To the Editor
We are writing regarding the 2006 publication of a new stable isotope labeling kinetics (SILK) protocol for assessing the fractional synthesis and clearance rates of amyloid β (Aβ) in the brain1. This protocol has been used to demonstrate the relatively accelerated rate of turnover of Aβ peptides in the brain1 and to assess the effect of drugs on Aβ production2. However, we are concerned that aspects of the protocol have not been fully appreciated, raising questions about the interpretation of findings from the protocol.
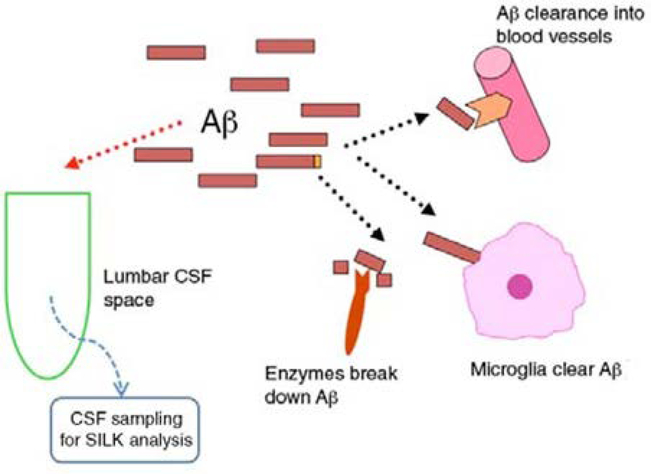
Our main concern is the estimation of fractional clearance rates for Aβ. In the Alzheimer’s disease literature, clearance usually refers to degradation or removal of Aβ by proteolytic enzymes, microglia and blood-brain barrier transport. Several studies have assumed that clearance rates measured by the SILK protocol reflect these factors1–3, but we suggest this is not the case. Understanding the distinction between clearance of Aβ and the phenomenon measured by the SILK protocol requires knowledge of details of the protocol. In brief, the protocol1 consists of a production phase, during which 13C6-labeled leucine (13C6-leucine) is infused intravenously and the proportion of synthesized and secreted Aβ labeled with 13C6-leucine at amino acid 17 is measured, and a clearance phase, during which the proportion of Aβ labeled with 13C6-leucine is monitored for some hours after cessation of the peripheral infusion. Cerebrospinal fluid (CSF) is sampled with a catheter inserted between the third and fourth lumbar vertebrae, with hourly sampling for 36 h. During peripheral 13C6-leucine infusion, approximately 10% of leucine available for protein synthesis is 13C6 labeled. The rate at which the proportion of Aβ labeled with 13C6-leucine approaches the saturation proportion of 10% provides an estimate of the synthesis rate of Aβ1. Interpretation of changes in this proportion after the production phase is less clear. The key observation in regard to our above concern is that the protocol does not measure the absolute amount of newly synthesized Aβ but rather the relative proportion of Aβ labeled with 13C6-leucine at amino acid 17 (ref. 1). Because the processes of degradation or removal of Aβ by proteolytic enzymes, microglia and blood-brain barrier transport do not distinguish between labeled and unlabeled Aβ, they do not influence the relative proportion of these molecules in fluids leaving the parenchyma (Fig. 1). The SILK protocol measures proportions, and therefore findings by the SILK protocol do not reflect the influence of parenchymal clearance on Aβ levels.
Figure 1.
During the infusion of 13C6-labeled leucine, the fraction of labeled Aβ secreted by neurons increases from 0% to a theoretical maximum of 10% at the neuronal fractional synthesis rate. Within the brain, secreted molecules are cleared by processes that do not discriminate labeled from unlabeled molecules and do not influence the fraction that is labeled. Labeled molecules leaving the brain are diluted by a large volume of CSF containing unlabeled Aβ before being sampled from the lumbar subarachnoid space.
What does the clearance rate obtained by the SILK protocol measure? We have implemented computer models to characterize this aspect of the protocol. (Supplementary Methods). As originally described1, clearance rates are estimated using data from hours 24 through 36 of the protocol. By hour 24 of the protocol, neuronal APP amyloid precursor protein and secreted will have turned over one or more times since the end of the infusion, and the proportion of newly secreted Aβ originating within the brain that is labeled will approach zero. Nonetheless, a measurable fraction of labeled Aβ remains within the substantial volume of CSF contained in the subarachnoid space at this time. This fraction declines logarithmically (as described in the original protocol1). Logarithmic decline is consistent with expectation for a process of dilution by bulk flow and turnover of CSF. For example, a person with a CSF volume of 150 ml and production of CSF of 20 ml h−1 at the choroid plexus and other brain sites will clear CSF labeled Aβ at a rate of 20/150 (13.33%) per hour (Supplementary Fig. 1). That is, rates measured at the clearance phase of the SILK protocol reflect CSF turnover. We conclude that there is little reason to extend CSF sampling beyond the production phase of the protocol and that time and resources could be saved, and study subject burden avoided, by ending the protocol at hour 15.
Our second concern is that fractional synthesis rate estimates by the protocol are underestimates of the actual synthesis rates. This is because the mixture of labeled and unlabeled Aβ molecules in fluids leaving the brain is diluted by a large volume of CSF containing only unlabeled Aβ before being sampled at the lumbar spine (Fig. 1). Dilution slows the rate at which the proportion of Aβ that is labeled approaches 10% saturation. We have demonstrated by computer simulation that this downward bias may be substantial (Supplementary Results). Moreover, the downward bias is a function of CSF volume, such that persons with larger CSF volume have a larger downward bias (Supplementary Fig. 1). This is relevant to the recent observation that synthesis rate estimates by the SILK protocol are comparable in individuals with sporadic Alzheimer’s disease and age-match controls3. On the basis of the greater volume of circulating CSF in people with Alzheimer’s disease, one would expect slower observed rates in affected individuals and faster observed rates in controls if synthesis rates were comparable in these two groups (Supplementary Fig. 2). The lack of an observed difference3 may simply reflect small sample size and type II error. Alternatively, if this finding holds in larger samples, it would be consistent with higher neuronal Aβ synthesis rates in sporadic Alzheimer’s disease.
Supplementary Material
ACKNOWLEDGMENTS
We would like to acknowledge support from US National Institutes of Health grants AG5131 and AG034439.
Footnotes
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
References
- 1.Bateman RJ et al. Nat. Med. 12, 856–861 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bateman RJ et al. Ann. Neurol. 66, 48–54 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mawuenyega KG et al. Science 330, 1774 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.