Abstract
Familial chylomicronemia syndrome (FCS) is a rare disorder associated with chylomicronemia (CM) and an increased risk of pancreatitis. Most individuals with CM do not have FCS but exhibit multifactorial CM (MCM), which differs from FCS in terms of risk and disease management. This study aimed to investigate clinical and gene expression profiles of FCS and MCM patients. Anthropometrics, clinical, and biochemical variables were analyzed in 57 FCS and 353 MCM patients. Gene expression analyses were performed in a subsample of 19 FCS, 28 MCM, and 15 normolipidemic controls. Receiver operating characteristic (ROC) curve analyses were performed to analyze the capacity of variables to discriminate FCS from MCM. Sustained fasting triglycerides ≥20 mmol/L (>15 mmol/L with eruptive xanthomas), history of pancreatitis, poor response to fibrates, diagnosis of CM at childhood, body mass index <22 kg/m2, and delipidated apolipoprotein B or glycerol levels <0.9 g/L and <0.05 mmol/L, respectively, had an area under the ROC curve ≥0.7. Gene expression analyses identified 142 probes differentially expressed in FCS and 32 in MCM compared with controls. Among them, 13 probes are shared between FCS and MCM; 63 are specific to FCS and 2 to MCM. Most FCS-specific or shared biomarkers are involved in inflammatory, immune, circadian, postprandial metabolism, signaling, docking systems, or receptor-mediated clearance mechanisms. This study reveals differential signatures of FCS and MCM. It opens the door to the identification of key mechanisms of CM expression and potential targets for the development of new treatments.
Keywords: gene expression, chylomicronemia, lipoprotein lipase deficiency
Chylomicronemia (CM) is a clinical condition associated with plasma accumulation of chylomicrons in the presence of severe hypertriglyceridemia, usually triglyceride (TG) levels >10 mmol/L). CM, whose the prevalence was estimated at 150 to 400 per 100 000 individuals in Caucasian populations, is associated with increased risk of abdominal pain, eruptive xanthomas, hepatosplenomegaly, lipemia retinalis, and acute, life-threatening pancreatitis [1, 2].
Chylomicrons are large TG-rich lipoproteins produced in the gut wall in the postprandial state. They are rapidly cleared from the bloodstream through TG hydrolysis by lipoprotein lipase (LPL), the major way for clearance of TG-rich lipoproteins, chylomicrons and very low-density lipoproteins (VLDLs). The impairment of LPL function will therefore frequently be the underlying mechanism of CM [3]. In the case of familial chylomicronemia syndrome (FCS), a rare autosomal recessive form of CM (0.1-0.2 per 100 000 individuals), LPL impairment is total and caused by null loss-of-function variants in LPL or LPL-related genes (GPIHBP1, APOA5, APOC2, and LMF1) [3, 4]. However, the vast majority of individuals with CM are not affected by FCS but present multifactorial CM (MCM) phenotypes in which the residual LPL activity is variable. MCM is a multifactorial disorder, most often polygenic, and frequently associated with overweight (or obesity) and other elements of the metabolic syndrome in addition to unhealthy life habits [5].
Although clinical risks associated with CM may appear similar whatever its etiology, pancreatitis and cardiometabolic risks, drug response, and disease management are different for MCM and FCS [4, 6, 7]. Both disorders differ according to genetic background [8]. However, because appropriate genetic testing may not always be available, it may be difficult to distinguish between these 2 diseases and rule out a FCS diagnosis.
The objective of this study was to explore gene expression, as well as functional and clinical signatures that could help to distinguish FCS from MCM, beyond patients’ genetic background, and identify key clinical discriminants, clinically useful algorithms, and potential targets for intervention.
1. Material and Methods
A. Subjects and Clinical Data
This study included 425 French Canadians adult subjects from the Saguenay–Lac-Saint-Jean founder population (Quebec, Canada). Fifty-seven were FCS patients with genetically and physiologically (postheparin LPL activity <5%) confirmed LPL deficiency, 353 had MCM, and 15 were healthy normolipidemic control subjects. History of pancreatitis and of plasma TG levels, response to fibrates, and age at diagnosis of severe hypertriglyceridemia were documented using questionnaires and the patient’s medical charts. Subjects gave their informed consent to participate in this study and were assigned a code that systematically de-identifies all clinical data [9]. This study, which was conducted as part of a research program on the natural history of severe hypertriglyceridemia (SMASH: Systems and Molecular Approaches of Severe Hyperlipidemias) was approved by IRB Services (now Advarra) and was conducted in accordance with the Declaration of Helsinki.
B. Biochemical Analyses
Blood samples were obtained after a 12-hour overnight fast from the antecubital vein into serum separation tubes. Total TG concentration was measured by enzymatic assays on a CX7Analyser (Beckman, Fullerton, CA, USA) [10]. Apolipoprotein (apo) B levels were determined in delipidated plasma using nephelometry. Plasma glycerol concentration, an orderable laboratory test usually available at low cost, was measured in delipidated plasma using an analyzer Technicon RA-500 (Bayer Corporation, Tarrytown, NY).
C. Gene Expression Analyses
Gene expression analyses were conducted in a subsample of 62 subjects; 19 FCS and 28 MCM subjects as well as 15 normolipidemic healthy controls (Table 1). Whole blood ribonucleic acid (RNA) was extracted from blood sampled using PAXgene RNA tubes (Qiagen, Valencia, CA, USA). RNA samples were hybridized on Affymetrix® Human Gene ST 2.0 microarrays (Santa Clara, CA, USA). Robust multi-array average (RMA) was applied on raw intensities [11]. Differential expression moderated t-tests between studied groups were performed using a linear model of the Bioconductor package Limma. The false discovery rate was controlled using the Benjamini–Hochberg procedure [12]. Data were analyzed using QIAGEN’s Ingenuity® Pathway Analysis (IPA®, QIAGEN Redwood City, CA, USA).
Table 1.
Characteristics of subjects included in gene expression analyses
| FCS (n = 19) | MCM (n = 28) | Control (n = 15) | P-value | |
|---|---|---|---|---|
| Age, years | 48.4 ± 13.9 | 55.6 ± 9.2 | 53.1 ± 12.1 | NS |
| Women, % | 63.2 | 35.7 | 46.7 | NS |
| Total triglyceride, mmol/L* | 22.7 (15.7-34.2) | 5.9 (4.1-11.5)a | 1.0 (0.9-1.5)a,b | <.001 |
| Body mass index, kg/m2 | 22.7 ± 4.5 | 29.4 ± 3.8a | 25.0 ± 3.2b | <.001 |
Data are mean ± SD, unless otherwise specified.
*Median (interquartile range) and P-value obtained after log10 transformation of data.
Abbreviations: FCS, familial chylomicronemia syndrome; MCM, multifactorial chylomicronemia; NS, P > .1.
Significantly different (P < .05) from aFCS or bMCM.
D. Statistical Analyses
Categorical variables were compared using the Pearson chi-square statistic or Fisher’s exact tests, and group differences for continuous variables were compared with unpaired 2-tailed Student-t tests or 1-way analysis of variance, using log10-transformed data, followed by Bonferroni post hoc tests. Univariate receiver operating characteristic (ROC) curve analyses were performed to analyze the capacity of each variable to discriminate FCS from MCM. An area under the curve (AUC) ≥0.7 was used to identify variables with a fair discriminant capacity [13]. Statistical analyses were performed with Stata/MP package (release 13.1, Tx, USA).
2. Results
Table 2 presents the characteristics of all FCS and MCM participants. Age, total TG, history of pancreatitis, response to fibrates, age at CM diagnosis, body mass index as well as apo B and glycerol concentrations are all significantly different between the groups. Prevalence (%) of overweight and obesity are also significantly lower among the FCS group. More than half (53%) of the subjects with FCS and only 4% of subjects with MCM have a body mass index <22 kg/m2 (P < .001).
Table 2.
Characteristics of patients with FCS and patients with MCM
| FCS(n = 57) | MCM(n = 353) | P-value | |
|---|---|---|---|
| Age, years | 33.8 ± 14.2 | 46.9 ± 11.2 | <.001 |
| Women, % | 44.8 | 32.3 | NS |
| Total triglyceridea, mmol/L* | 28.0 (18.3-41.4) | 14.0 (10.9-21.6) | <.001 |
| History of pancreatitisb, % | 83.0 | 10.8 | <.001 |
| Poor response to fibratesc, % | 100 | 5.2 | <.001 |
| Severe hyperTG diagnosed <18 yearsd, % | 50.0 | 0 | <.001 |
| High blood pressured, % | 40.9 | 42.3 | NS |
| Coronary artery diseased, % | 29.5 | 25.3 | NS |
| Type 2 diabetesd, % | 31.8 | 27.6 | NS |
| BMIe, kg/m2 | 22.9 ± 4.5 | 29.6 ± 5.2 | <.001 |
| BMI < 22 kg/m2, % | 53.3 | 4.4 | <.001 |
| Overweight (BMI > 27 kg/m2), % | 13.3 | 69.7 | <.001 |
| Obesity (BMI > 30 kg/m2), % | 8.9 | 40.1 | <.001 |
| Apolipoprotein Bf, g/L† | 0.73 ± 0.35 | 1.21 ± 0.40 | <.001 |
| Free glycerolg, mmol/L*† | 0.04 (0.02-0.08) | 0.08 (0.06-0.12) | <.001 |
Data are mean ± standard deviation, unless otherwise specified.
Abbreviations: NS, P > .1. FCS, familial chylomicronemia syndrome; HyperTG, hypertriglyceridemia; MCM, multifactorial chylomicronemia.
*Median (interquartile range) and P-value obtained after log10 transformation of data. †Measured in delipidated plasma.
aFCS: n = 50; bFCS: n = 47; cMCM: n = 115; dFCS: n = 44; eFCS: n = 45; fFCS: n = 39, MCM: n = 245; gFCS: n = 41, MCM: n = 222.
An iterative process based on the area under the ROC curve has led to the selection of a subset of significant variables and to the thresholds selected for the continuous ones. ROC curve analyses of these variables used to discriminate FCS from MCM (not considering the genotype) revealed that sustained fasting TG ≥20 mmol/L (or >15 mmol/L with history of eruptive xanthomas) based on all historical available values, a positive history of pancreatitis, a poor response to fibrates (<20% TG decrease, which corresponds to the lowest reduction of TG expected with fibrates [14]), a diagnosis of CM at childhood (before 18 years) with history of recurrent colicky pain and/or failure to thrive, a body mass index <22 as well as delipidated plasma apo B or free glycerol levels <0.9 g/L and <0.05 mmol/L, respectively, correspond to an AUC ≥0.7 (Table 3). Although few FCS patients simultaneously presented all of these characteristics, most presented at least 3 of them (Table 4).
Table 3.
Univariate ROC curve analysis to document the ability of selected candidate clinical markers to discriminate FCS from MCM
| AUC | 95% CI | |
|---|---|---|
| Sustained TG ≥20 mmol/L or TG >15 mmol/L and eruptive xanthomas | 0.78 | (0.72-0.83) |
| History of pancreatitis | 0.86 | (0.80-0.92) |
| Poor response to fibrates | 0.97 | (0.95-0.99) |
| Severe hyperTG diagnosed <18 years | 0.75 | (0.68-0.82) |
| Body mass index < 22 kg/m2 | 0.74 | (0.67-0.82) |
| Apolipoprotein B <0.9 g/La | 0.83 | (0.78-0.89) |
| Free glycerol <0.05 mmol/La | 0.70 | (0.61-0.77) |
Abbreviations: AUC, area under the curve; CI, confidence interval; FCS, familial chylomicronemia syndrome; hyperTG, hypertriglyceridemia; MCM, multifactorial chylomicronemia; ROC, receiver operating characteristic; TG, total triglyceride.
aMeasured in delipidated plasma.
Table 4.
Distribution (%) of patients with FCS and patients with MCM according to the number of candidate clinical markers presenta
| MCM | FCS | |
|---|---|---|
| 0 | 44 (47.3%) | 0 |
| 1 | 30 (32.3%) | 0 |
| 2 | 15 (16.1%) | 1 (3.2%) |
| 3 | 4 (4.3%) | 6 (19.4%) |
| 4 | 0 | 4 (12.9%) |
| 5 | 0 | 7 (22.6%) |
| 6 | 0 | 9 (29.0%) |
| 7 | 0 | 4 (12.9%) |
| Total | 93 (100%) | 31 (100%) |
FCS, familial chylomicronemia syndrome; MCM, multifactorial chylomicronemia.
aAmong patients for which information was available for all candidate clinical markers.
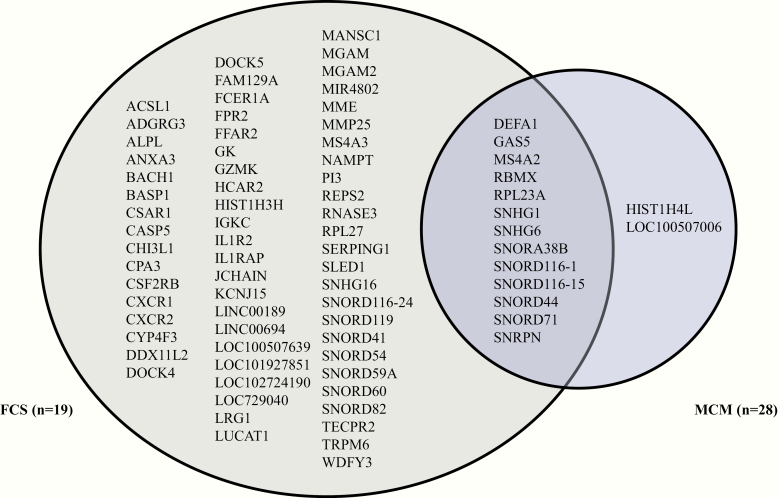
Gene expression analyses were then conducted and identified 142 probes differentially expressed in FCS, and 32 in MCM compared with controls. Among them, 13 annotated probes are shared between FCS and MCM, 63 are specific to FCS and 2 to MCM (Fig. 1). Most FCS-specific or shared probes are biomarkers involved in inflammatory, immune, circadian, postprandial metabolism, signaling, docking systems or receptor-mediated clearance mechanisms.
Figure 1.
Venn diagram representing the distribution of differentially expressed biomarkers among subjects with familial chylomicronemia syndrome (FCS) and multifactorial chylomicronemia (MCM) (|fold change| ≥2 at P < .01 and a false discovery rate >0.05).
3. Discussion
Results of our study suggest that simple and affordable clinical variables, beyond a patients’ genetic background, could adequately discriminate genetically and physiologically proven FCS from MCM in this sample. Most variables are the same as those of previously published studies [7, 15]. However, the current study further highlighted the discriminant capacity of apo B, a well-recognized correlate of metabolic syndrome and obesity which tends to be higher in MCM [15], and identified low delipidated glycerol levels as another promising clinical marker of FCS. Glycerol, as a subproduct of TG breakdown, is expected to be reduced in conditions associated with significant reduction of lipolysis [16]. It was therefore thought that it could be an additional easily measured and available variable that may help distinguish between FCS and MCM among patients with severe hypertriglyceridemia.
This study also led to the identification of genetic biomarkers specifically expressed among patients with FCS and others biomarkers only expressed among patients with MCM. This study suggests differential gene expression signatures for both disorders. Most of these biomarkers are known to be involved in inflammatory, immune, circadian, postprandial metabolism, signaling, docking systems, or receptor-mediated clearance mechanisms. Differences in gene expression levels between FCS and MCM, induced by or simply associated with the disorders, could then differentially affect their physiopathology and account, at least, in part, for the dissimilarities between them. Interestingly, while FCS is associated with a greater risk of recurrent acute pancreatitis than MCM [6,7,17], some genes previously associated with pancreatic cancer and/or chronic pancreatitis (IL1RAP, RPL27, and SERPING1) are differentially expressed in FCS patients [18, 19]. Others genes, for their part, are well known to be involved in lipid and fatty acids metabolism, such as GPR43/FFAR2 and ACSL1 [20, 21].
FCS and MCM are CM-related diseases that differ in terms of incidence, pancreatitis risk, cardiometabolic risk, and response to TG-lowering interventions. Several new or emerging therapies have been specifically developed for FCS while others target MCM, increasing the need for precise diagnosis [22]. These results suggest that clinical and gene expression profiling may contribute to the identification of key mechanisms and lead to the identification of potential targets for the development of new treatments. Our next steps will be to work on the development of a comprehensive and accessible diagnosis scoring system, based on the clinical signature of both disorders and on the analysis of all previous studies. We also plan to identify genetic variants using exome sequencing and to perform functional studies with top candidate genes, including plasmatic RNA expression validation and protein quantification.
Acknowledgments
We are thankful to all participants and the ECOGENE-21 staff. Microarrays were performed by the Genome Quebec and McGill University Innovation Center (Montréal, QC, Canada). The Canadian Center for Computational Genomics (C3G) is a Genomics Technology Platform (GTP) supported by the Canadian Government through Genome Canada.
Financial Support: This study was supported by ECOGENE-21, a non-for-profit research organization.
Glossary
Abbreviations
- apo
apolipoprotein
- AUC
area under the curve
- CM
chylomicronemia
- FCS
familial chylomicronemia syndrome
- LPL
lipoprotein lipase
- MCM
multifactorial chylomicronemia
- RNA
ribonucleic acid
- ROC
receiver operating characteristic
- TG
triglyceride
- VLDL
very low-density lipoprotein
Additional Information
Disclosure Summary: Authors have no conflict of interest to disclose.
Data Availability: Gene expression data are deposited at: https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE149607.
References
- 1. Johansen CT, Kathiresan S, Hegele RA. Genetic determinants of plasma triglycerides. J Lipid Res. 2011;52(2):189-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ford ES, Li C, Zhao G, Pearson WS, Mokdad AH. Hypertriglyceridemia and its pharmacologic treatment among US adults. Arch Intern Med. 2009;169(6):572-578. [DOI] [PubMed] [Google Scholar]
- 3. Brunzell JD, Deeb SS. Familial lipoprotein lipase deficiency, apo CII deficiency, and hepatic lipase deficiency. In: Scriver CR, Beaudet AL, Sly WS, Valle D, eds. The Metabolic and Molecular Bases of Inherited Disease. Volume 2 New York, NY: McGraw-Hill; 2001:2789-2816. [Google Scholar]
- 4. Brahm AJ, Hegele RA. Chylomicronaemia–current diagnosis and future therapies. Nat Rev Endocrinol. 2015;11(6):352-362. [DOI] [PubMed] [Google Scholar]
- 5. Chait A, Eckel RH. The Chylomicronemia syndrome is most often multifactorial: a narrative review of causes and treatment. Ann Intern Med. 2019;170(9):626-634. [DOI] [PubMed] [Google Scholar]
- 6. Gaudet D, Blom D, Bruckert E, et al. Acute pancreatitis is highly prevalent and complications can be fatal in patients with familial chylomicronemia: results from a survey of lipidologists. J Clin Lipidol. 2016;10(3):680-681. [Google Scholar]
- 7. Paquette M, Bernard S, Hegele RA, Baass A. Chylomicronemia: differences between familial chylomicronemia syndrome and multifactorial chylomicronemia. Atherosclerosis. 2019;283:137-142. [DOI] [PubMed] [Google Scholar]
- 8. Dron JS, Hegele RA. Genetics of triglycerides and the risk of atherosclerosis. Curr Atheroscler Rep. 2017;19(7):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gaudet D, Arsenault S, Bélanger C, et al. Procedure to protect confidentiality of familial data in community genetics and genomic research. Clin Genet. 1999;55(4):259-264. [DOI] [PubMed] [Google Scholar]
- 10. McNamara JR, Schaefer EJ. Automated enzymatic standardized lipid analyses for plasma and lipoprotein fractions. Clin Chim Acta. 1987;166(1):1-8. [DOI] [PubMed] [Google Scholar]
- 11. Irizarry RA, Hobbs B, Collin F, et al. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics. 2003;4(2):249-264. [DOI] [PubMed] [Google Scholar]
- 12. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Statist Soc B. 1995;57(1):289-300. [Google Scholar]
- 13. Metz CE. Basic principles of ROC analysis. Semin Nucl Med. 1978;8(4):283-298. [DOI] [PubMed] [Google Scholar]
- 14. Keech AC, Jenkins AJ. Triglyceride-lowering trials. Curr Opin Lipidol. 2017;28(6):477-487. [DOI] [PubMed] [Google Scholar]
- 15. Moulin P, Dufour R, Averna M, et al. Identification and diagnosis of patients with familial chylomicronaemia syndrome (FCS): expert panel recommendations and proposal of an “FCS score”. Atherosclerosis. 2018;275:265-272. [DOI] [PubMed] [Google Scholar]
- 16. Coppack SW, Persson M, Judd RL, Miles JM. Glycerol and nonesterified fatty acid metabolism in human muscle and adipose tissue in vivo. Am J Physiol. 1999;276(2):E233-E240. [DOI] [PubMed] [Google Scholar]
- 17. Brown WV, Goldberg I, Duell B, Gaudet D. Roundtable discussion: familial chylomicronemia syndrome: diagnosis and management. J Clin Lipidol. 2018;12(2):254-263. [DOI] [PubMed] [Google Scholar]
- 18. Thomas JK, Kim MS, Balakrishnan L, et al. Pancreatic cancer database: an integrative resource for pancreatic cancer. Cancer Biol Ther. 2014;15(8):963-967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pan S, Chen R, Stevens T, et al. Proteomics portrait of archival lesions of chronic pancreatitis. PLoS One. 2011;6(11):e27574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Brown AJ, Goldsworthy SM, Barnes AA, et al. The orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J Biol Chem. 2003;278(13):11312-11319. [DOI] [PubMed] [Google Scholar]
- 21. Ellis JM, Bowman CE, Wolfgang MJ. Metabolic and tissue-specific regulation of acyl-CoA metabolism. Plos One. 2015;10(3):e0116587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Laufs U, Parhofer KG, Ginsberg HN, Hegele RA. Clinical review on triglycerides. Eur Heart J. 2020;41(1):99-109c. [DOI] [PMC free article] [PubMed] [Google Scholar]