Abstract
Aims:
The objective of this study was to investigate the outcomes of leflunomide (LEF) compared with those of cyclophosphamide (CYC) as induction against active Takayasu’s arteritis (TA) in Chinese patients.
Methods:
This was an observational study based on a prospective cohort that included TA patients diagnosed in large third-level first-class general hospitals in East China from January 2009 to September 2018. LEF- or CYC-induced active patients were enrolled for comparative effectiveness analysis. One-to-more paired cohorts of LEF versus CYC were derived by propensity-score matching (PSM). The primary outcome was complete remission (CR) at 9-month follow up, and secondary endpoints included partial remission (PR) and effectiveness rate (ER). Multivariable logistic regression was used to identify statistical significance.
Results:
A total of 131 enrolled patients with at least 3-months treatment included 53 receiving a regimen of glucocorticoid (GC) and LEF and 78 receiving GC and CYC. Compared with the CYC group, the LEF group showed higher CR rate {LEF versus CYC: 84.6% [95% confidence interval (CI) 74.5–94.8%] versus 59.0% (47.8–70.1%); relative risk (RR) = 0.3 (0.1–0.6), p = 0.002} and lower daily GC dose [10.0 (5.0–12.5) versus 12.5 (10.0–15.0) mg, p = 0.043] at the end of the 9-month induction. In the matched analysis, the LEF group (n = 23) still indicated a higher CR rate than the CYC group (n = 54) after PSM [RR = 0.1 (0.0–0.6), p = 0.003]. Four LEF-treated patients had mild side effects, and one died unrelated to LEF.
Conclusion:
LEF could be an alternative induction therapy against TA, showing good effectiveness and tolerance compared with CYC.
Keywords: leflunomide, cyclophosphamide, Takayasu’s arteritis, treatment
Introduction
Takayasu’s arteritis (TA) is a type of large-vessel vasculitis involving the aorta and its branches, and is characterized by granulomatous inflammation.1 TA prevalence in Asian countries has been estimated 12.9–40.0 cases per million hospital-based population.2,3 Persistent inflammation in full-thickness vessel walls often leads to stenosis and/or occlusion of local involved arteries, which induces ischemic manifestations as well as organ dysfunction.4,5 Thus, timely and effective anti-inflammatory treatment is essential to control the inflammatory response, delay or prevent TA progression, and to improve long-term prognosis.
Standards or guidelines for TA treatment are lacking. Glucocorticoids (GC) have been considered to be the mainstay for induction therapy against TA. A Chinese questionnaire-based investigation on TA management indicated that, according to 83% of rheumatologists, GC should be administrated initially.6 However, 72% of TA patients undergoing GC mono-therapy experienced recurrence within 6 months after tapering the prednisolone dose to <10 mg/day.7 Conventional immunosuppressant (IS) and biologic agents combined with GC have been shown to induce rapid remission and successful GC reduction.8–11
Cyclophosphamide (CYC) is a commonly used IS for TA induction, and a remission rate of 40.0–82.1% has been reported upon its use in different populations.12–15 In China, CYC has been chosen as first-choice IS by 78% of rheumatologists, and combination of GC and CYC remains the most preferred regimen for induction therapy, particularly in patients with important organs involved.6 However, radiologic improvements do not match clinical remission.16 Moreover, the side effects of gonadal and reproductive toxicity have been observed more commonly than hemorrhagic cystitis in Asians upon CYC use.15 This factor should be taken into consideration if prescribing CYC to women of childbearing-age suffering from TA.
Leflunomide (LEF) is another type of IS that inhibits pyrimidine biosynthesis, activation of the nuclear factor-kappa B (NF-κB) signaling pathway, as well as production of pro-inflammatory cytokines such as interleukin (IL)-1, IL-17, and adhesion molecules.17–19 LEF has been successfully used as induction against rheumatoid arthritis, lupus nephritis, and giant cell arteritis.20–23 Moreover, LEF has been shown to protect the fertility of female patients with lupus nephritis.22 With regard to TA, LEF has been thought to be a second-line IS because of uncertainty around its efficacy and safety in induction. A small-sample open-label study of 15 TA patients from South America showed the promising response of LEF in induction, and good safety.24,25 Treated with LEF of 20 mg/day, 12 patients (80%) reached clinical response after 9.1 months and 5 (41.6%) achieved sustained remission after 43.0 ± 7.6 months follow up.24,25 Seven patients discontinued LEF treatment including six relapses and one side effect.25 Thus, LEF might be an alternative to CYC as induction against TA.
In this study, we aimed to investigate the effectiveness and safety of LEF as induction therapy for Chinese patients with TA.
Materials and methods
Study design
This was a retrospective observational study. We aimed to investigate effectiveness of LEF compared with that of CYC as induction in patients with active TA, using data from the East China TA (ECTA) cohort. The prospective cohort has been established by the ECTA collaboration group since 2009, which consists of hospital-based centers in East China and group leader of Zhongshan Hospital in Shanghai. The protocols for the ECTA cohort have been approved by the Ethics Committee of Zhongshan Hospital affiliated to Fudan University (B2016-168), and conform to the Declaration of Helsinki. All patients provided written informed consent before entrance into the ECTA cohort.
All eligible patients were enrolled in the ECTA cohort according to the classification criteria by the 1990 American College of Rheumatology for TA.26 Disease activity was assessed using the criteria of the National Institutes of Health (NIH) (Bethesda, MD, USA).5 Angiographic types were judged by the Numano classification.27 Patients enrolled from 1 January 2009 to 31 December 2015 were treated with a regimen of GC and CYC, while patients enrolled from 1 January 2016 to the present date received a revised regimen of GC and LEF. Prednisone was initiated (0.8–1.0 mg/kg/day, p.o.) for 4 weeks in induction, and tapered gradually to a small dose of 0.1–0.2 mg/kg/day within the next 5 months. CYC (0.5–0.75 g/m2, i.v.gtt) was given every 4 weeks. LEF was administered at 20 mg/day, p.o. The duration of induction was 9 months.
Participant enrollment
By 30 September 2018, 627 TA patients had been enrolled in the ECTA cohort. Inclusion criteria were patients: (a) with TA in the active phase (NIH score ⩾2); (b) who had undergone induction therapy of GC combined with LEF or CYC; (c) who had not been exposed to any other IS in the past 3 months. After screening by inclusion criteria, 488 patients were excluded, including inactive patients (n = 149) and active patients who received neither LEF nor CYC, nor any IS concomitantly within 3 months (n = 339). Among these patients with active TA, 44 obtained monotherapy of GC and others received combined therapy of GC and IS (hydroxychloroquine 121, methotrexate 45, azathioprine 30, thalidomide 27, mycophenolate mofetil 19, cyclosporine 4, tacrolimus 2, tocilizumab 21, etanercept 6, infliximab 4, adalimumab 2, rituximab 2).
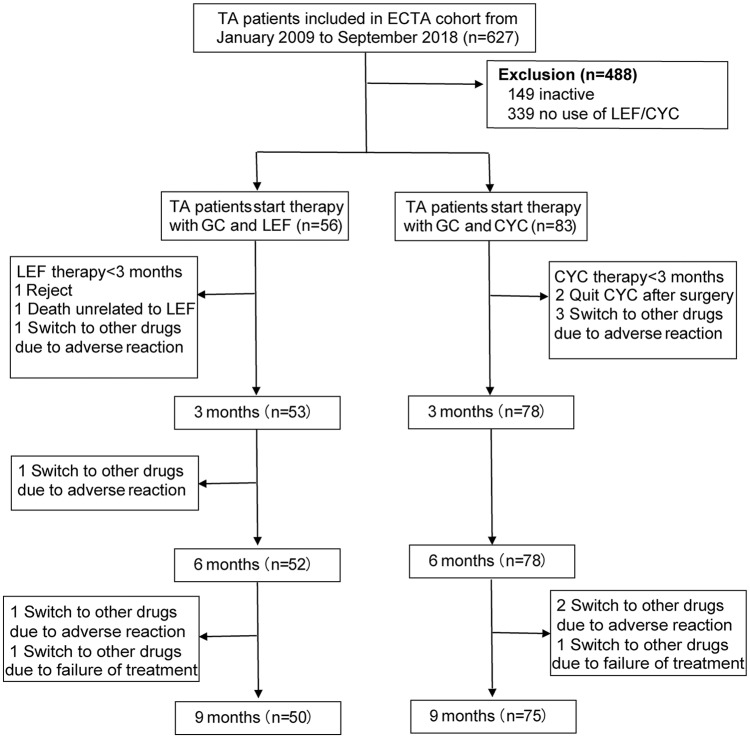
A total of 139 patients were eligible and recruited into our study: 56 patients in the LEF group and 83 in the CYC group. The number of patients treated with LEF and CYC was 53 and 78 by the end of 3 months, 52 and 78 by the end of 6 months, and 50 and 75 by the end of 9 months, respectively (Figure 1).
Figure 1.
Flowchart of participants.
TA, Takayasu’s arteritis; ECTA, East China Takayasu’s Arteritis; LEF, leflunomide; CYC, cyclophosphamide; GC, glucocorticoid.
Follow up and assessment
The frequency of follow-up visits was once per month during induction therapy, but was extended to every 3 months during remission therapy. Comprehensive evaluation of disease activity was completed at baseline and every 3 months at follow ups, including new-onset or aggravated symptoms or signs, laboratory profiles, and NIH scores.
Radiological imaging was carried out at baseline and at the end of 6 months. Whole-body enhanced magnetic resonance angiography, computed tomography angiography, or positron emission tomography replaced the invasive modality of digital subtraction angiography. Data were collected and inputted into an electronic database by specially assigned persons.
Outcomes
The primary outcome was complete remission (CR), and the secondary endpoints were partial remission (PR) and effectiveness rate (ER) 9 months after induction. All the following criteria should be satisfied for CR: (a) no new/worsened systemic symptoms; (b) no new/worsened vascular symptoms or signs; (c) erythrocyte sedimentation rate (ESR) was normal (⩽40 mm/h); (d) GC dose ⩽15 mg/day. PR was denoted if item (b) was satisfied combined with at least one of the other three items. ER referred to the total rate of patients receiving CR or PR by the end of 9 months after induction therapy. Other outcomes included safety such as side effects, adverse events, and death.
Statistical analyses
This study was a real-world analysis. The sample size depended mainly on the number of patients whose data were included in the ECTA cohort. Data were analyzed using STATA v11.2 SE (Stata, College Station, TX, USA). Continuous variables were described as the mean and standard deviation (SD) for normal distributions, or median and interquartile range (IQR) for data with a non-normal distribution. Categorical variables were described as numbers and percentages.
Clinical data between different groups were analyzed with Student’s t tests or Wilcoxon rank sum tests, as appropriate; χ2 tests were used to compare differences in categorical variables between each group, and Fisher’s exact test was used if the sample size was <5. For the primary endpoint of CR rates and secondary endpoints of PR rates and ER, multivariable logistic regression models were used to assess between-group differences in CR, PR rates and ER. Covariates included age at diagnosis, gender, duration, ESR, and complications (headache/dizziness, chest pain/distress, hypertension, cardiac dysfunction, renal dysfunction, and/or cerebral infarction).
Given the limited sample size and multiple unbalanced characteristics at baseline between the two treatment groups, a propensity-score analysis was applied to adjust for potential confounders at baseline.28 Propensity score matching (PSM) was carried out using a specific approach29: (1) the confounding factors considered for the PSM program included age at diagnosis, gender, duration, ESR, and complications (headache/dizziness, chest pain/distress, hypertension, cardiac dysfunction, renal dysfunction, and/or cerebral infarction); (2) nearest-neighbor matching was according to a ratio of one-to-more to overcome bias arising from lack of randomization as a consequence of the different co-variable distribution among patients who were in LEF or CYC, and the caliper value was set at 0.035; (3) the predicted values were then used to obtain nearest-neighbor matching. Finally, 78 cases were selected (24 cases with LEF treatment and 54 cases with CYC treatment). Propensity scores were generated based on a logistic regression with received treatment types as dependent variable, and with the confounding factors listed above at baseline as covariates. In order to reduce the number of covariates, the propensity scores were then included in the following multivariate logistic regression model as a covariate to test the difference of treatment effects. Effectiveness was estimated using absolute differences in CR rates and 95% CIs; p < 0.05 was considered significant.
Results
General characteristics at baseline
The demographic and general characteristics of 131 new-onset TA patients with at least 3-month treatments were shown in Table 1. Type V (37.6%) and type I (24.8%) were the most common angiographic types. These 131 patients were evaluated in the active phase with a baseline NIH score of 2 (2–3). The initial daily dose of prednisone was 40 (30–45) mg, and the LEF group had significantly lower level (LEF versus CYC: 30 (20–40) versus 40 (40–50) mg, p < 0.01). There were significant differences between the two groups at baseline including the diagnosis age, ESR levels, and rates of cardiac dysfunction. After matching of propensity scores, 24 patients receiving LEF were matched with 54 patients receiving CYC, with standardized differences <10% for all variables (Table 1).
Table 1.
Demographics and clinical parameters of patients with TA at baseline before and after PSM.
| Baseline characteristics | Total (n = 131) | Before matching |
After matching |
||||
|---|---|---|---|---|---|---|---|
| LEF group (n = 53) | CYC group (n = 78) | p | LEF group (n = 24) | CYC group (n = 54) | p | ||
| Diagnosis age, year | 34.5 ± 13.6 | 31.3 ± 12.4 | 37.0 ± 14.0 | 0.020 | 34.0 ± 12.2 | 36.9 ± 14.7 | 0.395 |
| Female, n (%) | 102 (78.5%) | 43 (81.1%) | 59 (75.6%) | 0.457 | 19 (79.2%) | 41 (75.9%) | 0.754 |
| Disease duration, month | 20 (5.0–50.0) | 18 (4.5–48.0) | 20.5 (5.8–53.3) | 0.659 | 11.5 (2.5–45.0) | 18.0 (4.0–54.8) | 0.314 |
| Complication, n (%) | 79 (58.1%) | 33 (60.0%) | 46 (59.0%) | 0.706 | 15 (62.5%) | 38 (70.4%) | 0.492 |
| Headache/dizziness | 46 (35.4%) | 23 (32.4%) | 23 (29.5%) | 0.102 | 10 (41.7%) | 19 (35.2%) | 0.585 |
| Chest pain/distress | 24 (18.5%) | 9 (17.0%) | 15 (19.2%) | 0.744 | 5 (20.8%) | 11 (20.4%) | 0.963 |
| Hypertension | 26 (20.0%) | 11 (20.8%) | 15 (19.2%) | 0.830 | 5 (20.8%) | 15 (27.8%) | 0.517 |
| Cardiac dysfunction | 5 (3.8%) | 5 (9.4%) | 0 (0.0%) | 0.010 | 1 (4.2%) | 0 (0.0%) | 0.308 |
| Renal dysfunction | 3 (2.3%) | 2 (3.8%) | 1 (1.3%) | 0.565 | 1 (4.2%) | 1 (1.9%) | 0.523 |
| Cerebral infarction | 4 (3.1%) | 3 (5.7%) | 1 (1.3%) | 0.303 | 3 (12.5%) | 1 (1.9%) | 0.084 |
| ESR, mm/h | 15.0 (7.0–41.0) | 28.0 (10.3–51.5) | 11.0 (5.0–21.0) | 0.000 | 16.0 (5.0–41.8) | 11.0 (4.8–21.0) | 0.268 |
TA, Takayasu’s arteritis; PSM, propensity score matching; LEF, leflunomide; CYC, cyclophosphamide; ESR, erythrocyte sedimentation rate.
p < 0.05 was considered there was significant different between the LEF group and CYC group.
Treatment responses at 3 and 6 months
At the end of 3-month induction treatments, 131 patients continued the regimen (LEF group = 53, CYC group = 78) (seen in Figure 1). The overall CR and PR rates in patients with active TA were 28.2% (37/131) and 29.0% (38/131), respectively. The total ER was 57.3% (75/131). There were no significant differences in the CR rate between the two groups [LEF versus CYC: 22.6% (12/53) versus 32.1% (25/78), p = 0.240]. The LEF group showed similar levels of ESR [LEF versus CYC: 13.0 (4.0–28.0) versus 16.0 (7.0–27.5) mm/h, p = 0.508], c-reactive protein (CRP) [LEF versus CYC: 4.6 (0.5–17.6) versus 5.7 (1.6–16.0) mg/L, p = 0.653], IL-6 [LEF versus CYC: 3.4 (2.0–8.0) versus 2.7 (2.0–5.9) pg/mL, p = 0.379], immunoglobulin (Ig) G [LEF versus CYC: 9.0 (6.7–12.0) versus 9.4 (7.0–11.0) g/L, p = 0.847], and GC daily dose [LEF versus CYC: 15.0 (10.0–25.0) versus 20.0 (15.0–25.0) mg, p = 0.101] with those of the CYC group.
At the end of 6-month induction treatments, there were 130 patients continuing regimens (LEF group = 52, CYC group = 78) (seen in Figure 1). The overall CR and PR rates in patients with active TA were 42.0% (55/131) and 22.1% (29/131), respectively. The total ER was 64.1% (84/131). The median daily dose of prednisone was 13.8 (10–15) mg. There were no significant differences between two groups in the CR rate [LEF versus CYC: 45.3% (24/53) versus 39.7% (31/78), p = 0.257] and the daily prednisone dose [LEF versus CYC: 15.0 (10.0–20.0) versus 12.5 (10.0–15.0) mg, p = 0.620].
The LEF group had significantly lower levels of ESR (18.0 ± 13.3 mm/h), CRP [3.4 (0.8–9.3) mg/L], IL-6 [2.6 (2–5.6) pg/mL], IgG (9.3 ± 3.8 g/L) and daily prednisone dose [15.0 (10.0–20.0) mg] than those at baseline (p = 0.001, p = 0.018, p = 0.046, p = 0.003 and p = 0.013, respectively). The CYC group also indicated significantly lower levels of ESR [17.0 (10.5–38.0) mm/h], CRP [5.2 (2.8–15.3) mg/L] and daily prednisone dose [12.5 (10.0–15.0) mg] compared with those at baseline (p = 0.023, p = 0.002, and p = 0.000, respectively). The LEF group showed significantly greater decreases in levels of ESR [9.0 (−1.5–27.0) versus −5.0 (−19.5–2.5) mm/h, p = 0.000] as well as CRP [0.8 (−1.9–12.0) versus −2.4 (−8.4–0.8) mg/L, p = 0.000], IgG (6.9 ± 0.4 versus −0.8 ± 3.3 g/L, p = 0.000), and IL-6 [3.0 (0.0–9.6) versus 0.0 (−1.5–2.0) pg/mL, p = 0.020] than the CYC group.
Primary and secondary outcomes at 9 months
At the end of 9-month induction treatments, there were 125 patients continuing the regimens (LEF group = 50, CYC group = 75) and 5 patients switched to other drugs due to three adverse reactions and two failures (seen in Figure 1). The mean daily dose of prednisone was 10.0 (8.8–15.0) mg/day. The overall CR and PR rates in patients with active TA were 69.2% (90/130) and 13.1% (17/130), respectively. The total ER was 82.3% (107/130). The LEF group showed more sustainable increases in remission than the CYC group through the follow ups until 9-month treatments (Figure 2).
Figure 2.
Percentages of TA patients with variable disease activity at 3-, 6- and 9-month follow ups after LEF and CYC induction.
CYC, cyclophosphamide; LEF, leflunomide; TA, Takayasu’s arteritis.
In the LEF group, the CR and PR rates were 84.6% (44/52) and 9.6% (5/52), and the ER rate was 94.2% (49/52), respectively. After 9-month LEF treatment, levels of ESR [11.0 (6.3–13.8) mm/h] as well as CRP [1.5 (0.7–3.8) mg/L], IL-6 [9.5 (6.2–11.2) pg/mL], IgG (8.9 ± 2.7 g/L) and daily prednisone dose [10.0 (5.0–12.5) mg] were significantly lower than those at baseline (p = 0.003, p = 0.021, p = 0.039, p = 0.041 and p = 0.000, respectively).
In the CYC group, the CR and PR rates were 59.0% (46/78) and 15.4% (12/78) and the ER was 74.4% (58/78), respectively. After 9-month CYC treatment, levels of ESR [13.0 (4.0–42.5) mm/h], CRP [5.4 (1.3–9.0) mg/L], IgG (9.5 ± 1.7 g/L) and daily prednisone dose [12.5 (10.0–15.0) mg] decreased (p = 0.491, p = 0.016, p = 0.001, and p = 0.000, respectively) while IL-6 [9.5 (8.2–10.5) pg/mL] increased (p = 0.001) compared with those at baseline. However, there was no significant difference between two groups in levels of ESR (p = 0.299), CRP (p = 0.053), IL-6 (p = 0.113), and IgG (p = 0.447).
In multivariable analysis, the confounding factors adjusted were age, sex, TA course, and complications such as hypertension, cardiac dysfunction, renal dysfunction, and/or cerebral infarction. LEF treatment was associated with a higher possibility of reaching CR [LEF versus CYC: 84.6% (95% CI 74.5–94.8%) versus 59.0% (47.8–70.1%); relative risk (RR) = 0.3 (0.1–0.6), p = 0.002] and ER [94.2% (87.7–100.0%) versus 74.4% (64.5–84.3%); RR = 0.2 (0.1–0.6), p = 0.004], and lower daily prednisone dose [10.0 (5.0–12.5) versus 12.5 (10.0–15.0) mg, p = 0.043] compared with CYC treatment (Table 2). After adjustment of propensity scores in 78 matched patients, 77 patients finished the 9-month treatment (LEF group = 23, CYC group = 54). The LEF group still indicated significantly higher CR rate (p = 0.003) and ER (p = 0.037) than those in the CYC group.
Table 2.
Adjusted RRs of reaching primary and secondary outcomes stratified by LEF/CYC treatment after 9 months in patients with TA.
| Before matching |
After matching |
|||||
|---|---|---|---|---|---|---|
| LEF group (n = 52) |
CYC group (n = 78) |
P | LEF group (n = 23) |
CYC group (n = 54) |
P | |
| CR % (95% CI) |
84.6 (74.5–94.8) |
59.0 (47.8–70.1) |
0.002* | 95.7 (86.6–100.0) |
63.0 (49.7–76.3) |
0.003* |
| RR (95% CI) |
1 | 0.3 (0.1–0.6) |
1 | 0.1 (0.0–0.6) |
||
| PR % (95% CI) |
9.6 (1.3–17.9) |
15.4 (7.2–23.6) |
0.339 | 4.3 (0–13.4) |
20.4 (9.3–31.5) |
0.095 |
| RR (95% CI) |
1 | 1.7 (0.6–5.2) |
1 | 5.6 (0.7–46.4) |
||
| ER % (95% CI) |
94.2 (87.7–100.0) |
74.4 (64.5–84.3) |
0.004* | 100 (100–100) |
83.3 (73.1–93.6) |
0.037* |
| RR (95% CI) |
1 | 0.2 (0.1–0.6) |
1 | 0.8 (0.3–2.1) |
||
RR, relative risk; TA, Takayasu’s arteritis; LEF, leflunomide; CYC, cyclophosphamide; CI, confidence interval; CR, complete remission; PR, partial remission; ER, effectiveness rate.
p < 0.05 was considered a significant different between the LEF and CYC groups.
Follow up after 9 months
After 9-month treatments, all the patients in the LEF group (n = 50) continued LEF treatment. Only one patient discontinued LEF for considering pregnancy after 12 months. In the CYC group (n = 75), 10.5% of the patients discontinued CYC treatment owing to failure of remission and side effects during the 9–24 months of CYC treatment. The majority of patients obtained remission from CYC treatment in different time ranges (36.8% within 3–9 months, 26.3% within 10–12 months, 21.1% within 12–24 months, and 5.3% after 24 months) and then switched CYC to other IS treatment (azathioprine 38.7%, LEF 32.3%, methotrexate 12.9%, hydroxychloroquine 6.5%, cyclosporine 6.5%, thalidomide 3.1%).
Safety
Side effects were noted in four LEF-treated patients (4/53, 7.5%). One patient suffered from a rash after 3 months and switched to azathioprine. The second patient had mild liver dysfunction after 1-month LEF treatment, which was reversed after suspension of LEF. This patient reused LEF induction and achieved remission so that she continued LEF for a 5-month continuation without any further side effects. The third patient complained of hypomenorrhea. The fourth patient suffered from diarrhea and discontinued LEF treatment at the eighth month. Besides, one patient of type V suffered from disease progression and died from a cerebrovascular accident 2-months after LEF treatment.
Out of 78 patients, 34 (43.6%) treated with CYC had side effects. Three had pulmonary infection after 1, 3, and 7 months, respectively, and switched to LEF and mycophenolate mofetil treatment separately. One patient suffered from fever and arthralgia after 1 month and switched to methotrexate, while another patient discontinued CYC owing to dizziness after 6 months. The other 29 patients had hypomenorrhea to varying degrees but no amenorrhea. There was a significant difference in the rates of hypomenorrhea between the two groups [LEF versus CYC: 1.9% (1/53) versus 37.2% (29/78); RR = 30.8 (4.0–234.6), P < 0.010].
Discussion
In our study, the regimen of LEF combined with GC was considered effective in TA induction. We observed that 60.4% of LEF-treated patients achieved clinical effectiveness after 6-month treatment, increasing to 94.2% by 9 months. The trend of sustained remission was consistent with data from South America, and it was even a little higher than the 80% reported at 9.1-month follow-up.24 LEF induction treatment seemed to show encouraging effectiveness in Chinese patients with active and severe TA. On one hand, our TA patients were evaluated in more severe conditions at baseline, with wider lesions involvement (type-V in 29.4% of patients) and more clinical manifestations (58.1%). On the other hand, the definition of CR and PR in this study might be overly strict, comprehensively including improvements or stabilization in symptoms, signs, ESR, and GC tapering.
Furthermore, the effectiveness of LEF induction was higher than that of CYC in this study. Even if our LEF-treated patients were characterized at baseline with more pulselessness, higher CRP levels, and more severe situations than those of the CYC group, they had significantly greater decreases after induction in levels of ESR, CRP, and IL-6. In a further step of multivariable analysis, after adjustment for possible confounding factors, LEF treatment continued to demonstrate higher CR rate than CYC treatment in active-TA patients.
In addition, LEF showed advantages over CYC in the aspect of safety. More patients in the CYC group complained of hypomenorrhea during induction than those in the LEF group, and this difference was significant. Careful consideration should be taken if prescribing CYC to women of childbearing age suffering from TA. In the LEF group, only one patient complained of hypomenorrhea, and other side effects of LEF were mild without treatment interruption. Currently, there has been no human evidence of LEF-related increase in risks of adverse pregnancy outcomes and congenital abnormalities.30–32 Although LEF may not be a human teratogen, it has been confirmed teratogenic in rodents.33 Thus, it is recommended to discontinue LEF before a planning pregnancy, and it should be avoided in pregnancy.34,35 Cholestyramine or active charcoal washout could quickly lower plasma levels to under 0.02 mg/l or even undetectable.36,37
The present study had two main limitations. First, this was a non-randomized observational pilot study, and there was unavoidable selection bias and ascertainment bias despite propensity-score matching. Second, the study cohort was small because TA is rare. Nevertheless, our study was the largest one reported with regard to induction therapy against TA using LEF. However, our data with narrow 95% CIs were credible and illustrative to some extent. Further and solid confirmation at a larger scale and with longer follow ups will be needed.
In a word, we suggest that LEF might be a promising alternative to CYC in induction therapy against TA, with regards to good performance in clinical effectiveness and fertility considerations in female patients of childbearing age. Currently, a multi-center and double-blinded randomized, placebo-controlled clinical trial (TACTIC) is underway in an East Chinese population with TA aimed at prospectively investigating the efficacy and safety of LEF as induction therapy against TA.
Footnotes
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Natural Science Foundation of China [grant number 81771730, 8180060345], and the Zhongshan Hospital Clinical Trial Funding [grant number 2016ZSLC06].
ORCID iD: Lindi Jiang  https://orcid.org/0000-0002-4326-6517
https://orcid.org/0000-0002-4326-6517
Contributor Information
Xiaomin Dai, Department of Rheumatology, Zhongshan Hospital, Fudan University, Shanghai, China.
Xiaomeng Cui, Department of Rheumatology, Zhongshan Hospital, Fudan University, Shanghai, China.
Ying Sun, Department of Rheumatology, Zhongshan Hospital, Fudan University, Shanghai, China.
Lili Ma, Department of Rheumatology, Zhongshan Hospital, Fudan University, Shanghai, China.
Lindi Jiang, Department of Rheumatology, Zhongshan Hospital, Fudan University, No. 180, Road Fenglin, Xuhui District, Shanghai 200032, P. R. China Evidence-Based Medicine Center, Fudan University, Shanghai, China.
References
- 1. Watanabe Y, Miyata T, Tanemoto K. Current clinical features of new patients with Takayasu arteritis observed from cross-country research in Japan: age and sex specificity. Circulation 2015; 132: 1701–1709. [DOI] [PubMed] [Google Scholar]
- 2. Toshihiko N. Current status of large and small vessel vasculitis in Japan. Int J Cardiol 1996; 54(Suppl.): S91–S98. [DOI] [PubMed] [Google Scholar]
- 3. Birlik M, Kücükyavas Y. Epidemiology of Takayasu’s arteritis in Turkey. Clin Exp Rheumatol 2016; 34(3 Suppl. 97): S33–S39. [PubMed] [Google Scholar]
- 4. Numano F, Kakuta T. Takayasu arteritis—five doctors in the history of Takayasu arteritis. Int J Cardiol 1996; 54(Suppl): S1–S10. [DOI] [PubMed] [Google Scholar]
- 5. Kerr GS, Hallahan CW, Giordano J, et al. Takayasu arteritis. Ann Intern Med 1994; 120: 919–929. [DOI] [PubMed] [Google Scholar]
- 6. Dai X, Dong Z, Chen S, et al. Chinese expert investigation on diagnosis and disease activity evaluation in Takayasu’s arteritis. Fudan Univ J Med Sci 2017; 44: 127–133. [Google Scholar]
- 7. Kathleen MM, Tiffany MC, Hoffman GS. Limitations of therapy and a guarded prognosis in an American cohort of Takayasu arteritis patients. Arthritis Rheum 2007; 56: 1000–1009. [DOI] [PubMed] [Google Scholar]
- 8. Hoffman GS, Leavitt RY, Kerr GS, et al. Treatment of glucocorticoid-resistant or relapsing Takayasu arteritis with methotrexate. Arthritis Rheum 1994; 37: 578–582. [DOI] [PubMed] [Google Scholar]
- 9. Liang P, Hoffman GS. Advances in the medical and surgical treatment of Takayasu arteritis. Curr Opin Rheumatol 2005; 17: 16–24. [DOI] [PubMed] [Google Scholar]
- 10. Shinjo SK, Pereira RM, Tizziani VA, et al. Mycophenolate mofetil reduces disease activity and steroid dosage in Takayasu arteritis. Clin Rheumatol 2007; 26: 1871–1875. [DOI] [PubMed] [Google Scholar]
- 11. Ohigashi H, Haraguchi G, Konishi M, et al. Improved prognosis of Takayasu arteritis over the past decade-comprehensive analysis of 106 patients. Circ J 2012; 76: 1004–1011. [DOI] [PubMed] [Google Scholar]
- 12. Henes JC, Muller M, Pfannenberg C, et al. Cyclophosphamide for large-vessel vasculitis: assessment of response by PET/CT. Clin Exp Rheumatol 2011; 29: S43–S48. [PubMed] [Google Scholar]
- 13. Stern S, Clemente G, Reiff A, et al. Treatment of pediatric Takayasu arteritis with infliximab and cyclophosphamide: experience from an American-Brazilian cohort study. J Clin Rheumatol 2014; 20: 183–187. [DOI] [PubMed] [Google Scholar]
- 14. Hahn D, Thomson PD, Kala U, et al. A review of Takayasu’s arteritis in children in Gauteng, South Africa. Pediatr Nephrol 1998; 12: 668–675. [DOI] [PubMed] [Google Scholar]
- 15. Sun Y, Ma L, Ma L, et al. Cyclophosphamide could be a better choice than methotrexate as induction treatment for patients with more severe Takayasu’s arteritis. Rheumatol Int 2017; 37: 2019–2026. [DOI] [PubMed] [Google Scholar]
- 16. Ozen S, Duzova A, Bakkaloglu A, et al. Takayasu arteritis in children: preliminary experience with cyclophosphamide induction and corticosteroids followed by methotrexate. J Pediatr 2007; 150: 72–76. [DOI] [PubMed] [Google Scholar]
- 17. Manna SK, Mukhopadhyay A, Aggarwal BB. Leflunomide suppresses TNF-induced cellular responses: effects on NF-kappa B, activator protein-1, c-Jun N-terminal protein kinase and apoptosis. J Immunol 2000; 165: 5962–5969. [DOI] [PubMed] [Google Scholar]
- 18. Burger D, Begue-Pastor N, Benavent S, et al. The active metabolite of leflunomide, A77 1726, inhibits the production of prostaglandin E(2), matrix metalloproteinase 1 and interleukin 6 in human fibroblast-like synoviocytes. Rheumatology (Oxford) 2003; 42: 89–96. [DOI] [PubMed] [Google Scholar]
- 19. Yao Y, Ding CZ, Fang Y. Combination of MTX and LEF attenuate inflammatory bone erosion by down-regulation of receptor activator of NF-kB lignad and interleukin-17 in type II collagen-induced arthritis rats. Rheumatol Int 2013; 33: 1845–1853. [DOI] [PubMed] [Google Scholar]
- 20. American College of Rheumatology Subcommittee on Rheumatoid Arthritis Guidelines. Guidelines for the management of rheumatoid arthritis: 2002 update. Arthritis Rheum 2002; 46: 328–346. [DOI] [PubMed] [Google Scholar]
- 21. Saag KG, Teng GG, Patkar NM, et al. American College of Rheumatology 2008 recommendations for the use of nonbiologic and biologic disease-modifying antirheumatic drugs in rheumatoid arthritis. Arthritis Rheum 2008; 59: 762–784. [DOI] [PubMed] [Google Scholar]
- 22. Wang HY, Cui TG, Hou FF, et al. Induction treatment of proliferative lupus nephritis with leflunomide combined with prednisone: a prospective multi-centre observational study. Lupus 2008; 17: 638–644. [DOI] [PubMed] [Google Scholar]
- 23. Diamantopoulos AP, Hetland H, Myklebust G. Leflunomide as a corticosteroid- sparing agent in giant cell arteritis and polymyalgia rheumatica: a case series. Biomed Res Int 2013; 2013: 120638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. de Souza AW, da Silva MD, Machado LS, et al. Short-term effect of leflunomide in patients with Takayasu’s arteritis: an observational study. Scand J Rheumatol 2012; 41: 227–230. [DOI] [PubMed] [Google Scholar]
- 25. de Souza AW, de Almeida Agustinelli R, de Cinque Almeida H, et al. Leflunomide in Takayasu arteritis- A long term observational study. Rev Bras Rheumatol 2016; 56: 371–375. [DOI] [PubMed] [Google Scholar]
- 26. Arend WP, Michel BA, Bloch DA, et al. The American College of Rheumatology 1990 criteria for the classification of Takayasu arteritis. Arthritis Rheum 1990; 33: 1129–1134. [DOI] [PubMed] [Google Scholar]
- 27. Moriwaki R, Noda M, Yajima M, et al. Clinical manifestations of Takayasu arteritis in India and Japan-new classification of angiographic findings. Angiology 1997; 48: 369–379. [DOI] [PubMed] [Google Scholar]
- 28. Rosenbaum PR, Rubin DB. Reducing bias in observational studies using subclassification on the propensity score. J Am Stat Assoc 1984; 79: 516–524. [Google Scholar]
- 29. Leuven E, Sianesi B. PSMATCH2: Stata module to perform full Mahalanobis and propensity score matching, common support graphing, and covariate imbalance testing. Statistical Software Components S432001, Boston College Department of Economics, https://ideas.repec.org/c/boc/bocode/s432001.html (2003, accessed 1 February 2018). [Google Scholar]
- 30. Berard A, Zhao JP, Shui I, et al. Leflunomide use during pregnancy and the risk of adverse pregnancy outcomes. Ann Rheum Dis 2018; 77: 500–509. [DOI] [PubMed] [Google Scholar]
- 31. Chambers CD, Johnson DL, Robinson LK, et al. Birth outcomes in women who have taken leflunomide during pregnancy. Arthritis Rheum 2010; 62: 1494–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cassina M, Johnson DL, Robinson LK, et al. Pregnancy outcome in women exposed to leflunomide before or during pregnancy. Arthritis Rheum 2012; 64: 2085–2094. [DOI] [PubMed] [Google Scholar]
- 33. Fukushima R, Kanamori S, Hirashiba M, et al. Teratogenicity study of the dihydroorotate-dehydrogenase inhibitor and protein tyrosine kinase inhibitor Leflunomide in mice. Reprod Toxicol 2007; 24: 310–316. [DOI] [PubMed] [Google Scholar]
- 34. Gotestam Skorpen C, Hoeltzenbein M, Tincani A, et al. The EULAR points to consider for use of antirheumatic drugs before pregnancy, and during pregnancy and lactation. Ann Rheum Dis 2016; 75: 795–810. [DOI] [PubMed] [Google Scholar]
- 35. Flint J, Panchal S, Hurrell A, et al. BSR and BHPR guideline on prescribing drugs in pregnancy and breastfeeding–Part I : standard and biologic disease modifying anti-rheumatic drugs and corticosteroids. Rheumatology (Oxford) 2016; 55: 1693–1697. [DOI] [PubMed] [Google Scholar]
- 36. Weber-Schoendorfer C, Beck E, Tissen-Diabate T, et al. Leflunomide – A human teratogen? A still not answered question. An evaluation of the German Embryotox pharmacovigilance database. Reprod Toxicol 2017; 71: 101–107. [DOI] [PubMed] [Google Scholar]
- 37. Brent RL. Teratogen update: reproductive risks of leflunomide (Arava); a pyrimidine synthesis inhibitor: counseling women taking leflunomide before or during pregnancy and men taking leflunomide who are contemplating fathering a child. Teratology 2001; 63: 106–112. [DOI] [PubMed] [Google Scholar]