Abstract
F-box proteins, such as F-box/WD repeat-containing protein 7 (FBW7), are essential components of the SKP1-CUL1-F-box (SCF) E3 ubiquitin ligases. They bind to S-phase kinase-associated protein 1 (SKP1) through the F-box motif and deliver their protein substrate to the E3 ligase complex for ubiquitination and subsequent degradation. F-box and leucine-rich repeat protein 16 (FBXL16) is a poorly studied F-box protein. Because it does not interact with the scaffold protein cullin 1 (CUL1), we hypothesized that FBXL16 might not form a functional SCF-E3 ligase complex. In the present study, we found that FBXL16 up-regulates the levels of proteins targeted by SCF-E3 ligases, such as C-MYC, β-catenin, and steroid receptor coactivator 3 (SRC-3). Focusing on C-MYC, a well-known oncoprotein overexpressed in most human cancers, we show that FBXL16 stabilizes C-MYC by antagonizing FBW7-mediated C-MYC ubiquitination and degradation. Further, we found that, although FBXL16 does not interact with CUL1, it interacts with SKP1 via its N-terminal F-box domain and with its substrate C-MYC via its C-terminal leucine-rich repeats (LRRs) domain. We found that both the F-box domain and the LRR domain are important for FBXL16-mediated C-MYC stabilization. In line with its role in up-regulating the levels of the C-MYC and SRC-3 oncoproteins, FBXL16 promoted cancer cell growth and migration and colony formation in soft agar. Our findings reveal that FBXL16 is an F-box protein that antagonizes the activity of another F-box protein, FBW7, and thereby increases C-MYC stability, resulting in increased cancer cell growth and invasiveness.
Keywords: Myc (c-Myc), E3 ubiquitin ligase, protein stability, ubiquitylation (ubiquitination), cancer, cell migration, F-box and leucine-rich repeat protein 16 (FBXL16), F-box protein, F-box/WD repeat-containing protein 7 (FBW7), protein homeostasis, proto-oncogene C-Myc (C-MYC)
Introduction
Protein degradation is a crucial biological process mainly mediated by the ubiquitin-proteasome system. The ubiquitin-proteasome system is a selective process in which the proteins are covalently conjugated with multiple ubiquitin proteins prior to degradation by the 26S proteasome complex (1, 2). Protein ubiquitination is achieved by an enzymatic cascade catalyzed by a ubiquitin-activating enzyme (E1), a ubiquitin-conjugating enzyme (E2), and a ubiquitin ligase (E3). E3 ligases are responsible for the selectivity of the substrate (1, 2). More than 500 E3 ligases are categorized into four major classes: HECT-type, RING finger–type, U-box–type, and PHD finger–type (3, 4). Among the RING finger–type, the subfamily of the cullin-based E3 is the largest one and includes the SKP1-CUL1-F-box protein (SCF) complex.
The SCF ubiquitin ligase complex is composed of the adaptor protein SKP1, the scaffold protein Cullin1 (CUL1) and a specific F-box protein (5). CUL1, as a scaffold protein, interacts with SKP1 and the RING finger protein RBX1 to form an E3 ubiquitin ligase platform. The F-box protein binds to SKP1 through its F-box motif and brings the bound substrate to the E3 ligase complex, followed by RBX1-mediated ubiquitination and the subsequent degradation of the substrate (5). Thus far, 69 F-box proteins have been identified in humans and fall into three subfamilies, depending on their substrate recognition domains: FBXLs (leucine-rich repeats or LRR), FBXWs (WD40 repeats), and FBXOs (other domains) (6, 7). For example, FBW7 recognizes its substrates such as c-myc through its WD40 repeats (8–10); FBXL1, also known as SKP2 (S-phase kinase-associated protein 2), binds to its substrates such as p27 through its LRRs (11, 12). Many F-box proteins have critical functions in crucial biological processes such as proliferation, apoptosis, cell cycle regulation or migration and are often dysregulated during tumorigenesis (13). For instance, FBW7 functions as a tumor suppressor in multiple cancers by mediating the degradation of several oncoproteins, including c-myc and cyclin E1 (14, 15).
FBXL16 is a poorly studied F-box protein which harbors a N-terminal proline-rich domain, an F-box motif, and a C-terminal LRR domain. Interestingly, homozygous knockout of FBXL16 gene in mice leads to perinatal lethality (International Mouse Phenotype Consortium, Ref. 16), and depletion of FBXL16 promoted differentiation of mouse embryonic stem cell along cardiomyocyte lineage (17), suggesting essential physiological roles for FBXL16. FBXL16 was shown to be a transcriptional target of E2F1 (18). Unlike other F-box proteins, including FBW7 and SKP2 (FBXL1), FBXL16 does not show detectable interaction with CUL1 and may not form a functional SCF-E3 ubiquitin ligase complex (17, 19). Surprisingly, it was shown to interact with protein phosphatase 2A (PP2A) complex and might regulate the latter's activity (17). Despite these interesting preliminary findings about FBX16, little is known about the biochemical roles of FBXL16 as an F-box protein.
In this study, we have found that FBXL16 up-regulates the protein levels of several substrates of SCF E3-ligases, in particular c-myc. Mechanistically, FBXL16 increases c-myc protein stability by antagonizing FBW7-mediated polyubiquitination and the subsequent degradation of c-myc. Both the F-box domain and the LRR domain are important for FBXL16 in stabilizing c-myc. In line with its role in up-regulating the level of c-myc and other oncoproteins, we found that FBXL16 promotes cancer cell growth and migration. Taken together, our findings reveal FBXL16 as a unique F-box protein antagonizing the activity of FBW7 and as a new positive regulator of c-myc.
Results
FBXL16 up-regulates the levels of several substrates of SCF-E3 ligases
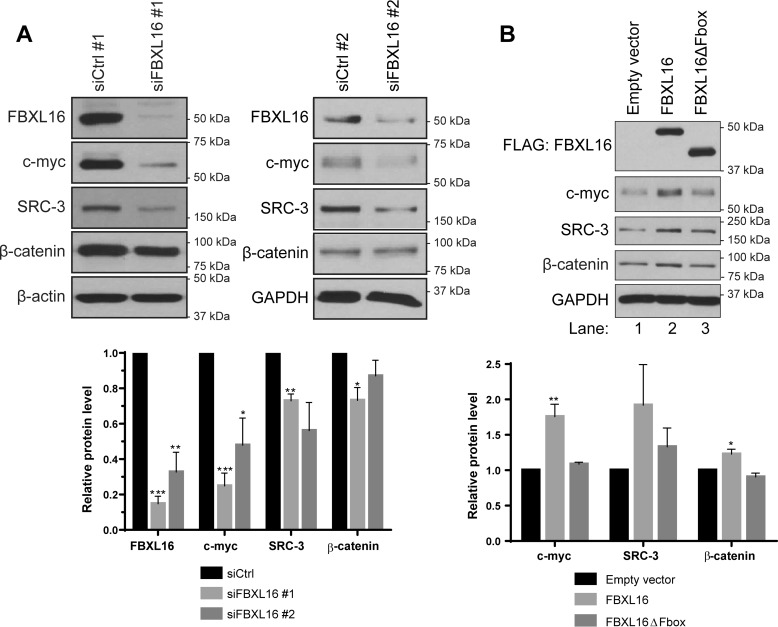
Although FBXL16 is structurally an F-box protein and interacts with SKP1, two previous studies suggest that it does not show detectable interaction with CUL1 and thus might not form a functional SCF ubiquitin ligase complex (17, 19). It is virtually unknown whether or not FBXL16 has a role in regulating the levels of the substrates of SCF-E3 ligases. We postulated that FBXL16 may play a role different from those of other F-box proteins such as FBW7 given that it has interaction with SKP1 but is incapable of forming a stable SCF ubiquitin ligase complex. To test this, we first knocked down FBXL16 separately by using two different siRNAs and then investigated its effect on the levels of several known substrates of SCF E3 ligases, including c-myc, steroid receptor coactivator 3 (SRC-3) (20) and β-catenin (21–23). Interestingly, knockdown of FBXL16 led to a remarkable reduction of c-myc protein level in A549 lung cancer cells (Fig. 1A). Similarly, the levels of SRC-3 and β-catenin were significantly decreased upon FBXL16 depletion (Fig. 1A), albeit to a lesser level than that of c-myc. To confirm that these results were not because of potential off-target effects of the siRNAs, we overexpressed FLAG-tagged FBXL16 in H1299 lung cancer cells, in which endogenous FBXL16 protein is barely detectable. As the activity of most F-box proteins depends on their F-box domains, we also tested a mutant of FBXL16 lacking its F-box domain (FBXL16ΔFbox). In line with the effects of FBXL16 knockdown in A549 cells, overexpression of FBXL16 increased the levels of c-myc, β-catenin, and SRC-3 protein whereas overexpression of FBXL16ΔFbox did not (Fig. 1B). Taken together, these results clearly suggest that opposite to the down-regulating roles of FBW7 on c-myc and SRC-3 and that of β-TRCP on β-catenin, FBXL16 up-regulates the levels of these substrates of SCF E3 ligases.
Figure 1.
FBXL16 up-regulates the protein levels of SCF E3 ligase substrates. A, A549 cells were transiently transfected with a negative control siRNA (siCtrl #1 or siCtrl #2) or an siRNA targeting FBXL16 (siFBXL16 #1 or siFBXL16 #2). Western blot analysis and quantification shows a decrease in c-myc, SRC-3, and β-catenin protein levels upon FBXL16 knockdown. B, Western blotting analyses of FLAG-FBXL16 (or FLAG-FBXL16ΔFbox) using an anti-FLAG Ab, c-myc, SRC-3, β-catenin, and β-actin in H1299 cells transiently transfected with a control empty vector, a vector expressing FLAG-tagged FBXL16 protein, or a vector expressing FLAG-tagged FBXL16 mutant with the deletion of F-box domain (FBXL16ΔFbox). H1299 cells were treated with CHX for 15 min prior to being lysed. Western blots are representative of three independent experiments. Values in graph bars represent mean ± S.E. and statistical significances were determined by Student's t test (*, p < 0.05; **, p < 0.01; ***, p < 0.001).
FBXL16 stabilizes c-myc protein
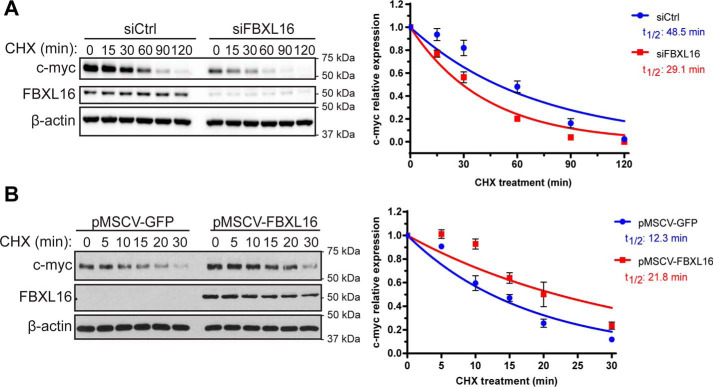
We observed that FBXL16 had a greater effect on c-myc protein level as compared with SRC-3 and β-catenin. We therefore focused on c-myc to elucidate how FBXL16 up-regulates its protein level. First, we investigated the effect of FBXL16 on c-myc protein stability. Indeed, FBXL16 knockdown decreased c-myc protein stability (a 1.7-fold decrease in half-life) (Fig. 2A). As the effect of FBXL16 on the total c-myc protein level (Fig. 1) appears to be higher than on c-myc protein stability, we wondered whether FBXL16 also affects c-myc mRNA expression level. Indeed, we found that FBXL16 knockdown also reduced c-myc mRNA expression (Fig. S1). To confirm the role of FBXL16 on c-myc protein stability, we generated MDA-MB-231 cells with stable expression of either a GFP control or FBXL16. Consistently, FBXL16 overexpression induced a 1.8-fold increase of c-myc protein half-life (Fig. 2B). As such, these results demonstrate that FBXL16 up-regulates and stabilizes c-myc protein.
Figure 2.
FBXL16 increases c-myc protein stability. A, A549 cells were transiently transfected with a negative control siRNA (siCtrl) or an siRNA targeting FBXL16 (siFBXL16). 48 h post transfection, protein translation was inhibited with CHX (100 μg/ml) for different times as indicated, followed by cell lysis and Western blot analysis of protein levels. c-myc protein level at each time point was normalized to that of β-actin, and the normalized c-myc protein level at 0 min time point was arbitrarily set as 1. c-myc half-life (t½) was determined from the exponential curve equation calculated using the one-phase exponential decay model (GraphPad Prism 6 software). B, MDA-MB-231 stable cells overexpressing pMSCV-GFP or pMSCV-FBXL16 were treated with CHX for different times and the half-life of endogenous c-myc protein was determined as in (A).
FBXL16 antagonizes SCFFBW7 activity and decreases c-myc ubiquitination
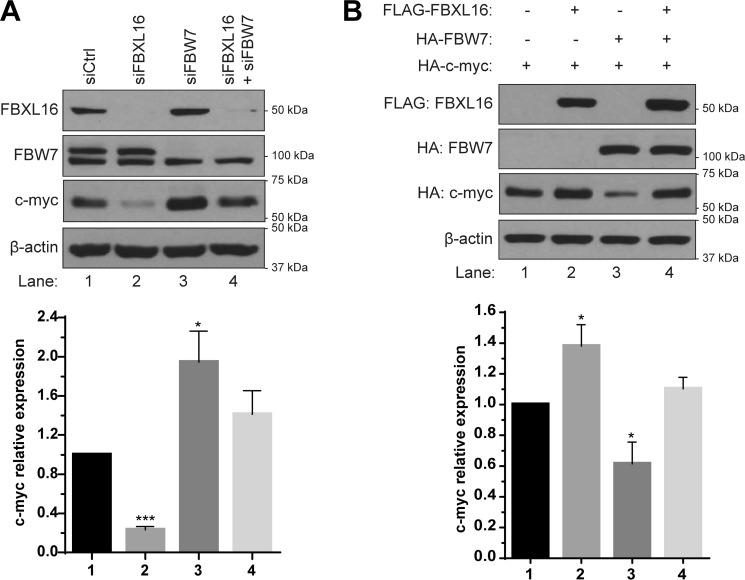
SCFFBW7 and SCFSKP2 are major E3-ligases responsible for c-myc polyubiquitination and proteasomal degradation (9, 24–26). As FBXL16 has opposite effect on c-myc, we postulated that FBXL16 may counteract the down-regulation of c-myc stability by other F-box proteins. To test this, first we knocked down FBXL16, FBW7, or both in A549 cells to see their effects on c-myc protein level. Although FBXL16 knockdown significantly decreased c-myc (Fig. 3A, lane 2), FBW7 depletion significantly increased c-myc protein level (Fig. 3A, lane 3). However, when both proteins were knocked down, no significant effect on c-myc protein was observed (Fig. 3A, lane 4). To confirm this, we co-overexpressed c-myc, along with either FBXL16, FBW7, or both proteins in 293T cells. c-myc protein level was up-regulated when co-overexpressed with FBXL16, but was down-regulated when co-overexpressed with FBW7 (Fig. 3B). Similar to the effect under the condition of the depletion of both FBXL16 and FBW7, co-overexpression of FBXL16 and FBW7 had no significant effect on c-myc level (Fig. 3B, bar 4 versus bar 1). On the contrary, we found that knockdown of FBXL16 had similar effect on c-myc in the presence or absence of SKP2, suggesting that FBXL16 regulates c-myc independently of SKP2 (Fig. S2). Altogether, these results indicate that FBXL16 antagonizes FBW7's function to increase c-myc protein level.
Figure 3.
FBXL16 regulates c-myc by antagonizing FBW7. A, Western blot analysis and quantification of c-myc protein level in A549 cells transiently transfected with negative control siRNAs (siCtrl), siRNA targeting FBW7 (siFBW7), siRNA targeting FBXL16 (siFBXL16), or both siFBW7 and siFBXL16. B, 293T cells were transiently co-transfected with HA–c-myc together with either empty plasmid controls, FLAG-FBXL16, HA-FBW7, or both FLAG-FBXL16 and HA-FBW7, followed by Western blot analysis using an anti-FLAG Ab for measuring FBXL16 and an anti-HA Ab for measuring both c-myc and FBW7. Western blots are representative of three independent experiments. Values in graph bars represent mean ± S.E. and statistical significances were determined by Student's t test (*, p < 0.05; **, p < 0.01; ***, p < 0.001).
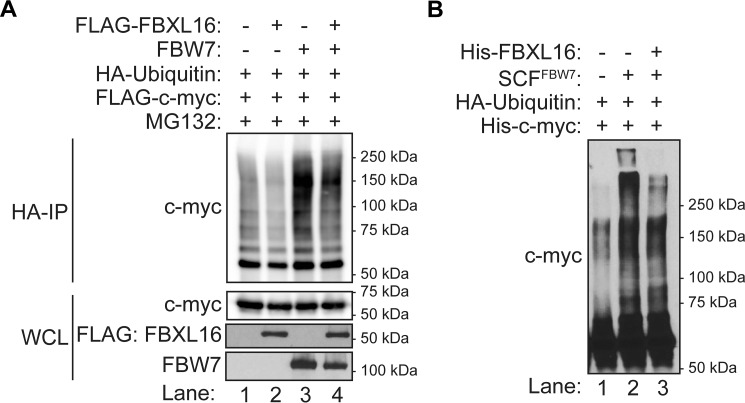
We then wanted to determine whether FBXL16 antagonizes FBW7's activity on c-myc ubiquitination. For this purpose, first we co-overexpressed c-myc and HA-ubiquitin along with either an empty vector control, FBXL16, FBW7, or both F-box proteins. As expected, FBW7 overexpression led to a remarkable increase of c-myc polyubiquitination (Fig. 4A, lane 3 versus lane 1). In contrast, FBXL16 overexpression greatly reduced c-myc polyubiquitination (Fig. 4A, lane 4 versus lane 3 and lane 2 versus lane 1). To test whether the role of FBXL16 in inhibiting FBW7-induced c-myc ubiquitination was direct, we performed an in vitro ubiquitination assay. Importantly, we found that recombinant FBXL16 protein decreased FBW7-induced polyubiquitination of c-myc in vitro (Fig. 4B). Thus, we conclude that FBXL16 antagonizes the effect of FBW7, leading to a decrease in ubiquitination and increase in stability of c-myc protein.
Figure 4.
FBXL16 decreases FBW7-depandant c-myc ubiquitination. A, 293T cells were co-transfected with pMT-HA-ubiquitin and pCMV-FLAG-c-myc plasmids together with either pSG5-FLAG-FBXL16, pcDNA-FBW7, or both as indicated. HA-ubiquitin conjugates were immunoprecipitated using anti-HA affinity gel and then immunoblotted with anti–c-myc antibody to detect the ubiquitinated c-myc proteins. The expression levels of c-myc, FBXL16, and FBW7 in whole cell lysate (WCL) were immunoblotted with anti–c-myc, anti-FLAG, and anti-FBW7 antibodies, respectively. B, in vitro ubiquitination assay was performed by incubating the purified recombinant c-myc protein with HA-ubiquitin, E1 and E2 enzymes together with or without SCFFBW7, and/or FBXL16 for 1 h at 37 °C, followed by Western blot analysis using anti–c-myc antibody.
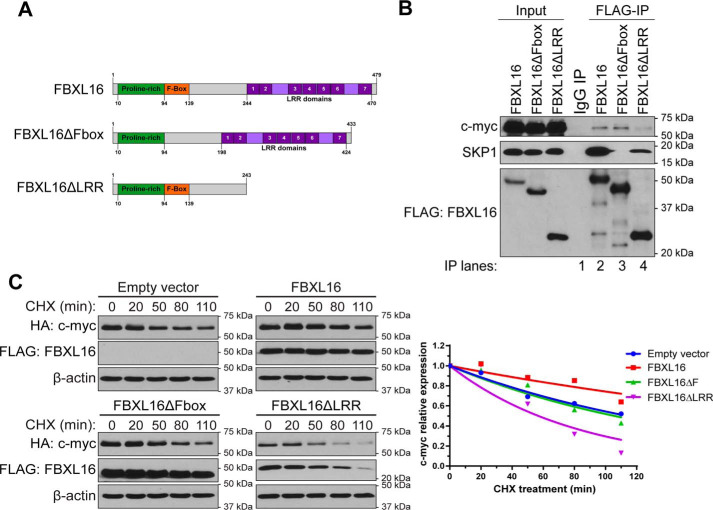
Both LRR and F-box domains are important for FBXL16 in stabilizing c-myc
For FBXL proteins, the F-box domain is responsible for interacting with SKP1, the adaptor protein which brings CUL1 and RBX1 to form the SCF-E3 ligase complex; LRR domains are more variable and usually involved in the substrate binding. FBXL16 also harbors an F-box domain and a 7-LRR domain (Fig. 5A). To determine the importance of the F-box and LRR domains for FBXL16's activity, we generated FBXL16ΔFbox that lacks the F-box motif and FBXL16ΔLRR that lacks the C terminus containing the 7 LRRs (Fig. 5A). We first analyzed the interaction of the different forms of FBXL16 with c-myc and SKP1 (Fig. 5B) by performing co-immunoprecipitation/Western blot assays. We found that FBXL16 interacts with both c-myc and SKP1 (compare IP lane 2 with lane 1). The deletion of the F-box domain abolished the interaction of FBXL16 with SKP1 (IP lane 3 versus lane 2), and the deletion of the LRR domain greatly reduced the interaction with c-myc (IP lane 4 versus lane 2). In addition, compared with the full-length FBXL16, FBXL16ΔLRR has reduced interaction with SKP1, indicating that LRR domain, besides its importance in binding with the substrate c-myc, may also contribute to the interaction with SKP1. We then determined the effects of these two deletion mutants on c-myc stability compared with the full-length FBXL16. The results indicated that although c-myc has increased stability when co-overexpressed with FBXL16 (compared with the empty vector control), its stability showed no clear change when co-overexpressed with FBXL16ΔFbox. Intriguingly, c-myc stability was even decreased when co-overexpressed with FBXL16ΔLRR (Fig. 5C), suggesting that this mutant might have a dominant-negative role. These results indicate that both the F-box and LRR domains are important for FBXL16 to regulate c-myc protein stability.
Figure 5.
Both F-box and LRR domains of FBXL16 are important for the regulation of c-myc. A, schematic structures of full-length FBXL16 protein, FBXL16 with the deletion of F-box domain (FBXL16ΔFbox), and FBXL16 with the deletion of the c-terminal LRRs (FBXL16ΔLRR). The numbers below or above the structures indicate the positions of the amino acids. B, FLAG-tagged FBXL16, FBXL16ΔFbox, or FBXL16ΔLRR was overexpressed in 293T cells, followed by immunoprecipitation using anti–FLAG-Ab conjugated agarose beads (FLAG-IP). Western blot analysis was then performed to examine the interactions of FBXL16 or the deletion mutant with endogenous SKP1 and c-myc proteins. C, HeLa cells were transiently co-transfected with HA–c-myc together with either an empty vector control, FLAG-FBXL16, FLAG-FBXL16ΔFbox, or FLAG-FBXL16ΔLRR. 32 h post transfection, protein translation was inhibited with CHX (100 μg/ml) for different times (minutes), followed by cell lysis and Western blot analysis of protein levels. c-myc protein level at each time point was normalized to that of β-actin, and the normalized c-myc protein level at 0 min time point was arbitrarily set as 1. Exponential curves were extrapolated using the one-phase exponential decay model (GraphPad Prism 6 software).
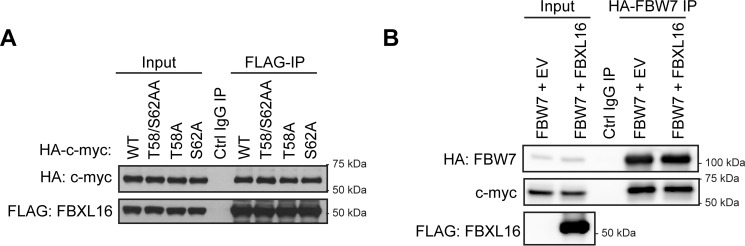
FBXL16 does not compete with FBW7 for c-myc binding
FBW7 binds to the phospho-degron pT58-X-X-X-pS62 on c-myc and induces its ubiquitination (9, 24). To determine the importance of these residues on FBXL16 activity toward c-myc, we generated T58A, S62A, and T58/S62AA mutants in which the threonine 58 or/and serine 62 were replaced by alanine(s). We first analyzed the interaction between FBXL16 and each different mutant of c-myc compared with WT c-myc by immunoprecipitation and Western blot analysis (Fig. 6A). Surprisingly, we found that the mutation of either Thr-58, Ser-62, or both showed little effect on the binding of FBXL16 to c-myc. These results indicate that, unlike the interaction between FBW7 and c-myc (24), the binding of FBXL16 to c-myc does not require the phosphorylation on any of these residues. Nevertheless, to determine whether FBXL16 may affect the FBW7/c-myc interaction, we co-overexpressed FBW7 along with FBXL16 or an empty plasmid in 293T cells (Fig. 6B). We found that the binding of FBW7 to c-myc was not changed by FBXL16 overexpression. Taken together, these results indicate that FBXL16 does not compete with FBW7 for binding to c-myc.
Figure 6.
FBXL16 does not compete with FBW7 for c-myc binding. A, 293T cells were co-transfected with pSG5-FLAG-FBXL16 and either WT, T58/S62AA, T58A, or S62A of HA-tagged c-myc constructs. FLAG-FBXL16 was immunoprecipitated using anti–FLAG-Ab conjugated agarose beads (FLAG-IP), and then Western blot analysis was performed to examine the interactions of FBXL16 with different HA-tagged c-myc protein. B, HA-tagged FBW7 was co-overexpressed with either an empty plasmid or pSG5-FLAG-FBXL16 in 293T cells. HA-FBW7 was then immunoprecipitated using anti–HA-Ab conjugated agarose beads (HA-FBW7 IP). Western blot analysis was then performed to examine the interactions of FBW7 with c-myc.
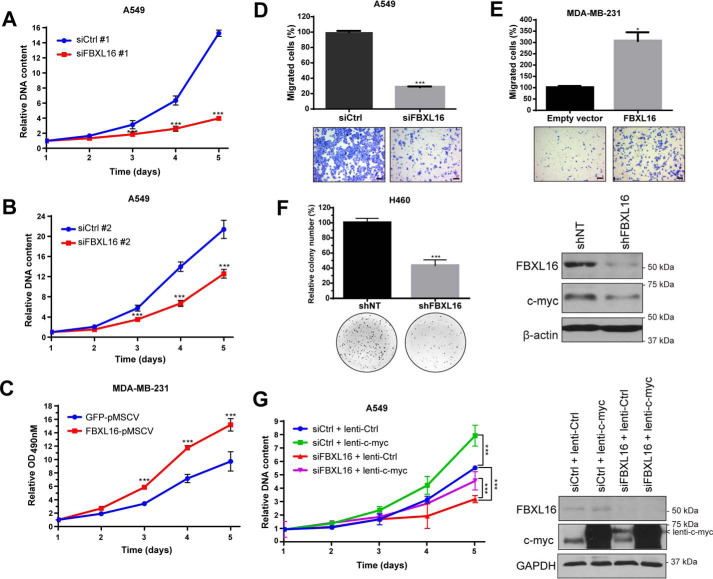
FBXL16 promotes cancer cell growth and migration
As FBXL16 up-regulates the levels of oncoproteins c-myc and SRC-3 (Fig. 1), we wanted to determine the roles of FBXL16 in cancer cell growth and migration. First, by dsDNA quantification assay, we found that transient knockdown of FBXL16 in A549 induced a remarkable decrease in cell proliferation (Fig. 7, A and B). To confirm these results, we performed a trypan blue exclusion assay to determine the number of viable cells after transient knockdown of FBXL16. Again, we found that FBXL16 knockdown greatly inhibited A549 cell growth (Fig. S3). The importance of FBXL16 on cancer cell growth was confirmed by the finding that stable expression of FBXL16 significantly promoted MDA-MB-231 cell proliferation (Fig. 7C). We then investigated the role of FBXL16 in cancer cell migration. Knockdown of FBXL16 drastically reduced (about 70%) A549 cell migration (Fig. 7D), whereas FBXL16 overexpression greatly increased MDA-MB-231 cell migration (Fig. 7E). Finally, we examined the role of FBXL16 on anchorage-independent cell growth in soft agar, an indicator of transformation and tumorigenic potential of cells. We decided to use H460 lung cancer cell line as it showed a good and consistent colony formation ability. We were actually incapable of generating cell line or pool with stable depletion of FBXL16, likely because of its essential role on cell growth and/or survival. We then generated H460 stable cell line with tetracycline-inducible knockdown of FBXL16 (Fig. 7F). We found that FBXL16 knockdown greatly decreased the capacity of H460 to form colonies in soft agar (Fig. 7F). Taken together, these results demonstrate that FBXL16 promotes both growth and migration of cancer cells.
Figure 7.
FBXL16 promotes cancer cell growth and migration. A, cell growth was determined by dsDNA quantification. After transient transfection of A549 cells with a negative control siRNA (siCtrl #1) or an siRNA targeting FBXL16 (siFBXL16 #1), DNA content was measured every 24 h and values were normalized to day 1. B, A549 cells were transiently transfected with siCtrl#2 or siFBXL16#2 and cell growth was assessed as in (A). C, an MTS proliferation assay was performed for MDA-MB-231 stable cells overexpressing pMSCV-GFP or pMSCV-FBXL16. Cell viability was determined by measuring A490 nm every 24 h and values were normalized to day 1. A–C, values in graphs represent mean ± S.D. of a representative experiment and statistical significances were determined by two-way ANOVA (*, p < 0.05; **, p < 0.01; ***, p < 0.001). D, A549 cells were transfected with siCtrl or siFBXL16 and their ability to migrate was analyzed by a two-chamber Transwell assay. Migrated cells were stained and photographed for quantification by counting. The migration ability of cells treated with siFBXL16 is presented as the percentage of the number of migrated cells per field in siFBXL16 over that of siCtrl. E, MDA-MB-231 cells were transiently transfected with an empty vector control or FBXL16 and their ability to migrate was analyzed as in (D). D and E, values in the bar graphs represent mean ± S.E. of three independent experiments. Statistical significance was determined by Student's t test (*, p < 0.05; **, p < 0.01; ***, p < 0.001). Representative images are shown at the bottom of the graphs. Scale bars, 100 μm. F, stable H460 cell lines with inducible expression of a nontargeting control shRNA (shNT) or a shRNA targeting FBXL16 (shFBXL16) were grown in soft agar in presence of doxycycline (0.5 μg/ml). After 8 days, colonies were stained and quantified using ImageJ software. The colony formation relative to shNT was determined from the average of two experiments with three replicates each time. Values in bar graphs represent mean ± S.E. Representative wells are shown at the bottom of the graphs. On the right, FBXL16 knockdown was confirmed by Western blot analysis after 5 days of doxycycline treatment (0.5 μg/ml). G, A549 cells were transiently transfected with siCtrl or siFBXL16 and transduced with a control lentivirus (lenti-Ctrl) or lentiviral FLAG-tagged c-myc (lenti-c-myc). Cell growth was assessed by dsDNA quantification as in (A). Values in graph represent mean ± S.D. of a representative experiment. Statistical significance on day 5 was determined by two-way ANOVA (*, p < 0.05; **, p < 0.01; ***, p < 0.001). On the right, knockdown of FBXL16 and lentiviral overexpression of FLAG-tagged c-myc (lenti-c-myc) were confirmed by Western blot analysis 2 days post transfection.
As c-myc is an essential regulator of cell growth (27), we thought that the role of FBXL16 in promoting cancer cell growth would be at least partially through the regulation of c-myc. To test this, we determined if exogenous overexpression of c-myc can rescue cell growth inhibition induced by FBXL16 knockdown. We found that c-myc overexpression was able to partially restore A549 cell growth after FBXL16 knockdown (siFBXL16 + lenti–c-myc versus siFBXL16 + lenti-ctrl, Fig. 7G). These results indicate that FBXL16 promotes cell growth partly through the up-regulation of c-myc.
Discussion
It is virtually unknown what the biochemical activities/functions of FBXL16 are. Given that FBXL16 harbors a conserved F-box domain and a LRR domain but does not form a stable SCF ubiquitin ligase complex, it may have distinct activities/roles as compared with other F-box proteins (e.g. FBW7) which can form functional SCF E3 ligase complex. Indeed, our study shows that opposite to the roles of FBW7 and β-TRCP, FBXL16 up-regulates the levels of several proteins targeted by SCF-E3 ligases, including c-myc and SRC-3, both of which are substrates of FBW7 (9, 20, 24), as well as β-catenin, a substrate of β-TRCP (21–23). With a focus on c-myc, we have found that FBXL16 increases c-myc protein stability by inhibiting the latter's polyubiquitination mediated by FBW7. As the antagonistic effect of FBXL16 on FBW7-mediated c-myc polyubiquitination also occurs in vitro, FBXL16 may act as a unique F-box protein to directly antagonize FBW7's activity toward the substrates. Although we are endeavoring to elucidate the detailed aspects of the underlying mechanism, we have found that both the F-box domain and the LRRs are important for FBXL16 to stabilize c-myc.
Another new finding from our study is that FBXL16 plays oncogenic roles. FBXL16 was shown to be a transcript target of E2F1 (18), a transcriptional factor with tumor promoting roles (28–30), but nothing is known about the function of FBXL16 in cancers. We have found that in line with its role in up-regulating c-myc by antagonizing FBW7, FBXL16 promotes cancer cell growth, migration, and colony formation in soft agar. Importantly, in cBioPortal for Cancer Genomics (31, 32), we found that FBXL16 is shown to be gene amplified and/or highly expressed in 9% of lung adenocarcinomas and 14% of invasive breast carcinomas (The Cancer Genome Atlas datasets, Fig. S4), further suggesting the oncogenic roles for FBXL16. FBW7 acts as a tumor suppressor in multiple cancers by inducing degradation of various oncoproteins, including c-myc, cyclin E, Jun, and SRC-3 (15). Thus, it is important to study the effects of FBXL16 on other substrates of FBW7 and to determine whether FBXL16 has a general antagonistic effect on FBW7 or its effect is substrate specific.
In summary, our study identifies FBXL16 as a unique F-box protein that inhibits FBW7's activity and thus up-regulates the levels of FBW7 substrates, including c-myc. FBXL16 promotes cancer cell growth and migration by antagonizing FBW7 tumor suppressor. Future work is warranted to verify the oncogenic roles of FBXL16 in vivo and determine whether FBXL16 is a therapeutic target of cancers.
Experimental procedures
Cell culture
A549, H1299, and H460 cell lines were maintained in RPMI 1640 medium (Gibco 22400-089). HEK293T, HeLa, and MDA-MB-231 cell lines were maintained in DMEM (Gibco 11965-092). All media were supplemented with 10% FBS (Gibco 26140-095) and 1% antibiotics (penicillin/streptomycin) (Gibco 15070-063).
Plasmids
The N-terminal FLAG-tagged human FBXL16 cDNA expressing construct (pSG5-FLAGFBXL16) was generated by PCR amplification of the coding region of FBXL16 using FBXL16 EST clone (Accession: BC036680, Clone ID: 5262152, Dharmacon MHS6278–202807943) as template and the PCR primers FBXL16-KpnI-F (CCAGGTACCATGTCGAGCCCGGGCATC) and FBXL16-KpnI-R (CCTGGTACCCTACTCAATGACGAGGCAGCGG) (KpnI restriction sites are underlined), followed by cloning the PCR product into a pSG5-KF2M1 vector using KpnI site. All the deletions and mutations were generated using the QuikChange II XL Site-Directed Mutagenesis Kit (Agilent Technologies). pSG5-FLAGFBXL16 was used as the template to generate pSG5-FLAGFBXL16ΔFbox with the deletion of F-box domain and pSG5-FLAGFBXL16ΔLRR with the deletion of the c-terminal LRRs. FBXL16ΔFbox was generated by deletion using FBXL16del94-139-F primer (GGCATGCAGCACCGGCCGCTCCGC) and its reverse complement. FBXL16ΔLRR was generated by replacing I244 with a stop codon using FBXL16I244Stop-F (ACGCTCAGCGAGGTCTAGCGCGCGCTCAGGC) and its reverse complement. pCDNA3-HA-c-myc was a gift from Martine Roussel (Addgene plasmid no. 74164) (33). FLAG-tagged c-myc was generated by digestion of pCDNA3-HA-c-myc with BamHI and EcoRI restriction enzymes and the insertion of the digested c-myc fragment into pCMVTag2B plasmid containing FLAG tag (Stratagene). The lentiviral expression construct of FLAG-tagged c-myc was generated by digestion of pCMVTag2B-c-myc with NheI and EcoRI restriction enzymes and the insertion of the digested FLAG-c-myc fragment into pLJM1 lentiviral plasmid (Addgene plasmid no. 34611) (34). T58A, S62A, and T58/S62AA mutants of c-myc were generated by site-directed mutagenesis using c-myc-T58A-F (CAGGGGCGGGGCGGGCAGCAGCT) and c-myc-S62A-F (GCGGCTAGGGGCCAGGGGCGGGG) primers and their reverse complements. pCDNA-HA-FBW7 was a gift from Yadi Wu (University of Kentucky-Lexington). This plasmid was used as template to generate a nontagged FBW7 by deletion of the HA-tag using FBW7delHA-F primer (CGCGTATGGCTTCTAGCTGGGAGGACCTTCTA) and its reverse complement. pMT-HA-ubiquitin was described previously (35). The TRIPZ inducible lentiviral nonsilencing shRNA control (RHS4743) was used as a template to insert the shRNA targeting FBXL16 following the manufacturer instruction (Horizon Discovery). pMSCV-GFP and pMSCV-FBXL16 were kindly provided by Wade Harper at Harvard Medical School (36).
For bacterial expression, His-tagged FBXL16 was generated by PCR amplification using the cDNA in pSG5 vector as the template and the PCR primers FBXL16-BamHI-F (GCATGGATCCTATGTCGAGCCCGGGCATCGAC) and FBXL16-HindIII-R (CTGCAAGCTTCTACTCAATGACGAGGCAGCGG) primers (restriction sites are underlined). After digestion, the PCR products were inserted into a pET-28b(+) vector (Novagen).
Transient siRNA and plasmid transfections
AllStars Negative Control siRNA (Qiagen SI03650318) or ON-TARGETplus Nontargeting Control Pool (Horizon D-001810-10) was used as nonsilencing control. Hs_FBXL16_8 FlexiTube siRNA (Qiagen SI04287276) or ON-TARGETplus Human FBXL16 siRNA (Horizon L-016797-00) was used to target FBXL16. ON-TARGETplus Human FBXW7 siRNA (Horizon L-004264-00) was used to target FBW7, and Hs_SKP2_5 FlexiTube siRNA (Qiagen SI00287819) was used to target SKP2. Dharmafect 1 (Dharmacon T-2001) was used to transiently knock down mRNA following manufacturer instructions. Transient plasmid transfections were performed using FuGene HD (Promega) or Lipofectamine 3000 (Invitrogen) following manufacturer instructions.
Western blot analysis
Cells were lysed with EBC buffer (50 mm Tris, pH 7.5, 150 mm NaCl, 0.5% Nonidet P-40, 1 mm cOmplete Protease Inhibitors (Roche Diagnostics), and 1 mm phosphatase inhibitor mixture III (Sigma-Aldrich)). Western blotting was performed by SDS-PAGE followed by transferring the proteins onto nitrocellulose membranes and blocking the membranes with 5% nonfat milk in PBS with Tween 20 following the procedures as described previously (37). The following antibodies were used: anti-FBXL16 (GeneTex, GTX31424), anti–β-actin (Sigma-Aldrich, A5316), anti-GAPDH (Cell Signaling Technologies, no. 2118), anti-c-myc (Cell Signaling Technologies, no. 13987 or Invitrogen 13-2500), anti–SRC-3 (BD Transduction Laboratories, 611105), anti–β-catenin (Cell Signaling Technologies, no. 8480), anti-HA (Sigma-Aldrich, H3663), anti-FLAG (Sigma-Aldrich, F1804), anti-FBW7 (Bethyl, A301-720), anti-SKP1 (Cell Signaling Technologies, no. 12248), anti-SKP2 (Cell Signaling Technologies, no. 2652), anti–mouse-HRP (Bio-Rad, 170-6516), and anti–rabbit-HRP (Bio-Rad, 170-6515).
RNA extraction and RT-qPCR
Total RNA was extracted from cells using TRIzolTM reagent (Ambion, 15596018). Reverse transcription (RT) was then performed using SuperScriptTM IV VILOTM Master Mix (Invitrogen 11756500) following manufacturer instructions. Quantitative PCR (qPCR) was performed using TaqManTM Universal Master Mix II, no UNG (Applied Biosystems, 4440040), Universal ProbeLibrary System (Roche), and the 7500 Real-Time PCR System Instrument (Applied Biosystems). GAPDH was used as the internal control. Relative expression was calculated using the ΔΔCT method.
Protein stability assays
A stock solution (10 mg/ml) of cycloheximide (CHX) (Sigma-Aldrich, C7698) was prepared in sterile water. 36 to 48 h after transient transfection, cells were treated with 100 μg/ml CHX for different time periods indicated in the experiments. After Western blot analysis, c-myc protein level at each time point was normalized to that of β-actin, and the normalized c-myc protein level at 0 min time point was arbitrarily set as 1. The protein half-life was calculated with GraphPad Prism 6 software using the one-phase exponential decay model.
Immunoprecipitation
Cell lysates were precleared by incubation with EZViewTM Red Protein A Affinity gel (Sigma-Aldrich, P6486) before incubation with EZviewTM Red Anti-HA Affinity Gel (Sigma-Aldrich, E6779), or EZviewTM Red FLAG® M2 Affinity Gel (Sigma-Aldrich, F2426) depending on the tag (HA or FLAG) that the immunoprecipitated proteins had. After 3 h, beads were washed three times with lysis buffer. The immunoprecipitated proteins were then eluted off the beads with 2× Laemmli sample buffer, followed by the Western blot analysis.
Recombinant protein expression in Escherichia coli
Competent Escherichia coli BL21 (DE3) cells were transformed with pET-28b(+) construct expressing FBXL16. A single colony was inoculated in Luria Broth (LB) medium and cultured overnight at 37 °C, followed by a 100-fold dilution and incubation at 37 °C. Once the A600 of bacterial culture reached 0.6, protein synthesis was induced with 1 mm isopropyl β-d-1-thiogalactopyranoside for 3 h. Bacteria were harvested and lysed in a denaturing buffer (20 mm Tris, 300 mm NaCl, 20 mm imidazole, 6 m guanidine hydrochloride, 0.1 mm PMSF, 1 kilounit rLyzozymeTM/milliliter, pH 7.5). After filtration, the lysate was incubated with nickel-nitrilotriacetic acid beads for 2 h at 4 °C. Beads were washed five times with denaturing buffer and recombinant proteins were eluted in elution buffer (20 mm Tris, 300 mm NaCl, 250 mm imidazole, 6 m guanidine hydrochloride, pH 7.5). Purified proteins were then refolded by dialysis (20 mm Tris, 300 mm NaCl, pH 7.5).
Ubiquitination assays
293T cells were co-transfected with HA-ubiquitin plasmid and other plasmids as indicated in the experiment. 24 h after plasmids transfection, 293T cells were treated with 20 μm of MG132 (Calbiochem, 474790) for 3 h. Cells were then lysed in a modified RIPA buffer (50 mm Tris, pH 7.5, 150 mm NaCl, 1 mm EDTA, 1% Nonidet P-40, 1% sodium deoxycholate, 0.1% SDS, 1 mm cOmplete Protease Inhibitors (Roche Diagnostics), and 1 mm phosphatase inhibitor mixture III (Sigma-Aldrich)) containing 25 mm N-ethylmaleimide (Sigma-Aldrich, E3876). Ubiquitinated proteins were then immunoprecipitated using anti-HA affinity gel and analyzed by Western blotting analysis.
For in vitro ubiquitination assays, 200 ng of recombinant c-myc protein (Abcam, ab84132) were mixed with 25 ng UBE1 (Millipore, 23-021), 50 ng UbcH3 (Millipore, 23-022), 50 ng UbcH5a (Millipore, 23-029), 150 ng SCFFBW7 (Millipore, 23-030), 20 μm ubiquitin (Boston Biochem, U-110), and 1 mm Mg/ATP in ubiquitination reaction buffer (Boston Biochem, SK-10). The reaction mix was incubated for 1 h at 37 °C, followed by Western blot analysis using anti–c-myc antibody (Invitrogen, 13-2500).
Two-chamber Transwell cell migration assay
Cell migration was analyzed by using a modified two-chamber Transwell system (BD Biosciences) following the manufacturer's instructions. The bottom well was filled with cOmplete medium containing 10% fetal bovine serum. Cells resuspended in serum-free medium were added into each Transwell insert and allowed to migrate in a 37 °C cell incubator for different period of times as indicated in each specific experiment. Next, cells on the upper surface of the insert membrane were removed with cotton swabs. The migrated cells attached to the undersurface of the insert membrane were then fixed in 4% paraformaldehyde for 15 min and stained with 0.5% crystal violet solution for 10 min. Migrated cells were assessed under a microscope (×50).
Proliferation assays
Cell proliferation was determined either using the CellTiter 96® AQueous One Solution Cell Proliferation Assay Kit (Promega, G3580) or FluoReporterTM Blue Fluorometric dsDNA Quantitation Kit (Invitrogen, F2962) following the manufacturer's instructions.
Soft agar colony formation assay
Anchorage-independent colony formation assay was performed using the Cell Transformation Detection Assay Kit (Sigma-Aldrich, ECM570) following the manufacturer's protocol. 1000 H460 cells/well of a 24-well plate were grown in 250 μl of 0.3% agarose in cOmplete medium containing 0.5 μg/ml doxycycline in a 37 °C humidified cell culture incubator. Fresh cOmplete medium containing 0.5 μg/ml doxycycline was added to replace the old medium every 2 days to maintain the induction of the shRNA for a total of 8 days. Cell colonies in agarose were then stained with 1 mg/ml of Cell Stain Solution overnight. Cell colony formation was quantified using Image J software.
Statistics
Data are expressed as mean ± S.D. or S.E., as specified in the figure legends. All experiments were repeated at least three times, and a representative figure is presented. Statistical significance was determined by Student's t test, one-way analysis of variance (ANOVA), or two-way ANOVA, as indicated in each figure legend, and a p value of less than 0.05 was considered statistically significant (*, p < 0.05; **, p < 0.01; ***, p < 0.001).
Data availability
All data are contained within the article and supporting materials.
Supplementary Material
This article contains supporting information.
Author contributions—M. M. and W. L. conceptualization; M. M. and W. L. data curation; M. M. and W. L. formal analysis; M. M. and W. L. validation; M. M., K. N. S., and W. L. investigation; M. M. and W. L. methodology; M. M. and W. L. writing-original draft; W. L. resources; W. L. supervision; W. L. funding acquisition; W. L. project administration.
Funding and additional information— This work was supported by NCI, National Institutes of Health Grant 1R01CA193264–01 (to W. L.). This work was also supported by a start-up fund of Wright State University. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflict of interest—The authors declare that they have no conflicts of interest with the contents of this article.
- LRR
- leucine-rich repeats
- IP
- immunoprecipitation
- CHX
- cycloheximide
- ANOVA
- analysis of variance.
References
- 1. Hershko A., and Ciechanover A. (1998) The ubiquitin system. Annu. Rev. Biochem. 67, 425–479 10.1146/annurev.biochem.67.1.425 [DOI] [PubMed] [Google Scholar]
- 2. Pickart C. M. (2001) Mechanisms underlying ubiquitination. Annu. Rev. Biochem. 70, 503–533 10.1146/annurev.biochem.70.1.503 [DOI] [PubMed] [Google Scholar]
- 3. Dye B. T., and Schulman B. A. (2007) Structural mechanisms underlying posttranslational modification by ubiquitin-like proteins. Annu. Rev. Biophys. Biomol. Struct. 36, 131–150 10.1146/annurev.biophys.36.040306.132820 [DOI] [PubMed] [Google Scholar]
- 4. Zheng N., and Shabek N. (2017) Ubiquitin ligases: Structure, function, and regulation. Annu. Rev. Biochem. 86, 129–157 10.1146/annurev-biochem-060815-014922 [DOI] [PubMed] [Google Scholar]
- 5. Petroski M. D., and Deshaies R. J. (2005) Function and regulation of cullin-RING ubiquitin ligases. Nat. Rev. Mol. Cell Biol. 6, 9–20 10.1038/nrm1547 [DOI] [PubMed] [Google Scholar]
- 6. Jin J., Cardozo T., Lovering R. C., Elledge S. J., Pagano M., and Harper J. W. (2004) Systematic analysis and nomenclature of mammalian F-box proteins. Genes Dev. 18, 2573–2580 10.1101/gad.1255304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Skaar J. R., Pagan J. K., and Pagano M. (2013) Mechanisms and function of substrate recruitment by F-box proteins. Nat. Rev. Mol. Cell Biol. 14, 369–381 10.1038/nrm3582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Orlicky S., Tang X., Willems A., Tyers M., and Sicheri F. (2003) Structural basis for phosphodependent substrate selection and orientation by the SCFCdc4 ubiquitin ligase. Cell 112, 243–256 10.1016/S0092-8674(03)00034-5 [DOI] [PubMed] [Google Scholar]
- 9. Welcker M., Orian A., Jin J., Grim J. A., Harper J. W., Eisenman R. N., and Clurman B. E. (2004) The Fbw7 tumor suppressor regulates glycogen synthase kinase 3 phosphorylation-dependent c-Myc protein degradation. Proc. Natl. Acad. Sci. U.S.A. 101, 9085–9090 10.1073/pnas.0402770101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Welcker M., and Clurman B. E. (2008) FBW7 ubiquitin ligase: A tumour suppressor at the crossroads of cell division, growth and differentiation. Nat. Rev. Cancer. 8, 83–93 10.1038/nrc2290 [DOI] [PubMed] [Google Scholar]
- 11. Kobe B., and Kajava A. V. (2001) The leucine-rich repeat as a protein recognition motif. Curr. Opin. Struct. Biol. 11, 725–732 10.1016/S0959-440X(01)00266-4 [DOI] [PubMed] [Google Scholar]
- 12. Hao B., Zheng N., Schulman B. A., Wu G., Miller J. J., Pagano M., and Pavletich N. P. (2005) Structural basis of the Cks1-dependent recognition of p27 Kip1 by the SCF Skp2 ubiquitin ligase. Mol. Cell 20, 9–19 10.1016/j.molcel.2005.09.003 [DOI] [PubMed] [Google Scholar]
- 13. Heo J., Eki R., and Abbas T. (2016) Deregulation of F-box proteins and its consequence on cancer development, progression and metastasis. Semin. Cancer Biol. 36, 33–51 10.1016/j.semcancer.2015.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cremona C. A., Sancho R., Diefenbacher M. E., and Behrens A. (2016) Fbw7 and its counteracting forces in stem cells and cancer: Oncoproteins in the balance. Semin. Cancer Biol. 36, 52–61 10.1016/j.semcancer.2015.09.006 [DOI] [PubMed] [Google Scholar]
- 15. Shimizu K., Nihira N. T., Inuzuka H., and Wei W. (2018) Physiological functions of FBW7 in cancer and metabolism. Cell. Signal. 46, 15–22 10.1016/j.cellsig.2018.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dickinson M. E., Flenniken A. M., Ji X., Teboul L., Wong M. D., White J. K., Meehan T. F., Weninger W. J., Westerberg H., Adissu H., Baker C. N., Bower L., Brown J. M., Brianna Caddle L., Chiani F., et al. (2016) High-throughput discovery of novel developmental phenotypes. Nature. 537, 508–514 10.1038/nature19356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Honarpour N., Rose C. M., Brumbaugh J., Anderson J., Graham R. L. J., Sweredoski M. J., Hess S., Coon J. J., and Deshaies R. J. (2014) F-box protein FBXL16 binds PP2A-B55α and regulates differentiation of embryonic stem cells along the FLK1+ lineage. Mol. Cell. Proteomics 13, 780–791 10.1074/mcp.M113.031765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sato K., Kusama Y., Tategu M., and Yoshida K. (2010) FBXL16 is a novel E2F1-regulated gene commonly upregulated in p16INK4A- and p14ARF-silenced HeLa cells. Int. J. Oncol. 36, 479–490 10.3892/ijo_00000522 [DOI] [PubMed] [Google Scholar]
- 19. Liu X., Reitsma J. M., Mamrosh J. L., Zhang Y., Straube R., and Deshaies R. J. (2018) Cand1-mediated adaptive exchange mechanism enables variation in F-box protein expression. Mol. Cell 69, 773–786.e6 10.1016/j.molcel.2018.01.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wu R. C., Feng Q., Lonard D. M., and O'Malley B. W. (2007) SRC-3 coactivator functional lifetime is regulated by a phospho-dependent ubiquitin time clock. Cell 129, 1125–1140 10.1016/j.cell.2007.04.039 [DOI] [PubMed] [Google Scholar]
- 21. Latres E., Chiaur D. S., and Pagano M. (1999) The human F box protein β-Trcp associates with the Cul1/Skp1 complex and regulates the stability of β-catenin. Oncogene 18, 849–854 10.1038/sj.onc.1202653 [DOI] [PubMed] [Google Scholar]
- 22. Winston J. T., Strack P., Beer-Romero P., Chu C. Y., Elledge S. J., and Harper J. W. (1999) The SCF(β-TRCP)-ubiquitin ligase complex associates specifically with phosphorylated destruction motifs in IκBα and β-catenin and stimulates IκBα ubiquitination in vitro. Genes Dev. 13, 270–283 10.1101/gad.13.3.270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kitagawa M., Hatakeyama S., Shirane M., Matsumoto M., Ishida N., Hattori K., Nakamichi I., Kikuchi A., Nakayama K., and Nakayama K. (1999) An F-box protein, FWD1, mediates ubiquitin-dependent proteolysis of β-catenin. EMBO J. 18, 2401–2410 10.1093/emboj/18.9.2401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yada M., Hatakeyama S., Kamura T., Nishiyama M., Tsunematsu R., Imaki H., Ishida N., Okumura F., Nakayama K., and Nakayama K. I. (2004) Phosphorylation-dependent degradation of c-Myc is mediated by the F-box protein Fbw7. EMBO J. 23, 2116–2125 10.1038/sj.emboj.7600217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. von der Lehr N., Johansson S., Wu S., Bahram F., Castell A., Cetinkaya C., Hydbring P., Weidung I., Nakayama K., Nakayama K. I., Söderberg O., Kerppola T. K., and Larsson L. G. (2003) The F-box protein Skp2 participates in c-Myc proteosomal degradation and acts as a cofactor for c-Myc-regulated transcription. Mol. Cell 11, 1189–1200 10.1016/S1097-2765(03)00193-X [DOI] [PubMed] [Google Scholar]
- 26. Kim S. Y., Herbst A., Tworkowski K. A., Salghetti S. E., and Tansey W. P. (2003) Skp2 regulates Myc protein stability and activity. Mol. Cell 11, 1177–1188 10.1016/S1097-2765(03)00173-4 [DOI] [PubMed] [Google Scholar]
- 27. Eilers M., and Eisenman R. N. (2008) Myc's broad reach. Genes Dev. 22, 2755–2766 10.1101/gad.1712408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Johnson D. G., Cress W. D., Jakoi L., and Nevins J. R. (1994) Oncogenic capacity of the E2F1 gene. Proc. Natl. Acad. Sci. U.S.A. 91, 12823–12827 10.1073/pnas.91.26.12823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lukas J., Petersen B. O., Holm K., Bartek J., and Helin K. (1996) Deregulated expression of E2F family members induces S-phase entry and overcomes p16INK4A-mediated growth suppression. Mol. Cell. Biol. 16, 1047–1057 10.1128/MCB.16.3.1047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Poppy Roworth A., Ghari F., and La Thangue N. B. (2015) To live or let die—complexity within the E2F1 pathway. Mol. Cell. Oncol. 2, e970480 10.4161/23723548.2014.970480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cerami E., Gao J., Dogrusoz U., Gross B. E., Sumer S. O., Aksoy B. A., Jacobsen A., Byrne C. J., Heuer M. L., Larsson E., Antipin Y., Reva B., Goldberg A. P., Sander C., and Schultz N. (2012) The cBio Cancer Genomics Portal: An open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2, 401–404 10.1158/2159-8290.CD-12-0095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gao J., Aksoy B. A., Dogrusoz U., Dresdner G., Gross B., Sumer S. O., Sun Y., Jacobsen A., Sinha R., Larsson E., Cerami E., Sander C., and Schultz N. (2013) Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci. Signal. 6, pl1 10.1126/scisignal.2004088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Vo B. H. T., Wolf E., Kawauchi D., Gebhardt A., Rehg J. E., Finkelstein D., Walz S., Murphy B. L., Youn Y. H., Han Y. G., Eilers M., and Roussel M. F. (2016) The interaction of Myc with Miz1 defines medulloblastoma subgroup identity. Cancer Cell 29, 5–16 10.1016/j.ccell.2015.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zoncu R., Bar-Peled L., Efeyan A., Wang S., Sancak Y., and Sabatini D. M. (2011) mTORC1 senses lysosomal amino acids through an inside-out mechanism that requires the vacuolar H+-ATPase. Science 334, 678–683 10.1126/science.1207056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Li C., Wu R.-C., Amazit L., Tsai S. Y., Tsai M.-J., and O'Malley B. W. (2007) Specific amino acid residues in the basic helix-loop-helix domain of SRC-3 are essential for its nuclear localization and proteasome-dependent turnover. Mol. Cell. Biol. 27, 1296–1308 10.1128/MCB.00336-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tan M. K. M., Lim H. J., Bennett E. J., Shi Y., and Harper J. W. (2013) Parallel SCF adaptor capture proteomics reveals a role for SCFFBXL17 in NRF2 activation via BACH1 repressor turnover. Mol. Cell 52, 9–24 10.1016/j.molcel.2013.08.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Elkhadragy L., Alsaran H., Morel M., and Long W. (2018) Activation loop phosphorylation of ERK3 is important for its kinase activity and ability to promote lung cancer cell invasiveness. J. Biol. Chem. 293, 16193–16205 10.1074/jbc.RA118.003699 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are contained within the article and supporting materials.