Abstract
Simple Summary
Methane produced by enteric fermentation contributes to the emission of greenhouse gases (GHG) into the atmosphere. Methane is one of the GHG arising from anthropogenic activities with the greater contribution to global warming. This paper provides a brief introduction to the potential use of tropical foliage trees, pods, and secondary metabolites to reduce methane emissions from ruminant supply chains. A better knowledge of the available strategies for efficient foliage use in the tropics is essential in order to ensure increasing livestock production while preserving the environment. The mitigation of rumen methane production through the use of the foliage and metabolites of tropical trees represents an interesting challenge for scientists working in the field of ruminant nutrition.
Abstract
Methane produced by enteric fermentation contributes to the emission of greenhouse gases (GHG) into the atmosphere. Methane is one of the GHG resulting from anthropogenic activities with the greater global warming contribution. Ruminant production systems contribute between 18% and 33% of methane emissions. Due to this, there has been growing interest in finding feed alternatives which may help to mitigate methane production in the rumen. The presence of a vast range of secondary metabolites in tropical trees (coumarins, phenols, tannins, and saponins, among others) may be a valuable alternative to manipulate rumen fermentation and partially defaunate the rumen, and thus reduce enteric methane production. Recent reports suggest that it is possible to decrease methane emissions in sheep by up to 27% by feeding them saponins from the tea leaves of Camellia sinensis; partial defaunation (54%) of the rumen has been achieved using saponins from Sapindus saponaria. The aim of this review was to collect, analyze, and interpret scientific information on the potential of tropical trees and their secondary metabolites to mitigate methane emissions from ruminants.
Keywords: climate change, ruminants, secondary metabolites, saponins, volatile fatty acids
1. Introduction
Methane (CH4) gas is a byproduct of the anaerobic microbial fermentation of carbohydrates in the rumen [1,2], and it is one of the six greenhouse gases (GHG) included in the Kyoto Protocol, with a global warming potential 23 times that of Carbon dioxide (CO2) [3,4]. Among agricultural activities, ruminant production is one of the major sources of GHG emissions, contributing about 18% to 33% of the total CH4 emitted into the environment [4,5,6,7]. This is due to the fact that between 2% and 12% of the gross energy consumed by the ruminant is converted into CH4 during rumen fermentation [8]. Over recent years, there has been growing interest in predicting CH4 emissions from ruminant species in order to reduce emissions [9,10]. New strategies include the use of plant secondary metabolites [11,12].
Ruminant production systems in the tropics are characterized by grazing native and introduced grasses which present fluctuations in quantity and quality throughout the year [13]. The relatively low quality of tropical forages determines, to a large extent, an increasing fibrous material intake and, therefore, the production of rumen CH4 [14,15]. In this sense, tropical trees (TT) may contribute to an improvement in ruminants’ feeding due to their high nutritive value (136 to 325 g crude protein (CP/kg) dry matter (DM) and 50 to 60% apparent digestibility) [16]. Furthermore, TT contain a range of secondary metabolites [17,18], which could alter rumen fermentation [19,20], partially defaunate the rumen [21], and consequently reduce CH4 emissions [22,23].
The aim of this review was to collect, analyze, and interpret scientific information on the potential of using tropical trees and their secondary metabolites to mitigate CH4 emissions from ruminants.
2. Greenhouse Gases and Animal Production
Carbon dioxide (CO2), nitrous oxide (N2O), hydrofluorocarbons (HFCs), perfluorocarbons (PFCs), sulphur hexafluoride (SF6), and methane (CH4) are the main greenhouse gases (GHG) produced by global livestock [24]. The Intergovernmental Panel on Climate Change [3] reported for the period of 1970 to 2004 increases of 70% and 40% in the emission of CO2 and CH4, respectively. According to current data, the world human population has reached ≈ seven billion; however, it is expected to rise to nine billion by 2050 [25,26]. The projected population growth will drive up global demand for food and livestock production. In particular, it is estimated that meat consumption will increase from 229 to 465 million tons between the years 2000 and 2050, and the demand for dairy products will likely reach 1045 million tons [5]. As a result of the increased demand for animal-based protein, CH4 emissions are predicted to rise exponentially [27].
For example, studies conducted in Mexico showed that in 2015, CH4 emissions reached a magnitude of 70 567.60 Gg CO2e, with enteric fermentation making up 76% of the total CH4 released into the environment [28]. This was partly due to the growing livestock population reported for the period of 2006 to 2015 (33.5 million cattle, nine million goats, and nine million sheep) [29].
In 2015, García-Apaza et al. [30] forecast a linear growth rate of CH4 emissions deriving from the livestock sector in Bolivia. The aforementioned values were calculated following the Intergovernmental Panel on Climate Change (IPCC) recommendations [3], which in turn are based on estimates of cattle inventories. As a consequence, the estimate’s precision strongly depends on the availability and reliability of such information.
In Mexico, in order to establish the most appropriate strategies towards CH4 mitigation, it is necessary to develop precise emission factors with the purpose of having a reliable inventory of the magnitude of enteric CH4 emissions and a well-established livestock policy.
3. Overview of Methanogenesis in the Ruminants
Methane production by ruminants is a natural process which originates in the rumen during feed digestion [31]. In this process, several microorganism species known as methanogens convert feed such as proteins and starch into amino acids and sugars which are then fermented to become volatile fatty acids, while molecular hydrogen (H2) released during the production of acetate and butyrate in the rumen [32,33] and CO2 are reduced to CH4 [34].
The amount of methane produced in the rumen depends on the characteristics of the diet consumed by the animals [35,36]. By knowing the exact dry matter intake [37,38] and, consequently, the quantity of volatile fatty acids produced in the rumen, it is possible to calculate the total amount of methane that ruminants will emit [39]. Further studies on ruminal function and metabolic variables are needed in order to gain deeper insights into the effects of tropical plant foliage and secondary metabolites on livestock-derived GHG emissions.
4. Potential of Tropical Trees for the Feeding of Ruminants
A large diversity of tropical tree species could potentially be used to feed ruminants and improve livestock production [40,41,42]. The content of crude protein deriving from tropical tree foliage and fruit has a range of 136–325 g/kg dry matter (DM) and 79–429 g/kg DM, respectively, with a digestibility rate of 50–60% (Table 1) [20]. The productive performance (weight gain, milk yield) of ruminants is the best reflection of feed quality.
Table 1.
Chemical composition (g/kg of dry matter) of foliage, fruits, and leaves of forage trees.
| Species | Fraction | OM | CP | NDF | ADF | References |
|---|---|---|---|---|---|---|
| Acacia pennatula | Foliage | 929 | 125 | 590 | 358 | [41] |
| Cratylia argentea | Foliage | - | 273 | 587 | [43] | |
| Erithryna berteroana | Foliage | 901 | 243 | - | - | [44] |
| Gliricidia sepium | Foliage | 894 | 238 | 385 | 247 | [45] |
| Guazuma ulmifolia | Foliage | 862 | 104 | 425 | 295 | [46] |
| Guazuma ulmifolia | Foliage | - | 110 | 520 | 344 | [46] |
| Hibiscus rosasinensis | Foliage | - | 266 | 367 | 223 | [41] |
| Leucaena leucocephala | Foliage | 898 | 201 | 275 | 191 | [41] |
| Leucaena leucocephala | Foliage | - | 245 | 452 | 255 | [41] |
| Morus alba | Foliage | - | 176 | 260 | 228 | [47] |
| Trichantera gigantea | Foliage | - | 199 | 407 | 339 | [48] |
| Acalipha villosa | Foliage | 899 | 162 | 361 | 291 | [46] |
| Ampelocissus erduendbergiana | Foliage | 934 | 157 | 494 | 332 | [46] |
| Brosimum alicastrum | Foliage | - | 142 | 375 | 260 | [41] |
| Crecopia obstusifolia | Foliage | 896 | 165 | 394 | 271 | [46] |
| Dalbergia glabra | Foliage | 941 | 187 | 629 | 415 | [46] |
| Galactia multiflora | Foliage | 925 | 137 | 409 | 232 | [46] |
| Guazuma ulmifolia | Foliage | 919 | 137 | 451 | 288 | [46] |
| Piscidia piscipula | Foliage | 905 | 126 | 500 | 346 | [46] |
| Psichotria nervosa | Foliage | 889 | 165 | 326 | 193 | [46] |
| Spondias mombim | Foliage | 892 | 148 | 283 | 197 | [46] |
| Tropis racemosa | Foliage | 878 | 130 | 345 | 297 | [46] |
| Acacia pennatula | Fruits | 955 | 85 | 720 | 487 | [41] |
| Enterolobium cyclocarpum | Fruits | 907 | 109 | 251 | - | [49] |
| Enterolobium cyclocarpum | Fruits | 966 | 164 | 339 | 221 | [41] |
| Guazuma ulmifolia | Fruits | 947 | 58 | 461 | 354 | [41] |
| Leucaena leucocephala | Fruits | 942 | 186 | 519 | 370 | [41] |
| Pithecellobium saman | Fruits | 920 | 147 | 291 | - | [49] |
| Enterolobium cyclocarpum | Leaves | - | 204 | 640 | 382 | [50] |
| Gliricidia sepium | Leaves | - | 195 | 526 | 299 | [50] |
| Leucaena leucocephala | Leaves | - | 216 | 687 | 412 | [50] |
| Moringa oleifera | Leaves | - | 254 | 632 | 411 | [50] |
CP: crude protein; OM: organic matter; NDF: neutral detergent fiber; ADF: acid detergent fiber.
In the literature, many studies support this correlation. In Pelibuey lambs, for example, a moderate weight gain (90 g/head/day) has been observed after including 12% of Acacia farnesiana fruit in their diet [51]. Brown et al. [52] found that adding around 40% to 50% of Acacia karroo foliage in the Pedia goat diet based on Setaria verticillata leads to a higher DM, organic matter (OM), neutral detergent fibre (NDF), and acid detergent fibre (ADF) digestibility compared to the results obtained by including only 20%, 25%, and 30% of A. karroo foliage. Similarly, it has been shown that the use of 15% and 30% of Gliricidia sepium and Enterolobium cyclocarpum foliage, respectively, in the cross-heifer ration improves animal productivity due to their crude protein (CP), tannin, and saponin content [53].
In another study on bull diet, it was observed that replacing cotton seeds with Morus alba (0%, 5%, 10%, and 15% of the total ration) resulted in significant weight gain (554, 583, 565, 568 g/head/day, respectively) [54]. However, the substitution of milled sorghum with milled E. cyclocarpum fruits (0%, 12%, 24%, and 36% of the DM ration) had no significant effects on the productive performance of hair sheep [55].
Regarding the consumption rate, the incorporation of 45% of the ground fruits such as Acacia pennatula (group one) or E. cyclocarpum (group two) added to the commercial concentrated feed in the Pelibuey sheep ration significantly increased the consumption rate compared to group three fed only with commercial concentrate feed (1155, 1123 vs. 933 g DM/day, respectively)[56]. On the other hand, the addition of 0%, 20%, 30%, 40%, and 50% of the ground fruit of E. cyclocarpum in the ration of hair sheep significantly decreased the digestibility of DM in the treatment with the highest amount of fruit (50%). This result could be explained by a higher NDF intake despite similar DM intakes among the various treatments (73, 87, 88, 94 and 91 g/kg0.75/day) [57].
Lastly, Ansari, Mohammadabadi and Sari [58] found that adding Albizzia lebbeck in the humpback camel diet did not affect the digestibility of dry matter and NDF; similar results were observed for the conventional alfalfa diet.
5. Secondary Metabolites in Tropical Forage Trees
Trees are part of a complex set of interactions between plants, animals, and insects [59]. Given those interactions, trees have developed mechanisms of defense such as spikes, fibrous foliage, growth patterns, and the presence of secondary metabolites against herbivory, pathogens, pests, and defoliation [60]. Secondary metabolites, for example, are known to reduce the palatability and voluntary feed intake as well as the dry matter and protein digestibility of forages [61]. The most commonly present secondary metabolites in tropical trees are: tannins, alkaloids, cyanogenic glycosides, and saponins (Table 2).
Table 2.
Concentration of the main secondary metabolites in foliage of tropical trees (g/kg DM).
| Species | Fraction | TF | CT | SAP | References |
|---|---|---|---|---|---|
| Acacia pennatula | Foliage | 29.0 | 40.0 | - | [41] |
| Albizia lebbeck | Foliage | 9.4 | 5.3 | - | [62] |
| Enterolobium cyclocarpum | Foliage | 1.4 | 1.5 | 8.0 | [21] |
| Erithrina variegata | Foliage | 2.2 | 0.2 | - | [62] |
| Gliricidia sepium | Foliage | 3.0 | - | - | Laboratory * |
| Leucaena leucocephala | Foliage | 5.0 | 1.8 | - | [62] |
| Moringa oleifera | Foliage | 4.0 | 2.9 | - | [62] |
| Enterolobium cyclocarpum | Pods | - | 52 | 19.0 | Laboratory * |
| Sapindus saponaria | Pods | - | 32 | 120.0 | [49] |
TF: total phenols; CT: condensed tannins; SAP: saponins; - without information; * laboratory analysis of experimental samples.
6. Effect of Secondary Metabolites of Tropical Trees on Rumen Fermentation
Due to public concerns for the dramatic increase in the use of chemical compounds such as ionophores and antibiotics in the ruminant production industry, there has been growing interest in finding alternative feed additives [60]. In this regard, secondary metabolites represent a valuable and sustainable option as they may be used to manipulate rumen fermentation (i.e., alter the molar proportions of volatile fatty acids and reduce biohydrogenation of unsaturated fatty acids) [60].
Among secondary metabolites, tannins and especially saponins seem to be the most promising alternative feed additives [8,60]. Condensed tannins (CT) comprise a diverse group of polyphenols found in a large number of plant species in which they are responsible for bounding and precipitating proteins. While a low concentration of CT has a beneficial effect on nitrogen utilization due to the protection of proteins against microbial degradation in the rumen, a high concentration of CT has a detrimental effect on the intake, digestibility, and weight gain [63].
Saponins are found in many plant species and consist of bioorganic compounds classified as glycoside steroids, triterpenoids, and steroidal alkaloids. More specifically, they are defined as glycosides of high molecular weight, with one or more hydrophilic sugar chains (glucose, galactose, xylose, arabinose, ramnose, or glucuronic acid) combined with lipophilic aglycones which are either triterpene or steroid molecules. The aglycone moiety is also known as sapogenin [61,64].
Given their vast biological role as emulsifiers and detergents, as well as their pharmacological hemolytic [65] and antiprotozoal properties [17,66], saponins have recently been proposed as a means of manipulating rumen fermentation. For example, interactions between saponins and membrane-bound cholesterol lead to unsuitability, lysis, and death of the cell [59]. Additionally, in vivo and in vitro experiments using tropical trees such as Sapindus saponaria, Pithecellobium saman, Tithonia diversifolia, and E. cyclocarpum have highlighted the effects of saponins as defaunating agents and modifiers of rumen fermentation [21,49,67].
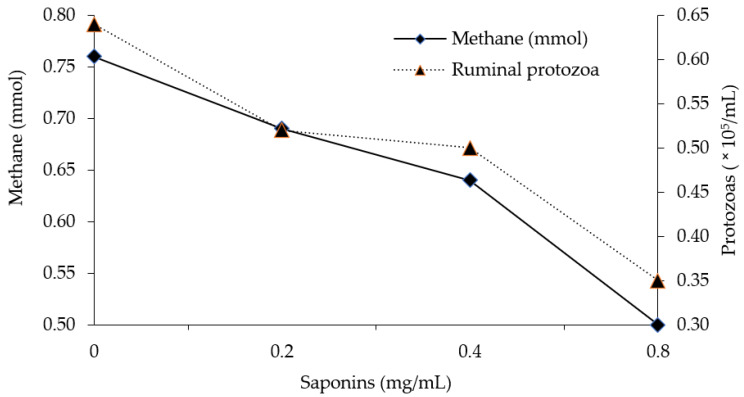
Thus, the use of saponins as feed additives would highly benefit the environment and ruminant productivity as it has been shown that a reduction in the protozoa rumen decreases the total production of enteric CH4 while the use of dietary energy is increased (Figure 1) [53,68,69].
Figure 1.
Effect of the inclusion of saponins on protozoa population and rumen methane (CH4) in vitro (Adapted from Hu et al., 2006 [70]).
The potential of the forages and fruits of tropical trees for CH4 reduction, rumen defaunation, and changes in the molar proportions of volatile fatty acid (VFA) in the rumen has been demonstrated [20] (Table 3 and Table 4); however, conclusions are sometimes still contradictory.
Table 3.
Potential of foliage of tropical trees for methane (CH4) mitigation, rumen defaunation, and changes in the molar proportions of volatile fatty acids in vitro.
| Species | CH4 | CH4/Total Gas | Protozoa | VFA l/100 moL) | Reference | ||
|---|---|---|---|---|---|---|---|
| (mL) | (v:v) | (104/mL) | Ac | Pr | Bu | ||
| Pennisetum purpureum | 6.53 | 0.184 | - | - | - | - | [20] |
| Sesbania sesban 10865 | 0.75 | 0.068 | 3.01 | 68 | 20 | 9 | |
| Samanea saman | 1.14 | 0.052 | 2.39 | 63 | 25 | 9 | |
| Acacia angustissima 459 | 1.25 | 0.075 | 4.01 | 69 | 20 | 8 | |
| Acacia nilotica | 2.2 | 0.064 | 3.25 | 72 | 16 | 9 | |
| Leucaena leucocephala | 5.57 | 0.112 | 2.82 | 73 | 20 | 6 | |
| Sasbania sesban 15019 | 6.56 | 0.144 | 3.77 | 70 | 20 | 7 | |
| Gliricidia sepium | 7.33 | 0.147 | 2.15 | 70 | 21 | 7 | |
| Moringa stenopetala | 7.72 | 0.15 | 2.72 | 71 | 20 | 7 | |
Ac: acetate; Pr: propionate; Bu: butyrate; CH4: methane; VFA: volatile fatty acids.
Table 4.
Potential of foliage and seeds of tropical trees for methane (CH4) mitigation, rumen defaunation, and changes in the molar proportions of volatile fatty acids in vitro.
| Species | CH4 | CH4/total gas | Protozoa | VFA (moL/100 moL) | Reference | ||
|---|---|---|---|---|---|---|---|
| (mL) | (v:v) | (104/mL) | Ac | Pr | Bu | ||
| Pennisetum purpureum | 6.53 | 0.184 | - | - | - | - | [20] |
| Sapindus saponaria | 5.14 | 0.12 | 1.86 | 65 | 25 | 8 | |
| Leucaena leucocephala | 7.32 | 0.133 | 3.68 | 66 | 24 | 7 | |
| Albizia lebbeck | 7.95 | 0.137 | 0.62 | 64 | 23 | 10 | |
| bracteolate | 10.68 | 0.163 | 2.72 | 67 | 22 | 9 | |
| Enterolobium cyclocarpum | 12.71 | 0.175 | 2.1 | 63 | 27 | 9 | |
| Albizia saman | 16.01 | 0.205 | 5.16 | 69 | 21 | 8 | |
Ac: acetate; Pr: propionate; Bu: butyrate; CH4: methane; VFA: volatile fatty acids.
Lila et al. [71] observed under in vitro conditions a linear decrease in the production of rumen CH4 as the level of saponins of Yucca schidigera in the ration was increased, with values ranging between 13.87, 10.96, 9.57, 7.25, and 5.82 mmol of CH4 for 0, 1.2, 1.8, 2.4, and 3.2 g/L of Y. schidigera, respectively. Another in vitro experiment using Sapindus mukorossi in diets based on wheat flour (80%) and wheat straw (20%) revealed a reduction of 22.68%, 11.48%, and 0% of methane in buffalo ruminal fluid when extracts of water, ethanol, and methanol were modified, respectively (Table 5) [72].
Table 5.
Effect of metabolites from tropical trees on molar proportions of volatile fatty acids and CH4 production in the rumen.
| Diet/Conditions and Quantity of Substrate | Source of Metabolites | Dose | Molar Proportion | CH4 | References | ||
|---|---|---|---|---|---|---|---|
| Acetate | Propionate | Butyrate | mmoL/day | ||||
| RUSITEC (14 g/day of mix grass: legume, 80: 20 in fermenters). | Samanea saman 14884 | ND | 63 | 27 | 7 | 3.61 | [73] |
| Acacia angustissima 459 | ND | 64 | 26 | 7 | 2.02 | ||
| Sesbania sesban 10865 | ND | 63 | 28 | 7 | 1.55 | ||
| Basal diet | Sheep fed with concentrates | 0:3 | 73 | 19 | 7 | 1.85 | [74] |
| B. brizantha: Cratylia argentea | 1:3 | 72 | 21 | 7 | 1.81 | ||
| 2:1 | 68 | 23 | 7 | 1.73 | |||
| Basal diet | Sheep fed with concentrates plus S. saponaria (7.71 g crude saponin/lamb/day) in each proportion | 0:3 | 72 | 21 | 6 | 1.63 | |
| Cratylia argentea: B. brizantha | 1:3 | 70 | 23 | 6 | 1.68 | ||
| 2:1 | 69 | 23 | 7 | 1.64 | |||
| Isoenergetic and isoproteic balanced diets |
Neomillspaughia emargiata
Tabernaemontana amygdalifolia Caesalpinia gaumeri Piscidia piscipula Leucaena leucocephala Havardia albicans |
1:3 | 61 | 25.8 | 13.92 | 1.73 | [75] |
| Water flour (80%) Water straw (20%) |
Sapindus mukurossi Water extract |
20 g/100 mL of solvent | 53.12 | 34.20 | 12.67 | 22.68 | [72] |
| Control | 0 | 60.26 | 21.71 | 17.97 | |||
|
Sapindus mukurossi Methanol extract |
20 g/100 mL of solvent | 61.21 | 27.24 | 11.56 | 0 | ||
| Control | 0 | 61.48 | 27.4 | 11.21 | |||
|
Sapindus mukurossi Ethanol extract |
20 g/100 mL of solvent | 60.60 | 29.11 | 10.29 | 11.48 | ||
| Control | 0 | 61.10 | 29.92 | 8.97 | |||
| Control | 0 | 3.92 | 0.94 | 0.30 | 2.35 | ||
| HFD 80:20 |
Myristica fragrans | 1 mL extract/100 mL | 2.90 | 0.76 | 0.31 | 1.97 | [76] |
| Control | 0 | 4.09 | 1.13 | 0.38 | 2.57 | ||
| LFD 20:80 |
Myristica fragrans | 1 mL extract/100 mL | 3.06 | 0.96 | 0.41 | 2.01 | |
RUSITEC: Ruminal simulation technique system; CH4: methane; ND: not determinate; HFD: high fiber diet; LFD: low fiber diet.
Conversely, studies on tropical plants such as G. sepium and E. cyclocarpum, and Y. schidigera, concluded that saponins did not reduce CH4 production under in vitro conditions [77], and no significant effects were reported on ruminal methane production under in vivo conditions when Pelibuey sheep were fed with P. purpureum and supplemented with increasing levels of Yucca schidigera saponins (0, 1.5, 3.0, and 4.5 g/day) [78]. Akanmu et al. [79] reported under in vitro conditions that the addition of 50 mg/kg of Moringa oleifera and Tithonia diversifolia extracts to a forage-based diet reduced CH4 production without adverse effects on feed digestibility.
Pen et al. [80] found that using 2 to 6 mL/L liquid extract of Y. schidigera and Quillaja saponaria induced a partial defaunation of the rumen, a change in the proportion of propionate, a reduction of the ratio of acetate to propionate, and a decrease in CH4 production from 32% to 42%. Similar results have been reported by Bekele et al. [73], who observed a reduction of CH4 of 13% and 34% when adopting Acacia angustisima and Sesbania sesban, respectively. A reduction in CH4 emissions has also been recorded with the use of saponins from Y. schidigera and Q. saponaria as a result of the negative effect on the digestibility of NDF [81], mainly caused by the reduced activity of rumen bacteria during NDF fermentation [59]. Furthermore, a decrease of 10% and 27% of CH4 production was documented in the rumen of goats and sheep, respectively, when saponins from tea leaves were added to their diet [23,70,82].
The daily use of 880 and 2640 mg of saponin from powdered Y. schidigera in bulls increased the proportion of propionate (2.8 y 3.0 mmol) compared to a diet without saponins, which in turn leads to a lower CH4 production. Additionally, it has been demonstrated that the use of 880 mg of saponins reduces protozoa population by 42%, while at a higher dose (2640 mg) no further effects on defaunation were recorded [83]. Likewise, it was recorded that by introducing 187 g DM of leaves of E. cyclocarpum in the ration (14.96 g of saponins) of sheep fed barley silage and concentrate (60:40), it was possible to diminish the protozoa population in the rumen by 25% [21].
CH4 emissions can be reduced up to 70% when feeding goats (8 kg live weight) with G. sepium as a basal ration (214 g DM/day) compared to a control ration [84]. However, the use of 45% of Acacia pennatula and E. cyclocarpum in sheep’s diets did not result in lower CH4 emissions (237 and 219 vs. 196 kJ/mol of the control group) [56].
Experiments on dairy cows recorded no reduction in rumen CH4 when saponins from Y. schidigera and Q. saponaria were added in doses of 10 g/kg of DM [81]. Probably, this is related to the type of saponins since previous studies reported a significant effect on CH4 production using similar doses of another type of saponin [81]. Several authors suggested that the lack of long-term effects of saponins is likely due to the adaptation of rumen microorganisms to these metabolites [85,86]. This finding is supported by the results obtained in steers fed a basal ration (corn and maize silage) with the addition of 1.5%, 1.5%, and 0.5% of saponins from Y. schidigera, Q. Saponaria, and Camelia sinensis, respectively, which indicate that those levels and types of saponins did not affect the daily emission of CH4 [87].
However, the use of Leucaena leucocephala caused a reduction in the daily CH4 emission of 11–31.56% when the legume was increased from 22% to 44% of the total DM intake [42,75,88]. Table 5 and Table 6 show evidence of the effects of saponins from tropical trees on rumen fermentation, rumen microbial population, and CH4 emissions. Diversity of the results are reported in the literature regarding the effect of tropical tree metabolites on ruminal microorganisms and methane emission. Studies are still needed to better understand the action of these compounds in ruminal physiology.
Table 6.
Effect of metabolites of foliage of tropical trees on the rumen microbial population and CH4 reduction.
| Species | Method | Treatments | Protozoa | Bacteria | Metanogens | References |
|---|---|---|---|---|---|---|
| CFU/mL | ||||||
| Basal diet | Sheep fed with concentrate | 00:03 | 138 | 2930 | 452 | [74] |
| B. brizantha: Cratylia argente | 01:02 | 207 | 2530 | 484 | ||
| 02:01 | 154 | 2510 | 517 | |||
| Basal diet | Sheep fed with concentrate plus S. saponaria (7.71 g crude saponins/lamb/day) in each proportion | 00:03 | 50 | 3530 | 493 | |
| Cratylia argentea: B. brizantha | 01:02 | 71 | 4010 | 697 | ||
| 02:01 | 91 | 4180 | 703 | |||
| Control | RUSITEC | 0 | 6.3 | 3500 | 220 | [49] |
| Sapindus saponaria (100 mg fruits/g diet) | 120 mg saponins/g fruit | 12 | 2.9 | 3300 | 210 | |
| Enterolobium cyclocarpum (200 mg fruits/g diet) | 19 mg saponins/g fruit | 3.8 | 9.7 | 3300 | 210 | |
| Pithecellobium saman (200 mg fruit/g diet) | 17 mg saponins/g fruit | 3.4 | 9.7 | 3400 | 230 | |
RUSITEC: Ruminal simulation technique system; CFU: colony forming units. Protozoa numbers × 103; Bacteria and metanogen numbers × 106; Diet: grass hay (620, 555, 498, 494), Arachis pintoi (248, 222, 194, 195), barley straw (120, 112, 100, 100), and urea (12, 11, 8, 11). Control diet (first value) and (second, third, and fourth value) represents inclusion levels g/kg DM diet ingredients in each tropical fruit tree.
7. Conclusions
This paper shows that the use of foliage and fruits from tropical trees as feed for ruminants represents a valuable and sustainable alternative in the developing countries of Latin America, particularly during those seasons characterized by lower forage quality and availability. The presence of secondary metabolites in tropical forage trees, especially saponins and tannins, may be used to manipulate rumen fermentation, partially defaunate the rumen, and, consequently, reduce the emission of enteric CH4 into the environment.
Acknowledgments
We are grateful to the Tecnológico Nacional de México for the facilities granted to hold the meetings that allowed the discussions of this manuscript.
Author Contributions
Conceptualization of review, J.C.-S.; Investigation and redaction A.C.-C., J.C.-S., L.C.-S, F.C.-L. and A.P.-V.; data curation M.C.-N., M.B.-R.; writing—original draft preparation, L.C.-S., M.C.-N and J.C.-M. All authors have read and agreed to the published version of the manuscript. All the authors have been involved in developing, writing, and commenting on the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Flachowsky G. Carbon-footprints for food of animal origin, reduction potentials and research need. J. Appl. Anim. Res. 2011;39:2–14. doi: 10.1080/09712119.2011.570047. [DOI] [Google Scholar]
- 2.Meale S.J., McAllister T.A., Bauchemin K.A., Harstad O.M., Chaves A.V. Strategies to reduce greenhouse gases from ruminant livestock. Acta Agric. Scand. Sect. A Anim. Sci. 2012;62:199–211. doi: 10.1080/09064702.2013.770916. [DOI] [Google Scholar]
- 3.Pachauri R.K., Reisinger A. IPCC Fourth Assessment Report; Proceedings of the 27th Session of the Intergovernmental Panel on Climate Change; Valencia, Spain. 12–17 November 2007. [Google Scholar]
- 4.Gerber P.J., Hristov A.N., Herderson B., Makkar H., Oh J., Lee C., Meinen R., Montes F., Ott T., Firkins J., et al. Technical options for the mitigation of direct methane and nitrous oxide emissions from livestock: A review. Animal. 2013;7:220–234. doi: 10.1017/S1751731113000876. [DOI] [PubMed] [Google Scholar]
- 5.Steinfeld H., Gerber P., Wassenaar T., Castel V., Rosales M., De Haan C. La larga Sombra del Ganado. Problemas Ambientales y Opciones. FAO; Rome, Italy: 2009. p. 431. [Google Scholar]
- 6.McAllister T.A., Newbold C.J. Redirecting rumen fermentation to reduce methanogenesis. Aust. J. Exp. Agric. 2008;48:7–13. doi: 10.1071/EA07218. [DOI] [Google Scholar]
- 7.Eckard R.J., Grainger C., De Klein C.A.M. Options for the abatement of methane and nitrous oxide from ruminant production: A review. Livest. Sci. 2010;130:47–56. doi: 10.1016/j.livsci.2010.02.010. [DOI] [Google Scholar]
- 8.Bonilla C.J.A., Lemus F.C.L. Emisión de metano entérico por rumiantes y su contribución al cambio climático global. Revisión. Rev. Mex. Cienc. Pecu. 2012;3:215–246. [Google Scholar]
- 9.Ramin M., Huhtanen P. Development of equations for predicting methane emissions from ruminants. J. Dairy Sci. 2013;96:2476–2493. doi: 10.3168/jds.2012-6095. [DOI] [PubMed] [Google Scholar]
- 10.McCartney C.A., Bull I.D., Yan T., Dewhurst R.J. Assesment of archaeol as a molecular proxy for methane production in cattle. J. Dairy Sci. 2013;96:1211–1217. doi: 10.3168/jds.2012-6042. [DOI] [PubMed] [Google Scholar]
- 11.Morgavi D.P., Martin C., Boudra H. Fungal secondary metabolites from Monascus spp. reduce rumen methane production in vitro and in vivo. J. Anim. Sci. 2013;91:848–860. doi: 10.2527/jas.2012-5665. [DOI] [PubMed] [Google Scholar]
- 12.Vélez-Terranova M., Campos-Ganoa R., Sánchez-Guerrero H. Use of plant secondary metabolites to reduce ruminal methanogenesis. Trop. Subtrop. Agroecosyst. 2014;17:489–499. [Google Scholar]
- 13.Becholie D., Tamir B., Terrill T.H., Singh B.P., Kassa H. Suitability of tagasaste (Chamaecytisus palmensis L.) as a source of protein supplement to a tropical grass hay fed to lambs. Small Rumin. Res. 2005;56:55–64. doi: 10.1016/j.smallrumres.2004.02.012. [DOI] [Google Scholar]
- 14.Martin C., Morgavi D.P., Doreau M. Methane mitigation in ruminants: From microbe to the farm scale. Animal. 2010;4:351–365. doi: 10.1017/S1751731109990620. [DOI] [PubMed] [Google Scholar]
- 15.DeRamus H.A., Clement T.C., Giampola D.D., Dickison P.C. Methane emissions of beef cattle on forages: Efficiency of grazing management systems. J. Environ. Qual. 2003;32:269–277. doi: 10.2134/jeq2003.2690. [DOI] [PubMed] [Google Scholar]
- 16.Zamora S., García J., Bonilla G., Aguilar H., Harvey C.A., Ibrahim M. Uso de frutos y follaje arbóreo en la alimentación de vacunos en la época seca en Boaco, Nicaragua. Agroforesteria en las Américas. 2001;8:31–38. [Google Scholar]
- 17.Goel G., Makkar H.P.S. Methane mitigation from ruminants using tannins and saponins. Trop. Anim. Health Prod. 2012;44:729–739. doi: 10.1007/s11250-011-9966-2. [DOI] [PubMed] [Google Scholar]
- 18.Phaikaew C., Suksaran W., Ted-Arsen J., Nakamanee G., Saichuer A., Seejundee S., Kotprom N., Shelton H.M. Incidenc of subclinical toxicity in goats and dairy cows consuming leucaena (Leucaena leucocephala) in Thailand. Anim. Prod. Sci. 2012;52:283–286. doi: 10.1071/AN11239. [DOI] [Google Scholar]
- 19.Kamra D.N., Patra A.K., Chatterjee P.N., Kumar R., Agarwal N., Chaudhary L.C. Effect of plant extracts on methanogenesis and microbial profile of the rumen of buffalo: A brief overview. Aust. J. Exp. Agric. 2008;48:175–178. doi: 10.1071/EA07268. [DOI] [Google Scholar]
- 20.Soliva C.R., Zeleke A.B., Clement C., Hess H.D., Fievez V., Kreuzer M. In vitro screening of various tropical foliages, seeds, fruits and medicinal plants for low methane and high ammonia generating potentials in the rumen. Anim. Feed. Sci. Technol. 2008;147:53–71. doi: 10.1016/j.anifeedsci.2007.09.009. [DOI] [Google Scholar]
- 21.Koenig K.M., Ivan M., Teferedegne B.T., Morgavi D.P., Rode L.M., Ibrahim I.M., Newbold C.J. Effect of dietary Enterolobium cyclocarpum on microbial protein flow and nutrient digestibility in sheep maintained fauna-free, with total mixed fauna or with Entodinium caudatum monofauna. Br. J. Nutr. 2007;98:504–516. doi: 10.1017/S0007114507723930. [DOI] [PubMed] [Google Scholar]
- 22.Patra A.K., Saxena J. Dietary phytochemicals as rumen modifiers: A review of the effects on microbial populations. Anthonie Van Leeuwenhoek. 2009;96:363–375. doi: 10.1007/s10482-009-9364-1. [DOI] [PubMed] [Google Scholar]
- 23.Mao H.L., Wang J.K., Zhou Y.Y., Liu J.X. Effects of addition of tea saponins and soybean oil on methane production, fermentation and microbial population in the rumen of growing lambs. Livest. Sci. 2010;129:56–62. doi: 10.1016/j.livsci.2009.12.011. [DOI] [Google Scholar]
- 24.IPCC . A Report of Working Group II of the Intergovernmental Panel on Climate Change. In: McCarthy J.J., Canziani O.F., Leary N.A., Dokken D.J., White K.S., editors. Climate Change 2001: Impacts, Adaptation, and Vulnerability. 1st ed. Cambridge University Press; Cambrige, UK: 2001. pp. 3–18. [Google Scholar]
- 25.Raney T. The State of Food and Agriculture-Livestock in the Balance. Food and Agriculture Organization of the United Nations; Rome, Italy: 2009. p. 164. [Google Scholar]
- 26.Godfray H.C.J., Beddington J.R., Crute I.R., Haddad L., Lawrence D., Muir J., Pretty J., Robinson S., Thomas S., Toulmin C. Food security: The challenge of feeding 9 billion people. Science. 2010;327:812–818. doi: 10.1126/science.1185383. [DOI] [PubMed] [Google Scholar]
- 27.Smith J., Sones K., Grace D., Macmillan S., Tarawali S., Herrero M. Beyond milk, meat, and eggs: Role of livestock in food and nutrition security. Anim. Front. 2013;3:6–13. doi: 10.2527/af.2013-0002. [DOI] [Google Scholar]
- 28.INECC . Inventario Nacional de Emisiones de Gases y Compuestos de Efecto Invernadero 1990–2015 en México. INECC; Mexico City, Mexico: 2015. [Google Scholar]
- 29.SIAP Resumen Nacional 2006–2015. Servicio de Información Agroalimentaria y Pesquera. [(accessed on 28 April 2020)];2016 Available online: https://www.gob.mx/siap/documentos/poblacion-ganadera.
- 30.García-Apaza E., Paz O., Arana I. Greenhouse gas emissions from enteric fermentation of livestock in Bolivia: Values for 1990-2000 and future projections. Aust. J. Exp. Agric. 2008;48:255–259. doi: 10.1071/EA07247. [DOI] [Google Scholar]
- 31.Piñeiro-Vázquez A.T., Canul-Solis J.R., Alayón-Gamboa J.A., Chay-Canul A.J., Ayala-Burgos A.J., Aguilar-Pérez C.F., Solorio-Sánchez F.J., Ku-Vera J.C. Potential of condensed tannins for the reduction of emissions of enteric methane and their effect on ruminant productivity. Archivos de Medicina Veterinaria. 2015;47:263–272. doi: 10.4067/S0301-732X2015000300002. [DOI] [Google Scholar]
- 32.Greening C., Geier R., Wang C., Woods L.C., Morales S.E., McDonald M.J., Rushton-Green R., Morgan X.C., Koike S., Leahy S.C., et al. Diverse hydrogen production and consumption pathways influence methane production in ruminants. ISME J. 2019;13:2617–2632. doi: 10.1038/s41396-019-0464-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Czerkawski J.W. An Introduction to Rumen Studies. 1st ed. Pergamon Press; New York, NY, USA: 1986. p. 236. [Google Scholar]
- 34.Hegarty R., Nolan J. Estimation of Ruminal Methane Production From Measurement of Volatile Fatty Acid Production. In: Makkar H.P., Vercoe P.E., editors. Measuring Methane Production From Ruminants. 1st ed. Springer; Dordrecht, The Netherlands: 2007. pp. 69–92. [Google Scholar]
- 35.Hales K.E., Cole N.A., MacDonald J.C. Effects of increasing concentrations of wet distillers grains with solubles in steam-flaked corn-based diets on energy metabolism, carbon-nitrogen balance, and methane emissions of cattle. J. Anim. Sci. 2013;91:819–828. doi: 10.2527/jas.2012-5418. [DOI] [PubMed] [Google Scholar]
- 36.Kurihara M., Magner T., Hunter R.A., McCrabb G.J. Methane production and energy partition of cattle in the tropics. Br. J. Nutr. 1999;81:227–234. doi: 10.1017/S0007114599000422. [DOI] [PubMed] [Google Scholar]
- 37.Shibata M., Terada F. Factors affecting methane production and mitigation in ruminants. Anim. Sci. J. 2010;81:2–10. doi: 10.1111/j.1740-0929.2009.00687.x. [DOI] [PubMed] [Google Scholar]
- 38.Smith P., Nkem J., Calvin K., Campbell D., Cherubini F., Grassi G., Korotkov V., Hoang A.L., Lwasa S., McElwee P., et al. Interlinkages Between Desertification, Land Degradation, Food Security and Greenhouse Gas Fluxes: Synergies, Trade-offs and Integrated Response Options. In: Shukla P.R., Skea J., Calvo Buendia E., Masson-Delmotte V., Portner H.-O., Roberts D.C., Zhai P., Slade R., Connors S., van Diemen R., et al., editors. Climate Change and Land: An IPCC Special Report on Climate Change, Desertification, Land Degradation, Sustainable Land Management, Food Security, and Greenhouse Gas Fluxes in Terrestrial Ecosystems. 2019. [(accessed on 29 April 2020)]. Available online: https://www.ipcc.ch/srccl/chapter/chapter-6/ [Google Scholar]
- 39.Williams S.R.O., Hannah M.C., Jacobs J.L., Wales W.J., Moate P.J. Volatile fatty acids in ruminal fluid can be used to predict methane yield of dairy cows. Animals. 2019;9:1006. doi: 10.3390/ani9121006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Topps J.H. Potential, composition and use of legume shrubs and trees as fodders for livestock in the tropics. J. Agric. Sci. 1992;118:1–8. doi: 10.1017/S0021859600067940. [DOI] [Google Scholar]
- 41.Ku-Vera J.C., Ayala-Burgos A.J., Solorio-Sánchez F.J., Briceño-Poot; E.G., Ruiz-González A., Piñeiro-Vázquez A.T., Barros-Rodríguez M., Soto-Aguilar A., Espinosa-Hernández J.C.L., Albores-Moreno S., et al. Tropical Tree Foliages and Shrubs As Feed Additives in Ruminants Rations. In: Salem A., editor. Nutritional Strategies of Animal Feed Additives. 1st ed. Nova Science Publishers; New York, NY, USA: 2013. pp. 59–76. [Google Scholar]
- 42.Albores-Moreno S., Alayón-Gamboa J.A., Miranda-Romero L.A., Alarcón-Zúñiga B., Jiménez-Ferrer G., Ku-Vera J.C., Piñeiro-Vázquez A.T. Effect of supplementation with tree foliage on in vitro digestibility and fermentation, synthesis of microbial biomass and methane production of cattle diets. Agrofor. Syst. 2019;51:1–12. doi: 10.1007/s10457-019-00416-1. [DOI] [PubMed] [Google Scholar]
- 43.Valles-de la Mora B., Castillo-Gallegos E., Alonso-Díaz M.Á., Ocaña-Zavaleta E., Jarillo-Rodríguez J. Live-weight gains of Holstein× Zebu heifers grazing a Cratylia argentea/Toledo-grass (Brachiaria brizantha) association in the Mexican humid tropics. Agrofor. Syst. 2017;91:1057–1068. doi: 10.1007/s10457-016-9980-5. [DOI] [Google Scholar]
- 44.González E., Cáceres O. Valor nutritivo de árboles, arbustos y otras plantas forrajeras para los rumiantes. Pastos y Forrajes. 2002;25:15–20. [Google Scholar]
- 45.Pinto-Ruiz R., Hernández D., Gómez H., Cobos M.A., Quiroga R., Pezo D. Árboles forrajeros de tres regiones ganaderas de Chiapas, México: Usos y características nutricionales. Universidad y Ciencia. 2010;26:19–31. [Google Scholar]
- 46.López M.A., Rivera J.A., Ortega L., Escobedo J.G., Magaña M.A., Sanginés J.R., Sierra A.C. Contenido nutritivo y factores antinutricionales de plantas nativas forrajeras del norte de Quintana Roo. Técnica Pecuaria en México. 2008;46:205–215. [Google Scholar]
- 47.Azim A., Khan A.G., Ahmad J., Ayaz M., Mirza I.H. Nutritional evaluation of fodder tree leaves with goats. Asian Australas. J. Anim. Sci. 2002;15:34–37. doi: 10.5713/ajas.2002.34. [DOI] [Google Scholar]
- 48.Delgado D.C., Galindo J., González R., González N., Scull I., Dihigo L., Cairo J., Aldama A.I., Moreira O. Feeding of tropical trees and shrub foliages as a strategy to reduce ruminal methanogenesis: Studies conducted in Cuba. Trop. Anim. Health Prod. 2012;44:1097–1104. doi: 10.1007/s11250-011-0045-5. [DOI] [PubMed] [Google Scholar]
- 49.Hess H.D., Monsalve L.M., Lascano C.E., Carulla J.E., Díaz T.E., Kreuzer M. Supplementation of a tropical grass diet with forage legumes and Sapindus saponaria fruits: Effects on in vitro ruminal nitrogen turnover and methanogenesis. Aust. J. Agric. Res. 2003;54:703–713. doi: 10.1071/AR02241. [DOI] [Google Scholar]
- 50.Fasae O.A., Sowande O.S., Popoola A.A. Evaluation of selected of trees and foliage of shrubs as fodder in ruminant production. J. Agric. Sci. Environ. 2010;10:36–44. [Google Scholar]
- 51.García-Winder L.R., Goñi-Cedeño S., Olguin-Lara P.A., Díaz-Salgado G., Arriaga-Jordan C.M. Huizache (Acacia farnesiana) whole pods (flesh and seed) as an alternative feed for sheep in Mexico. Trop. Anim. Health Prod. 2009;41:1615–1621. doi: 10.1007/s11250-009-9355-2. [DOI] [PubMed] [Google Scholar]
- 52.Brown D., Ng’ambi J.W., Norris D. Effect of tanniniferous Acacia karroo leaf meal inclusion level on feed intake, digestibility and live weight gain of goats fed a Setaria verticillata grass hay-based diet. J. Appl. Anim. Res. 2017;46:248–253. doi: 10.1080/09712119.2017.1289939. [DOI] [Google Scholar]
- 53.Molina-Botero I.C., Arroyave-Jaramillo J., Valencia-Salazar S., Barahona-Rosales R., Aguilar-Pérez C.F., Ayala-Burgos A., Arango J., Ku-Vera J.C. Effects of tannins and saponins contained in foliage of Gliricidia sepium and pods of Enterolobium cyclocarpum on fermentation, methane emissions and rumen microbial population in crossbred heifers. Anim. Feed Sci. Technol. 2019;251:1–11. doi: 10.1016/j.anifeedsci.2019.01.011. [DOI] [Google Scholar]
- 54.Vu C.C., Verstegen M.W.A., Hendriks W.H., Pham K.C. The nutritive Value of Mulberry leaves (Morus alba) and partial replacement of cotton seed in rations on the performance of growing vietnamese cattle. Asian Australas. J. Anim. Sci. 2011;24:1233–1242. doi: 10.5713/ajas.2011.90328. [DOI] [Google Scholar]
- 55.Moscoso C., Vélez M., Flores A., Agudelo N. Effects of Guanacaste tree (Enterolobium cyclocarpum Jacq. Griseb.) fruit as replacement for sorghum grain and cotton-seed meal in lamb diets. Small Rumin. Res. 1995;18:121–124. doi: 10.1016/0921-4488(95)00677-D. [DOI] [Google Scholar]
- 56.Briceño-Poot E.G., Ruiz-González A., Chay-Canul A.J., Ayala-Burgos A.J., Aguilar-Pérez C.F., Solorio-Sánchez F.J., Ku-Vera J.C. Voluntary intake, apparent digestibility and prediction of methane production by rumen stoichiometry in sheep fed pods of tropical legumes. Anim. Feed Sci. Technol. 2012;176:117–122. doi: 10.1016/j.anifeedsci.2012.07.014. [DOI] [Google Scholar]
- 57.Piñeiro-Vázquez A.T., Ayala-Burgos A.J., Chay-Canul A.J., Ku-Vera J.C. Dry matter intake and digestibility of rations replacing concentrates with graded levels of Enterolobium cyclocarpum in Pelibuey lambs. Trop. Anim. Health Prod. 2013;45:577–583. doi: 10.1007/s11250-012-0262-6. [DOI] [PubMed] [Google Scholar]
- 58.Ansari K., Mohammadabadi T., Sari M. The effect of feeding of Albizia lebbeck leaf on fermentation, gas production, digestibility and rumen protozoa of one-humped camel. J. Rumin. Res. 2017;5:117–128. [Google Scholar]
- 59.Francis G., Kerem Z., Makkar H.P., Becker K. The biological action of saponins in animal systems: A review. Br. J. Nutr. 2002;88:587–605. doi: 10.1079/BJN2002725. [DOI] [PubMed] [Google Scholar]
- 60.Wallace R.J., McEwan N.R., McIntosh F.M., Teferedegne B., Newbold C.J. Natural products as manipulators of rumen fermentation. Asian Australas. J. Anim. Sci. 2002;15:10–21. doi: 10.5713/ajas.2002.1458. [DOI] [Google Scholar]
- 61.Wina E., Muetzel S., Becker K. The impact of saponins or saponin-containing plant materials on ruminant production A Review. J. Agric. Food Chem. 2005;53:8093–8105. doi: 10.1021/jf048053d. [DOI] [PubMed] [Google Scholar]
- 62.Galindo J., Marrero Y. Manipulación de la fermentación microbiana ruminal. Revista Cubana de Ciencia Agrícola. 2005;39:439–450. [Google Scholar]
- 63.Ibrahim M., t’Mannetje L., Ospina S. Prospects and Problems in the Utilization of Tropical Herbaceous and Woody Leguminous Forages. In: Ramírez L., Sandoval C., Ku J., editors. VI International Symposium on the Nutrition of Herbivores. Universidad Autónoma de Yucatán; Merida, Mexico: 2003. pp. 35–55. [Google Scholar]
- 64.Podolak I., Galanty A., Sobolewska D. Saponins as cytotoxic agents: A review. Phytochem. Rev. 2010;9:425–474. doi: 10.1007/s11101-010-9183-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hostettmann K., Marston A. Saponins. 1st ed. Cambridge University Press; New York, NY, USA: 2005. pp. 4–20. [Google Scholar]
- 66.Makkar H.P.S., Francis G., Becker K. Bioactivity of phytochemicals in some lesser-known plants and their effects and potential applications in livestock and aquaculture production systems. Animal. 2007;1:1371–1391. doi: 10.1017/S1751731107000298. [DOI] [PubMed] [Google Scholar]
- 67.Galindo J., González N., Sosa A., Ruiz T., Torres V., Aldana A.I., Díaz H., Moreira O., Sarduy L., Noda A.C. Efecto de Tithonia diversifolia (Helms.) Gray (Botón de oro) en la población de protozoos y metanógenos ruminales en condiciones in vitro. Revista Cubana de Ciencia Agrícola. 2011;45:33–37. [Google Scholar]
- 68.Morgavi D.P., Forano E., Martin C., Newbold C.J. Microbial ecosystem and methanogenesis in ruminants. Animal. 2010;4:1024–1036. doi: 10.1017/S1751731110000546. [DOI] [PubMed] [Google Scholar]
- 69.Morgavi D.P., Martin C., Jouany J.P., Ranilla M.J. Rumen protozoa and methanogenesis: Not a simple cause-effect relationship. Br. J. Nutr. 2012;107:388–397. doi: 10.1017/S0007114511002935. [DOI] [PubMed] [Google Scholar]
- 70.Hu W., Liu J., Wu Y., Guo Y., Ye J. Effects of tea saponins on in vitro ruminal fermentation and growth performance in growing Boer goat. Arch. Anim. Nutr. 2006;60:89–97. doi: 10.1080/17450390500353119. [DOI] [PubMed] [Google Scholar]
- 71.Lila Z.A., Mohammed N., Kanda S., Kamada T., Itabashi H. Effect of sarsaponin on ruminal fermentation with particular reference to methane production in vitro. J. Dairy Sci. 2003;86:3330–3336. doi: 10.3168/jds.S0022-0302(03)73935-6. [DOI] [PubMed] [Google Scholar]
- 72.Agarwal N., Kamra D.N., Chaudhary L.C., Patra A.K. Effect of Sapindus mukorossi extracts on in vitro methanogenesis and fermentation characteristics in buffalo rumen liquor. J. Appl. Anim. Res. 2006;30:1–4. doi: 10.1080/09712119.2006.9706814. [DOI] [Google Scholar]
- 73.Bekele A.Z., Clément C., Kreuzer M., Soliva C. Efficiency of Sesbania sesban and Acacia angustissima in limiting methanogenesis and increasing ruminally available nitrogen in a tropical grass-based diet depends on accession. Anim. Prod. Sci. 2009;49:145–153. doi: 10.1071/EA08202. [DOI] [Google Scholar]
- 74.Hess H.D., Beuret R.A., Lötscher M., Hindrichsen I.K., Machmüller A., Carulla J.E., Lasscano C.E., Kreuzer M. Ruminal fermentation, methanogenesis and nitrogen utilization of sheep receiving tropical grass hay-concentrate diets offered with Sapindus saponaria fruits and Cratylia argentea foliage. Anim. Sci. 2004;79:177–189. doi: 10.1017/S1357729800054643. [DOI] [Google Scholar]
- 75.Albores-Moreno S., Alayón-Gamboa J.A., Miranda-Romero L.A., Alarcón-Zúñiga B., Jiménez-Ferrer G., Ku-Vera J.C., Piñeiro-Vázquez A.T. Effect of tree foliage supplementation of tropical grass diet on in vitro digestibility and fermentation, microbial biomass synthesis and enteric methane production in ruminants. Trop. Anim. Health Prod. 2019;51:893–904. doi: 10.1007/s11250-018-1772-7. [DOI] [PubMed] [Google Scholar]
- 76.Sirohi S.K., Goel N., Pandey P. Efficacy of different methanolic plant extracts on anti-methanogenesis, rumen fermentation and gas production kinetics in vitro. Open Vet. J. 2012;2:72–77. [PMC free article] [PubMed] [Google Scholar]
- 77.Canul-Solis J.R., Piñeiro-Vazquez A.T., Chay-Canul A.J., Castillo-Sánchez L.E., Alayón-Gamboa J.A., Ayala-Burgos A.J., Aguilar-Pérez C.F., Pedraza-Beltran P., Castelán-Ortega O.A., Ku-Vera J.C. Effect of the source and concentration of saponins on in vitro and ruminal methane production. Archivos de Zootecnia. 2019;68:362–369. doi: 10.21071/az.v68i263.4194. [DOI] [Google Scholar]
- 78.Canul-Solis J.R., Piñeiro-Vázquez A.T., Briceño-Poot E.G., Chay-Canul A.J., Alayón-Gamboa J.A., Ayala-Burgos A.J., Aguilar-Pérez C.F., Solorio-Sánchez F.J., Castelán-Ortega O.A., Ku-Vera J.C. Effect of supplementation with saponins from Yucca schidigera on ruminal methane production by Pelibuey sheep fed Pennisetum purpureum grass. Anim. Prod. Sci. 2014;54:1834–1837. doi: 10.1071/AN14296. [DOI] [Google Scholar]
- 79.Akanmu A.M., Hassen A., Adejoro F.A. Gas Production, Digestibility and Efficacy of Stored or Fresh Plant Extracts to Reduce Methane Production on Different Substrates. Animals. 2020;10:146. doi: 10.3390/ani10010146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pen B., Takaura K., Yamaguchi S., Asa R., Takahashi J. Effects of Yucca schidigera and Quillaja saponaria with or without β 1–4 galacto-oligosaccharides on ruminal fermentation, methane production and nitrogen utilization in sheep. Anim. Feed Sci. Technol. 2007;138:75–88. doi: 10.1016/j.anifeedsci.2006.11.018. [DOI] [Google Scholar]
- 81.Holtshausen L., Chaves A.V., Beauchemin K.A., McGinn S.M., McAllister T.A., Odongo N.E., Cheeke P.R., Benchaar C. Feeding saponin-containing Yucca schidigera and Quillaja saponaria to decrease enteric methane production in dairy cows. J. Dairy Sci. 2009;92:2809–2821. doi: 10.3168/jds.2008-1843. [DOI] [PubMed] [Google Scholar]
- 82.Zhou Y.Y., Mao H.L., Jiang F., Wang J.K., Liu J.X., McSweeney C.S. Inhibition of rumen methanogenesis by tea saponins with reference to fermentation pattern and microbial communities in Hu sheep. Anim. Feed Sci. Technol. 2011;166:93–100. doi: 10.1016/j.anifeedsci.2011.04.007. [DOI] [Google Scholar]
- 83.Hristov A.N., Mcallister T.A., Van Herk F.H., Cheng K.J., Newbold C.J., Cheeke P.R. Effect of Yucca schidigera on ruminal fermentation and nutrient digestion in heifers. J. Anim. Sci. 1999;77:2554–2563. doi: 10.2527/1999.7792554x. [DOI] [PubMed] [Google Scholar]
- 84.Silivong P., Xaykham O., Aloun O., Preston T.R. Effect of potassium nitrate and urea on feed intake, digestibility, N balance and methane production of goats fed a basal diet of Gliricidia (Gliricidia sepium) and Mimosa (Mimosa pigra) foliages supplemented with molasses. Livest. Res. Rural. Dev. 2012;24 [Google Scholar]
- 85.Newbold C.J., El Hassan S.M., Wang J.M., Ortega M.E., Wallace R.J. Influence of foliage from African multipurpose trees on activity of rumen protozoa and bacteria. Br. J. Nutr. 1997;78:237–249. doi: 10.1079/BJN19970143. [DOI] [PubMed] [Google Scholar]
- 86.Teferedegne B., Mcinthosh F., Osuji P.O., Odenyo A., Wallace R.J., Newbold C.J. Influence of foliage from different accessions of the subtropical leguminous tree, Sesbania sesban, on ruminal protozoa in Ethiopian and Scotish sheep. Anim. Feed Sci. Technol. 1999;78:11–20. doi: 10.1016/S0377-8401(98)00272-7. [DOI] [Google Scholar]
- 87.Li W., Powers W. Effects of saponin extracts on air emissions from steers. J. Anim. Sci. 2012;90:4001–4013. doi: 10.2527/jas.2011-4888. [DOI] [PubMed] [Google Scholar]
- 88.Kennedy P.M., Charmley E. Methane yields from Brahman cattle fed tropical grasses and legumes. Anim. Prod. Sci. 2012;52:225–239. doi: 10.1071/AN11103. [DOI] [Google Scholar]