Abstract
Background
Mesoporous calcium-silicate nanoparticles (MCSNs) have good prospects in the medical field due to their great physicochemical characteristics, antibacterial activity and drug delivery capacity. This study was to analyze the antibiofilm activity and mechanisms of silver (Ag) and zinc (Zn) incorporated MCSNs (Ag/Zn-MCSNs) with different percentages of Ag and Zn.
Methods
Ag/Zn(1:9, molar ratio)-MCSNs and Ag/Zn(9:1, molar ratio)-MCSNs were prepared and characterized. Endocytosis of nanoparticles by Enterococcus faecalis (E. faecalis) treated with Ag/Zn-MCSNs was observed using TEM to explore the antibacterial mechanisms. The antibiofilm activity of Ag/Zn-MCSNs with different ratios of Ag and Zn was tested by E. faecalis biofilm model in human roots. The human roots pretreated by different Ag/Zn-MCSNs were cultured with E. faecalis. Then, SEM and CLSM were used to observe the survival of E. faecalis on the root canal wall. Cytotoxicity of the nanoparticles was tested by CCK8 kits.
Results
The Ag/Zn-MCSNs release Ag+ and destroy the cell membranes to kill bacteria. The MCSNs containing Ag showed antibacterial activity against E. faecalis biofilms in different degrees, and they can adhere to dentin surfaces to get a continuous antibacterial effect. However, MTA, MCSNs and Zn-MCSNs could not disrupt the bacterial biofilms obviously. MCSNs, Ag/Zn(1:1, molar ratio)-MCSNs and Ag/Zn(1:9)-MCSNs showed no obvious cytotoxicity, while Ag-MCSNs and Ag/Zn(9:1)-MCSNs showed cytotoxicity. Zn-MCSNs can slightly promote cell proliferation.
Conclusion
Ag/Zn-MCSNs have good antibiofilm activity. They might achieve an appropriate balance between the antibacterial activity and cytotoxicity by adjusting the ratio of Ag and Zn. Ag/Zn-MCSNs are expected to be a new type of root canal disinfectant or sealer for root canal treatment.
Keywords: antibacterial mechanism, antibiofilm, mesoporous calcium-silicate nanoparticles, silver, zinc
Introduction
Pulp and periapical diseases are mainly attributed to bacterial biofilm infection. Due to the anatomical complexity of the root canal system, dentin structure and composition and limitations of chemical disinfectants, bacteria and bacterial biofilms can persist in the root canal and dentin tubules for a long time, which are difficult to be completely removed even after thoroughly chemo-mechanical preparation.1 Under appropriate conditions, residual bacteria in irregular areas of the root canal or dentin tubules may cause reinfection of the root canal and lead to failure of root canal treatment.2 Intracanal medication is an important disinfection way used between appointments of endodontic treatment. Calcium hydroxide (CH) is the commonly used intracanal medication, and it releases hydroxyl in liquid, resulting in antimicrobial and endotoxin neutralizing effects. However, dentin, exudates from the periapical area, microbial biomass and residual necrotic pulp can inactivate the antimicrobial activity of CH.3 CH has low solubility and diffusibility, which make it difficult to eliminate the bacteria located within dentinal tubules and anatomical variations of canals.4 In addition, dentin exposed to CH over time may show reduced flexural strength and low fracture resistance, which will increase the risk of tooth fracture and endodontic treatment failure.5,6
Nanomaterials and nanotechnology have been widely studied in the medical field. Nanoparticles can efficiently transport drugs to the target sites and be used in targeted therapies for a variety of cancers.7,8 As antibiotic resistance has become a global issue, in order to avoid or reduce the use of antibiotics, nanomaterials have recently been widely studied to find an alternative to antibiotics due to that microorganisms are unlikely to develop resistance against nanoparticles.9,10 Mesoporous calcium-silicate nanoparticles (MCSNs) are newly synthesized advanced materials with multi-functions for root canal filling because of their special nanostructure, injectability, mineralization of apatite, antibacterial capability and drug delivery.11 Recent studies have shown that nano-size MCSNs can infiltrate into bacteria and continuously release calcium ions (Ca2+) and silicon ions (SiO44−), creating a weak alkaline microenvironment to achieve antibacterial effects. MCSNs can also promote bone generation and defect repair. They are considered as excellent platforms for the efficient delivery of drugs and osteogenesis.12 However, the antibacterial activity of MCSNs is limited, which cannot completely remove the bacteria and their biofilms in the root canals, such as Enterococcus faecalis (E. faecalis) and Staphylococcus aureus (S. aureus).13,14
Silver ions (Ag+) are a great substitution to antibiotics because they have broad-spectrum antimicrobial activity and show no resistance to target bacteria.15 Silver nanoparticles (AgNPs) have been used for medical and dental field because of its outstanding antibacterial characteristics. Incorporation of AgNPs to biomaterials can prevent or reduce the formation of biofilms without affecting the mechanical properties of the materials.16 Zinc (Zn) is an important trace element for protein and DNA synthesis, cell mitosis and proliferation and the proliferation and differentiation of osteoblasts.17 Zinc ions (Zn2+) can inhibit enzymatic activity and prevent cell metabolism above the threshold of ion concentration.18 In addition, Zn2+ can depolarize the cell membrane of bacteria, and Ag+ and Zn2+ may have synergic antibacterial effects on E. faecalis and its biofilms.19
Ag and Zn can incorporate with MCSNs and release in a sustained manner. Ag/Zn-MCSNs can adhere to the root canal walls well and infiltrate into the dentinal tubules. They will not have a negative impact on the mechanical properties of dentin after sealing in the root canal for 30 days.5 However, the antibacterial effects and mechanisms of Ag/Zn-MCSNs in different proportions of Ag and Zn are still unclear. The aim of this study was to analyze the cytotoxicity, antibacterial activity and mechanisms of Ag/Zn-MCSNs with different percentages of Ag and Zn. The experimental findings will provide reference for the application of Ag/Zn-MCSNS in root canal infection.
Materials and Methods
Synthesis of the Nanoparticles
MCSNs, Ag-MCSNs, Zn-MCSNs and Ag/Zn(1:1, molar ratio)-MCSNs were synthesized and characterized in our previous studies.5 Ag/Zn(1:9, molar ratio)-MCSNs and Ag/Zn(9:1, molar ratio)-MCSNs were synthesized by the template method according to previous methods.5,13 Different weights of silver nitrate and zinc nitrate (Reagent No.1 Factory of Shanghai Chemical Reagent Co. Ltd, China) were added (Table 1).
Table 1.
The Molar Ratio of Ag/Zn and the Weight of Silver Nitrate and Zinc Nitrate Added in the Synthesis of the Nanoparticles
| Nanoparticles | Ag:Zn (Molar) | Silver Nitrate | Zinc Nitrate |
|---|---|---|---|
| Ag/Zn-MCSNs | 1:9 | 0.238 g | 3.75 g |
| Ag/Zn-MCSNs | 9:1 | 2.14g | 0.416g |
Abbreviation: Ag/Zn-MCSNs, nanosilver- and nanozinc-incorporated MCSNs.
The prepared Ag/Zn(1:9)-MCSNs and Ag/Zn(9:1)-MCSNs were characterized by transmission electron microscopy (TEM, JEM-2100, JEOL, Tokyo, Japan), field emission scanning electron microscopy (FE-SEM, 1530 VP, LEO, Germany) and energy dispersive spectrometry (EDS, I MCA 300, OXFORD, UK). The surface area, pore volume and pore size distribution according to N2 adsorption-desorption isotherms (ASAP 2020 Micromeritics, Norcross, GA, USA) were measured using Brunauer-Emmett-Teller (BET) and Barrett-Joyner-Halenda (BJH) analyses. The ions release (ICP-AES, Prodigy 7; Leeman, USA) and pH measurement (SIN-PH100, Sinomeasure, China) of the prepared nanoparticles were tested according to our previous methods.5
Endocytosis of Nanoparticles by E. faecalis
One milliliter E. faecalis suspension (1×108 CFUs/mL) was co-cultured with 10 mg of MCSNs, Ag-MCSNs, Zn-MCSNs, Ag/Zn(1:1)-MCSNs, Ag/Zn(1:9)-MCSNs and Ag/Zn(9:1)-MCSNs for 24 hrs, respectively. After diluting, the nanoparticle-treated culture was gained by centrifugation at 3000 rpm for 10 mins. Each sample was prepared for TEM observation using standard processes, including fixation, staining, dehydration, infiltration with polymer resin, oven curing, and slicing via ultra-microtome (LEICA EM UC7, Leica, Germany). Ultrathin sections were stained with uranyl acetate and lead citrate and were observed using the TEM (JEOL JEM-1010, JEOL, Japan) at 80 kV.
Antimicrobial Effects of the Nanoparticles
E. faecalis (ATCC 29212, Manassas, VA, USA) suspension was diluted to 1×104 colony forming units (CFUs)/mL. Then, 1 mL suspension was mixed with 10 mg of all prepared nanoparticles and CH, respectively and incubated at 4°C for 24 hrs. Then, 10 μL inoculums were plated on brain heart infusion (BHI, OXOID, Basingstoke, UK) agar plate and incubated at 37°C for 24 hrs. Finally, CFUs of E. faecalis were counted by Automatic colony counter (Scan 1200, Interscience, France). The test was repeated 6 times for each group.
Antibiofilm Activity of the Nanoparticles Against E. faecalis Biofilms
Mature human mandibular premolars with single root were collected under the protocol approved by the Ethical Committee Department, the Affiliated Stomatological Hospital of Nanjing Medical University (PJ 2017–055-001). All clinical investigations have been conducted according to the principles expressed in the Declaration of Helsinki, and the written informed consents have been obtained from the participants. The crowns were removed, and roots were standardized to 12 mm long from root apex. The root canals were prepared using ProTaper NiTi rotary instruments (Dentsply Maillefer, Tochigi, Japan) to F3 size according to standard processes. For sterilization, they were autoclaved at 121ºC for 20 mins. Then, they were placed in 3 mL of E. faecalis suspension (1×108 CFUs/mL) and cultured under anaerobic conditions for 4 weeks at 37°C. The BHI broth was refreshed every second day to remove dead cells and to ensure bacterial viability. CH and MTA pastes (mixed with sterile ddH2O=1:1.5), MCSNs, Ag-MCSNs, Zn-MCSNs, Ag/Zn(1:1)-MCSNs, Ag/Zn(1:9)-MCSNs and Ag/Zn(9:1)-MCSNs pastes (mixed with sterile ddH2O=1:3) were prepared. The pastes were filled into the root canals by lentulo-spirals (Mani Inc, Tochigi-ken, Japan). Then, the roots were placed in sterile tubes. Five specimens were tested in each group. After 7 days, each canal was gently washed with 10 mL phosphate buffer saline (PBS, Gibco, USA) to clear the intracanal paste and dried with sterile paper points.
Afterwards, the specimens from each group were split into two halves. One root-half randomly selected from each root was scanned with a SEM (HITACHI SU3500, Tokyo, Japan) or a FE-SEM (QUANTA 200F, Fei, USA) according to previously described method.13 The other root-half was stained with fluorescent LIVE/DEAD BacLight Bacterial Viability stain (Molecular Probes, Eugene, OR, USA) according to the instructions of manufacturer. Three randomly selected canal wall areas of each root were scanned by a confocal laser scanning microscope (CLSM, LSM 710, Carl Zeiss, Germany) with a 5 μm step size at 20×lens. The excitation/emission wavelengths were 488/525 nm for SYTO® 9 and 561/642 nm for PI. Simultaneous dual-channel imaging was used to display the green fluorescence (live bacteria) and red fluorescence (dead bacteria) using the ZEN software (Carl Zeiss, Germany).
Bacterial Colonization on Root Canal Walls Pretreated with the Nanoparticles
Forty roots were prepared and autoclaved as described above. Each prepared canal was filled with 10 mg/mL suspension of CH, MCSNs, Ag-MCSNs, Zn-MCSNs, Ag/Zn(1:1)-MCSNs, Ag/Zn(1:9)-MCSNs and Ag/Zn(9:1)-MCSNs and activated by the ultrasonic device (P5XS, Satelec, Cedex, France). The ultrasonic device was set at scale 4, and two 30-second sessions of vibration were applied. All roots were stored in a 100% humid environment at 37°C for 7 days. Then, each canal was washed with 10 mL PBS to remove the intracanal medication. All pretreated specimens were immersed in 3 mL E. faecalis suspension (1 × 108 CFUs/mL) at 37°C for 7 days. Afterwards, each root was split into two halves, one root-half randomly selected from each root was observed by the FE-SEM (QUANTA 200F, Fei, USA) and the other root-half was assessed using the CLSM (LSM 710, Carl Zeiss, Germany) according to the methods mentioned above.
Cytotoxicity Test of the Nanoparticles
The extracts were prepared by adding the nanoparticles to α-MEM medium (Gibco/Thermo Scientific, Grand island, USA) at 10 mg/mL and incubated at 37°C for 24 hrs, respectively. Then, the supernatant was sterilized using a 0.22 μm filter (Merck Millipore Ltd., Darmstadt, Germany) after centrifugated at 1000 rpm for 5 mins, and supplemented with 10% fetal bovine serum (FBS, ScienCell, SanDiego, USA).
Mouse pre-osteoblast cell line (MC3T3-E1, ATCC) cells were cultured in α-MEM medium containing 10% FBS and 100 IU/mL penicillin-streptomycin (HyClone, Utah, USA) at 37°C in a 5% CO2 atmosphere. MC3T3-E1 cells were inoculated at density of 2×103 cells/well in 100 μL fresh medium for 48 hrs. Then, they were treated with different extracts, respectively. After 1, 3 and 7 d, the cells were washed with fresh α-MEM. They were incubated with 100 μL α-MEM and 10 μL CCK-8 solution (Dojindo Laboratories, Kumamoto, Japan) in each well at 37°C and 5% CO2 for 4 hrs. The absorbance at 450 nm was measured by a spectrophotometer (Spectramax190, Molecular Devices, USA). The cells not treated by extracts were used as controls. The results of optical density (OD) were obtained in sextuplicate.
Statistical Analysis
All data were showed as means ± standard deviation (SD) and analyzed using One-Way ANOVA with a Post Hoc test by SPSS 22.0 (SPSS Inc., Chicago, IL). The level of significance was set at P < 0.05.
Results
Characterizations
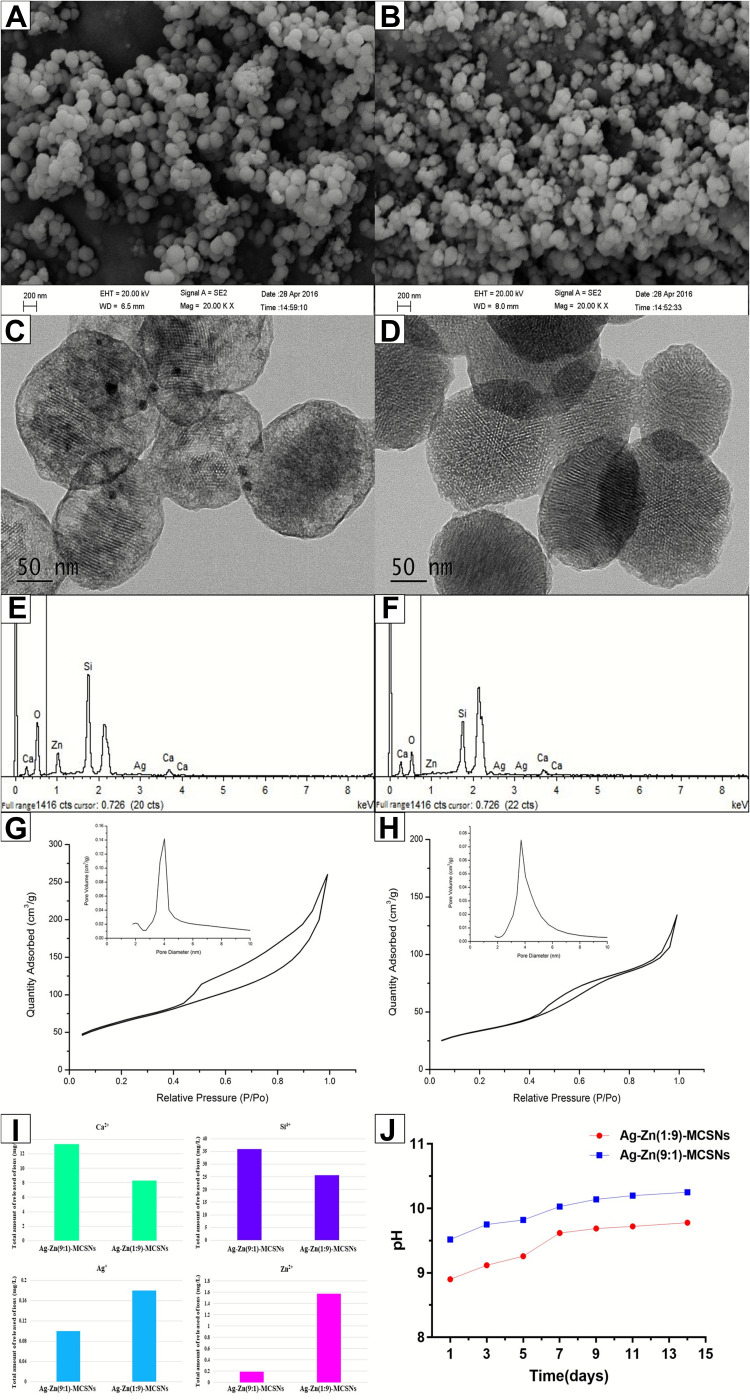
Ag/Zn(1:9)-MCSNs and Ag/Zn(9:1)-MCSNs possessed spherical morphology with 200–250 nm diameter (Figure 1A and B), well-ordered nanopores and channel structures (Figure 1C and D). Ca, Si, Ag and Zn elements present in both nanoparticles (Figure 1E and F). The nanoparticles showed type IV isotherms with H1-type hysteresis loops (Figure 1G and H). The surface area, pore volume and mean pore size of the nanoparticles are showed in Table 2. Ag/Zn(9:1)-MCSNs showed a faster release of Ag+ and a slower release of Zn2+ than Ag/Zn(1:9)-MCSNs (Figure 1I). The pH values of the nanoparticles gradually increased within 14 days and were stabilized at 10. The addition of Ag did not significantly affect the pH while Zn slightly reduced the pH of the MCSNs (Figure 1J).
Figure 1.
Physicochemical properties of the prepared nanoparticles.
Notes: FE-SEM images of Ag/Zn(1:9)-MCSNs and Ag/Zn(9:1)-MCSNs (A and B); TEM images of Ag/Zn(1:9)-MCSNs and Ag/Zn(9:1)-MCSNs (C and D); EDS of Ag/Zn(1:9)-MCSNs and Ag/Zn(9:1)-MCSNs (E and F); nitrogen adsorption-desorption isotherm test and pore size distribution of Ag/Zn(1:9)-MCSNs and Ag/Zn(9:1)-MCSNs (G and H); ions release of Ag/Zn(1:9)-MCSNs and Ag/Zn(9:1)-MCSNs (I); pH measurement of Ag/Zn(1:9)-MCSNs and Ag/Zn(9:1)-MCSNs (J).
Abbreviations: Ag/Zn-MCSNs, nanosilver- and nanozinc-incorporated mesoporous calcium-silicate nanoparticles; FE-SEM, field emission scanning electron microscopy; TEM, transmission electron microscopy; EDS, energy dispersive spectrometry.
Table 2.
Surface Area (SBET), Pore Volume (VP), and Mean Pore Size (DP) of the Nanoparticles
| Nanoparticles | SBET (m²/g) | VP (cm³/g) | DP (nm) |
|---|---|---|---|
| Ag/Zn(9:1)-MCSNs | 117.90 | 0.21 | 7.05 |
| Ag/Zn(1:9)-MCSNs | 225.96 | 0.40 | 7.12 |
Abbreviation: Ag/Zn-MCSNs, nanosilver- and nanozinc-incorporated MCSNs.
Endocytosis of Nanoparticles by E. faecalis
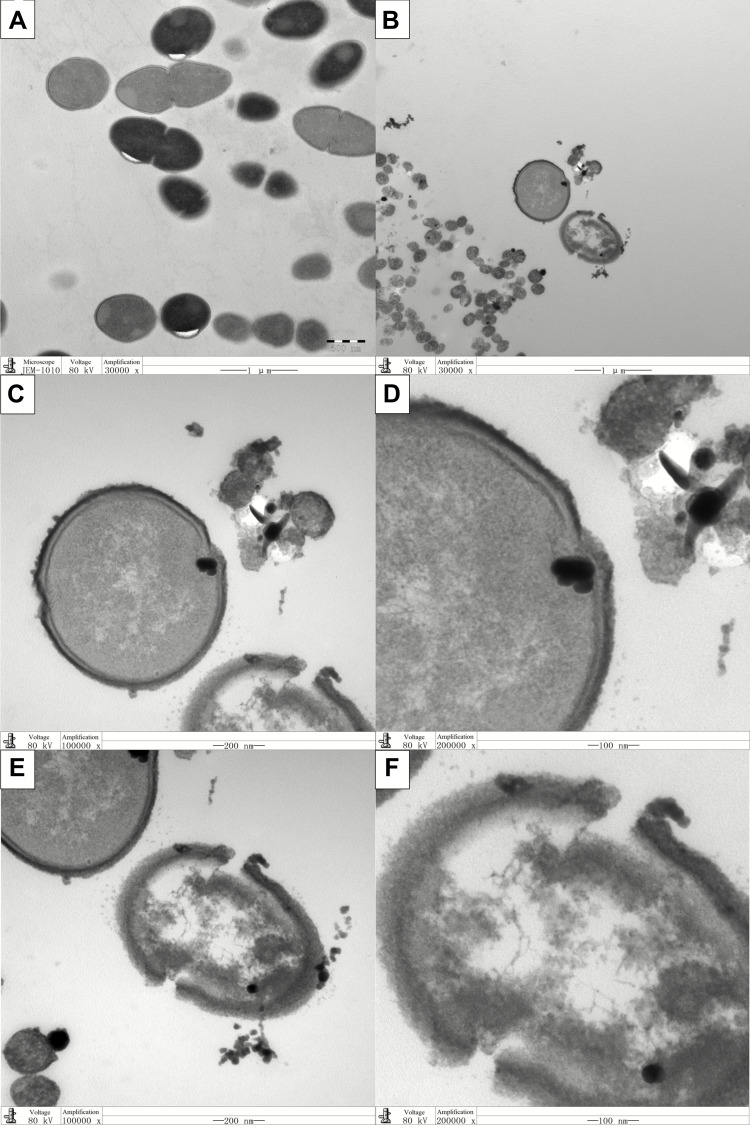
Live E. faecalis showed typical spherical structure and intact cell membrane in the TEM images (Figure 2A). For coculture groups, the destruction of cell walls and cell membranes was observed in Ag-MCSNs, Ag/Zn(1:1)-MCSNs, Ag/Zn(1:9)-MCSNs and Ag/Zn(9:1)-MCSNs groups. Meanwhile, Ag particles were observed to break through the cell membrane and enter the cell. The cell contents leaked from the rupture of the cell membrane (Figure 2B-F). Vacuolar cells without cellular contents can be observed. However, these phenomena were not distinctly observed in the MCSNs and Zn-MCSNs groups.
Figure 2.
Representative TEM images of endocytosis of nanoparticles by E. faecalis.
Notes: (A) E. faecalis (×30,000); (B–F) E. faecalis cocultured with Ag/Zn(1:1)-MCSNs. ×30,000 (B); ×100,000 (C and E); ×200,000 (D and F).
Abbreviations: TEM, transmission electron microscopy; Ag/Zn-MCSNs, nanosilver- and nanozinc-incorporated mesoporous calcium-silicate nanoparticles.
Antibacterial Effects of the Nanoparticles
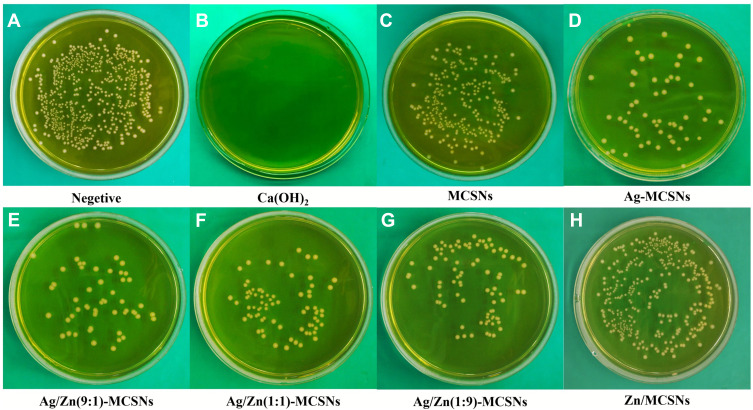
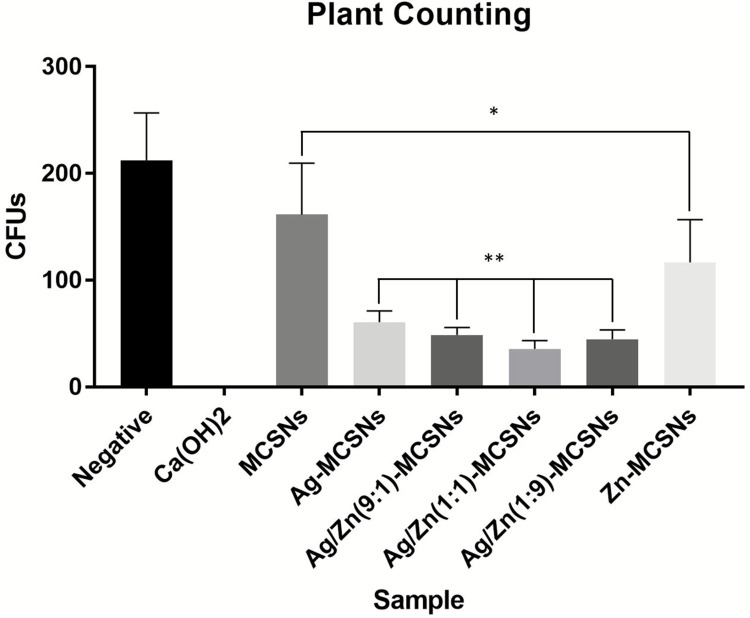
Negative control group showed numerous bacteria on the BHI agar plate (Figure 4A). CH showed the best antibacterial activity among the experimental groups (Figures 3 and 4B). Ag-MCSNs, Ag/Zn(1:1)-MCSNs, Ag/Zn(1:9)-MCSNs and Ag/Zn(9:1)-MCSNs revealed better antimicrobial activity than MCSNs and Zn-MCSNs (P<0.05) (Figures 3 and 4C–H).
Figure 4.
Representative colonial pictures of the materials against planktonic E.faecalis.
Notes: (A) negative control group; (B) CH group; (C) MCSNs group; (D) Ag-MCSNs group; (E) Ag/Zn(9:1)-MCSNs group; (F) Ag/Zn(1:1)-MCSNs group; (G) Ag/Zn(1:9)-MCSNs group; (H) Zn-MCSNs group.
Abbreviations: CH, calcium hydroxide; MCSNs, mesoporous calcium-silicate nanoparticles; Ag-MCSNs, nanosilver-incorporated MCSNs; Ag/Zn-MCSNs, nanosilver- and nanozinc-incorporated MCSNs; Zn-MCSNs, nanozinc-incorporated MCSNs.
Figure 3.
Antibacterial effects of the materials against planktonic E. faecalis.
Notes: Comparisons of CFUs count among the groups, including Negative, CH, MCSNs, Ag-MCSNs, Ag/Zn(9:1)-MCSNs, Ag/Zn(1:1)-MCSNs, Ag/Zn(1:9)-MCSNs and Zn/MCSNs. *P<0.05 when compared with the negative control group. **P<0.05 when compared with the MCSNs group.
Abbreviations: CFUs, colony forming units; CH, calcium hydroxide; MCSNs, mesoporous calcium-silicate nanoparticles; Ag-MCSNs, nanosilver-incorporated MCSNs; Ag/Zn-MCSNs, nanosilver- and nanozinc-incorporated MCSNs; Zn-MCSNs, nanozinc-incorporated MCSNs.
Antibiofilm Activity of the Nanoparticles Against E. faecalis Biofilms
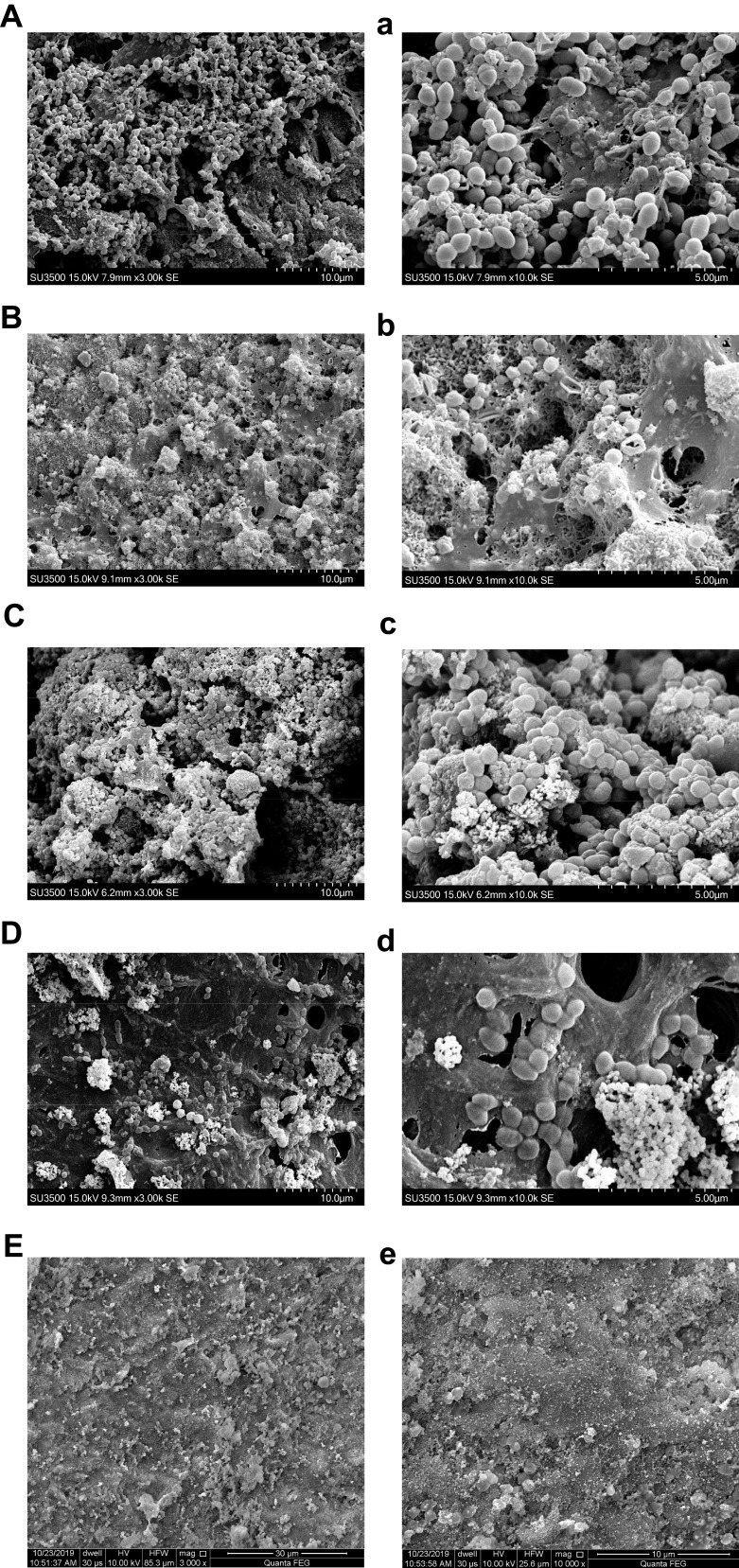
SEM images showed homogenous and dense E. faecalis biofilms on dentin surface in the negative group (Figure 5A and a). E. faecalis biofilms treated with CH showed deformation and rupture (Figure 5B and b), while MTA did not destroy the E. faecalis biofilm structures (Figure 5C and c). The biofilms treated with MCSNs, Ag-MCSNs, Zn-MCSNs, Ag/Zn(1:1)-MCSNs, Ag/Zn(1:9)-MCSNs and Ag/Zn(9:1)-MCSNs exhibited different degrees of structural damages (Figure 5D–I and d–i). Ag-MCSNs, Ag/Zn(1:1)-MCSNs, Ag/Zn(1:9)-MCSNs and Ag/Zn(9:1)-MCSNs can destroy most of E. faecalis and its biofilms. But there are still intact E. faecalis biofilms observed in the MCSNs and Zn-MCSNs groups.
Figure 5.
Continued.
Figure 5.
Representative E. faecalis biofilms (4-week old) on dentin surface after medication were scanned by SEM or FE-SEM (capital letters: ×3000 magnification; small letters: ×10,000 magnification).
Notes: (A and a) Negative control group; (B and b) CH group; (C and c) MTA group; (D and d) MCSNs group; (E and e) Ag-MCSNs group; (F and f) Zn-MCSNs group; (G and g) Ag/Zn(1:9)-MCSNs group; (H and h) Ag/Zn(1:1)-MCSNs group; (I and i) Ag/Zn(9:1)-MCSNs group.
Abbreviations: SEM, scanning electron microscopy; FE-SEM, field emission scanning electron microscopy; CH, calcium hydroxide; MTA, mineral trioxide aggregate; MCSNs, mesoporous calcium-silicate nanoparticles; Ag-MCSNs, nanosilver-incorporated MCSNs; Ag/Zn-MCSNs, nanosilver- and nanozinc-incorporated MCSNs; Zn-MCSNs, nanozinc-incorporated MCSNs.
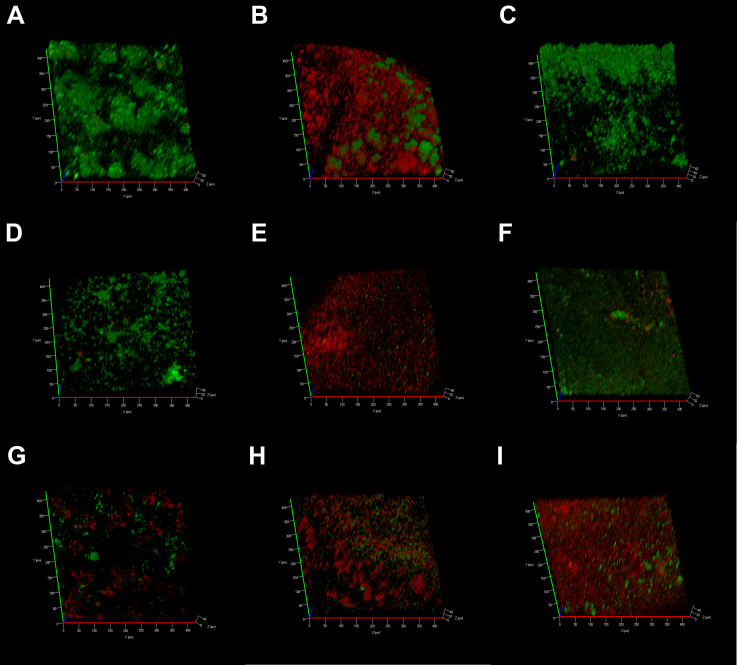
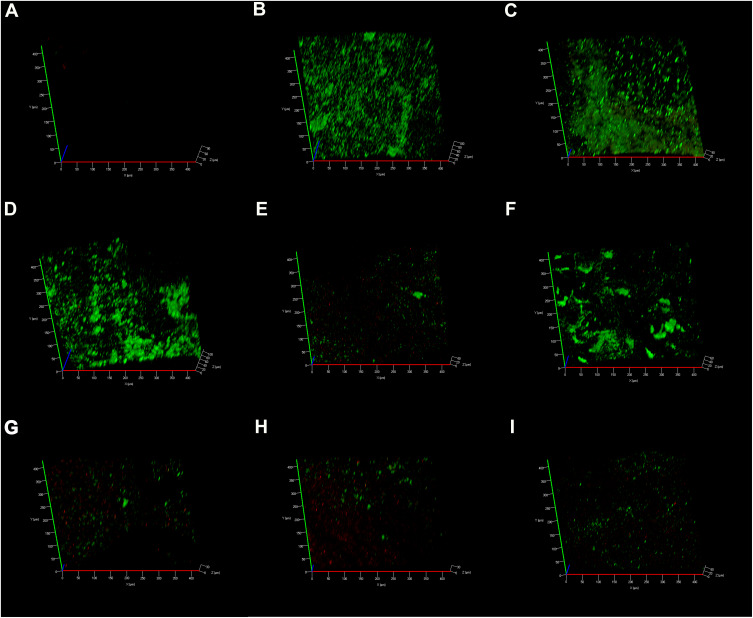
In the negative control group, CLSM images displayed a high percentage of live bacteria in the E. faecalis biofilms (Figure 6A). Most dead bacteria with few live bacteria were observed in CH group (Figure 6B). The treatment with MTA, MCSNs and Zn-MCSNs revealed a high percentage of green fluorescence in the biofilms (Figure 6C, D and F). Large areas of red fluorescence and a small amount of scattered green fluorescence were observed in Ag-MCSNs, Ag/Zn(1:1)-MCSNs and Ag/Zn(9:1)-MCSNs groups (Figure 6E, H and I). The staggered existing red fluorescence and green fluorescence were observed in Ag/Zn(1:9)-MCSNs group showing a certain antibiofilm ability (Figure 6G).
Figure 6.
CLSM 3-dimensional reconstructions of E. faecalis biofilms (4-week old) after medication (green: live cells; red: dead cells).
Notes: (A) Negative control group; (B) CH group; (C) MTA group; (D) MCSNs group; (E) Ag-MCSNs group; (F) Zn-MCSNs group; (G) Ag/Zn(1:9)-MCSNs group; (H) Ag/Zn(1:1)-MCSNs group; (I) Ag/Zn(9:1)-MCSNs group.
Abbreviations: CLSM, confocal laser scanning microscope; CH, calcium hydroxide; MTA, mineral trioxide aggregate; MCSNs, mesoporous calcium-silicate nanoparticles; Ag-MCSNs, nanosilver-incorporated MCSNs; Ag/Zn-MCSNs, nanosilver- and nanozinc-incorporated MCSNs; Zn-MCSNs, nanozinc-incorporated MCSNs.
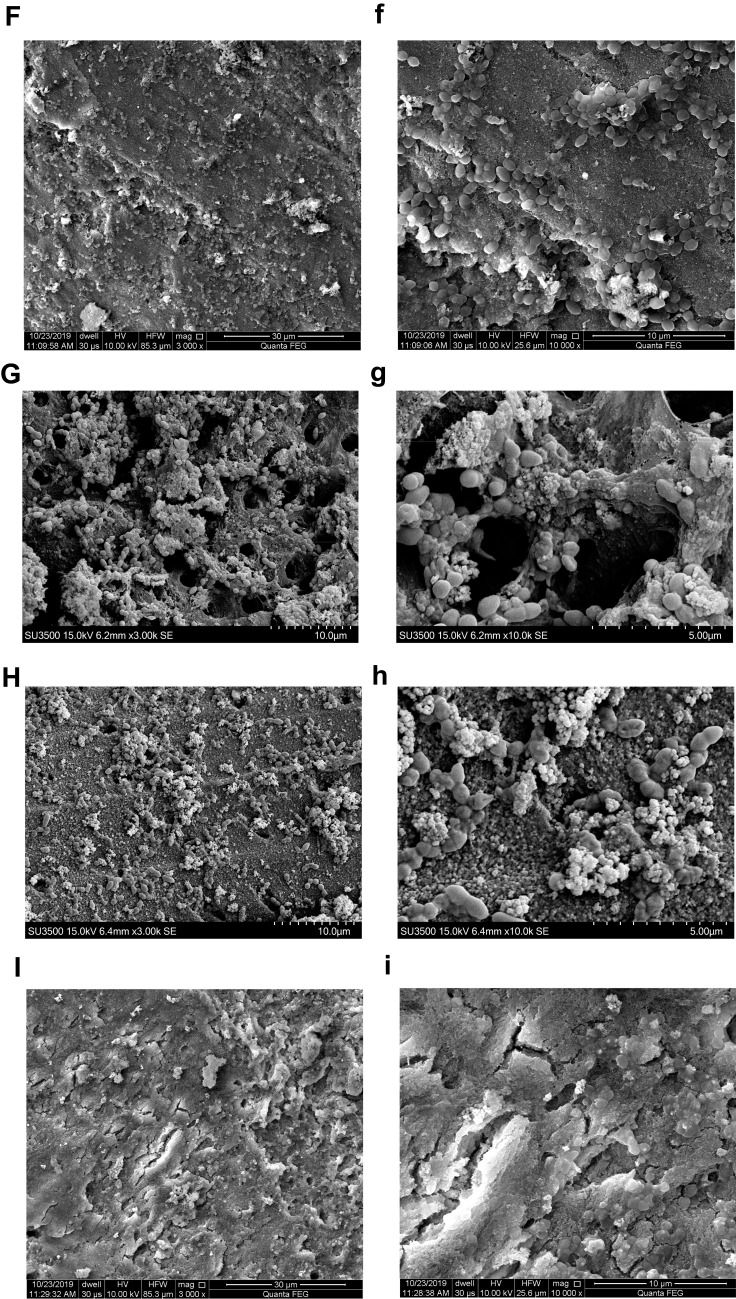
Bacterial Colonization on Root Canal Walls Pretreated with the Nanoparticles
FE-SEM images showed that innumerable E. faecalis colonized on the canal walls in negative control, CH, MCSNs and Zn-MCSNs groups (Figure 7A–C, E and a–c, e). Furthermore, the canal pretreated with CH and Zn-MCSNs seemed to attract more E. faecalis than negative group (Figure 7B, E and b, e). Few bacteria colonization was observed in Ag-MCSNs, Ag/Zn(1:9)-MCSNs, Ag/Zn(1:1)-MCSNs and Ag/Zn(9:1)-MCSNs groups (Figure 7D, F–H and d, f–h). Many nanoparticles adhered to dentin on root canal walls in experimental groups (Figure 7C–H and c–h).
Figure 7.
Representative FE-SEM images showing colonization of E. faecalis on the pretreated root canal walls (capital letters: ×3000 magnification; small letters: ×10,000 magnification).
Notes: (A and a) Negative control group; (B and b) CH group; (C and c) MCSNs group; (D and d) Ag-MCSNs group; (E and e) Zn-MCSNs group; (F and f) Ag/Zn(1:9)-MCSNs group; (G and g) Ag/Zn(1:1)-MCSNs group; (H and h) Ag/Zn(9:1)-MCSNs group.
Abbreviations: FE-SEM, field emission scanning electron microscopy; CH, calcium hydroxide; MCSNs, mesoporous calcium-silicate nanoparticles; Ag-MCSNs, nanosilver-incorporated MCSNs; Ag/Zn-MCSNs, nanosilver- and nanozinc-incorporated MCSNs; Zn-MCSNs, nanozinc-incorporated MCSNs.
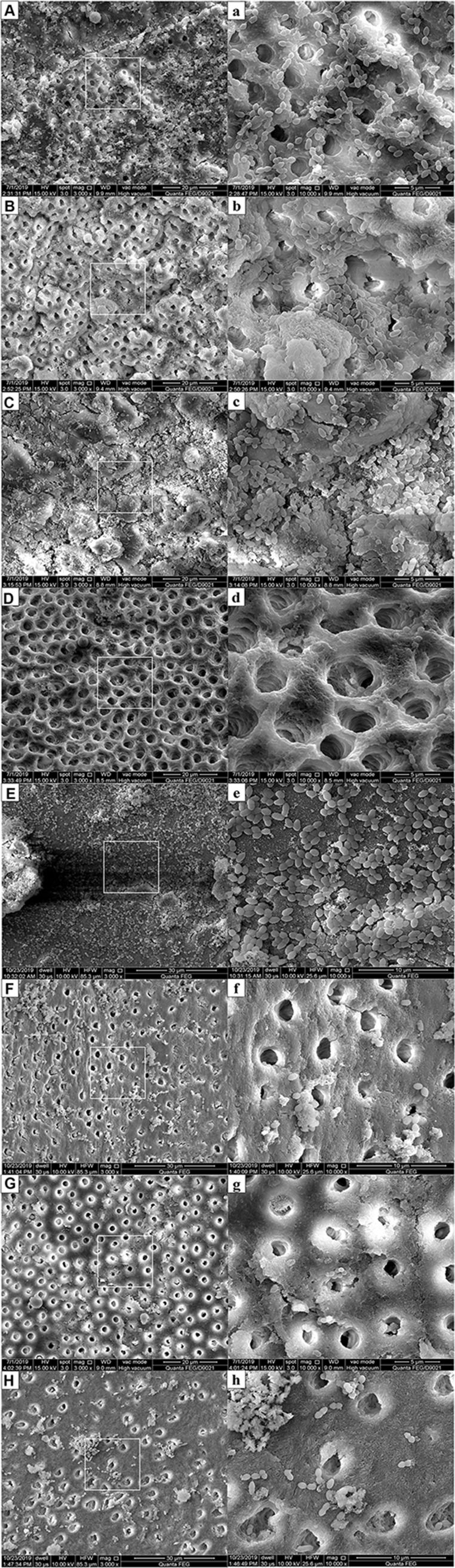
CLSM images showed no background fluorescence produced by the root canal wall (Figure 8A), strong green fluorescence was observed on the canal walls in negative control, CH and MCSNs groups (Figure 8B–D). Weak green fluorescence and scattered red fluorescence were observed in Ag-MCSNs, Ag/Zn(1:9)-MCSNs, Ag/Zn(1:1)-MCSNs and Ag/Zn(9:1)-MCSNs groups, which indicated very few bacteria survival and colonization (Figure 8E, G–I). For Zn-MCSNs group, some patches of strong green fluorescence can be seen on the pretreated root canal walls (Figure 8F).
Figure 8.
E. faecalis adherence and colonization on the pretreated root canal walls observed by CLSM (green: live cells; red: dead cells).
Notes: (A) Blank control group (no background fluorescence produced by dentin); (B) Negative group; (C) CH group; (D) MCSNs group; (E) Ag-MCSNs group; (F) Zn-MCSNs group; (G) Ag/Zn(1:9)-MCSNs group; (H) Ag/Zn(1:1)-MCSNs group; (I) Ag/Zn(9:1)-MCSNs group.
Abbreviations: CLSM, confocal laser scanning microscope; CH, calcium hydroxide; MCSNs, mesoporous calcium-silicate nanoparticles; Ag-MCSNs, nanosilver-incorporated MCSNs; Ag/Zn-MCSNs, nanosilver- and nanozinc-incorporated MCSNs; Zn-MCSNs, nanozinc-incorporated MCSNs.
Cytotoxicity of the Nanoparticles
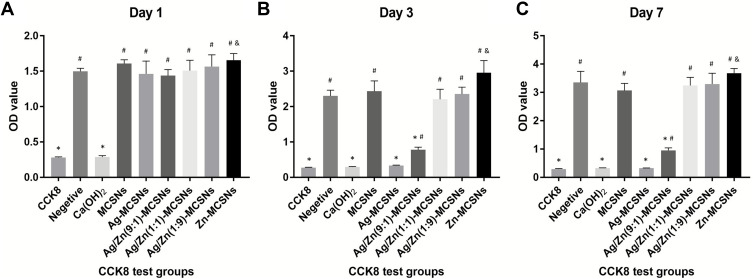
After 1 d incubation, MCSNs, Ag-MCSNs, Ag/Zn(1:1)-MCSNs, Ag/Zn(1:9)-MCSNs and Ag/Zn(9:1)-MCSNs showed no obvious cytotoxicity to MC3T3-E1 cells (P>0.05), while Zn-MCSNs can slightly promote cell proliferation (P<0.05). However, CH showed obvious cytotoxicity (P<0.05) (Figure 9A). After 3 d incubation, Ag-MCSNs showed distinct cytotoxicity to MC3T3-E1 cells, Ag/Zn(9:1)-MCSNs also significantly inhibited cell growth (P<0.05). MCSNs, Ag/Zn(1:1)-MCSNs and Ag/Zn(1:9)-MCSNs showed no obvious cytotoxicity, while Zn-MCSNs still promote cell proliferation (P<0.05) (Figure 9B). The results of incubation for 7 d were same as these results of 3 d (Figure 9C).
Figure 9.
CCK8 test on the MC3T3-E1 cells.
Notes: (A) Culture for 1 d; (B) culture for 3 d; (C) culture for 7 d. *P<0.05 when compared with the negative control (decrease); &P<0.05 when compared with the negative control (increase); #P<0.05 when compared with the CCK8 reagent only group.
Abbreviations: CH, calcium hydroxide; MCSNs, mesoporous calcium-silicate nanoparticles; Ag-MCSNs, nanosilver-incorporated MCSNs; Ag/Zn-MCSNs, nanosilver- and nanozinc-incorporated MCSNs; Zn-MCSNs, nanozinc-incorporated MCSNs.
Discussion
Nano-antibacterial agents have been proposed as a choice for intracanal disinfections because they can disrupt bacterial biofilm and prevent bacterial adhesion to dentin.13 In this study, the synthesized Ag/Zn(1:9)-MCSNs and Ag/Zn(9:1)-MCSNs are similar to the previously synthesized nanoparticles which possess representative mesoporous structures where the Ag and Zn are distributed inside. The nanoparticles were nano-scale and had high surface areas and pore volumes. They release Ca,2+ SiO4,4− Ag+ and Zn2+ in aqueous solution, producing a weak alkaline micro-environment and maintaining a high pH value over time. These characteristics are consistent with previous studies on calcium silicate-based materials.20
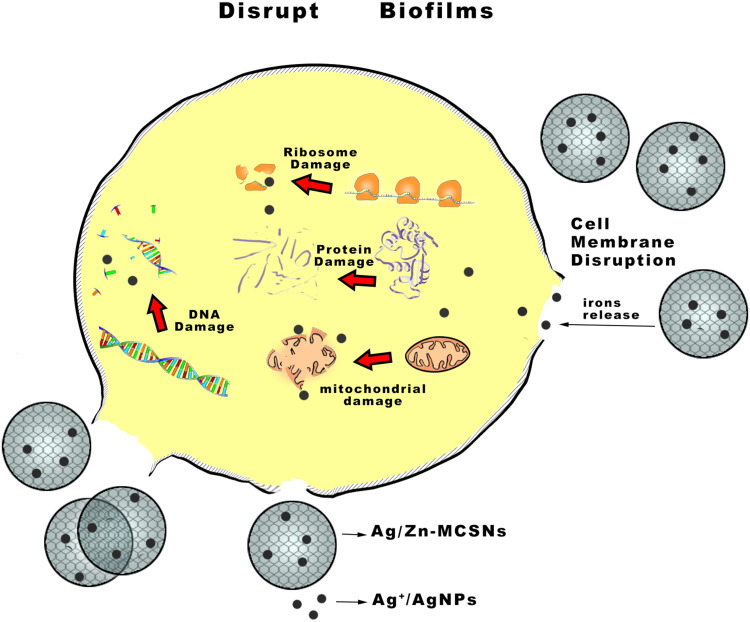
Nanoparticles can enter bacterial cells through endocytosis, and damage their intracellular structures such as mitochondria, vacuoles, ribosomes and lysosomes, leading to cell lysis.21 Metallic nanoparticles may obtain antibacterial effects by destroying membrane proteins, producing superoxide radicals and generating ions that interfere with the cell granules causing particles aggregation.22 In this study, the results indicated that the pH and osmotic pressure of solution may rise due to ions releasing of the nanoparticles which made its surroundings harmful to bacterial survival, but the Ag+ released by Ag-MCSNs or Ag/Zn-MCSNs can directly damage bacterial cell walls and membranes and enter the cells, causing cell contents to leak out and destruction of intracellular structures, leading to bacteria death (Figure 10). Ag+ has a high affinity to electron-giving groups, which are extensively found in cell membranes or proteins such as sulfhydryl, carbonyl, amino, imidazole and phosphate groups. Ag+ can bind to thiol groups (ASH) of proteins to obtain stable AS-Ag bonds, which can change the 3D structure of proteins and block the active binding sites.23 Ag+ has high reactivity, which can bind to tissue proteins and cause structural changes in the cell wall and nuclear membrane of bacteria, leading to cell deformation and death. Ag+ can also denature and inhibit bacterial replication by binding to bacterial DNA and RNA.24 The release of Ag+ may be the reason why Ag-MCSNs and Ag/Zn-MCSNs showed better antibacterial ability than MCSNs in the experiments. Ag+ can be efficiently transported through mesoporous channels of MCSNs and continuously release to achieve better antibacterial effects.
Figure 10.
Antimicrobial mechanism pattern diagram of Ag/Zn-MCSNs.
Abbreviation: Ag/Zn-MCSNs, nanosilver- and nanozinc-incorporated mesoporous calcium-silicate nanoparticles.
In this study, MCSNs showed a slight antimicrobial activity, which agrees with the previous study.13 The nano-dimension of MCSNs may interfere with bacteria metabolism, especially when they are in direct contact with bacteria wall in a liquid environment.25 Zn2+ had antibacterial activity that might destroy the bacteria membrane structure, intracellular enzymes and the replication of DNA.26 However, Zn-MCSNs showed a weak antimicrobial activity. This may be due to the low proportions of Zn contained in Zn-MCSNs and its slow release. Ag-MCSNs, Ag/Zn(1:1)-MCSNs, Ag/Zn(1:9)-MCSNs and Ag/Zn(9:1)-MCSNs showed evident higher antibacterial effects than the MCSNs and Zn-MCSNs groups. Ag incorporated into MCSNs could be released in the form of Ag+ in liquid environment.13 Guo et al reported that Ag+ had great antibacterial effects on Escherichia coli (E. coli), Pseudomonas aeruginosa (P. aeruginosa) and Staphylococcus aureus (S. aureus).27 Therefore, these phenomena indicated that the antibacterial mechanism of Ag/Zn-MCSNs is mainly associated with the release of Ag+ rather than Ca2+ or SiO4.4− In addition, the synergistic antibacterial effects of Ag and Zn are not obvious. It may be necessary to set up more different concentration groups in later studies to verify the synergistic effects of Ag and Zn.
The antibiofilm activity of the nanoparticles was studied for the potential use in root canal disinfection. MTA is the most representative calcium-silicate cements used in clinical, which can induce the generation of hard tissue and achieve ideal apical seal.28 However, MTA has no obvious antibacterial activity and cannot effectively kill E. faecalis, S. aureus, E. coli or other anaerobic bacteria, and it is difficult to enter the narrow and curved root canal and dentin tubules to remove the bacteria.29 Pompermayer et al found that MTA was not effective against multispecies microcosm biofilms including E. faecalis, Streptococcus salivarius (S. salivarius), Streptococcus mutans (S. mutans) and so on.30 In this study, the E. faecalis biofilms treated with MTA was still intact, and the proportion of live bacteria was high, suggesting that MTA had no obvious antibacterial effect on E. faecalis biofilms, which was similar to previous studies. MCSNs has antibacterial effect on planktonic E. faecalis, but the antibacterial effect of MCSNs on E. faecalis biofilms is obviously lower than that of CH. It may be attributed to the changes in interaction between MCSNs and bacteria in bacteria biofilms instead of planktonic bacteria, which compromised the antibacterial effect of MCSNs.13 Previous studies reported that nano-sized ZnO may obtain good antibacterial activity by entering the cell membrane of bacteria and releasing Zn2+ to destroy lipids, proteins and important metabolic pathways.31 However, Zn-MCSNs did not show significant antibiofilm ability in this study. The possible reasons may be the low Zn2+ concentration and slow release of Zn2+ of Zn-MCSNs that cannot reach the desired threshold for antibiofilm effect. Ag-MCSNs, Ag/Zn(1:9)-MCSNs, Ag/Zn(1:1)-MCSNs and Ag/Zn(9:1)-MCSNs have improved antibiofilm activity than MTA, MCSNs and Zn-MCSNs. These results confirm the above-mentioned possible explanations that the antimicrobial mechanism of Ag/Zn-MCSNs is mainly associated with the released Ag+. Saravanan et al reported that gram-negative (G−) bacteria were more sensitive to AgNPs, which were rapidly disguised through bacterial thin cell walls (low content of peptidoglycan), thus resulting in maladjustment of protein structure and promoting cell death.32 However, gram-positive (G+) bacteria have a thick cell wall containing peptidoglycan, which requires a higher concentration of materials to achieve antibacterial effect. In this study, the Ag-MCSNs, Ag/Zn(1:1)-MCSNs, Ag/Zn(9:1)-MCSNs groups that have higher Ag content were observed more biofilm destruction and more dead bacteria than the Ag/Zn(1:9)-MCSNs group. Therefore, the higher the content of Ag+, the greater the antibiofilm effect.
Substantivity on dentin surface is another significant feature of intracanal medications. Fan et al reported that CH paste has very weak substantivity on dentin surface, and there was no residual antimicrobial activity after CH paste being removed.33 The findings of this study also showed that CH had no substantivity ability, and the root canal surface treated with CH seemed to have enrichment effect on E. faecalis. The reason may be that the high pH value caused by CH changes the organic components such as proteins in dentin, affecting the adhesion and colonization of E. faecalis on the root canal wall. MCSNs can adhere to dentin that may be attributed to the mineralization of apatite and the capability of calcium silicate to directly bind to living tissue.34 Nevertheless, MCSNs had no obvious resistance to bacterial adhesion and proliferation, while Ag-MCSNs and Ag/Zn-MCSNs adhered to dentin and infiltrated into dentinal tubules, which will release Ag+ continuously and get a long-term antibiofilm effect. Studies have shown that doping Zn to nanoparticles can show better antibacterial activity against E. coli (G−) and S. aureus (G+) than original materials.35 However, Zn-MCSNs showed no obvious antibiofilm activity in this study. On the contrary, Zn-MCSNs showed an enrichment effect on E. faecalis and seem to encourage E. faecalis to form biofilms. This phenomenon may be explained by that the low content of Zn2+ in Zn-MCSNs which is not enough for the antibacterial effect and Zn promote cell proliferation, which is consistent with the cytotoxicity results of this study.
CH showed very strong cytotoxicity to MC3T3 cells while MCSNs, Ag/Zn(1:1)-MCSNs and Ag/Zn(1:9)-MCSNs have no obvious cytotoxicity. However, Ag-MCSNs and Ag/Zn(9:1)-MCSNs significantly inhibited cell growth, and Ag-MCSNs even showed the same strong cytotoxicity as CH. The results indicated that the cytotoxicity of Ag/Zn-MCSNs depends on the amount of Ag, the higher the content of Ag, the greater the cytotoxicity. These results were comparable as previous studies that Ag have adverse effects on human health.25 It was interesting to find that Zn-MCSNs has advantage to promote cell proliferation, the higher the content of Zn in Ag/Zn-MCSNs, the less cytotoxicity of them. As a cofactor and a structural and regulatory ion, Zn2+ takes major biological roles and involves in homeostasis, immune responses, oxidative stress and apoptosis.36 Previous studies demonstrated that Zn takes the active part in bone metabolism and has a stimulatory effect on osteogenesis.37,38 Ag/Zn(1:1)-MCSNs and Ag/Zn(1:9)-MCSNs possess wonderful antibacterial capability without significant cytotoxicity. Therefore, MCSNs containing Zn and low concentrations of Ag can exert excellent antibacterial effects and increase cell proliferation. Further studies will be performed to evaluate the optimal proportions of Zn and Ag.
Conclusion
MCSNs containing different proportions of Ag and Zn were successfully synthesized which have ideal physicochemical properties and obvious antibiofilm activity. They destroy the cell membranes to kill bacteria by releasing Ag+. Ag/Zn-MCSNs might achieve an appropriate balance between the antibacterial effects and cytotoxicity by adjusting the ratio of Ag and Zn. Therefore, Ag/Zn-MCSNs is expected to be a new type of root canal disinfectant or sealant for root canal treatment. However, the synergistic antibacterial effects of Ag and Zn contained in Ag/Zn-MCSNs is not clear, and other possible antibacterial mechanisms need further exploration.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (31800843), the Scientific Research of Jiangsu Commission of Health (H2017050) and the Southeast University-Nanjing Medical University Cooperative Research Project (2242018K3DN15).
Disclosure
The authors report no conflicts of interest in this study.
References
- 1.Ye WH, Fan B, Purcell W, et al. Anti-biofilm efficacy of root canal irrigants against in-situ Enterococcus faecalis biofilms in root canals, isthmuses and dentinal tubules. J Dent. 2018;79:68–76. doi: 10.1016/j.jdent.2018.10.002 [DOI] [PubMed] [Google Scholar]
- 2.Baras BH, Sun J, Melo MAS, et al. Novel root canal sealer with dimethylaminohexadecyl methacrylate, nano-silver and nano-calcium phosphate to kill bacteria inside root dentin and increase dentin hardness. Dent Mater. 2019;35:1479–1489. doi: 10.1016/j.dental.2019.07.014 [DOI] [PubMed] [Google Scholar]
- 3.Louwakul P, Saelo A, Khemaleelakul S. Efficacy of calcium oxide and calcium hydroxide nanoparticles on the elimination of Enterococcus faecalis in human root dentin. Clin Oral Investig. 2017;21(3):865–871. doi: 10.1007/s00784-016-1836-x [DOI] [PubMed] [Google Scholar]
- 4.Heling I, Chandler NP. The antimicrobial effect within dentinal tubules of four root canal sealers. J Endod. 1996;22(5):257–259. doi: 10.1016/s0099-2399(06)80144-5 [DOI] [PubMed] [Google Scholar]
- 5.Zhu J, Liang R, Sun C, et al. Effects of nanosilver and nanozinc incorporated mesoporous calcium-silicate nanoparticles on the mechanical properties of dentin. PLoS One. 2017;12:e0182583. doi: 10.1371/journal.pone.0182583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marending M, Stark WJ, Brunner TJ, Fischer J, Zehnder M. Comparative assessment of time-related bioactive glass and calcium hydroxide effects on mechanical properties of human root dentin. Dent Traumatol. 2009;25(1):126–129. doi: 10.1111/j.1600-9657.2008.00735.x [DOI] [PubMed] [Google Scholar]
- 7.Pugazhendhi A, Edison TNJI, Karuppusamy I, et al. Inorganic nanoparticles: a potential cancer therapy for human welfare. Int J Pharm. 2018;25; 539(1–2):104–111. doi: 10.1016/j.ijpharm.2018.01.034 [DOI] [PubMed] [Google Scholar]
- 8.Samuel MS, Jose S, Selvarajan E, et al. Biosynthesized silver nanoparticles using Bacillus amyloliquefaciens; application for cytotoxicity effect on A549 cell line and photocatalytic degradation of p-nitrophenol. J Photochem Photobiol B. 2020;202:111642. doi: 10.1016/j.jphotobiol.2019.111642 [DOI] [PubMed] [Google Scholar]
- 9.Saravanan M, Barik SK, Mubarakali D, et al. Synthesis of silver nanoparticles from Bacillus brevis (NCIM 2533) and their antibacterial activity against pathogenic bacteria. Microb Pathog. 2018;116:221–226. doi: 10.1016/j.micpath.2018.01.038 [DOI] [PubMed] [Google Scholar]
- 10.Gao M, Chang R, Wang D, et al. Short communication: fructose-enhanced antibacterial activity of self-assembled nano-peptide amphiphiles for treating antibiotic-resistant bacteria. Int J Nanomed. 2020;15:513–519. doi: 10.2147/IJN.S200505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu C, Chang J, Fan W. Bioactive mesoporous calcium-silicate nanoparticles with excellent mineralization ability, osteostimulation, drug-delivery and antibacterial properties for filling apex roots of teeth. J Mater Chem. 2012;22(33):16801–16809. doi: 10.1039/c2jm33387b [DOI] [Google Scholar]
- 12.Huang CY, Huang TH, Kao CT, et al. Mesoporous calcium silicate nanoparticles with drug delivery and odontogenesis properties. J Endod. 2017;43(1):69–76. doi: 10.1016/j.joen.2016.09.012 [DOI] [PubMed] [Google Scholar]
- 13.Fan W, Wu D, Tay FR, Ma T, Wu Y, Fan B. Effects of adsorbed and templated nanosilver in mesoporous calcium-silicate nanoparticles on inhibition of bacteria colonization of dentin. Int J Nanomed. 2014;9:5217–5230. doi: 10.2147/IJN.S73144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mubina MSK, Shailajha S, Sankaranarayanan R, et al. In vitro bioactivity, mechanical behavior and antibacterial properties of mesoporous SiO-CaO-NaO-PO nano bioactive glass ceramics. J Mech Behav Biomed Mater. 2019;100:103379. doi: 10.1016/j.jmbbm.2019.103379 [DOI] [PubMed] [Google Scholar]
- 15.Rai M, Yadav A, Gade A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol Adv. 2009;27(1):76–83. doi: 10.1016/j.biotechadv.2008.09.002 [DOI] [PubMed] [Google Scholar]
- 16.Bapat RA, Chaubal TV, Joshi CP, et al. An overview of application of silver nanoparticles for biomaterials in dentistry. Mater Sci Eng C Mater Biol Appl. 2018;91:881–898. doi: 10.1016/j.msec.2018.05.069 [DOI] [PubMed] [Google Scholar]
- 17.Beyersmann D, Haase H. Functions of zinc in signaling, proliferation and differentiation of mammalian cells. Biometals. 2001;14(3–4):331–341. doi: 10.1023/a:1012905406548 [DOI] [PubMed] [Google Scholar]
- 18.Sirelkhatim A, Mahmud S, Seeni A, et al. Review on zinc oxide nanoparticles: antibacterial activity and toxicity mechanism. Nanomicro Lett. 2015;7(3):219–242. doi: 10.1007/s40820-015-0040-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fan W, Sun Q, Li Y, et al. Synergistic mechanism of Ag+-Zn2+ in anti-bacterial activity against Enterococcus faecalis and its application against dentin infection. J Nanobiotechnology. 2018;16(1):10. doi: 10.1186/s12951-018-0336-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu YJ, Guo XX, Sham TK. Calcium silicate-based drug delivery systems. Expert Opin Drug Deliv. 2017;14(2):215–228. doi: 10.1080/17425247.2016.1214566 [DOI] [PubMed] [Google Scholar]
- 21.Li Y, Hu Q, Miao G, et al. Size-dependent mechanism of intracellular localization and cytotoxicity of mono-disperse spherical mesoporous nano-and micron-bioactive glass particles. J Biomed Nanotechnol. 2016;12(5):863–877. doi: 10.1166/jbn.2016.2235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shaikh S, Nazam N, Rizvi SMD, et al. Mechanistic insights into the antimicrobial actions of metallic nanoparticles and their implications for multidrug resistance. Int J Mol Sci. 2019;20(10):2468. doi: 10.3390/ijms20102468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tang S, Zheng J. Antibacterial activity of silver nanoparticles: structural effects. Adv Healthc Mater. 2018;7(13):e1701503. doi: 10.1002/adhm.201701503 [DOI] [PubMed] [Google Scholar]
- 24.Pugazhendhi A, Prabakar D, Jacob JM, et al. Synthesis and characterization of silver nanoparticles using Gelidium amansii and its antimicrobial property against various pathogenic bacteria. Microb Pathog. 2018;114:41–45. doi: 10.1016/j.micpath.2017.11.013 [DOI] [PubMed] [Google Scholar]
- 25.Shrestha A, Kishen A. Antibacterial nanoparticles in endodontics: a review. J Endod. 2016;42(10):1417–1426. doi: 10.1016/j.joen.2016.05.021 [DOI] [PubMed] [Google Scholar]
- 26.Alavi M, Nokhodchi A. An overview on antimicrobial and wound healing properties of ZnO nanobiofilms, hydrogels, and bionanocomposites based on cellulose, chitosan, and alginate polymers. Carbohydr Polym. 2020;227:115349. doi: 10.1016/j.carbpol.2019.115349 [DOI] [PubMed] [Google Scholar]
- 27.Guo Y, Wang S, Du H, et al. Silver ion-histidine interplay switches peptide hydrogel from antiparallel to parallel β-Assembly and enables controlled antibacterial activity. Biomacromolecules. 2019;20(1):558–565. doi: 10.1021/acs.biomac.8b01480 [DOI] [PubMed] [Google Scholar]
- 28.Torabinejad M, Parirokh M, Dummer PMH. Mineral trioxide aggregate and other bioactive endodontic cements: an updated overview-part II: other clinical applications and complications. Int Endod J. 2018;51(3):284–317. doi: 10.1111/iej.12843 [DOI] [PubMed] [Google Scholar]
- 29.Dalmia S, Gaikwad A, Samuel R, et al. Antimicrobial efficacy of different endodontic sealers against Enterococcus faecalis: an in vitro study. J Int Soc Prev Community Dent. 2018;8(2):104–109. doi: 10.4103/jispcd.JISPCD_29_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pompermayer JA, Francisco M, Martins QR, et al. Antimicrobial effect of bioceramic cements on multispecies microcosm biofilm: a confocal laser microscopy study. Clin Oral Investig. 2019;23:1367–1372. doi: 10.1007/s00784-018-2551-6 [DOI] [PubMed] [Google Scholar]
- 31.Vimbela GV, Ngo SM, Fraze C, Yang L, Stout DA. Antibacterial properties and toxicity from metallic nanomaterials. Int J Nanomed. 2017;12:3941–3965. doi: 10.2147/IJN.S134526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saravanan M, Arokiyaraj S, Lakshmi T, et al. Synthesis of silver nanoparticles from Phenerochaete chrysosporium (MTCC-787) and their antibacterial activity against human pathogenic bacteria. Microb Pathog. 2018;117:68–72. doi: 10.1016/j.micpath.2018.02.008 [DOI] [PubMed] [Google Scholar]
- 33.Fan W, Wu Y, Ma T, Li Y, Fan B. Substantivity of Ag-Ca-Si mesoporous nanoparticles on dentin and its ability to inhibit Enterococcus faecalis. J Mater Sci Mater Med. 2016;27(1):16. doi: 10.1007/s10856-015-5633-x [DOI] [PubMed] [Google Scholar]
- 34.Nilormi B, Aniruddha S, Soumik P, et al. Phase pure, high hardness, biocompatible calcium silicates with excellent anti-bacterial and biofilm inhibition efficacies for endodontic and orthopaedic applications. J Mech Behav Biomed Mater. 2018;86:264–283. doi: 10.1016/j.jmbbm.2018.06.046 [DOI] [PubMed] [Google Scholar]
- 35.Gupta VK, Fakhri A, Tahami S, et al. Zn doped CdO nanoparticles: structural, morphological, optical, photocatalytic and anti-bacterial properties. J Colloid Interface Sci. 2017;504:164–170. doi: 10.1016/j.jcis.2017.05.026 [DOI] [PubMed] [Google Scholar]
- 36.Toshiyuki F, Taiho K. Welcome to the world of zinc signaling. Int J Mol Sci. 2018;19(3):785. doi: 10.3390/ijms19030785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang B, Yang M, Liu L, et al. Osteogenic potential of Zn-passivated carbon dots for bone regeneration in vivo. Biomater Sci. 2019;7:5414–5423. doi: 10.1039/c9bm01181a [DOI] [PubMed] [Google Scholar]
- 38.Xiong K, Zhang J, Zhu Y, Chen L, Ye J. Zinc doping induced differences in the surface composition, surface morphology and osteogenesis performance of the calcium phosphate cement hydration products. Mater Sci Eng C Mater Biol Appl. 2019;105:110065. doi: 10.1016/j.msec.2019.110065 [DOI] [PubMed] [Google Scholar]