Abstract
Background:
Cardiac rehabilitation programs provide a comprehensive framework for the institution of secondary preventive measures. Smartphone technology can provide a platform for the delivery of such programs and is a promising alternative to hospital-based services. However, there is limited evidence to date supporting this approach. Accordingly, we performed a systematic review and meta-analysis examining smartphone-based secondary prevention programs to traditional cardiac rehabilitation in patients with established coronary artery disease to ascertain the feasibility and effectiveness of these interventions.
Methods:
A systematic search of PubMed, MEDLINE, EMBASE, and the Cochrane Library was conducted. A meta-analysis was performed using a random-effects model with the outcomes of interest being 6-minute walk test (6MWT) distance, systolic blood pressure, low-density lipoprotein (LDL) cholesterol, and body mass index (BMI).
Results:
A total of 8 studies with 1120 patients across 5 countries were included in the quantitative analysis. Follow-up ranged from 6 weeks to 12 months. Five studies examined all patients post acute coronary syndrome, 2 studies examined only patients undergoing percutaneous coronary intervention, and 1 study examined all patients with a diagnosis of coronary artery disease, independent of intervention. Exercise capacity, as measured by the 6MWT, was significantly greater in the smartphone group (20.10 meters, 95% confidence interval [CI] 7.44-33.97; P < .001; I2 = 45.58). There was no significant difference in BMI reduction, systolic blood pressure, or LDL cholesterol levels between groups (P value for all > .05).
Conclusion:
Publicly available smartphone-based cardiac rehabilitation programs are a convenient and easily disseminated intervention which show merit in exercise promotion in patients with established coronary artery disease. Further research is required to establish the clinical significance of recent findings favoring their use.
Keywords: mHealth, smartphone, cardiac rehabilitation, cardiovascular risk factors, secondary prevention
Introduction
Coronary artery disease (CAD) is the leading cause of morbidity and mortality in the developed world. The rapid rise in CAD burden over recent decades can be attributed to socioeconomic changes, increase in life expectancy, and acquisition of lifestyle-related risk factors.1 Patients with established CAD are at increased risk of premature death, myocardial infarction, and rehospitalization, and as such, international guidelines advocate for adherence to secondary prevention strategies following diagnosis.2
Cardiac rehabilitation programs provide a comprehensive framework for the institution of secondary preventive measures, such as evidence-based pharmacotherapy, cardiac risk factor optimization, and diet and physical activity recommendations.3 There is extensive literature to date supporting these interventions, with a recent Cochrane review revealing a 13% decrease in all-cause mortality and a 26% decrease in cardiovascular mortality in participants of structured rehabilitation programs.4 Despite this, cardiac rehabilitation programs remain generally underutilized, with poor referral and completion rates.5-9 When referred, the commencement of cardiac rehabilitation can be delayed weeks to months post the index event, and can cause a delay in the resumption of work.10 Furthermore, the opportunity to immediately reinforce the importance of physical activity and lifestyle change is lost. As such, novel models of care have been considered in this population.
Digital health interventions for secondary prevention offer an effective alternative to traditional cardiac rehabilitation and can be implemented immediately.11,12 Early research in this field predominantly focused on telehealth interventions, defined as provision of healthcare via telephone calls, Internet, and videoconferencing,13 which although effective, still pose a strain on resource utilization. Smartphone technology is an advance on previous telehealth interventions and can provide an automated platform for a patient-centered program with the capacity to incorporate education, motivation, reminders, and support.3
Although promising, most of the available evidence in this field is based on pilot and feasibility studies with widely varying treatment lengths and digital intervention types.14-21 The exponential growth and availability of smartphone technology may provide a novel tool to optimize secondary prevention of coronary heart disease. This form of digital intervention can be accessed anywhere, at any time, overcoming geographic and resource associated limitations of healthcare delivery and as such has the potential to revolutionize the landscape of secondary prevention. However, the overall value of this approach remains unclear. Accordingly, we have performed a systematic review and meta-analysis comparing the effectiveness of smartphone-based secondary prevention to traditional cardiac rehabilitation programs in the optimization of secondary prevention in patients with CAD.
Methods
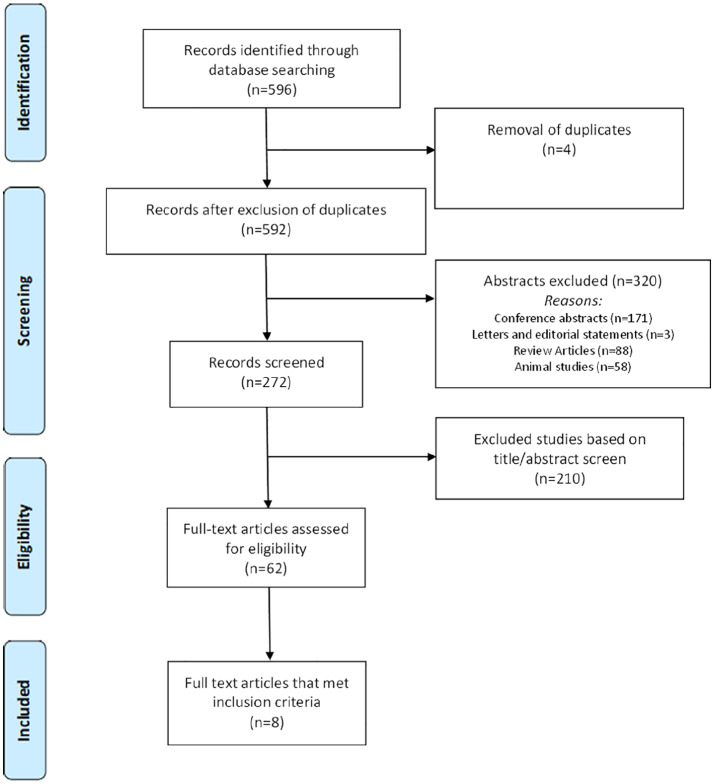
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses Protocols (PRISMA-P) Statement22 was followed (Figure 1) (Table S3). A comprehensive literature search was performed through MEDLINE, EMBASE, PubMed, and the Cochrane Library from establishment to April 2019 using the following search areas: “smartphone,” “rehabilitation,” and “coronary artery disease” (Table S1). Articles were screened based on title and abstract. Full text of the articles meeting the inclusion criteria based on initial screening were then reviewed. Two reviewers (A.C.M. and G.M.) independently extracted the data and resolved conflicts by consensus. Reference lists of reviewed articles were screened to identify further relevant studies.
Figure 1.
Preferred reporting items for systematic reviews and meta-analyses (PRISMA) flow diagram.
Studies included were not limited by date, location of study, study design, or publication status. Inclusion criteria were as follows: (1) studies performed in adult patients with established CAD; (2) study design involving a comparison of patients undergoing smartphone-based cardiac rehabilitation against patients undergoing traditional outpatient-based cardiac rehabilitation; (3) studies published in English. In the case of multiple publications involving the same patient cohort, we used the longest available follow-up. A quality assessment of both randomized and nonrandomized controlled trials was performed using the van Tulder scale (Table S2).23 The van Tulder scale evaluates 11 components, including randomization, allocation concealment, baseline characteristics, patient blinding, caregiver blinding, observer blinding, cointervention, compliance, dropout rate, end point assessment time point, and intention to treat analysis. Studies scoring 5 or more points on the 11-point scale were deemed to be of high quality. Two reviewers (A.C.M., G.M.) independently extracted the data and resolved conflicts by consensus.
The outcomes of interest were exercise capacity as measured by the 6-minute walk test (6-MWT, meters), systolic blood pressure (SBP, mmHg), low-density lipoprotein cholesterol (LDL-C, mmol/L), and body mass index (BMI, kg/m2). Diastolic blood pressure (DBP), high-density lipoprotein cholesterol (HDL-C), total cholesterol, medication adherence, clinical events (acute myocardial infarction [AMI], unplanned revascularization, stroke, mortality, and recurrent cardiac hospitalization), weight, waist circumference (WC), resting heart rate (RHR), smoking status, glucose levels, and glycated hemoglobin (HbA1c) levels were evaluated on secondary analyses.
Statistical analysis
Descriptive statistics are presented as mean and standard deviation for continuous variables and absolute and relative frequencies for categorical variables. Continuous outcomes were analyzed using an inverse variance method based on a Der Simonian and Laird random-effects model to estimate the mean differences with 95% confidence intervals (CIs). Random-effect analysis was prespecified in preference to a fixed-effects model in this study due to clinical variations in study types and populations assessed. Statistical heterogeneity was quantified using the I2 statistic. The I2statistic provides an estimate of the amount of variance due to heterogeneity rather than chance and is based on the traditional measure of variance, the Cochrane Q statistic. I2 ⩾ 75% was considered to indicate significant interstudy heterogeneity.24 As the number of included studies was less than 10, we did not perform tests to ascertain publication bias.25 We considered a P value < .05 as statistically significant. Statistical analyses were performed and Forest plots generated using comprehensive meta-analysis using the reported study data (Version 3, Biostat, Englewood, NJ).
Results
A total of 8 studies (7 randomized controlled trials and 1 observational case-control study) examining 1120 patients were included in the quantitative analysis.14-20,26 Overall study quality was deemed high in all eight studies. Follow-up ranged from 6 weeks to 12 months and the indication for cardiac rehabilitation varied. Five studies examined all patients post acute coronary syndrome (ACS),14-18 2 studies examined only patients undergoing percutaneous coronary intervention (PCI),20,26 and 1 study examined all patients with a diagnosis of CAD, independent of intervention.19 Compliance with follow-up assessments varied from 83% to 100% in the smartphone group compared with 44% to 100% in the control group. Study and patient characteristics are presented in Table 1. A summary of the smartphone interventions has been included in Table 2. Due to significant heterogeneity in outcome reporting, clinical events such as acute myocardial infarction, unplanned revascularization, stroke, mortality, and recurrent cardiac hospitalization were not analyzed.
Table 1.
Study and patient characteristics.
| Blasco 2012 | Varnfield 2014 | Widmer 2015 | Johnston 2016 | Widmer 2017 | Maddison 2019 | Fang 2019 | Dorje 2019 | |
|---|---|---|---|---|---|---|---|---|
| Smartphone intervention (n) | 102 | 53 | 25 | 86 | 34 | 82 | 33 | 156 |
| Traditional cardiac rehabilitation (n) | 101 | 41 | 19 | 80 | 37 | 80 | 34 | 156 |
| Design | RCT | RCT | Case-Control | RCT | RCT | RCT | RCT | RCT |
| Program duration | 12 months | 6 months | 3 months | 6 months | 3 months | 3 months | 6 weeks | 6 months |
| Population | ACS | ACS | ACS | ACS | ACS | CAD | Post PCI | Post PCI |
| Follow-up time point | 12 months | 6 months | 6 months | 6 months | 6 months | 6 months | 6 weeks | 12 months |
| Follow-up completion (%) | ||||||||
| Smartphone CR | 88 | 77 | 89 | 93 | 92 | 94 | 83 | 100 |
| Traditional CR | 91 | 43 | 44 | 93 | 85 | 98 | 85 | 100 |
| Age (years) | ||||||||
| Smartphone CR | 60.6 ± 11.5 | 54.9 ± 9.6 | 60.2 ± 12.1 | 56.8 ± 8.0 | 61.0 ± 13.2 | 62.5 ± 10.7 | 60.2 ± 9.4 | 59.1 ± 9.4 |
| Traditional CR | 61 ± 12.1 | 56.2 ± 10.1 | 70.4 ± 9.9 | 58.4 ± 8.6 | 61.5 ± 12.2 | 63.6 ± 10.9 | 61.4 ± 10.2 | 61.9 ± 8.7 |
| Male (%) | ||||||||
| Smartphone CR | 81 | 91 | 76 | 83 | 84 | 78 | 64 | 82 |
| Traditional CR | 80 | 83 | 89 | 79 | 88 | 85 | 62 | 81 |
| BMI (kg/m2) | ||||||||
| Smartphone CR | 28.2 ± 5.3 | NR | 29.2 ± 4.4 | 28.9 ± 5.6 | 29.1 ± 4.6 | 31.4 ± 5 | NR | 25.3 ± 3.0 |
| Traditional CR | 27.7 ± 3.5 | NR | 30.6 ± 5.6 | 28.4 ± 4.7 | 27.9 ± 3.5 | 30.5 ± 6.0 | NR | 25.4 ± 3.5 |
| Hypertension (%) | ||||||||
| Smartphone CR | NR | 42 | 95 | 46.5 | 65 | 82 | 46 | NR |
| Traditional CR | NR | 51 | 93 | 47.5 | 61 | 70 | 41 | NR |
| Diabetes (%) | ||||||||
| Smartphone CR | NR | 15 | 27 | 9.3 | 18 | 32 | 27 | NR |
| Traditional CR | NR | 20 | 33 | 16.3 | 18 | 13 | 38 | NR |
| Hyperlipidemia (%) | ||||||||
| Smartphone CR | NR | 55 | 68 | 27.9 | 77 | 97 | NR | NR |
| Traditional CR | NR | 46 | 71 | 16.3 | 88 | 93 | NR | NR |
| Smoker (%) | ||||||||
| Smartphone CR | 77 | 5 | 14 | NR | 0 | 3 | NR | 56 |
| Traditional CR | 74 | 10 | 11 | NR | 1 | 15 | NR | 57 |
Abbreviations: ACS, acute coronary syndrome; BMI, body mass index; CAD, coronary artery disease; CR, cardiac rehabilitation; NR, not recorded; PCI, percutaneous coronary intervention; RCT, randomized controlled trial.
Table 2.
Smartphone interventions.
| Author | Date | Smartphone intervention |
|---|---|---|
| Blasco | 2012 | Patient-measured blood pressure, lipid profile, and glucose levels are entered into an app-based questionnaire. The medical team analyze the data and send management advice via the app. |
| Varnfield | 2014 | The inbuilt health system in the smartphone monitors daily activity. The platform allows for delivery of motivational and educational materials to participants. The program is connected to a web portal and weekly data are entered for monitoring by the medical team. |
| Widmer | 2015 | The inbuilt health system in the smartphone monitors daily activity and further information is inputted by the patient. The platform allows for delivery of motivational and educational materials to participants. Automated messages are sent to encourage compliance with the program. |
| Johnston | 2016 | Patients are given an exercise program and drug adherence diary to complete and data regarding these behaviors are inputted into the app. The platform allows for delivery of motivational and educational materials to participants. Automated messages are sent to encourage compliance with the program. |
| Widmer | 2017 | Patients are given an exercise program to complete and data regarding dietary and exercise behaviors are inputted into the app. The platform allows for delivery of motivational and educational materials to participants. Automated messages are sent to encourage compliance with the program. |
| Maddison | 2019 | The inbuilt health system in the smartphone monitors daily activity. Patients are given an individualized exercise program to complete and data regarding dietary and exercise behaviors are inputted into the app. The platform allows for delivery of motivational and educational materials to participants. Automated messages are sent to encourage compliance with the program. |
| Fang | 2019 | Patients were fitted with remote monitoring system which communicated with the inbuilt health system in the smartphone to monitor daily activity. The platform allows for delivery of motivational and educational materials to participants. |
| Dorje | 2019 | The inbuilt health system in the smartphone monitors daily activity. The platform allows for delivery of motivational and educational materials to participants. The program is connected to a web portal and weekly data are entered for monitoring by the medical team. |
The results of the meta-analysis of the outcomes of interest are presented in Figure 2. Exercise capacity, as measured by the 6MWT, was significantly greater in the smartphone group (20.10 meters, 95% CI 7.44-33.97; P < 0.001; I2 = 45.58). There was a numerical reduction in BMI in the smartphone group seen across studies, but this was not statistically significant (–0.53 kg/m2, 95% CI –1.10 to 0.05; P = .07, I2 = 32.0%). There was no significant difference in systolic blood pressure or LDL cholesterol levels between groups(–0.63 mmHg, 95% CI –3.41 to 2.15; P = .66, I2 = 0% and 0.01 mmol/L, 95% CI –3.41 to 2.15; P = .97, I2 = 90% respectively). Similarly DBP, HDL-C, total cholesterol, medication adherence, clinical events, weight, WC, RHR, smoking status, or glycemic control were comparable between groups (P value for all > .05) as assessed on secondary analyses.
Figure 2.
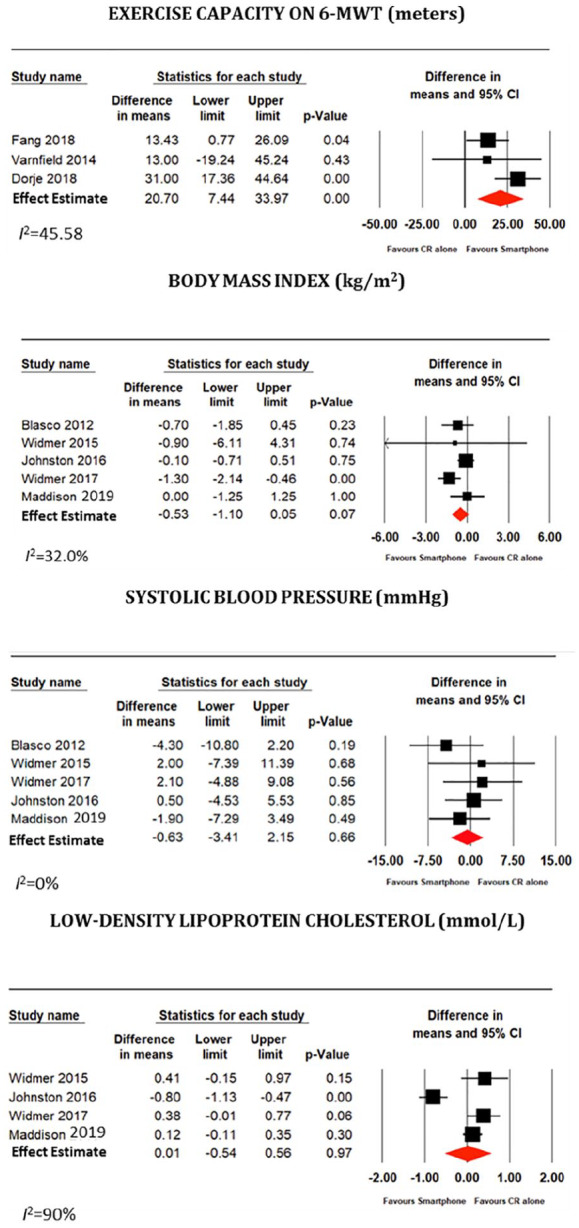
Meta-analysis of the primary endpoints.
Discussion
The purpose of this study was to compare the effectiveness of smartphone-based interventions to traditional cardiac rehabilitation for secondary prevention in patients with CAD. On review of the literature, smartphone technology appears well received by patients. The results of the meta-analysis demonstrate that the application of smartphone-based interventions in studies ranging from 6 weeks to 12 months has a beneficial effect on 6MWT capacity. Both smartphone-based and traditional cardiac rehabilitation had comparable outcomes in the other measured secondary prevention parameters.
The number of smartphone users today surpasses 3 billion and is predicted to grow by several hundred million.27 Smartphone users can now access interactive applications from anywhere at any time. As such, there is great potential for the delivery of digital health interventions via smartphone technology. Traditional cardiac rehabilitation programs require dedicated multidisciplinary involvement and expend significant hospital resources. Furthermore, compliance is reliant on patient availability to attend multiple visits per week which may be impacted by employment and household commitments.10 Unfortunately, specific compliance with the smartphone application was not reported in the included studies, despite discussion comments made of general acceptability and usability of the technology. Follow-up rates were largely similar between groups, except for 2 studies where follow-up in the smartphone group was roughly double that of the traditional cardiac rehabilitation group.15,17 Although follow-up assessment cannot be used as a surrogate for compliance, there may be a potential improvement in patient engagement with a smartphone-based platform.
Physical inactivity is independently associated with 12.2% of the global burden of acute myocardial infarction.28 Accordingly, physical activity is a cornerstone of secondary prevention, with a dose-response relationship existing between 6-MWT and the risk of future cardiovascular events.29 In this analysis, smartphone-based cardiac rehabilitation was associated with a greater improvement in exercise capacity when compared with traditional programs. Dorje et al26 have attributed this benefit to the high level of participant engagement, achieved as a result of the system’s remote activity monitoring, timely feedback, and positive reinforcement measurements. In contrast, compliance with exercise recommendations is still far from desirable in traditional cardiac rehabilitation programs and may contribute to poorer long-term outcomes.30
Blood pressure and cholesterol control are important secondary prevention targets. A meta-analysis by Ettehad et al31 of over 600 000 adults indicated that a 10 mmHg reduction in systolic blood pressure is associated with a 20% reduction in major cardiovascular events and 13% reduction in all-cause mortality. In addition, with every 1 mmol/L decline in LDL cholesterol concentration, there is a 21% reduction in cardiovascular events.32 As such, close monitoring and aggressive treatment of these parameters are paramount to the optimal management of patients with CAD. Although the pooled analysis in this study did not show a significant advantage of smartphone technology over traditional cardiac rehabilitation in the management of cardiac risk factors, the authors acknowledge the positive results of the large-scale SMART-CR/SP trial by Dorje et al26 published in 2019. This was the first randomized controlled trial to show the efficacy of smartphone-based home cardiac rehabilitation in all outcome domains. Although further research is required to support the clinical significance of these findings, this study highlights the potential of smartphone technology as an effective and accessible alternative to traditional rehabilitation programs.
The majority of available evidence in the field of digital health intervention for secondary prevention is based on pilot and feasibility studies with widely varying treatment lengths and intervention types. To our knowledge, this is the first meta-analysis examining only smartphone-based interventions, which is a strength of this study. Furthermore, the assessment of study quality was high which increases the generalizability of our findings. A significant limitation is the intermediate duration of follow-up. In addition, outcome assessments in some studies may be subject to bias due to them being unblinded to the treatment type. As we did not have access to individual study data, we were unable to stratify study outcomes at a specific time period. The variability in patient follow-up may have affected the results of our analysis. As such, the results of this meta-analysis are merely hypothesis generating rather than conclusive. Further studies are required to examine the impact of smartphone-based intervention on clinical events such as acute myocardial infarction, unplanned revascularization, stroke, mortality, and recurrent cardiac hospitalization as these are important measures of success of a secondary prevention program.
Conclusion
Publicly available smartphone-based cardiac rehabilitation programs are a convenient and easily disseminated intervention which show merit in exercise promotion in patients with established CAD. Further research is required to establish the clinical significance of recent findings favoring their use. In particular, larger studies which are adequately powered for clinical endpoints and with longer follow-up are needed to ascertain the effectiveness and sustainability in the long term. In the interim, implementation of these strategies should be considered for the many cardiac patients without access to cardiac rehabilitation and secondary prevention services.
Supplemental Material
Supplemental material, mHealth_Supplement for Efficacy of Smartphone-Based Secondary Preventive Strategies in Coronary Artery Disease by Alexandra C Murphy, Georgina Meehan, Anoop N Koshy, Phelia Kunniardy, Omar Farouque and Matias B Yudi in Clinical Medicine Insights: Cardiology
Footnotes
Funding:The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of Conflicting Interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: All authors had access to the data and a role in writing the manuscript.
ORCID iDs: Alexandra C Murphy  https://orcid.org/0000-0002-4248-7537
https://orcid.org/0000-0002-4248-7537
Georgina Meehan  https://orcid.org/0000-0002-1365-7461
https://orcid.org/0000-0002-1365-7461
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Gaziano TA, Bitton A, Anand S, Abrahams-Gessel S, Murphy A. Growing epidemic of coronary heart disease in low- and middle-income countries. Curr Probl Cardiol. 2010;35:72-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Smith SC, Benjamin EJ, Bonow RO, et al. AHA/ACCF secondary prevention and risk reduction therapy for patients with coronary and other atherosclerotic vascular disease: 2011 update: a guideline from the American Heart Association and American College of Cardiology Foundation. Circulation. 2011;124:2458-2473. [DOI] [PubMed] [Google Scholar]
- 3. Yudi MB, Clark DJ, Tsang D, et al. SMARTphone-based, early cardiac REHABilitation in patients with acute coronary syndromes [SMART-REHAB trial]: a randomized controlled trial protocol. BMC Cardiovasc Disord. 2016;16:170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Heran BS, Chen JM, Ebrahim S, et al. Exercise-based cardiac rehabilitation for coronary heart disease. Cochrane Database Syst Rev. 2011;7:CD001800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Suaya JA, Shepard DS, Normand SL, Ades PA, Prottas J, Stason WB. Use of cardiac rehabilitation by Medicare beneficiaries after myocardial infarction or coronary bypass surgery. Circulation. 2007;116:1653-1662. [DOI] [PubMed] [Google Scholar]
- 6. Johnson N, Fisher J, Nagle A, Inder K, Wiggers J. Factors associated with referral to outpatient cardiac rehabilitation services. J Cardiopulm Rehabil. 2004;24:165-170. [DOI] [PubMed] [Google Scholar]
- 7. Chew DP, French J, Briffa TG, et al. Acute coronary syndrome care across Australia and New Zealand: the SNAPSHOT ACS study. Med J Aust. 2013;199:185-191. [DOI] [PubMed] [Google Scholar]
- 8. Bunker S, McBurney H, Cox H, Jelinek M. Identifying participation rates at outpatient cardiac rehabilitation programs in Victoria, Australia. J Cardiopulm Rehabil. 1999;19:334-338. [DOI] [PubMed] [Google Scholar]
- 9. Scott IA, Lindsay KA, Harden HE. Utilisation of outpatient cardiac rehabilitation in Queensland. Med J Aust. 2003;179:341-345. [DOI] [PubMed] [Google Scholar]
- 10. Worcester MU, Elliott PC, Turner A, et al. Resumption of work after acute coronary syndrome or coronary artery bypass graft surgery. Heart Lung Circ. 2014;23:444-453. [DOI] [PubMed] [Google Scholar]
- 11. Huang K, Liu W, He D, et al. Telehealth interventions versus center-based cardiac rehabilitation of coronary artery disease: a systematic review and meta-analysis. Eur J Prev Cardiol. 2015;22:959-971. [DOI] [PubMed] [Google Scholar]
- 12. Neubeck L. Telehealth-based cardiac rehabilitation: a solution to the problem of access? Eur J Prev Cardiol. 2015;22:957-958. [DOI] [PubMed] [Google Scholar]
- 13. Neubeck L, Redfern J, Fernandez R, Briffa T, Bauman A, Freedman SB. Telehealth interventions for the secondary prevention of coronary heart disease: a systematic review. Eur J Cardiovasc Prev Rehabil. 2009;16:281-289. [DOI] [PubMed] [Google Scholar]
- 14. Blasco A, Carmona M, Fernandez-Lozano I, et al. Evaluation of a telemedicine service for the secondary prevention of coronary artery disease. J Cardiopulm Rehabil Prev. 2012;32:25-31. [DOI] [PubMed] [Google Scholar]
- 15. Varnfield M, Karunanithi M, Lee CK, et al. Smartphone-based home care model improved use of cardiac rehabilitation in postmyocardial infarc-tion patients: results from a randomised controlled trial. Heart. 2014;100:1770-1779. [DOI] [PubMed] [Google Scholar]
- 16. Widmer RJ, Allison TG, Lennon R, Lopez-Jimenez F, Lerman LO, Lerman A. Digital health intervention during cardiac rehabilitation: a randomized controlled trial. Am Heart J. 2017;188:65-72. [DOI] [PubMed] [Google Scholar]
- 17. Widmer RJ, Allison TG, Lerman LO, Lerman A. Digital health intervention as an adjunct to cardiac rehabilitation reduces cardiovascular risk factors and rehospitalizations. J Cardiovasc Transl Res. 2015;8:283-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Johnston N, Bodegard J, Jerstrom S, et al. Effects of interactive patient smartphone support app on drug adherence and lifestyle changes in myocardial infarction patients: a randomized study. Am Heart J. 2016;178:85-94. [DOI] [PubMed] [Google Scholar]
- 19. Maddison R, Rawstorn JC, Stewart RAH, et al. Effects and costs of real-time cardiac telerehabilitation: randomised controlled non-inferiority trial. Heart. 2019;105:122-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fang J, Huang B, Xu D, Li J, Au WW. Innovative application of a home-based and remote sensing cardiac rehabilitation protocol in Chinese patients after percutaneous coronary intervention. Telemed J E Health. 2019;25:288-293. [DOI] [PubMed] [Google Scholar]
- 21. Dorje T, Zhao G, Tso K, et al. Smartphone and social media-based cardiac rehabilitation and secondary prevention in China (SMART-CR/SP): a parallel-group, single-blind, randomised controlled trial. Lancet Digit Health. 2019;1:e363-e374. [DOI] [PubMed] [Google Scholar]
- 22. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS MED. 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. van Tulder M, Furlan A, Bombardier C, Bouter L, Editorial Board of the Cochrane Collaboration Back Review G. Updated method guidelines for systematic reviews in the Cochrane Collaboration back review group. Spine (Phila Pa 1976). 2003;28:1290-1299. [DOI] [PubMed] [Google Scholar]
- 24. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539-1558. [DOI] [PubMed] [Google Scholar]
- 25. Rao G, Lopez-Jimenez F, Boyd J, et al. Methodological standards for meta-analyses and qualitative systematic reviews of cardiac prevention and treatment studies: a scientific statement from the American Heart Association. Circulation. 2017;136:e172-e194. [DOI] [PubMed] [Google Scholar]
- 26. Dorje T, Zhao G, Scheer A, et al. SMARTphone and social media-based cardiac rehabilitation and secondary prevention (SMART-CR/SP) for patients with coronary heart disease in China: a randomised controlled trial protocol. BMJ Open. 2018;8:e021908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Number of mobile phone users worldwide from 2016 to 2021. statista.com. Published 2019. Accessed December 3, 2019.
- 28. Lloyd-Jones D, Adams RJ, Brown TM, et al. Heart disease and stroke statistics—2010 update: a report from the American Heart Association. Circulation. 2009;121:e46-e215. [DOI] [PubMed] [Google Scholar]
- 29. Beatty AL, Schiller NB, Whooley MA. Six-minute walk test as a prognostic tool in stable coronary heart disease: data from the heart and soul study. Arch Intern Med. 2012;172:1096-1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Goertzen L, Halas G, Rothney J, et al. Mapping a decade of physical activity interventions for primary prevention: a protocol for a scoping review of reviews. JMIR Res Protoc. 2015;4:e91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ettehad D, Emdin CA, Kiran A, et al. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet. 2016;387:957-967. [DOI] [PubMed] [Google Scholar]
- 32. Yusuf S, Lonn E, Bosch J. Lipid lowering for primary prevention. Lancet. 2009;373:1152-1155. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, mHealth_Supplement for Efficacy of Smartphone-Based Secondary Preventive Strategies in Coronary Artery Disease by Alexandra C Murphy, Georgina Meehan, Anoop N Koshy, Phelia Kunniardy, Omar Farouque and Matias B Yudi in Clinical Medicine Insights: Cardiology