Abstract
The plasma membrane phospholipid phosphatidylinositol 4,5-bisphosphate (PIP2) regulates the activity of diverse ion channels to include the epithelial Na+ channel ENaC. Whether PIP2 regulation of ENaC is due to a direct phospholipid-protein interaction, remains obscure. To date, possible interaction of PIP2 with ENaC primarily has been tested indirectly through assays of channel function. A fragment-based biochemical analysis approach is used here to directly quantify possible PIP2-ENaC interactions. We find using the CIBN-CRY2 optogenetic dimerization system that the phosphoryl group positioned at carbon 5 of PIP2 is necessary for interaction with ENaC. Previous studies have implicated conserved basic residues in the cytosolic portions of β- and γ-ENaC subunits as being important for PIP2-ENaC interactions. To test this, we used synthetic peptides of these regions of β- and γ-ENaC. Steady-state intrinsic fluorescence spectroscopy demonstrated that phosphoinositides change the local conformation of the N terminus of β-ENaC, and two sites of γ-ENaC adjacent to the plasma membrane, suggesting direct interactions of PIP2 with these three regions. Microscale thermophoresis elaborated PIP2 interactions with the N termini of β- (Kd ∼5.2 μm) and γ-ENaC (Kd ∼13 μm). A weaker interaction site within the carboxyl terminus of γ-ENaC (Kd ∼800 μm) was also observed. These results support that PIP2 regulates ENaC activity by directly interacting with at least three distinct regions within the cytoplasmic domains of the channel that contain conserved basic residues. These interactions are probably electrostatic in nature, and are likely to bear a key structural role in support of channel activity.
Keywords: hypertension, phosphoinositide, epithelial sodium channel (ENaC), ion channel, transport, epithelial ion transport, microscale thermophoresis, phospholipid signaling, sodium excretion, steady state intrinsic spectroscopy
Introduction
The activity of the amiloride-sensitive, epithelial Na+ channel (ENaC) is limiting for sodium transport across many absorptive epithelial tissues to include those lining the renal distal nephron, the lungs and colon. ENaC is expressed in the apical plasma membranes of polarized epithelial cells that form these tissues (1). This function and location in the kidney makes ENaC the final arbiter of renal sodium excretion. As such, the activity of ENaC plays a key role in the normal control of blood pressure by affecting renal sodium excretion (2). Mutation of ENaC, consequently, causes inheritable forms of hyper- and hypotension (1, 3). Moreover, because of its function and expression in epithelial cells of the airways and lungs, ENaC activity is critical to normal hydration of airway mucus and fluid clearance from alveolar spaces (4). ENaC dysfunction in these latter tissues, thus, can contribute to the pathology of cystic fibrosis and respiratory distress (5).
The bulk of the protein structure of ENaC, as elaborated by cryo-EM, is known (6). Similar to the related acid-sensing ion channel 1 (ASIC1), the first member of the ENaC/Deg ion channel family to be crystallized (7), ENaC is a trimeric ion channel. Specifically, ENaC is a heterotrimer consisting of related α, β, and γ subunits, which are encoded by distinct genes (6). The overall structure of ENaC/Deg ion channels, as exemplified by ENaC and ASIC1, is similar to that of the unrelated ionotropic P2X receptors (8). Each ENaC subunit contains two transmembrane domains, a large extracellular domain, and relatively short cytosolic amino and carboxyl termini. ENaC subunits fit together to form a chalice-like structure with an internal pore that spans the plasma membrane. The bulk of the channel is extracellular and formed of large globular domains. The channel pore is formed by the helical second transmembrane domains of each subunit as they run perpendicular to the plasma membrane. Similar to that for crystallization of ASIC1, the cytoplasmic regions of ENaC were truncated to facilitate the resolution of structure. Thus, the structure of the intracellular domains of ENaC are currently obscure. Strong evidence, although, supports that these cytoplasmic domains are key cell signaling targets during the normal regulation of channel activity. Signaling to cytoplasmic domains within the ENaC influence channel expression, turnover, and gating (9–12). A physiologically important regulator of ENaC activity that is thought to influence the channel through interactions with intracellular domains is the plasma membrane phospholipid, phosphatidylinositol 4,5-bisphosphate (PIP2) (2, 13, 14).
PIP2 effects on ion channels were first reported in the early 1990's where PIP2 was shown to stimulate Ca2+ current through ionotropic ryanodine receptors, and prevent rundown of KATP channels in excised membrane patches (15, 16). The dependence on local PIP2 of the inward rectifying K+ channel, Kir, for normal channel activity was clearly demonstrated a few years later by Hilgemann et al. (17, 18). In these studies, depletion of PIP2 reduced the activity of Kir, whereas, exogenous PIP2 increased channel activity. PIP2 was found to directly interact with critical cationic residues within cytoplasmic domains of Kir (18). Since these pioneering studies, PIP2 has been shown to be a key regulator of many distinct ion channels to include voltage-gated K+ channels, 2-pore K+ channels, P2X receptors, TRP channels, and N-type and P/Q-type voltage gated Ca2+ channels (19, 20). Many of these channels contain clusters of cationic residues within cytoplasmic domains critical to their PIP2 dependence. These cationic sites resemble those of the pleckstrin homology and ENTH PIP2-binding domains (21), and are suspected to bind the exposed phosphoryl groups of PIP2, commonly referred to as the “PIP2 headgroup.” Electrostatic PIP2-ion channel interactions have been directly observed in the cryo-EM and crystal structures of TRPM8, TMEM16F, Kir2.2, and Kir3.2 (22–25). However, the precise mechanism by which PIP2 regulates diverse ion channels is still debated. Although PIP2 is reported to inactivate some channels, including hEAG1 (26) and TRPL (27), most channels sensitive to this phospholipid are activated by it (19). To date, ENaC is the only Na+-selective ion channel reported to be activated by PIP2 (14, 28).
The effects of PIP2 on ENaC have primarily been characterized in electrophysiology studies of channel function. For instance, manipulation of phospholipid levels in excised, inside-out cell patches containing heterologously expressed ENaC revealed that PIP2 stabilizes ENaC currents and prevents channel rundown (29). Moreover, stimulation of G protein–coupled receptors, which are coupled via Gq to PLC, facilitates hydrolysis of PIP2 leading to a decrease in ENaC activity in native renal tubules (20). Co-immunoprecipitation studies combined with site-directed mutagenesis are consistent with, but not definitive of, PIP2 directly interacting with clusters of basic residues within the cytoplasmic regions of the β- and γ-, but not α-ENaC subunits (14, 30). More recent evidence suggests that PIP2 upon interacting with the channel may increase the helical content of the N terminus of γ-ENaC (31). Although substantial proof exists for ENaC's dependence on PIP2 and probable interaction of this phospholipid with the channel, specific details about the strength and exact channel domains involved in such interactions have remained obscure.
The current study addresses this deficiency by directly quantifying PIP2-ENaC interactions using a quantitative biophysical approach capable of elaborating the binding affinities of such interactions as well as precisely defining the domains within the channel involved in these interactions. We hypothesized that the headgroups of PIP2 directly interact with multiple clusters of cationic residues conserved within the cytoplasmic termini of β- and γ-ENaC subunits. Optogenetic manipulation of PIP2 demonstrated that the PIP2 headgroup is necessary for effects on ENaC. Steady-state intrinsic fluorescence spectroscopy and microscale thermophoresis in conjunction with mutagenesis identified three distinct clusters of cationic residues conserved in the cytoplasmic termini of β- and γ-ENaC as being necessary for channel interactions with PIP2. These three PIP2-binding sites within ENaC interact with the phospholipid with low to moderate affinity in the micromolar range.
Results
Blue light-recruitment of 5-ptase reduces membrane PIP2 levels
We hypothesized, as others have before (14, 30), that phosphoryl groups at carbons 4 and 5 (C4 and C5) of PIP2, illustrated in Fig. S1, are necessary for PIP2-ENaC interactions. Targeted removal of the C5 phosphoryl group of PIP2 was accomplished using the recently developed CIBN-CRY2–5-phosphataseOCRL optogenetics system. This system has been described previously in the study of PIP2 regulation of KCNQ K+ channels (32). In brief, proteins in this system have been engineered such that the CAAX-fused CIBN is localized to the plasma membrane, and mCherry-CRY2 fused to the 5-phosphatase OCRL (mCh-CRY2–5-ptase) is expressed in the cytoplasm. CRY2 dimerizes with CIBN upon exposure to blue light. Upon blue light illumination (BLI), the 5-ptase translocates to the membrane where it dephosphorylates PIP2 at C5.
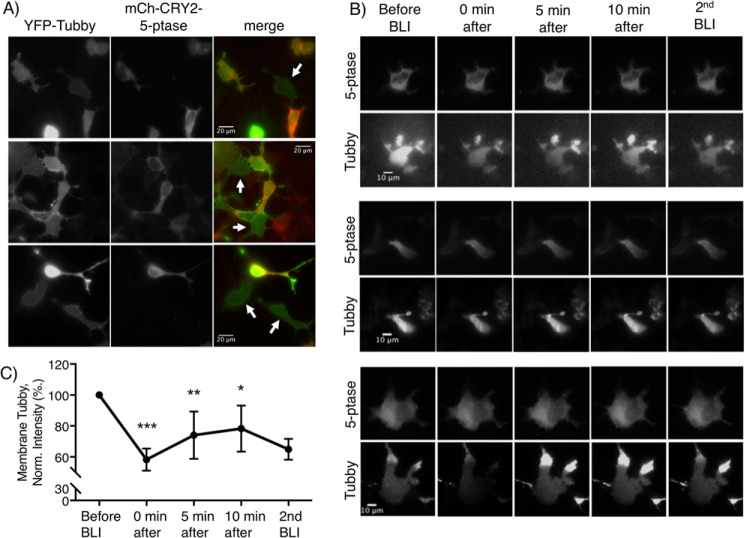
To confirm the functionality of the CIBN/CRY2–5-ptase dimerization system on PIP2 in our hands, we followed the localization pattern of the cellular PIP2 reporter YFP-Tubby. This reporter selectively binds PIP2 over inositol triphosphate (IP3) (33, 34). Blue light activation of CIBN/mCh-CRY2–5-ptase was achieved using brief global illumination through a standard CFP fluorescence filter. Diffuse, cytoplasmic YFP-Tubby (green) was consistently observed in cells exposed to BLI that also expressed mCh-CRY2–5ptase (red) (Fig. 1A). In contrast, even with BLI, cells expressing only YFP-Tubby displayed stronger membrane localization, consistent with brighter fluorescence along the edges of the cells. This is indicated by the white arrows in the merged images of Fig. 1A. This change in localization is consistent with Tubby being recruited to the membrane via association with PIP2 but translocating to the cytoplasm when PIP2 is depleted. TIRF imaging was used to more specifically monitor the effects of BLI-induced recruitment of mCh-CRY2–5-ptase to the membrane on the location of YFP-Tubby. This strategy, combined with finding cells that express mCherry with 561 nm illumination, and imaging Tubby at 514 nm, enabled us to selectively activate cells independent of each other. Although the mCh-CRY2–5ptase changes tended to be subtle following BLI, YFP-Tubby membrane fluorescence immediately dropped to 58 ± 7% of starting levels, suggesting a significant loss of intact phosphoinositide at the membrane (Fig. 1, B and C). Tubby levels recovered in 5–10 min to 78 ± 15% of starting levels. This experiment is consistent with the CIBN/CRY2–5-ptase dimerization system causing a rapid and significant reduction in membrane PIP2 upon BLI.
Figure 1.
BLI-induced 5-ptase recruitment leads to translocation of the Tubby PIP2-binding domain from the membrane to cytoplasm. A, three representative fluorescence micrographs of HEK 293 cells transfected with YFP-Tubby (left), CIBN and mCh-CRY2–5-ptase (middle) after BLI. The merged images are shown to the right, with white arrows indicating cells expressing only YFP-Tubby. B, three representative cells imaged under TIRF microscopy showing mCh-CRY2–5-ptase (top panels) and YFP-Tubby (bottom panels) before and 0, 5, and 10 min after BLI, then immediately following a second BLI. C, summary graph of membrane YFP-Tubby normalized to the TIRF fluorescence intensity of membrane YFP-Tubby before BLI. Data represent a mean ± S.E. of n = 5 or 6 cells of n = 2 independent experiments; ***, p < 0.0001; **, p < 0.005; *, p < 0.05 compared with the cells before BLI.
Na+ flux through ENaC depends on the C5 phosphoryl group of PIP2
The activity of ENaC in excised, inside-out patches is influenced by the levels of PIP2 in the plasma membrane (14, 29). To further elaborate PIP2 effects on ENaC, we quantified changes in Na+ flux through human ENaC (hENaC) before and after the targeted removal of the C5 phosphoryl group of PIP2 using the CIBN/CRY2–5-ptase dimerization system. CoroNa Green fluorescent sodium-selective indicator was used to monitor changes in intracellular Na+ levels in HEK 293 cells transfected with mCh-CRY2–5-ptase and CIBN-CAAX, with and without hENaC before and after BLI. We recorded changes in intracellular Na+ levels as a function of normalized change of CoroNa Green fluorescence using a method similar to that used previously to detect intracellular Na+ changes in cortical neurons (35). The sensitivity of CoroNa Green to Na+ changes affected by ENaC activity was first tested using the selective blocker amiloride (Fig. S2, black bars). Basal Na+ levels in HEK 293 cells were initially estimated in the presence of 10 μm amiloride. Removal of amiloride by perfusion of PBS resulted in a significant increase of Corona Green fluorescence by ∼68%, consistent with an increase of intracellular Na+ through ENaC upon removal of the channel blocker. Reapplication of 10 μm amiloride significantly decreased Corona Green fluorescence (∼63%) to levels near starting values in amiloride. In contrast, removing amiloride had no effect on Corona Green fluorescence in control cells not expressing ENaC (Fig. S2, purple bars). These results support the use of CoroNa Green for detecting changes in intracellular Na+ levels via ENaC. Thus, a reduction in the magnitude of green fluorescence indicates a decrease in intracellular [Na+] consistent with a decrease in Na+ influx upon inhibition of ENaC.
We next tested the effects of depleting PIP2 on ENaC activity using this system. Because of the quick depletion of PIP2 and its subsequent recovery observed in Fig. 1, we imaged CoroNa at 488 nm, with an additional flash at 5 min to retain membrane-localized mCh-CRY2–5-ptase for the duration of the experiment. The representative wide-field fluorescence micrographs in Fig. 2A show that prior to BLI, the mCh-CRY2–5-ptase fluorescence is diffuse throughout the cell (left). Following BLI, mCh-CRY2–5-ptase fluorescence increases at the plasma membrane (middle). Recruitment of mCh-CRY2–5-ptase to the membrane was quantified by subtracting mCherry fluorescence across each cell prior to BLI from that 10 min after BLI shown in the differential fluorescence micrographs (right). Intensity plots for regions of interest across each cell document that significant peak-BLI-sensitive changes in mCherry fluorescence occur at the edges of these cells consistent with the plasma membrane (Fig. 2, B and C). In contrast, there was no significant difference in the overall fluorescence of the cytoplasmic (middle) region of each cell (Fig. 2C). The summary graph in Fig. 2D shows that changes in membrane mCh-CRY2–5-ptase following BLI occur independent of co-expression of hENaC. However, as shown in the representative fluorescence micrographs in Fig. 2E and summarized in Fig. 2F, the magnitude of CoroNa Green fluorescence decreases as a function of hENaC expression and translocation of mCh-CRY2–5-ptase to the plasma membrane following BLI. In cells expressing hENaC, normalized (to starting values) CoroNa Green fluorescence was significantly decreased by 25.2 ± 4.6% 10 min after BLI. In contrast, CoroNa Green fluorescence slightly, but not significantly, increased by 4.5 ± 8.5% 10 min after BLI in control cells that were not transfected with hENaC, suggesting that this decrease was not due to leakage or bleaching of CoroNa Green. These results are consistent with the C5 phosphoryl headgroup of membrane PIP2 being necessary for normal ENaC function.
Figure 2.
Depletion of membrane PIP2 slows the influx of Na+ into HEK 293 cells in the presence but not absence of hENaC. A, HEK 293 cells transfected with human ENaC, mCh-CRY2–5-ptase and CIBN-CAAX before (left) and 10 min after (middle) BLI pulses. Representative micrographs are shown (right) for hENaC expressing cells for which fluorescence from mCh-CRY2–5-ptase before BLI was subtracted and 10 min after BLI. Note that panels B and E correspond to the representative cells in A. B, summary graphs comparing the relative fluorescence of mCh-CRY2–5-ptase across the indicated cell to the left, marked by a line in the micrographs of subtracted CRY2 levels shown in A. The fluorescence across each cell before BLI is shown in purple, and 10 min after shown in cyan. Changes at the plasma membrane are noted by peaks and indicated by black arrows. C, summary of changes of mCh-CRY2–5-ptase membrane fluorescence at the right and left edge of each cell compared with the middle of the cell before (purple) and 10 min after (cyan) BLI. D, summary graph of the relative change in membrane mCh-CRY2–5-ptase fluorescence 10 min after BLI, as compared with before, of cells with and without ENaC. E, representative micrographs of CoroNa Green fluorescence before (left) and 10 min after (right) BLI, corresponding to the cells shown in A. F, summary graph comparing intracellular CoroNa Green levels before and 10 min after BLI in HEK 293 cells not transfected with hENaC (left) and in those expressing the channel (right). Data are from n = 10–11 cells for each group; **, p < 0.005. n.s., not significant.
Cationic residues within the cytosolic termini of β- and γ-ENaC potentially cluster to form PIP2-binding sites
Based upon our previous findings that clusters of cationic residues within the cytoplasmic termini of β- and γ-mENaC (mouse ENaC) are involved in the regulation of the channel by PIP2 (14), we designed synthetic peptides corresponding to these regions of hENaC. Table S1 shows the peptide sequences used for this study and their corresponding mutants studied in our prior work (14). The positions of these peptides within β- and γ-ENaC subunits are shown in the illustrations (A) and sequence alignments (B and C) of Fig. 3. Notably, these domains within ENaC are highly conserved. In a recent study (31), it was suggested that PIP2 promoted assumption of a helical structure by the N terminus of γ-ENaC. JPred4 online software was used to identify potential regions of helical content within the amino and carboxyl termini of β- and γ-ENaC, indicated by a series of red “H” below the sequence alignments in Fig. 3. The candidate peptides studied here predicted to have the greatest helical content are βN1, γN1, and γC.
Figure 3.
Putative PIP2-binding sites within ENaC subunits. A, cartoons demarking putative PIP2-binding sites in the cytosolic termini of β- and γ-ENaC subunits. B and C, alignment of the cytosolic termini of the β- and γ-ENaC subunits from human and mouse: * indicates conserved residues. Blue and green boxes indicate the human sequences used as synthetic peptides in these studies. Also shown below each alignment are the results of a JPred4/Jnet analysis indicating sites of predicted helical structure. Red H and orange E indicate that these domains within ENaC subunits have a high and moderate probability, respectively, of forming an α helix.
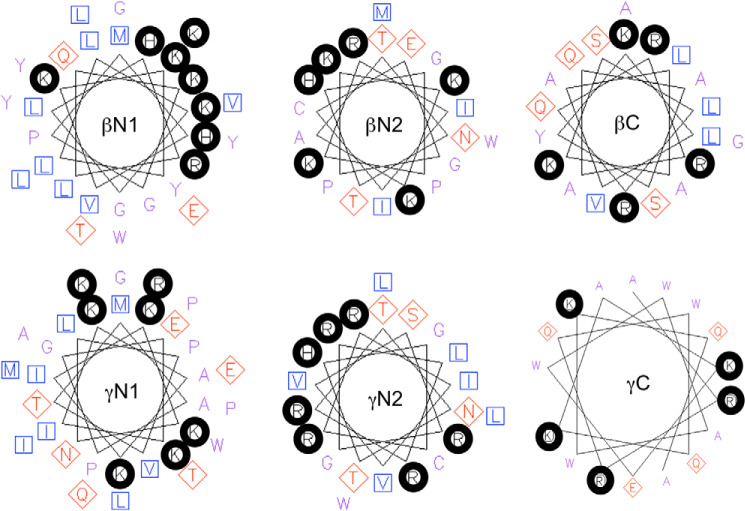
Helical wheel diagrams were generated, as shown in Fig. 4, to evaluate the potential distribution of the positively charged residues of these peptides if they were to form helices. Clustering of cationic residues secondary to protein folding may increase the likelihood for electrostatic interactions with the negatively charged phosphoryl groups of PIP2. The βN1 helix model displays a cluster of 7 basic residues to one side of the putative helix. Smaller clusters are observed within γN1, γN2, and βN2. γC and βC each present at least one doublet of basic residues, which may also serve as phosphoinositide interaction sites. These bioinformatics data are consistent with helical structures within the cytoplasmic domains of ENaC subunits, should they occur, forming electropositive regions that would likely be receptive to interaction with the negative phosphoryl headgroups of PIP2.
Figure 4.
Clustering of cationic residues by helical formation of hENaC cytoplasmic termini. Helical wheel projections of the sequences for each peptide corresponding to the putative PIP2 sites in β- and γ-hENaC subunits. The peptide label is shown inside each wheel. Residues with basic side chains, H, K, and R, are circled with thick black lines. Hydrophobic residues marked with blue squares, and acidic residues and hydroxyl- and nitrogen-containing residues marked with red diamonds. Helical projections were made using the online tool EMBOSS Pepwheel.
Phosphoinositides cause a change in emission spectra of βN1, γN2, and γC ENaC peptides
Ponchynyuk et al. (14) found that mutations and deletions within the extreme N termini of β- and γ-mENaC, βND (corresponding to the βN1 region in this study), and γNS (γN1 in the current study), abrogated ENaC responses to depletion of PIP2, suggesting these regions are important for PIP2-sensitive responses. Likewise, channels with mutations in the carboxyl termini adjacent to the membrane, β2D (βC in the current study) and γ2S/D (γC in the current study), did not respond to phosphatidylinositol 3-kinase and increases of the related phospholipid, phosphatidylinositol 3,4,5-bisphosphate (PIP3) suggesting these sites were perhaps also important for responses to changes in phospholipid levels. ENaC containing mutants in the N termini adjacent to the membrane, γ1D/S (γN2 in the current study) and β1D/S (βN2 in the current study) did not produce detectable Na+ currents in this earlier work, and consequently, could not be tested for phospholipid sensitivity. It has been reported previously that this region of γ-ENaC is also important for interacting with PIP2 (31). Here we studied the direct biochemical interactions of peptides corresponding to these regions of ENaC with soluble analogs of PIP2 and PIP3. We first examined the steady-state intrinsic fluorescence (SSIF) of each ENaC peptide in the absence and presence of diC4-PIP2 and diC4-PIP3. The latter are synthetic phospholipids that have shortened fatty acyl tails to improve their solubility in physiological salt solutions. The simplified molecular structures shown in Fig. S1 highlight the positions of critical functional groups in these phospholipids. The PIP2 and PIP3 analogs used for this study do not absorb at the same wavelengths (250 and 280 nm) as aromatic amino acids (Fig. S3, A and B). This fact enabled us to evaluate PIP2- and PIP3-dependent changes in the emission spectra of ENaC peptides as a faithful readout for phosphoinositide binding.
Fluorescence emission spectra were recorded for each ENaC peptide (0.25 μm) before and after addition of PIP2 (Fig. 5) and PIP3 (Fig. 6) with excitation at 250 and 280 nm. The prior wavelength documents changes in the local environment following emissions from regions containing tyrosine (Tyr) residues and the latter from those containing tryptophan (Trp) residues (36). Although 250 nm typically is also used to follow local changes in structure in regions that include phenylalanine (Phe) (36, 37), none of the ENaC peptides studied here contained Phe. Consequently, changes observed at 250 nm reflect local changes around Tyr. Phosphoinositides were added to each peptide in excess (10 μm) and incubated at room temperature at least 1 min prior to recording to facilitate binding.
Figure 5.
PIP2 effects on the steady-state intrinsic fluorescence of hENaC cytosolic fragments. Emission spectra of hENaC peptides excited at 250 (left panel) and 280 nm (right panel) in the absence (black line) and presence (cyan line) of diC4-PIP2. Emissions spectra for βN1, βN2, and βC peptides in A; γN1, γN2, and γC peptides in B; and mutant peptides in C. Quantification of the changes in peak emissions with excitation at 250 (D) and 280 nm (E). Summary results for WT and mutant peptides are shown as black and white histograms, respectively. Significant differences between the WT and mutants were noted for βN1, γN2, and γC (multiple t tests; *, p < 0.05; **, p < 0.005). In contrast, no significant difference was noted between βN2, βC, γN1, and mutant peptides for all PIP2 data sets (2-way ANOVA; interaction: F(5,24) = 0.7758, p = 0.5767; Fwavelength(1,24) = 1.188, p = 0.2866; Fwt vs mutant(5,24) = 1.282, p =0.3041. Data are presented as mean ± S.D. from 3 replicates.
Figure 6.
PIP3 effects on the steady-state intrinsic fluorescence of hENaC cytosolic fragments. Emission spectra of hENaC peptides excited at 250 (left panel) and 280 nm (right panel) in the absence (black line) and presence (green line) of diC4-PIP3. Spectra for βN1, βN2, and βC are shown in A; for γN1, γN2, and γC in B; and mutant peptides in C. Changes in peak emissions with excitation at 250 (D) and 280 nm (E). Summary results for WT and mutant peptides shown as black and white histograms, respectively. Significant peak changes were determined by multiple t tests (*, p < 0.05; **, p < 0.005). Peak changes of 5% or less were compared with mutant peak changes and analyzed by 2-way ANOVA (FInteraction(5,24) = 0.2827, p = 0.9180; Fwavelength(1,24) = 0.0144, p = 0.9054; Fwt vs mutant(5,24) = 2.453, p = 0.0624), showing no significant difference. Data are presented as mean ± S.D. from 3 replicates.
Addition of diC4-PIP2 (Fig. 5) caused peak changes of 9–16% for βN1, γN2, and γC after excitation at 250 nm, and 6–14% at 280 nm. As documented in Fig. 5, D and E, these peak changes were significantly greater in WT peptides as compared with peptides that contained mutations of their basic residues. Because charge-neutralized mutants were insoluble, all mutations used in these studies consisted of anionic glutamate in the place of cationic residues, resulting in charge reversal (Table S1, blue text). Mutant peptides had peak changes in response to PIP2 of only ∼1–3%. Addition of diC4-PIP3 (Fig. 6) also caused greater changes in emissions from βN1, γN2, and γC peptides (∼7–14%) as compared with their mutants; however, only βN1 and γN2 excited at 280 nm had changes significantly greater than their corresponding mutants. No significant difference was observed in responses between βC, βN2, γN1, and their mutants at either excitation wavelength but a clear trend is apparent with mutants having lower peak changes in response to PIP3 compared with WT peptides. Similar to effects on mutants, inositol, which does not contain phosphoryl groups, caused minimal changes (∼2–4%) in peak intrinsic fluorescence of βN1, γN2, and γC peptides, compared with changes induced by diC4-PIP2 (Fig. S3, C and D). These changes in intrinsic fluorescence emission spectra suggest that the membrane-adjacent cytosolic fragments of γ-ENaC, γC, and γN2, and the proximal half of the N terminus of β-ENaC, which includes βN1, directly interact with PIP2.
The PIP2 headgroup directly interacts with βN1, γN2, and γC domains within ENaC
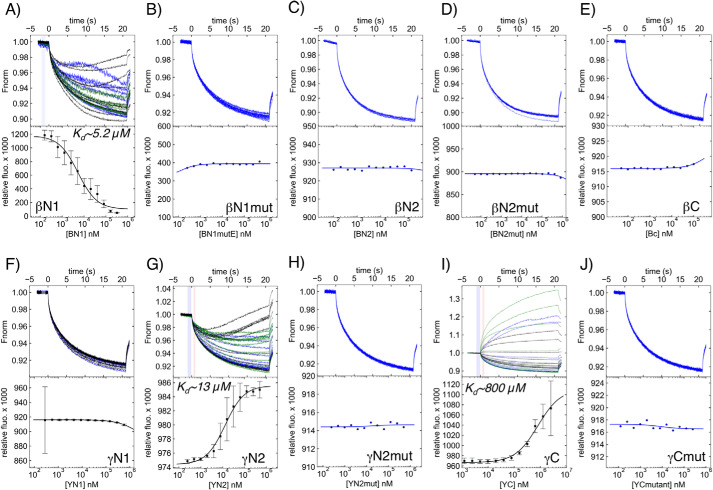
Microscale thermophoresis (MST) was used to quantify the affinities of each of these peptides for the PIP2 headgroup. A 1:1 serial dilution of each ENaC peptide was combined with 100 nm fluorescein-PIP2-HG. In this molecule, the fluorescein is attached to the C1 phosphoryl group of the PIP2 headgroup instead of the two acyl chains. This maintains similar chemistry to the native PIP2 headgroup, which has fatty acyl chains attached to the C1 phosphoryl group, not thought to interact with intracellular domains of channel proteins (see Fig. S1). MST data were analyzed using PALMIST software (38, 39). βN1 had moderate interaction with the fluorescein-PIP2-HG with a Kd equal to 5.2 μm at a confidence interval of 3.2–8.6 μm. γN2 similarly had moderate interactions with a Kd equal to 13 μm and a confidence interval of 10–16 μm. γC had weak interactions with a Kd equal to 800 μm and a confidence interval of 600–1100 μm (Fig. 7). No interaction was detected with the other ENaC peptides or with mutants of βN1, γN2, and γC. These MST results in combination with the above SSIF results are consistent with PIP2 directly interacting with the proximal half of the β-ENaC N terminus, and the membrane-adjacent cytosolic amino and carboxyl termini of γ-ENaC.
Figure 7.
Microscale thermophoresis ligand binding data for the PIP2 headgroup with hENaC cytosolic fragments. Sample traces (top) and summarized results calculating respective Kd values (bottom) for the binding of 100 nm PIP2 headgroup (fluorescein-PIP2-HG) with WT and mutant βN1 (A and B), βN2 (C and D), βC (E), γN1 (F), γN2 (G and H), and γC (I and J) peptides as measured by MST. Peptide concentrations ranged from 0.1 to 1000 μm. For experiments where it was possible to determine Kd, results from three independent trials are shown as different colored raw traces (black, green, and blue). A single representative trace is shown when binding was not detected. Time domains analyzed in these thermographs are denoted with light blue and red bars, where blue is resting fluorescence before application of a heat gradient and red is fluorescence in the present of applied heat. βN1 binding was determined using the cold fluorescence method. γN2 and γC binding was determined using the regions of cold and Tjump fluorescence, which is the fluorescence level immediately after applying the heat gradient. All data were fit using a 1:1 binding model in PALMIST.
Discussion
This is the first study that we are aware of, that directly quantifies the binding affinity of ENaC for phospholipids. Phospholipid binding to ENaC is thought to be critical to the proper function of the channel. Here, cellular and in vitro biophysical methods demonstrate that PIP2 regulates hENaC activity through direct interactions with at least three distinct intracellular domains of the channel. Each of these domains is rich in cationic residues. Results from the CRY2-CIBN optogenetics cellular assay show that depletion of PIP2 reduces intracellular Na+ levels in HEK cells transfected with ENaC, whereas Na+ levels remain constant in control cells lacking ENaC. Bioinformatics analysis was consistent with several cationic-rich regions within β- and γ-ENaC cytoplasmic tails forming amphipathic helices and the clustering of positively charged residues to one side of these helices. Such clustering would form electropositive sites attractive to the negative phosphoryl groups of PIP2. Such phosphoryl groups, as demonstrated by results shown in Fig. 2, are necessary for PIP2 regulation of ENaC. Results from SSIF ligand-binding experiments were consistent with both PIP2 and PIP3 changing the secondary structure of intracellular domains of the channel proteins, specifically the extreme N terminus of β-ENaC (βN1) and a membrane adjacent region in the N terminus of γ-ENaC (γN2); whereas, only PIP2 significantly altered the membrane adjacent to the carboxyl terminus of γ-ENaC (γC). Results from MST experiments demonstrate that PIP2 binds to βN1 and γN2 with dissociation constants in the lower micromolar range and γC in the high micromolar range. In contrast, charge reversal of the cationic residues in these domains obliterated phospholipid binding. Together these results indicate that PIP2, and possibly PIP3, regulate ENaC activity by directly interacting with the βN1, γN2, and γC domains of the channel, and that such interactions are dependent on phosphoryl groups within the phospholipid, and the cationic residues within these domains of ENaC. Overall, these findings are consistent with our prior work (14) reporting phospholipid interaction with the βN1 and γC regions of ENaC, but also circumvented the limitations of this earlier study, which was constrained by the necessity of functional expressing ENaC. Consequently, the current study was able to reveal, in addition, that γN2 is also involved in interactions with PIP2.
These results are consistent with the hypothesis that PIP2 interacts with ENaC at three discrete cationic sites, without the need for an adapter molecule. Like ENaC, Kir and TRP channels also contain multiple PIP2-interaction sites that are distant from each other. Interestingly, these sites are joined together by a single PIP2 molecule. For example, homology modeling of TRPV1 revealed that basic residues on the C terminus and the S4–S5 linker interact with a single PIP2 headgroup (40). Cryo-EM analysis of TRPM8 shows a single PIP2 molecule binding distant cationic residues within the pre-S1, S4–S5, TRP, and MHR4′ domains (25). Likewise, X-ray crystallography of GIRK/Kir3.2 shows a single PIP2 headgroup interacting with cationic residues at the C terminus-membrane interface and a more distant N-terminal lysine (22). If this binding mode generalizes to ENaC then the apparent presence of multiple PIP2-binding sites within ENaC may actually reflect the possibility that distant domains come together to form a single binding site. In the absence of a resolved structure of the full channel with PIP2, the formation of such a larger PIP2-binding pocket remains speculative, and we can only conclude from the current results that there are at least three discrete PIP2 interaction sites within ENaC.
Another key observation of the PIP2-TRP and PIP2-Kir structures is the high helical content at the PIP2 interaction sites. As noted in Fig. 4, helical formation of the PIP2-ENaC sites may form an electropositive region to enhance binding with the phosphoryl groups of PIP2. A recent study reported that the purified γN terminus of ENaC adopted a helical conformation upon addition of a PIP2 analog, consistent with PIP2 inducing or stabilizing a helical conformation in γN-ENaC (31). The extreme N terminus of β-ENaC is also predicted to be helical. The βN1 site shares sequence homology with the N terminus of the Caenorhabditis elegans Mec-4 ion channel, which also is a member of the ENaC/Deg ion channel family (Fig. S4). This sequence similarity is notable because the solution NMR structure of Mec-4 displays several α helices in its N terminus in the absence of PIP2 (PDB ID 2K2B) (48). This suggests that the related βN1 region of hENaC might also be helical prior to binding PIP2, whereas the γN2 region may first be disordered then become helical after coming into contact with PIP2. The impact of such structural changes is not yet obvious, but one thought is that any structural changes caused by PIP2 to the cytoplasmic termini of ENaC could impact the open probability of the channel. This would be consistent with previous work where ENaC open probability was noted to be tied in a positive manner to membrane PIP2 levels (14).
The major contribution of this work is the quantification of PIP2-ENaC interactions. The low to moderate affinities of PIP2-ENaC interactions revealed by MST experiments provide insight about the control of ENaC activity by membrane phospholipids. ENaC is constitutively active, requiring PIP2 for this activity. This is known because ENaC activity quickly declines in response to a reduction in membrane PIP2 levels, with all channels closed within 5 min (14, 28, 29). The moderate and low binding affinities of ENaC for PIP2 documented in the current study possibly explain this critical sensitivity of ENaC for PIP2. If Kd values had reflected more stringent binding, perhaps in the picomolar to nanomolar range, then PIP2 would likely remain associated with the channel as unbound PIP2 is depleted within the membrane, and the current rundown would not be observed, or would take much longer. The lower to moderate affinities reported here favor PIP2 more quickly dissociating from ENaC in response to changes in membrane PIP2 levels allowing such regulation to be dynamic.
Having only moderate affinity for PIP2 might also contribute to tight spatiotemporal regulation of ENaC. Enrichment of PIP2 in the plasma membrane perhaps is an effective method to maintain nascent channels quiescent until they reach their destination at the plasma membrane (19). Because other phosphoinositides, but not PIP2, are present in intracellular vesicles, ENaC must have a high enough affinity to be selective for PIP2 once it reaches the plasma membrane, yet not so high that it cannot properly respond to changes in PIP2 levels. Thus, the low to moderate Kd values discovered by this study are consistent with patch clamp studies and explain why ENaC activity is highly responsive to membrane levels of PIP2.
Tubby, the phospholipid reporter used in experiments reported in Fig. 1, can interact with other phospholipids, including PIP3 (21, 41). Therefore the significant reduction we observed of membrane Tubby after BLI may also represent the depletion of other membrane phospholipids. PIP3 is also present at the plasma membrane, and earlier studies had suggested a role for both PIP2 and PIP3 in the regulation of ENaC in cultured nonpolarized cells. Similar to PIP2 effects on ENaC, addition of PIP3 to a bath solution containing excised patches of ENaC enhances ENaC currents (14, 28). The results in this study are consistent with ENaC binding both phospholipids. The structure of PIP3 is similar to that of PIP2, with the former differing from the latter only by the presence of an additional phosphoryl group at carbon 3 of the inositol ring. This extra phosphoryl group gives PIP3 an additional net −2e charge at neutral pH, suggesting PIP3 might bind more strongly to ENaC than PIP2. However, despite these observations, strong evidence contradicts physiological interactions between PIP3 and ENaC in polarized kidney cells, due to their distinct subcellular localization. For instance, storm imaging shows PIP2 segregated into nanoclusters, or lipid rafts, distinct from PIP3 nanoclusters (42). This might suggest that PIP2 and PIP3 would not simultaneously bind ENaC, and that ENaC would have to be in the same lipid raft as either phospholipid to support interactions. The same study reported, like others, that PIP3 is present at much lower concentrations than PIP2 in the plasma membrane, bolstering the case that PIP2 would be more likely to interact with ENaC than PIP3. Other studies performed in polarized Madin-Darby canine kidney cells and LLC-PK1 cells found that PIP2 preferentially localizes to the apical membrane in polarized cells, whereas PIP3 predominates in the basolateral membrane (43, 44). Because ENaC is also located in the apical membrane (10, 45), PIP2, but not PIP3, would most likely be the phospholipid that affects ENaC activity in polarized epithelial cells, such as the principal cells of the cortical collecting. Although we, and others, observe PIP3-ENaC interactions in vitro, these subcellular localization patterns suggest that PIP2 is the physiologically relevant binding partner, and careful interpretation of phospholipid interaction should be emphasized when studying these interactions in nonpolarized cells.
Because the dysfunction of ENaC contributes to hypertension and other diseases, such as cystic fibrosis, understanding the molecular mechanism of phospholipid regulation of ENaC is important. However, the difficulty of studying the full channel and lipid-based molecules in aqueous solutions has inhibited our ability to fully understand the dynamics of phospholipid-ion channel interactions. In this study, we used peptides and soluble phospholipid analogs to quantify PIP2 interactions with ENaC. Quantifying such interactions as done here possibly explains how a low to moderate PIP2 binding affinity for ENaC precisely controls channel activity at the apical plasma membrane of polarized epithelial cells.
Experimental procedures
Plasmids, synthetic peptides, and reagents
Plasmids containing mCherry-CRY2–5-phosphataseOCRL (mCh-CRY2–5-ptase) and CIBN-CAAX were gifts from Pietro De Camilli (Addgene plasmid numbers 66836 and 79574). The YFP-Tubby plasmid was a gift from Andrew Tinker (Queen Mary University of London, UK). Human ENaC constructs in pMT3 capable of expressing channel subunits in mammalian systems have been described previously (46, 47). For these studies, the cDNA sequence for α-hENaC was cloned in-frame into the pECFP-C1 (Clontech/Takara, USA) plasmid. The resulting eCFP-α-hENaC fusion protein was used to track expression.
Synthetic peptides of the cytoplasmic regions of β- and γ-hENaC subunits were designed according to a previous study of PIP2 regulation of ENaC with mutated or deleted amino acids (14) with the corresponding sequences shown in Table S1. Peptides were prepared at 95% purity (Peptide 2.0, Chantilly, VA). Lyophilized peptides were reconstituted in HBS buffer (150 mm NaCl and 20 mm HEPES, pH 7.4). The peptide concentration was quantified by amino acid analysis (Protein Chemistry Lab, Texas A&M, College Station, TX) or absorbance at 280 nm corrected for the appropriate extinction coefficient. Peptides were named for subunit isoform and relative location. Mutants of ENaC peptides containing alanine (Ala) substitutions in place of cationic residues had very low solubility and were unstable. Consequently, they could not be tested in the current work. In contrast, the “charge-reversed” mutants substituted with glutamic acid (E) used here showed good stability and were soluble in the same buffer as WT peptides. Thus, the charged reversed mutants were used in this study.
FuGENE HD (Promega) was used for transient transfection. HEK 293 cells were from ATCC (CRL-1573). The cell permeant sodium indicator, CoroNa Green AM (ThermoFisher Scientific), was used to monitor intracellular Na+ levels. Phosphoinositides, including fluorescein-PIP2-HG were purchased from Echelon Biosciences Inc. (Salt Lake City, UT) and dissolved in HBS or chelexed MilliQ water. Phosphoinositide solutions were sonicated for 3–5 min prior to use. All experiments involving phospholipids were performed at concentrations below that of their critical micelle concentration.
Quantification of changes in intracellular [Na+] with optogenetics and live cell imaging
HEK 293 cells were seeded in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum on 35-mm uncoated glass bottom dishes. A day after seeding, cells were transfected with 0.25 μg/construct of pECFP-C1-α-hENaC, pMT3-β-hENaC, pMT3-γ-hENaC, CIBN-CAAX and mCh-CRY2–5-ptase per dish using FuGENE HD following standard protocols. Control cells were transfected with YFP-Tubby + CIBN-CAAX and mCh-CRY2–5-ptase or only ENaC constructs. Transfected cells expressing CIBN-CAAX and mCh-CRY2–5-ptase with and without ENaC were maintained in standard tissue culture conditions in the dark and in the presence of 10 μm amiloride for 24 h. Prior to imaging, cells were incubated in the dark at 37 °C for 30 min in serum-free Dulbecco's modified Eagle's medium containing 10 μm amiloride (Sigma), 5 μm CoroNa Green (Molecular Probes), and 0.04% Pluronic F-127 (Biotium). Cells were rinsed with PBS and then imaged using a ×60/1.45 TIRF oil objective or ×10/0.13 objective with ×1.5 amplification on an inverted Nikon Eclipse TE2000-U fluorescence microscope. To obviate premature dimerization of CRY2 with CIBN, spurious illumination in the blue wavelength was avoided with cells positive for expression of these heterologous proteins identified initially with a mercury light source for excitation through a mCherry filter set, ET560/40 (Chroma number 96365). OBIS FP fiber pigtailed lasers (Coherent, Inc.) were used at 2% power for imaging and BLI, unless otherwise noted. TIRF imaging of YFP-Tubby was used to quantify the fluorescence at the membrane. YFP-Tubby was monitored at 514 nm and the CIBN/CRY2 dimerization was induced at 445 nm at 10% power for 10 s. For quantitation of ENaC-facilitated Na+ entry under TIRF, CRY2 dimerization with CIBN was driven with BLI pulses for 30 s at 445 nm with 300 ms illumination at a 1-Hz frequency. CoroNa Green and mCh-CRY2–5-ptase were imaged at 488 and 561 nm, respectively, before, immediately following, 5 and 10 min after BLI. The presence of ENaC expression as assayed with eCFP-α-hENaC fluorescence was confirmed during BLI pulsing. Images were captured with an Andor iXon Ultra camera and evaluated using Metamorph software (Molecular Devices). Fluorescence intensity data were analyzed using ImageJ with images presented as total cellular fluorescence corrected for background noise. Membrane mCh-CRY2–5-ptase was compared in cells with and without ENaC by subtracting the total fluorescence of the cell before BLI from that 10 min after BLI using ImageJ. The change in fluorescence was plotted in arbitrary units normalized to the starting fluorescence of each experiment. Analysis of each cellular experiment was performed on n = 10–12 individual cells.
Steady-state intrinsic fluorescence
Steady-state intrinsic fluorescence of synthetic peptides (at 250 nm in HBS) was quantified in the absence and presence of 10 μm phosphoinositide (diC4-PIP3 or diC4-PIP2) or inositol by exciting at 250 and 280 nm and scanning emissions between 260 and 450 nm through 6-nm band passes using a Photon Technologies International (PTI) Quantamaster spectrophotometer. Neither diC4-PIP3 nor diC4-PIP2 absorbs or emits at these wavelengths. Data were collected using Felix32 software version 1.10 (PTI/HORIBA Instruments) and then compiled in Excel using an R Studio text file organizer created by Aaron Horning (Stanford University, CA). Final data analysis was performed in GraphPad Prism 7. Steady-state intrinsic fluorescence data are presented as corrected intensity, protein fluorescence minus buffer fluorescence. Changes in peak intensities were determined by dividing the highest point of the larger peak by the highest point of the smaller peak. Statistical significance of SSIF data were determined by multiple t tests and nonadjusted p values are reported. Peptides with peak changes of 5% or less were compared with mutant peptides using 2-way ANOVA followed by Tukey's test to confirm no significant changes in fluorescence.
Microscale thermophoresis
For MST experiments, synthetic peptides were serially diluted with equal parts HBS up to 16 times. Peptide concentrations ranged from 0.1 to 1000 μm. Following addition of fluorescein-PIP2-HG (final concentration 100 nm), samples were loaded into premium, low protein-binding glass capillaries (catalog number MO-K005 or MO-AK005, Nanotemper Technologies). Microscale thermophoresis was then measured using a Nanotemper Monolith 1.115 (Case Western Reserve University, Cleveland, OH) or a Nanotemper NT.Automated (UT Health San Antonio, TX). Data were recorded using the auto-detect functions of IR laser power to apply the heat gradient to each capillary and light emitting diode power to track fluorescence migration along the heat gradient. Data were fit to a 1:1 binding model in PALMIST using the “cold” function, which compares fluorescence data before application of the heat gradient, or the “Tjump” function, which is the difference between the cold fluorescence and fluorescence immediately after application of the heat gradient (38). Data plots were rendered in GUSSI (39).
Bioinformatics and statistical analysis
ClustalOmega (RRID: SCR_001591) was used to generate alignments of mouse and human ENaC subunits (Uniprot IDs: P37088, P51168, and P51170). Secondary structures for β- and γ-hENaC subunits were predicted using Jpred4 (RRID: SCR_016504) and HHPred (RRID: SCR_010276). Helical projection models were created using EMBOSS Pepwheel (RRID: SCR_018398).
The mean of 10–12 independent experiments of cell assays is shown as mean ± S.D., or S.E. for grouped experiments of n = 4–6 cells in each independent experiment, where indicated. SSIF data are shown as a mean ± S.D. of 3 independent experiments. MST data are shown as mean ± S.D. and dissociation constants represent the mean of 3 experiments, with error reported as parametric confidence interval derived from the error surface projection method, previously described in detail (38). Statistical significance for each experiment was determined using GraphPad Prism 7 using either a paired, two-tailed t test, multiple t test using the Holm-Sidak method, 2-way ANOVA followed by Dunnet's or Tukey's test, where appropriate; p < 0.05 (*), p < 0.005 (**), or p < 0.0001 (***).
Data availability
The data supporting the findings of this study are contained within the article and its supplementary materials. The datasets generated during the current study will be freely provided from the corresponding author upon request.
Supplementary Material
Acknowledgments
We thank Chad Brautigam, Ph.D., UT Southwestern Dallas, TX, and Aaron Horning, Ph.D., Stanford University, CA, for expert assistance with the analysis of MST and SSIF data; Yinghua Chen, Ph.D., of the Protein Core Facility, Case Western Reserve University, Cleveland, OH, for sharing expertise in MST data collection; and Jennifer Torrez, St. Mary's University, San Antonio, TX, for technical assistance with raw SSIF data collection. We thank Mark S. Shapiro, Ph.D., for his guidance in designing the YFP-Tubby microscopy experiments. The MST data were collected at the Protein Core Facility, Case Western Reserve University, Cleveland OH and the Center for Innovative Drug Discovery at University of Texas Health, San Antonio, TX.
This article contains supporting information.
Author contributions—C. R. A., B. T. E., and C. M. C. data curation; C. R. A. formal analysis; C. R. A. investigation; C. R. A. methodology; C. R. A. writing-original draft; B. T. E. and J. D. S. writing-review and editing; J. D. S. conceptualization; J. D. S. funding acquisition.
Funding and additional information—This work was supported by American Heart Association Grant 20POST35210746 and National Institutes of Health, NHLBI Grant T32 HL007446 (to C. R. A.), NCATS Grant TL1 TR002647 and NIGMS Grant K12 GM11726 (to B. T. E.), and NIDDK Grant R01 DK113816 (to J. D. S.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the American Heart Association or National Institutes of Health.
Conflict of interest—The authors declare that they have no conflicts of interest with the contents of this article.
- ENaC
- epithelial Na+ channel
- PIP2
- phosphatidylinositol 4,5-bisphosphate
- PIP3
- phosphatidylinositol 3,4,5-bisphosphate
- BLI
- blue light illumination
- 5-ptase
- 5-phosphatase
- SSIF
- steady-state intrinsic fluorescence assay
- MST
- microscale thermophoresis
- PDB
- Protein Data Bank
- TIRF
- total internal reflection fluorescence
- ASIC1
- acid-sensing ion channel 1
- Kir
- inward rectifying K+ channel
- YFP
- yellow fluorescent protein
- ANOVA
- analysis of variance
- TRP
- transient receptor potential.
References
- 1. Hanukoglu I., and Hanukoglu A. (2016) Epithelial sodium channel (ENaC) family: phylogeny, structure-function, tissue distribution, and associated inherited diseases. Gene 579, 95–132 10.1016/j.gene.2015.12.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mironova E., Boiko N., Bugaj V., Kucher V., and Stockand J. D. (2015) Regulation of Na+ excretion and arterial blood pressure by purinergic signalling intrinsic to the distal nephron: consequences and mechanisms. Acta Physiol. (Oxf.) 213, 213–221 10.1111/apha.12372 [DOI] [PubMed] [Google Scholar]
- 3. Pavlov T. S., and Staruschenko A. (2017) Involvement of ENaC in the development of salt-sensitive hypertension. Am. J. Physiol. Renal Physiol. 313, F135–F140 10.1152/ajprenal.00427.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhou-Suckow Z., Duerr J., Hagner M., Agrawal R., and Mall M. A. (2017) Airway mucus, inflammation and remodeling: emerging links in the pathogenesis of chronic lung diseases. Cell Tissue Res. 367, 537–550 10.1007/s00441-016-2562-z [DOI] [PubMed] [Google Scholar]
- 5. Shei R. J., Peabody J. E., Kaza N., and Rowe S. M. (2018) The epithelial sodium channel (ENaC) as a therapeutic target for cystic fibrosis. Curr. Opin. Pharmacol 43, 152–165 10.1016/j.coph.2018.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Noreng S., Bharadwaj A., Posert R., Yoshioka C., and Baconguis I. (2018) Structure of the human epithelial sodium channel by cryo-electron microscopy. Elife 7, e39340 10.7554/eLife.39340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jasti J., Furukawa H., Gonzales E. B., and Gouaux E. (2007) Structure of acid-sensing ion channel 1 at 1.9 Å resolution and low pH. Nature 449, 316–323 10.1038/nature06163 [DOI] [PubMed] [Google Scholar]
- 8. Kasuya G., Yamaura T., Ma X. B., Nakamura R., Takemoto M., Nagumo H., Tanaka E., Dohmae N., Nakane T., Yu Y., Ishitani R., Matsuzaki O., Hattori M., and Nureki O. (2017) Structural insights into the competitive inhibition of the ATP-gated P2X receptor channel. Nat. Commun. 8, 876 10.1038/s41467-017-00887-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Eaton D. C., Malik B., Bao H. F., Yu L., and Jain L. (2010) Regulation of epithelial sodium channel trafficking by ubiquitination. Proc. Am. Thorac. Soc. 7, 54–64 10.1513/pats.200909-096JS [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Klemens C. A., Edinger R. S., Kightlinger L., Liu X., and Butterworth M. B. (2017) Ankyrin G expression regulates apical delivery of the epithelial sodium channel (ENaC). J. Biol. Chem. 292, 375–385 10.1074/jbc.M116.753616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Alli A. A., Bao H. F., Liu B. C., Yu L., Aldrugh S., Montgomery D. S., Ma H. P., and Eaton D. C. (2015) Calmodulin and CaMKII modulate ENaC activity by regulating the association of MARCKS and the cytoskeleton with the apical membrane. Am. J. Physiol. Renal Physiol. 309, F456–F463 10.1152/ajprenal.00631.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Weixel K. M., Edinger R. S., Kester L., Guerriero C. J., Wang H., Fang L., Kleyman T. R., Welling P. A., Weisz O. A., and Johnson J. P. (2007) Phosphatidylinositol 4-phosphate 5-kinase reduces cell surface expression of the epithelial sodium channel (ENaC) in cultured collecting duct cells. J. Biol. Chem. 282, 36534–36542 10.1074/jbc.M703970200 [DOI] [PubMed] [Google Scholar]
- 13. Pochynyuk O., Bugaj V., Vandewalle A., and Stockand J. D. (2008) Purinergic control of apical plasma membrane PI(4,5)P2 levels sets ENaC activity in principal cells. Am. J. Physiol. Renal Physiol. 294, F38–F46 10.1152/ajprenal.00403.2007 [DOI] [PubMed] [Google Scholar]
- 14. Pochynyuk O., Tong Q., Medina J., Vandewalle A., Staruschenko A., Bugaj V., and Stockand J. D. (2007) Molecular determinants of PI(4,5)P2 and PI(3,4,5)P3 regulation of the epithelial Na+ channel. J. Gen. Physiol. 130, 399–413 10.1085/jgp.200709800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chu A., and Stefani E. (1991) Phosphatidylinositol 4,5-bisphosphate-induced Ca2+ release from skeletal muscle sarcoplasmic reticulum terminal cisternal membranes: Ca2+ flux and single channel studies. J. Biol. Chem. 266, 7699–7705 [PubMed] [Google Scholar]
- 16. Furukawa T., Yamane T., Terai T., Katayama Y., and Hiraoka M. (1996) Functional linkage of the cardiac ATP-sensitive K+ channel to the actin cytoskeleton. Pflugers Arch. 431, 504–512 10.1007/BF02191896 [DOI] [PubMed] [Google Scholar]
- 17. Hilgemann D. W., and Ball R. (1996) Regulation of cardiac Na+, Ca2+ exchange and KATP potassium channels by PIP2. Science 273, 956–959 10.1126/science.273.5277.956 [DOI] [PubMed] [Google Scholar]
- 18. Huang C. L., Feng S., and Hilgemann D. W. (1998) Direct activation of inward rectifier potassium channels by PIP2 and its stabilization by Gβγ. Nature 391, 803–806 10.1038/35882 [DOI] [PubMed] [Google Scholar]
- 19. Suh B. C., and Hille B. (2008) PIP2 is a necessary cofactor for ion channel function: how and why? Annu. Rev. Biophys. 37, 175–195 10.1146/annurev.biophys.37.032807.125859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hansen S. B. (2015) Lipid agonism: the PIP2 paradigm of ligand-gated ion channels. Biochim. Biophys. Acta 1851, 620–628 10.1016/j.bbalip.2015.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. McLaughlin S., Wang J., Gambhir A., and Murray D. (2002) PIP2 and proteins: interactions, organization, and information flow. Annu. Rev. Biophys. Biomol Struct. 31, 151–175 10.1146/annurev.biophys.31.082901.134259 [DOI] [PubMed] [Google Scholar]
- 22. Whorton M. R., and MacKinnon R. (2011) Crystal structure of the mammalian GIRK2 K+ channel and gating regulation by G proteins, PIP2, and sodium. Cell 147, 199–208 10.1016/j.cell.2011.07.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hansen S. B., Tao X., and MacKinnon R. (2011) Structural basis of PIP2 activation of the classical inward rectifier K+ channel Kir2.2. Nature 477, 495–498 10.1038/nature10370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Feng S., Dang S., Han T. W., Ye W., Jin P., Cheng T., Li J., Jan Y. N., Jan L. Y., and Cheng Y. (2019) Cryo-EM studies of TMEM16F calcium-activated ion channel suggest features important for lipid scrambling. Cell Rep. 28, 567–579.e4 10.1016/j.celrep.2019.06.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yin Y., Le S. C., Hsu A. L., Borgnia M. J., Yang H., and Lee S. Y. (2019) Structural basis of cooling agent and lipid sensing by the cold-activated TRPM8 channel. Science 363, eaav9334 10.1126/science.aav9334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Han B., He K., Cai C., Tang Y., Yang L., Heinemann S. H., Hoshi T., and Hou S. (2016) Human EAG channels are directly modulated by PIP2 as revealed by electrophysiological and optical interference investigations. Sci. Rep. 6, 23417 10.1038/srep23417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Estacion M., Sinkins W. G., and Schilling W. P. (2001) Regulation of Drosophila transient receptor potential-like (TrpL) channels by phospholipase C-dependent mechanisms. J. Physiol. 530, 1–19 10.1111/j.1469-7793.2001.0001m.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pochynyuk O., Tong Q., Staruschenko A., Ma H. P., and Stockand J. D. (2006) Regulation of the epithelial Na+ channel (ENaC) by phosphatidylinositides. Am. J. Physiol. Renal Physiol. 290, F949–F957 10.1152/ajprenal.00386.2005 [DOI] [PubMed] [Google Scholar]
- 29. Pochynyuk O., Bugaj V., and Stockand J. D. (2008) Physiologic regulation of the epithelial sodium channel by phosphatidylinositides. Curr. Opin. Nephrol. Hypertens. 17, 533–540 10.1097/MNH.0b013e328308fff3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yue G., Malik B., Yue G., and Eaton D. C. (2002) Phosphatidylinositol 4,5-bisphosphate (PIP2) stimulates epithelial sodium channel activity in A6 cells. J. Biol. Chem. 277, 11965–11969 10.1074/jbc.M108951200 [DOI] [PubMed] [Google Scholar]
- 31. Kota P., Buchner G., Chakraborty H., Dang Y. L., He H., Garcia G. J., Kubelka J., Gentzsch M., Stutts M. J., and Dokholyan N. V. (2014) The N-terminal domain allosterically regulates cleavage and activation of the epithelial sodium channel. J. Biol. Chem. 289, 23029–23042 10.1074/jbc.M114.570952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Idevall-Hagren O., Dickson E. J., Hille B., Toomre D. K., and De Camilli P. (2012) Optogenetic control of phosphoinositide metabolism. Proc. Natl. Acad. Sci. U.S.A. 109, E2316–E2323 10.1073/pnas.1211305109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Balla T., and Varnai P. (2009) Visualization of cellular phosphoinositide pools with GFP-fused protein-domains. Curr. Protoc. Cell Biol. Chapter 24, Unit 24.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Szentpetery Z., Balla A., Kim Y. J., Lemmon M. A., and Balla T. (2009) Live cell imaging with protein domains capable of recognizing phosphatidylinositol 4,5-bisphosphate: a comparative study. BMC Cell Biol. 10, 67 10.1186/1471-2121-10-67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tu P., Kunert-Keil C., Lucke S., Brinkmeier H., and Bouron A. (2009) Diacylglycerol analogues activate second messenger-operated calcium channels exhibiting TRPC-like properties in cortical neurons. J. Neurochem. 108, 126–138 10.1111/j.1471-4159.2008.05752.x [DOI] [PubMed] [Google Scholar]
- 36. (2006) Protein fluorescence. in Principles of Fluorescence Spectroscopy (Lakowicz J. R., ed) pp. 529–575, Springer, Boston, MA [Google Scholar]
- 37. VanScyoc W. S., Sorensen B. R., Rusinova E., Laws W. R., Ross J. B., and Shea M. A. (2002) Calcium binding to calmodulin mutants monitored by domain-specific intrinsic phenylalanine and tyrosine fluorescence. Biophys. J. 83, 2767–2780 10.1016/S0006-3495(02)75286-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Scheuermann T. H., Padrick S. B., Gardner K. H., and Brautigam C. A. (2016) On the acquisition and analysis of microscale thermophoresis data. Anal. Biochem. 496, 79–93 10.1016/j.ab.2015.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Brautigam C. A. (2015) Calculations and publication-quality illustrations for analytical ultracentrifugation data. Methods Enzymol. 562, 109–133 10.1016/bs.mie.2015.05.001 [DOI] [PubMed] [Google Scholar]
- 40. Brauchi S., Orta G., Mascayano C., Salazar M., Raddatz N., Urbina H., Rosenmann E., Gonzalez-Nilo F., and Latorre R. (2007) Dissection of the components for PIP2 activation and thermosensation in TRP channels. Proc. Natl. Acad. Sci. U.S.A. 104, 10246–10251 10.1073/pnas.0703420104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Carroll K., Gomez C., and Shapiro L. (2004) Tubby proteins: the plot thickens. Nat. Rev. Mol. Cell Biol. 5, 55–63 10.1038/nrm1278 [DOI] [PubMed] [Google Scholar]
- 42. Wang J., and Richards D. A. (2012) Segregation of PIP2 and PIP3 into distinct nanoscale regions within the plasma membrane. Biol. Open 1, 857–862 10.1242/bio.20122071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mahon M. J. (2011) Apical membrane segregation of phosphatidylinositol-4,5-bisphosphate influences parathyroid hormone 1 receptor compartmental signaling and localization via direct regulation of ezrin in LLC-PK1 cells. Cell Signal. 23, 1659–1668 10.1016/j.cellsig.2011.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Martin-Belmonte F., and Mostov K. (2007) Phosphoinositides control epithelial development. Cell Cycle 6, 1957–1961 10.4161/cc.6.16.4583 [DOI] [PubMed] [Google Scholar]
- 45. Butterworth M. B., Weisz O. A., and Johnson J. P. (2008) Some assembly required: putting the epithelial sodium channel together. J. Biol. Chem. 283, 35305–35309 10.1074/jbc.R800044200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. McDonald F. J., Price M. P., Snyder P. M., and Welsh M. J. (1995) Cloning and expression of the β- and γ-subunits of the human epithelial sodium channel. Am. J. Physiol. 268, C1157–C1163 10.1152/ajpcell.1995.268.5.C1157 [DOI] [PubMed] [Google Scholar]
- 47. Tong Q., Menon A. G., and Stockand J. D. (2006) Functional polymorphisms in the alpha-subunit of the human epithelial Na+ channel increase activity. Am. J. Physiol. Renal Physiol. 290, F821–F827 10.1152/ajprenal.00312.2005 [DOI] [PubMed] [Google Scholar]
- 48. Everett J.K., Liu G., Driscoll M.A., Montelione G.T., New York Consortium on Membrane Protein Structure (NYCOMPS). (2008) Sparse-constraint solution NMR structure of micelle-solublized cytosolic amino terminal domain of C. elegans mechanosensory ion channel subunit MEC-4. New York Consortium on Membrane Protein Structure (NYCOMPS) (CASP TARGET). Protein Data Bank, 10.2210/pdb2K2B/pdb [DOI]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting the findings of this study are contained within the article and its supplementary materials. The datasets generated during the current study will be freely provided from the corresponding author upon request.