
Results
A total of 7169 participants were enrolled in this study. The overall prevalence of H. pylori infection was 55.2%. Participants with H. pylori infection were more likely to be older than those without H. pylori infection. For male participants with H. pylori infection, multivariable logistic regression analysis indicated that both smoking (odds ratio (OR): 1.61; 95% confidence interval (CI): 1.41–1.83) and alcohol consumption (OR: 1.30; 95% CI: 1.10–1.52) were independently positively associated with H. pylori infection. For female participants, multivariable logistic regression analysis indicated that both smoking (OR: 0.03; 95% CI: 0.02–0.07) and alcohol consumption (OR: 0.20; 95% CI: 0.12–0.33) were inversely significantly associated with H. pylori infection after adjustment for age.
Conclusions
Smoking and alcohol consumption were risk factors for male participants but these were protective factors for female individuals with H. pylori infection.
Keywords: Epidemiology, prevalence, Helicobacter pylori, smoking, alcohol, sex differences
Introduction
The routes of transmission of Helicobacter pylori include gastro–oral, oral–oral and faecal–oral routes.1 Person-to-person transmission and intrafamilial spread appear to be the main routes, based on observed intra-familial clustering.2 Transmission events are more frequent between close relatives and between individuals living in the same household.3 In developing countries, horizontal transmission may have a concomitant role with intrafamilial infection, leading to a higher prevalence.4
Among the primary related lifestyle habits,3 smoking and alcohol consumption show discordant results. In most studies, there is no significant association with H. pylori infection. Shi et al.5 reported finding no association between H. pylori prevalence and smoking or drinking. Cheng et al.6 reported that no significant differences were noted for age, sex, alcohol consumption, or smoking between H. pylori-positive and H. pylori-negative individuals. Den Hollander et al.7 indicated that among different ethnicities, age, smoking, and alcohol use were not associated with H. pylori colonization. Zhu et al.8 reported finding no association between H. pylori prevalence and smoking or drinking; there was no association between the prevalence of H. pylori infection and the use of tobacco or alcohol.9 However, Ozaydin et al.10 reported that regular smokers were at higher risk of developing H. pylori infection than non-smokers in Turkey. However, this association did not hold for female participants; regular alcohol consumption was a protective factor against H. pylori infection in women.10
On the basis of the above mentioned findings, in this study, we aimed to investigate the relationship of H. pylori infection with alcohol and smoking.
Methods
Study design and participant selection
We conducted a cross-sectional study at the First Affiliated Hospital of Wenzhou Medical University of mainland China. All participants who had undergone annual routine health check-ups between January 2013 and March 2017 were eligible for inclusion in this study.11 H. pylori infection was assessed using the 13C urea breath test (UBT) after a minimum 6-hour fast.11 Citric acid was not used. The test was performed using a HCBT-01 Breath Test Tester (Shenzhen Zhonghe Headway Bio-Sci & Tech Co., Ltd. Shenzhen, China.) For the UBT, each participant was requested to swallow a tablet containing 75 mg 13C-urea, and the delta over baseline value of 4.0 was used as a cut-off point for the diagnosis of H. pylori infection.11 The study protocol was approved by the Ethics Committee of the First Affiliated Hospital of Wenzhou Medical University. This study was performed according to the principles expressed in the Declaration of Helsinki and informed consent was obtained from all participants.
Inclusion, exclusion criteria, and data collection
The inclusion criteria were asymptomatic individuals who underwent at least one UBT between January 2013 and March 2017. Exclusion criteria were repeated 13C-urea breath tests in the same participant during January 2013 and March 2017 (the period during which only the first UBT was included in our study), unavailability of the results of 13C-urea breath tests, and no information on smoking and alcohol drinking. Sex, age, and results of 13C-urea breath tests were recorded. Smoking and alcohol drinking exposure status was determined using standardized self-administered questionnaires.12 Participants were classified as alcohol drinkers (alcohol consumption) if they had regularly consumed any alcoholic beverage one or more times per week during the preceding 6 months.13,14 Participants were classified as smokers if they had smoked 10 or more cigarettes per week during the preceding 6 months.13,14 The overall prevalence of H. pylori infection was calculated as follows: (all individuals with a positive H. pylori test)/(all individuals who underwent an H. pylori test).11
Statistical analysis
Categorical variables are presented as number and percentage and compared using the χ2 test. A Shapiro–Wilk test was used to evaluate whether the continuous data had a normal distribution. According to the results of the Shapiro–Wilk test, continuous variables are expressed as mean ± standard deviation (SD) or median and interquartile range (IQR) and compared using the independent-samples t-test or Kruskal–Wallis nonparametric test.15
Logistic regression analysis was used to evaluate the relationship of H. pylori infection with alcohol and smoking. The odds ratio (OR) was calculated with the 95% confidence interval (CI).16 Two-sided P-values < 0.05 were considered statistically significant. All analyses were performed using Stata version 12.0 (StataCorp LLC, College Station, TX, USA).
Results
Baseline characteristics of participants
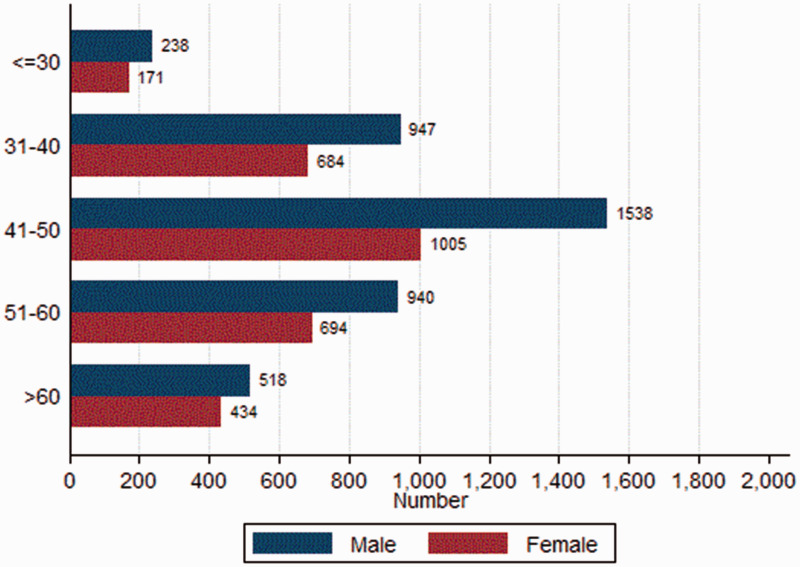
A total of 7169 participants (58.3% men) were enrolled in this study (Figure 1 and Table 1), among which 1358 participants had undergone two or more UBTs. The overall prevalence of H. pylori infection was 55.2%. Participants with H. pylori infection were more likely to be older than those without H. pylori infection (mean age: 47.7 ± 10.7 vs. 46.8 ± 12.1 years, P = 0.001). There was no significant difference in the prevalence of H. pylori infection with regard to male sex (59.3% vs. 57.1%). Of the total, 1900 (26.5%) and 1022 (14.3%) individuals consumed alcohol and smoked, respectively. Of the 7169 participants, 623 both smoked and consumed alcohol. In male participants, the proportions of alcohol use and smoking were 20.5% (855/4181) and 38.8% (1624/4181), respectively. In female participants, the proportions of alcohol use and smoking were 5.6% (167/2988) and 9.2% (276/2988), respectively.
Figure 1.
Distribution of sex and age groups among 7169 participants.
Table 1.
Demographic and clinical characteristics of 7169 patients.
| Characteristic | H. pylori(N = 3955) | Non-H. pylori(N = 3214) | P-value |
|---|---|---|---|
| Age (years), mean ± SD | 47.7 ± 10.7 | 46.8 ± 12.1 | 0.001 |
| Male sex, n (%) | 2345 (59.3%) | 1836 (57.1%) | 0.064 |
| Smoking, n (%) | 1048 (26.5%) | 852 (26.5%) | 0.992 |
| Alcohol use, n (%) | 564 (14.3%) | 458 (14.3%) | 0.990 |
SD, standard deviation.
Alcohol, smoking, and H. pylori infection
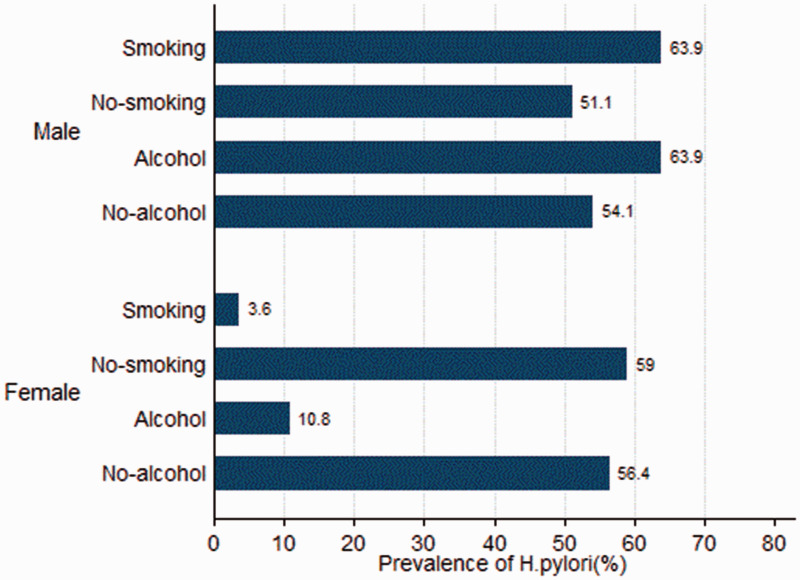
When we analysed participants according to sex, there were no significant differences between individuals with and without H. pylori infection with respect to proportions of alcohol use and smoking (Table 1). For the male subgroup, those who smoked (63.9% vs. 51.1%, P < 0.001) and consumed alcohol (63.9% vs. 54.1%, P < 0.001) had higher prevalence of H. pylori infection than their counterparts who did not smoke or use alcohol (Figure 2). For the female subgroup, individuals who smoked (3.6% vs. 59.0%, P < 0.001) and consumed alcohol (10.8% vs. 56.4%, P < 0.001) had lower prevalence of H. pylori infection than their non-smoking and non-drinking counterparts (Figure 2). Among 623 participants who were both smokers and consumed alcohol, men (63.8%, 339/531) had a higher prevalence of H. pylori infection than women (4.4%, 4/92; P < 0.001).
Figure 2.
Prevalence of Helicobacter pylori infection stratified by sex in participants with or without alcohol use and smoking.
For the male subgroup, multivariable logistic regression analysis indicated that both smoking (OR: 1.61; 95% CI: 1.41–1.83; P < 0.001) and alcohol consumption (OR: 1.30; 95% CI: 1.10–1.52; P = 0.002) were independently positively associated with H. pylori infection, after adjusting for age. In the female subgroup, multivariable logistic regression analysis indicated that both smoking (OR: 0.03; 95% CI: 0.02–0.07; P < 0.001) and alcohol use (OR: 0.20; 95% CI: 0.12–0.33; P < 0.001) were inversely and significantly associated with H. pylori infection after adjusting for age.
Discussion
The prevalence of H. pylori infection is associated with family size, education level, and low socioeconomic status including low family income, limited education, living in a rural area, living in crowded housing, and difficult access to sanitized water;3,5 these represent risk factors for H. pylori infection. With improved socioeconomic conditions and hygiene, H. pylori infection rates show decreasing trends in many regions worldwide.11 The overall prevalence of H. pylori infection in our study was 48.4%, which was lower than that in Korea (51.0%).17 These differences in H. pylori prevalence likely reflect differences in the level of urbanization, sanitation, access to clean water, and socioeconomic status.11
Data on the association among alcohol, smoking, and H. pylori infection are somewhat conflicting. Alcohol has strong antimicrobial activity and stimulates gastric acid secretion.18 Alcohol consumption may therefore compromise the living conditions of H. pylori in the stomach.18 In 1999, Brenner et al.18 reported that there was a clear inverse dose–response relationship between reported alcohol consumption and H. pylori infection, based on H. pylori immunoglobulin G antibodies. Our data suggested that women who smoked (OR: 0.03; 95% CI: 0.02–0.07) or consumed alcohol (OR: 0.20; 95% CI: 0.12–0.33) had significant inverse associations with H. pylori infection, after adjusting for age. These findings support the hypothesis that moderate alcohol consumption may facilitate spontaneous elimination of H. pylori infection in adults.18
On the contrary, Wang et al.19 suggested that patients who consumed alcohol had a higher prevalence of active H. pylori infection than non-drinkers (OR: 1.139; 95% CI: 1.025–1.290; P = 0.0407). Zhang et al.20 reported that in patients with functional dyspepsia, there is no significant association between active H. pylori infection and smoking. However, other studies have found that alcohol consumption appears to be associated with H. pylori infection.20 Amaral et al.9 indicated that no association was found between the prevalence of H. pylori infection and the use of tobacco, alcohol, and coffee or other dietary factors. Findings of a recent individual participant pooled analysis by Ferro et al.21 did not support an association between smoking and H. pylori seropositivity. Our data indicated when participants of both sexes were analysed, there were no significant differences between individuals with and without H. pylori infection with respect to the proportion who consumed alcohol and smoked. However, subgroup analysis based on sex suggested that both smoking (OR: 1.61; 95% CI: 1.41–1.83) and alcohol consumption (OR: 1.30; 95% CI: 1.10–1.52) were independently associated with H. pylori infection in men.
The way in which sex contributes to differing prevalence of H. pylori infection with respect to alcohol and smoking is unclear, although it is becoming widely recognized that there are important sex differences in many diseases.22,23 For most autoimmune diseases, there are clear sex differences in prevalence, with female individuals generally more frequently affected than male individuals.24 Twice as many women as men are affected by irritable bowel syndrome in Western countries, suggesting a role of sex hormones in the pathophysiology of this disorder.25 Female sex is an independent risk factor for worse outcomes in coronary heart disease.26 Kim et al.27 reported sex differences in the association between self-reported stress and cigarette smoking among Korean adolescents. Yue et al.28 suggested that there were sex differences in the association among cigarette smoking, alcohol consumption, and depressive symptoms in Chinese adolescents. Female sex has also been found to affect H. pylori eradication failure in chronic gastritis.29
Among the strengths of the present study, we included a large sample size; thus, the study had sufficient statistical power. We performed stratified analyses by sex, leading to rigorous conclusions. All participants underwent health check-ups and all H. pylori infections were diagnosed using UBTs, which may make our study more homogeneous.11 Several limitations of the study must be mentioned. First, we investigated smoking and alcohol drinking exposure status during the preceding 6 months. Therefore, participants who stopped both drinking and smoking just prior to 6 months before enrolment would be subjectively misclassified. In addition, the number of female participants who drank and smoked was very low in comparison with male participants. In view of the above, our findings should be interpreted with caution, although we believe that they do not substantially influence the overall results. Second, we could not examine in detail why the same factors were adverse in the male group and protective in the female group, as well as the dose-related effect of smoking and alcohol consumption on damage to the gastric mucosa and the relationship between severity of gastric mucosal changes and H. pylori colonization. We will consider investigating these mechanisms in depth in the future. Third, because of the retrospective study design, we had no detailed data for the type and quantity of alcohol consumed or the number of cigarettes smoked, as well as the frequency of alcohol consumption and smoking. It would be useful to divide the groups of alcohol drinkers and smokers into subgroups in the future. Finally, the 13C-urea breath test was used in this study to avoid false positive and false negative cases as much as possible. According to the results of a meta-analysis, the UBT achieves sensitivity of 97% and specificity of 96% in Asian populations;30 therefore, the number of potential false positive and false negative results are negligible in our study population.11,31
In conclusion, smoking and alcohol consumption were risk factors for male participants but these were protective factors for female individuals with H. pylori infection. Therefore, health practitioners may need to adopt different screening and eradication strategies for H. pylori infection according to sex.
Author contributions
All authors contributed toward data analysis and drafting and critically revising the paper and agree to be accountable for all aspects of the work. All of the authors read and approved the manuscript.
Availability of data and materials
The datasets used and/or analysed in the current study are available from the corresponding author on reasonable request.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This work was supported by lzp-2018/1-0135 in Latvia.
ORCID iD
Wandong Hong https://orcid.org/0000-0001-6857-4252
References
- 1.Kayali S, Aloe R, Bonaguri C, et al. Non-invasive tests for the diagnosis of helicobacter pylori: state of the art. Acta Biomed 2018; 89: 58–64. DOI: 10.23750/abm.v89i8-S.7910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Calvet X, Ramirez Lazaro MJ, Lehours P, et al. Diagnosis and epidemiology of Helicobacter pylori infection. Helicobacter 2013; 18: 5–11. DOI: 10.1111/hel.12071. [DOI] [PubMed] [Google Scholar]
- 3.Eusebi LH, Zagari RM, Bazzoli F. Epidemiology of Helicobacter pylori infection. Helicobacter 2014; 19: 1–5. DOI: 10.1111/hel.12165. [DOI] [PubMed] [Google Scholar]
- 4.Nagy P, Johansson S, Molloy-Bland M. Systematic review of time trends in the prevalence of Helicobacter pylori infection in China and the USA. Gut Pathog 2016; 8: 8. DOI: 10.1186/s13099-016-0091-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shi R, Xu S, Zhang H, et al. Prevalence and risk factors for Helicobacter pylori infection in Chinese populations. Helicobacter 2008; 13: 157–165. DOI: 10.1111/j.1523-5378.2008.00586.x. [DOI] [PubMed] [Google Scholar]
- 6.Cheng H, Hu F, Zhang L, et al. Prevalence of Helicobacter pylori infection and identification of risk factors in rural and urban Beijing, China. Helicobacter 2009; 14: 128–133. DOI: 10.1111/j.1523-5378.2009.00668.xHEL668 [pii]. [DOI] [PubMed] [Google Scholar]
- 7.Den Hollander WJ, Holster IL, Den Hoed CM, et al. Ethnicity is a strong predictor for Helicobacter pylori infection in young women in a multi-ethnic European city. J Gastroenterol Hepatol 2013; 28: 1705–1711. DOI: 10.1111/jgh.12315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu Y, Zhou X, Wu J, et al. Risk Factors and Prevalence of Helicobacter pylori Infection in Persistent High Incidence Area of Gastric Carcinoma in Yangzhong City. Gastroenterol Res Pract 2014; 2014: 481365. DOI: 10.1155/2014/481365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amaral O, Fernandes I, Veiga N, et al. Living Conditions and Helicobacter pylori in Adults. Biomed Res Int 2017; 2017: 9082716. DOI: 10.1155/2017/9082716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ozaydin N, Turkyilmaz SA, Cali S. Prevalence and risk factors of Helicobacter pylori in Turkey: a nationally-representative, cross-sectional, screening with the (1)(3)C-Urea breath test. BMC Public Health 2013; 13: 1215. DOI: 10.1186/1471-2458-13-1215 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hong W, Tang H, Dong X, et al. Prevalence of Helicobacter pylori infection in a third-tier Chinese city: relationship with gender, age, birth-year and survey years. Microb Health Dis 2019; 1: e150. [Google Scholar]
- 12.Breitling LP, Raum E, Muller H, et al. Synergism between smoking and alcohol consumption with respect to serum gamma-glutamyltransferase. Hepatology 2009; 49: 802–808. DOI: 10.1002/hep.22727. [DOI] [PubMed] [Google Scholar]
- 13.Hong W, Geng W, Chen B, et al. Predictors of acute pancreatitis with low elevation of serum amylase. Ther Clin Risk Manag 2017; 13: 1577–1584. DOI: 10.2147/TCRM.S147594tcrm-13-1577 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng KH, Chu CS, Lin TH, et al. Lipid paradox in acute myocardial infarction-the association with 30-day in-hospital mortality. Crit Care Med 2015; 43: 1255–1264. DOI: 10.1097/CCM.0000000000000946. [DOI] [PubMed] [Google Scholar]
- 15.Hong W, Zimmer V, Basharat Z, et al. Association of total cholesterol with severe acute pancreatitis: a U-shaped relationship. Clin Nutr 2019; 39: 250–257. DOI: 10.1016/j.clnu.2019.01.022. [DOI] [PubMed] [Google Scholar]
- 16.Hong W, Lillemoe KD, Pan S, et al. Development and validation of a risk prediction score for severe acute pancreatitis. J Transl Med 2019; 17: 146. DOI: 10.1186/s12967-019-1903-6 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee JH, Choi KD, Jung HY, et al. Seroprevalence of Helicobacter pylori in Korea: a multicenter, nationwide study conducted in 2015 and 2016. Helicobacter 2018; 23: e12463. DOI: 10.1111/hel.12463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brenner H, Berg G, Lappus N, et al. Alcohol consumption and Helicobacter pylori infection: results from the German National Health and Nutrition Survey. Epidemiology 1999; 10: 214–218. DOI: 00001648-199905000-00001 [pii]. [PubMed] [Google Scholar]
- 19.Wang W, Jiang W, Zhu S, et al. Assessment of prevalence and risk factors of helicobacter pylori infection in an oilfield Community in Hebei, China. BMC Gastroenterol 2019; 19: 186. DOI: 10.1186/s12876-019-1108-8 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang L, Eslick GD, Xia HH, et al. Relationship between alcohol consumption and active Helicobacter pylori infection. Alcohol Alcohol 2010; 45: 89–94. DOI: 10.1093/alcalc/agp068 [pii]. [DOI] [PubMed] [Google Scholar]
- 21.Ferro A, Morais S, Pelucchi C, et al. Smoking and Helicobacter pylori infection: an individual participant pooled analysis (Stomach Cancer Pooling-StoP Project). Eur J Cancer Prev 2019; 28: 390–396. DOI: 10.1097/CEJ.0000000000000471. [DOI] [PubMed] [Google Scholar]
- 22.Hong W, Geng W, Wang C, et al. Prevalence of colonic diverticulosis in mainland China from 2004 to 2014. Sci Rep 2016; 6: 26237. DOI: 10.1038/srep26237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hong W, Dong L, Stock S, et al. Prevalence and characteristics of colonic adenoma in mainland China. Cancer Manag Res 2018; 10: 2743–2755. DOI: 10.2147/CMAR.S166186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ngo ST, Steyn FJ, McCombe PA. Gender differences in autoimmune disease. Front Neuroendocrinol 2014; 35: 347–369. DOI: 10.1016/j.yfrne.2014.04.004 S0091-3022(14)00046-6 [pii]. [DOI] [PubMed] [Google Scholar]
- 25.Meleine M, Matricon J. Gender-related differences in irritable bowel syndrome: potential mechanisms of sex hormones. World J Gastroenterol 2014; 20: 6725–6743. DOI: 10.3748/wjg.v20.i22.6725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maas AH, Appelman YE. Gender differences in coronary heart disease. Neth Heart J 2010; 18: 598–602. DOI: 10.1007/s12471-010-0841-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim K, Park H. Gender differences in the association between self-reported stress and cigarette smoking in Korean adolescents. Tob Induc Dis 2016; 14: 19. DOI: 10.1186/s12971-016-0084-984 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yue Y, Hong L, Guo L, et al. Gender differences in the association between cigarette smoking, alcohol consumption and depressive symptoms: a cross-sectional study among Chinese adolescents. Sci Rep 2015; 5: 17959. DOI: 10.1038/srep17959 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chang YW, Ko WJ, Oh CH, et al. Clarithromycin resistance and female gender affect Helicobacter pylori eradication failure in chronic gastritis. Korean J Intern Med 2018; 34: 1022–1029. DOI: 10.3904/kjim.2018.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abd Rahim MA, Johani FH, Shah SA, et al. 13C-Urea Breath Test Accuracy for Helicobacter pylori Infection in the Asian Population: a Meta-Analysis. Ann Glob Health 2019; 85: 110. DOI: 10.5334/aogh.2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kwon YH, Kim N, Yoon H, et al. Effect of Citric Acid on Accuracy of (13)C-Urea Breath Test after Helicobacter pylori Eradication Therapy in a Region with a High Prevalence of Atrophic Gastritis. Gut Liver 2019; 13: 506–514. DOI: 10.5009/gnl18398 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed in the current study are available from the corresponding author on reasonable request.