Abstract
This review is intended to help clinicians and patients understand the present state of peripheral artery disease, appreciate the progression and presentation of critical limb ischemia/chronic limb-threatening ischemia, and make informed decisions regarding inflow and outflow endovascular revascularization and surgical treatment options within the context of current debates in the medical community. A controlled literature search was performed to obtain research on outcomes of critical limb ischemia patients undergoing complete leg revascularization for peripheral artery disease inflow and outflow disease. Data for this review were identified by queries of medical and life science databases, expert referral, and references from relevant papers published between 1997 and 2019, resulting in 48 articles. The literature review herein indicates that endovascular revascularization—including ballooning, stenting, and atherectomy—is an effective peripheral artery disease therapy for both above the knee and below the knee disease, and can safely and effectively treat both inflow and outflow disease. As such, it plays a leading role in the therapy of lower extremity artery disease.
Keywords: Peripheral artery disease, revascularization, critical limb ischemia, limb salvage
Search and selection methods
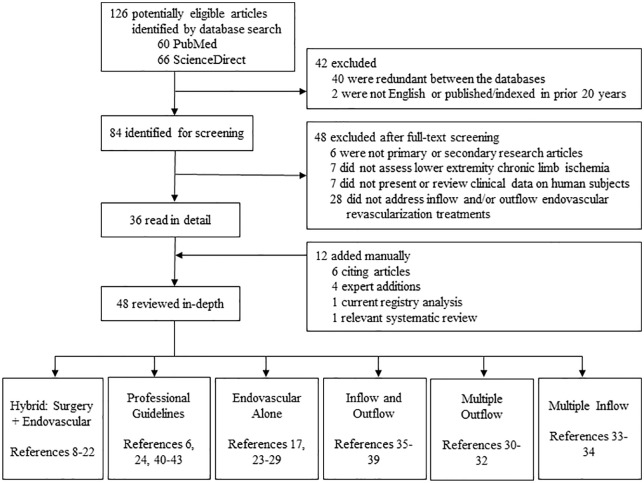
A controlled literature search was performed to obtain research on outcomes of critical limb ischemia (CLI) patients undergoing complete leg revascularization for peripheral artery disease (PAD) inflow and outflow disease (Figure 1). Data for this review were identified by queries of medical and life science databases, expert referral, and references from relevant articles published between 1997 and 2019. PubMed and ScienceDirect databases were searched as follows: PubMed User Query: (“complete leg” or “inflow” or “hybrid” or “outflow”) AND revascularization AND endovascular AND (CLI or “critical limb ischemia”) AND English (Language) and ScienceDirect User Query: (“complete leg” OR inflow OR outflow OR hybrid) AND revascularization AND (CLI or “critical limb ischemia”) AND English (Language) AND humans AND angioplasty. Only primary and secondary research articles, published in English, involved lower extremity CLI PAD in human subjects, and focused on endovascular inflow and/or outflow revascularization treatments, were included.
Figure 1.
A controlled literature search algorithm on outcomes of patients undergoing complete leg revascularization for peripheral artery inflow and outflow disease.
Introduction
PAD is on the rise worldwide, with rates both of claudication and CLI, also known as chronic limb-threatening ischemia (CLTI), steadily increasing. PAD made headlines in 2013 when it was referred to as a global pandemic, which affected six times as many individuals as did the HIV pandemic, with a worldwide burden of nearly a quarter billion people at that time.1 This number was considered a conservative estimate, based upon a systematic literature review spanning the first decade of this millennium.2 In the ensuing years, not only has the global burden of PAD continued to rise, but also the WHO has cited a lack of support for patients with PAD and stressed the need for greater awareness and treatment.3 While efforts have focused on the prevention of PAD, those patients whose disease has already progressed to CLI often face a lack of knowledge in regard to existing treatment options. CLI represents an advanced clinical presentation of PAD and is associated with a 25% 1-year mortality rate and a 25% 1-year major amputation rate.4 Of all US PAD patients, approximately 1% will progress to CLI,5 and of subjects currently diagnosed with claudication, 10%–15% will progress to CLI.6 Historically, bypass surgery was considered the appropriate first-line therapy for CLI; however, guidelines and practice have changed dramatically in recent years.
CLI patients tend to have multilevel disease and, unlike for claudication patients, inflow-only revascularization is often insufficient.7,8 For CLI, it is typically preferred to obtain in-line flow to the foot with at least one patent tibial/peroneal artery. This requires complete revascularization of the limb to treat both inflow and outflow lesions. Specifically for patients with common femoral artery (CFA) disease, complete revascularization has been shown to yield a higher postoperative ankle brachial index (ABI) than does incomplete revascularization, and treating both inflow and outflow disease may contribute to improved limb salvage rates.7 There is disagreement on the optimal approach to achieve either incomplete or complete revascularization in CLI patients. The aim of this article is to review the published literature of the past 20 years regarding lower extremity PAD revascularization methods, for both inflow and outflow, in order to discern best practices for future therapy.
Hybrid: endovascular + surgery
Hybrid revascularization is a dual technique that typically utilizes endovascular methods to treat inflow disease and surgical revascularization for femoral or infrainguinal disease.9 In CLI patients, iliac stenting is a standard endovascular route, coupled with common femoral endarterectomy or femoral–popliteal bypass as the adjunctive surgical procedure.8 Heart disease, renal insufficiency, and diabetes are comorbidities commonly seen in the PAD population. In comorbid patients with multilevel disease, the hybrid technique is an attractive option that allows low complication and mortality rates and a high limb salvage rate.10 As of 2013, it was estimated that 5%–21% of all limb revascularization procedures performed in the United States were hybrid interventions, with iliac stenting and CFA endarterectomy as the most common case types.11 More recent technologies have allowed endovascular therapies to include interventions on many arteries such as the superficial femoral, popliteal, proximal and distal tibial vessels as well as the pedal arterial circulation. The primary target population for these interventions are subjects with multilevel arterial occlusive disease. Approximately one-fourth of these patients will require both aortoiliac and infrainguinal revascularization; however, with hybrid surgery, either inflow or outflow arteries may be revascularized (Figure 2). In fact, in a series of cases reported by Kavanagh et al., hybrid-based external iliac and femoral endarterectomy yielded such significant increases in inflow, that outflow revascularization was often unnecessary.12 This, of course, is dependent on the presenting pathology and Rutherford classification (Figure 3).
Figure 2.

Anatomic location of inflow, outflow, and runoff vessels.
Figure 3.
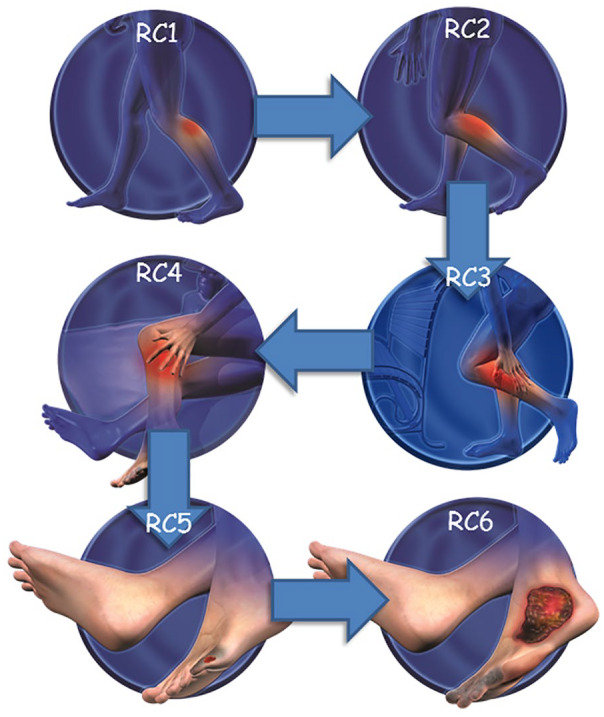
Rutherford classification (RC) of peripheral artery disease and presenting pathology.
RC1: mild claudication; RC2: moderate claudication; RC3: severe claudication; RC4: ischemic rest pain; RC5: minor tissue loss—nonhealing ulcer, focal gangrene with diffuse pedal ischemia; RC6: major tissue loss—extending above transmetatarsal level.
Hybrid procedures may offer the advantages of noninvasive endovascular techniques without the consequences of invasive surgical methods. For example, treating TransAtlantic Inter-Society Consensus (TASC) C and D lesions of the iliofemoral arteries entirely by open bypass surgery correlates with substantial morbidity and mortality, but the mid-term and long-term outcomes are superior to those of solely percutaneous revascularization procedures.13 Huynh and Bechara11 showed that hybrid procedures yield much lower morbidity and mortality rates than surgical bypass for inflow/outflow disease, with comparably high limb salvage rates. Zhou et al. performed hybrid procedures in which open revascularization was performed to correct inflow, outflow, or both diseases. They concluded that treating multilevel infrainguinal artery occlusive disease by the hybrid method yielded shorter hospitalization stays, less perioperative morbidity, and similar early- and long-term efficacy compared with open revascularization.14 For TASC C/D iliac lesions, endovascular procedures are now the first line of treatment, combined with endarterectomy for patients with common femoral disease.15 This approach agrees with the findings of other practitioners in regard to endovascular treatment of TASC C/D iliac lesions.16,17
While many studies reveal comparable or increased long-term patency for endovascular-included interventions relative to open surgical interventions,12,14,15,18 this outcome may be anatomy-specific. For example, Huded et al.19 reported that, for femoral–femoral bypass (FFB), adding inflow endovascular revascularization (EVR) actually decreased patency while maintaining 5-year limb salvage and mortality rates. However, it is important to note that Huded’s group reviewed clinical, operative, and demographic data on patients treated at one surgical center, restricting their review to FFB subjects with adjunctive inflow procedures. Schamp et al.20 similarly reported their finding that limb salvage rates are comparable between infrapopliteal angioplasty and bypass surgery via a literature review, but indicated that patency may be better after surgery. The Huded and Schamp studies, however, are limited in their retrospective nature. In addition, a more comprehensive look into the procedure itself could help to elucidate factors that contribute to specific outcomes. Dougherty et al. evaluated hybrid procedures in order to determine whether endovascular interventions are the cause of graft failure in these procedures. Not only did they find this was not the case, but also they demonstrated success in hybrid interventions when the long, severe lesion was treated surgically, and the focal, less severe lesion was treated endovascularly.21
Dosluoglu and colleagues also took a detailed look into hybrid procedures. Their analysis was based upon the TASC classification of the disease segment that is treated endovascularly. When the lesion classification was A/B, the hybrid intervention was labeled as “simple” and when the classification was C/D, it was labeled as “complex.” While Dougherty’s report showed success in the endovascular portion of hybrid procedures when that portion was TASC A/B, Dosluoglu showed success in both simple and complex interventions, demonstrating exceptional outcomes in endovascular interventions on TASC C/D lesions as well.16 Furthermore, in a direct comparison, Dosluoglu’s team revealed that 5-year primary patency, assisted-primary patency, and secondary patency rates were all higher in their endovascular group, relative to the open bypass group.22 They also reported a notable difference in the baseline characteristics of these two populations, which should be taken into account. Subjects in the endovascular group were older with more comorbidities, while subjects in the open group had more multilevel disease and infrapopliteal intervention. Thirty-day mortality was lower, and need for inflow/outflow reintervention was higher, in the endovascular population.22
Endovascular intervention
Venkatachalam et al. performed a controlled literature search on the exclusive usage of endovascular techniques in the treatment of CLI.23 Over the two decades covered by their summary, the reported technical success rate of endovascular procedures on infrainguinal arterial disease ranged 70%–100% and on suprainguinal arterial disease 84%–100%. Survival at 1 year ranged 71%–97% for infrainguinal arterial disease and 69%–96% in suprainguinal arterial disease. Considering that infrainguinal disease is far more difficult to treat than suprainguinal disease,17 these procedural and safety outcome rates of endovascular intervention are remarkable.
Venkatachalam and colleagues’ comprehensive review allows a more accurate comparison between surgical and endovascular outcomes in CLI treatment than does more limited analysis, such as the Bypass versus Angioplasty in Severe Ischemia of the Leg (BASIL) Trial. BASIL considered only balloon angioplasty as the endovascular intervention. Despite this, the study was still able to show that percutaneous transluminal angioplasty (PTA) is an effective alternative to open surgery for CLI patients.24 Furthermore, in a prospective, randomized, all-male trial, no significant difference was found in survival, patency, or limb salvage between PTA and bypass surgery treatment of iliac, femoral, or popliteal occlusions.17 In the case of iliac disease specifically, PTA yields technical success rates >90% with patency rates out to 5 years of 80%.17 These rates are generally improved when adjunctive stenting is performed. In a cohort-based study, Mustapha et al. investigated Medicare claims of beneficiaries with initial CLI diagnosis. In comparing propensity score–matched patients who had undergone revascularization via endovascular or surgical means, they discovered that long-term survival and cost in CLI management is comparable between these revascularization techniques, with lower major amputation rates following EVR.25
CFA lesions tend to be heavily calcified and complex, with surgery historically considered to be the optimal treatment option, although newer endovascular treatment strategies now exist and yield favorable outcomes.26–28 Thiney et al.29 looked at patients with disabling claudication or CLI who exclusively underwent CFA PTA. Of these procedures, 13% involved inflow vessels and 23% involved outflow vessels. The overall procedural success rate was 96%, freedom from total lesion revascularization (TLR) 97%, and the rate of restenosis 7.5%. These outcomes are comparable with those of surgical revascularization procedures, with only restenosis being significantly lower in some surgical studies. Furthermore, morbidity and mortality, local infections, and lymphatic problems are all greater in surgical procedures, suggesting endovascular intervention is a valid alternative in CFA treatment. Thiney et al.29 also reported that, for CLI patients with multilevel disease, it is insufficient to treat a femoral lesion in isolation. To resolve the CLI, outflow disease must also be addressed.
Multiple outflow
An angiosome is an anatomic unit of tissue that is perfused by a specific source artery. According to the angiosome concept in PAD, revascularization of the source artery to the critical angiosome may result in greater wound healing and limb salvage rates.30 While there is controversy surrounding this theory, recent support was found in a group of patients who underwent endovascular therapy alone, without bypass surgery. Those who were revascularized according to the angiosome concept experienced significantly higher rates of amputation-free survival, freedom from major adverse limb events (MALEs), and freedom from major amputation, relative to those who underwent indirect revascularization.31 Even more recently, Okazaki et al. described their experience with angiosome-directed revascularization in CLI patients. They noted that obtaining in-line blood flow to ischemic tissue is complicated by the fact that matched vessels are often small in diameter and severely calcified. In their two reported cases, they handled this dilemma by performing bifurcated dual runoff bypass.32 This added a secondary outflow channel to the original bypass outflow, and achieved successful perfusion. An alternative approach in these heavily calcified cases is to modify the lesion by endovascular methods and thereby prepare the target artery for bypass anastomosis.30
Multiple inflow
Kobayashi et al.33 recently reported that revascularization of both the anterior and posterior tibial arteries, as opposed to single inflow revascularization to either artery alone, resulted in superior outcomes. In double-inflow–treated patients, the wound healing rate was higher, repeat intervention rate lower, and the time to wound healing shorter. Although these are both inflow procedures, this illuminates the advantages for CLI patients of increasing blood flow through simultaneous interventions. Manzi et al. demonstrated the feasibility of achieving multiple inflow in a single intervention to recanalize foot arteries. Using the pedal–plantar loop technique, both tibial arteries could be treated in one procedure, restoring nearly complete antegrade–retrograde blood flow to the foot vessels.34 The operators took advantage of anatomic anastomoses in the collaterals below the ankle, which allowed them to complete these procedures entirely by PTA, without involving bypass.
Inflow and outflow
As summarized herein, numerous trials and reports have revealed the safety and efficacy of endovascular intervention on CLI patients. Khanolkar and Ephrem35 confirmed these findings in the challenging popliteal and infrapopliteal arterial beds, and further investigated the parameters that specifically contribute to successful clinical outcomes. They discovered that obtaining normal inflow and outflow are essential to wound healing and amputation prevention. In the case of below the knee (BTK) disease, it is critical that at least one of the three infrapopliteal arteries is patent. In approximately one-third of the patients they studied, there was additionally a lesion in the femoral segment. In all of these cases, revascularization was successfully performed in order to improve inflow. Approximately, 18% of the patients in the study had single vessel runoff; the majority of these were successfully revascularized and linear flow to the foot was achieved.35
Troisi et al. studied runoff disease in diabetic CLI patients with foot wounds. They discovered it was pedal arch patency, and not direct-angiosome revascularization, that determined successful outcomes.36 EVR was performed on the anterior tibial artery, the posterior tibial artery, or the peroneal artery. Both the estimated 1-year limb salvage rate and the estimated 1-year survival rate were 100% for those patients who had a complete pedal arch.36 Higashimori et al.37 similarly reported that BTK EVR is most successful when straight-line flow is established to a patent pedal arch, yielding 12-month amputation-free survival and limb salvage rates of 88.2% and 98.4%, respectively.
Dorros et al. performed a prospective analysis of hundreds of CLI patients undergoing tibioperoneal vessel angioplasty (TPVA) for outflow disease. The majority of these patients had ipsilateral inflow lesion balloon angioplasty, followed by infrapopliteal tibioperoneal vessel outflow balloon angioplasty.38 It was found that dilatation was possible in all of the inflow lesions and in 92% of the outflow lesions. The distinct improvement in distal perfusion pressure experienced by patients with successful inflow lesion angioplasty was similar to that which might have been attained by femoropopliteal bypass. However, those patients who also had angioplasty of the outflow lesion experienced an enhancement in distal perfusion pressure to their ischemic foot that could not have been readily achieved with surgery.38 Furthermore, the complication rates were low, with only one incidence of compartment syndrome (0.4%), one amputation (0.4%), and one death (0.4%) related to the procedure. At hospital discharge, 94% of subjects had improved their clinical status. Five-year follow-up was possible with 97% of these subjects, and revealed a 91% limb salvage rate.38
Dorros et al.38 also qualitatively compared these endovascular outcomes with published longitudinal surgical data of five studies. They found that both the procedural and follow-up success of the TPVA cohort exceeded those of the historical surgical series, and stated “the differences in endovascular and surgical survival rates, however, are even more dramatic when the surgical procedural operative mortality is considered.”38 Restoration of blood flow, relief of rest pain, and enhanced wound healing outcomes were all superior via angioplasty than bypass surgery; these data provide evidence that angioplasty is the primary therapy for patients with CLI. As an alternative to surgery, Dorros et al. conclude that “endovascular therapy has an important and definitive role for patients with infrapopliteal arterial occlusive disease and CLI by easily revascularizing inflow and outflow lesions with minimal morbidity and mortality, which significantly improved distal extremity perfusion pressure.”38
Joint inflow and outflow revascularization is beneficial in other CLI scenarios as well. In patients with chronic occlusion of popliteal artery aneurysm (PPA), poor outflow independently correlates with worse primary patency and limb salvage rates.39 Endovascular intervention allows simultaneous repair of the occlusive PPA and the downstream infrapopliteal lesion(s), yielding significantly better outcomes.
Professional guidelines
Per the 2016 AHA/ACC Guideline on the Management of Patients With Lower Extremity Peripheral Artery Disease,6 both EVR and surgical procedures are recommended to “establish in-line blood flow to the foot in patients with nonhealing wounds or gangrene.” However, the level of evidence in support of the EVR recommendation is considerably higher (Level B-Randomized) than that in support of surgical intervention (Level C-Limited Data).6 In PAD patients with lifestyle-limiting claudication, EVR is recommended for revascularization in hemodynamically significant disease, both aortoiliac occlusive and femoropopliteal.24 The effectiveness of EVR in isolated infrapopliteal artery disease has not been established.
In terms of minimizing tissue loss in CLI patients, the 2016 Guideline recommends evaluation of revascularization options by an interdisciplinary care team. Amputation should not be performed first.6 In its evaluation, the care team would consider lesion characteristics as well as patient comorbidities, such as congestive heart failure, severe lung disease, and chronic kidney disease. An EVR-first approach is recommended in cases where: comorbidities put the patient at increased risk of surgical complications, the patient has rest pain or multilevel disease, or the patient lacks a suitable autologous vein for bypass grafting.24 For patients with multilevel disease, all diseased segments can be treated in one setting, unless clinical factors (e.g. ischemic rest pain) or patient safety would be compromised. In those cases, the Guideline recommends inflow disease to be first addressed, and then outflow disease to be treated in a staged manner.6
In the updated BTK supplement to the Inter-Society Consensus for the Management of PAD (TASC II), the steering committee determined that hybrid intervention is best for the patient with both inflow aortoiliac disease and infrainguinal disease.40 In CLI patients particularly, the committee generally recommends inflow procedures to be managed by endovascular techniques, followed by infrainguinal surgical revascularization. Aho and Venermo41 reported that, from 2004 to 2011, the number of hybrid procedures performed annually increased from 4 to 73, with inflow interventions accounting for 60%, and outflow for 40% of the cases. They noted that hybrid methods result in similar treatment outcomes to surgical intervention, but with less morbidity and shorter hospital stays. Slovut and Sullivan42 agreed that EVR procedures yield lower morbidity and mortality with decreased length of hospitalization, and additionally that they are less invasive, but potentially less durable, than surgical bypass. In discussing EVR for treating inflow lesions of the aortoiliac or superficial femoral artery, they found that such intervention does not compromise the long-term patency of a downstream bypass graft. They maintained that EVR procedures can be performed successfully for inflow disease, outflow disease, and inflow/outflow combinations.42
Finally, three major global vascular surgical societies, the European Society for Vascular Surgery (ESVS), the Society for Vascular Surgery (SVS), and the World Federation of Vascular Societies (WFVS), joined efforts to launch the Global Vascular Guidelines (GVG) initiative.43 They recently decided that the term chronic limb-threatening ischemia (CLTI) is preferred over critical limb ischemia (CLI), as the latter implies threshold values of impaired perfusion rather than a continuum. The GVG also states that evidence-based revascularization (EBR) depends on three independent axes: Patient risk, Limb severity, and ANatomic complexity (PLAN). In addition, the working group of GVG proposed a new Global Anatomic Staging System (GLASS), which involves defining a preferred target artery path (TAP) and then estimating limb-based patency (LBP), resulting in three stages of complexity for intervention. The GVG also gives recommendations for EBR strategies for CLTI that are based on best available data—specifically stating that vein bypass may be preferred for average-risk patients with advanced limb threat and high complexity disease, while those with less complex anatomy, intermediate severity limb threat, or high patient risk may be favored for endovascular intervention.43 The GVG further states that the decision to perform staged or multilevel revascularization for patients with combined inflow and outflow disease should be individualized on the basis of severity of limb threat (especially if tissue loss), anatomic complexity, and patient risk.43 Finally, similar to the other guidelines mentioned above, the GVG stresses the importance of multidisciplinary teams and centers of excellence for amputation prevention as a key health system initiative.
Conclusion
Over the past 30 years, endovascular interventions have seen dramatic improvement in technique, operator skill, treatment devices, safety, and procedural success. Not surprisingly, recent years have shown a sharp increase in the percentage of bypass procedures hybridized with endovascular methods, outpacing bypass-exclusive procedures for the first time in 2010.19 EVR encompasses less invasive measures, including PTA, atherectomy, and stenting, than does open bypass. However, many contend that EVR is only appropriate for TASC A and B lesions, and surgery should be employed for TASC C and D lesions. Yet, when surgical and EVR interventions were compared for TASC B and C aortoiliac lesions no difference in mortality or limb salvage at 5 years was found.17 In fact, the acute success rates of EVR in all anatomic territories are >95%, regardless of TASC lesion severity.44
Another objection to the primary use of EVR in treatment of PAD is the occurrence of restenosis, particularly after stenting. It was found that limited or compromised runoff predicted higher failure rates of EVR procedures.44 These conclusions were generally drawn prior to the introduction of adjuvant methods, such as modern atherectomy, to prepare lesions for PTA. Since decreased blood flow has been shown to increase the rate of restenosis, current techniques such as orbital atherectomy, which maintain blood flow after PTA, may facilitate improved outcomes and remove substantial limitations to EVR success.44,45 In BTK disease, several studies have reported that PTA is feasible, successful, and safe when combined with adjuvant atherectomy and stenting.17 A 2019 analysis of the XLPAD registry revealed that the addition of atherectomy in BTK interventions is associated with lower rates of 1-year repeat target limb revascularization.46 In above the knee (ATK) disease, multiple randomized controlled trials (RCTs) have shown no difference in 1- or 3-year death, amputation, or revascularization failure rates after surgery versus angioplasty.17
A major limitation in comparing the effectiveness of various treatment modalities, however, is the variability among research studies. As pointed out by Antoniou and colleagues in their meta-analysis of femoral–popliteal therapy, PTA could range from plain old balloon angioplasty or bare metal stenting in some trials to endoluminal reconstruction of inflow and outflow vessels in others.47 Similarly, there was variation in open revascularization modalities in the reported studies, from prosthetic vein grafting to common femoral endarterectomy. It is critical to be specific and consistent in describing procedures and outcomes measured when performing such comparisons. The most recently published systematic review to compare evidence for bypass surgery and endovascular interventions in CLI patients covered nine cohort or RCT studies, none of which include data obtained in the past decade.48 As concluded by Dominguez et al. in their expert review of endovascular therapy, “catheter-based technologies are rapidly evolving at a rate that is outpacing large-scale studies evaluating relevant clinical outcomes.”4
This review comprises a breadth of studies in an attempt to avoid such limitations and provide a critical summary of PAD research findings. In the literature reviewed herein, EVR—including ballooning, stenting, and atherectomy—is shown to be an efficacious therapy for CLI. When used to augment collateral inflow, limb salvage benefits are realized, and when used to treat runoff disease, tissue healing is promoted.17 EVR is an effective PAD therapy for both ATK and BTK, and can safely and effectively treat both inflow and outflow disease. As such, it plays a leading role in the therapy of lower extremity artery disease.44
Footnotes
Author contributions: J.A.M. and B.J.M. contributed to the conceptualization of the study. B.M.A., B.J.M., and J.A.M. contributed to methodology of the study. B.J.M. and B.M.A. contributed to visualization of the study. B.M.A. contributed to the literature search for the study. B.M.A. and B.J.M. contributed to presenting figures. B.M.A., B.J.M., and J.A.M. contributed to writing—original draft preparation. All the authors contributed to writing—review and editing.
Declaration of conflicting interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: J.A.M. has consulting agreements with Boston Scientific (BSc), Cardiovascular Systems, Inc. (CSI), Bard Peripheral Vascular (BPV), Terumo Medical, and Philips. G.P. is a consultant for CSI. C.W.B. is a lecturer for Penumbra and Cook, and an advisory committee member for BSc. G.A. has consulting agreements with BSc, CSI, Cook Medical, and Abbott Vascular. F.S. has consulting agreements with BSc, CSI, Terumo, BPV, Philips, and Medtronic. B.M.A. and B.J.M. are employees of and hold stock in CSI. M.S.L. and J.R. have no declarations of interest.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Jihad A Mustapha  https://orcid.org/0000-0002-6351-8080
https://orcid.org/0000-0002-6351-8080
Bynthia M Anose  https://orcid.org/0000-0002-0739-5549
https://orcid.org/0000-0002-0739-5549
References
- 1. Hirsch AT, Duval S. The global pandemic of peripheral artery disease. Lancet 2013; 382: 1312–1314. [DOI] [PubMed] [Google Scholar]
- 2. Fowkes FGR, Rudan D, Rudan I, et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in2000 and 2010: a systematic review and analysis. Lancet 2013; 382: 1329–1340. [DOI] [PubMed] [Google Scholar]
- 3. Peripheral arterial disease (PAD): European population-based action plan for early diagnosis, http://www.euro.who.int/en/health-topics/Life-stages/healthy-ageing/news/news/2014/03/peripheral-arterial-disease-pad-european-population-based-action-plan-for-early-diagnosis (2014, accessed 21 February 2018).
- 4. Dominguez A, III, Bahadorani J, Reeves R, et al. Endovascular therapy for critical limb ischemia. Expert Rev Cardiovasc Ther 2015; 13: 429–444. [DOI] [PubMed] [Google Scholar]
- 5. Shean KE, Soden PA, Schermerhorn ML, et al. Lifelong limb preservation: a patient-centered description of lower extremity arterial reconstruction outcomes. J Vasc Surg 2017; 66: 1117–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gerhard-Herman MD, Gornik HL, Barrett C, et al. 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: a report of the American college of cardiology/American heart association task force on clinical practice guidelines. Circulation 2017; 135: e726–e779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Takayama T, Matsumura J. IP191. Patients with critical limb ischemia from complex arterial lesions: is complete revascularization necessary? J Vasc Surg 2016; 63: 115S–116S. [Google Scholar]
- 8. Kinlay S. Management of critical limb ischemia. Circ Cardiovasc Interv 2016; 9: e001946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Matsagkas M, Kouvelos G, Arnaoutoglou E, et al. Hybrid procedures for patients with critical limb ischemia and severe common femoral artery atherosclerosis. Ann Vasc Surg 2011; 25(8): 1063–1069. [DOI] [PubMed] [Google Scholar]
- 10. Grandjean A, Iglesias K, Dubuis C, et al. Surgical and endovascular hybrid approach in peripheral arterial disease of the lower limbs. Vasa 2016; 45(5): 417–422. [DOI] [PubMed] [Google Scholar]
- 11. Huynh TTT, Bechara CF. Hybrid Interventions in Limb Salvage. Methodist Debakey Cardiovasc J 2013; 9(2): 90–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kavanagh CM, Heidenreich MJ, Albright JJ, et al. Hybrid external iliac selective endarterectomy surgical technique and outcomes. J Vasc Surg 2016; 64(5): 1327–1334. [DOI] [PubMed] [Google Scholar]
- 13. Schrijver AM, Moll FL, De Vries JP. Hybrid procedures for peripheral obstructive disease. J Cardiovasc Surg 2010; 51(6): 833–843. [PubMed] [Google Scholar]
- 14. Zhou M, Huang D, Liu C, et al. Comparison of hybrid procedure and open surgical revascularization for multilevel infrainguinal arterial occlusive disease. Clin Interv Aging 2014; 9: 1595–1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Patel SD, Donati T, Zayed H. Hybrid revascularization of complex multilevel disease: a paradigm shift in critical limb ischemia treatment. J Cardiovasc Surg 2014; 55(5): 613–623. [PubMed] [Google Scholar]
- 16. Dosluoglu HH, Lall P, Cherr GS, et al. Role of simple and complex hybrid revascularization procedures for symptomatic lower extremity occlusive disease. J Vasc Surg 2010; 51(6): 1425–1435. [DOI] [PubMed] [Google Scholar]
- 17. Allaqaband S, Kirvaitis R, Jan F, et al. Endovascular treatment of peripheral vascular disease. Curr Probl Cardiol 2009; 34: 359–476. [DOI] [PubMed] [Google Scholar]
- 18. Cotroneo AR, Iezzi R, Marano G, et al. Hybrid therapy in patients with complex peripheral multifocal steno-obstructive vascular disease: two-year results. Cardiovasc Intervent Radiol 2007; 30(3): 355–361. [DOI] [PubMed] [Google Scholar]
- 19. Huded CP, Goodney PP, Powell RJ, et al. The impact of adjunctive iliac stenting on femoral-femoral bypass in contemporary practice. J Vasc Surg 2012; 55(3): 739–745; discussion 744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schamp KBC, Meerwaldt R, Reijnen Geelkerken RH, et al. The ongoing battle between infrapopliteal angioplasty and bypass surgery for critical limb ischemia. Ann Vasc Surg 2012; 26(8): 1145–1153. [DOI] [PubMed] [Google Scholar]
- 21. Dougherty MJ, Young LP, Calligaro KD. One hundred twenty-five concomitant endovascular and open procedures for lower extremity arterial disease. J Vasc Surg 2003; 37(2): 316–322. [DOI] [PubMed] [Google Scholar]
- 22. Dosluoglu HH, Lall P, Harris LM, et al. Long-term limb salvage and survival after endovascular and open revascularization for critical limb ischemia after adoption of endovascular-first approach by vascular surgeons. J Vasc Surg 2012; 56(2): 361–371. [DOI] [PubMed] [Google Scholar]
- 23. Venkatachalam S, Shishehbor MH, Gray BH. Basic data related to endovascular management of peripheral arterial disease in critical limb ischemia. Ann Vasc Surg 2012; 26(7): 1039–1051. [DOI] [PubMed] [Google Scholar]
- 24. Gerhard-Herman MD, Gornik HL, Barrett C, et al. 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: executive summary. Circulation 2016; 22: NP1–NP43. [DOI] [PubMed] [Google Scholar]
- 25. Mustapha JA, Katzen BT, Neville RF, et al. Determinants of long-term outcomes and costs in the management of critical limb ischemia: a population-based cohort study. J Am Heart Assoc 2018; 7: E009724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lee MS, Srivastava PK, Al Yaseen S, et al. Acute procedural outcomes of orbital atherectomy for the treatment of profunda femoris artery disease: subanalysis of the CONFIRM registries. J Invasive Cardiol 2018; 30: 177–181. [PubMed] [Google Scholar]
- 27. Mehta M, Zhou Y, Paty PSK, et al. Percutaneous common femoral artery interventions using angioplasty, atherectomy, and stenting. J Vasc Surg 2016; 64(2): 369–379. [DOI] [PubMed] [Google Scholar]
- 28. Azéma L, Davaine JM, Guyomarch B, et al. Endovascular repair of common femoral artery and concomitant arterial lesions. Eur J Vasc Endovasc Surg 2011; 41(6): 787–793. [DOI] [PubMed] [Google Scholar]
- 29. Thiney P-O, Millon A, Boudjelit T, et al. Angioplasty of the common femoral artery and its bifurcation. Ann Vasc Surg 2015; 29: 960–967. [DOI] [PubMed] [Google Scholar]
- 30. Brodmann M. The angiosome concept in clinical practice: implications for patient-specific recanalization procedures. J Cardiovasc Surg (Torino) 2013; 54(5): 567–571. [PubMed] [Google Scholar]
- 31. Iida O, Soga Y, Hirano K, et al. Long-term results of direct and indirect endovascular revascularization based on the angiosome concept in patients with critical limb ischemia presenting with isolated below-the-knee lesions. J Vasc Surg 2012; 55(2): 363–370. [DOI] [PubMed] [Google Scholar]
- 32. Okazaki J, Ishida M, Kuma S, et al. Infrapopliteal bifurcated dual run-off bypass in critical limb ischemia: a report of 2 cases. Ann Vasc Surg 2015; 29(5): 1020e17–1020e21. [DOI] [PubMed] [Google Scholar]
- 33. Kobayashi N, Hirano K, Yamawaki M, et al. Clinical effects of single or double tibial artery revascularization in critical limb ischemia patients with tissue loss. J Vasc Surg 2017; 65: 744–753. [DOI] [PubMed] [Google Scholar]
- 34. Manzi M, Fusaro M, Ceccacci T, et al. Clinical results of below-the knee intervention using pedal-plantar loop technique for the revascularization of foot arteries. J Cardiovasc Surg 2009; 50(3): 331–337. [PubMed] [Google Scholar]
- 35. Khanolkar UB, Ephrem B. Endovascular reconstruction of popliteal and infrapopliteal arteries for limb salvage and wound healing in patients with critical limb ischemia: a retrospective analysis. Indian Heart J 2016; 68(1): 77–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Troisi N, Turini F, Chisci E, et al. Pedal arch patency and not direct-angiosome revascularization predicts outcomes of endovascular interventions in diabetic patients with critical limb ischemia. Int Angiol J Int Union Angiol 2017; 36: 438–444. [DOI] [PubMed] [Google Scholar]
- 37. Higashimori A, Iida O, Yamauchi Y, et al. Outcomes of One straight-line flow with and without pedal arch in patients with critical limb ischemia. Catheter Cardiovasc Interv off J Soc Card Angiogr Interv 2016; 87: 129–133. [DOI] [PubMed] [Google Scholar]
- 38. Dorros G, Jaff MR, Dorros AM, et al. Tibioperoneal (outflow lesion) angioplasty can be used as primary treatment in 235 patients with critical limb ischemia: five-year follow-up. Circulation 2001; 104: 2057–2062. [DOI] [PubMed] [Google Scholar]
- 39. Giaquinta A, Veroux P, D’Arrigo G, et al. Endovascular treatment of chronic occluded popliteal artery aneurysm. Vasc Endovascular Surg 2016; 50: 16–20. [DOI] [PubMed] [Google Scholar]
- 40. Jaff MR, White CJ, Hiatt WR, et al. An update on methods for revascularization and expansion of the TASC lesion classification to include below-the-knee arteries: a supplement to the inter-society consensus for the management of peripheral arterial disease (TASC II). TASC steering committee. J Endovasc Ther 2015; 22: 663–677. [DOI] [PubMed] [Google Scholar]
- 41. Aho P-S, Venermo M. Hybrid procedures as a novel technique in the treatment of critical limb ischemia. Scand J Surg 2012; 101(2): 107–113. [DOI] [PubMed] [Google Scholar]
- 42. Slovut DP, Sullivan TM. Critical limb ischemia: medical and surgical management. Vasc Med 2008; 13(3): 281–291. [DOI] [PubMed] [Google Scholar]
- 43. Conte MS, Bradbury AW, Kolh P, et al. Global vascular guidelines on the management of chronic limb-threatening ischemia. J Vasc Surg 2019; 69: 3S–125Se40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Malyar NM, Reinecke H, Freisinger E. Restenosis after endovascular revascularization in peripheral artery disease. VASA Z Gefasskrankheiten 2015; 44: 257–270. [DOI] [PubMed] [Google Scholar]
- 45. Lee M, Généreux P, Shlofmitz R, et al. Orbital atherectomy for treating de novo, severely calcified coronary lesions: 3-year results of the pivotal ORBIT II trial. Cardiovasc Revasc Med 2017; 18(4): 261–264. [DOI] [PubMed] [Google Scholar]
- 46. Khalili H, Jeon-Slaughter H, Armstrong EJ, et al. Atherectomy in below-the-knee endovascular interventions: one-year outcomes from the XLPAD registry. Catheter Cardiovasc Interv off J Soc Card Angiogr Interv 2019; 93: 488–493. [DOI] [PubMed] [Google Scholar]
- 47. Antoniou GA, Chalmers N, Georgiadis GS, et al. A meta-analysis of endovascular versus surgical reconstruction of femoropopliteal arterial disease. J Vasc Surg 2013; 57(1): 242–253. [DOI] [PubMed] [Google Scholar]
- 48. Abu Dabrh AM, Steffen MW, Asi N, et al. Bypass surgery versus endovascular interventions in severe or critical limb ischemia. J Vasc Surg 2016; 63: 244–253. e11. [DOI] [PubMed] [Google Scholar]