Abstract
Primary adrenal leiomyosarcoma (PAL) is a rare, high-grade proliferating mesenchymal tumor with a considerable risk of metastasis, deriving from the smooth muscle wall of a central adrenal vein, or its tributaries. Roughly 40 patients with PAL have been reported in the literature. Herein, we present 3 patients with incidentally discovered PAL, along with an overview of the current knowledge on the clinical, radiological, and histopathological characteristics of PAL.
Keywords: adrenal carcinoma, leiomyosarcoma, adrenal incidentaloma, vena cava occlusion
Primary adrenal leiomyosarcoma (PAL) is a rare mesenchymal tumor that derives from the smooth muscle wall of a central adrenal vein or its tributaries [1, 2]. PAL is a high-grade proliferating tumor with a considerable risk of metastasis. PAL does not express or secrete adrenal hormones and characteristic tumor biomarkers have not been identified. Hence, a preoperative diagnosis of PAL is difficult, and the tumor is often discovered at an advanced stage.
The first patient with PAL was reported by Choi and Liu in 1981 [3]. To our knowledge, some 40 additional cases have since then been reported in the literature. Herein, we present 3 patients with incidentally discovered PAL, along with an overview of the current knowledge of the clinical, radiological, and histopathological characteristics of PAL.
Subject #1
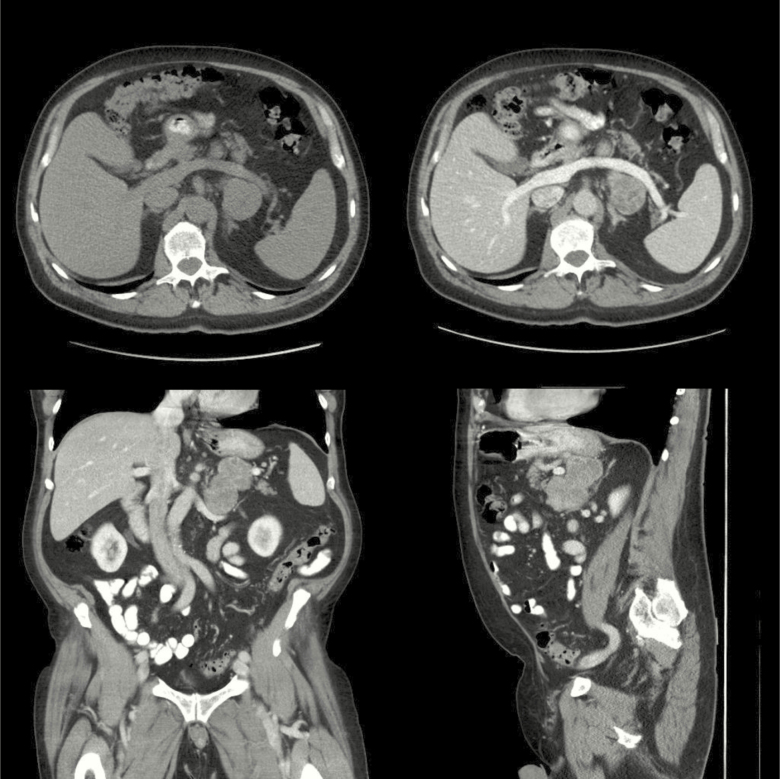
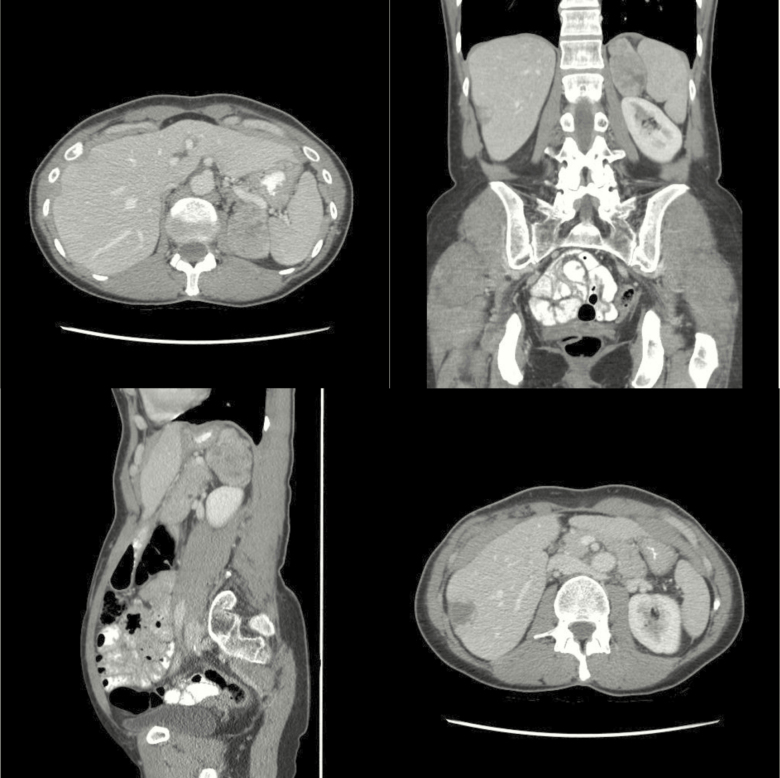
A 72-year-old man, with a previous medical history of mild hypertension, presented with a sudden onset abdominal pain and a feeling of uneasiness. He had no history of weight loss or fever. Computed tomography (CT) of the abdomen, performed due to suspected bowel perforation, revealed mild diverticulitis and a 4 × 8.5 × 10 cm left-sided adrenal mass with a native attenuation of 32 Hounsfield units (HU) (Fig. 1). There were no signs of invasion into the renal veins or infiltration into adjacent organs. The liver, pancreas, and spleen had an ordinary appearance and there were no signs of enlarged lymph nodes or skeletal metastases.
Figure 1.
Computed tomography from subject #1, demonstrating a 4 × 8.5 × 10 cm left-sided adrenal mass with a native attenuation of 32 Hounsfield units.
The patient had no clinical or biochemical signs of pheochromocytoma, Cushing syndrome or Conn syndrome. The following tumor markers in serum were within the normal reference range; chromogranin-A 49 μg/L (upper limit of normal [ULN] 102 μg/L), placental alkaline phosphatase (PLAP) < 30 mU/L (ULN 100 mU/L), carcinoembryonic antigen (CEA) < 0.5 µg/L (ULN 5.0 µg/L), lactate dehydrogenase (LDH) 2.85 µkat/L (ULN 4.2 µkat/L) and beta-human chorionic gonadotropin (beta-hCG) < 5 U/L (ULN 5 U/L). The primary differential diagnoses were lymphoma and a non-functioning adrenocortical carcinoma. The patient underwent open adrenalectomy, the tumor was located at the site of the left adrenal gland with no signs of invasion to adjacent organs. There were no postoperative complications and the patient was discharged 4 days after the surgery.
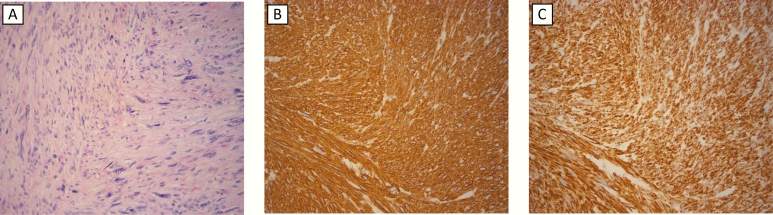
Gross pathological examination revealed a 9 × 8 × 4 cm lobulated white-grey tumor. Microscopic examination revealed an infiltrative tumor with leiomyomatous differentiation with alternating areas of low- and high-grade cytological atypia, with the latter demonstrating pleomorphism (Fig. 2). Multiple necrotic foci were seen, comprising less than 50% of the total tumor area. Frequent mitotic figures were seen (10 per high-power field [HPF]; 0,1734 mm2), including some atypical mitoses in the pleomorphic areas. The excision was histologically complete, with a narrow surgical margin measuring less than 1 mm. The tumor showed positive immunoreactivity for alpha smooth muscle actin (SMA), desmin, and h-caldesmon. The Ki-67 proliferation index was 40%. Based on these findings, the diagnosis of grade 3 PAL was confirmed, according to the Fédération Nationale des Centres de Lutte Contre Le Cancer (FNCLCC) classification [4], with 3 points for tumor differentiation, 2 points for mitoses and 1 point for tumor necrosis.
Figure 2.
Microscopic features of primary adrenal leiomyosarcoma from subject #1. (A) Hematoxylin Eosin staining showcasing atypical spindle cells and pleomorphic cell nuclei; (B) Tumor cells staining positive for smooth muscle actin; and (C) Positive immunostaining for Desmin.
Due to high proliferation grade and narrow margins, adjuvant chemotherapy was recommended after a discussion by a multidisciplinary team. However, in agreement with the patient, treatment with local conventional fractionated radiotherapy was instead initiated, with a total dose of 50 Gray units (Gy). Postoperatively, the patient has been followed every 3 to 6 months, without local recurrence. However, 31 months after the operation, metastases in the liver, sternum, mesentery, and gluteal muscle were discovered. After reassessment, the patient was again offered palliative chemotherapy but chose to decline it. Today, 48 months after the primary operation, the patient is still alive.
Subject #2
A 66-year-old man, with a history of type 2 diabetes, hyperlipidemia, and benign prostate hyperplasia, presented with macroscopic hematuria. Nephrolithiasis was suspected. CT-urography revealed no abnormalities in the urinary tract. Instead, a 7.5 × 6 × 4 cm retroperitoneal tumor with a native attenuation of 38 HU was found at the right suprarenal pole (Fig. 3). Adrenal hormones were normal. An endoscopic ultrasound-guided fine-needle aspiration biopsy, on the premise of suspected lymphoma, revealed an area of necrosis and spindle cells with an immunophenotype with a positive reaction towards SMA, desmin, and CD163. Supplementary imaging showed no signs of metastasis. A soft tissue sarcoma was suspected, and the patient underwent an open adrenalectomy. Perioperatively, tumor invasion into the inferior vena cava was discovered; hence, a surgical resection and reconstruction was performed. Postoperatively, the patient developed renal failure due to renal ischemia. Consequently, he had a prolonged recovery time, being discharged 2 weeks postoperatively.
Figure 3.
Computer tomography from subject #2, demonstrating a 7.5 × 6 × 4 cm right-sided adrenal lesion, with a native attenuation of 38 Hounsfield units.
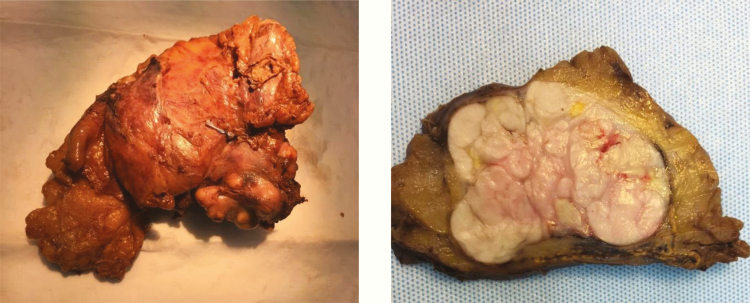
Gross pathological examination of the 12.0 × 10.0 × 6.0 cm surgical specimen revealed a whitish, encapsulated tumor measuring 4.3 × 6.7 × 7.0 cm (Fig. 4). The surgical margins were generally narrow, with a 2.5 × 2.5 cm area showing an exposed tumor capsule without covering tissue. Parts of adrenal tissue were also seen adjacent to the tumor. Histological examination showed an encapsulated tumor with both well-differentiated leiomyomatous areas and pleomorphic areas with strong nuclear pleomorphism and bizarre nuclei. Mitotic figures were frequent (17 per HPF), with atypical mitotic figures seen in the pleomorphic areas. Multiple necrotic foci were seen, constituting less than 50% of the tumor area. No growth outside the tumor capsule was seen, indicating a complete excision with a narrow margin. The adrenal parenchyma proper was negative for malignancy and separated from the tumor by a capsule. The tumor was positive for SMA, desmin, h-caldesmon, and CD163. The Ki-67 index was 30%. The findings were consistent with a grade 2 PAL, with 2 points for tumor differentiation, 2 points for mitoses and 1 point for tumor necrosis.
Figure 4.
Macroscopic features of the tumor from subject #2, demonstrating a whitish, encapsulated tumour measuring 4.3 × 6.7 × 7.0 cm.
Two months postoperatively, metastases were discovered in the thoracic wall, right femur, left gluteus maximus muscle, and the right lateral vastus muscle. A multidisciplinary team did not recommend adjuvant therapy due to the presence of impaired renal function. Instead, the patient underwent surgical excision of the metastases in the chest wall and femur. Since then, further metastatic deposits have been discovered subcutaneously, in the lungs, skeletal muscle, and bone. Due to slowly progressing disease, treatment with trofosfamide 50 mg once daily was initiated 15 months postoperatively, increased to 50 mg twice daily after 19 months, but discontinued after 21 months due to worsening renal function. The patient is alive 23 months postoperatively.
Subject #3
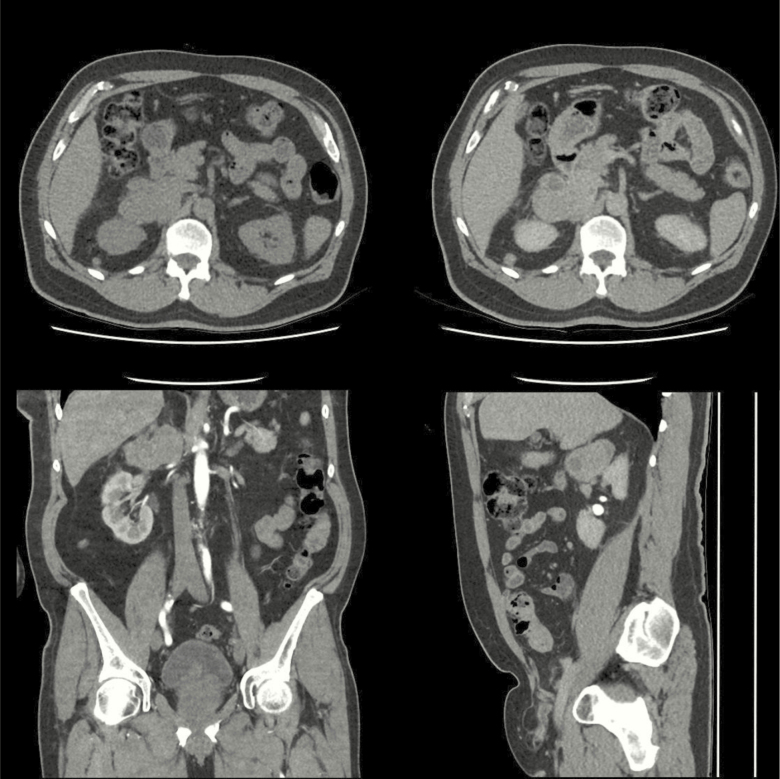
A 58-year-old woman, with no previous medical history, presented with severe intermittent pain in the upper right abdomen. Cholelithiasis was suspected but an abdominal ultrasound revealed no abnormalities in the gallbladder. Instead, a malignant lesion in the liver was suspected. Subsequent CT of the abdomen revealed a 3.5 × 5 × 6 cm left adrenal tumor with heterogeneous contrast enhancement (Fig. 5a, 5b, and 5c), together with multiple liver lesions, suspected to be metastases (Fig. 5d). A liver biopsy revealed spindle shaped cells that were positive for SMA, desmin, h-caldesmon, and vimentin, confirming the diagnosis of PAL. The Ki-67 index was 40%.
Figure 5.
Computer tomography from subject #3, showing a 3.5 × 5 × 6 cm left-sided adrenal tumor and metastatic lesions in the liver.
Due to multiple liver metastases, chemotherapy with dacarbazine and doxorubicin was initiated. After 4 months of treatment, disease progression was noted and second-line treatment with gemcitabine and docetaxel were initiated instead. Today, 13 months after chemotherapy was initiated, the patient is alive with no signs of further tumor progression.
Discussion
PAL is a rare malignant tumor, representing only 0.1% to 0.2% of all retroperitoneal soft tissue sarcomas in adults [5]. Some 40 cases have been reported in the English-language literature to date [1-3, 5-36] (Table 1). The age at diagnosis ranges from 14 to 78 years, with 80% of the patients diagnosed after the age of 40 [1-3, 5-36]. PAL occurs both in males and females. The size of the tumor varies between 3 and 27 cm, with the vast majority (> 80%) being ≥ 4 cm in the largest diameter [1-3, 5-36]. Most patients present with unilateral PAL, and only 2 patients with bilateral PAL have been reported [11, 21]. PAL can be asymptomatic and discovered incidentally during imaging performed for reasons unrelated to the PAL itself, as was the case in all of our patients. The most common symptom at presentation is abdominal [1, 7, 9, 10, 18, 21, 23, 24, 26, 30, 32-36] or flank pain [2, 5, 12, 17, 19, 27, 31, 37]. Other symptoms include weight loss [9, 12, 19, 20, 27, 36] and peripheral edema due to tumor growth into the inferior vena cava [7, 16, 23]. Concomitant immunodeficiency has been reported in 2 cases [5, 10].
Table 1.
A Ssummary of the Clinical Characteristics in Patients with Primary Adrenal Leiomyosarcoma Reported in the Literature
| Author(s) | Year of Publication | Age (years) /Gender (M/F) | Size (cm) /Laterality (R/L) | Symptom(s) |
|---|---|---|---|---|
| Choi, Liu. | 1981 | 50/F | 16/L | - |
| Lack et al. | 1991 | 49/M | 11/R | Flank pain |
| Zetler et al. | 1995 | 30/M | 11/L | Immunodeficiency |
| Etten et al. | 2001 | 73/F | 27/R | Abdominal pain and vena cava syndrome |
| Matsui et al. | 2002 | 61/F | -/R | Chest pain, vena cava thrombosis |
| Thamboo et al. | 2003 | 68/F | 12/R | Abdominal pain, fever, weight loss |
| Kato et al. | 2004 | 59/M | 10/L | Abdominal pain |
| Linos et al. | 2004 | 14/F | 3,5/R, 4/L | Immunodeficiency |
| Candanedo-González et al. | 2005 | 59/F | 16/L | Flank pain, weight loss |
| Wong et al. | 2005 | 57/M | -/L | Groin pain, cyanosis, cold feet |
| Lee et al. | 2006 | 49/M | 3/L | - |
| Mohanty et al. | 2007 | 47/F | 10/L | Abdominal pain |
| Wang et al. | 2007 | 64/F | 13/R | Edema, hepatomegaly |
| Goto et al. | 2008 | 73/F | 8/R | Flank pain |
| Mencoboni et al. | 2008 | 75/F | 5/R | Epigastric pain |
| Tomasich et al. | 2008 | 48/F | 9/L | Flank pain, weight loss |
| Van Laarhoven et al. | 2009 | 78/M | -/L | Weight loss, skeletal pain |
| Hamada et al. | 2009 | 62/F | 8/R, 4/L | Abdominal pain |
| Nanpo et al. | 2009 | 78/F | 10/L | Fever, nausea |
| Karaosmanoglu, Gee. | 2010 | 63/M | -/R | Abdominal pain, edema |
| Kanthan et al. | 2012 | 28/F | 16.5/L | Abdominal pain |
| Shao et al. | 2012 | 66/M | 9.5/L | Nausea, abdominal fullness |
| Deshmukh et al. | 2013 | 60/F | 5.2/L | Abdominal pain |
| Lee et al. | 2014 | 28/M | 13.8/R | Flank pain, weight loss |
| Wei et al. | 2014 | 57/F | 7.7/L | Asymptomatic, routine medical examination |
| Gulpinar et al. | 2014 | 48/M | 8.6/R | Symptoms from the urinary tract |
| Ozturk. | 2014 | 70/- | 7.8/R | Abdominal pain |
| Alam et al. | 2014 | 35/F | 8.5/L | Flank pain |
| Bhalla et al. | 2014 | 45/M | 11/R | Abdominal pain |
| Nagaraj et al. | 2015 | 61/M | 16/L | Flank pain |
| Zhou et al. | 2015 | 49/F | 6/L | Abdominal pain, back pain |
| Quildrian et al. | 2015 | 44/F | 7.4/R | Abdominal pain |
| Onishi et al. | 2016 | 34/M | 5.2/R | Abdominal pain |
| Taniguchi et al. | 2017 | 61/M | 7/L | Abdominal pain |
| Mulani et al. | 2018 | 50/M | 8.1/L | Abdominal pain, weight loss |
| Doppalapudi et al. | 2019 | 70/M | 12/R | Edema, abdominal varices |
| Nerli et al. | 2020 | 27/M | 9//L | Back pain |
| Sakellariou et al. | 2020 | 62/M | 10/L | Asymptomatic, routine medical examination |
There are no distinct biochemical tests that are useful to identify and confirm PAL preoperatively. Adrenal hormones are consistently normal [1, 5, 16-18, 21, 22, 24, 29, 30, 33-36], and no specific tumor markers have been identified. Elevated concentration of neuron-specific enolase, which normalized postoperatively, was recently noted in one patient with PAL [17]. Neuron-specific enolase has not been reported elsewhere and further research on this potential biomarker is therefore warranted.
Imaging is important in patients with suspected malignant adrenal lesion for grading of the tumor, to determine the possibility of surgical resection, to discover metastases, and to estimate prognosis. PAL are most commonly discovered by using CT, typically showing lobulated mass with heterogeneous contrast enhancement [1, 5, 33-36]. Surprisingly, the radiological characteristics of PAL in terms of attenuation before and after injection of contrast have not been discussed previously. High native attenuation of 32 and 38 HU, respectively, was seen in both of our patients who had been investigated with unenhanced CT before treatment. Other imaging modalities that may revealed PAL are abdominal ultrasound, demonstrating hypoechoic lesion that may be of either homogeneous or heterogeneous texture [9, 10, 22, 29, 30, 36, 38], and magnetic resonance imaging showing a mass with a low-signal intensity on T1-weighted images, high-signal intensity on T2-weighted images, heterogeneous contrast uptake and absence of loss-of-signal on an out-of-phase sequence [7, 16, 23, 39].
Currently, the diagnosis of PAL is based on histopathological and immunohistochemical findings, showing a neoplasm consisting of spindle cells that stain positively for one or more of the following smooth muscle cell markers; SMA [1, 2, 6-11, 16-18, 20, 21, 24-30, 32-34, 40], desmin [14-16, 19, 23, 25-28, 32], and vimentin [1, 5, 19, 20, 24, 26, 28, 33]. In addition to SMA and desmin, the tumors in our patients showed positive immunoreactivity for h-caldesmon, also a marker of smooth muscle cells, consistent with previous reports in patients with leiomyosarcomas of other origins [41]. The proliferation index Ki-67 was high (≥ 20) in all of our patients, which is in agreement with previous cases showing an index between 20% and 75% [1, 18, 30, 33, 34, 40].
As with other retroperitoneal leiomyosarcomas, PAL is a high-grade tumor with a significant risk of metastasis [42]. Delayed diagnosis, combined with high risk of metastasis, are the main reasons behind the overall poor prognosis. The longest reported survival in a patient treated with an adrenalectomy, without any known metastases, is 36 months [33]. The longest reported survival in a patient with a disseminated disease (liver and lymph nodes), treated with adrenalectomy, nephrectomy, and chemotherapy, is 53 months [19]. The most common sites for metastasis are the skeleton [2, 10, 20, 21, 40], liver [10, 12, 19, 32, 36, 40], lymph nodes [8, 19, 32, 34], and lungs [8, 36, 40]. Several cases have been reported where the tumor has invaded the inferior vena cava and caused a thrombosis [7, 10, 13, 16, 23, 30, 39]. In our patients, dissemination to liver, muscles, and bones occurred. Muscle metastases are in general rare, and usually derive from lung cancer, gastrointestinal, or urogenital tumors. One study found muscle metastases in roughly 5% of patients with sarcoma [43]. Two of our patients had muscle metastases, which have not been reported previously in patients with PAL.
The primary treatment for PAL is radical surgery with or without adjuvant chemotherapy or/and radiotherapy. The role of adjuvant therapy has not been evaluated in randomized trials due to the rarity of the disease. PAL should be assessed and managed by a multidisciplinary team. A thorough follow-up is important due to the high grade proliferating nature of the tumor where metastases can occur late, as seen in 1 of our patients and 5 other patients from previous reports [2, 12, 13, 21, 30].
Conclusions
PAL is a rare highly malignant tumor that is often diagnosed at an advanced stage. The most common symptoms at diagnosis are abdominal, flank, or back pain, weight loss, or peripheral edema due to occlusion of the inferior vena cava. On imaging, PAL almost always presents with characteristics compatible with malignant lesions, where the main differential diagnoses are adrenocortical carcinoma, metastasis, and lymphoma. The primary treatment is surgery, often with subsequent adjuvant chemotherapy and/or radiotherapy. Unfortunately, the overall prognosis for patients with PAL is, however, poor and the vast majority eventually develop a therapy-resistant and incurable metastatic disease.
Acknowledgments
Financial Support: This work has not received any specific grants.
Glossary
Abbreviations
- CT
computed tomography
- HPF
high-power field
- HU
Hounsfield units
- PAL
primary adrenal leiomyosarcoma
- SMA
smooth muscle actin
- ULN
upper limit of normal
Additional Information
Disclosure Summary: The authors have nothing to declare.
Data Availability: All data generated or analyzed during this study are included in this published article or in the data repositories listed in References.
References
- 1. Zhou Y, Tang Y, Tang J, Deng F, Gong G, Dai Y. Primary adrenal leiomyosarcoma: a case report and review of literature. Int J Clin Exp Pathol. 2015;8(4):4258-4263. [PMC free article] [PubMed] [Google Scholar]
- 2. Lack EE, Graham CW, Azumi N, et al. Primary leiomyosarcoma of adrenal gland. Case report with immunohistochemical and ultrastructural study. Am J Surg Pathol. 1991;15(9):899-905. [DOI] [PubMed] [Google Scholar]
- 3. Choi SH, Liu K. Leiomyosarcoma of the adrenal gland and its angiographic features: a case report. J Surg Oncol. 1981;16(2):145-148. [DOI] [PubMed] [Google Scholar]
- 4. Fletcher CDM. WHO Classification of Tumours of Soft Tissue and Bone. Lyon: IARC Press; 2013. [Google Scholar]
- 5. Nagaraj V, Mustafa M, Amin E, Ali W, Naji Sarsam S, Darwish A. Primary adrenal leiomyosarcoma in an arab male: a rare case report with immunohistochemistry study. Case Rep Surg. 2015;2015:702541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zetler PJ, Filipenko JD, Bilbey JH, Schmidt N. Primary adrenal leiomyosarcoma in a man with acquired immunodeficiency syndrome (AIDS). Further evidence for an increase in smooth muscle tumors related to Epstein-Barr infection in AIDS. Arch Pathol Lab Med. 1995;119(12):1164-1167. [PubMed] [Google Scholar]
- 7. Etten B, van Ijken MG, Mooi WJ, Oudkerk M, van Geel AN. Primary leiomyosarcoma of the adrenal gland. Sarcoma. 2001;5(2):95-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Matsui Y, Fujikawa K, Oka H, Fukuzawa S, Takeuchi H. Adrenal leiomyosarcoma extending into the right atrium. Int J Urol. 2002;9(1):54-56. [DOI] [PubMed] [Google Scholar]
- 9. Thamboo TP, Liew LC, Raju GC. Adrenal leiomyosarcoma: a case report and literature review. Pathology. 2003;35(1):47-49. [PubMed] [Google Scholar]
- 10. Kato T, Kato T, Sakamoto S, et al. Primary adrenal leiomyosarcoma with inferior vena cava thrombosis. Int J Clin Oncol. 2004;9(3):189-192. [DOI] [PubMed] [Google Scholar]
- 11. Linos D, Kiriakopoulos AC, Tsakayannis DE, Theodoridou M, Chrousos G. Laparoscopic excision of bilateral primary adrenal leiomyosarcomas in a 14-year-old girl with acquired immunodeficiency syndrome (AIDS). Surgery. 2004;136(5):1098-1100. [DOI] [PubMed] [Google Scholar]
- 12. Candanedo-González FA, Vela Chávez T, Cérbulo-Vázquez A. Pleomorphic leiomyosarcoma of the adrenal gland with osteoclast-like giant cells. Endocr Pathol. 2005;16(1):75-81. [DOI] [PubMed] [Google Scholar]
- 13. Wong C, Von Oppell UO, Scott-Coombes D. Cold feet from adrenal leiomyosarcoma. J R Soc Med. 2005;98(9):418-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lee CW, Tsang YM, Liu KL. Primary adrenal leiomyosarcoma. Abdom Imaging. 2006;31(1):123-124. [DOI] [PubMed] [Google Scholar]
- 15. Mohanty SK, Balani JP, Parwani AV. Pleomorphic leiomyosarcoma of the adrenal gland: case report and review of the literature. Urology 2007;70(3):591.e5-7 doi: 10.1016/j.urology.2007.07.029 [DOI] [PubMed] [Google Scholar]
- 16. Wang TS, Ocal IT, Salem RR, Elefteriades J, Sosa JA. Leiomyosarcoma of the adrenal vein: a novel approach to surgical resection. World J Surg Oncol. 2007;5:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Goto J, Otsuka F, Kodera R, et al. A rare tumor in the adrenal region: neuron-specific enolase (NSE)-producing leiomyosarcoma in an elderly hypertensive patient. Endocr J. 2008;55(1):175-181. [DOI] [PubMed] [Google Scholar]
- 18. Mencoboni M, Bergaglio M, Truini M, Varaldo M. Primary adrenal leiomyosarcoma: a case report and literature review. Clin Med Oncol. 2008;2:353-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tomasich FD, Luz Mde A, Kato M, et al. [Primary adrenal leiomyosarcoma]. Arq Bras Endocrinol Metabol. 2008;52(9):1510-1514. [DOI] [PubMed] [Google Scholar]
- 20. Van Laarhoven HW, Vinken M, Mus R, Flucke U, Oyen WJ, Van der Graaf WT. The diagnostic hurdle of an elderly male with bone pain: how 18F-FDG-PET led to diagnosis of a leiomyosarcoma of the adrenal gland. Anticancer Res. 2009;29(2):469-472. [PubMed] [Google Scholar]
- 21. Hamada S, Ito K, Tobe M, et al. Bilateral adrenal leiomyosarcoma treated with multiple local therapies. Int J Clin Oncol. 2009;14(4):356-360. [DOI] [PubMed] [Google Scholar]
- 22. Nanpo Y, Kuramoto T, Mori T, et al. [Primary adrenal leiomyosarcoma: a case report]. Nihon Hinyokika Gakkai Zasshi. 2009;100(6):640-645. [DOI] [PubMed] [Google Scholar]
- 23. Karaosmanoglu AD, Gee MS. Sonographic findings of an adrenal leiomyosarcoma. J Ultrasound Med. 2010;29(9):1369-1373. [DOI] [PubMed] [Google Scholar]
- 24. Kanthan R, Senger JL, Kanthan S. Three uncommon adrenal incidentalomas: a 13-year surgical pathology review. World J Surg Oncol. 2012;10:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shao IH, Lee WC, Chen TD, Chiang YJ. Leiomyosarcoma of the adrenal vein. Chang Gung Med J. 2012;35(5):428-431. [DOI] [PubMed] [Google Scholar]
- 26. Deshmukh SD, Babanagare SV, Anand M, Pande DP, Yavalkar P. Primary adrenal leiomyosarcoma: a case report with immunohistochemical study and review of literature. J Cancer Res Ther. 2013;9(1):114-116. [DOI] [PubMed] [Google Scholar]
- 27. Lee S, Tanawit GD, Lopez RA, Zamuco JT, Cheng BG, Siozon MV. Primary leiomyosarcoma of adrenal gland with tissue eosinophilic infiltration. Korean J Pathol. 2014;48(6):423-425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wei J, Sun A, Tao J, Wang C, Liu F. Primary adrenal leiomyosarcoma: case report and review of the literature. Int J Surg Pathol. 2014;22(8):722-726. [DOI] [PubMed] [Google Scholar]
- 29. Gulpinar MT, Yildirim A, Gucluer B, et al. Primary leiomyosarcoma of the adrenal gland: a case report with immunohistochemical study and literature review. Case Rep Urol. 2014;2014:489630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Oztürk H. Vena Cava ınvasion by Adrenal Leiomyosarcoma. Rare Tumors. 2014;6(2):5275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Alam MM, Naser MF, Islam MF, Rahman MA. Primary adrenal leiomyosarcoma in an adult female. Mymensingh Med J. 2014;23(2):380-383. [PubMed] [Google Scholar]
- 32. Bhalla A, Sandhu F, Sieber S. Primary adrenal leiomyosarcoma: a case report and review of the literature. Conn Med. 2014;78(7):403-407. [PubMed] [Google Scholar]
- 33. Quildrian S, Califano I, Carrizo F, Daffinoti A, Calónico N. Primary adrenal leiomyosarcoma treated by laparoscopic adrenalectomy. Endocrinol Nutr. 2015;62(9):472-473. [DOI] [PubMed] [Google Scholar]
- 34. Onishi T, Yanagihara Y, Kikugawa T, et al. Primary adrenal leiomyosarcoma with lymph node metastasis: a case report. World J Surg Oncol. 2016;14(1):176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Taniguchi A, Ujike T, Fujita K, et al. [A Case of Adrenal Leiomyosarcoma]. Hinyokika Kiyo. 2017;63(11):465-469. [DOI] [PubMed] [Google Scholar]
- 36. Mulani SR, Stoner P, Schlachterman A, Ghayee HK, Lu L, Gupte A. First reported case of endoscopic ultrasound-guided core biopsy yielding diagnosis of primary adrenal leiomyosarcoma. Case Rep Gastrointest Med. 2018;2018:8196051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nerli RB, Ghagane S, Dixit NS, Hiremath MB, Deole S. Adrenal leiomyosarcoma in a young adult male. Int Cancer Conf J. 2020;9(1):14-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gong X, Yu Y, Zhan W. Ultrasonographic findings of 1385 adrenal masses: a retrospective study of 1319 benign and 66 malignant masses. J Ultrasound Med. 2019;38(9):2249-2257. [DOI] [PubMed] [Google Scholar]
- 39. Doppalapudi SK, Shah T, Fitzhugh VA, Bargman V. Primary adrenal leiomyosarcoma with inferior vena cava extension in a 70-year-old man. BMJ Case Rep. 2019;12(3) doi: 10.1136/bcr-2018-227670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sakellariou M, Dellaportas D, Grapsa E, et al. Primary adrenal leiomyosarcoma: a case report. Mol Clin Oncol. 2020;12(4):317-320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hisaoka M, Wei-Qi S, Jian W, Morio T, Hashimoto H. Specific but variable expression of h-caldesmon in leiomyosarcomas: an immunohistochemical reassessment of a novel myogenic marker. Appl Immunohistochem Mol Morphol. 2001;9(4):302-308. [DOI] [PubMed] [Google Scholar]
- 42. Chen F, Li W. Retroperitoneal Leiomyosarcoma. In: Luo C-H, ed. Retroperitoneal Tumors: Clinical Management. Dordrecht: Springer Netherlands; 2018:163–167. [Google Scholar]
- 43. Surov A, Köhler J, Wienke A, et al. Muscle metastases: comparison of features in different primary tumours. Cancer Imaging. 2014;14:21. [DOI] [PMC free article] [PubMed] [Google Scholar]