Abstract
Trigeminal neuralgia is a common neuropathic pain in the head and face. The pathogenesis of trigeminal neuralgia is complex, and so far, the pathogenesis of trigeminal neuralgia involving peripheral and central nervous inflammation theory has not been explained clearly. The loss of dopamine neurons in striatum may play an important role in the development of trigeminal nerve, but the reason is not clear. C-Abl is a nonreceptor tyrosine kinase, which can be activated abnormally in the environment of neuroinflammation and cause neuron death. We found that in the rat model of infraorbital nerve ligation trigeminal neuralgia, the pain threshold decreased, the expression of c-Abl increased significantly, the downstream activation product p38 was also activated abnormally and the loss of dopamine neurons in striatum increased. When treated with imatinib mesylate (STI571), a specific c-Abl family kinase inhibitor, the p38 expression was decreased and the loss of dopaminergic neurons was reduced. The mechanical pain threshold of rats was also improved. In conclusion, c-abl-p38 signaling pathway may play an important role in the pathogenesis of trigeminal neuralgia, and it is one of the potential targets for the treatment of trigeminal neuralgia.
Keywords: Trigeminal neuralgia, c-Abl, p38, dopamine neuron, neuroinflammation
Introduction
Trigeminal neuralgia (TN) is the most common facial neuralgia. TN is typically characterized by transient, intense electroshock pain, seizures and terminations that range from a few seconds to a few minutes at a time.1–3 Epidemiological studies show that the global incidence of TN is about 4 to 28.9/100,000, and TN patients had a significantly higher risk of depression and anxiety disorders,4 which seriously affects the quality of life of patients. Unfortunately, the pathogenesis of TN has not yet been elucidated.5
At present, the research shows that the pathogenesis of TN involves peripheral and central nerve sensitization as well as microglia abnormal activation.6 After peripheral nerve injury, reactive oxygen species (ROS), colony stimulating factor-1 (CSF-1), adenosine triphosphate (ATP) and other substances can be induced to release, leading to oxidative stress and other central neuroinflammatory cascade reactions.7,8 Studies have shown that oxidative stress can cause the loss of dopaminergic neurons in striatum. Strittmatter et al.9 and Dieb et al.10 both believe that the central mechanism of TN is related to the damage of dopaminergic neurons in striatum. Dopamine can regulate pain at different levels of the body and play a key role in the downward inhibition of pain.11–13 The decrease of dopamine level may lead to the occurrence of pain.14,15 Although dopaminergic neuron loss plays an important role in the development of TN, the specific mechanism is still unclear. C-Abl is a nonreceptor tyrosine kinase, which is closely related to neuroinflammation and neuronal death. C-Abl plays an important role in the process of neural development and maintains a relatively static state in highly differentiated neurons.16,17 Activated c-Abl has a variety of biological functions and participates in signal transduction of various signal pathways, including growth factor signal transduction, cell adhesion, oxidative stress and DNA damage response.18 Schlatterer16 observed that the overexpression of c-Abl can cause neuron loss and the occurrence of neuroinflammatory response in two kinds of transgenic mice (Ablpp/TTA and Argpp/TTA). It was observed that the activation of c-Abl under oxidative stress can mediate the loss of dopaminergic neurons in Parkinson’s disease (PD) animal model. Whether the activation of c-Abl mediates the loss of dopaminergic neurons to participate in the pathogenesis of TN has not been reported.
In this study, we found that c-Abl was activated abnormally in the rat model of TN after infraorbital nerve ligation, which promoted the loss of dopamine neurons in striatum by phosphorylation of p38 downstream. The inhibition of c-Abl expression by imatinib mesylate (STI571) can improve the pain threshold of TN rats and produce dopaminergic neuron protection. In conclusion, we found that c-Abl-p38 signaling may play an important role in the pathogenesis of TN.
Methods
Animals and groups
Sixty adult male SD rats were purchased from the animal center of Southwest Medical University (Luzhou, China). This experiment was approved by the ethics committee of the Affiliated Hospital of Southwest Medical University, strictly in accordance with the principles of animal ethical protection. Before the experimental group, the animals needed adaptive training for seven days, and those with pain threshold of more than or equal to 26 g for three consecutive days and complete hair and tentacles at the mouth and nose were randomly divided into Sham group: only the right infraorbital nerve was separated without ligation; ION-CCI group: infraorbital nerve chronic constriction injury (ION-CCI) was performed. ION-CCI+Saline group and ION-CCI+STI571 group were injected intraperitoneally with saline or STI571 after the successful establishment of the TN model.
Establishment of animal model of TN
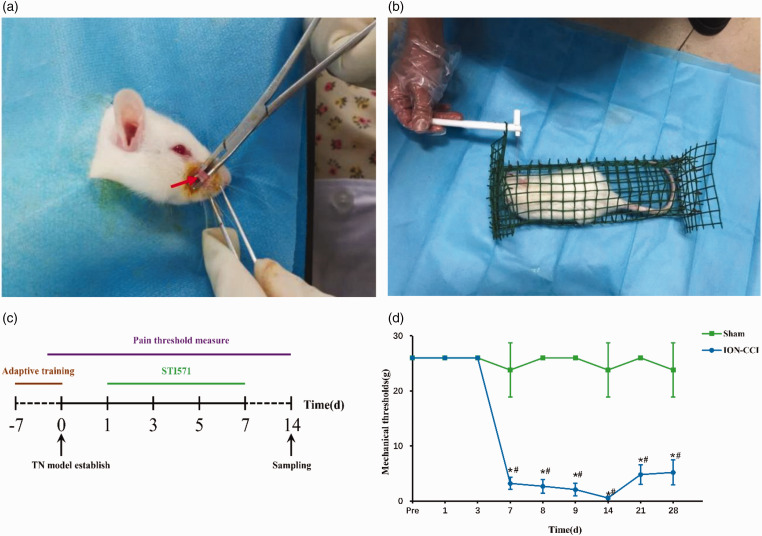
Rats were anesthetized by intraperitoneal injection of pentobarbital sodium (30 mg/kg). Blunt-separation of muscle peripheral fascia, until the exposure of infraorbital nerve, continues to separate the exposure of infraorbital foramen. The length of the suborbital nerve from the proximal part of the suborbital foramen to the distal part is about 4–5 mm. The nerve was ligated with two chrome gut wires (4–0) at a distance of about 2 mm between the proximal and distal ends (Figure 1(a)). It can be seen that the ligation line only slightly thinned the diameter of the infraorbital nerve but did not completely block its conduction and the blood circulation of the nerve adventitia.19 In the Sham operation group, only the infraorbital nerve was exposed but not ligated.
Figure 1.
(a) Infraorbital nerves were isolated during the establishment of the TN model. The arrows indicate the isolated orbital nerves, (b) measurement of mechanical pain threshold in rats by Von Frey filaments, (c) processing timeline of experimental animals plotted (made with Adobe-Photoshop-CC2019 software, color in RGB), (d) changes of mechanical pain threshold in rats of Sham group and ION-CCI group, data are shown as Mean ± SEM (SNK, *P < 0.05 indicates comparison with preoperative; ANOVA, #P < 0.05 indicates comparison with Sham group).
Mechanical pain threshold
The mechanical pain threshold was measured according to the method described in the previous study.19,20 Rats were fixed in a self-made cage, after the rats were acclimated and quiet, the subjects held the Von Frey filaments (the stimulation intensity was 0.008, 0.02, 0.04, 0.07, 0.16, 0.4, 0.6, 1.0, 1.4, 2.0, 4.0, 6.0, 8.0, 10.0, 15.0 and 26.0 g, respectively) to stimulate the right palpebral pad of rats for three times (Figure 1(b)). The stimulation standard is to make the filaments just bend. The thresholds of mechanical stimulation were recorded on the 1st, 3rd, 7th, 8th, 9th, 14th, 21st and 28th day after operation. The pain threshold was measured between 10:00 and 12:00. Any one of the reactions can be judged as positive: When there are at least three times of non-simultaneous scratching of the tentacle pad, sudden grasping and biting of the stimulator, quick curling of the body to the cage wall, hiding the head and face or turning the head to the other side to avoid stimulation. When the positive behavior is more than or equal to two times in three times of stimulation, the corresponding gram is the threshold of facial mechanical pain of rats. If the stimulation intensity is ≥26 g, 26 g was recorded.
Sampling and sample preparation
Fourteenth day after operation, 10 rats from each group were randomly selected and killed by pentobarbital sodium (30 mg/kg) for intraperitoneal injection anesthesia. After heart perfusion in phosphate buffer saline (PBS) (4°C, pH7.4), the brain was cut off, and the right striatum was quickly taken out in eppendorf (EP) tube. After grinding, the rat striatum was homogenated at 4°C to 13,000 r/min, centrifuged for 15 min, the supernatant and protein were measured by bicinchoninic acid (BCA) method and the supernatant was used to measure Western Blotting (n = 5). In addition, five rats in each group were perfused with 4% paraformaldehyde after heart perfusion in PBS (4°C, pH7.4). After perfusion, the brain was cutoff, and the whole brain was taken out and fixed in 4% paraformaldehyde for 24 h, dehydrated, soaked in wax, embedded and sliced continuously in coronal position with paraffin slicer. The slice thickness is 5 µm; then the slice is pasted on the adhesive glass slide, baked overnight at 60°C, taken out and stored at room temperature for standby (Figure 1(c)).
Western Blotting: Detection of c-Abl, p38, p-p38 and TH expression in striatum
The sections were dewaxed, rehydrated with gradient ethanol, and then distilled water was used. The sections were then placed in an antigen retrieval solution (generic powerful antigen retrieval solution, Shanghai Biyuntian, China) and incubated in a medium temperature water bath for 20 min. Once cooled to room temperature, the sections were removed with 0.2% Triton X-100, and the solution was broken (Amresco, USA) for 15 min. The sections were sealed in 1% BSA for 60 min and then incubated with primary antibody (rabbit anti-c-Abl polyclonal antibody, 1:100, American CST Company; rabbit anti-p38MAPK polyclonal antibody, 1:100, American CST Company; rabbit anti-p-p38MAPK polyclonal antibody, 1:100, American CST Company; rabbit anti-TH polyclonal antibody, 1:50, American Abcam company) overnight at 4°C. and with different fluorescently labeled secondary antibodies (1:100; Kangwei Century Biotechnology, China) for 1 h. Then the sections were contaminated with 4',6-diamidino-2-phenylindole (DAPI) (DAPI staining solution, Shanghai Biyuntian, China) for 5 min. The striatum (Olympus, Japan) was observed under a fluorescence microscope and an automatic camera system (magnification 400×).
Immunofluorescence: Detection of c-Abl, p38, p-p38 and TH expression and expression localization in striatum
The striatum tissue was taken out in a refrigerator (Panasonic, Japan) at −80°C, weighed and placed in a mill. Then it was homogenized in a RIPA Lysis buffer (RIPA) containing a protease inhibitor. RIPA protein lysate (Shanghai Biyuntian, China): Protease inhibitor phenylmethylsulfonyl fluoride (PMSF) (100:1; Shanghai Biyuntian, China). When extracting p-p38 protein, a phosphorylase inhibitor (Solarbio, USA) needs to be added (RIPA protein lysate: protease inhibitor PMSF: phosphorylase inhibitor = 100:1:1) and then the above mixture was added to the tissue in the mill; the tissue was milled up and down 10 times, placed on ice for half an hour to make the two fully function, and then 3K15 high speed low temperature centrifuge was used (Sigma, USA) to centrifuge (4°C, 14,000 r/min, 15 min). The concentration of the protein extract was determined using a BCA protein detection kit (Shanghai Biyuntian, China). The transfer membrane buffer solution was prepared according to the conventional formula and pre-cooled in a refrigerator at 4°C to avoid the heat generated during the membrane transfer process from causing the gel to shrink and cause the transfer band to deform. An equal amount of protein (10 µg) was separated by electrophoresis and transferred to a polyvinylidene fluoride (PVDF) membrane (Millipore company, USA) using a Western Blot vertical electrophoresis and transfer membrane system (Bio-Rad company, USA). At room temperature, the membrane was blocked with 5% albumin from bovine serum (BSA) for 2 h and incubated at room temperature overnight at 4°C. Main antibodies include: rabbit anti-c-Abl polyclonal antibody, rabbit anti-p38MAPK polyclonal antibody, rabbit anti-p-p38MAPK polyclonal antibody (1:1000; American CST Company, USA), rabbit anti-TH polyclonal antibody (1:1000; Abcam, USA) and rabbit-derived GAPDH monoclonal antibody (1:5000; Proteintech, USA). The membrane was washed in Tris Buffered Saline Tween (TBST) and incubated with hrp-labeled goats for 1 h at room temperature with goat anti-rabbit IgG (1:5000; Proteintech, USA). Protein levels were enhanced by chemiluminescence (Biyuntian Company, China) and automatic radiography (Electrophoresis gel image analyzer, Bio-Rad Corporation, USA). Finally, the obtained bands were analyzed by Image J software, and the band gray value was compared with the internal reference band GAPDH for statistical analysis. The number of TH-positive cells was counting by Image J software.
Statistical analysis
SPSS21.0 statistical software was used for processing. The change of pain threshold before and after operation at each time point, c-Abl, TH, p38, p-p38 expression and TH-positive neurons count in each group were expressed as means ± SEM. Differences in pain threshold, c-Abl, TH, expression and TH-positive neurons count between groups were analyzed by one-way analysis of variance (ANOVA). Pairwise comparisons between time points were performed by the Student–Newman–Keuls (SNK) test, and protein comparisons among multiple groups were performed by the SNK test. P < 0.05 means statistically significant.
Results
The expression of c-Abl was increased, and the loss of dopaminergic neurons was increased in TN rats
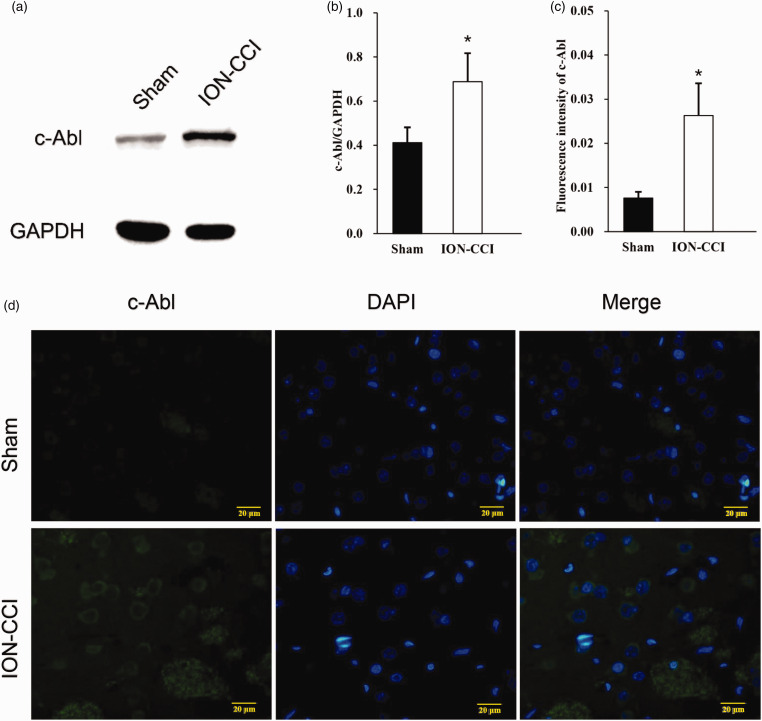
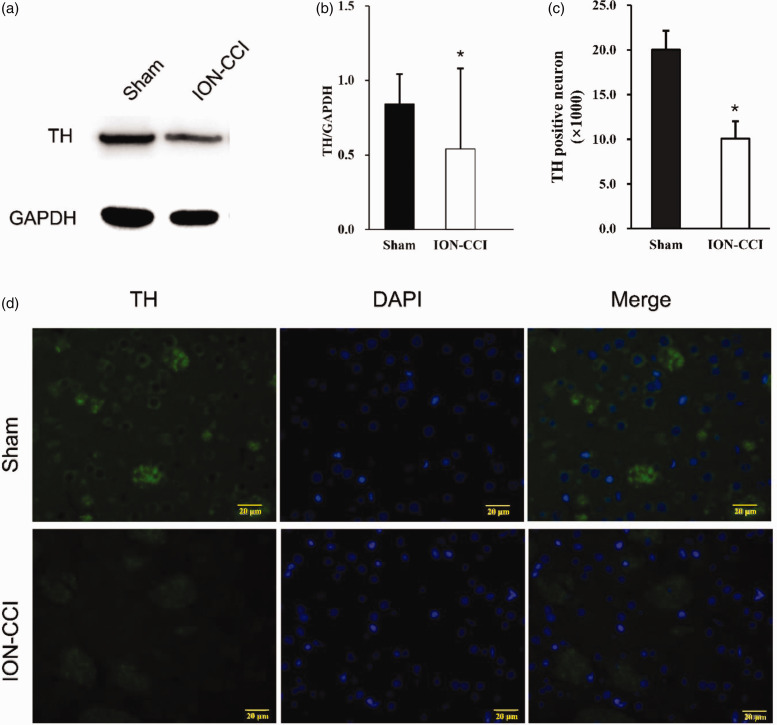
In order to verify whether c-Abl promotes the death of dopamine neurons and participates in the pathogenesis of TN, we established a rat infraorbital nerve ligation model, measured the change of pain threshold, the expression of c-Abl and TH in the right striatum 14 days after modeling by Western blot and Immunofluorescence. After the establishment of the model, the pain threshold of ION-CCI group was significantly lower than that of Sham group at the 7th day after operation, and reached the lowest level at the 14th day. And then, the pain threshold was slightly improved, but still significantly lower than that of sham group (Figure 1(d)). Western blotting showed that the expression level of c-Abl in the right striatum of ION-CCI group rats increased nearly 1.7 times (Figure 2(a) and (b)), and immunofluorescence also showed similar results (Figure 2(c)). The expression site was mainly in the striatum specific patch structure (Figure 2(d)). At the same time, the expression of TH in the striatum of rats in ION-CCI group decreased significantly (Figure 3(a) and (b)). The results of immunofluorescence at 14th day after operation showed that the TH-positive neurons in the right striatum of rats with infraorbital nerve ligation decreased significantly (Figure 3(c) and (d)).
Figure 2.
(a) On the 14th day after surgery, the expression of c-Abl in the right striatum of the Sham group and ION-CCI group rats was detected by Western blotting, (b) Western blot results of c-Abl in Sham and IONCCI group based on quantification using Image J software, data are shown as Mean ± SEM (ANOVA, *P < 0.05 indicates comparison with Sham group), (c) semi-quantitative of fluorescence intensity results of c-Abl in Sham and ION-CCI group based on quantification using Image J software, data are shown as Mean ± SEM (ANOVA, *P < 0.05 indicates comparison with Sham group), (d) C-Abl expression of Sham and IONCCI group (the green fluorescence represents the c-Abl protein, and the blue fluorescence represents DAPI, 400×).
Figure 3.
(a) On the 14th day after surgery, the expression of c-Abl in the right striatum of the Sham group and ION-CCI group rats was detected by Western blotting, (b)Western blot results of TH protein in Sham group and ION-CCI group based on quantification using Image J software, showed as Mean ± SEM (ANOVA, *P < 0.05 indicates comparison with Sham group), (c) TH-positive neurons in Sham and ION-CCI group based on quantification using Image J software, showed as Mean ± SEM (ANOVA, *P < 0.05 indicates comparison with Sham group), (d) TH expression of Sham and ION-CCI group (the green fluorescence represents the TH protein, and the blue fluorescence represents DAPI, 400×).
C-Abl inhibitor STI571 can reduce the loss of dopaminergic neurons and improve the mechanical pain threshold of TN rats
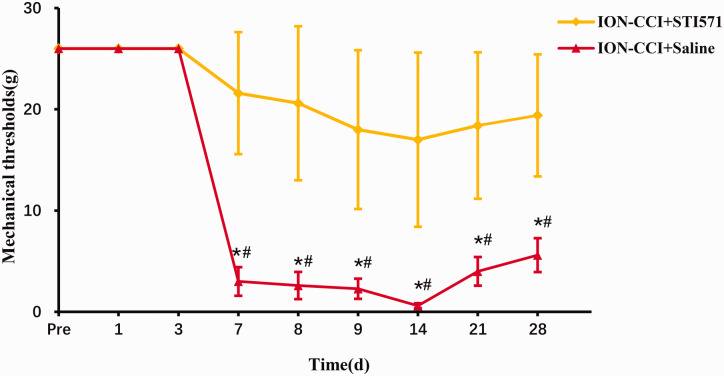
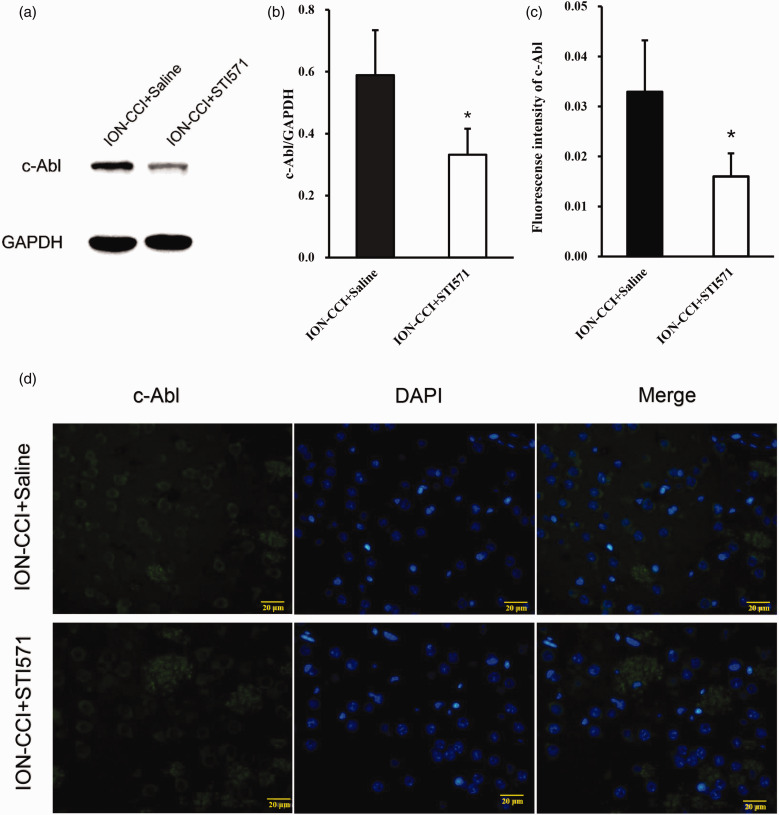
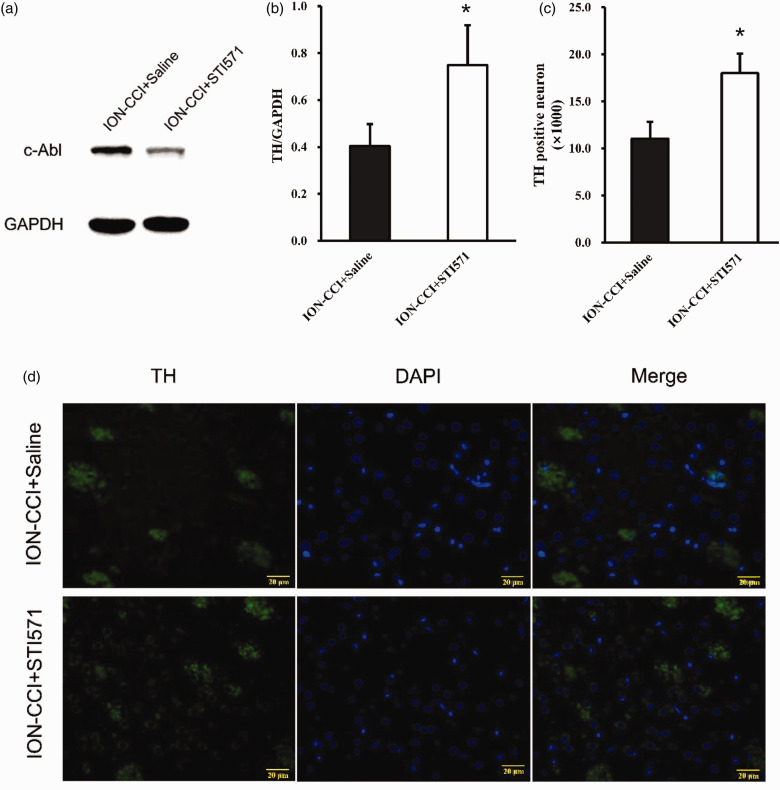
Further to verify whether c-Abl causes the loss of dopaminergic neurons in the TN model, we injected saline or STI571 into the abdominal cavity of the two groups of TN model rats on the 1st, 3rd, 5th and 7th days after the establishment of the TN model, respectively. STI571 was dissolved in normal saline, with a concentration of 6 mg/ml for standby, and the dose of intraperitoneal injection was 30 mg/kg as Schindler reported.17 The results of the pain threshold of the ION-CCI+Saline group and ION-CCI-STI571 group showed that the pain threshold of the STI571 group was significantly higher than that of the saline group on the 7th day after surgery and reached a minimum after 14th day, but still significantly higher than that of the saline group (Figure 4). In addition, 14 days after the operation, Western blotting and immunofluorescence were performed on the right striatum of rats. STI571 significantly reduced the expression of c-Abl (Figure 5(a)–(d)). The results of western blotting showed that TH expression increased (Figure 6(a) and (b)) after STI571 treatment and the fluorescence showed that dopamine neuron loss decreased (Figure 6(c) and (d)). C-Abl inhibitor can produce significantly protective effect on dopamine neurons in TN rats. These results further prove that c-Abl indeed mediates the loss of dopamine neurons in TN rats and plays a key role in the maintenance of pain. Therefore, specific inhibition or block of c-Abl expression in patients with TN may be one of the effective ways to treat it.
Figure 4.
Changes of mechanical pain threshold in rats of ION-CCI+Saline and ION-CCI+STI571 group, data are shown as Mean ± SEM (SNK, *P < 0.05 indicates comparison with preoperative; ANOVA, #P < 0.05 indicates comparison with ION-CCI+Saline group).
Figure 5.
(a) On the 14th day after surgery, the expression of c-Abl in the right striatum of the ION-CCI+Saline and ION-CCI+STI571 group rats was detected by Western blotting, (b) saline or STI571 was injected into the abdominal cavity of the two groups of TN model rats on the 1st, 3rd, 5th and 7th days after the establishment of the TN model, respectively (STI571, 30 mg/kg). Western blot results of c-Abl in IONCCI+Saline and ION-CCI+STI571 group based on quantification using Image J software, showed as Mean ± SEM (ANOVA, *P < 0.05 indicates comparison with ION-CCI+Saline), (c) semi-quantitative of fluorescence intensity results of c-Abl in ION-CCI+Saline and ION-CCI+STI571 group based on quantification using Image J software, data are shown as Mean ± SEM (ANOVA, *P < 0.05 indicates comparison with IONCCI+Saline), (d) C-Abl expression in ION-CCI+Saline and ION-CCI+STI571 group (the green fluorescence represents the c-Abl protein, the blue fluorescence represents DAPI, 400×).
Figure 6.
(a) On the 14th day after surgery, the expression of TH in the right striatum of the ION-CCI+Saline and ION-CCI+STI571 group rats was detected by Western blotting, (b) Western blot results of TH in IONCCI+Saline and ION-CCI+STI571 group based on quantification using Image J software, showed as Mean ± SEM (ANOVA, *P < 0.05 indicates comparison with ION-CCI+Saline), (c) TH-positive neurons in IONCCI+Saline group and ION-CCI+STI571 group based on quantification using ImageJ software, data are shown as Mean ± SEM (ANOVA, *P < 0.05 indicates comparison with ION-CCI+Saline), (d) TH expression in ION-CCI+Saline and ION-CCI+STI571 group (the green fluorescence represents the TH, and the blue fluorescence represents DAPI, 400×).
C-Abl may be involved in the pathogenesis of TN by activating p38 and leading to the loss of dopamine neurons
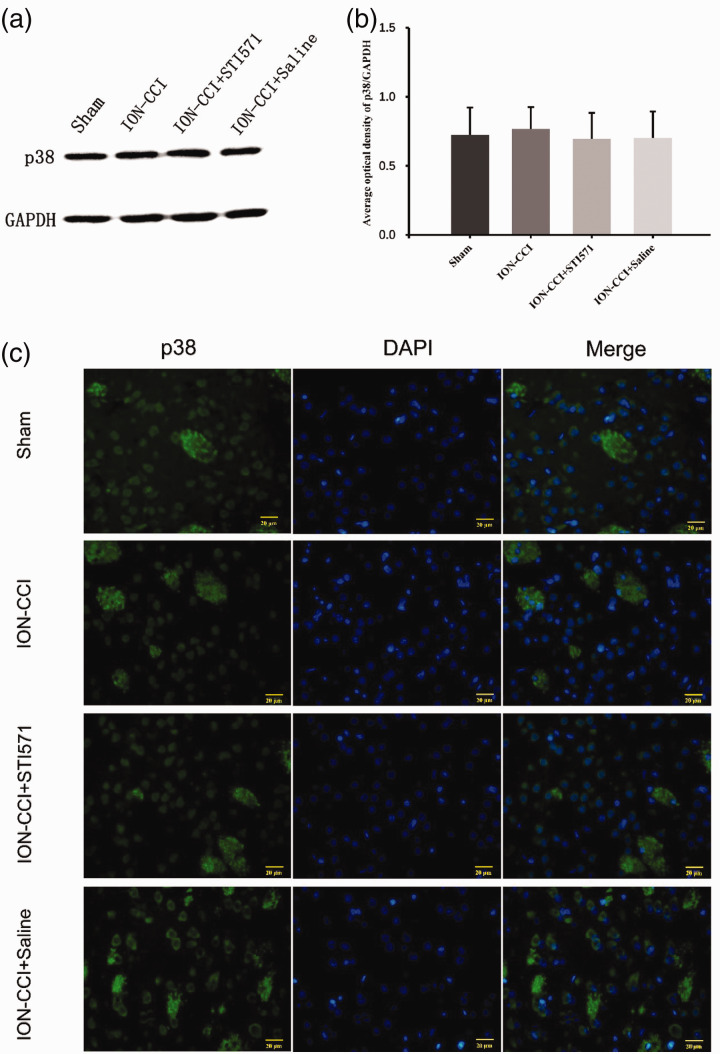
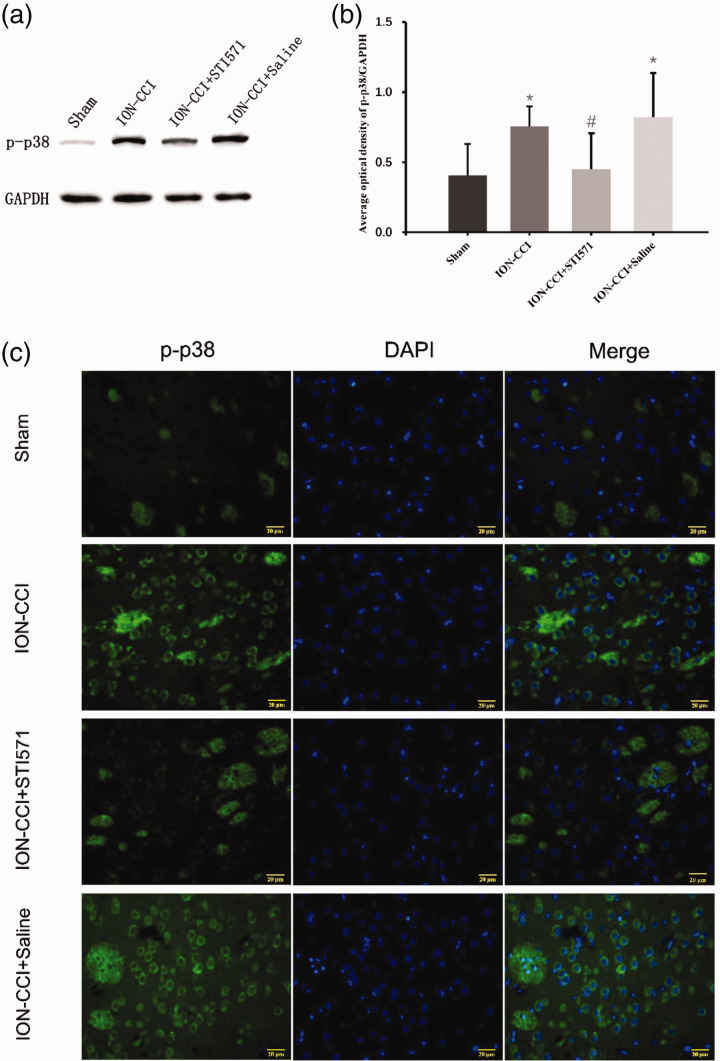
Based on the above results, we further explored how c-Abl caused the loss of dopaminergic neurons in TN rats. Wu et al.21 reported that since c-Abl is a tyrosine kinase, its substrate can be determined by SILAC technology to recognize tyrosine phosphorylated peptide, and finally c-Abl can activate p38 specifically by tyrosine182 (Y182) and tyrosine323 (Y323). Combined with the characteristics of p38 inducing neural cell death,22,23 we further detected the expression of p38 and phosphorylated p38 (p-p38) in the right striatum of TN model rats. The results showed that there was no difference in p38 expression between four groups (Figure 7(a)–(c)), while its activation product p-p38 expression in striatum of ION-CCI group was significantly increased compared with Sham group, and its expression site was similar in c-Abl, suggesting that there might be a specific relationship between them. We further found that the p-p38 of TN rats using STI571 decreased significantly (Figure 8(a)–(c)). These results show that c-Abl mediates the loss of dopaminergic neurons in the striatum of TN rats by activating p38. However, the molecular mechanism of p38 activation by c-Abl in TN rat model needs further explore. To further explore the role of c-Abl-p38 in TN under the condition of knocking out p38 gene may be the direction of our further research.
Figure 7.
(a) On the 14th day after surgery, the expression of p38 in the right striatum of the four groups of rats was detected by Western blotting, (b) Western blot results of p38 protein in four groups based on quantification using Image J software, showed as Mean ± SEM (SNK, no statistical difference between the four groups), (c) p38 expression in four groups (the green fluorescence represents the p38, and the blue fluorescence represents DAPI, 400×).
Figure 8.
(a) On the 14th day after surgery, the expression of p-p38 in the right striatum of the four groups of rats was detected by Western blotting, (b) Western blot results of p-p38 protein in four groups based on quantification using Image J software, showed as Mean ± SEM (SNK, *P < 0.05 indicates comparison with Sham group; #P < 0.05 indicates comparison with ION-CCI+Saline group), (c) p-p38 expression in four groups (the green fluorescence represents the p38 and the blue fluorescence represents DAPI, 400×).
Discussion
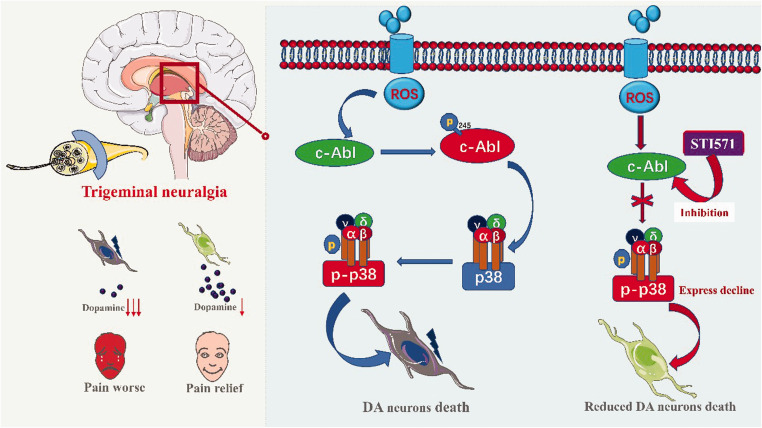
The central mechanism of TN is related to the damage of dopamine neurons in the substantia striatum, and the decrease of dopamine production leads to the decrease of its anti-injury sensitivity.9,10 Watanabe et al.24 believe that the maintenance of neuropathic pain is related to the decrease of dopaminergic neurons. In this study, we found that the c-Abl-p38 signaling pathway mediates the loss of dopaminergic neurons in the striatum of TN rats to participate in the occurrence and development of TN (Figure 9). At first, the expression of c-Abl was increased in TN rats, and activation of p38 resulted in the decrease of TH expression in the striatum of TN rats. Second, the protective effect of c-Abl inhibitor STI571 on dopaminergic neurons in TN rats was better, and the pain threshold of TN rats was significantly improved. These results suggest that c-Abl-p38 plays an important role in the pathogenesis of TN and provide a new basis for clarifying the pathogenesis of TN. C-Abl-p38 may be one of the important targets in the treatment of TN.
Figure 9.
Based on our results, the mechanism of the c-Abl-p38 pathway in TN plotted (made with Adobe Illustrator-CC-2019 software, color in RGB).
In our study, we copied the TN model established by Love et al.,25 which simulates the clinical trigeminal ganglion vascular compression theory, and currently more modeling methods are used. The pain threshold of rats with suborbital nerve ligation decreased significantly 7th day after operation, the lowest 14th day after operation, and then slightly improved, which is considered to be the most serious cause of myelin sheath injury during 7–14 days after operation.26 On the 14th day after operation, the pathological study of TN rats showed that there were obvious changes of myelinated fiber demyelination in the compressed area, and at the same time, the pain threshold of the animals decreased to the lowest level, which was similar to the TN and its pathological changes observed in clinic.27 Therefore, we choose to detect the expression of related indexes in striatum 14 days after operation, which has better representativeness.
Activation of c-Abl under oxidative pressure was first reported in the pathogenesis of chronic myeloid leukemia.28 Later, scholars found that in PD, abnormal activation of c-Abl played an important role in the death of dopaminergic neurons, which was related to the occurrence of pain in PD patients.21 STI571 can specifically enter the striatum to inhibit c-Abl but has little effect on other neurons.17,29 C-Abl inhibition can effectively reduce the loss of dopaminergic neurons in PD model. It is suggested that c-Abl may also be activated abnormally in neuropathic pain and participate in the occurrence and maintenance of pain. The abnormal expression of c-Abl in TN rats was confirmed by our experiment. The possible reason is that a large number of ROS, CSF-1 and ATP were released after peripheral nerve injury, which caused abnormal activation of c-Abl under oxidative stress. Furthermore, we can study the specific mechanism of inducing abnormal activation of c-Abl in TN model. P38 is a member of mitogen-activated protein kinase (MAPKs) family, which plays an important role in regulating inflammation, neurodegeneration and cell death.30 In general, p38 is activated by MAPK kinases such as MKK3 or MKK6 in the upstream,31 and p38 is closely related to neuropathic pain.32 Wu et al.21 found that p38 can be activated abnormally by c-Abl in the neuroinflammatory environment, thus causing neuron death. C-Abl inhibitor can effectively reduce the expression of p-p38 and produce the protective effect of dopaminergic neurons. Immunofluorescence showed that p-p38 was similar to c-Abl and TH, which further indicated that c-Abl mediated the loss of dopaminergic neurons in the striatum of TN rats by activating p38. In addition, as is well known, some inhibitory neurons get lost under chronic pain states, which in turn contributes to pain chronicity via loss of inhibition,33 whether the c-Abl-p38 pathway is also involved in this mechanism may be the next research direction.
Our results suggest that c-abl-p38 may be an important target for the treatment of TN. At present, c-Abl inhibitors, including STI571, have shown good therapeutic effects in animal models, but they generally have less dose into the striatum. Therefore, the development of blocking drugs with higher efficiency through the blood–brain barrier will be more conducive to the treatment of neuropathic pain such as TN.
Conclusion
In conclusion, this study confirmed that c-Abl plays an important role in the loss of dopaminergic neurons in TN rats. The possible mechanism is that c-Abl participates in the occurrence and development of TN by activating p38.
Author Contributions
JF designed the study, performed experiments, analyzed data and prepared figures. GM designed the study, performed experiments, prepared figures, prepared and revised the manuscript. LQ and JZ performed part of the experiments and analyzed data. CO designed the study, accepts responsibility for conduct of research and final approval.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work has been supported by Sichuan Provincial Health and Family Planning Commission (16PJ567).
ORCID iDs
References
- 1.Sindou M, Brinzeu A. Topography of the pain in classical trigeminal neuralgia: insights into somatotopic organization. Brain 2020; 143: 531–540. [DOI] [PubMed] [Google Scholar]
- 2.Moore D, Chong MS, Shetty A, Zakrzewska JM. A systematic review of rescue analgesic strategies in acute exacerbations of primary trigeminal neuralgia. Br J Anaesth 2019; 123: e385–e396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang WB, Zeng YY, Chang BW, Min LZ, Sun QY, Li B, Tao BB, Wang XQ. Prognostic nomogram for microvascular decompression-treated trigeminal neuralgia. Neurosurg Rev 2020; DOI: 10.1007/s10143-020-01251-0. [DOI] [PubMed]
- 4.Zakrzewska JM, Linskey ME. Trigeminal neuralgia. Am Fam Physician 2016; 94: 133–135. [PubMed] [Google Scholar]
- 5.Montano N, Conforti G, Di Bonaventura R, Meglio M, Fernandez E, Papacci F. Advances in diagnosis and treatment of trigeminal neuralgia. Ther Clin Risk Manag 2015; 11: 289–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ji RR, Chamessian A, Zhang YQ. Pain regulation by nonneuronal cells and inflammation. Science 2016; 354: 572–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guo R, Chen LH, Xing C, Liu T. Pain regulation by gut microbiota: molecular mechanisms and therapeutic potential. Br J Anaesth 2019; 123: 637–654. [DOI] [PubMed] [Google Scholar]
- 8.Inoue K, Tsuda M. Microglia in neuropathic pain: cellular and molecular mechanisms and therapeutic potential. Nat Rev Neurosci 2018; 19: 138–152. [DOI] [PubMed] [Google Scholar]
- 9.Strittmatter M, Grauer M, Isenberg E, Hamann G, Fischer C, Hoffmann KH, Blaes F, Schimrigk K. Substance P, somatostatin and monoaminergic transmitters in the cerebrospinal fluid of patients with chronic idiopathic trigeminal neuralgia. Schmerz 1996; 10: 261–268. [DOI] [PubMed] [Google Scholar]
- 10.Dieb W, Ouachikh O, Durif F, Hafidi A. Lesion of the dopaminergic nigrostriatal pathway induces trigeminal dynamic mechanical allodynia. Brain Behav 2014; 4: 368–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wawrzczak-Bargieła A, Ziółkowska B, Piotrowska A, Starnowska-Sokół J, Rojewska E, Mika J, Przewłocka B, Przewłocki R. Neuropathic pain dysregulates gene expression of the forebrain opioid and dopamine systems. Neurotox Res 2020; 37: 800–814. DOI: 10.1007/s12640-020-00166-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Serafini RA, Pryce KD, Zachariou V. The mesolimbic dopamine system in chronic pain and associated affective comorbidities. Biol Psychiatry 2020; 87: 64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grenier P, Mailhiot MC, Cahill CM, Olmstead MC. Blockade of dopamine D1 receptors in male rats disrupts morphine reward in pain naïve but not in chronic pain states. J Neurosci Res 2019. DOI: 10.1002/jnr.24553. [DOI] [PubMed]
- 14.Vegas-Suarez S, Paredes-Rodriguez E, Aristieta A, Lafuente JV, Miguelez C, Ugedo L. Dysfunction of serotonergic neurons in Parkinson’s disease and dyskinesia. Int Rev Neurobiol 2019; 146: 259–279. [DOI] [PubMed] [Google Scholar]
- 15.Jarcho JM, Mayer EA, Jiang ZK, Feier NA, London ED. Pain, affective symptoms, and cognitive deficits in patients with cerebral dopamine dysfunction. Pain 2012; 153: 744–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schlatterer SD, Tremblay MA, Acker CM, Davies P. Neuronal c-Abl overexpression leads to neuronal loss and neuroinflammation in the mouse forebrain. J Alzheimers Dis 2011; 25: 119–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schindler T, Bornmann W, Pellicena P, Miller WT, Clarkson B, Kuriyan J. Structural mechanism for STI-571 inhibition of abelson tyrosine kinase. Science 2000; 289: 1938–1942. [DOI] [PubMed] [Google Scholar]
- 18.Gonfloni S, Maiani E, Di Bartolomeo C, Diederich M, Cesareni G. Oxidative stress, DNA damage, and c-Abl signaling: at the crossroad in neurodegenerative diseases. Int J Cell Biol 2012; 2012: 683097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xia L, Liu MX, Zhong J, Dou NN. Pain threshold monitoring during chronic constriction injury of the infraorbital nerve in rats. Br J Neurosurg 2019; 33: 409–412. [DOI] [PubMed] [Google Scholar]
- 20.Stern E, Forsythe AB, Youkeles L, Dixon WJ. A cytological scale for cervical carcinogenesis. Cancer Res 1974; 34: 2358–2361. [PubMed] [Google Scholar]
- 21.Wu R, Chen H, Ma J, He Q, Huang Q, Liu Q, Li M, Yuan Z. c-Abl-p38α signaling plays an important role in MPTP-induced neuronal death. Cell Death Differ 2016; 23: 542–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin X, Wang M, Zhang J, Xu R. p38 MAPK: a potential target of chronic pain. Curr Med Chem 2014; 21: 4405–4418. [DOI] [PubMed] [Google Scholar]
- 23.Zhang L, Stüber F, Lippuner C, Schiff M, Stamer UM. ERK and p38 contribute to the regulation of nociceptin and the nociceptin receptor in human peripheral blood leukocytes. Mol Pain 2019; 15: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Watanabe M, Narita M, Hamada Y, Yamashita A, Tamura H, Ikegami D, Kondo T, Shinzato T, Shimizu T, Fukuchi Y, Muto A, Okano H, Yamanaka A, Tawfik V L, Kuzumaki N, Navratilova E, Porreca F, Narita M. Activation of ventral tegmental area dopaminergic neurons reverses pathological allodynia resulting from nerve injury or bone cancer. Mol Pain 2018; 14: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Love S, Coakham HB. Trigeminal neuralgia: pathology and pathogenesis. Brain 2001; 124: 2347–2360. [DOI] [PubMed] [Google Scholar]
- 26.Liu CY, Lu ZY, Li N, Yu LH, Zhao YF, Ma B. The role of large-conductance, calcium-activated potassium channels in a rat model of trigeminal neuropathic pain. Cephalalgia 2015; 35: 16–35. [DOI] [PubMed] [Google Scholar]
- 27.Devor M, Govrin-Lippmann R, Rappaport ZH. Mechanism of trigeminal neuralgia: an ultrastructural analysis of trigeminal root specimens obtained during microvascular decompression surgery. J Neurosurg 2002; 96: 532–543. [DOI] [PubMed] [Google Scholar]
- 28.Marley SB, Deininger MW, Davidson RJ, Goldman JM, Gordon MY. The tyrosine kinase inhibitor STI571, like interferon-alpha, preferentially reduces the capacity for amplification of granulocyte-macrophage progenitors from patients with chronic myeloid leukemia. Exp Hematol 2000; 28: 551–557. [DOI] [PubMed] [Google Scholar]
- 29.Dai H, Marbach P, Lemaire M, Hayes M, Elmquist WF. Distribution of STI-571 to the brain is limited by P-glycoprotein-mediated efflux. J Pharmacol Exp Ther 2003; 304: 1085–1092. [DOI] [PubMed] [Google Scholar]
- 30.Chen Y, Li G, Huang LY. p38 MAPK mediates glial P2X7R-neuronal P2Y1R inhibitory control of P2X3R expression in dorsal root ganglion neurons. Mol Pain 2015; 11: 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mittelstadt PR, Yamaguchi H, Appella E, Ashwell JD. T cell receptor-mediated activation of p38α by mono-phosphorylation of the activation loop results in altered substrate specificity. J Biol Chem 2009; 284: 15469–15474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kiyomoto M, Shinoda M, Honda K, Nakaya Y, Dezawa K, Katagiri A, Kamakura S, Inoue T, Iwata K. p38 phosphorylation in medullary microglia mediates ectopic orofacial inflammatory pain in rats. Mol Pain 2015; 11: 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hara R, Asaoka Y, Takahashi D, Nomura H, Amano T, Minami M. Disappearance of the inhibitory effect of neuropeptide Y within the dorsolateral bed nucleus of the stria terminalis in rats with chronic pain. Neurosci Lett 2020; 728: 134958 DOI: 10.1016/j.neulet.2020.134958. [DOI] [PubMed] [Google Scholar]