Abstract
The mammalian brain goes through final maturation during late adolescence and early adulthood with sex differences in timing. The key cellular processes, including changes in neurotransmitter receptor density and synaptic pruning, make this age uniquely vulnerable to neurotoxic insults. Teenagers and young adults are the major consumers of energy drinks, which contain high levels of taurine and caffeine. Taurine is one of the most abundant amino acids in the central nervous system, but the effects of supplemental taurine consumption during adolescence has not been well studied. We conducted an initial short-term exposure study with 0.12% taurine in drinking water and a long-term dose-response study using 0.06 and 0.12% taurine in male and female C57BL/6J mice.r We examined a broad range of cognitive functions and behaviors and measured neurotransmitter levels. We found no significant differences in anxiety, open field locomotor activity, or sensorimotor gating. However, we found impairments in novel object recognition and sex differences in Morris water maze. When taurine treatment stopped before behavioral experiments began, male mice had significant impairments in spatial learning and memory. In the dose-response study when taurine treatment continued throughout behavioral experiments, females had significant impairments. We also found sex differences in neurotransmitter levels with females having higher levels of glutamate, DOPAC and 5-HIAA. We conclude that both females and males are at risk from excess taurine consumption during final brain maturation.
1. Introduction
The mammalian brain does not completely mature until early adulthood with multiple changes occurring during the adolescent period (Gogtay et al. 2004). These include synaptic pruning and stabilization in the prefrontal cortex (Panagiotakopoulos & Neigh 2014, Selemon 2013), maturation of brain regions associated with the stress response (Roberts & Lopez-Duran 2019, Rincón-Cortés et al. 2019), and myelination of the basal ganglia and cortex (Paquola et al. 2019, Eminian et al. 2018). Neurotransmission is also changing during adolescence. Jiang et al. (2019) used PET imaging to demonstrate that gene expression of the vesicular monoamine transporter 2 (VMAT2) and the dopamine transporter (DAT) increase in the striatum of Wistar rats during adolescence with gene expression changes continuing until 4 months of age. Anderson et al. (2000) reported age and sex-dependent changes in dopamine D1 and D2 receptors in the prefrontal cortex, nucleus accumbens and striatum from postnatal day 25 (P25) to P120 in Sprague-Dawley rats. Therefore, it is increasingly clear that the adolescent brain could be uniquely vulnerable to toxic insults.
Energy drink sales exceeded $53 billion in 2018 and are expected to reach nearly $85 billion worldwide by 2026 with the highest consumption by teenagers and young adults (AMR 2019, Radian Insights 2018). Energy drinks and shots typically contain 80-320 mg of caffeine per serving and a mixture of additives including the amino acid taurine (Curran & Marczinski 2017, Marczinski 2015). Caffeine is the only psychoactive compound that can legally be sold to minors. Reissig et al. (2009) reported caffeine levels in energy drinks as high as 505 mg per serving, and Thompson & Scheiss (2010) estimated that 40% of teenagers would exceed the adverse effect level of 3 mg/kg/day by consuming a single energy drink.
Taurine is one of the most abundant amino acids in the mammalian central nervous system (CNS), and taurine can alter signaling through multiple pathways including GABAergic (Sava 2014), serotonergic (Aldegunde et al.1983), dopaminergic (Hashimoto-Kitsukawa et al. 1988a, 1988b)and noradrenergic signaling (Huxtable 1989). Taurine levels in the most popular energy drinks typically range from 750 to 1,000 mg per serving (McLellan & Lieberman 2012), is sold as a nutritional supplement with common formulations at 1,000 mg per dose. The Mayo Clinic recommends limiting taurine intake to 3,000 mg per day (Zeratatsky 2015). Since seafood, beef and dark meat poultry contain significant amounts of taurine (Wójcik et al. 2010), consumption of 1-2 energy drinks could push an individual’s daily intake above the accepted limits.
Given the continued popularity of energy drinks in the adolescent and young adult market and the lack of regulations in the United States regarding their sales to minors, we designed experiments to explore the effects of high taurine exposure during adolescence and early adulthood on cognitive function, behavior and neurochemistry. The studies described here represent a series of studies in mice that began with a short-term exposure to assess the impact of excess taurine during final brain maturation and expanded to consider dose response and long-term exposure through both adolescence and early adulthood. Since taurine has been promoted as a neuroprotectant (reviewed in Chen et al. 2019), but Giles et al. (2012) reported cognitive benefits only from the caffeine component of energy drinks, this also allowed us to explore potential benefits of taurine supplementation in healthy, young adult animals. To examine sex differences, males and females were included in both studies.
2. Methods
2.1. Animals.
All animal studies were conducted under protocols approved the Northern Kentucky University Institutional Animal Care and Use Committee and in accord with the ARRIVE guidelines (Kilkenny et al. 2010). Housing and animal care were consistent with requirements outlined in the Guide for the Care and Use of Laboratory Animals.
2.1.1. Animal source and husbandry.
Male and female C57BL/6J mice were purchased from The Jackson Laboratory (Bar Harbor, ME) at 21 days of age. Animals were housed 4 per cage by sex and treatment group in standard polysulfone shoebox cages with corncob bedding and a 12h:12h light-dark cycle. Water and Lab Diet 5015 chow (18.9% protein; 11% fat, 32.6% starch and < 0.01% taurine) were provided ad libitum. Animals were checked daily for health concerns, and cages were changed weekly. Animals were acclimated to the vivarium for > 1wk prior to beginning experimental treatments. Weights were recorded weekly during the treatment period.
2.1.2. Taurine treatments (Fig. 1).
Fig. 1.
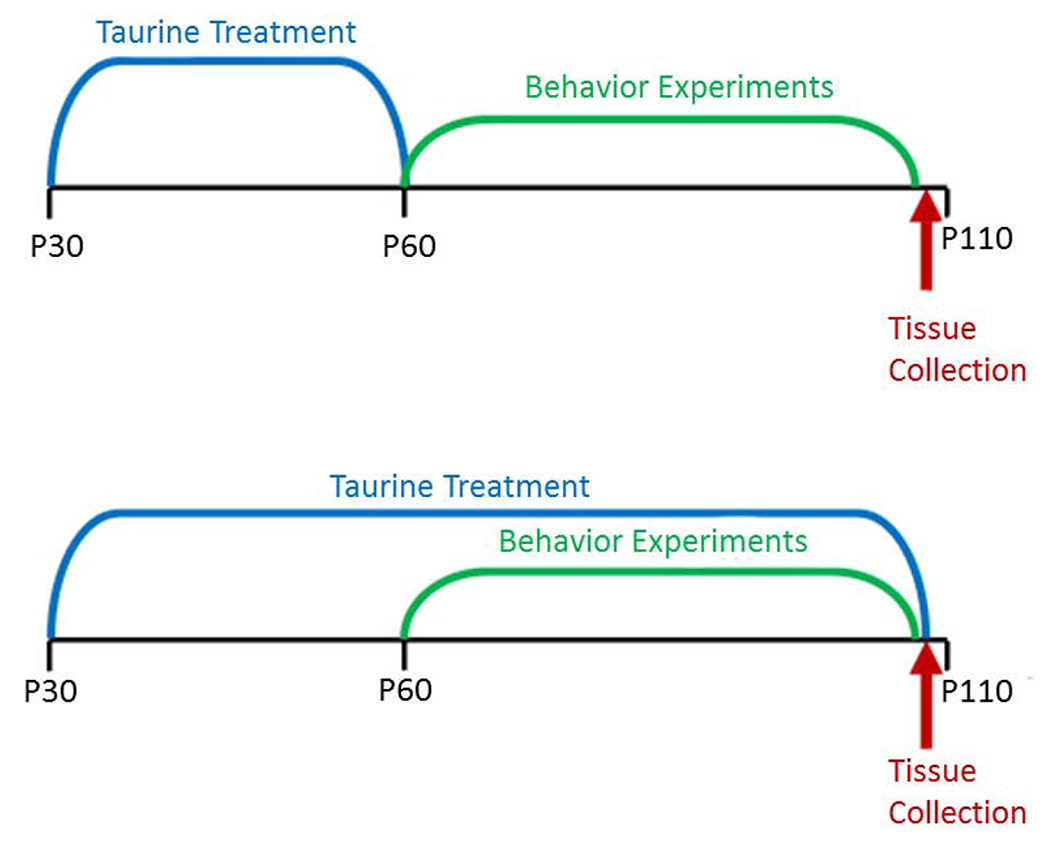
Experimental timelines illustrating the different exposure periods in the initial and dose-response studies.
Animals were randomly assigned to treatment groups (n = 16 per group) for all experiments. For the initial, short-term study, mice received either tap water or 0.12% taurine in water based on the studies of Suge et al. (2007) from P30 to P60 when behavioral experiments began. In the long-term, dose-response study, mice received 0, 0.06 or 0.12% taurine in drinking water from P30 until the end of behavioral experiments at ~P110 to better model known human consumption patterns from adolescence into early adulthood.
2.1.3. Caffeine challenge.
To explore the interaction between co-exposure to caffeine and taurine, animals were injected with 10/mg/kg caffeine (i.p.) or the equivalent volume of saline for control animals. The 10 mg/kg caffeine dose was previously determined to produce hyperactivity in mice (Zhang et al. 2011).
2.14. Euthanasia and tissue collection.
Immediately following the end of behavioral experiments, mice were euthanized by rapid decapitation, and brain tissues were dissected on ice and snap frozen at −80°C until analysis of neurotransmitters.
2.2. Behavior experiments.
Behavioral testing began at P60 for both studies. Experiments are described in the order in which they were performed with differences noted between the short-term exposure study and the follow-up long-term, dose-response study. Mice went through a single test per day during the light cycle, and testing was restricted to a 4h time block to avoid confounding by circadian rhythms. All investigators were blinded to treatment.
2.2.1. Elevated zero maze (short-term exposure study only).
Mice were placed in a closed quadrant of a circular maze (105 cm diameter) with two closed and two open quadrants under low-light conditions. Mice were videotaped for 5 min, and videos were scored for latency to enter an open quadrant, head dips over the side of the open quadrants, zone crossings, and time spent in the open.
2.2.2. Acoustic startle with pre-pulse inhibition (short-term exposure study only).
The SR-Lab apparatus (San Diego Instruments, San Diego, CA) was used to test baseline startle response and sensorimotor gating with a 120db startle stimulus and pre-pulses of 74 and 76db. A Latin Squares design was used with four trial types randomly presented over a total of 48 trials. Peak response amplitudes (Vmax) were recorded, and the percent attenuation following the pre-pulse tones was calculated.
2.2.3. Open field locomotor activity (both studies).
Mice were placed in square plexiglass chambers (41 cm x 41 cm) under low-light conditions. Baseline activity levels were monitored using the Photobeam Activity System (San Diego Instruments, San Diego, CA) for 60 min. Beam breaks and rearing were recorded in 5 min intervals.
2.2.4. Marble burying (both studies).
Polysulfone shoebox cages identical to those used for housing were filled with 5 cm of hardwood chip bedding. A 3 x 5 array of 15 blue marbles was arranged on top of the bedding using a template. Mice were placed in the cages for 20 min. Afterward, images were taken, and the number of marbles buried more than half-way was recorded.
2.2.5. Novel object recognition (both studies).
Mice were acclimated to circular arenas (91 cm diameter) 10 min per day for two days with no objects present and for two additional day with practice objects. Testing was conducted on the fifth day with a familiarization phase where mice accumulated 30 s of exploration of two identical objects (unique from practice objects). One hour later, mice were presented with a copy of the familiar object and a unique novel object until 30 s of exploration time was accumulated. Familiar and novel objects were counter-balanced to avoid confounding by object preference. The percent time spent exploring the novel object was calculated and compared.
2.2.6. Morris water maze (MWM) (both studies).
A four-week schedule of testing was completed using standard methods (Curran et al. 2012, 2011; Vorhees & Williams 2006). In the cued phased, an orange ping pong ball on a 10 cm tall pole was centered on a 10 cm diameter platform in a tank of water (122 cm diameter) with water temperature controlled at 22°C ± 2°C. The platform was slightly submerged and obscured by the addition of non-toxic white paint to the water. Curtains were closed around the tank, so the only visual cue was on the platform. Mice received 6 trials on day 1 with a fixed start and platform location. Mice received 2 trials on days 2-6 with random start and platform positions. During the hidden platform phases, the curtains were open to reveal visual cues along three walls of the test room. Platform size decreased from 10 cm to 7 cm and 5 cm in each phase. Start locations were randomized, and mice received 4 trials/day for 6 days followed by a 30 s probe trial on day 7 with the platform removed to assess reference memory. Latency to escape, distance traveled, and swim speed were recorded by AnyMaze™ software (Stoelting, Inc.). Mice that failed to find the platform in 60 s were guided to the platform and left there for 15 s. For probe trials, average distance to the target and target crossings were recorded.
2.2.7. Caffeine challenge (short-term exposure study).
Identical chambers and software were used as described in Section 2.2.3 to monitor mouse activity. Half of the control and taurine-treated mice were randomly assigned to receive either 10 mg/kg caffeine in saline or the saline vehicle (i.p. injection) 10 min prior to testing (n = 8 per group). Total activity and rearing was recorded for 60 min.
2.2.8. Caffeine challenge (dose-response study).
Identical chambers and software were used as described in Section 2.2.3 to monitor mouse activity. To increase sample size (n = 16 per group), baseline activity and rearing was recorded for 30 min for all mice. All control and taurine-treated mice received 10 mg/kg caffeine (i.p.) 10 min before being returned to the chambers for an additional 60 min. The response to caffeine was compared across groups and against baseline activity.
2.2.9. Social preference and recognition (dose-response study).
As described in Yang et al. (2016), stranger mice (age and sex-matched) were acclimated to wire cages and the test apparatus prior to the experiments. Experimental mice were placed in the center chamber of a 27cm x45.5cm x 27cm acrylic apparatus divided into three chambers with acrylic walls for a 5 min acclimation period with a 10.5cm x 10 cm circular wire cage placed in each end chamber. A stranger mouse was placed in one of the wire cages. Following the acclimation period, doors were removed to allow the test animal to explore all three chambers for 10 min while a live observer scored behavior. When the head and forepaws crossed into the chamber, the mouse was considered in the new chamber. The mice were removed from the apparatus and the stranger used in the first phase and a new stranger were placed into the wire cages, with positions alternating between test mice. The test mouse was placed in the center chamber and allowed to explore for 10 minutes while a live observer recorded movements. All trials were video-taped and rescored by a second observer. Data was averaged prior to analysis.
2.2.10. Alcohol preference (dose-response study).
Because energy drinks can increase alcohol consumption in humans (Marczinski et al. 2016, Marczinski 2015, Marczinski & Fillmore 2014, Marczinski et al. 2013), we examined alcohol preference following behavior experiments. Mice were separated into cages with 2 animals per cage and provided two water bottles in their home cages: one with an alcohol solution and one with water. During the first 48 h, mice were given a 2% ethanol solution. A 5% solution was given for the next 48 h, increasing to 10% ethanol for the last 72 h. The bottles were weighed twice daily, checked for leaks, and changed every 2 days. Initial and final bottle weights were used to calculate consumption.
2.3. Neurotransmitter analysis.
Frozen brain tissue was sent to the Vanderbilt University Neurochemistry Research Core for analysis of amino acids, monoamine neurotransmitters and their primary metabolites using high-performance liquid chromatography (amino acid analysis in short-term exposure study and monoamines in both studies) and gas chromatography mass spectrometry (amino acid analysis in dose-response study). Tissues sampled included cortex, hippocampus, and hypothalamus (n = 8 per group).
2.4. Data analysis.
Data were analyzed at the University of Kentucky by the Kentucky Biomedical Research Infrastructure Network (KBRIN) Applied Statistics Core using mixed-models analysis of variance (ANOVA) and SAS software (SAS Institute Inc., Cary, NC). For open field locomotor activity, interval was considered a repeated measure. For Morris water maze, day was considered a repeated measure. Swim speed was analyzed as a covariate for latency. Data are presented as least square means ± the standard error of the mean. Significance was defined as P < 0.05. Trends were defined as P < 0.1.
3. Results.
Data are presented individually when a behavioral test was conducted in a single study. For tests used in both the short-term exposure study and the long-term, dose-response study, data are presented together to highlight similar results and areas where the results diverged.
3.1. Animal weights.
There were no significant weight differences between taurine-treated mice and control mice. As expected, male mice weighed significantly more than female mice (P < 0.01).
3.2. Elevated zero maze (short-term exposure study).
There were no significant differences in the latency to leave the closed quadrants, heads dips, zone crossings or time spent in the open (P > 0.05).
3.3. Acoustic startle with pre-pulse inhibition (short-term exposure study).
There was a significant main effect of sex for the baseline startle response (P < 0.001) and attenuation for the 74 (P < 0.05) and 76db (P < 0.001) pre-pulse conditions with males having a larger amplitude of a response compared with females. These differences were expected due to the heavier weights of male mice. There was no effect of treatment and no sex x treatment interaction (P > 0.05).
3.4. Open field locomotor activity (both studies).
There was no effect of taurine treatment and no sex x treatment interaction in the initial study. Those findings were replicated in the dose-response study (P > 0.05).
3.5. Marble burying (both studies).
There was no effect of taurine treatment and no significant sex x treatment interaction in the initial study, and those findings were replicated in the dose-response study (P > 0.05).
3.6. Novel object recognition (both studies).
There was a trend in the short-term exposure study for taurine-treated mice to spend less time exploring the novel object (Fig. 2A), but the differences were not significant (P < 0.1). However, there was a significant impairment in novel object recognition in the dose-response study (P < 0.01) with both taurine-treated groups spending significantly less time exploring the novel object (Fig. 2B). This suggests that the longer term exposure to supplemental taurine was responsible for the impairment.
Fig. 2A. Novel object recognition.
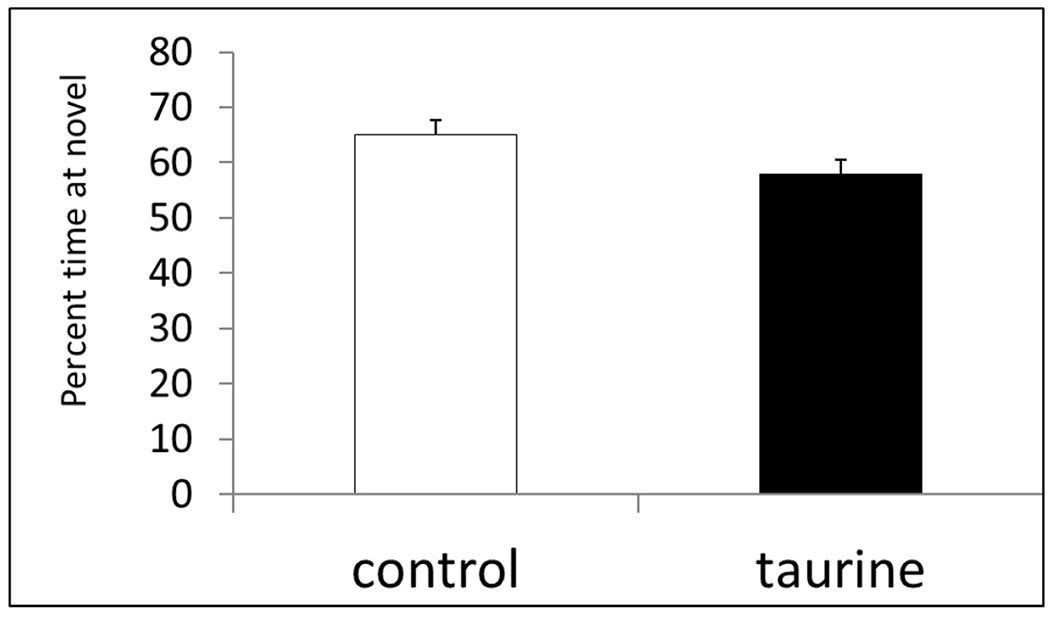
Control mice spent more time exploring the novel object compared with mice receiving 0.12% taurine from P30-P60; however the differences did not reach significance. P < 0.1
Fig. 2B. Novel object recognition.
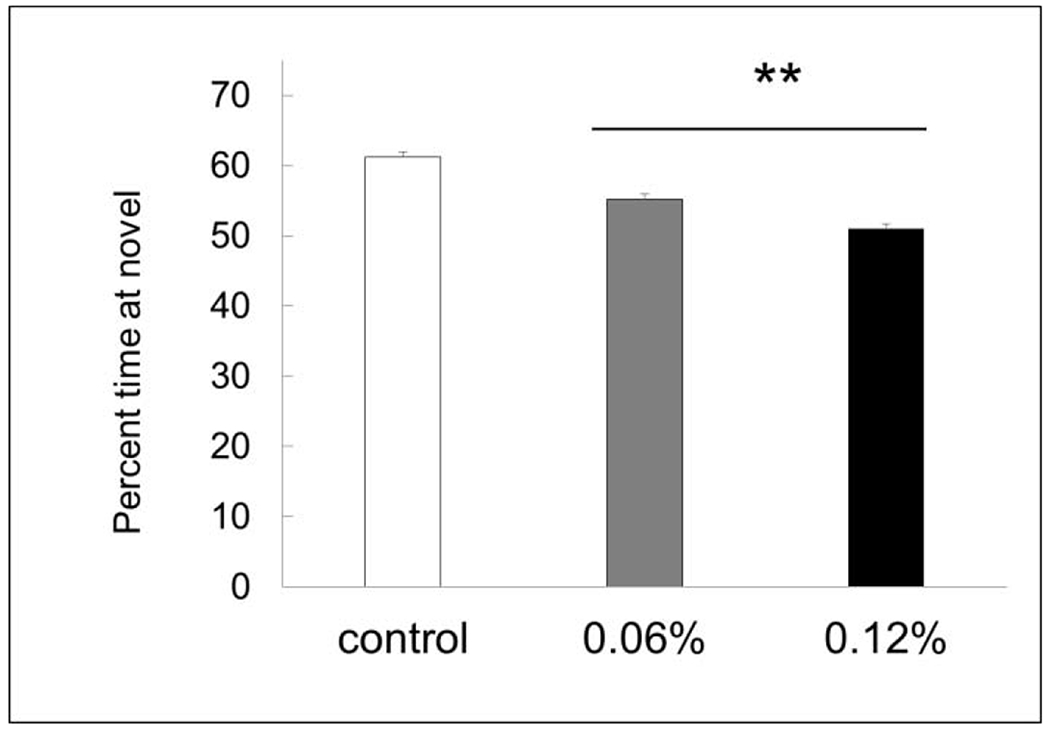
Mice treated with 0.06 and 0.12% taurine both spent significantly less time exploring the novel object.** P < 0.01
3.7. Morris water maze (both studies).
There were no significant differences between control and taurine-treated mice in the Cued Phase which trained mice to find the escape platform in an area of the pool away from the sides (Fig. 3A). This indicates all mice had normal swimming ability and the ability to navigate using visual cues. These findings were replicated in the dose-response study (Fig. 3B)
Fig. 3A. MWM Cued Phase.
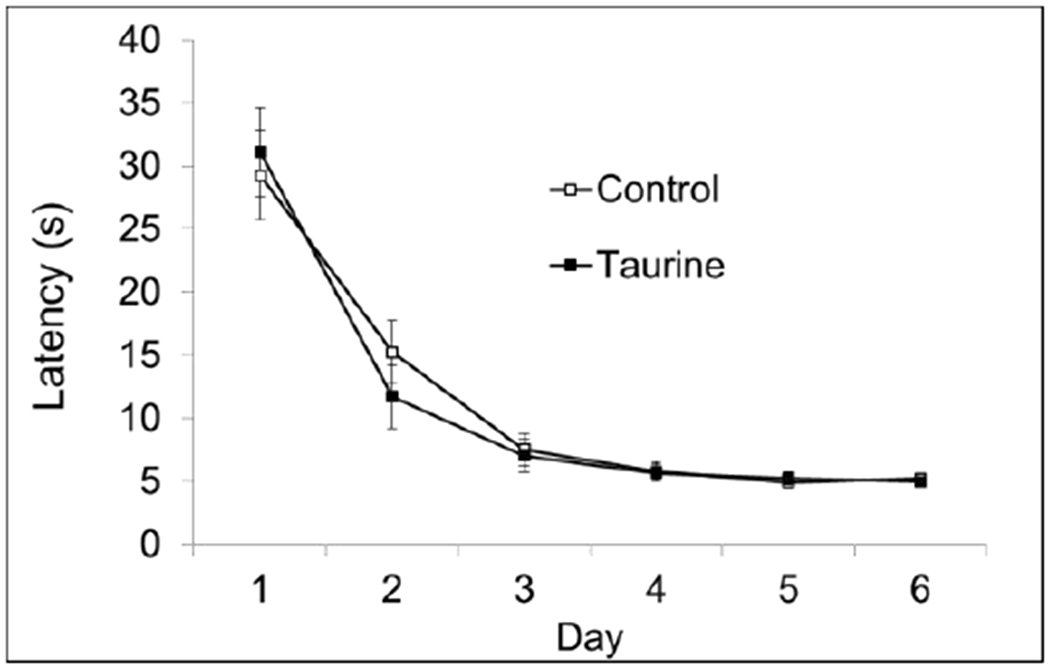
There were no significant differences on any day of testing during the initial studies. P > 0.05.
Fig. 3B. MWM Cued Phase.
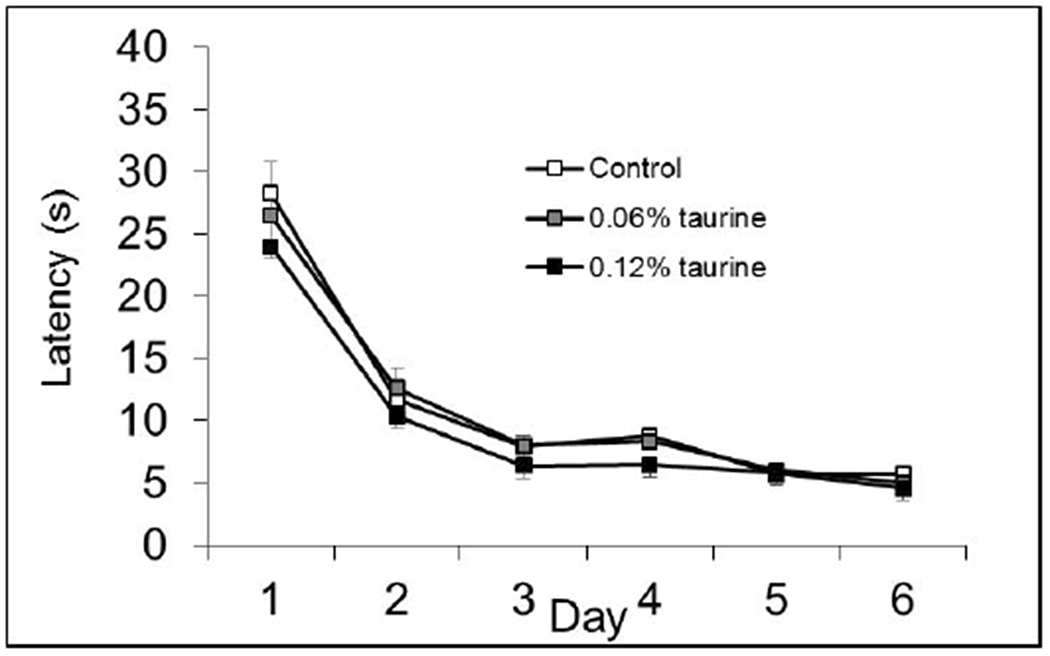
There were no significant differences on any day of testing during the follow-up dose-response studies. P > 0.05.
3.7.1. Hidden platform phases (short-term exposure study).
Path length is reported for all hidden platform phases of Morris water maze, because it is not affected by swim speed. There were no significant differences in the Acquisition Phase (10cm escape platform) based on treatment or sex (Fig. 4A–B)
Fig. 4A. MWM Acquisition Phase (males).
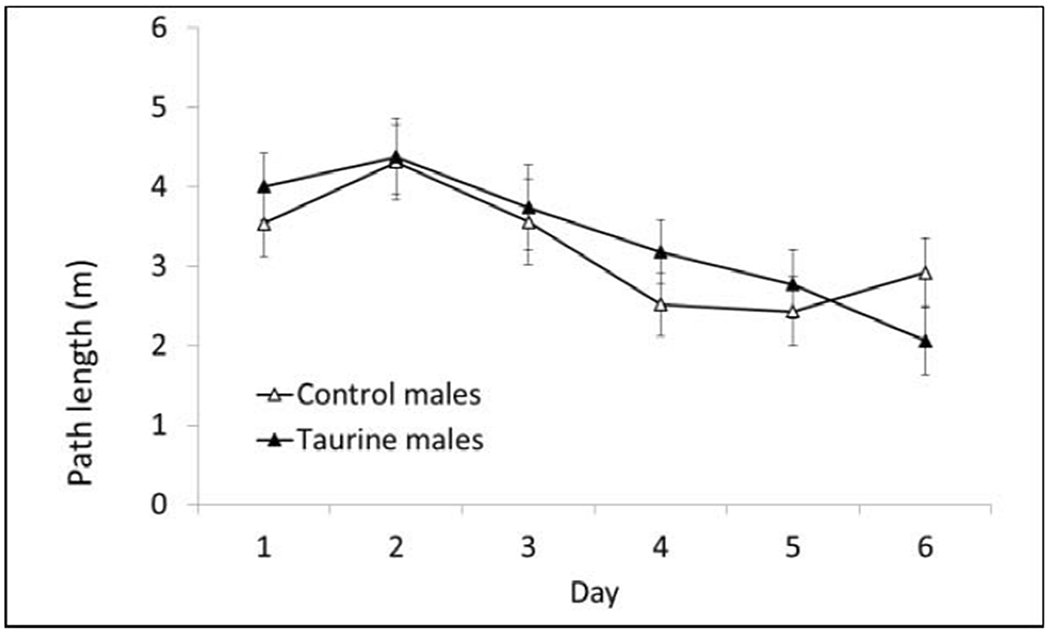
There were no significant differences on any day of testing during the initial studies. P > 0.05.
Fig. 4B. MWM Acquisition Phase (females).
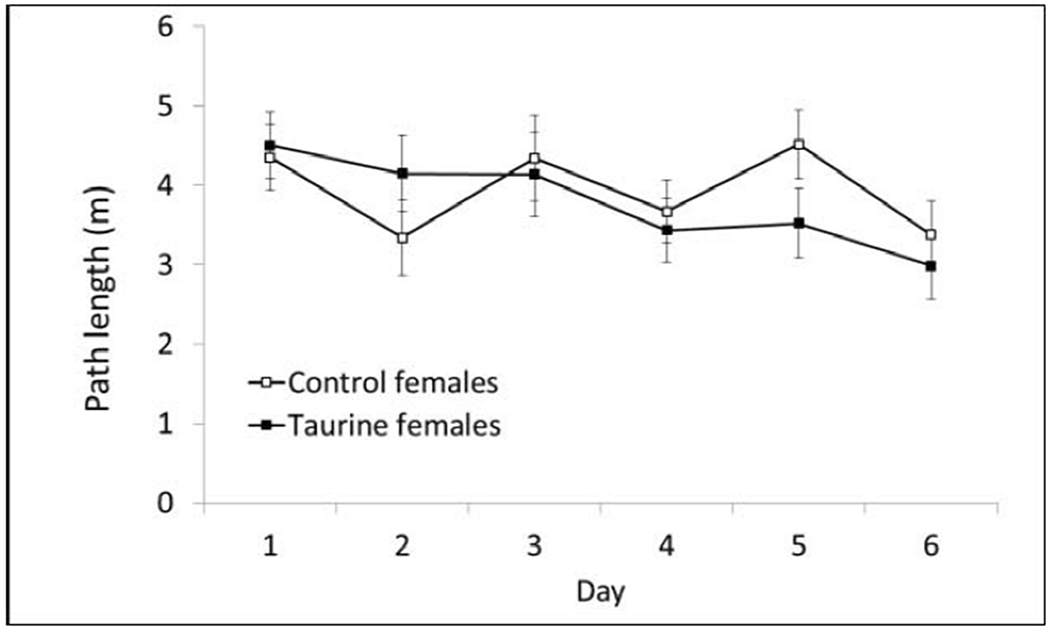
There were no significant differences on any day of testing during the initial studies. P > 0.05.
There was a significant sex x treatment interaction during the two more difficult phases of hidden platform testing. Taurine-treated males had longer path lengths compared with control males whereas taurine-treated females had shorter path lengths on multiple days of testing in the Reverse Phase (7 cm platform) and Shift-reduced Phase (5 cm platform). In these phases, the escape platform was smaller and moved to a new location. These results suggest males are more sensitive to high taurine consumption during adolescence and early adulthood (Figs. 5A–D).
Figs. 5A-D. MWM Reverse and Shift-reduced Phases.
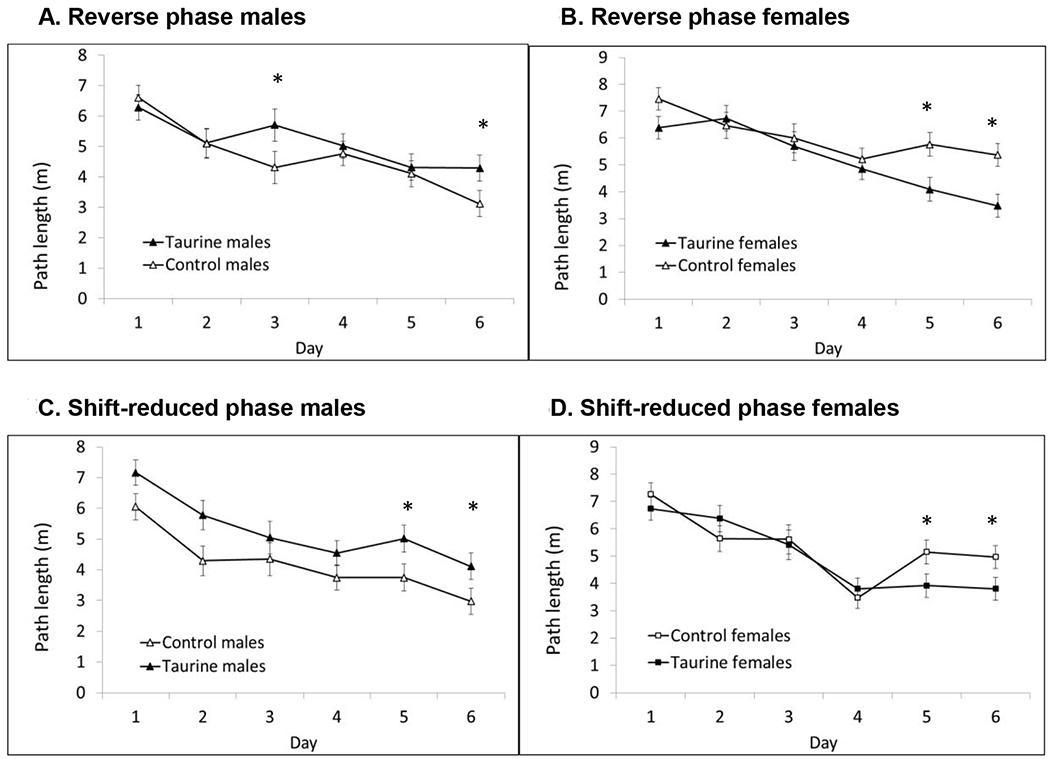
There was a significant sex x treatment interaction in the final two phases of hidden platform testing. The greatest impairments were seen in taurine-treated males in the most difficult Shift-reduced phase. In contrast, taurine-treated females out-performed control females. * P < 0.05
3.7.2. Probe trials (short-term exposure study).
A 30s probe trial was conducted on the day after each hidden platform phase ended. In the Acquisition Phase, taurine-treated females had significantly more platform crossings compared with control females. Taurine-treated males showed no impairments in reference memory in either the Acquisition or Reverse Phase; however, there was a significant sex x treatment interaction in the Shift-reduced phase (P < 0.05). Taurine-treated males had significantly fewer platform crossings whereas taurine-treated females had significantly more when compared with their respective controls (Figs. 6A–C).
Figs. 6A-C. MWM probe trials (short-term exposure study).
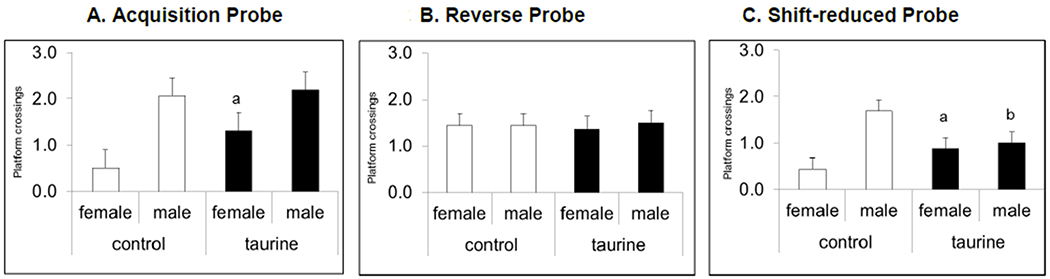
There was a significant sex main effect of sex in the Acquisition probe trials (P < 0.05) and a significant sex x treatment interaction in the Shift-reduced probe trials P < 0.05). Taurine-treated females out-performed control females in the first and third phases. Taurine-treated males showed impaired reference memory in the Shift-reduced probe trial.
a. significantly different from control females, b. significantly different from control males.
3.7.3. Hidden platform phases (dose-response study).
There were no significant differences in the first two hidden platform phases in the dose response study based on sex or treatment (Figs. 7A–B) and no sex x treatment interaction. All mice in all groups improved over the 6 days of testing and showed normal spatial learning and memory. In the most difficult Shift-reduced phase, we unexpectedly found the opposite pattern from the initial, short-term exposure study. Taurine-treated males outperformed controls on the last two days of testing whereas taurine-treated females showed impairments compared with control females (Figs. 7C–D). It’s important to note that taurine treatment continued during testing in the long-term, dose-response study while treatment stopped at P60 in the short-term exposure study. This strongly suggests that timing is important when assessing learning and memory in taurine-treated mice.
Figs. 7A-D. MWM hidden platform phases (long-term, dose-response study).
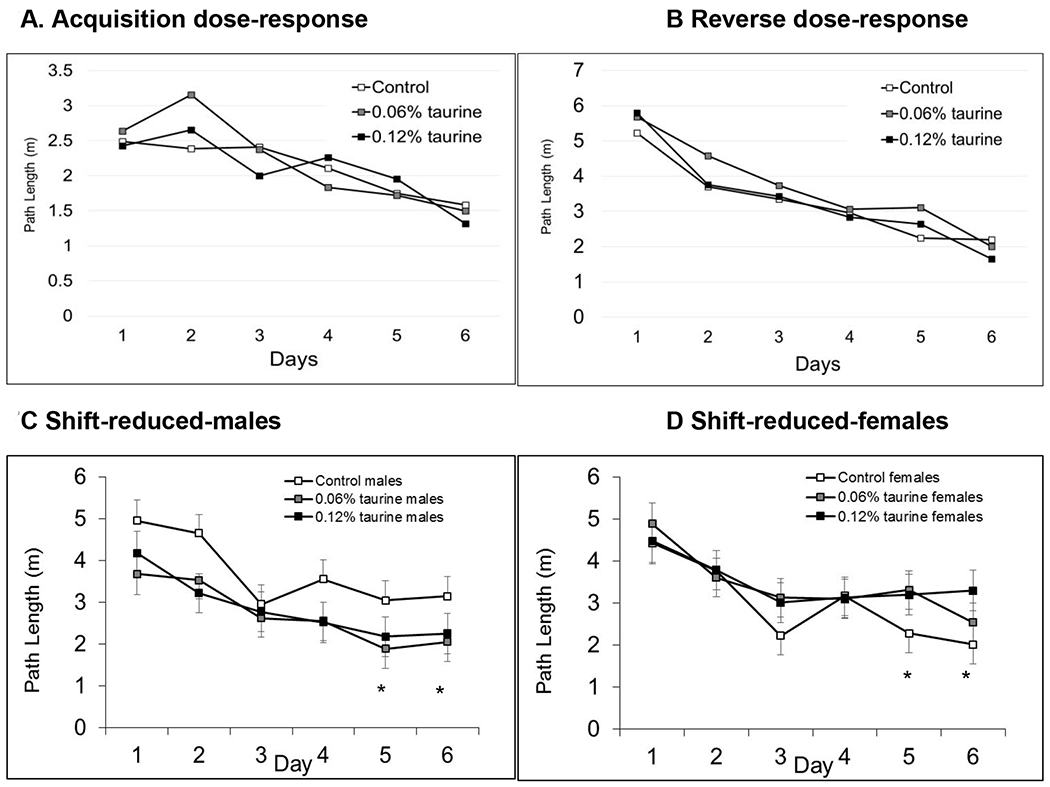
There no differences in the Acquisition and Reverse phases based on sex or treatment. There was a significant sex x treatment interaction in the Shift-reduced phase with both groups of taurine-treated males having significantly shorter path lengths compared with control males on Days 5-6. In contrast, taurine-treated females had significantly longer path lengths compared with control females. * P < 0.05.
3.7.4. MWM Probe trials (long-term, dose-response study).
There were no differences in the probe trial following the Acquisition phase; however, there was a significant sex x treatment interaction in the Reverse and Shift-reduced probe trials (Figs. 8A–C). Taurine-treated females had significantly longer average distances to the target whereas taurine-treated males had significantly shorter average distances to the target compared with controls (P < 0.05).
Figs. 8A-C. MWM probe trials (long-term, dose-response study).
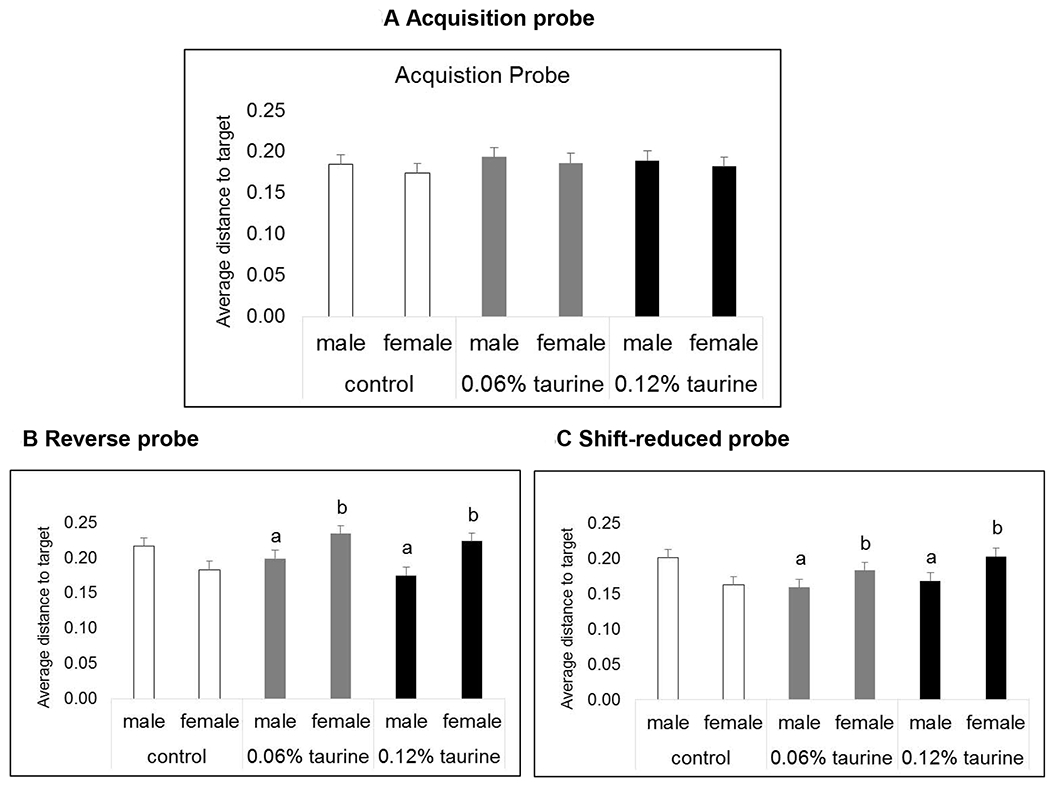
There were no differences in the Acquisition probe trial (P > 0.05). There was a significant sex x treatment interaction in both the Reverse and Shift-reduced probe trials. Taurine-treated males out-performed control males. Taurine-treated females showed impaired reference memory compared with control females.
a. significantly different from control males. b. significantly different from control females.
3.8. Caffeine challenge (short-term exposure study)
In theshort-term exposure study, we treated half of each group with 10mg/kg saline (i.p.) and the other half with the saline vehicle (Fig. 9). Saline-treated females exposed to 0.12% taurine from P30-60 were significantly more active than saline-treated control females (P < 0.05). Males treated with taurine showed an exaggerated response to caffeine compared with control males (P < 0.05). This suggests that supplemental taurine consumption during adolescence and early adulthood can alter the response to caffeine later in life for males and increase baseline locomotor activity in females.
Fig.9. Caffeine challenge (short-term exposure study).
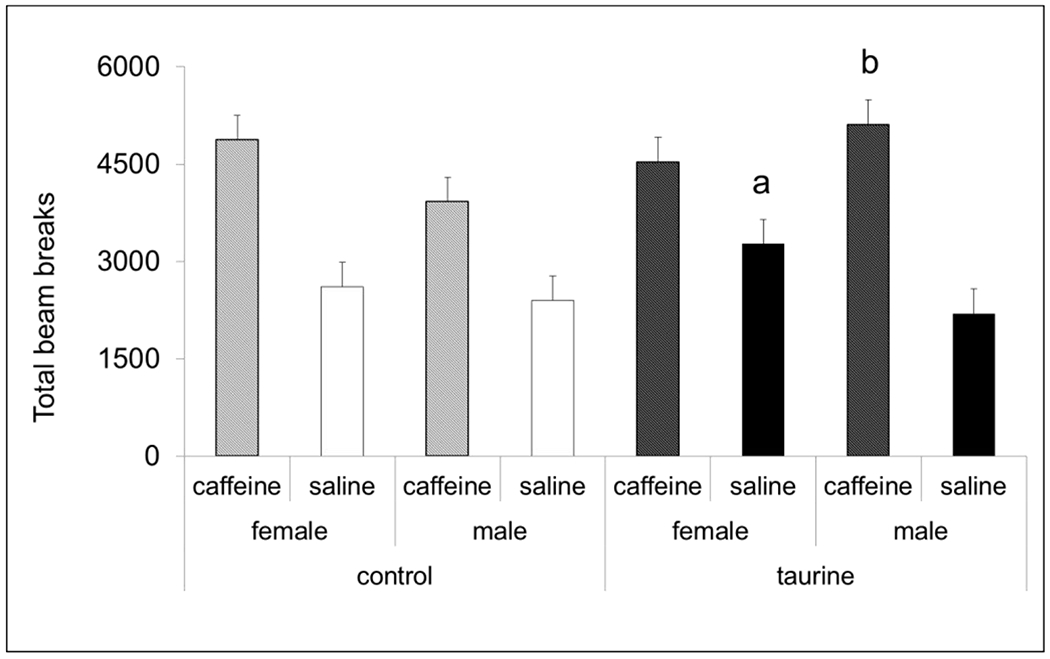
All groups showed increased activity in open field locomotor chambers following caffeine dosing. There was a significant sex x treatment interaction with taurine-treated females having higher baseline activity compared with control females when injected with saline. Taurine-treated males injected with caffeine had significantly higher locomotor activity compared with control males injected with caffeine. n = 8 per group.
a significantly different than control/saline females
b significantly different than control/caffeine males
3.8. Caffeine challenge (dose-response study)
To increase sample size in the dose-response study, baseline locomotor activity was recorded for 30 min for all animals. Each animal then received a 10mg/kg injection of caffeine (i.p.) and locomotor activity was recorded for another 60 min. For analysis, the number of photobeam breaks recorded during the baseline period was compared with the first 30 min of activity following the caffeine dosing. Control mice responded as expected with significantly increased activity following caffeine treatment (P < 0.01). Surprisingly, both taurine-treated groups showed no significant differences in activity before and after caffeine treatment (Fig. 10). This suggests that co-exposure to taurine blunts the effect of the caffeine stimulant.
Fig. 10. Caffeine challenge (dose-response study).
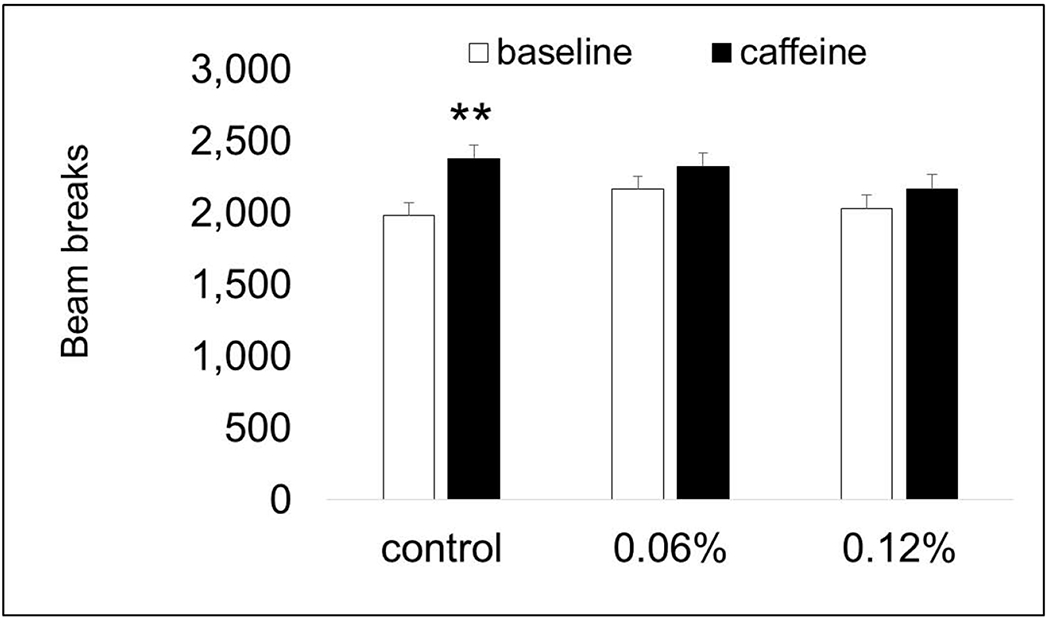
Only control mice showed significantly increased activity following treatment with 10mg/kg caffeine. Both taurine-treated groups had similar activity levels before and after the caffeine treatment. ** P < 0.01.
3.9. Social preference
We used a standard test of social preference and social recognition to determine if supplemental taurine consumption during adolescence and early adulthood affected social recognition memory, which is driven primarily by olfactory cues (Yang et al. 2016). In the first phase, mice are able to explore a three-chambered apparatus with an empty wire cup one side and a stranger mouse inside a wire cup on the opposite side. In the second phase, mice are exposed to the original stranger and a new stranger mouse. Unexpectedly, we found that only control mice spent significantly more time with the stranger mouse (P < 0.05) compared with the empty wire cup (Fig. 11A). Both groups of taurine-treated mice spent nearly equal amounts of time with the stranger mouse and the empty wire cup. In the second phase, however, all groups showed normal social recognition memory (Fig. 11B). The time spent with the new stranger was significantly more than the time spent with the original stranger mouse.
Fig. 11A. Social preference.
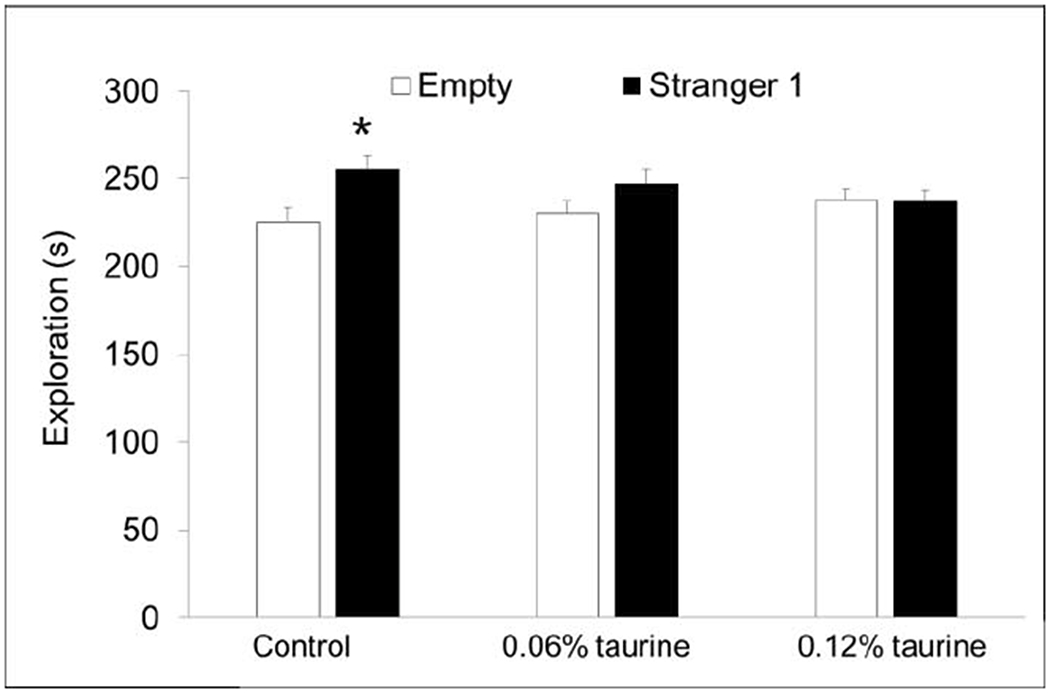
Only control mice showed normal social preference, spending significantly more time exploring the novel mouse. * P < 0.05.
Fig. 11B. Social recognition.
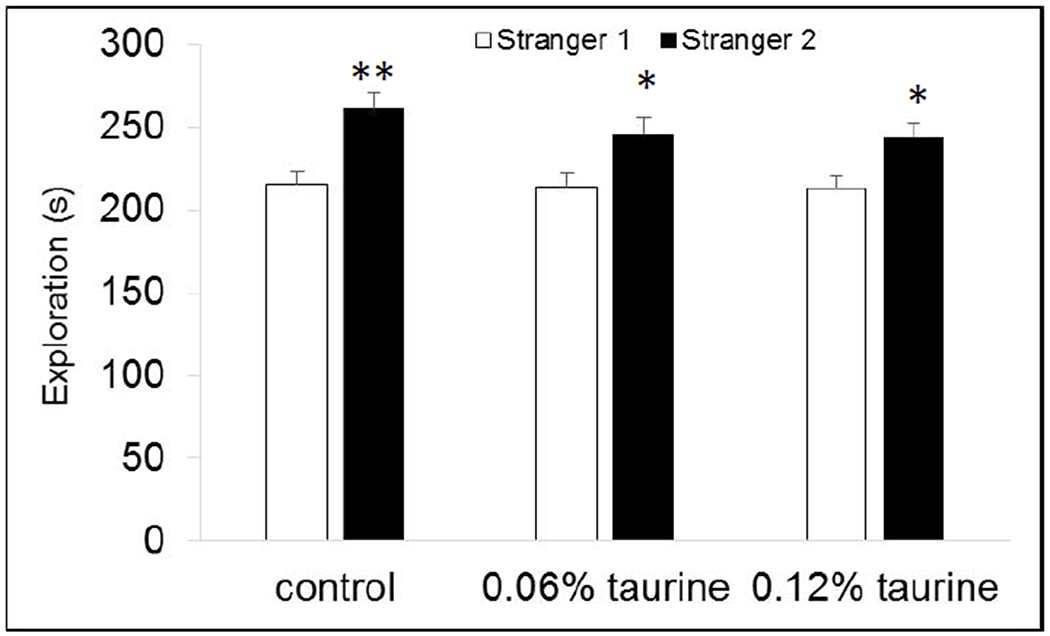
All mice had normal social recognition memory, spending significantly more time with the new stranger mouse than the original stranger. * P < 0.05. ** P < 0.01
3.10. Alcohol preference
There was a significant main effect of sex for alcohol consumption when 2% alcohol (P < 0.01), 5% alcohol (P < 0.05) were provided and for the first 48h when 10% alcohol was provided (P < 0.05). Under all three conditions, females consumed more alcohol compared with males. The differences did not reach significance for total 10% alcohol consumed in 72h (P = 0.064). Interestingly, the sex differences were only apparent in the taurine-treated groups (Fig. 12)
Fig 12. Alcohol preference.
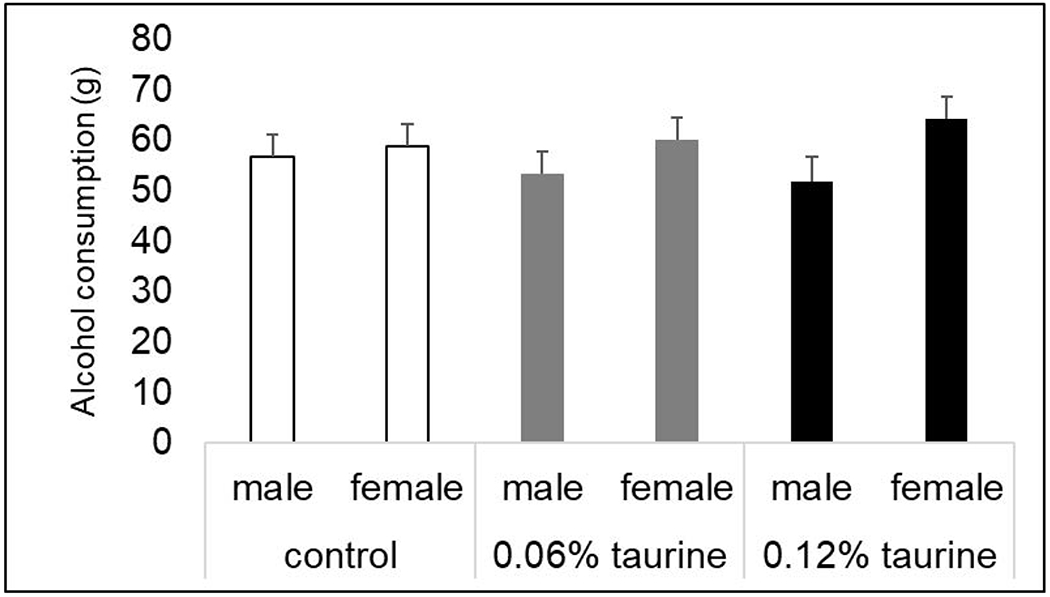
There was a trend for a significant main effect of sex when mice were provided with drinking water and 10% alcohol for 72h. P = 0.064. There was no effect of treatment and no significant sex x treatment interaction.
3.11. Neurotransmitter analysis
3.11.1. Amino acids.
In the initial, short-term study, we measured glutamate, GABA, and taurine levels in the cortex and hypothalamus (Table 1) because taurine reportedly can affect both glutamate Ye et al. 2013) and GABA signaling (Sava et al. 2014, Akita and Okada 2014, Jia et al. 2008). We found significant sex differences in the hypothalamus with females having higher glutamate levels (P < 0.001) and males having higher taurine levels (P < 0.05). There was a trend for females to have higher glutamate levels in the cortex (P < 0.1) and a trend for lower GABA levels in the cortex of taurine-treated mice (P < 0.1).
Table 1.
Amino acid evels (short-term exposure study).
| Tissue | Group | Glutamate | GABA | Taurine |
|---|---|---|---|---|
| Cortex | Control | 155.06 ± 4.32 | 37.34 ± 0.72 | 331.56 ± 6.93 |
| 0.12% taurine | 152.83 ± 4.32 | 35.55 ± 0.72a | 326.24 ± 6.93 | |
| female | 160.04 ± 4.32b | 36.72 ± 0.72 | 328.71 ± 6.93 | |
| male | 147.85 ± 4.32 | 36.17 ± 0.72 | 329.08 ± 6.93 | |
| Tissue | Group | Glutamate | GABA | Taurine |
| Hypothalamus | Control | 85.56 ± 2.57 | 54.79 ± 1.1 | 142.30 ± 2.26 |
| 0.12% taurine | 90.08 ± 2.57 | 56.85 ± 1.1 | 147.69 ± 2.26 | |
| female | 96.03 ± 2.57c | 56.66 ± 1.1 | 140.74 ± 2.26 | |
| male | 79.62 ± 2.57 | 54.98 ± 1.1 | 149.25 ± 2.26d | |
Values are reported as pmol/μg protein. Differences are indicated by bold text.
trend for lower levels in taurine-treated mice P < 0.1,
trend for higher levels in females P < 0.1,
significantly higher in females P < 0.001,
significantly higher in males P < 0.05.
In the long-term, dose-response study, we focused on the prefrontal cortex due its key role in learning and memory and decision-making (Table 2). Again, we found significant sex differences with higher glutamate levels in female mice (P < 0.01) and higher taurine levels in male mice (P < 0.01). There was a trend for a treatment effect (P < 0.1); however the dose response was unexpected. Mice on the lower dose of 0.06% taurine had higher levels than those on the higher 0.12% dose. This suggests some compensation is occurring with taurine transport or metabolism in the high-dose group.
Table 2.
Amino acid evels (long-term, dose-response study).
| Tissue | Group | Glutamate | GABA | Taurine |
|---|---|---|---|---|
| Prefrontal cortex | Control | 181.73 ± 11.85 | 5.96 ± 0.53 | 13.97 ± 0.62 |
| 0.06% taurine | 183.09 ± 11.85 | 4.94 ± 0.53 | 15.86 ± 0.62a | |
| 0.12% taurine | 176.20 ± 11.85 | 5.24 ± 0.53 | 14.24 ± 0.62 | |
| Group | Glutamate | GABA | Taurine | |
| female | 199.14 ± 9.68b | 5.92 ± 0.43c | 13.70 ± 0.51 | |
| male | 161.54 ± 9.68 | 4.85 ± 0.43 | 15.70 ± 0.51d | |
Values are reported as ng/μg protein. Differences are indicated by bold text.
trend for higher levels in 0.06% group. P < 0.1,
significantly higher in females P < 0.01,
trend for higher levels in females, P <0.1,
significantly higher in males P < 0.01.
3.11.2. Monoamine neurotransmitters.
In the initial, short-term exposure study, monoamine neurotransmitters and their primary metabolites were measured in cortex and hypothalamus (Table 3). There was a trend for a sex x treatment interaction for dopamine (P = 0.072) and HVA metabolite levels (P = 0.076) in the cortex with control males having higher levels than control females whereas taurine-treated females had higher levels than taurine-treated males. There was a significant main effect of sex for the serotonin metabolite 5-HIAA (P < 0.01) in both the cortex and hypothalamus with higher levels in females compared with males. There was a significant main effect of treatment (P < 0.05) for serotonin levels in the hypothalamus with higher levels in taurine-treated mice compared with controls.
Table 3.
Monoamine neurotransmitters and metabolites (short-term exposure).
| Tissue | Treatment | Sex | DA | DOPAC | HVA | 5-HT | 5-HIAA |
|---|---|---|---|---|---|---|---|
| Cortex | Control | female | 20.36 ± 2.78 | 2.42 ± 0.25 | 3.85 ± 0.37 | 8.60 ± 0.3 | 5.79 ± 0.2b |
| male | 27.29 ± 2.78 | 2.66 ± 0.25 | 4.63 ± 0.37 | 8.21 ± 0.3 | 4.77 ± 0.2 | ||
| 0.12% taurine | female | 23.24 ± 2.78a | 2.36 ± 0.25 | 4.37 ± 0.37a | 8.61 ± 0.3 | 5.74 ± 0.2b | |
| male | 19.76 ± 2.78a | 2.01 ± 0.25 | 3.80 ± 0.37a | 8.18 ± 0.3 | 4.30 ± 0.2 | ||
| Tissue | Treatment | Sex | DA | DOPAC | HVA | 5-HT | 5-HIAA |
| Hypothalamus | Control | female | 6.04 ± 0.58 | 2.13 ± 0.13 | 3.20 ± 0.21 | 18.26 ± 0.42 | 11.06 ± 0.40b |
| male | 4.76 ± 0.58 | 1.84 ± 0.13 | 3.28 ± 0.21 | 17.98 ± 0.42 | 9.04 ± 0.40 | ||
| 0.12% taurine | female | 5.65 ± 0.58 | 1.94 ± 0.13 | 3.63 ± 0.21c | 19.33 ± 0.42d | 11.74 ± 0.40b | |
| male | 6.16 ± 0.58 | 2.0 ± 0.13 | 3.6 ± 0.21c | 18.79 ± 0.42d | 8.32 ± 0.40 |
Values are reported as ng/mg protein. Differences are indicated by bold text.
trend for sex * treatment interaction with higher levels in females and lower values in males compared to controls ,P < 0.1,
significantly higher in females P < 0.001,
trend for higher values in treated mice P < 0.1
significantly higher in taurine-treated mice.
In the long-term, dose-response study, we compared monoamines in the prefrontal cortex, hippocampus and hypothalamus to further explore differences observed in learning, memory and behavior (Table 4). There was a trend for higher levels of the dopamine metabolite HVA in the prefrontal cortex in the high-dose mice (P = 0.068). There was a significant main effect of sex for the serotonin metabolite 5-HIAA (P < 0.01) in the hippocampus. There was a significant main effect of sex for the serotonin metabolite 5-HIAA (P < 0.001) in the hypothalamus with females having higher levels compared with males. These findings were consistent with the initial, short-term exposure study data. There was also a significant main effect of sex (P < 0.01) with females have having higher levels of the dopamine metabolite DOPAC and a trend for significance (P = 0.066) with females having higher levels of serotonin in the hypothalamus. There was a trend for a significant effect of treatment for HVA with higher levels in both taurine-treated group (P = 0.059)
Table 4.
Monoamine neurotransmitters and metabolites (long-term, dose-response study).
| Tissue | Treatment | Sex | DA | DOPAC | HVA | 5-HT | 5-HIAA |
|---|---|---|---|---|---|---|---|
| Prefrontal cortex | Control | female | 4.16 ± 2.15 | 2.61 ± 0.47 | 2.46 ± 0.49 | 23.33 ± 1.81 | 4.34 ± 0.56 |
| male | 4.03 ± 2.15 | 1.90 ± 0.47 | 1.62 ± 0.49 | 21.64 ± 1.81 | 3.11 ± 0.56 | ||
| 0.06% taurine | female | 2.54 ± 2.15 | 1.95 ± 0.47 | 1.76 ± 0.49 | 18.84 ± 1.81 | 3.29 ± 0.56 | |
| male | 3.71 ± 2.15 | 1.88 ± 0.47 | 1.91 ± 0.49 | 18.64 ± 1.81 | 2.87 ± 0.56 | ||
| 0.12% taurine | female | 6.57 ± 2.15 | 2.41 ± 0.47 | 2.85 ± 0.49a | 20.21 ± 1.81 | 3.68 ± 0.56 | |
| male | 8.52 ± 2.15 | 3.03 ± 0.47 | 3.0 ± 0.49a | 19.36 ± 1.81 | 2.86 ± 0.56 | ||
| Tissue | Treatment | Sex | DA | DOPAC | HVA | 5-HT | 5-HIAA |
| Hippocampus | Control | female | 1.64 ± 1.06 | 1.46 ± 0.16 | 0.80 ± 0.34 | 21.32 ± 1.61 | 6.80 ± 0.66b |
| male | 1.17 ± 1.06 | 1.40 ± 0.16 | 0.55 ± 0.34 | 23.31 ± 1.61 | 6.32 ± 0.66 | ||
| 0.06% taurine | female | 1.83 ± 1.06 | 3.68 ± 0.16 | 1.46 ± 0.34 | 23.38 ± 1.61 | 8.07 ± 0.66b | |
| male | 1.58 ± 1.06 | 1.57 ± 0.16 | 0.75 ± 0.34 | 24.41 ± 1.61 | 6.31 ± 0.66 | ||
| 0.12% taurine | female | 1.17 ± 1.06 | 1.50 ± 0.16 | 0.72 ± 0.34 | 25.75 ± 1.61 | 8.81 ± 0.66b | |
| male | 0.92 ± 1.06 | 1.54 ± 0.16 | 0.61 ± 0.34 | 25.0 ± 1.61 | 6.60 ± 0.66 | ||
| Tissue | Treatment | Sex | DA | DOPAC | HVA | 5-HT | 5-HIAA |
| Hypothalamus | Control | female | 6.22 ± 0.67 | 3.48 ± 0.22b | 3.38 ± 0.28 | 39.37 ± 1.65d | 11.70 ± 0.6e |
| male | 6.23 ± 0.67 | 3.08 ± 0.22 | 3.26 ± 0.28 | 36.46 ± 1.65 | 8.21 ± 0.6 | ||
| 0.06% taurine | female | 6.83 ± 0.67 | 3.91 ± 0.22b | 4.34 ± 0.28c | 39.94 ± 1.65d | 12.21 ± 0.6e | |
| male | 5.54 ± 0.67 | 2.87 ± 0.22 | 3.56 ± 0.28c | 38.61 ± 1.65 | 9.11 ± 0.6 | ||
| 0.12% taurine | female | 6.66 ± 0.67 | 3.54 ± 0.22b | 3.93 ± 0.28c | 42.17 ± 1.65d | 13.05 ± 0.6e | |
| male | 6.26 ± 0.67 | 3.47 ± 0.22 | 3.84 ± 0.28c | 38.75 ± 1.65 | 9.28 ± 0.6 |
Values are reported as ng/mg protein. Differences are indicated by bold text.
trend for higher values in 0.12% taurine group, P < 0.1,
significantly higher in females. P < 0.01,
trend for higher levels in taurine-treated mice P < 0.1,
trend for higher values in females P < 0.1,
significantly higher in females P < 0.001.
4. Discussion
4.1. Key findings.
We conducted an initial, short-term exposure study and follow-up, dose-response study to understand the effects of supplemental taurine consumption during adolescence and early adulthood using C57BL/6J male and female mice and a broad range of behavioral tests. We found no evidence that taurine exposure increased anxiety based on results from the zero maze and marble burying experiments. There was no evidence of hyperactivity except during the caffeine challenge in the short-term study where taurine-treated females injected with saline had significantly higher open field locomotor activity than control females injected with saline. Since this experiment had the smallest sample size (n = 8 per group), the results should be interpreted with caution. Sensorimotor gating was normal in all groups.
The greatest impact of taurine treatment was seen in tests of learning and memory. There was a trend for a significant main effect of treatment in novel object recognition in the short-term exposure study and a significant main effect of treatment in the long-term, dose-response study. There was a significant sex x treatment interaction in Morris water maze in both studies; however, the affected sex was different. In the initial study where taurine treatment stopped when behavior testing began, males were impaired in spatial learning and memory while females had improved performance over controls. However, in the dose-response study where taurine treatment continued throughout behavioral testing, females were impaired and males outperformed controls. This indicates that both males and females are susceptible to adverse effects of high-dose taurine during adolescence and early adulthood, depending on the timing and duration of exposure.
There were also sex and treatment effects in the response to caffeine. In the short-term exposure study, taurine-treated males had a heightened response to caffeine. In the long-term, dose-response study, both taurine treated groups had a blunted response to the stimulant. Taurine-treated mice showed normal social recognition memory; however, both taurine-treated groups exhibited impaired sociality in the social preference test. Females had higher alcohol consumption than males, a difference driven primarily by higher consumption in the two taurine-treated groups.
There were multiple differences in neurotransmitter levels and taurine levels in both studies. Females had higher levels of glutamate in the cortex and hypothalamus and greater dopamine and serotonin turnover, based on higher levels of the primary metabolites DOPAC and 5-HIAA. Males had higher levels of taurine. The key differences were replicated in the dose-response study, suggesting that underlying sex differences in neurotransmitter levels should be further explored to determine their role in taurine-induced neurotoxicity. Shortterm taurine exposure significantly increased serotonin levels in the hypothalamus, which could possibly explain the negative findings in tests of anxiety-like behavior. Long-term taurine exposure altered neurotransmitter levels in the cortex with a sex * treatment interaction for dopamine and HVA. Levels were higher in taurine-treated females compared with control females and taurine-treated males. Taurine-treated males had significantly lower levels compared with control males. These results may not be fully informative, because changes in dopamine signaling during brain development and maturation can also have long-term effects on cognition and behavior (Money and Stanwood 2013). The only significant difference in the hippocampus was higher 5-HIAA levels in females, which again, is not sufficient to explain changes in tests of spatial and non-spatial learning and memory.
4.2. Comparison with related studies
Our studies are consistent with the findings of Suge et al. (2007) who reported both beneficial and adverse effects in male C57BL/6 mice depending on the age when taurine treatment began and the length of exposure. Importantly, Suge also reported that the longest exposure periods had the most adverse effects. Neuwirth et al. (2013) reported an increased response in cued and contextual fear conditioning in FVB/NJ male mice treated with 0.05% taurine in drinking water from P28 to six months of age, but an attenuated response in mice treated with an acute dose of taurine (43 mg/kg/s.c.). FVB/NJ male mice treated with 0.05% taurine for 4 weeks also had impaired motor coordination on the rotarod test (Santora et al. 2013). Our work extends these findings by including both male and female mice, uncovering sex differences in multiple tests of learning and memory and behavior.
4.3. Relevance to taurine as a neuroprotectant
Numerous studies have provided strong evidence that taurine’s antioxidant and anti-inflammatory properties make it an effective neuro-protectant in the aged brain and following toxic insults to the nervous system (reviewed in Chen et al. 2019). Recent examples include the use of taurine to prevent or reverse damage caused by manganese exposure (Ahmadi et al. 2018, Ommati et al. 2018, Lu et al. 2014). Cui et al. (2019) showed that taurine supplementation can attenuate hyperactivity in mice exposed prenatally to PM2.5. Taurine supplementation improved learning and motor function in an Angelman’s mouse model (Guzzetti et al. 2018), lowered beta-amyloid levels in an Alzheimer’s disease mouse model (Zhu et al. 2019), and inhibited microglia-mediated neuroinflammation and protected dopaminergic neurons in mice treated with paraquat and maneb to mimic Parkinson’s disease (Hou et al. 2018). Taurine also restored levels of nerve growth factor and brain-derived neurotrophic factor in rats exposed to the flame retardant hexabromocyclododecane and rescued deficits in the Morris water maze (Zhang et al. 2017).
Human studies have demonstrated the effectiveness of taurine supplementation in maintaining blood-brain barrier integrity and reducing inflammation in elderly women (Chupel et al. 2018). However, there have been mixed results in clinical trials of the related compound homotaurine in patients with Alzheimer’s disease and mild cognitive impairment (Tsolaki 2019). These reports do not contradict our findings, because the effects were all reported under conditions of taurine depletion, oxidative stress and/or inflammation. Our findings of improved performance of taurine-treated female mice in the Morris water maze under the short-term exposure and improved performance of taurine-treated male mice under the long-term exposure does suggest some level of taurine may be beneficial in adolescence or young adulthood. However, the concern remains that novel object recognition was impaired regardless of sex.
4.4. Taurine changes over the lifespan
Taurine is found in high concentrations in the mammalian central nervous system (Huxtable 1989). Taurine levels are greatest in the developing brain and characteristically decrease throughout adulthood (Suárez et al. 2016, Huxtable 1992, Banay-Schwartz et al. 1989). Studies in mice found that cerebral taurine concentrations are maximal within one week of age and drastically decrease by four weeks of age (Kontro et al. 1984). Taurine is essential for neurological development and function, most notably as a trophic factor during CNS development (Ye et al. 2013). Taurine transporter (TauT) knockout mice lose the ability to accumulate necessary levels of taurine, resulting in numerous congenital anomalies including neurological defects (Lambert et al. 2015, Lotsch et al. 2014).
The importance of taurine to the normally developing brain cannot be overlooked, but the human brain continues to develop through adolescence and early adulthood (Gogtay et al. 2004). Since taurine levels are naturally at their highest during this period, it is unlikely that taurine supplementation is needed in a healthy individual. Instead, our findings suggest that excess taurine will have a negative and persistent effect on adult cognitive function and behavior.
4.5. Possible mechanisms of taurine neurotoxicity
There are numerous, biologically plausible mechanisms whereby excess taurine could interfere with adolescent brain maturation and adult brain function. In primary hippocampal neurons, taurine increased synaptic protein translation and in neural progenitor cells, taurine stimulated the formation of synapses and promoted neuronal proliferation in early postnatal development (Shivaraj et al 2012). Since adolescence is an important time period for synapse modification including pruning, supplemental taurine could alter the balance between new synapse formation and synaptic pruning during final cortical maturation.
Abrupt withdrawal from taurine supplementation should be considered for the initial, short-term exposure experiments. However, the consistency in novel object impairments across both studies and the fact that impairments were seen in the later tests conducted argues against that explanation. Withdrawal effects, if present, should have manifested most acutely during the first weeks of behavioral testing.
Taurine interacts with multiple neurotransmitters and brain regions. The hypothalamus is one of the most sensitive brain regions to oral taurine administration (Hruska 1976), and hypothalamic serotonin turnover was decreased by taurine administration in male rats (Aldegunde et al. 1983). Taurine was found to inhibit or stimulate norepinephrine release in a concentration and brain region dependent manner (Huxtable 1992). Striatal dopaminergic neuronal activity is decreased by taurine (Hashimoto-Kitsukawa et al. 1988a, 1988b). Still, the most likely targets of high-dose taurine are pathways related to glutamate and GABA.
Taurine stimulates action potentials in GABAergic neurons (Jia, et al. 2008). Taurine acts as a gliotransmitter and neurotransmitter by agonizing the inhibitory GABA type A (GABAAR), GABA type B (GABABR), and glycine (GlyR) receptors on postsynaptic and extrasynaptic binding sites in the brain (Sava et al 2014, Jia et al. 2008, Albrecht and Schousboe 2005). Taurine binding to these receptors triggers chloride ionic currents in GABAergic and glycinergic neurons (El Idrissi & Trenkner 2004). GABAaR and GlyR activation in response to taurine were reported in brain regions including cerebral cortex (Sava 2014), hippocampus (Mori et al. 2002, del Olmo et al. 2000), striatum (Chepkova et al. 2002), substantia nigra (Ye et al. 1997), thalamus (Jia et al. 2008), and cerebellum (El Idrissi and Trenkner 2004).
Taurine-mediated GABAAR activation is a possible mechanism for learning and memory deficits, because activation negates the effects of the glutamate N-methyl-D-aspartate (NMDA) receptor pathway. Additionally taurine has high specificity and affinity for the glycine receptor GlyR (Albrecht & Schousboe 2005). Taurine binding to GlyR mediates glia-to-neuron signaling in the hypothalamus, enhances synaptic transmission in the striatum, and mediates excitatory neurotransmission in early development of the neocortex and brain stem (El Idrissi & Trenkner 2004). Sava et al. (2014) found that taurine selectively initiates and increases frequency of excitatory GABAergic neurotransmission in the cortex of mice through binding at GlyR.
Together, these findings suggest that multiple mechanisms could be important in high-dose taurine neurotoxicity in the adolescent and young adult brain.
4.6. Limitations
Although there were many similarities across both studies, the differences in exposure period do limit cross comparisons. The long period between treatment cessation and neurotransmitter measurements in the initial study limited our ability to determine if taurine supplementation significantly altered levels of taurine in the brain; however, prior studies have demonstrated that 45% of dietary taurine is taken up by the brain (Huxtable and Lippincott 1982). There were no measurements of cell death, cell injury or brain histology to demonstrate an overt neurotoxic effects from supplemental taurine. Dietary taurine can also affect sodium intake, which could ultimately change neurochemistry and behavior. This potential confounder was not addressed in our studies.
4.7. Future directions
A strength of our studies was the use of male and female mice to identify sex differences following supplemental taurine exposure during adolescence and early adulthood. We found significant sex differences in glutamate levels and dopamine and serotonin turnover with the greatest changes in the hypothalamus. These are consistent with neurotransmitter systems know to be affected by taurine and offer insights in future directions. We were not able to identify a threshold where there were no adverse effects, so an important area of future study would be modifying the dosing protocol to include both lower doses and alternate exposure periods (e.g. intermittent v. daily dosing). Importantly, impairments in male mice were greatest when taurine supplementation ended. So, future studies should also include measurements of learning, memory and behavior in older mice exposed during adolescence and early adulthood. All of the tests used in these studies are amenable to testing aged mice. The hypothesis that taurine disrupts the normal glutamate-GABA balance in the brain can be tested by looking for changes in receptor levels and receptor binding. Because of the affinity of taurine for the glycine receptor, glycine and GlyR signaling should also be evaluated. It is also plausible that taurine transporters are down-regulated at the blood-brain barrier and elsewhere in the nervous system. This could prevent taurine from carrying out its normal physiological functions.
In the absence of regulation regarding sales of energy drinks to minors in the United States, it will also be important to further explore the co-exposure to taurine and caffeine in adolescents and young adults. Most of the studies have focused on exercise physiology to examine claims that energy drinks enhance performance (Scholey & Kennedy 2004). More work is needed to examine cognitive and behavioral endpoints and the individual and cumulative effects of the major ingredients. In conclusion, our findings suggest caution is warranted by both males and females when considering whether to regularly consume taurine supplements and/or energy drinks containing high levels of the amino acid.
Highlights.
Supplemental taurine consumption during adolescence alters behavior and cognitive function in mice
Supplemental taurine consumption during adolescence alters monoamine neurotransmitters
Effects vary depending on sex, dose, and the length of treatment
Impairments in object recognition were independent of dose and sex
Future studies are needed on the effects of energy drinks on adolescents and young adults
4.8. Acknowledgements
We gratefully acknowledge funding from NIH grants ES020053, GM103436 (Curran) and AA019795 (Marczinski) and NSF RSF-034-07 to support the purchase of neurobehavioral equipment and student research assistants. Additional funding was provided by the Northern Kentucky University Honors program, UR-STEM Fellowships, Dorothy Westerman Herrmann Fellowship, Faculty Development Project Grant and College of Arts and Sciences Collaborative Faculty-Student Project Award.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
5. References
- Ahmadi N, Ghanbarinejad V, Ommati MM, Jamshidzadeh A, Heidari R, 2018. Taurine prevents mitochondrial membrane permeabilization and swelling upon interaction with manganese: Implication in the treatment of cirrhosis-associated central nervous system complications. J. Biochem. Mol. Toxicol 32, e22216 10.1002/jbt.22216 [DOI] [PubMed] [Google Scholar]
- Akita T, Okada Y, 2014. Characteristics and roles of the volume-sensitive outwardly rectifying (VSOR) anion channel in the central nervous system. Neuroscience 275, 211–231. 10.1016/j.neuroscience.2014.06.015 [DOI] [PubMed] [Google Scholar]
- Albrecht J, Schousboe A, 2005. Taurine interaction with neurotransmitter receptors in the CNS: an update. Neurochem. Res 30, 1615–1621. 10.1007/s11064-005-8986-6 [DOI] [PubMed] [Google Scholar]
- Aldegunde M, Miguez I, Martin I, Fernando Otero MP, 1983. Changes in brain monoamine metabolism associated with hypothermia induced by intraperitoneally administered taurine in the rat. IRCS J. Med. Sci 11:3, 258–259 [Google Scholar]
- [AMR] Allied Market Research, 2019. Energy drink market by type and end user: Global opportunity analysis and industry forecast, 2019-2026. [Google Scholar]
- Andersen SL, Teicher MH, 2000. Sex differences in dopamine receptors and their relevance to ADHD. Neurosci Biobehav Rev 24, 137–141. 10.1016/s0149-7634(99)00044-5 [DOI] [PubMed] [Google Scholar]
- Banay-Schwartz M, Lajtha A, Palkovits M, 1989. Changes with aging in the levels of amino acids in rat CNS structural elements. II. Taurine and small neutral amino acids. Neurochem. Res 14, 563–570. 10.1007/bf00964919 [DOI] [PubMed] [Google Scholar]
- Chen C, Xia S, He J, Lu G, Xie Z, Han H, 2019. Roles of taurine in cognitive function of physiology, pathologies and toxication. Life Sci. 231, 116584 10.1016/j.lfs.2019.116584 [DOI] [PubMed] [Google Scholar]
- Chepkova AN, Doreulee N, Yanovsky Y, Mukhopadhyay D, Haas HL, Sergeeva OA, 2002. Long-lasting enhancement of corticostriatal neurotransmission by taurine. Eur. J. Neurosci 16, 1523–1530. 10.1046/j.1460-9568.2002.02223.x [DOI] [PubMed] [Google Scholar]
- Chupel MU, Minuzzi LG, Furtado G, Santos ML, Hogervorst E, Filaire E, Teixeira AM, 2018. Exercise and taurine in inflammation, cognition, and peripheral markers of blood-brain barrier integrity in older women. Appl Physiol Nutr Metab 43, 733–741. 10.1139/apnm-2017-0775 [DOI] [PubMed] [Google Scholar]
- Cui J, Fu Y, Lu R, Bi Y, Zhang L, Zhang C, Aschner M, Li X, Chen R, 2019. Metabolomics analysis explores the rescue to neurobehavioral disorder induced by maternal PM2.5 exposure in mice. Ecotoxicol. Environ. Saf 169, 687–695. 10.1016/j.ecoenv.2018.11.037 [DOI] [PubMed] [Google Scholar]
- Curran CP, Altenhofen E, Ashworth A, Brown A, Kamau-Cheggeh C, Curran M, Evans A, Floyd R, Fowler J, Garber H, Hays B, Kraemer S, Lang A, Mynhier A, Samuels A, Strohmaier C, 2012. Ahrd Cyplci2(−/−) mice show increased susceptibility to PCB-induced developmental neurotoxicity. Neurotoxicology 33, 1436–1442. 10.1016/j.neuro.2012.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran CP, Marczinski CA, 2017. Taurine, caffeine, and energy drinks: Reviewing the risks to the adolescent brain. Birth Defects Res 109, 1640–1648. 10.1002/bdr2.1177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran CP, Nebert DW, Genter MB, Patel KV, Schaefer TL, Skelton MR, Williams MT, Vorhees CV, 2011. In utero and lactational exposure to PCBs in mice: adult offspring show altered learning and memory depending on Cyp1a2 and Ahr genotypes. Environ. Health Perspect. 119, 1286–1293. 10.1289/ehp.1002965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Olmo N, Bustamante J, del Rio RM, Solis JM, 2000. Taurine activates GABA(A) but not GABA(B) receptors in rat hippocampal CA1 area. Brain Res. 864, 298–307. 10.1016/s0006-8993(00)02211-3 [DOI] [PubMed] [Google Scholar]
- El Idrissi A, Trenkner E, 2004. Taurine as a modulator of excitatory and inhibitory neurotransmission. Neurochem. Res 29, 189–197. 10.1023/b:nere.0000010448.17740.6e [DOI] [PubMed] [Google Scholar]
- Eminian S, Hajdu SD, Meuli RA, Maeder P, Hagmann P, 2018. Rapid high resolution T1 mapping as a marker of brain development: Normative ranges in key regions of interest. PLoS ONE 13, e0198250 10.1371/journal.pone.0198250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giles GE, Mahoney CR, Brunyé TT, Gardony AL, Taylor HA, Kanarek RB 2012. Differential cognitive effects of energy drink ingredients: Caffeine, taurine, and glucose. Pharmacol Biochem Behav. 102(4):569–77. 10.1016/j.pbb.2012.07.004. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF, Herman DH, Clasen LS, Toga AW, Rapoport JL, Thompson PM, 2004. Dynamic mapping of human cortical development during childhood through early adulthood. Proc. Natl. Acad. Sci. U.S.A 101, 8174–8179. 10.1073/pnas.0402680101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzzetti S, Calzari L, Buccarello L, Cesari V, Toschi I, Cattaldo S, Mauro A, Pregnolato F, Mazzola SM, Russo S, 2018. Taurine Administration Recovers Motor and Learning Deficits in an Angelman Syndrome Mouse Model. Int J Mol Sci 19 10.3390/ijms19041088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto-Kitsukawa S, Okuyama S, Aihara H, 1988a. Enhancing effect of taurine in the rat caudate spindle. II. Effect of bilateral 6-hydroxydopamine lesions of the nigro-striatal dopamine system. Pharmacol. Biochem. Behav 31, 417–423. 10.1016/0091-3057(88)90368-1 [DOI] [PubMed] [Google Scholar]
- Hashimoto-Kitsukawa S, Okuyama S, Aihara H, 1988b. Enhancing effect of taurine on the rat caudate spindle. I: Interaction of taurine with the nigro-striatal dopamine system. Pharmacol. Biochem. Behav 31, 411–416. 10.1016/0091-3057(88)90367-x [DOI] [PubMed] [Google Scholar]
- Hou L, Che Y, Sun F, Wang Q, 2018. Taurine protects noradrenergic locus coeruleus neurons in a mouse Parkinson’s disease model by inhibiting microglial Ml polarization. Amino Acids 50, 547–556. 10.1007/s00726-018-2547-1 [DOI] [PubMed] [Google Scholar]
- Hruska RE, Thut PD, Huxtable RJ, Bressler R, 1975. Suppression of conditioned drinking by taurine and related compounds. Pharmacol. Biochem. Behav 3, 593–599. 10.1016/0091-3057(75)90179-3 [DOI] [PubMed] [Google Scholar]
- Huxtable RJ Lippincott SE 1982. Diet and biosynthesis as sources of taurine in the mouse. J Nutr. 112(5): 1003–10. 10.1093/jn/112.5.1003 [DOI] [PubMed] [Google Scholar]
- Huxtable RJ, 1989. Taurine in the central nervous system and the mammalian actions of taurine. Prog. Neurobiol 32, 471–533. 10.1016/0301-0082(89)90019-1 [DOI] [PubMed] [Google Scholar]
- Huxtable RJ, 1992. Physiological actions of taurine. Physiol. Rev 72, 101–163. 10.1152/physrev.1992.72.1.101 [DOI] [PubMed] [Google Scholar]
- Jia F, Yue M, Chandra D, Keramidas A, Goldstein PA, Homanics GE, Harrison NL, 2008. Taurine is a potent activator of extrasynaptic GABA(A) receptors in the thalamus. J. Neurosci 28, 106–115. 10.1523/JNEUROSCI.3996-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang D, Lu X, Li Z, Rydberg N, Zuo C, Peng F, Hua F, Guan Y, Xie F, 2018. Increased Vesicular Monoamine Transporter 2 (VMAT2) and Dopamine Transporter (DAT) Expression in Adolescent Brain Development: A Longitudinal Micro-PET/CT Study in Rodent. Front Neurosci 12, 1052 10.3389/finins.2018.01052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG, 2010. Improving Bioscience Research Reporting: The ARRIVE Guidelines for Reporting Animal Research. PLOS Biology 8, el000412 10.1371/journal.pbio.1000412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kontro P, Mamela KM, Oja SS, 1984. GABA, taurine and hypotaurine in developing mouse brain. Acta Physiol Scand Suppl 537, 71–74. [PubMed] [Google Scholar]
- Lambert IH, Kristensen DM, Holm JB, Mortensen OH, 2015. Physiological role of taurine--from organism to organelle. Acta Physiol (Oxf) 213, 191–212. 10.1111/apha.12365 [DOI] [PubMed] [Google Scholar]
- Lötsch J, Hummel T, Warskulat U, Coste O, Häussinger D, Geisslinger G, Tegeder I, 2014. Congenital taurine deficiency in mice is associated with reduced sensitivity to nociceptive chemical stimulation. Neuroscience 259, 63–70. 10.1016/j.neuroscience.2013.11.037 [DOI] [PubMed] [Google Scholar]
- Lu C-L, Tang S, Meng Z-J, He Y-Y, Song L-Y, Liu Y-P, Ma N, Li X-Y, Guo SC, 2014. Taurine improves the spatial learning and memory ability impaired by sub-chronic manganese exposure. J. Biomed. Sci 21, 51 10.1186/1423-0127-21-51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marczinski CA, 2015. Can energy drinks increase the desire for more alcohol? Adv Nutr 6, 96–101. 10.3945/an.114.007393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marczinski CA, Fillmore MT, 2014. Energy drinks mixed with alcohol: what are the risks? Nutr. Rev 72 Suppl 1, 98–107. 10.1111/nure.12127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marczinski CA, Fillmore MT, Henges AL, Ramsey MA, Young CR, 2013. Mixing an energy drink with an alcoholic beverage increases motivation for more alcohol in college students. Alcohol. Clin. Exp. Res 37, 276–283. 10.1111/j.1530-0277.2012.01868.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marczinski CA, Fillmore MT, Stamates AL, Maloney SF, 2016. Desire to drink alcohol is enhanced with high caffeine energy drink mixers. Alcohol. Clin. Exp. Res 40, 1982–1990. 10.1111/acer.13152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan TM, Lieberman HR, 2012. Do energy drinks contain active components other than caffeine? Nutr. Rev 70, 730–744. 10.1111/j.1753-4887.2012.00525.x [DOI] [PubMed] [Google Scholar]
- Money KM., Standwood GD 2013. Developmental origins of brain disorders: roles for dopamine. Front Cell Neurosci. 7:260 10.3389/fincel.2013.00260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori M, Gähwiler BH, Gerber U, 2002. Beta-alanine and taurine as endogenous agonists at glycine receptors in rat hippocampus in vitro. J. Physiol. (Lond.) 539, 191–200. 10.1113/jphysiol.2001.013147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuwirth LS, Volpe NP, El Idrissi A, 2013. Taurine effects on emotional learning and memory in aged mice: neurochemical alterations and differentiation in auditory cued fear and context conditioning. Adv. Exp. Med. Biol 775, 195–214. 10.1007/978-1-4614-6130-2_17 [DOI] [PubMed] [Google Scholar]
- Ommati MM., Heidari R, Ghanbarinejad V, Abdoli N, Niknahad H, 2019. Taurine Treatment Provides Neuroprotection in a Mouse Model of Manganism. Biol Trace Elem Res 190, 384–395. 10.1007/s12011-018-1552-2 [DOI] [PubMed] [Google Scholar]
- Panagiotakopoulos L, Neigh GN, 2014. Development of the HPA axis: where and when do sex differences manifest? Front Neuroendocrinol 35, 285–302. 10.1016/j.yfrne.2014.03.002 [DOI] [PubMed] [Google Scholar]
- Paquola C, Bethlehem RA, Seidlitz J, Wagstyl K, Romero-Garcia R, Whitaker KJ, Vos de Wael R, Williams GB, NSPN Consortium, Vértes PE, Margulies DS, Bernhardt B, Bullmore ET, 2019. Shifts in myeloarchitecture characterise adolescent development of cortical gradients. Elife 8 10.7554/eLife.50482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radian Insights, 2018. Global sports & energy drinks market research report 2018. https://www.radiantinsights.com/research/global-sports-amp-energy-drinks-market-research-report-2018
- Reissig CJ, Strain EC, Griffiths RR, 2009. Caffeinated energy drinks—a growing problem. Drug Alcohol Depend 99, 1–10. 10.1016/j.drugalcdep.2008.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rincón-Cortés M, Herman JP, Lupien S, Maguire J, Shansky RM, 2019. Stress: Influence of sex, reproductive status and gender. Neurobiol Stress 10, 100155 10.1016/j.ynstr.2019.100155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AG, Lopez-Duran NL, 2019. Developmental influences on stress response systems: Implications for psychopathology vulnerability in adolescence. Compr Psychiatry 88, 9–21. 10.1016/j.comppsych.2018.10.008 [DOI] [PubMed] [Google Scholar]
- Santora A, Neuwirth LS, L’Amoreaux WJ, Idrissi AE, 2013. The effects of chronic taurine supplementation on motor learning. Adv. Exp. Med. Biol 775, 177–185. 10.1007/978-1-4614-6130-2_15 [DOI] [PubMed] [Google Scholar]
- Sava BA, Chen R, Sun H, Luhmann HJ, Kilb W, 2014. Taurine activates GABAergic networks in the neocortex of immature mice. Front. Cell. Neurosci 8 10.3389/fncel.2014.00026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholey AB, Kennedy DO, 2004. Cognitive and physiological effects of an “energy drink”: an evaluation of the whole drink and of glucose, caffeine and herbal flavouring fractions. Psychopharmacology (Berk) 176, 320–330. 10.1007/s00213-004-1935-2 [DOI] [PubMed] [Google Scholar]
- Selemon LD, 2013. A role for synaptic plasticity in the adolescent development of executive function. Transl Psychiatry 3, e238 10.1038/tp.2013.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivaraj MC, Marcy G, Low G, Ryu JR, Zhao X, Rosales FJ, Goh ELK, 2012. Taurine induces proliferation of neural stem cells and synapse development in the developing mouse brain. PLoS ONE 7, e42935 10.1371/journal.pone.0042935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suárez LM, Muñoz M-D, Martín Del Río R, Solís JM, 2016. Taurine content in different brain structures during ageing: effect on hippocampal synaptic plasticity. Amino Acids 48, 1199–1208. 10.1007/s00726-015-2155-2 [DOI] [PubMed] [Google Scholar]
- Suge R, Hosoe N, Furube M, Yamamoto T, Hirayama A, Hirano S, Nomura M, 2007. Specific timing of taurine supplementation affects learning ability in mice. Life Sci. 81, 1228–1234. 10.1016/j.lfs.2007.08.028 [DOI] [PubMed] [Google Scholar]
- Thomson B, Scheiss S, 2010. Risk profiles: Caffeine in energy drinks and energy shots. Available at: http://www.foodsafety.govt.nz/elibrary/industry/Risk_Profile_Caffeine-Science_Research.pdf
- Tsolaki M, 2019. Future strategies of management of Alzheimer’s Disease. The role of homotaurine. Hell J Nucl Med 22 Supp1, 82–94. [PubMed] [Google Scholar]
- Vorhees CV, Williams MT, 2006. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc 1, 848–858. 10.1038/nprot.2006.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wójcik OP, Koenig KL, Zeleniuch-Jacquotte A, Costa M, Chen Y, 2010. The potential protective effects of taurine on coronary heart disease. Atherosclerosis 208, 19–25. 10.1016/j.atherosclerosis.2009.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Silverman JL, Crawley JN, 2011. Automated three-chambered social approach task for mice. Curr Protoc Neurosci Chapter 8, Unit 826 10.1002/0471142301.ns0826s56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye G, Tse AC, Yung W, 1997. Taurine inhibits rat substantia nigra pars reticulata neurons by activation of GABA- and glycine-linked chloride conductance. Brain Res. 749, 175–179. 10.1016/s0006-8993(96)01427-8 [DOI] [PubMed] [Google Scholar]
- Ye H-B, Shi H-B, Yin S-K, 2013. Mechanisms underlying taurine protection against glutamate-induced neurotoxicity. Can J Neurol Sci 40, 628–634. 10.1017/s0317167100014840 [DOI] [PubMed] [Google Scholar]
- Zeratatsky, 2015. What is taurine? Is it safe? Mayo Clinic, http://www.mayoclinic.org/healthy-lifestyle/nutrition-and-healthy-eating/expert-answers/taurine/faq-20058177
- Zhang X, Wang X, Zhang J, Pan X, Jiang J, Li Y, 2017. Effects of taurine on alterations of neurobehavior and neurodevelopment key proteins expression in infant rats by exposure to hexabromocyclododecane. Adv. Exp. Med. Biol 975, 119–130. 10.1007/978-94-024-1079-211 [DOI] [PubMed] [Google Scholar]
- Zhang Q, Yu Y-P, Ye Y-L, Zhang J-T, Zhang W-P, Wei E-Q, 2011. Spatiotemporal properties of locomotor activity after administration of central nervous stimulants and sedatives in mice. Pharmacol. Biochem. Behav 97, 577–585. 10.1016/j.pbb.2010.09.011 [DOI] [PubMed] [Google Scholar]
- Zhu M, Akimana C, Wang E, Ng CK, 2019. 1H-MRS Quantitation of Age-Dependent Taurine Changes in Mouse Brain. Mol Imaging Biol 21, 812–817. 10.1007/s11307-019-01333-6 [DOI] [PubMed] [Google Scholar]


