Abstract
Purpose
The impact of endogenous androgen levels on the risk of type 2 diabetes in women remains uncertain. The objective was to investigate associations between endogenous androgen levels and risk of type 2 diabetes in young women without established comorbidity.
Methods
In this retrospective cohort study, women aged 18 to 50 years who underwent measurement of plasma testosterone, dehydroepiandrosterone-sulfate (DHEA-S), dihydrotestosterone (DHT), and sex hormone-binding globulin (SHBG) for the first time from January 2007 to December 2015 were included. Androgens were analyzed using tandem liquid chromatography mass spectrometry. Women with established comorbidity were excluded, using Danish healthcare registries. We calculated incidence rate ratios (IRRs, 95% confidence intervals) of type 2 diabetes according to quartiles of plasma androgens using multivariate Poisson regression models.
Results
A total of 8876 women, with a mean ± SD age of 38.5 ± 4.6 years and a median (interquartile range [IQR]) follow-up duration of 8.1 (6.6-9.4) years, were eligible for analyses. During 69 728 person-years, 69 women were diagnosed with type 2 diabetes. Women in the highest quartile of plasma total testosterone and calculated free testosterone displayed increased risk of type 2 diabetes compared with the lowest quartile: IRR 1.97 (1.01; 3.85), P = .048 and IRR 7.32 (2.84; 18.83), P < .001. SHBG was inversely associated with type 2 diabetes, Q4 versus Q1; IRR 0.06 (0.02; 0.21), P < .001. Plasma DHEA-S and DHT were not associated with incident type 2 diabetes.
Conclusions
Higher levels of plasma total and free testosterone were associated with increased risk of type 2 diabetes among women.
Keywords: testosterone, women, androgens, type 2 diabetes
The impact of endogenous androgens on risk of type 2 diabetes is well established in women with established hyperandrogenism such as polycystic ovary syndrome (PCOS) [1, 2], a condition strongly associated with increased insulin resistance, but knowledge on plasma testosterone level per se as a risk factor in women without established hyperandrogenism is warranted. Only few prospective studies have investigated the relation between and with conflicting results [3-8]. The available literature is predominantly based on postmenopausal women, whereas longitudinal studies among premenopausal women are scarce [3, 4]. Furthermore, information on the association between other endogenous androgens than testosterone and type 2 diabetes in women is virtually nonexistent. The impact of hyperandrogenism on insulin resistance in women has been investigated in several observational studies; although not fully understood, the potential mechanisms include hyperandrogenism induced: hepatic steatosis increased visceral adipose tissue, and dysfunctional adipose tissue [9-11]. In women, endogenous androgen levels decrease steadily following the age of 30 years [12-15]. When reaching menopause, women have lost approximately 60% of their total androgen pool [14], which, in theory, could limit the impact of androgens on the risk of type 2 diabetes among postmenopausal women. The female adrenal pool consist of various androgens which differ in plasma levels and affinity for the androgen receptor [16]. Plasma levels of testosterone and dihydrotestosterone (DHT) are very low in women but have high affinity for the androgen receptor and therefore hold solid androgenic properties whereas the adrenal androgen dehydroepiandrosterone-sulfate (DHEA-S) is found in high plasma concentrations but possesses low affinity for the androgen receptor and is mainly considered a precursor androgen [13].
The objective of this study was to investigate the relation between risk of incident type 2 diabetes and plasma levels of total testosterone (TT), calculated free testosterone (cFT), DHT, DHEA-S, and sexual hormone-binding globulin (SHBG) using a retrospective cohort of young women who had no established comorbidities.
1. Materials and Methods
A. Study cohort and setting
The study cohort included women 18 to 50 years of age residing in Denmark and who underwent first-time measurement of endogenous androgens at the nationally approved laboratory, the Danish State Serum Institute, Copenhagen, Denmark, from January 1, 2007, until December 31, 2015. During this period, the Danish State Serum Institute performed most endogenous androgen assessments in Denmark using state-of-the-art methods, such as tandem liquid chromatography mass spectrometry. Referrals for measurements of androgen levels were from general practitioners, physicians with a private clinic, or hospital departments. Follow-up was performed until December 31, 2017.
Women eligible for inclusion in the study were predefined as being without any known comorbidities. Therefore, we excluded women who at inclusion featured established chronic comorbidity or administered medications (which indicated chronic comorbidity) that could influence endogenous androgen levels in plasma or could impact risk of developing type 2 diabetes. The predefined comorbidities were identified from the Danish National Patient Registry using the International Classification of Diseases, 10th revision (ICD-10): established diabetes of any kind including previous gestational diabetes (ICD-10: DE10, DE11, DE13, DE14, and DO244); PCOS (ICD-10: DE282) or hirsutism (ICD-10: DL680), overweight or obesity (ICD-10: DE66), cardiac diseases including arrythmias (ICD-10: DI44-49), ischemic heart disease/nonfatal myocardial infarction (ICD-10: I20-I25), heart failure (ICD-10: DI110, DI500-501, DI509, DI420-422, DI426, DI429, DJ819) and valve diseases (ICD-10: DI05, DI06, DI34-36, DZ952); nonfatal hemorrhagic or ischemic stroke (ICD-10: DI60-64); venous thromboembolism (ICD-10: DI26, DI74, DI81, DI646, DI801-803, DI822-823, DI828-829); renal diseases (ICD-10: DI12-13, DN03-04, DN17-19, DR34, DT858-859); chronic obstructive pulmonary disease (ICD-10: DJ40-44); current or previous cancer of any kind (ICD-10: DC00-090); pituitary diseases (ICD-10: DE22-23); Addison’s disease (ICD-10: DE27); Turner’s disease (ICD-10: DQ96); thyroid diseases (ICD-10: DE02-03, DE05, DE062-63, DO905); anemia (ICD-10:DD500, DD509, DD629, DD638, DD649); dementia (ICD-10: DF00-03). Additionally women were excluded if the Charlson comorbidity index [17] was >0.
From the Danish National Prescription Registry, data on all prescribed medication were evaluated. We excluded women who at baseline (180 days before and 7 days after measurement of endogenous androgens) were treated with any glucose-lowering drugs (A10), contraceptive pills (G03A), diuretics (C03), statins (C10), androgens (G03B), analgesics (paracetamol (N02) or nonsteroidal anti-inflammatory drugs (M01A), or had established hypertension defined by use of antihypertensive drugs in combination treatment with a least 2 classes of antihypertensive drugs such as adrenergic alpha antagonists (C02CA), nonloop diuretics (C03AA), vasodilators (C08), beta-blockers (C07AB02), calcium-channel blockers (C08CA01), and renin–angiotensin system inhibitors (C09AA02).
B. Endogenous androgen assessments
Plasma testosterone was analyzed using liquid chromatography tandem mass spectrometry (LC-MS). An in-house method was used from 2007 to 2010. In 2011 and onwards, the Perkin Elmer CHS LC-MS Steroid kit (Perkin Elmer, Waltham, Massachusetts) was used for testosterone measurement. DHEA-S was initially analyzed using the Abbot Architect DHEA-S immunoassay (Abbott Diagnostics, Illinois) but was transferred to LC-MS when the Perkin Elmer kit was implemented. DHT was initially measured by an in-house radioimmunoassay. From 2012 an onwards DHT was measured by in an in-house LC-MS method. In general, intra- and interassay variations for androgen measurements were 8% and 10%, respectively. Plasma SHBG levels were assessed using the Abbot Architech SHBG immunoassay (Abbott Diagnostics, Illinois). Plasma free testosterone levels were calculated (cFT) using plasma TT and SHBG in methods described by Bartsch [18].
C. Outcome
The primary outcome, incident type 2 diabetes, was defined with ICD-10 codes according to the Danish National Patient Registry: type 2 diabetes (DE11). We did not include other types of diabetes (DE13), diabetes without specification (DE14), or gestational diabetes (DO244), in the primary outcome and we also did not consider individuals who were diagnosed with type 1 diabetes (DE10).
To minimize risk of confounding by indication, follow-up was started after a grace period of 6 months. Thus, we did not consider individuals who reached the primary outcome within 6 months from measurement of endogenous androgen levels.
D. Danish nationwide registries
All citizens of Denmark are given a permanent unique civil registry number allowing us to perform individual-level linkage in nationwide registries [19].
The Danish National Patient Registry holds records on hospital contacts at an individual level since 1978. Every hospital contact is given a primary diagnosis with an ICD-10 code and, if appropriate, secondary diagnoses are registered [20].
The Danish National Prescription Registry contains information on all prescribed medication (including dispensation date, doses, and quantity) among residents of Denmark since 1995 and has been validated as highly accurate [21].
E. Ethics
The study was approved by the Danish Data Protection Agency (2007-58-0015, GEH-2014–016, I-Suite no. 02734). A cohort study based on registries, at present, does not require approval from an ethics committee according to Danish law. All civil registry numbers were fully anonymized before linkage with the Danish registries on servers provided by Statistics Denmark.
F. Statistical analyses
Categorical variables are presented as percentages and compared using the chi-square test. Numerical variables are presented as means ± standard deviations (SD) and compared using analysis of variance. Incidence rates (IRs) are displayed as cases per 1000 person-years stratified for quartiles of androgens. We used a multivariate time-dependent Poisson regression model to calculate incidence rate ratios (IRRs) with 95% confidence intervals (95% CI) using the lowest quartile of each plasma androgen level, Q1, as a reference compared with the upper three quartiles, Q2, Q3, and Q4. The Poisson model was adjusted for the following covariates: age, plasma SHBG, average yearly income, healthcare referral setting, geographical region, and calendar year. Since we did not have measurements of body mass index (BMI) or body composition, which are important risk factors of type 2 diabetes, all multivariate Poisson models were adjusted for plasma SHBG, which is strongly inversely linked with abdominal obesity and insulin resistance [22]. Therefore, plasma SHBG is a relevant indirect parameter of body composition such as BMI. The Poisson model for SHBG was further adjusted for TT while the model for cFT was not adjusted for SHBG as the covariate is included in the estimation of cFT [18]. Participants were included at the first-time androgen measurement and followed until the primary outcome was reached or censored at the end of the follow-up period December 31, 2017, death, or migration (whichever came first). We performed 2 types of sensitivity analyses on plasma TT and cFT and risk of type 2 diabetes as these data were complete. (1) Extending the primary outcome to also include women without a hospital-based diagnosis of type 2 diabetes but confined to either (a) therapy with ≥2 glucose-lowering drugs or (b) monotherapy with metformin in women aged ≥40 years. These criteria were established to minimize risk of confounding by indication by the diagnosis of PCOS. Accordingly, metformin is first-line therapy for type 2 diabetes, but can also be used in therapy of PCOS, especially among younger women [23]. And (2) including subgroups who, a priori, were assumed to be associated with increased risk of type 2 diabetes: overweight/obesity and PCOS/hirsutism. P < .05 was considered statistically significant in all analyses. We used the statistical software Stata Software version 15 (StataCorp, College Station TX, USA) and SAS version 9.4 (SAS Institute, Gary NC, USA) to perform the statistical analyses.
2. Results
A. Characteristics of the study cohort
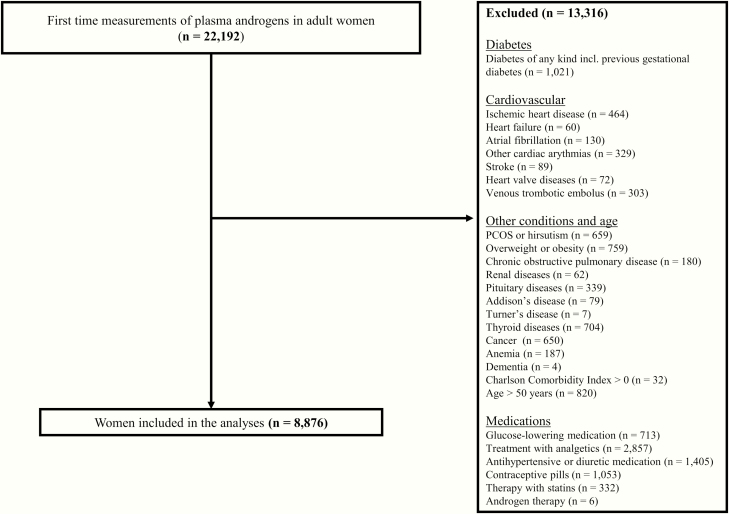
We identified 22 192 women, 18 years or above, in whom endogenous androgens were measured for the first time during the period 2007 to 2015. After exclusion of women who were older than 50 years of age and with established comorbidities or prescribed medication according to prespecifications, a total of 8876 women, with a mean (SD) age of 38.5 ± 4.6 years (range 31-50) were eligible for the study (Fig. 1). The median (interquartile range [IQR]) follow-up duration was 8.1 (6.6-9.4) years. Forty women (0.5%) died during the observation period and none migrated. Measurement of plasma TT, cFT, and SHBG were complete and thus available in all 8876 women whereas plasma DHEA-S and DHT were only available in approximately 83% and 51%, respectively.
Figure 1.
Flow chart displaying inclusion and exclusion procedures of the study cohort.
Characteristics of the study cohort are presented according to quartiles of plasma TT in Table 1. Women in Q1 of plasma TT were older than women in Q2, Q3, and Q4. Plasma SHBG was lower among women in Q1 of plasma TT than among women in Q2, Q3 and Q4.
Table 1.
Baseline characteristics of the included women according to quartiles of plasma total testosterone (total number of women in the cohort: 8876)
| Q1 | Q2 | Q3 | Q4 | P-value | |
|---|---|---|---|---|---|
| n (% of total cohort) | 2194 (24.7) | 2206 (24.9) | 2242 (25.3) | 2234 (25.2) | |
| Characteristics | |||||
| Age (years) | 39.9 (4.6) | 39.0 (4.6) | 38.2 (4.5) | 37.0 (4.2) | <.001 |
| Average yearly income past 5 years (US$) | 63 390 (50 120) | 60 440 (41 270) | 58 970 (32 400) | 57 490 (41 280) | <.001 |
| Endogenous androgens | |||||
| P-TT (nmol/L) | 0.65 (0.15) | 1.00 (0.10) | 1.38 (0.13) | 2.28 (0.90) | <.001 |
| P-cFT (pmol/L) | 10.1 (4.3) | 15.0 (5.4) | 20.6 (7.6) | 34.1 (18.0) | <.001 |
| P-SHBG (nmol/L) | 66.4 (37.9) | 71.3 (38.4) | 72.8 (40.9) | 75.5 (48.4) | <.001 |
| P-DHEA-S (nmol/L) | 4.39 (2.16) | 5.15 (2.43) | 5.81 (2.52) | 6.30 (2.83) | <.001 |
| P-DHT (nmol/L) | 0.47 (0.26) | 0.52 (0.25) | 0.58 (0.27) | 0.72 (0.33) | <.001 |
| Healthcare referral settings—n, (%) | <.001 | ||||
| General practitioners | 941 (42.9) | 986 (44.7) | 1091 (48.7) | 1051 (47.0) | |
| Physicians with private clinic | 982 (44.8) | 1007 (45.6) | 959 (42.8) | 990 (44.3) | |
| Hospital departments | 268 (12.2) | 211 (9.6) | 191 (8.5) | 192 (8.6) | |
| Other | 3 (0.1) | 2 (0.1) | 1 (0.0) | 1 (0.0) | |
| Region of Denmark—n, (%) | <.001 | ||||
| Capital | 1536 (70.0) | 1581 (71.7) | 1630 (72.7) | 1603 (71.8) | |
| Middle Jutland | 126 (5.7) | 99 (4.5) | 66 (2.9) | 63 (2.8) | |
| Southern Jutland (incl. Funen) | 176 (8.0) | 169 (7.7) | 206 (9.2) | 216 (9.3) | |
| Northern Jutland | 159 (7.2) | 135 (6.1) | 132 (5.9) | 101 (4.5) | |
| Zealand | 197 (9.0) | 222 (10.1) | 208 (9.3) | 251 (11.2) |
All values are means (SD) unless otherwise stated.
Abbreviations: cFT, calculated free testosterone; DHEA-S, dehydroepiandrosterone-sulfate; DHT, dihydrotestosterone; SHBG, sexual hormone-binding globulin; TT, total testosterone.
B. Risk of type 2 diabetes according to quartiles of endogenous androgens
Incidence rates of type 2 diabetes.
During a median (IQR) follow-up of 8.1 (6.6-9.4) years, 69 (0.8%) women were given a diagnosis of type 2 diabetes 6 months or later from inclusion in the study. Extending the primary outcome to include diagnosis type 2 diabetes based on therapy medical therapy, including monotherapy with metformin for women above 40 years of age, 240 (2.7%) developed type 2 diabetes during the observation period. In the entire cohort the unadjusted IR of being diagnosed with type 2 diabetes was 1/1000 person-years during the total observation period of 69 728 person-years (Table 2). Plasma cFT displayed an undeviating development from lowest IR of type 2 diabetes among women in Q1, 0.3/1000 person-years, to highest IR among women in Q4, 2/1000 person-years (Table 2). In contrast, plasma SHBG was inversely associated with risk of incident type 2 diabetes, with highest IR for type 2 diabetes was in Q1, 2.9/1000 person-years and lowest IR in Q4: 0.2/1000 person-years (Table 2).
Table 2.
Incident cases and incidence rates of type 2 diabetes according to quartiles of endogenous androgens
| Q1 | Q2 | Q3 | Q4 | Sample size | |
|---|---|---|---|---|---|
| TT | 8876 | ||||
| Incident type 2 diabetes (n, (%)) | 17 (0.8) | 14 (0.6) | 14 (0.6) | 24 (1.1) | |
| Time at risk (1000 PY) | 16.5 | 17.1 | 17.7 | 18.5 | |
| IR (cases per 1000 PY) | 1.0 | 0.8 | 0.8 | 1.3 | |
| Age-adjusted IR (per 1000 PY) | 0.9 | 0.8 | 0.8 | 1.3 | |
| cFT | 8876 | ||||
| Incident type 2 diabetes—n, (%) | 5 (0.2) | 8 (0.4) | 20 (0.9) | 36 (1.6) | |
| Time at risk (1000 PY) | 17.1 | 17.0 | 17.6 | 18.1 | |
| IR (cases per 1000 PY) | 0.3 | 0.5 | 1.1 | 2.0 | |
| Age-adjusted IR (per 1000 PY) | 0.3 | 0.4 | 1.0 | 1.9 | |
| DHT | 4516 | ||||
| Incident type 2 diabetes—n, (%) | 15 (0.7) | 12 (0.5) | 10 (0.4) | 7 (0.3) | |
| Time at risk (1000 PY) | 10.7 | 10.3 | 10.6 | 10.3 | |
| IR (cases per 1000 PY) | 1.4 | 1.2 | 0.9 | 0.7 | |
| Age-adjusted IR (per 1000 PY) | 1.4 | 1.1 | 0.9 | 0.7 | |
| DHEA-S | 7357 | ||||
| Incident type 2 diabetes—n, (%) | 12 (0.5) | 13 (0.6) | 20 (0.9) | 21 (0.9) | |
| Time at risk (1000 PY) | 15.4 | 15.3 | 15.5 | 15.7 | |
| IR (cases per 1000 PY) | 0.8 | 0.8 | 1.4 | 1.3 | |
| Age-adjusted IR (per 1000 PY) | 0.7 | 0.8 | 1.3 | 1.2 | |
| SHBG | 8876 | ||||
| Incident type 2 diabetes—n, (%) | 52 (2.4) | 10 (0.5) | 4 (0.2) | 3 (0.1) | |
| Time at risk (1000 PY) | 17.8 | 17.0 | 17.9 | 16.9 | |
| IR (cases per 1000 PY) | 2.9 | 0.6 | 0.2 | 0.2 | |
| Age-adjusted IR (per 1000 PY) | 2.8 | 0.6 | 0.2 | 0.2 |
Abbreviations: cFT, calculated free testosterone; DHEA-S, dehydroepiandrosterone-sulfate; DHT, dihydrotestosterone; IR, incidence rate; PY, person-years; SHBG, sexual hormone-binding globulin; TT, total testosterone.
C. Multivariate Poisson regression models
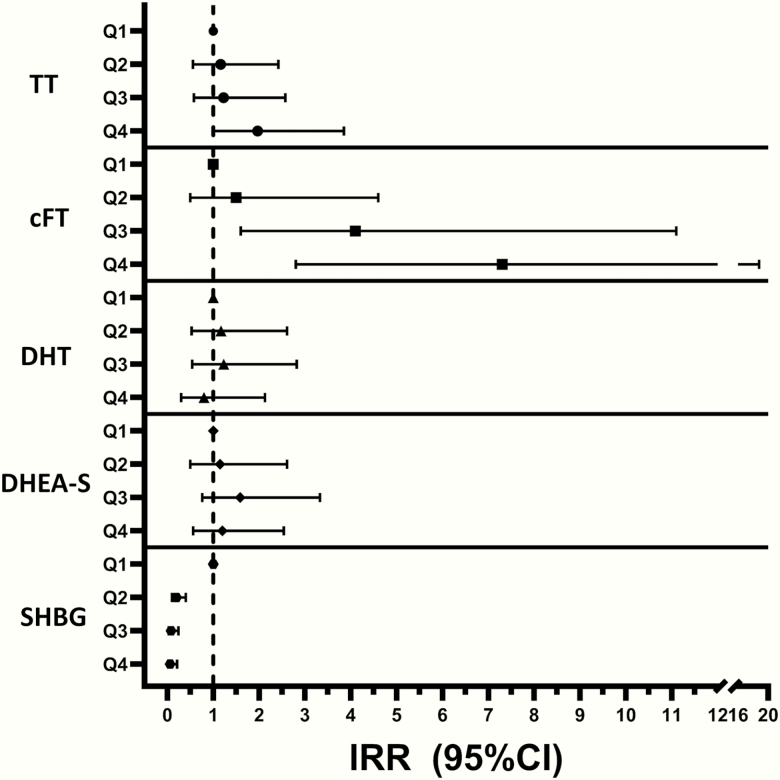
In multivariate Poisson models, adjusted for age, plasma SHBG, average yearly income, healthcare referral setting, geographical region, and calendar year, women in Q4 of plasma TT and cFT displayed increased risk of being diagnosed with type 2 diabetes compared with Q1: plasma TT, IRR 1.97 (1.01; 3.85), P = .048; and cFT, IRR 7.32 (2.84; 18.83), P < .001 (Fig. 2). There were no differences in the risk of incident type 2 diabetes among quartiles of plasma DHEA-S and DHT (Fig. 2). In contrast to plasma TT and cFT, plasma SHBG displayed an inverse association with risk of type 2 diabetes in Q4 compared with Q1: IRR, 0.06 (0.02; 0.21), P < .001 (Fig. 2).
Figure 2.
Risk of type 2 diabetes according to quartiles of endogenous androgens, with lowest quartile (Q1) as reference, presented as incidence rate ratios (IRRs) from multivariate Poisson regression models. 95%CI, 95% confidence interval; cFT, calculated free testosterone; DHEA-S, dehydroepiandrosterone-sulfate; DHT, dihydrotestosterone; SHBG, sexual hormone-binding globulin; TT, total testosterone.
In the multivariate Poisson models the following covariates were associated with increased risk of type 2 diabetes: higher age, lower plasma SHBG, higher plasma TT, and lower average yearly income.
D. Sensitivity analyses
Extending primary outcome to include diagnosis of type 2 diabetes based on medical therapy did not change the main findings; Q4 of plasma TT and cFT were associated with increased risk of type 2 diabetes compared with Q1 in multivariate Poisson models; IRRs 1.49 (1.06; 2.10) and 3.28 (2.26; 4.78).
In further sensitivity analyses using plasma TT, risk of type 2 diabetes was increased among women in Q4 compared with women in Q1 after including women who were diagnosed with either overweight/obesity, IRR 2.12 (1.15; 3.90) or PCOS/hirsutism, IRR 2.13 (1.16; 3.91). Using another estimate of plasma free testosterone, the free androgen index [24], calculated as plasma TT/SHBG × 100, in the multivariate Poisson model, risk of type 2 diabetes was increased among women in Q4 compared with Q1: IRR 9.78 (3.48; 27.54), P < .001.
To evaluate potential nonestablished comorbidity, which we did not assess in our initial exclusion criteria, the same multivariate Poisson models were used to assess risk of all-cause mortality or cancer of any kind (ICD-10 codes: DC00-090) among quartiles of plasma TT or cFT. Neither all-cause mortality nor incidence of cancer differed among plasma androgens quartiles (data not shown).
3. Discussion
In this large retrospective cohort study, the primary finding is that high plasma levels of TT and cFT were associated with a 2- to 7-fold increased risk of developing type 2 diabetes during approximately 8 years of follow-up among young women who did not feature established chronic comorbidity and were without continuing medication. Findings were consistent, with a gradual increase in risk of type 2 diabetes by each stepwise increase in quartile of plasma TT and cFT. The numbers of women being diagnosed with type 2 diabetes during the observation period were low in this young healthy cohort of women. Thus, our findings add information on the pathophysiological impact of endogenous androgens on risk of type 2 diabetes in young women without established comorbidity.
Androgens are associated with increased insulin resistance in women but the link is incompletely understood, although detrimental effects on adipose tissue have been suggested [1, 2, 25, 26]. Increased endogenous androgen levels induce adipocyte hypertrophy mediating adipocyte dysfunction and increased inflammation [25, 26]. Furthermore, hyperandrogenism has been associated with increased visceral adipose tissue in female-to-male transsexuals [10] and even male illicit users of anabolic androgens steroids, with supraphysiologic plasma androgen levels, seem to feature more visceral adipose tissue than matched nonusers [27].
Endogenous androgen levels decrease markedly with age, especially after menopause, and are therefore higher among younger women than older women [12, 14, 15]. Hence, androgen levels could have greater impact on type 2 diabetes risk among women before the age of menopause. In line with our findings, a recent primary care-based retrospective study demonstrated a doubled risk of type 2 diabetes, comparing women with plasma TT <1.0 and >3.5 nmol/L among women with a mean age of approximately 33 years [8]. Interestingly, a recent biobank study analyzed determinants of endogenous testosterone and noted that genetically determined higher testosterone increases the risk of type2 diabetes in women considerably [28].
A few cohort studies have investigated the relation between plasma TT and cFT and risk of type 2 diabetes in postmenopausal women without establishing a link [3, 5, 6] whereas we have demonstrated a strong association between plasma TT and cFT and risk of type 2 diabetes in a cohort of women who featured a mean age of approximately 40 years and, therefore, were considerably younger than in most previous studies among postmenopausal women in which mean age at baseline was >60 years [3, 5]. A recent study demonstrated that mean age at type 2 diabetes diagnosis was 63 years for women [29]. Therefore, studies on risk of type 2 diabetes among postmenopausal women could have underestimated the risk of type 2 diabetes as many cases could have been excluded at baseline.
In the present study, especially plasma cFT displayed a strong association with type 2 diabetes, increasing incrementally, from lowest risk of type 2 diabetes among women in the lowest quartile, to highest risk of type 2 diabetes among women in the upper quartile. Free testosterone levels, which make up a minority of plasma TT, is considered the active form of testosterone [30] and could represent the pathophysiological impact of testosterone on risk of type 2 diabetes in women. Of interest, free testosterone is not considered to be influenced by BMI and, thus, may represent the androgen effect per se in women. In contrast, plasma DHEA-S and DHT were not related to risk of type 2 diabetes. Regarding DHEA-S, a reason could be that DHEA-S is considered a precursor androgen and features low affinity for the androgen receptor and, therefore, has limited androgenic properties as opposed to testosterone, which features high affinity leading to potent androgenic effects [16]. Furthermore, since DHEA-S has low biochemical activity, it is not tightly regulated, creating a large biological variation, and therefore could be difficult to observe any statistically significant findings. Consistent with our findings, a recent cohort study did not establish a relation between plasma DHEA-S and type 2 diabetes among postmenopausal women [7]. DHT is considered the most potent androgen in humans but was not associated with risk of type 2 diabetes. An explanation could be that the effects of DHT on body composition and lipid metabolism is somewhat negligible compared with testosterone, as control mechanisms in adipose and muscle tissue efficiently maintain local equilibrium of DHT limiting it influence in these tissues [16]. To our knowledge, the relation between plasma DHT and risk of type 2 diabetes has not previously been investigated among women.
Plasma levels of SHBG displayed a strong inverse association with risk of type 2 diabetes. Therefore, low plasma levels of SHBG could be a solid risk marker for type 2 diabetes in women. Obesity and increased insulin resistance are well-known parameters to suppress plasma SHBG, although the mechanisms are not fully elucidated [22]. In accordance with our findings, a few studies have demonstrated an inverse association between plasma SHBG and type 2 diabetes among women [5, 6, 31].
This study holds several limitations which should be addressed. The nature of the study limits firm conclusions on causality. Confounding by indication or selection bias may have hampered the results in a study design as the present; for example, women being examined for endogenous androgen abnormalities might be more prone to be examined for diabetes. We aimed at accommodating the risk of these biases by using a grace period of 6 months, creating a homogeneous cohort without comorbidity and by using the lowest quartiles of plasma androgens as reference, since this is the most clinically relevant. We had no information on whether the women were pre- or postmenopausal, but a recent study showed that the mean age of menopause is 50 years [32]. We did not hold information on which time during the day blood for measurement of endogenous androgens was drawn or whether the participants were in a fasting state; neither did we hold information on timing of blood collection in relation to the premenopausal women’s menstrual cycle. Plasma testosterone increases from the early follicular to luteal phase of the menstrual cycle, but a recent study demonstrated the increase in plasma testosterone is minor and would most likely not lead to reallocation of individuals among the quartiles of plasma testosterone [15]. We did not measure free testosterone levels in plasma biochemically, but recently cFT levels have shown good agreement with measured free testosterone levels [24].
We had information on the women’s diagnoses, medication, and endogenous androgen levels, but we had limited information on the women’s body composition and BMI which should be considered as a limitation and would have been of interest. Nevertheless, plasma SHBG levels are strongly inversely related to BMI and insulin resistance among women [22], meaning that we have adjusted for body composition indirectly. Interestingly, a recent study demonstrated that endogenous androgens are inversely related to BMI in healthy women [15]. In line with this, we noted plasma SHBG was lowest among women in Q1 and highest among women in Q4 of plasma TT. Thus, suggesting that women in the lowest quartile of plasma TT could feature the highest insulin resistance and BMI at baseline, while women in the highest quartile of plasma TT potentially feature the lowest insulin resistance and BMI. Accordingly, we may have underestimated, rather than overestimated, the influence of testosterone on the risk of developing type 2 diabetes in women.
In conclusion, the highest quartiles of plasma TT and cFT were associated with increased risk of type 2 diabetes within approximately 8 years of follow-up among younger women who featured no established comorbidity. The findings of this study suggest testosterone could play a role in the pathogenesis of type 2 diabetes among young women. Further studies are needed to clarify how testosterone influences insulin resistance and risk of type 2 diabetes in women.
Acknowledgments
Financial Support: None.
Author Contributions: Conception and design: J.J.R., C.S.., CK. Acquisition of data: J.J.R., C.S., C.K., C.T.P., L.K., G.G., D.M.H., A.S.C.. Analysis of data: J.J.R., C.S., C.K. Interpretation of data: J.J.R., C.S., C.K., M.S., S.F., J.F., L.K., G.G., C.T.P., A.S.C., D.M.H.. Drafting of manuscript: J.J.R. Revising manuscript of critically important intellectual content: J.J.R., C.S., C.K., M.S., S.F., J.F., L.K., G.G., C.T.P., A.S.C., D.M.H. Final approval of manuscript: J.J.R., C.S., C.K., M.S., S.F., J.F., L.K., G.G., C.T.P., A.S.C., D.M.H.
Glossary
Abbreviations
- cFT
calculated free testosterone
- BMI
body mass index
- CI
confidence interval
- DHEA-S
dehydroepiandrosterone-sulfate
- DHT
dihydrotestosterone
- ICD-10
International Classification of Diseases 10th revision
- IRR
incidence rate ratio
- LC-MS
liquid chromatography tandem mass spectrometry
- PCOS
polycystic ovary syndrome
- SD
standard deviation
- SHBG
sex hormone-binding globulin
- TT
total testosterone
Additional Information
Disclosure Summary: JJR, CS, CK, MS, SF, JF, LK, GG, CTP, ASC, DMH have no conflicts of interest in relation to the present topic.
Data Availability: Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
- 1. Rubin KH, Glintborg D, Nybo M, Abrahamsen B, Andersen M. Development and risk factors of type 2 diabetes in a nationwide population of women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2017;102(10):3848-3857. [DOI] [PubMed] [Google Scholar]
- 2. Kakoly NS, Earnest A, Teede HJ, Moran LJ, Joham AE. The impact of obesity on the incidence of type 2 diabetes among women with polycystic ovary syndrome. Diabetes Care. 2019;42(4):560-567. [DOI] [PubMed] [Google Scholar]
- 3. Fenske B, Kische H, Gross S, et al. Endogenous androgens and sex hormone-binding globulin in women and risk of metabolic syndrome and type 2 diabetes. J Clin Endocrinol Metab. 2015;100(12):4595-4603. [DOI] [PubMed] [Google Scholar]
- 4. Ding EL, Song Y, Manson JE, Rifai N, Buring JE, Liu S. Plasma sex steroid hormones and risk of developing type 2 diabetes in women: a prospective study. Diabetologia. 2007;50(10):2076-2084. [DOI] [PubMed] [Google Scholar]
- 5. Muka T, Nano J, Jaspers L, et al. Associations of steroid sex hormones and sex hormone-binding globulin with the risk of type 2 diabetes in women: a population-based cohort study and meta-analysis. Diabetes. 2017;66(3):577-586. [DOI] [PubMed] [Google Scholar]
- 6. Kalyani RR, Franco M, Dobs AS, et al. The association of endogenous sex hormones, adiposity, and insulin resistance with incident diabetes in postmenopausal women. J Clin Endocrinol Metab. 2009;94(11):4127-4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brahimaj A, Muka T, Kavousi M, Laven JS, Dehghan A, Franco OH. Serum dehydroepiandrosterone levels are associated with lower risk of type 2 diabetes: the Rotterdam Study. Diabetologia. 2017;60(1):98-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. O’Reilly MW, Glisic M, Kumarendran B, et al. Serum testosterone, sex hormone-binding globulin and sex-specific risk of incident type 2 diabetes in a retrospective primary care cohort. Clin Endocrinol (Oxf). 2019;90(1):145-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kumarendran B, O’Reilly MW, Manolopoulos KN, et al. Polycystic ovary syndrome, androgen excess, and the risk of nonalcoholic fatty liver disease in women: a longitudinal study based on a United Kingdom primary care database. Plos Med. 2018;15(3):e1002542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Elbers JM, Asscheman H, Seidell JC, Megens JA, Gooren LJ. Long-term testosterone administration increases visceral fat in female to male transsexuals. J Clin Endocrinol Metab. 1997;82(7):2044-2047. [DOI] [PubMed] [Google Scholar]
- 11. Ek I, Arner P, Bergqvist A, Carlström K, Wahrenberg H. Impaired adipocyte lipolysis in nonobese women with the polycystic ovary syndrome: a possible link to insulin resistance? J Clin Endocrinol Metab. 1997;82(4):1147-1153. [DOI] [PubMed] [Google Scholar]
- 12. Labrie F, Bélanger A, Cusan L, Gomez JL, Candas B. Marked decline in serum concentrations of adrenal C19 sex steroid precursors and conjugated androgen metabolites during aging. J Clin Endocrinol Metab. 1997;82(8):2396-2402. [DOI] [PubMed] [Google Scholar]
- 13. Labrie F, Martel C, Bélanger A, Pelletier G. Androgens in women are essentially made from DHEA in each peripheral tissue according to intracrinology. J Steroid Biochem Mol Biol. 2017;168:9-18. [DOI] [PubMed] [Google Scholar]
- 14. Labrie F, Bélanger A, Bélanger P, et al. Androgen glucuronides, instead of testosterone, as the new markers of androgenic activity in women. J Steroid Biochem Mol Biol. 2006;99(4-5):182-188. [DOI] [PubMed] [Google Scholar]
- 15. Skiba MA, Bell RJ, Islam RM, Handelsman DJ, Desai R, Davis SR. Androgens during the reproductive years: what is normal for women? J Clin Endocrinol Metab. 2019;104(11):5382-5392. [DOI] [PubMed] [Google Scholar]
- 16. Swerdloff RS, Dudley RE, Page ST, Wang C, Salameh WA. Dihydrotestosterone: biochemistry, physiology, and clinical implications of elevated blood levels. Endocr Rev. 2017;38(3):220-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. [DOI] [PubMed] [Google Scholar]
- 18. Bartsch W. Interrelationships between sex hormone-binding globulin and testosterone, 5 alpha-dihydrotestosterone and oestradiol-17 beta in blood of normal men. Maturitas. 1980;2(2):109-118. [DOI] [PubMed] [Google Scholar]
- 19. Pedersen CB. The Danish civil registration system. Scand J Public Health. 2011;39(7 Suppl):22-25. [DOI] [PubMed] [Google Scholar]
- 20. Lynge E, Sandegaard JL, Rebolj M. The Danish national patient register. Scand J Public Health. 2011;39(7 Suppl):30-33. [DOI] [PubMed] [Google Scholar]
- 21. Kildemoes HW, Sørensen HT, Hallas J. The Danish national prescription registry. Scand J Public Health. 2011;39(7 Suppl):38-41. [DOI] [PubMed] [Google Scholar]
- 22. Wallace IR, McKinley MC, Bell PM, Hunter SJ. Sex hormone binding globulin and insulin resistance. Clin Endocrinol (Oxf). 2013;78(3):321-329. [DOI] [PubMed] [Google Scholar]
- 23. Sam S, Ehrmann DA. Metformin therapy for the reproductive and metabolic consequences of polycystic ovary syndrome. Diabetologia. 2017;60(9):1656-1661. [DOI] [PubMed] [Google Scholar]
- 24. Keevil BG, Adaway J, Fiers T, Moghetti P, Kaufman JM. The free androgen index is inaccurate in women when the SHBG concentration is low. Clin Endocrinol (Oxf). 2018;88(5):706-710. [DOI] [PubMed] [Google Scholar]
- 25. Gambineri A, Pelusi C. Sex hormones, obesity and type 2 diabetes: is there a link? Endocr Connect. 2019;8(1):R1-R9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Faulds G, Rydén M, Ek I, Wahrenberg H, Arner P. Mechanisms behind lipolytic catecholamine resistance of subcutaneous fat cells in the polycystic ovarian syndrome. J Clin Endocrinol Metab. 2003;88(5):2269-2273. [DOI] [PubMed] [Google Scholar]
- 27. Rasmussen JJ, Schou M, Selmer C, et al. Insulin sensitivity in relation to fat distribution and plasma adipocytokines among abusers of anabolic androgenic steroids. Clin Endocrinol (Oxf). 2017;87(3):249-256. [DOI] [PubMed] [Google Scholar]
- 28. Ruth KS, Day FR, Tyrrell J, et al. ; Endometrial Cancer Association Consortium Using human genetics to understand the disease impacts of testosterone in men and women. Nat Med. 2020;26(2):252-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jacobs E, Rathmann W, Tonnies T, et al. Age at diagnosis of type 2 diabetes in Germany: a nationwide analysis based on claims data from 69 million people. Diabet Med. 2019. [DOI] [PubMed] [Google Scholar]
- 30. Kanakis GA, Tsametis CP, Goulis DG. Measuring testosterone in women and men. Maturitas. 2019;125:41-44. [DOI] [PubMed] [Google Scholar]
- 31. Onat A, Hergenç G, Bulur S, Uğur M, Küçükdurmaz Z, Can G. The paradox of high apolipoprotein A-I levels independently predicting incident type-2 diabetes among Turks. Int J Cardiol. 2010;142(1):72-79. [DOI] [PubMed] [Google Scholar]
- 32. de Kat AC, van der Schouw YT, Eijkemans MJC, Broer SL, Verschuren WMM, Broekmans FJM. Can menopause prediction be improved with multiple AMH measurements? results from the prospective Doetinchem cohort study. J Clin Endocrinol Metab. 2019;104(11):5024-5031. [DOI] [PubMed] [Google Scholar]