Abstract
Raffinose and its precursor galactinol accumulate in plant leaves during abiotic stress. RAFFINOSE SYNTHASE (RAFS) catalyzes raffinose formation by transferring a galactosyl group of galactinol to sucrose. However, whether RAFS contributes to plant drought tolerance and, if so, by what mechanism remains unclear. In this study, we report that expression of RAFS from maize (or corn, Zea mays) (ZmRAFS) is induced by drought, heat, cold, and salinity stresses. We found that zmrafs mutant maize plants completely lack raffinose and hyper-accumulate galactinol and are more sensitive to drought stress than the corresponding null-segregant (NS) plants. This indicated that ZmRAFS and its product raffinose contribute to plant drought tolerance. ZmRAFS overexpression in Arabidopsis enhanced drought stress tolerance by increasing myo-inositol levels via ZmRAFS-mediated galactinol hydrolysis in the leaves due to sucrose insufficiency in leaf cells and also enhanced raffinose synthesis in the seeds. Supplementation of sucrose to detached leaves converted ZmRAFS from hydrolyzing galactinol to synthesizing raffinose. Taken together, we demonstrate that ZmRAFS enhances plant drought tolerance through either raffinose synthesis or galactinol hydrolysis, depending on sucrose availability in plant cells. These results provide new avenues to improve plant drought stress tolerance through manipulation of the raffinose anabolic pathway.
Keywords: Arabidopsis, carbohydrate metabolism, carbohydrate biosynthesis, carbohydrate function, galactosyltransferase, drought stress, galactinol hydrolysis, maize, raffinose synthase, raffinose synthesis
Introduction
Raffinose is thought to play a role in plant drought stress-tolerance based on positive correlations between raffinose accumulation in leaves when plants encounter drought stress (1–3). GALACTINOL SYNTHASE (GOLS) and RAFFINOSE SYNTHASE (RAFS) are two key enzymes responsible for raffinose biosynthesis (4, 5). Using myo-inositol and UDP-galactose, GOLS (EC 2.4.1.123) catalyzes the production of galactinol (6); whereas RAFS (EC 2.4.1.82) uses sucrose and galactinol to synthesize raffinose (4).
The expression and function of GOLS in response to plant abiotic stress have been well-elucidated in many plant species. Expression of some GOLS genes is induced by abiotic stress, such as drought, heat shock, salinity, and osmotic shock (7, 8), whereas others are induced by pathogen attack (9). Overexpression of GOLS increased the galactinol and raffinose content, whereas concurrently enhancing the abiotic stress tolerance of transgenic plants (7, 10–12). Because both galactinol and raffinose are increased by overexpression of GOLS genes, it is unclear whether galactinol, raffinose, or both directly enhance abiotic stress tolerance. Further complicating this situation, GALACTINOL SYNTHASE has, as one of its substrates, myo-inositol, a sugar alcohol with abiotic stress alleviating properties of its own (13) and whose utilization to make galactinol, necessarily draws down its concentration in the cell.
Unlike GOLS, very few RAFS have been characterized in vitro or in vivo (4, 5, 14–17). RAFS genes are induced by dehydration stress (17), and their transcripts accumulate in the late stages of seed maturation and desiccation, coincident with increased raffinose content (18, 19). There are six putative RAFFINOSE SYNTHASE genes (AtRS1–6) in Arabidopsis thaliana (Arabidopsis) (20). AtRS5 is the only genuine raffinose synthase that has been shown to have RAFS activity (16). The mutation of AtRS5 abolishes raffinose in the leaves and reduces the raffinose content in seeds. AtRS4, identified as a STACHYOSE SYNTHASE (AtSTS), has some raffinose synthetic capacity and the atrs4 mutant is devoid of stachyose in the seeds (5). In addition to AtRS5 and AtRS4 in Arabidopsis, AtRS6 is predicted to be a drought-induced (through alternative splicing) RAFS (21). Knockout mutants in genes involved in raffinose biosynthesis in Arabidopsis have failed to show any plant-level phenotypic changes during normal growth other than perturbations in raffinose family oligosaccharides amounts and a repression of seed germination in darkness (5, 16). Fewer RAFS genes have been predicted in the maize (Zea mays) genome based on bioinformatics studies (22), and some of these, such as GRMZM2G340656, have already been experimentally identified and annotated as encoding ALKALINE ALPHA GALACTOSIDASE (23). We recently characterized the unique maize raffinose synthase gene and found that it plays an important role in maize seed vigor and longevity (19). Currently, no evidence has shown that over-expression of RAFS can enhance the raffinose content in plant vegetative tissues. Despite extensive studies, the function of raffinose in regulation of, or response to, vegetative abiotic stress has not been distinguished from that of galactinol or myo-inositol and, thus, the role raffinose plays in plant drought stress tolerance remains unclear. There are three hypothetical mechanisms addressing how raffinose performs its function when plants are in, or are recovering from, adverse conditions. 1) As a protective agent, raffinose could stabilize membranes during dehydration and prevent leakage of cellular contents and membrane fusion after rehydration (24). 2) As a ROS scavenger, raffinose (and galactinol) may mitigate oxidative damage under abiotic stress conditions (20). 3) Raffinose could be transported into chloroplasts, protecting thylakoids and stabilizing PSII (25, 26).
In this study, we demonstrated that maize zmrafs mutants lacking raffinose and hyper-accumulating galactinol, but with WT amounts of sucrose and myo-inositol were more sensitive to drought stress than null-segregant lines in two maize inbred lines. Similar to the ZmGOLS2-overexpressing Arabidopsis plants, ZmRAFS-overexpressing Arabidopsis plants displayed a significantly increased tolerance to drought stress. However, whereas the galactinol and raffinose amounts in ZmGOLS2-overexpressing plants were greater, both were significantly decreased in ZmRAFS-overexpressing Arabidopsis leaves. The ZmRAFS in ZmRAFS-overexpressing Arabidopsis leaves did not seemingly contribute to net raffinose accumulation but rather hydrolyzed galactinol without transfer of the galactose to sucrose, thereby generating large amounts of myo-inositol under drought stress. This study demonstrates that ZmRAFS enhances plant drought tolerance through either synthesis of more raffinose or by generation of more myo-inositol via hydrolysis of galactinol.
Results
ZmRAFS is induced by abiotic stress
ZmRAFS transcription was rapidly and dramatically up-regulated in leaves when the V3 stage (three leaves stage) seedlings were treated with heat shock, dehydration, or subjected to increased salt within 2–3 h, but returned to background levels with prolonged treatment (Fig. 1A). Under cold stress, ZmRAFS exhibited a weakly increased level of transcript in V3 stage seedlings treated for 8 h (Fig. 1A). ZmRAFS protein was undetectable in untreated leaves of V3 stage seedlings, but accumulated nearly concomitantly with transcript under heat shock and salt stress, but showed a gradual increase under dehydration treatment (Fig. 1B). No ZmRAFS protein could be detected in leaves under cold stress (Fig. 1B). The ZmRAFS mRNA abundance and its protein accumulation are imperfectly correlated in maize seedlings under abiotic stress. These data suggest that ZmRAFS expression might be regulated at both transcriptional and translational levels. Subcellular localization of ZmRAFS protein was determined by transient expression of a fusion protein comprising ZmRAFS and YFP in maize leaf protoplasts. The fluorescence signal from YFP was detected throughout the cytoplasm and chloroplasts but was not in the vacuole, whereas the fluorescence of the ZmRAFS-YFP fusion protein was only detected in the cytoplasm (Fig. 1C). Western blot analysis of ZmRAFS-YFP fusion protein expression showed that the fusion protein was present and intact in transformed maize leaf protoplasts as was endogenous RAFS (Fig. 1D).
Figure 1.
ZmRAFS is responsive to abiotic stress and its encoded protein is localized to the cytosol. A and B, ZmRAFS expression was induced by different abiotic stresses in maize B73 seedling leaves (V3 stage). The maize seedlings were treated with heat shock (42 °C), dehydration (dry on blot paper), NaCl (200 mm), and cold stress (4 °C), respectively, for certain periods of time as indicated in the graph, and the leaf samples were collected for detection of the mRNA or protein expression of ZmRAFS. A, the ZmRAFS mRNA accumulation in the second leaf was determined by real-time RT-PCR. The expression of ZmRAFS mRNA was normalized to the expression of maize GAPDH and is presented relative to the untreated, control samples (0 h). The x-axis represents the time course of treatment. The y-axis indicates relative ZmRAFS expression. The mixture of the 2nd leaf of three different seedlings were applied for RNA extraction and qRT-PCR as one biological replicate, there are three biological replicates. Each circle represents one biological replicate, and the horizontal bars denote the means. Different lowercase letters above the bars denote significant differences among different times of treatments (ANOVA test, p < 0.05). B, ZmRAFS protein accumulation was determined by Western blot analysis. Top panel, Western blot analysis of ZmRAFS protein; bottom panel, Western blot analysis of GAPDH protein demonstrating equal protein loading. C, ZmRAFS was localized to the cytoplasm of maize protoplast cells. YFP expression vector or a ZmRAFS-YFP fusion protein expression vector were transiently transformed into maize protoplasts. The YFP fluorescence and chloroplast autofluorescence signals were detected by confocal microscopy and then merged (bar = 10 μm). BF, bright field; YFP, YFP signal; Chl, chloroplast autofluorescence signal; Merge, merged signal of YFP and Chl. D, Western blot analysis of ZmRAFS-YFP fusion protein in transiently transformed maize protoplast cells as in C using ZmRAFS antibody. Both protoplast samples have endogenous RAFS (Native) but only the transiently transformed cells also have signal from the chimeric ZmRAFS-YFP fusion. From the same samples, Western blot analysis of ZmGAPDH protein in the lower panels demonstrating equal protein loading.
zmrafs maize mutants, containing no raffinose but accumulating galactinol, are more sensitive to drought stress than NS
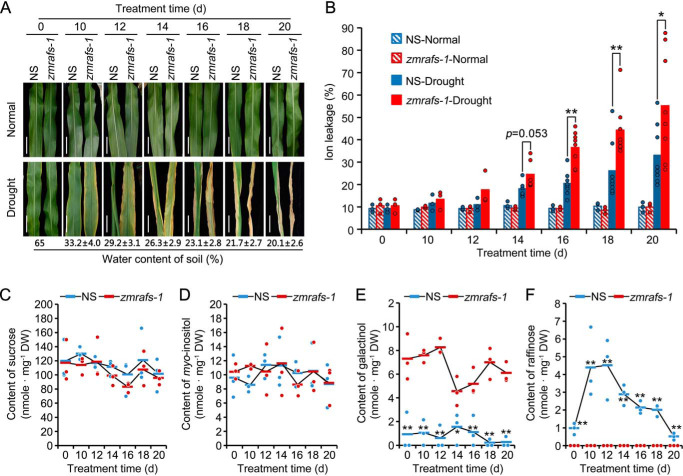
We next investigated the drought stress tolerance of the two zmrafs mutant lines in which ZmRAFS expression was interrupted by mutator insertion (19) (Fig. S1). The 7th leaf of maize plants was repeatedly photographed every other day during water withholding (Fig. 2A and Fig. S2A). The zmrafs-1 mutant plants exhibited more severe injury compared with null-segregant (NS) plants (Fig. 2A). zmrafs-2 leaves showed greater longitudinal folding than NS when the rolled leaves were spread open (Fig. S2A). Electrolyte leakage, an indicator of cellular injury (27), of the mutant leaves was significantly greater than that of NS leaves (Fig. 2B) on the 16th, 18th, and 20th day of the drought stress, but not during normal watering. A similar electrolyte leakage phenotype was observed in zmrafs-2 mutant plants relative to their NS, occurring even earlier at day 14 of the stress (Fig. S2B). Sucrose and myo-inositol amounts in leaves prior to and during the drought were similar between NS and mutant plants except for myo-inositol at 10 days after the start of the drought stress (Fig. 2, C and D). Galactinol accumulated to greater amounts in mutant leaves were compared with those of the NS; whereas the reverse was true of raffinose which, as expected, was undetected in the mutant (Fig. 2, E and F).
Figure 2.
Mutator interrupted zmrafs-1 mutant plants were more sensitive to drought stress than null-segregant plants. A, representative leaves of NS or zmrafs-1 plants, grown under normal or drought stress conditions, were photographed at the start of and during the treatment. Bar = 10 cm. One NS and one mutant plant were grown in the same pot under normal conditions in a greenhouse for 50 days (V15 stage). Plants were then grown under 65% water content of the soil's capacity or under drought stress conditions (water withheld) for 20 days. B, a comparison of electrolyte leakage of leaves between NS (W22 background; blue) and zmrafs-1 (red) plants that were grown under normal (hatched bars) or drought stress (filled bars) conditions as in A. The middle part of the 7th leaves from different plants were collected for testing of the electrolyte leakage. Each circle represents one biological replicate (one single plant), there are three to eight biological replicates for each treatment. The horizontal bars denote the means. *, p < 0.05; **, p < 0.01 as compared by Student's t test. C–F, soluble sugar profiles of leaves from NS and zmrafs-1 plants at the start and during drought stress. Sucrose (C), myo-inositol (D), galactinol (E), and raffinose (F) amounts were measured for the null-segregant (blue circles) and the maize raffinose synthase mutant-1 (zmrafs-1; red diamonds). The middle part (1 g) of the 7th leaves from different plants were collected for sugar extraction and HPLC-ELSD detection. Each circle represents one biological replicate (one single plant), there are three biological replicates. The horizontal bars denote the means. *, p < 0.05; **, p < 0.01 denotes a significant difference between NS and zmrafs-1 (Student's t test). There was no significant difference of sucrose and myo-inositol between NS and zmrafs-1 mutant.
Overexpression of ZmRAFS in Arabidopsis enhanced galactinol hydrolysis rather than raffinose synthesis in leaves yet still enhanced drought stress tolerance
To further investigate the consequences of altered raffinose amounts on plant drought stress tolerance, three Arabidopsis lines overexpressing the ZmRAFS gene were characterized for drought stress tolerance. In addition to WT control, the GFP- and ZmGOLS2-overexpressing Arabidopsis lines (12) were also included as controls. The ZmRAFS and ZmGOLS2 protein accumulation was determined by Western blot analysis (Fig. S3A). The Arabidopsis raffinose synthase (atrs5) mutant was characterized by PCR (Fig. S3, B and C) and also used as a drought-susceptible control. After drought stress and re-watering, the 3 ZmRAFS-overexpressing lines were obviously more resistant to drought stress than any of the control lines except for the ZmGOLS2-overexpressing plants known to possess superior drought tolerance (12) (Fig. 3A). The survival percentage of ZmRAFS- and ZmGOLS2-overexpressing lines after drought stress was similar and both were significantly greater than that of the wildtype (WT), GFP expressing (OEGFP) or atrs5 plants (Fig. 3B). For unstressed seedlings, there was no difference in leaf ion leakage percentages regardless of genotype (Fig. 3C). However, upon drought stress, those percentages for all ZmRAFS- and ZmGOLS2-overexpressing plants were similarly low, and both are significantly lower than that of the WT, GFP expressing, or atrs5 plants (Fig. 3C).
Figure 3.
In Arabidopsis, overexpression of ZmGOLS2 and ZmRAFS increased and decreased, respectively, the galactinol and raffinose contents; and yet, both enhanced the drought stress tolerance of those Arabidopsis plants. A, photographs of Arabidopsis plants without drought stress (Normal), at the end of the drought stress (drought; 2 weeks without watering), and after re-watering (Re-water). Bar = 2 cm. Abbreviations are as follows: WT, WT Col-0 Arabidopsis plants; OEGFP, GFP-overexpressing Arabidopsis plants; OEZmRAFS-8, -21, or -25, ZmRAFS-overexpressing Arabidopsis plants; ZmGOLS2, ZmGOLS2-overexpressing Arabidopsis plants; atrs5, Arabidopsis raffinose synthase5 knockout mutant plants. B, the percentage survival of both the ZmRAFS- and ZmGOLS2-overexpressing Arabidopsis plants was greater than that of WT plants when water was withheld from the plants. The survival rate was calculated from three to six pots of plants for each Arabidopsis line in one independent experiment. There are three independent experiments. Each circle represents the data of one independent experiment. The horizontal bars denote the means. *, p < 0.05; **, p < 0.01, as compared with WT control (ANOVA test). C, the leaf ion leakage of all three ZmRAFS- and the ZmGOLS2-overexpressing Arabidopsis plants was less than that of WT plants at the end of the drought stress treatment. Leaves form different plants that were grown under normal and drought conditions were collected for ion leakage test. Each circle represents one biological replicate (one single plant), there are 3–12 biological replicates for each tested line. The horizontal bars denote the means. **, p < 0.01 compared with WT control (ANOVA test). D, comparison of soluble sugar related to raffinose biosynthesis of the different Arabidopsis plants as in A, which were grown under well-watered or drought conditions. Total soluble sugar was extracted from 0.5 g of leaves that were collected from different Arabidopsis plants for each line (one biological replicate) for HPLC-ELSD detection. Each circle represents one biological replicate and there are three to six biological replicates for each tested line. The horizontal bars denote the means. The colored asterisks are indicative of significant differences from WT under normal (blue) or drought (red) conditions (*, p < 0.05 and **, p < 0.01, ANOVA test). A black asterisk (*, p < 0.05 and **, p < 0.01) indicates significant differences between the normal and drought condition of each line (Student's t test). E, characterization of the raffinose synthetic activity of the leaf extracts from different Arabidopsis plants as described in A. Raffinose and myo-inositol were determined using cell-free leaf extracts of three ZmRAFS-overexpressing lines incubated with galactinol and sucrose, as detected by HPLC-ELSD. The enzymatic activity was determined by raffinose amounts (nmol) produced per mg of protein/min. The crude enzyme extracts that were extracted from the leaf mixture of three different plants were determined for its raffinose synthetic activity (one biological replicate). There are three biological replicates. Each triangle represents one biological replicate, and the horizontal bars denote the means. **, p < 0.01 compared with WT (ANOVA test). F, characterization of the galactinol hydrolytic activity of the leaf extracts from the Arabidopsis plants as described in A. Galactose and myo-inositol were determined using cell-free leaf extracts of three ZmRAFS-overexpressing lines incubated with galactinol as detected by HPLC-ELSD. The enzymatic activity was determined by myo-inositol amounts (nmol) produced per mg of protein/min. The crude enzyme extracts that were extracted from the leaf mixture of three different plants were determined for its galactinol hydrolytic activity (one biological replicate). There are three biological replicates. Each triangle represents one biological replicate, and the horizontal bars denote the means. **, p < 0.01 compared with WT (ANOVA test).
Drought stress enhanced the sucrose content in the leaves of all plant genotypes; increased the myo-inositol content in the 3 ZmRAFS-overexpressing plants and in the atrs5 mutant; increased the galactinol content in all leaves apart from those of the 3 ZmRAFS plants; and increased the raffinose content in all leaves excluding those of 2 of the 3 ZmRAFS plants (Fig. 3D). Overexpression of ZmGOLS2 enhanced both galactinol and raffinose contents, regardless of stress, compared with control lines (WT and OEGFP) (Fig. 3D). The raffinose content was decreased, whereas the galactinol content was increased in atrs5 mutant leaves as compared with WT (Fig. 3D). Unexpectedly, the raffinose and galactinol content in leaves of the 3 ZmRAFS lines were significantly decreased compared with WT and OEGFP whether the plants were well-watered or under drought conditions (Fig. 3D). Surprisingly, given what was observed in the leaves, the raffinose content in seeds of ZmRAFS- overexpressing Arabidopsis lines was significantly greater than that of other lines (19). To investigate whether ZmRAFS protein expressed in OEZmRAFS leaves was able to synthesize raffinose using sucrose and galactinol, enzyme activities of leaf extracts from all Arabidopsis lines were tested in vitro for raffinose-synthetic (transferase) and nonraffinose producing, galactinol-hydrolytic activities. Under the conditions tested, the leaf extracts of all three of the ZmRAFS-expressing Arabidopsis lines had both dramatically greater transferase (Fig. 3E) as well as hydrolytic (Fig. 3F) activities relative to the leaf extracts of the other genotypes.
Characterization of the substrate-binding site of ZmRAFS
RAFFINOSE SYNTHASE binds two substrates (galactinol and sucrose) and it has both raffinose synthetic and galactinol hydrolytic activities. To determine the substrate-binding sites of the ZmRAFS, enzyme activity assay of mutated ZmRAFS was performed (Fig. 4). According to its closely related templates, the most probable ligand-binding sites of ZmRAFS were predicted using the I-TASSER online resource (28) (Table S1). To determine the putative substrate-binding residues of ZmRAFS, those predicted amino acids (AA) were mutated to alanine to minimize potential structural alterations of the mutated protein (Fig. 4A). The solubility of the mutant ZmRAFS-His6 recombinant proteins were demonstrated using Western blot analysis of an aliquot of the supernatant for each mutant (Fig. 4B). The bacterial-expressed, WT ZmRAFS protein showed both raffinose synthetic and unproductive galactinol hydrolytic activities as determined by in vitro assay (Fig. 4C). Although mutation of many residues decreased or abolished either raffinose-synthetic activity or both raffinose-synthetic and unproductive galactinol-hydrolytic activity, none enhanced raffinose-synthetic activity (Fig. 4C). The Asp-264–Ala mutant had greatly increased (∼2-fold) unproductive galactinol-hydrolytic activity but had no detectable raffinose-synthetic activity. These results are suggestive that the galactinol-hydrolytic activity of ZmRAFS is independent of forming raffinose, supportive of the previous characterization of the catalytic mechanism as a Ping-Pong, Bi-Bi reaction (4).
Figure 4.
Characterization of enzyme activity of ZmRAFS and its amino acid-point mutants. A, schematic representation of the bacterial expression vector of ZmRAFS and its mutants. The residue number of each of the alanine point mutations is indicated. Letters in red and blue indicate the mutation of the AA and the corresponding nucleotide(s), respectively. B, using the ZmRAFS antibody, Western blotting characterization of the presence of each mutated ZmRAFS protein (arrow denotes the expected signal; * denotes a cross-reacting protein) in the soluble E. coli extract. A sister gel was stained with Coomassie Brilliant Blue-250 (CBB). Abbreviations are as follows: VC, pET-21d empty vector control; ZmRAFS-WT, unmutated ZmRAFS-His6 fusion protein; and all designated residues, alanine mutants. C, in vitro assay of the raffinose-synthetic and galactinol-hydrolytic activity of ZmRAFS and its alanine-mutated forms calculated as a ratio of the amount of product to enzyme, then converted to a percentage of the WT ZmRAFS activity. Crude enzyme extracts were extracted from bacteria cultures of ZmRAFS-WT, VC, or ZmRAFS mutant clones for the enzymatic activity assay. Each circle represents one biological replicate (cultures from one flask), there are three biological replicates. The horizontal bars denote the means. Using the ANOVA test, significant differences as compared with the WT ZmRAFS are denoted with * and **, representing p < 0.05 and <0.01, respectively). n.d. indicates not detected under the conditions used.
A relative amount of galactinol and sucrose to ZmRAFS, determines whether ZmRAFS completes the transferase reaction, forming raffinose, after hydrolyzing galactinol
To investigate the effects of galactinol or sucrose on the ZmRAFS enzyme, the activity of Escherichia coli-expressed ZmRAFS-His6 recombinant protein was characterized in substrate concentration control systems (Fig. 5, A and B). Myo-inositol is generated by both raffinose synthesis and galactinol hydrolysis; whereas raffinose is only generated when the enzyme completes its transferase function. By this logic, the molar ratio of myo-inositol to raffinose is expected to reflect whether ZmRAFS performs the entire synthesis reaction or only hydrolyzes galactinol without transfer of galactose to sucrose. When sucrose was saturating (29.2 mm) and the galactinol concentration was low (1.1–2.6 mm), similar amounts of myo-inositol and raffinose were generated (Fig. 5A: the ratio (myo-inositol/raffinose) was approximately 1). As the galactinol concentration increased (≥5.3 mm compared with the apparent Km = 2.93 mm; Table S2), the amount of myo-inositol that was generated was significantly greater than that of raffinose and so the ratio became statistically significantly greater than 1 (Fig. 5A). When galactinol was saturating (15.9 mm), regardless of the sucrose concentration, the amount of myo-inositol that was generated was always greater than that of raffinose produced; however, the ratio of myo-inositol to raffinose gradually decreased as the sucrose concentration was increased (Fig. 5B). These results show that the relative amount of the two substrates, sucrose and galactinol, determines whether ZmRAFS predominantly hydrolyzed galactinol unproductively or predominantly completed the synthesis of raffinose, suggesting that greater raffinose production in Arabidopsis leaves expressing ZmRAFS may be possible by boosting substrate amounts. The kinetic assays determined that ZmRAFS exhibited apparent lower affinities of the substrates than that of AtRS5 (Table S2) (29). Under the conditions used, ZmRAFS has a higher affinity for galactinol (apparent Km = 2.93) than sucrose (apparent Km = 7.40) when both substrates were present; and exhibited higher apparent affinity for galactinol when sucrose was absent (Table S2).
Figure 5.

The relative amounts of sucrose and galactinol determines whether RAFS completed the transferase reaction. A, amount of sugar products of the transferase reaction as detected by HPLC-ELSD (top panel) and the molar ratio of those products (myo-inositol/raffinose; bottom panel). Sucrose was kept at saturating levels (29.2 mm) as galactinol concentration was varied as noted on the x axis. Crude enzyme extracts were extracted from BL21 (expressing ZmRAFS) bacteria cultures for the enzymatic activity assay. Each circle (in the top panel) or triangle (in the bottom panel) represents one biological replicate (cultures from one flask), there are three biological replicates. Sugar amount data represent the mean of three replicates and significant differences between the two products were indicated (** = p < 0.01) using a Student's t test. Different lowercase letters denote significant differences among the ratios within each data set (Tukey's test). B, same as in A except galactinol concentration was kept constant and saturating (15.9 mm) as the sucrose concentration was varied.
To attempt to boost raffinose amounts and to further investigate why ZmRAFS predominantly hydrolyzed galactinol without completing the transferase reaction to produce raffinose in ZmRAFS-expressing Arabidopsis leaves, ZmGOLS2/ZmRAFS-double expressing plants were generated to test whether ZmRAFS would synthesize raffinose if the galactinol content was increased in leaves. The paradigm for this experiment was the ZmGOLS2 overexpressing plants, which produce greater amounts of both galactinol and raffinose (Fig. 3D). As characterized by Western blot analysis, the accumulation of ZmRAFS protein in leaves of ZmRAFS- and ZmGOLS2/ZmRAFS-double expressing plants were, as expected, greater than that of WT, GFP, or the ZmGOLS2 line (Fig. S4). Similarly, the ZmGOLS protein accumulation in leaves of ZmGOLS2- and ZmGOLS2/ZmRAFS-double expressing plants was greater than that of WT, GFP, or the ZmRAFS line (Fig. S4). Comparison of the plant morphology, percentage survival, and the ion leakage of these different Arabidopsis genotypes after drought stress showed that the ZmGOLS2/ZmRAFS-, ZmRAFS-, and ZmGOLS2-expressing lines were more tolerant of drought stress compared with WT plants (Fig. 6, A–C).
Figure 6.
Supplementation of sucrose to the detached leaves of ZmGOLS2/ZmRAFS-overexpressing Arabidopsis plants permitted ZmRAFS to complete the transferase activity and form raffinose. A, photographs of Arabidopsis plants before drought stress, after drought stress, and after re-watering. Plant genotypes, as described in the legend to Fig. 3, included WT, OEGFP, OEZmRAFS-25, and OEZmGOLS2 and the additional OEZmGOLS2/OEZmRAFS-double overexpressing Arabidopsis plants. B, the percentage survival of different Arabidopsis lines after re-watering following drought stress. Survival rate was determined by counting the plants from five pots of each Arabidopsis line for one independent experiment. There are six independent experiments. Each circle represents data from one independent experiment. The horizontal bars denote the means. Significance as compared with the WT control was determined using ANOVA test and is denoted (**, p < 0.01). C, comparison of the leaf ion leakage of different Arabidopsis lines, which were under normal growth condition or after drought stress (before re-watering) treatments. Leaves from different individual plant under normal and drought conditions were collected for ion leakage test. Each circle represents one biological replicate (one plant) and there are three to eight biological replicates for each Arabidopsis line. The horizontal bars denote the means. Significance is indicated (**,p < 0.01) for the comparisons made against WT plants (ANOVA test). D, comparison of sucrose, myo-inositol, galactinol, and raffinose content of leaves among different Arabidopsis lines, which were grown under normal or drought conditions. Total soluble sugar was extracted from 0.5 g of leaves from different plants and the sugar content was detected by HPLC-ELSD. Each circle represents one biological replicate (independent sugar sample from different individual plant), there are three to six biological replicates for each Arabidopsis line. The horizontal bars denote the means. ANOVA test was performed to detect the significant difference between different lines (*, p < 0.05; **, p < 0.01), as show with colored asterisks. Student's t test was using for analyze significant differences between the normal and drought condition of each line (*, p < 0.05, **, p < 0.01, showed by black asterisk). E–G, comparison of the sucrose (E), galactinol (F), and raffinose (G) content in detached leaves, infiltrated with either water or 30 mm sucrose for 1 h, among the various Arabidopsis genotypes. The leaves of different Arabidopsis lines (4 weeks-old) were then subsequently infiltrated in the same solution for 2 h and then incubated for another 22 h under light, the sugar content of leaves was determined by HPLC-ELSD. Each circle represents one biological replicate (leaves from 5 plants of each Arabidopsis line for each treatment), there are there biological replicates. The horizontal bars denote the means; *, p < 0.05; **, p < 0.01 (Student's t test). An asterisk indicates a significant difference between sugar- and water-treated groups of each plant genotype. Different lowercase letters denote significance among different plant genotypes within the treatment group (Tukey's test).
Drought stress increased sucrose accumulation in leaves of all lines; and increased myo-inositol in OEZmRAFS-25 and two double over-expressing lines (OEZmGOLS2/ZmRAFS-1 and -3) (Fig. 6D). Expression of ZmGOLS2 in Arabidopsis significantly increased the galactinol and raffinose content in leaves, whether the plants were grown under normal or drought stress conditions, as compared with WT (Fig. 6D). The galactinol content in the leaves of ZmGOLS2/ZmRAFS expressing plants was significantly less than that of the ZmGOLS2 expressing plants, whether the plants were grown under either normal or drought conditions (Fig. 6D). The raffinose content in the leaves of ZmGOLS2/ZmRAFS expressing plants was similar to that of the ZmGOLS2 expressing line when the plants were grown under well-watered conditions, but was less than that of the ZmGOLS2 line when the plants were grown under drought conditions, although it was still superior to WT or GFP control plants (Fig. 6D). These data indicate that ZmRAFS still unproductively hydrolyzed galactinol in ZmGOLS2/ZmRAFS expressing plants. Infiltration of sucrose into the detached leaves of all the Arabidopsis genotypes, compared with that of infiltration of detached leaves with water, increased the sucrose content in the leaf (Fig. 6E). Of the water-treated group, galactinol was only detected in ZmGOLS2 plants (Fig. 6F). The galactinol content was significantly increased in detached leaves of both the ZmGOLS2- and -1 of 3 ZmGOLS2/ZmRAFS-1 expressing plants upon sucrose treatment compared with water treatment, but remained constant in ZmGOLS2/ZmRAFS-2 and -3 lines (Fig. 6F). Even with water infiltration, the raffinose content in ZmGOLS2 plants was significantly greater than that of other plants (Fig. 6G). Interestingly, the raffinose content in the ZmGOLS2/ZmRAFS-1 and -2 plants was significantly greater than that, not only of the control plants, but also that of ZmGOLS2 plants when sucrose treated (Fig. 6G) despite the seemingly different ratios of protein of the two enzymes accumulated in those plants (Fig. S4). These results are consistent with the greater accumulation of raffinose in the seeds of the ZmRAFS plants (19), these data suggested that infiltration of sucrose to the detached leaves of ZmGOLS2/ZmRAFS-expressing Arabidopsis plants provided sufficient sucrose substrate for ZmRAFS to complete the transferase reaction after hydrolyzing galactinol and subsequently, to accumulate raffinose.
Overexpression of ZmMIPS increased myo-inositol accumulation in leaves and enhanced plant drought tolerance
Over-expression of ZmRAFS in Arabidopsis decreased galactinol and raffinose content, whereas it increased myo-inositol content in leaves and enhanced the plant drought tolerance (Figs. 3, A–D, and 6, A–D). To further demonstrate that it is myo-inositol that functions in this scenario to protect the plants from drought stress, two maize homologues of AtMIPS (myo-inositol-1-phosphate synthase, the key enzyme for myo-inositol synthesis in Arabidopsis) were cloned and named ZmMIPS1 (GRMZM2G155242) and ZmMIPS2 (GRMZM2G004528). The expression of ZmMIPS1 and ZmMIPS2 proteins were induced in bacteria and purified (Fig. S5A). Enzyme activity assay of the purified N-terminal His tag fusion proteins of ZmMIPS1 or ZmMIPS2 showed that both ZmMIPS fusion proteins were able to generate myo-inositol-1-phosphate (Fig. S5B). Plant expression vectors for ZmMIPS1 or ZmMIPS2 were constructed and transformed into Arabidopsis. The mRNA and protein accumulation of ZmMIPS in Arabidopsis was determined by RT-PCR and Western blotting, respectively (Fig. 7A). Over-expressing ZmMIPS substantially increased myo-inositol content in leaves under both normal and drought conditions (Fig. 7B). The 3 ZmMIPS-overexpressing Arabidopsis lines were more resistant to drought stress than any of the control lines (Fig. 7C). After re-watering, the survival percentage of the 3 ZmMIPS expressing lines were significantly higher than that of control lines (Fig. 7D). These results demonstrated that increasing myo-inositol amounts was able to enhance plant drought tolerance.
Figure 7.
Over-expression of ZmMIPS1 or ZmMIPS2 increased myo-inositol accumulation in Arabidopsis leaves and enhanced plant drought tolerance. A, characterization of ZmMIPS1 and ZmMIPS2 overexpressing Arabidopsis plants. Top panel, RT-PCR analysis of mRNA accumulation of ZmMIPS, AtACTIN2 expression was used as a control; bottom panel, Western blot analysis of ZmMIPS protein accumulation. RUBISCO CBB staining was used as a loading control. WT, WT Col-0 Arabidopsis plants; OEGFP, GFP-overexpressing Arabidopsis plants; OEZmMIPS1–6, ZmMIPS1 over-expressing line; OEZmMIPS2–8/-16, ZmMIPS2 over-expressing line. B, comparison of myo-inositol content in leaves among different Arabidopsis lines, which were grown under normal and drought conditions. Total soluble sugar was extracted from 0.5 g of leaves from different plants for myo-inositol measurement by HPLC-ELSD. Each circle represents one biological replicate (sugars extracted from different plant of the same Arabidopsis line), there are three biological replicates. The horizontal bars denote the means. **, p < 0.01 (compared with WT, ANOVA test). C, photographs of Arabidopsis plants before drought stress, after drought stress, and after re-watering. Bar = 2 cm. D, the percentage survival of different Arabidopsis lines after re-watering following the drought stress. Survival rate was determined by counting the plants from five pots of each Arabidopsis line for one independent experiment. There are three independent experiments. Each circle represents data from one independent experiment. The horizontal bars denote the means. *, p < 0.05; **, p < 0.01 (ANOVA test).
Discussion
Raffinose, but not galactinol, directly contributes to plant drought stress tolerance
The function of raffinose and galactinol in plant drought stress tolerance and the molecular mechanism by which they act remains unclear despite extensive studies. Galactinol is a direct substrate for raffinose biosynthesis (Fig. 8). Both galactinol and raffinose are accumulated to higher levels in plants in response to abiotic stresses (12). Knockout mutants of relevant genes involved in raffinose biosynthesis have failed to show any abnormalities in Arabidopsis during normal growth other than perturbations in raffinose amounts and decreased completion of germination in the dark (5, 16, 29). Over-expression of GOLS in Arabidopsis increased both galactinol and raffinose content, decreased myo-inositol (Fig. 3D), whereas concurrently enhancing the abiotic stress tolerance of plants (7, 10–12). Until now, no evidence had shown that over-expression of RAFS could regulate the raffinose content in plant vegetative tissues. In short, despite extensive studies, the function of raffinose in response to plant abiotic stress has not been distinguished from that of galactinol and the role raffinose plays in plant drought stress tolerance remains unknown. By characterization of the maize knockout mutant (zmrafs) and ZmRAFS-expressing Arabidopsis plants, we found that raffinose, but not galactinol, directly and positively regulates plant drought stress tolerance.
Figure 8.
Simplified raffinose synthetic metabolism and its effects on drought stress tolerance in maize and Arabidopsis. RAFFINOSE SYNTHASE enhances plant drought tolerance by either synthesizing raffinose, or by hydrolyzing galactinol, which will generate more myo-inositol, depending on the relative amount of its substrates, galactinol and sucrose. GOLS, GALACTINOL SYNTHASE; RAFS, RAFFINOSE SYNTHASE; MIPS, myo-INOSITOL PHOSPHATE SYNTHASE. RFO, Raffinose family oligosaccharides.
myo-Inositol generated from ZmRAFS-mediated galactinol hydrolysis in ZmRAFS overexpressing Arabidopsis enhances plant drought tolerance
ZmGOLS2 overexpressing Arabidopsis plants with increased galactinol and raffinose enhanced plant drought stress tolerance (Figs. 3 and 6) (12). However, ZmRAFS overexpressing Arabidopsis plant leaves contained much less raffinose and galactinol, yet the plants were more resistant to drought stress compared with the WT or GFP plants (Fig. 3, A–D). These results indicate that ZmGOLS2- and ZmRAFS-overexpressing Arabidopsis plants utilize a different mechanism to protect plants from drought stress. ZmRAFS protein in leaf extracts of ZmRAFS overexpressing Arabidopsis plants was able to catalyze two different reactions: 1) to synthesize raffinose using exogenous sucrose and galactinol, or 2) to hydrolyze galactinol to produce myo-inositol and galactose when sucrose is absent (Fig. 3, E and F). The myo-inositol was significantly increased in ZmRAFS overexpressing plants under drought stress (Fig. 3D), indicating that ZmRAFS hydrolyzed galactinol instead of synthesizing raffinose in ZmRAFS overexpressing Arabidopsis leaves. It has been proposed that overexpression of myo-inositol phosphate synthase (MIPS) enhanced dehydration, salt, and cold stress tolerance in several plant species (13, 30–33). Overexpression of two maize MIPSs in Arabidopsis increased myo-inositol accumulation and enhanced the plant drought tolerance (Fig. 7). These data support that myo-inositol contribute to plant drought tolerance. myo-Inositol, either synthesized from Glc-6-P by MIPS in ZmMIPS overexpressing plants, or generated by the hydrolysis of galactinol in ZmRAFS overexpressing plant leaves, contributes to the plant drought tolerance. Galactinol functions as a storage or a supply of myo-inositol or galactose. ZmRAFS may either hydrolyze galactinol to generate myo-inositol in ZmRAFS-expressing lines, or use galactinol to synthesize raffinose in ZmGOLS2 lines, and in both cases, enhance the plant drought stress tolerance.
Taken together, we propose a model to elucidate the function and mechanism of raffinose metabolism in plant drought stress tolerance (Fig. 8). The drought stress signal activates transcription factors responsible for up-regulating genes encoding key enzymes, such as GOLS, RAFS, or MIPS in the raffinose biosynthetic pathway, and their action stimulates the accumulation of myo-inositol, galactinol, and raffinose. Both myo-inositol and raffinose directly and positively regulate plant drought stress tolerance, at least in Arabidopsis and maize.
Availability of sucrose determines whether ZmRAFS is to synthesize raffinose or hydrolyze galactinol
The ZmRAFS has been proven to have both raffinose synthetic and galactinol hydrolytic activity as determined either in vitro or in vivo (Figs. 3–5) (19). Either synthesis of raffinose or hydrolysis of galactinol by ZmRAFS would generate myo-inositol. Based on our data and the findings that the pea RAFS showed attributes of catalyzing a Ping-Pong Bi-Bi reaction (4), we propose here that RAFS binds and hydrolyzes galactinol first, releasing myo-inositol and retaining the galactose for some period until RAFS either: 1) binds sucrose and makes raffinose; or 2) releases free galactose exhibiting a galactinol hydrolytic activity. The lower the sucrose concentration, the greater the probability of RAFS hydrolyzing galactinol and the less opportunity RAFS has to make raffinose (Fig. 5B). With increased sucrose, ZmRAFS tends to synthesize raffinose rather than to hydrolyze galactinol (Fig. 5B). This hypothesis is also supported by the point mutation data of the ZmRAFS protein. Mutation of certain AAs of ZmRAFS abolished both galactinol hydrolytic and raffinose synthetic activity of ZmRAFS (Fig. 4). These sites are possibly critical for galactinol binding because the RAFS would not synthesize raffinose if it loses the ability of binding and hydrolyzing galactinol. Mutation of AA 264 from aspartic acid (Asp) to alanine (Ala) abolished the raffinose synthetic activity entirely while enhancing the galactinol hydrolytic activity about 2-fold, indicating that this site is likely critical either for sucrose binding or for catalytic activity of RAFFINOSE SYNTHASE, but not for galactinol binding. Supplementation of sucrose converted the ZmRAFS from hydrolyzing galactinol to synthesizing raffinose in ZmGOLS2/ZmRAFS double expressors (Fig. 6G). These data also suggest that ZmRAFS hydrolyzes galactinol first and then starts synthesizing raffinose if free sucrose is available.
Over-expression of GOLS has been reported to enhance the galactinol and raffinose content and abiotic stress tolerance of the plants (10, 12, 34). In those studies, more galactinol was produced by a combination of endogenous and heterologous GOLS gene expression, whereas raffinose was produced by endogenous RAFS. These data suggest that the substrate galactinol is a limiting factor for RAFS to synthesize raffinose in vivo and the higher molar ratio of galactinol to RAFS would stimulate raffinose biosynthesis. The myo-inositol, which was generated from galactinol hydrolysis, regardless of raffinose synthesis, was quickly recycled back to galactinol by a greater titer of GOLS enzyme.
ZmRAFS exhibited lower affinity to sucrose than it did with galactinol (Table S2). AtRS5 has dramatically higher affinity to sucrose than with galactinol (Table S2) (29). Thus, the high level of galactinol could promote raffinose synthesis catalyzed by AtRS5 without being hampered by sucrose insufficiency in the GOLS-overexpressing Arabidopsis line (Figs. 3D and 6D) (10, 12, 34). The sucrose content in Arabidopsis leaves is very high regardless of genotype (Fig. 3D), however, it is preferentially exported to the sink tissues loaded into companion cells or sieve elements by sucrose transporters. The Km of AtSUC9 for sucrose is 0.006 (35), whereas the Km of AtRS5 for sucrose is 0.35 (29) and the ZmRAFS to sucrose is 7.40 (Table S2). These data show that the sucrose transporter in leaves has a much higher affinity for sucrose than RAFS. Once sucrose is synthesized in leaves, it is immediately bound by the sucrose transporters and transported to the sink tissues. The remaining sucrose in leaves is presumably insufficient for RAFS to synthesize raffinose in ZmRAFS-overexpressing Arabidopsis leaves. Once sucrose is unloaded at the sink tissue, it is free for raffinose synthesis. We have previously reported that ZmRAFS-expressing Arabidopsis seeds has much greater raffinose content than that of control lines (19).
To our knowledge, the effects of over-expressing RAFS have not been reported except in one case of a cucumber RAFS gene, along with a muskmelon GOLS gene and the Alonsoa meridionalis STACHYOSE SYNTHASE (STS) gene all co-transformed into Arabidopsis plants. Transgenic lines homozygous for the 3 genes accumulated substantial galactinol, raffinose, and stachyose (36). Because over-expression of the GOLS gene itself may also cause these biological effects, it is unclear whether or not the foreign RAFS gene functions and it is difficult to draw a conclusion as to its contribution to raffinose production. The scarcity of publications of overexpression of RAFS genes might be due to its unanticipated function hydrolyzing galactinol instead of synthesizing raffinose in plant leaves by RAFS when it is overexpressed in plants.
Regulation of raffinose metabolism pathway for improving plant drought stress tolerance
Our data indicate that raffinose is beneficial to plant drought stress tolerance. We and other groups have confirmed that overexpression of GOLS genes enhanced the abiotic stress tolerance of transgenic plants (11, 12, 34, 37). It is worthwhile mentioning that constitutive expression of ZmGOLS2 genes in Arabidopsis did not cause any adverse effects to the plant under normal conditions (12). Similarly, overexpression of ZmRAFS enhanced plant drought stress tolerance without causing adverse effects to the plants under normal conditions (Fig. 3, A–C). Furthermore, seed vigor can also be improved through manipulation of the raffinose family oligosaccharides metabolic pathway (19). Taken together, it has become promising to manipulate raffinose in crop plants to enhance both seed longevity and plant drought stress tolerance.
Experimental procedures
Plant material
Maize (Z. mays L.) inbred line B73 was maintained in the laboratory. The method of genotyping, Mu-insertional site identification, and characterization of seed sugar profile of the zmrafs mutants and NS counterparts were described previously (19). The Arabidopsis mutant atrs5 (CS318250, T-DNA inserted in At5g40390) was purchased from the Arabidopsis Biological Resource Center (ABRC) through the Arabidopsis Information Resource (TAIR; RRID:CR_004618). The transgenic Arabidopsis lines expressing ZmGOLS2, ZmRAFS, and ZmMIPS were obtained by using floral dip method as previously described (19).
Vector construction
For construction of the ZmRAFS-YFP fusion protein expression vector, the Rluc CDS, the second 2× 35S-promoter, and the Fluc CDS in a dual luciferase control vector (38) were replaced by a MCS sequence (containing XhoI, PstI, NheI, HindIII, SacI, MluI, BglII, NcoI, BamHI, EcoRI, and XbaI). The YFP CDS was obtained by PCR from pmEYFP-1 vector (number 38773, Addgene) using a pair of primers (PYFP-F and PYFP-R; Table S3) and then inserted between the BamHI and EcoRI sites. Upstream of the YFP CDS, a double-stranded linker DNA fragment (5′-(CCATGG)GGAGGATCTGGAGGAGGAGGATCTGGAGGA(GGATCC)-3′) was inserted between NcoI and BamHI sites. The ZmRAFS CDS, without the stop codon, was amplified using a pair of primers (ZmRAFS-CRF-HindIII and ZmRAFS-CRR-MluI; Table S3) together with the template, pET-ZmRAFS, and inserted between the HindIII and MluI sites. The construction of expression vectors for expressing ZmRAFS in Arabidopsis followed the published protocol (19). For construction of bacterial expression vector of mutated ZmRAFS protein, a QuikChange II XL Site-directed Mutagenesis Kit (Agilent Technologies, USA) was used according to the instruction manual utilizing the ZmRAFS:His6 expressing vector pET-ZmRAFS (19) as the PCR template. Listed in Table S3, the primers were named by both the original AA, its position in the ZmRAFS protein, and the mutated AA (e.g. S115A-F and S115A-R are the forward and reverse primers, respectively, for mutating serine 115 of the ZmRAFS to alanine).
For expression of ZmMIPS protein in bacteria, prokaryotic expression vectors were constructed (pET-ZmMIPS1 and pET-ZmMIPS2). The cDNA of ZmMIPS1 and ZmMIPS2 were amplified from cDNA synthesized from mRNA that was isolated from maize leaf (B73 inbred line) using a pair of primers (for ZmMIPS1, pCSGFPBT-MIPS1-F×pCSGFPBT-MIPS1-R; for ZmMIPS2, pCSGFPBT-MIPS2-F×pCSGFPBT-MIPS2-R). Then the coding region of ZmMIPS1 and ZmMIPS2 were amplified from the first PCR amplicon using primers pET-ZmMIPS-F and pET-ZmMIPS-R (conservative sequence of both ZmMIPS1 and ZmMIPS2). The coding region of ZmMIPS1 or ZmMIPS2 was ligated into the EcoRI-HindIII site of the pET-21d vector. For construction of Arabidopsis over-expression vector of ZmMIPS, pET-ZmMIPS1 and pET-ZmMIPS2 were used as templates for amplification of the coding region of ZmMIPS1 or ZmMIPS2 using the same primer pair (MIPS1/2-ara-F and MIPS1/2-ara-R). The PCR products were ligated into MluI-XbaI sites of pCsGFPBT.
Abiotic stress treatment and sampling
The three-leaf stage of hydroponically-grown B73 maize seedlings (under 12 h light (890 ± 54 μmol m−2 s−1) and 12 h darkness; constant temperature of 28 ± 1 °C) were used for abiotic stress treatment. For heat shock treatment, plants were transferred to 42 °C for 0, 2, 4, and 8 h. For dehydration treatment, plants were transferred to filter paper and dried for 0, 2, 4, 8, and 16 h. For salt stress treatment, plants were transferred to 200 mm NaCl solution and grown for 0, 1.5, 3, 6, 12, and 24 h. For cold stress treatment, plants were transferred to 4 °C for 0, 2, 4, and 8 h. The second leaf was harvested for RNA and protein analysis. For testing maize drought stress tolerance, one zmrafs mutant and one NS plant were grown in the same pot (15,000 cm3) in a greenhouse for 50 days (V15 stage) under 18 h light (900 ± 54 μmol m−2 s−1) at ambient temperature (about 20 °C at night and 25 to 30 °C during the day). Starting on day 51, water was withheld from the experimental group (10 randomly chosen pots), whereas the control group (the other 10 pots) was grown with continued watering to the same content (65% weight ratio of water/soil). After 10 days of dehydration, 3 to 8 replications (pots) were randomly chosen and the blade in the same position of NS and zmrafs plants were photographed. The middle part of the blades (about 8 cm long) were collected and tested for the electrolyte leakage following a published protocol (27). Leaf samples for sugar detection (HPLC) were collected at the same time of the day (12:00 noon) every 2 days during the dehydration stress treatment. For testing the drought stress tolerance of Arabidopsis plants, seeds of different genotypes were germinated on growth medium agar (MS salt, 2.2 g liter−1; MES, 0.4 g liter−1, pH 5.8; sucrose, 10 g liter−1) plates at 22 °C for 2 weeks, then the plants were transferred to soil and grown at 22 °C for 2 weeks. Water was then withheld for 2 weeks before the plants were re-watered and allowed to recover. Just before re-watering, the electrolyte leakage was determined as previously described (27). After re-watering, the percentage of survival of each line was calculated from 8 plants per pot for each genotype. The experiment was repeated 3 times.
RNA extraction and qPCR
RNA isolation and qPCR were performed following a published protocol (12). The expression level of tested genes was normalized to the expression level of constitutively expressed genes, specifically AtACTIN2 in Arabidopsis and ZmGAPDH in maize (Table S3). The experiment was repeated at least three times with independent biological samples. The real-time RT-PCR was performed using primers as listed in Table S3.
Purification of ZmMIPS protein and its enzyme activity assay
Prokaryotic expression vector of ZmMIPS1 (pET-ZmMIPS1) and ZmMIPS2 (pET-ZmMIPS2) were transformed into E. coli (Rosetta gami2, DE3, EMD Millipore) cells. The bacterial cultures were grown in Luria-Bertani medium to A600 nm = 0.6 under 37 °C, then supplemented with 0.4 mm isopropyl β-d-thiogalactoside and growth was continued for 12 h under 18 °C. Bacteria was collected by centrifugation (4 °C, 8000 × g, 10 min) and then lysed in Tris-HCl buffer (20 mm, pH 7.5) using ultrasonication, and the homogenates were centrifuged (4 °C, 12,000 × g, 20 min). The clear supernatants were loaded onto pre-equilibrated (20 mm Tris-HCl, pH 7.5, 100 mm imidazole) nickel-nitrilotriacetic acid columns. The columns were then washed by 10-fold column volumes of 20 mm Tris-HCl (pH 7.5, containing 100 mm imidazole). The ZmMIPS-His6 fusion proteins were eluted by 20 mm Tris-HCl (pH 7.5, containing 400 mm imidazole, 10-fold column volumes). Protein concentration was determined by Bradford assay (39).
Activity assay was performed by a published method (40). The 100-μl reaction systems containing 100 mm Tris acetate (pH 7.5), 28 mm NH4Cl, 1.6 mm NAD+, 10 mm β-mercaptoethanol, 10 mm Glc-6-P, and 2 μg of ZmMIPS-His6 fusion protein or BSA, were incubated at 37 °C for 1 h. Reactions were terminated by addition of 40 μl of 20% TCA (w/v). After centrifugation (12,000 × g, 10 min), the supernatants were supplied by equivalent volumes (130 μl) of NaIO4 (200 mm) and incubated at 37 °C for 1 h to remove Pi. Redundant NaIO4 was eliminated by application of 140 μl of 1 m Na2SO3. The released inorganic phosphates were measured by a colorimetric method (41). A 0.56-ml Pi reagent (prepared by adding 1 volume of 3 m H2SO4, 1 volume of 2.5% (NH4)6Mo7O24) and 2 volumes of H2O followed by 1 volume of 10% ascorbic acid) was added into sample and re-incubated under 37 °C for 1 h. A820 nm of samples were detected and used for to quantify the released phosphates using the Pi standard curve.
Western blot analysis of ZmGOLS2, ZmRAFS, and ZmMIPS protein accumulation
Polyclonal antiserum against ZmGOLS2 and ZmMIPS was generated in immunized rabbits. ZmGOLS2-His6 fusion protein expressed in E. coli BL21 (DE3) cells was purified using an electrodialysis method as previously described (19). The equal volume mixture of Freund's complete adjuvant (Sigma, USA) of purified ZmGOLS2-/ZmMIPS2-His6 fusion protein (150 μg) was then used for the first immunization. Two weeks after the first immunization, equal volume mixtures of Freund's incomplete adjuvant (Sigma, USA) and purified ZmGOLS2-/ZmMIPS2-His6 fusion protein (150 μg) were used to inject the rabbit every 2 weeks for a total of 4 times antigen injections. Serum was extracted 2 weeks following the final boost and used for Western blotting hybridization. The polyclonal antibody for ZmRAFS was prepared as previously described (19). The polyclonal antibody for ZmGAPDH was purchased from CWBIO (China). For determining whether ZmRAFS protein accumulation was regulated by abiotic stress, the second leaf of the B73 seedlings treated by the different abiotic stresses were used for protein extraction. For determination of ZmGOLS2, ZmRAFS, and ZmMIPS protein expression in transgenic Arabidopsis lines, the 2-week-old seedlings were used for protein extraction and subsequent Western blots as previously performed (12).
Localization of ZmRAFS-YFP fusion proteins in leaf protoplasts
The ZmRAFS-YFP fusion expression vector was transformed into maize (B73) leaf protoplasts using the PEG-Ca2+ method (38). The transformed protoplasts were visualized with a confocal microscope (Nikon A1R, Japan). ZmRAFS-YFP protein production in the transformed protoplasts was also probed using ZmRAFS antibody.
Soluble carbohydrate extraction and HPLC-evaporative light-scattering detector (ELSD) analysis of sugar components
Soluble sugar extraction was performed following a published protocol with minor revision (23). A Waters X-bridge amide column (Waters, USA) was used with methanol:H2O (90:10) as the mobile phase at a speed of 0.5 ml min−1 for separation of soluble sugar components. An ELSD (Waters 2424) monitored the sugar signal.
Enzyme activity assay of ZmRAFS
The expression vector of ZmRAFS or its mutants (mZmRAFS) was transformed into E. coli cells (Rosetta-gamiTM 2 (DE3), EMD Millipore). Protein expression was induced by isopropyl β-d-thiogalactoside and the activity reaction was performed as previously described (19). The lysate of ZmRAFS or mZmRAFS expressing strains were used for Western blotting to confirm the soluble expression of the protein. The products catalyzed by each ZmRAFS or mZmRAFS were detected by HPLC-ELSD. The enzyme activity was calculated by the products divided by the intensity of the RAFS protein as determined by Western blot analysis. The relative activity of mZmRAFS was normalized to WT ZmRAFS. For kinetic analysis of ZmRAFS in raffinose synthesis, the substrate galactinol was saturated (16 mm), whereas the concentration of sucrose was provided from 2.8 to 28 mm; or the substrate sucrose was saturated (28 mm), whereas the concentration of galactinol ranged from 1 to 16 mm. For kinetic analysis of ZmRAFS in galactinol hydrolysis, the concentration of galactinol ranged between 1 and 16 mm, although no sucrose was supplied in the reaction system. For testing the raffinose-synthetic and galactinol-hydrolytic activities in leaves of over-expressing ZmRAFS Arabidopsis, 0.5 g of fresh leaves were ground into a powder in liquid nitrogen and transferred into 1 ml of extraction buffer (50 mm HEPES, 5 mm MgCl2, 1 mm EDTA, 20 mm DTT, 50 mm sodium ascorbate, 0.1% Triton X-100, 2% polyvinylpyrrolidone-40, and 1 mm phenylmethylsulfonyl fluoride, pH 7.5, adjusted with KOH). After incubating for 30 min on ice, the supernatant was collected by centrifuging for 20 min at 12,000 × g at 4 °C. The protein concentration was determined by Bradford assay (39). The transferase and hydrolase reactions (50 μl) consisted of 25 μl of crude extract together with 20 mm HEPES-KOH, pH 7.0, 16 mm galactinol with and without 28 mm sucrose, respectively. The reaction mixtures were incubated at 37 °C for 2 h prior to the addition of 500 μl of 80% ethanol and boiling for 10 min to terminate the reaction. After recovery of the supernatant from a 10-min centrifugation at 15,000 × g at room temperature, a dilution of the ethanol with 2.75 ml of H2O permitted the extract to freeze prior to lyophilizing to dryness under vacuum. The sugar powder was dissolved in 100 μl of water for HPLC-ELSD detection.
Infiltration of exogenous sugar
To investigate whether the sucrose concentration can determine the completion of the ZmRAFS transferase activity, exogenous sucrose were applied to the leaves from 4-week-old WT, OEGFP, OEZmRAFS-25, OEZmGOLS2, and three ZmGOLS2/ZmRAFS Arabidopsis plants. Leaves of different lines were submerged in 30 mm sucrose solution or H2O and then infiltrated for 1 h. All samples in their respective liquids were incubated under light (100 μmol m−2 s−1) at 23 °C for 24 h before being washed with deionized water 3 times and stored at −80 °C until HPLC-ELSD analysis of sugar content.
Statistical analysis
The significance among means was analyzed by the Student's t test. In either case, if the comparison contained multiple datasets, the ANOVA test and Tukey's test (α = 0.05) were used to determine the significance. The significance analysis was processed by using IBM SPSS Statistics 19 software.
Accession numbers
The sequence data can be found in Maize GDB or GenBankTM under the following accession numbers: ZmRAFS: GRMZM2G150906 or BT063253; ZmGOLS2: GRMZM5G872256 or AF497509; ZmGAPDH: GRMZM2G046804 or XM NM_008679567; and AtRS5: NM_123403.
Data availability
All data are included in the manuscript.
Supplementary Material
Acknowledgments
We acknowledge Dr. Hongchang Cui from Northwest A&F University for useful discussions about the manuscript. We thank the Maize Genetics COOP Stock Center for providing the maize mutants and the Arabidopsis Biological Resource Center for the Arabidopsis mutants.
This article contains supporting information.
Author contributions—T. L., A. B. D., G. W., and T. Z. conceptualization; T. L. and L. M. A. D. data curation; T. L., Y. Z., Y. L., X. L., G. H., Q. H., L. M. A. D., and J. W. investigation; T. L. methodology; T. L. and T. Z. writing-original draft; L. M. A. D., A. B. D., Y.-L. R., G. W., and T. Z. writing-review and editing; Y.-L. R. supervision; T. Z. resources; T. Z. funding acquisition; T. Z. project administration.
Funding and additional information—This work was supported by NSFC (National Science Foundation in China) Grant 31671776 and the Special fund for transgenic research from Ministry of Agriculture in China Grant 2014ZX0800920B (to T. Z.).
Conflict of interest—The authors declare that they have no conflicts of interest with the contents of this article.
- GOLS
- GALACTINOL SYNTHASE
- RAFS
- RAFFINOSE SYNTHASE
- NS
- null-segregant
- AA
- amino acid(s)
- MIPS
- myo-inositol phosphate synthase
- STS
- STACHYOSE SYNTHASE
- YFP
- yellow fluorescent protein
- ELSD
- evaporative light-scattering detector
- ANOVA
- analysis of variance
- qPCR
- quantitative PCR
- GAPDH
- glyceraldehyde-3-phosphate dehydrogenase
- OEGFP
- GFP-overexpressing.
References
- 1. Downie B., Gurusinghe S., Dahal P., Thacker R. R., Snyder J. C., Nonogaki H., Yim K., Fukanaga K., Alvarado V., and Bradford K. J. (2003) Expression of a GALACTINOL SYNTHASE gene in tomato seeds is up-regulated before maturation desiccation and again after imbibition whenever radicle protrusion is prevented. Plant Physiol. 131, 1347–1359 10.1104/pp.016386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Koster K. L., and Leopold A. C. (1988) Sugars and desiccation tolerance in seeds. Plant Physiol. 88, 829–832 10.1104/pp.88.3.829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Egert A., Eicher B., Keller F., and Peters S. (2015) Evidence for water deficit-induced mass increases of raffinose family oligosaccharides (RFOs) in the leaves of three Craterostigma resurrection plant species. Front. Physiol. 6, 206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Peterbauer T., Mach L., Mucha J., and Richter A. (2002) Functional expression of a cDNA encoding pea (Pisum sativum L.) raffinose synthase, partial purification of the enzyme from maturing seeds, and steady-state kinetic analysis of raffinose synthesis. Planta 215, 839–846 10.1007/s00425-002-0804-7 [DOI] [PubMed] [Google Scholar]
- 5. Gangl R., Behmüller R., and Tenhaken R. (2015) Molecular cloning of AtRS4, a seed specific multifunctional RFO synthase/galactosylhydrolase in Arabidopsis thaliana. Front. Plant Sci. 6, 789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Saravitz D. M., Pharr D. M., and Carter T. E. (1987) Galactinol synthase activity and soluble sugars in developing seeds of four soybean genotypes. Plant Physiol. 83, 185–189 10.1104/pp.83.1.185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Taji T., Ohsumi C., Iuchi S., Seki M., Kasuga M., Kobayashi M., Yamaguchi-Shinozaki K., and Shinozaki K. (2002) Important roles of drought- and cold-inducible genes for galactinol synthase in stress tolerance in Arabidopsis thaliana. Plant J. 29, 417–426 10.1046/j.0960-7412.2001.01227.x [DOI] [PubMed] [Google Scholar]
- 8. Santos T. B., de Lima R. B., Nagashima G. T., Petkowicz C. L., Carpentieri-Pípolo V., Pereira L. F., Domingues D. S., and Vieira L. G. (2015) Galactinol synthase transcriptional profile in two genotypes of Coffea canephora with contrasting tolerance to drought. Genet. Mol. Biol. 38, 182–190 10.1590/S1415-475738220140171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhou Y., Liu Y., Wang S., Shi C., Zhang R., Rao J., Wang X., Gu X., Wang Y., Li D., and Wei C. (2017) Molecular cloning and characterization of galactinol synthases in Camellia sinensis with different responses to biotic and abiotic stressors. J. Agric. Food Chem. 65, 2751–2759 10.1021/acs.jafc.7b00377 [DOI] [PubMed] [Google Scholar]
- 10. Himuro Y., Ishiyama K., Mori F., Gondo T., Takahashi F., Shinozaki K., Kobayashi M., and Akashi R. (2014) Arabidopsis galactinol synthase AtGolS2 improves drought tolerance in the monocot model Brachypodium distachyon. J. Plant Physiol. 171, 1127–1131 10.1016/j.jplph.2014.04.007 [DOI] [PubMed] [Google Scholar]
- 11. Shimosaka E., and Ozawa K. (2015) Overexpression of cold-inducible wheat galactinol synthase confers tolerance to chilling stress in transgenic rice. Breeding Sci. 65, 363–371 10.1270/jsbbs.65.363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gu L., Zhang Y., Zhang M., Li T., Dirk L. M., Downie B., and Zhao T. (2016) ZmGOLS2, a target of transcription factor ZmDREB2A, offers similar protection against abiotic stress as ZmDREB2A. Plant Mol. Biol. 90, 157–170 10.1007/s11103-015-0403-1 [DOI] [PubMed] [Google Scholar]
- 13. Majee M., Maitra S., Dastidar K. G., Pattnaik S., Chatterjee A., Hait N. C., Das K. P., and Majumder A. L. (2004) A novel salt-tolerant l-myo-inositol-1-phosphate synthase from Porteresia coarctata (Roxb.) Tateoka, a halophytic wild rice: molecular cloning, bacterial overexpression, characterization, and functional introgression into tobacco-conferring salt tolerance. J. Biol. Chem. 279, 28539–28552 10.1074/jbc.M310138200 [DOI] [PubMed] [Google Scholar]
- 14. Li S. H., Li T., Kim W. D., Kitaoka M., Yoshida S., Nakajima M., and Kobayashi H. (2007) Characterization of raffinose synthase from rice (Oryza sativa L. var. Nipponbare). Biotechnol. Lett. 29, 635–640 10.1007/s10529-006-9268-3 [DOI] [PubMed] [Google Scholar]
- 15. Sui X. L., Meng F. Z., Wang H. Y., Wei Y. X., Li R. F., Wang Z. Y., Hu L. P., Wang S. H., and Zhang Z. X. (2012) Molecular cloning, characteristics and low temperature response of raffinose synthase gene in Cucumis sativus L. J. Plant Physiol. 169, 1883–1891 10.1016/j.jplph.2012.07.019 [DOI] [PubMed] [Google Scholar]
- 16. Egert A., Keller F., and Peters S. (2013) Abiotic stress-induced accumulation of raffinose in Arabidopsis leaves is mediated by a single raffinose synthase (RS5, At5g40390). BMC Plant Biol. 13, 218 10.1186/1471-2229-13-218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lahuta L. B., Pluskota W. E., Stelmaszewska J., and Szablińska J. (2014) Dehydration induces expression of galactinol synthase and raffinose synthase in seedlings of pea (Pisum sativum L.). J. Plant Physiol. 171, 1306–1314 10.1016/j.jplph.2014.04.012 [DOI] [PubMed] [Google Scholar]
- 18. Lahuta L. B., and Gorecki R. J. (2011) Raffinose in seedlings of winter vetch (Vicia villosa Roth.) under osmotic stress and followed by recovery. Acta Physiol. Plant 33, 725–733 10.1007/s11738-010-0597-4 [DOI] [Google Scholar]
- 19. Li T., Zhang Y., Wang D., Liu Y., Dirk L. M. A., Goodman J., Downie A. B., Wang J., Wang G., and Zhao T. (2017) Regulation of seed vigor by manipulation of raffinose family oligosaccharides in maize and Arabidopsis thaliana. Mol. Plant 10, 1540–1555 10.1016/j.molp.2017.10.014 [DOI] [PubMed] [Google Scholar]
- 20. Nishizawa A., Yabuta Y., and Shigeoka S. (2008) Galactinol and raffinose constitute a novel function to protect plants from oxidative damage. Plant Physiol. 147, 1251–1263 10.1104/pp.108.122465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gangola M. P., Jaiswal S., Kannan U., Gaur P. M., Båga M., and Chibbar R. N. (2016) Galactinol synthase enzyme activity influences raffinose family oligosaccharides (RFO) accumulation in developing chickpea (Cicer arietinum L.) seeds. Phytochemistry 125, 88–98 10.1016/j.phytochem.2016.02.009 [DOI] [PubMed] [Google Scholar]
- 22. Zhou M. L., Zhang Q., Zhou M., Sun Z. M., Zhu X. M., Shao J. R., Tang Y. X., and Wu Y. M. (2012) Genome-wide identification of genes involved in raffinose metabolism in maize. Glycobiology 22, 1775–1785 10.1093/glycob/cws121 [DOI] [PubMed] [Google Scholar]
- 23. Zhao T. Y., Corum J. W., Mullen J., Meeley R. B., Helentjaris T., Martin D., and Downie B. (2006) An alkaline α-galactosidase transcript is present in maize seeds and cultured embryo cells, and accumulates during stress. Seed Sci. Res. 16, 107–121 10.1079/SSR2006243 [DOI] [Google Scholar]
- 24. Cacela C., and Hincha D. K. (2006) Monosaccharide composition, chain length and linkage type influence the interactions of oligosaccharides with dry phosphatidylcholine membranes. Biochim. Biophys. Acta Biomembr. 1758, 680–691 10.1016/j.bbamem.2006.04.005 [DOI] [PubMed] [Google Scholar]
- 25. Lineberger R. D., and Steponkus P. L. (1980) Cryoprotection by glucose, sucrose, and raffinose to chloroplast thylakoids. Plant Physiology 65, 298–304 10.1104/pp.65.2.298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schneider T., and Keller F. (2009) Raffinose in chloroplasts is synthesized in the cytosol and transported across the chloroplast envelope. Plant Cell Physiol. 50, 2174–2182 [DOI] [PubMed] [Google Scholar]
- 27. Li Z., Peng J., Wen X., and Guo H. (2013) Ethylene-insensitive3 is a senescence-associated gene that accelerates age-dependent leaf senescence by directly repressing miR164 transcription in Arabidopsis. Plant Cell 25, 3311–3328 10.1105/tpc.113.113340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yang J., Yan R., Roy A., Xu D., Poisson J., and Zhang Y. (2015) The I-TASSER suite: protein structure and function prediction. Nat. Methods 12, 7–8 10.1038/nmeth.3213, 10.1038/nmeth.3518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gangl R., and Tenhaken R. (2016) Raffinose family oligosaccharides act as galactose stores in seeds and are required for rapid germination of Arabidopsis in the dark. Front. Plant Sci. 7, 1115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Goswami L., and Sengupta S. (2014) Targeted expression of L-myo-inositol 1-phosphate synthase from Porteresia coarctata (Roxb.) Tateoka confers multiple stress tolerance in transgenic crop plants. J. Plant Biochem. Biotechnol. 23, 316–330 10.1007/s13562-013-0217-7 [DOI] [Google Scholar]
- 31. Joshi R., Ramanarao M. V., and Baisakh N. (2013) Arabidopsis plants constitutively overexpressing a myo-inositol 1-phosphate synthase gene (SaINO1) from the halophyte smooth cordgrass exhibits enhanced level of tolerance to salt stress. Plant Physiol. Biochem. 65, 61–66 [DOI] [PubMed] [Google Scholar]
- 32. Kaur H., Verma P., Petla B. P., Rao V., Saxena S. C., and Majee M. (2013) Ectopic expression of the ABA-inducible dehydration-responsive chickpea L-myo-inositol 1-phosphate synthase 2 (CaMIPS2) in Arabidopsis enhances tolerance to salinity and dehydration stress. Planta 237, 321–335 10.1007/s00425-012-1781-0 [DOI] [PubMed] [Google Scholar]
- 33. Tan J., Wang C., Xiang B., Han R., and Guo Z. (2013) Hydrogen peroxide and nitric oxide mediated cold- and dehydration-induced myo-inositol phosphate synthase that confers multiple resistances to abiotic stresses. Plant Cell Environ. 36, 288–299 10.1111/j.1365-3040.2012.02573.x [DOI] [PubMed] [Google Scholar]
- 34. Sun Z. B., Qi X. Y., Wang Z. L., Li P. H., Wu C. X., Zhang H., and Zhao Y. X. (2013) Overexpression of TsGOLS2, a galactinol synthase, in Arabidopsis thaliana enhances tolerance to high salinity and osmotic stresses. Plant Physiol. Biochem. 69, 82–89 10.1016/j.plaphy.2013.04.009 [DOI] [PubMed] [Google Scholar]
- 35. Sivitz A. B., Reinders A., Johnson M. E., Krentz A. D., Grof C. P., Perroux J. M., and Ward J. M. (2007) Arabidopsis sucrose transporter AtSUC9: high-affinity transport activity, intragenic control of expression, and early flowering mutant phenotype. Plant Physiol. 143, 188–198 10.1104/pp.106.089003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cao T., Lahiri I., Singh V., Louis J., Shah J., and Ayre B. G. (2013) Metabolic engineering of raffinose-family oligosaccharides in the phloem reveals alterations in carbon partitioning and enhances resistance to green peach aphid. Front. Plant Sci. 4, 263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Selvaraj M. G., Ishizaki T., Valencia M., Ogawa S., Dedicova B., Ogata T., Yoshiwara K., Maruyama K., Kusano M., Saito K., Takahashi F., Shinozaki K., Nakashima K., and Ishitani M. (2017) Overexpression of an Arabidopsis thaliana galactinol synthase gene improves drought tolerance in transgenic rice and increased grain yield in the field. Plant Biotechnol. J. 15, 1465–1477 10.1111/pbi.12731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gu L., Han Z., Zhang L., Downie B., and Zhao T. (2013) Functional analysis of the 5′ regulatory region of the maize GALACTINOL SYNTHASE2 gene. Plant Sci. 213, 38–45 10.1016/j.plantsci.2013.09.002 [DOI] [PubMed] [Google Scholar]
- 39. Kruger N. J. (1994) The Bradford method for protein quantitation. Methods Mol. Biol. 32, 9–15 [DOI] [PubMed] [Google Scholar]
- 40. Barnett J. E., Brice R. E., and Corina D. L. (1970) A colorimetric determination of inositol monophosphates as an assay for d-glucose 6-phosphate-1-l-myoinositol 1-phosphate cyclase. Biochem. J. 119, 183–186 10.1042/bj1190183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chen P. S., Toribara T. Y., and Warner H. (1956) Microdetermination of phosphorus. Anal. Chem. 11, 1756–1758 10.1021/ac60119a033 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are included in the manuscript.