Abstract
Many e-cigarette products contain cinnamaldehyde as a primary constituent of cinnamon flavorings. When used as a food additive, cinnamaldehyde is generally regarded as safe for ingestion. However, little is known about the effects of cinnamaldehyde or its degradation products, generated after heating and inhalation, which may lead to elevated circulatory exposure to the heart. Hence, in this study, we tested the in vitro cardiac toxicity of cinnamaldehyde and its thermal degradation products generated by heating at low (200 ± 50°C) and high temperatures (700 ± 50°C) on the contractility, rhythmicity and electrical signaling properties of human induced pluripotent stem cell derived cardiac myocytes (hiPSC-CMs). Cellular impedance measurements on spontaneously beating hiPSC-CMs revealed that cinnamaldehyde significantly alters contraction-dependent signal amplitude, beating rate, and cell morphology. These effects were attenuated after cinnamaldehyde was subjected to heating at low or high temperatures. Current clamp analysis of hiPSC-CM action potentials (APs) showed only modest effects of acute application of 1–100 µM cinnamaldehyde on resting membrane potential, while prolonged (~20 min) application of 100 µM cinnamaldehyde resulted in progressive depolarization and loss of rhythmic AP spiking activity. Collectively, these results suggest that micromolar levels of cinnamaldehyde could alter cardiac excitability, in part by impairing the processes that regulate membrane potential and depolarization. Our results further suggest that heating cinnamaldehyde by itself does not directly lead to the formation of products with greater cardiotoxicity in vitro.
Keywords: Electronic cigarettes, electronic nicotine delivery systems (ENDS), cellular impedance, cardiac action potential, arrhythmia, cytotoxicity
Introduction
Cinnamaldehyde is an aromatic aldehyde that confers the characteristic flavor and odor of cinnamon. As a primary constituent of cinnamon bark, cinnamaldehyde has been ingested by people for centuries and it is commonly heated at high temperatures in cooking and baking. More recently, with the increasing popularity of electronic cigarettes (e-cigarettes), cinnamaldehyde and its thermal degradation products are now routinely inhaled as this chemical is a major additive to commercially available e-liquid formulations (Behar et al., 2016). Chemical analyses of several of these e-liquids have measured cinnamaldehyde present at a range of concentrations, and as high as >1 M (Behar et al., 2016; Clapp et al., 2017). Although measurements of cinnamaldehyde and its metabolites in the plasma of e-cigarette users are lacking, pharmacokinetic analyses in rats suggest that cinnamaldehyde as well as products of cinnamaldehyde metabolism, cinnamyl alcohol and methyl cinnamate, persist in the plasma after oral and intravenous administration with a half-life of ~7 h (Zhao et al., 2014). These observations suggest that cinnamaldehyde enters the systemic circulation after these routes of exposure and can potentially affect the function of numerous cell types. Nevertheless, despite the increasing consumption of cinnamaldehyde via electronic nicotine delivery systems (ENDS), the cellular toxicity of cinnamaldehyde and its thermal degradation products remains unclear.
Previous work suggests that cardiovascular tissues may be particularly sensitive to chemical and xenobiotic exposure relative to other tissues, in part because cells of the heart and blood vessels have a low capacity for xenobiotic detoxification (Bhatnagar, 2004). This is consistent with studies showing that the heart may be highly vulnerable to cumulative injury subsequent to exposure of environmental toxins (Izzotti et al., 1999; Ping et al., 2003). Recently, it was reported that cinnamaldehyde reduces cardiac inflammation and fibrosis in fructose-fed rats (Kang et al., 2016) and that it attenuates LPS-induced cardiac dysfunction (Zhao et al., 2016), suggesting that anti-oxidant and anti-inflammatory actions of cinnamaldehyde can have a beneficial influence on cardiac function in the setting of induced inflammation. However, the direct effects of cinnamaldehyde on the function of cardiac myocytes have not been studied.
Despite potential beneficial effects of cinnamaldehyde on the heart, attributable to anti-inflammatory properties, cinnamaldehyde has been shown to affect ion channel activities (Alvarez-Collazo et al., 2014; Bandell et al., 2004) and the generation of reactive oxygen species (Ka et al., 2003; Noh et al., 2015). Hence, we tested the hypothesis that direct exposure of cardiac myocytes to cinnamaldehyde can acutely impact their function, independent of any potential chronic anti-inflammatory or anti-fibrotic effects. We also postulated that heating cinnamaldehyde, as during e-cigarette use and in cooking, could alter cinnamaldehyde toxicity and/or lead to the formation of new products that have their own unique cardiotoxicity profile. To test these hypotheses, we examined the effects of cinnamaldehyde and its thermal degradation products using spontaneously beating human induced pluripotent stem cell-derived cardiac myocytes (hiPSC-CM) as a model in vitro platform. These cells are stable and readily accessible for in vitro pharmacological and toxicological screening for potential cardiotoxic effects (Sharma et al., 2013). By using an integrated approach that combines cellular impedance measurements with electrophysiological evaluation of cellular action potential properties, we found that without heating, cinnamaldehyde impairs normal beating rhythmicity and contractility in hiPSC-CMs, before the onset of overt cytotoxicity, i.e., cell death. Significantly, we found that cells that were treated with an equivalent concentration of cinnamaldehyde, subjected to heating, did not exhibit the pattern of dysfunction seen with the parent cinnamaldehyde, suggesting that the cardiotoxicity of cinnamaldehyde may depend upon heating conditions used prior to exposure.
Methods
Chemical reagents and heating protocol:
Cinnamaldehyde was purchased from Sigma Aldrich (cat no. W228613; ≥95%). For some experiments, cinnamaldehyde was heated using a drop-tube furnace consisting of a quartz tube (Quartz Scientific, Inc.) configured in a vertical position and set at 200°C or 700°C (+/− 50°C), as previously described (Fetterman et al., 2018). The furnace (Thermocraft Inc.) was operated with a suspension air flow rate of 1.5 L/min to ensure suspension of the combustion products. Cinnamaldehyde was then added drop-wise into the heated area of the furnace, where it rapidly vaporized, which likely had a modest variable effect on the temperature of the quartz tube. The entire aerosol was then collected within a glass impinger (SKC Inc.) and subsequently eluted in an ethanol solution (55% in PBS). Stock concentrations of thermal product solutions that were used for experiments were determined by molar equivalents of cinnamaldehyde initially added to the furnace prior to heating and collection.
Use of human induced pluripotent stem cell-derived cardiac myocytes (hiPSC-CMs):
For in vitro testing, we used commercially-sourced human induced pluripotent stem cell-derived cardiac myocytes (hiPSC-CMs) obtained from NCardia (formerly Axiogenesis; Cor.4U.; Ax-C-HCO2–4M, lot CB458Cl_4M; used in impedance analyses) and Cellular Dynamics International (CDI; iCell Cardiomyocytes2; CMC-100–012-000.5, lot CMC031519; used for manual patch clamp and viability experiments). The Cor.4U hiPSC-CMs, obtained as differentiated cells from the manufacturer, underwent directed differentiation to cardiac myocytes involving intermediate cardiac gene-specific antibiotic selection using human iPSCs from a female donor. iCells2 hiPSC-CMs were also obtained as differentiated cells. These underwent retroviral transduction using fibroblast tissue from a female Caucasian donor source (#01434). Both cell sources were karyotyped and tested by qPCR for mycoplasma prior to use in experiments. hiPSC-CM differentiation is heterogeneous with respect to stage of development with genetic signatures of cardiac ion channels and cardiac markers that are common to both fetal and adult cardiomyocytes (Gelinas et al., 2017; Huo et al., 2017; Karakikes et al., 2015). Previous work has shown that iCells and Cor.4U cells have similar purity levels (91.4 ± 4.4% versus 89.2 ± 7.6% troponin-t positive cells, respectively) (Huo et al., 2017). These cells have been shown to consist of a mixed population of atrial-, nodal-, and ventricular-like cardiac myocytes (Koci et al., 2017; Ma et al., 2011).
Cellular impedance:
Cellular impedance was measured as previously described (Scott et al., 2014). Cells were seeded at 3 x 104 cells/well of a fibronectin-coated E-plate Cardio 96 well device (ACEA Biosciences). Prior to plating, background impedance was measured. On Day 2 after seeding, the medium was changed twice, ~8 h apart, by exchanging half of the well volume (90 µl) 4 times. Starting on Day 3 after seeding and throughout the remainder of the experiment, the medium was changed once per day and 2 h prior to test compound addition following the same regimen. All measurements were performed at 37°C/5% CO2 in a cell culture incubator using an ACEA xCELLigence RTCA Cardio Instrument. At the seeding density used, a monolayer syncytium of hiPSC-CMs is formed in each well where all cells spontaneously beat in unison. Any well in the 96 well plate that did not meet baseline beating stability/threshold amplitudes was excluded from analyses. Over a period of 48 h after addition of test compounds, we monitored changes in cell index (measure of electrical impedance; reduction relative to baseline is indicative of cardiomyocyte cytotoxicity) (Kustermann et al., 2013), beating frequency (number of positive or negative peaks in a time period of 20 s), and signal amplitude (difference in positive to negative cell index peaks). All hiPSC-CM impedance data are expressed as the vehicle (EtOH)-subtracted mean ± SEM change from well-matched baseline values for each condition.
Electrophysiology:
For electrophysiological measurements, cells were seeded at a density of 20,000 cells/cm2 on round glass coverslips (8 mm diameter) that were coated with 0.1% gelatin (Stem Cell Technologies). After seeding, maintenance medium (Cellular Dynamics International) was changed every other day and cells were maintained in culture for 7–10 days prior to patch clamp recordings. Whole cell recordings were performed in the current clamp mode using an AxoPatch 200B amplifier with a DigiData 1440A acquisition board and pClamp 10 software (Axon Instruments). Spontaneous rhythmic action potentials were recorded in gap-free mode using the perforated patch technique with a pipette solution containing (in mM): 5 NaCl, 5 MgATP, 150 KCl, 10 HEPES, 5 EGTA, 2 CaCl2, pH 7.2 adjusted with KOH. For membrane perforation, gramicidin (200 ng/mL) was added fresh to the pipette solution on the day of recording. Prior to, and during recording, the cells were continuously perfused (~1 mL/min in 0.25 mL recording chamber) at 35–37°C with saline solution containing (in mM): 150 NaCl, 5.4 KCl, 1 MgCl2, 1.8 CaCl2, 1 Na-Pyruvate, 15 glucose, 15 HEPES, pH 7.4 with NaOH. Data were acquired at 10 kHz and low pass filtered at 2 kHz. All action potential data were analyzed using Clampfit software.
Cell viability assays:
Relative viability was assessed using a 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) reduction assay as previously described (Leipnitz et al., 2018). Briefly, cells were plated, as described above for cellular impedance measurements, in a 96-well culture plate and allowed to equilibrate to culture conditions for a period of 7 days. The maintenance medium was changed every other day. After equilibration, the cells were treated with cinnamaldehyde (1–100 µM) or 0.1% ethanol in maintenance medium for a period of 6, 24, or 48 h. Cell viability was assessed using an MTS assay kit (Abcam) following the manufacturer’s instructions. Cells were incubated for 4 h in MTS prior to gentle shaking and measurement of absorbance (490 nm) using a BioTek Synergy microplate reader. Viability data for cells treated with cinnamaldehyde are expressed as background (medium control)-subtracted OD490 relative to cells treated with 0.1% ethanol for the same period (cell control).
Immunofluorescent staining and imaging:
Cells were plated as above for viability assays and fixed with 4% paraformaldehyde in PBS (10 min, room temp), permeabilized with 0.1% Triton-X 100, and washed (3x) with PBS. Non-specific binding was blocked using 1% bovine serum albumin (30 min) with 0.1% Tween-20 in PBS. Cells were then labeled with a rabbit polyclonal primary antibody raised against troponin-I (Santa Cruz Biotechnology; sc-15368; 1:200) overnight in blocking solution at 4°C. Cells were then washed and incubated in Alexa Fluor 488-conjugated anti-rabbit IgG secondary antibody (1:400; 1 hr, room temperature). Cells were imaged at 20x magnification using a Keyence BZX fluorescent microscopy system.
Statistics:
Data are mean ± SEM relative to baseline values for each condition. For impedance and AP measurements, data were analyzed using GraphPad Prism software using repeated measures comparisons between groups using the Friedman non-parametric test. P<0.05 was considered statistically significant.
Results
Response of hiPSC-CMs to known modulators of cardiac myocyte function.
To investigate the effects of cinnamaldehyde and its thermal degradation products, we examined changes in the contractility and rhythmicity of hiPSC-CMs using a cellular impedance assay (Koci et al., 2017). Initially, we performed tests to verify the responses of spontaneously beating hiPSC-CMs to known modulators of cardiomyocyte function. Table 1 summarizes the inotropic and chronotropic responses of hiPSC-CMs to these compounds. As indicated, the hERG Kv11.1 K+ channel inhibitor E4031 (30 nM), significantly reduced impedance signal amplitude and increased the beat rate (BR), yet did not significantly alter cell index (CI), a measure reflective of attachment quality, cell morphology and physiology of the cell membrane. The β-adrenergic agonist isoproterenol (ISO; 100 nM) caused a marked increase in beating rate and produced a small, yet significant reduction in signal amplitude. The oxidant hydrogen peroxide (H2O2; 50 µM) reduced the amplitude, BR and CI, consistent with well-known cytotoxic effects. The voltage-gated Na+ channel inhibitor tetrodotoxin (TTX) significantly lowered amplitude and BR, but did not alter CI. These data are consistent with previous reports (Khan et al., 2013) indicating that hiPSC-CMs respond in an expected manner to cardioactive agents. Our results are also in agreement with previous reports indicating that continuous measurement of hiPSC-CM impedance provides a useful in vitro model system for accurate identification and evaluation of distinct cardiotoxic effects of test compounds (Blinova et al., 2017; Millard et al., 2018).
Table 1:
Response of hiPSC-CMs to control compounds in cellular impedance measurements.
| Parameter | Baseline values |
|---|---|
| amplitude (a.u.) | 0.063 ± 0.002 |
| beat rate (bpm) | 65.192 ± 0.262 |
| cell index (a.u.) | 10.528 ± 0.563 |
| Test | Relative effect |
| H2O2 (50 µM) | |
| ∆ amplitude | 0.32 ± 0.19* |
| ∆ beat rate | 0.54 ± 0.32 |
| ∆ cell index | 0.48 ± 0.24 |
| TTX (250 nM) | |
| ∆ amplitude | 0.73 ± 0.02* |
| ∆ beat rate | 0.80 ± 0.01* |
| ∆ cell index | 1.04 ± 0.02 |
| Iso (100 nM) | |
| ∆ amplitude | 0.94 ± 0.01* |
| ∆ beat rate | 1.65 ± 0.04* |
| ∆ cell index | 1.01 ± 0.01 |
| E-4031 (30 nM) | |
| ∆ amplitude | 0.70 ± 0.05* |
| ∆ beat rate | 1.37 ± 0.08* |
| ∆ cell index | 1.00 ± 0.04 |
Data are mean ± SEM percent change from baseline relative to medium controls.
P<0.05
cessation of spontaneous beating activity.
Cinnamaldehyde disrupts hiPSC-CM rhythmicity and contractility.
We next performed a series of experiments to test the functional impact of cinnamaldehyde in its native (i.e., parent) form. We monitored changes in impedance amplitude, BR, and CI in the presence of 1, 10, and 100 µM cinnamaldehyde over a period of 48 h. While concentrations of cinnamaldehyde or its metabolites have not been reported in the blood after e-cig use, this concentration range is within expected values. Previous measurements made by others have shown that cinnamaldehyde is present in commercial e-liquid refills at levels exceeding 1 M (Behar et al., 2018; Clapp et al., 2017). For the most extreme case - assuming 100% transfer efficiency and minimal loss during inhalation - a 90 ml puff volume of 0.293 mg cinnamaldehyde, (Behar et al., 2018) following a 4-s puff spanning 4 heart beats (60 bpm), and a 400 ml volume of dilution (4 beats*100 ml/beat) would deliver ~6 μM cinnamaldehyde directly to the left heart cavities and myocardial tissue via the pulmonary venous blood. Thus, considering that the heart is the first downstream organ after completion of the pulmonary blood cycle, we reasoned that the heart is likely exposed to micromolar concentrations of cinnamaldehyde and/or it’s thermal products when inhaled. Depending on cinnamaldehyde metabolism and half-life, these levels likely accumulate with continued vaping (Zhao et al., 2014).
As shown in Figure 1, we found that parent cinnamaldehyde produced a concentration-dependent reduction in impedance amplitude when applied at 10 and 100 µM. At these concentrations, BR was also significantly reduced, and spontaneous beating activity was abolished after ~24 h in the presence of 100 µM cinnamaldehyde. At 1, 10, and 100 µM concentrations, we observed significant concentration-dependent effects of cinnamaldehyde on CI, with the greatest effect magnitude (>20%) at 100 µM cinnamaldehyde. Taken together, these results suggest that at micromolar concentrations, parent cinnamaldehyde induces time- and concentration-dependent deleterious effects on hiPSC-CM rhythmicity and contractility.
Figure 1: Cinnamaldehyde impairs human iPSC-CM function.
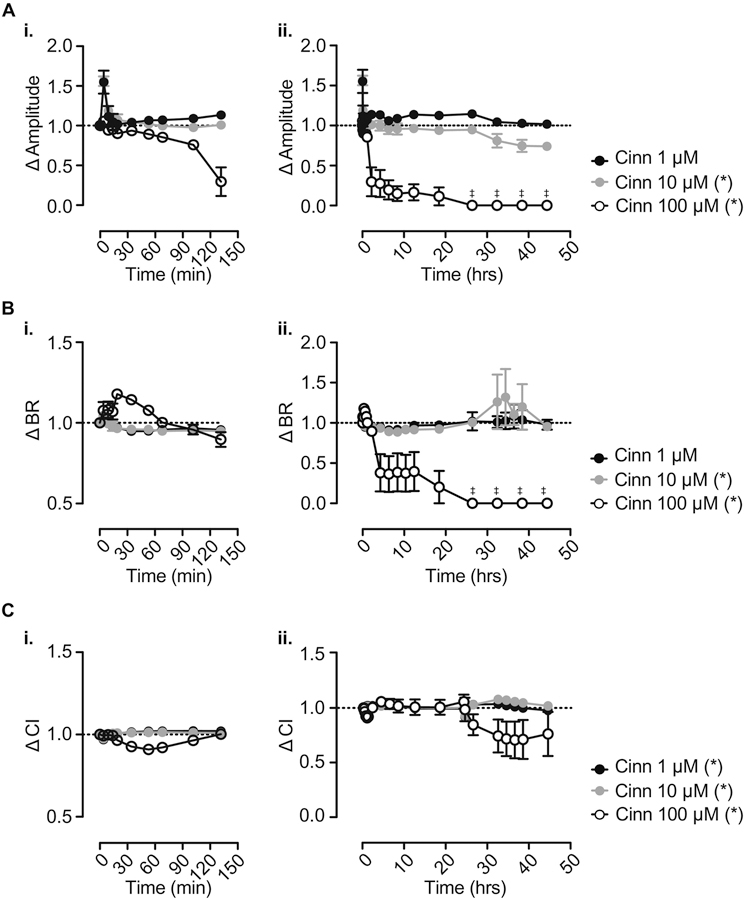
A-C. Change in hiPSC-CM impedance signal amplitude (A), beat rate (BR; B) and cell index (CI; C) at 0–2.5 h (i.) and 0–48 h (ii.) following application of 1 μM, 10 μM, and 100 μM cinnamaldehyde (n = 5). Media was changed at 24 h after initial treatment (t = 0 h). Data are expressed as relative change from baseline after subtraction of changes observed in wells treated with 0.1% ethanol as a vehicle control. Note that some error bars in summary graphs are masked by mean symbols. *P<0.05. ‡ denotes cessation of spontaneous beating activity.
Cinnamaldehyde causes time-dependent impairment of membrane potential regulation in hiPSC-CMs.
Cinnamaldehyde is known to possess cytotoxic properties and it can reduce the viability of a number of different cell types (Behar et al., 2016; Wani et al., 2014). Therefore, we assessed the cell viability of hiPSC-CMs treated with cinnamaldehyde using the MTS reduction assay. A fluorescence micrograph of hiPSC-CMs plated and separately labelled for the cardiac marker troponin-I is shown in Figure 2A. We found that incubation of cells with 1, 10, and 100 µM cinnamaldehyde for up to 6 h did not significantly reduce cell viability relative to vehicle-treated cells (Figure 2B). However, incubation of hiPSC-CMs for longer periods (24–48 h) in 100 µM cinnamaldehyde resulted in a modest but significant reduction (~30%) in cell viability, indicating that hiPSC-CM viability is sensitive to cinnamaldehyde only after prolonged incubation and therefore its observed functional effects (within ≤6 h) on rhythmicity and contractility may arise from cellular processes that are independent of acute cell death. Considering this, together with observed effects of parent cinnamaldehyde, in cellular impedance assays, we next tested whether acute application of cinnamaldehyde would alter the hiPSC-CM action potential (AP), as predicted if this compound alters cardiac ion channel activity. For this test, we performed current clamp recordings of spontaneous APs in individual hiPSC-CMs in the absence and presence of 1–100 µM cinnamaldehyde, applied for 2 min. As shown in Figure 2C–I, we found that, within the tested range of concentrations, cinnamaldehyde produced only modest effects on the AP of hiPSC-CMs. The primary effect of the 2 min exposure to 100 µM cinnamaldehyde was a small, but significant, depolarization of resting membrane potential, identified as a reduction in maximum diastolic potential. No significant effects were observed at any of the concentrations of cinnamaldehyde tested on AP amplitude, upstroke velocity (dV/dTmax) or AP duration at 10, 50, or 90% repolarization (APD10–90, respectively). As a positive control, we applied 100 nM E4031 in the presence of 100 μM cinnamaldehyde at the end of each experiment. As expected, application of E4031 significantly increased the APD90 and reduced AP amplitude and MDP (Figure 2C, I, D and F, respectively).
Figure 2: Acute application of 1–100 µM cinnamaldehyde has only modest effects on hiPSC-CM action potential properties.
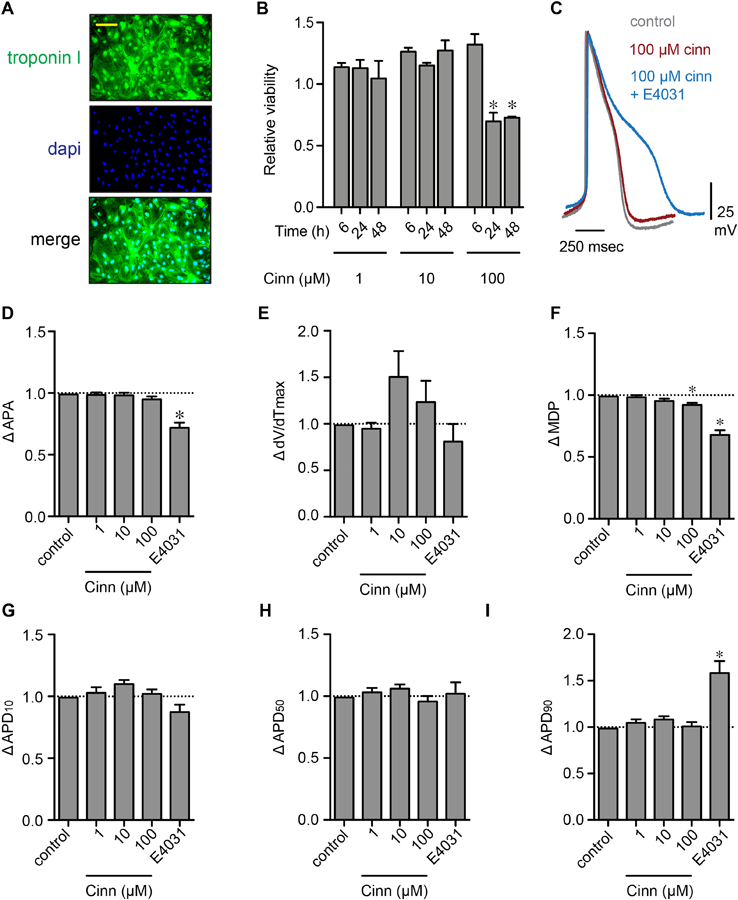
(A) Fluorescence images of hiPSC-CMs for troponin-I (green) and dapi (nuclear stain, blue). The merged image showing troponin-I and dapi labelling together is shown at the bottom. (B) Summary of viability of hiPSC-CMs treated with 1–100 µM cinnamaldehyde for 6, 24, and 48 h (n = 3 each). (C) Representative AP waveforms recorded from single hiPSC-CMs under control conditions (-cinn; gray) and in the presence of cinnamaldehyde (100 µM; red). Also shown, representative AP in the presence of cinnamaldehyde and the hERG K+ channel inhibitor E4031 (100 nM). (D-I) Summary bar plots showing mean ± SEM change in AP amplitude, upstroke velocity (dV/dTmax), resting maximum diastolic potential (MDP), and action potential duration at 10, 50, and 90% repolarization from peak potential (APD10–90-, respectively). Data are relative to baseline values obtained before the application of cinnamaldehyde. n = 5; *P<0.05.
Based on our results of impedance assays and our electrophysiological data, we postulated that parent cinnamaldehyde might affect the resting membrane potential of hiPSC-CMs after more prolonged exposures. To test this, we performed additional current clamp measurements of APs in the absence and presence of either 100 µM cinnamaldehyde or EtOH (0.2%) applied for ≥20 min in the perfusate. In contrast to findings of experiments in which cinnamaldehyde was applied acutely, perfusion of 100 µM cinnamaldehyde for this longer period of time lead to loss of rhythmic AP generation and irregular patterns of AP triggering and repolarization. After ~15 min of perfusion, 100 µM cinnamaldehyde caused progressive depolarization of the resting membrane potential, indicated by a significant reduction in MDP when compared with measurements made at similar time points in EtOH-perfused cells (Figure 3). Collectively, these data suggest that parent cinnamaldehyde causes time-dependent dysregulation of hiPSC-CM membrane potential that may ultimately impair the physiological regulation of cardiac myocyte rhythmicity and contractility.
Figure 3: Prolonged application of cinnamaldehyde impairs hiPSC-CM electrical signaling.
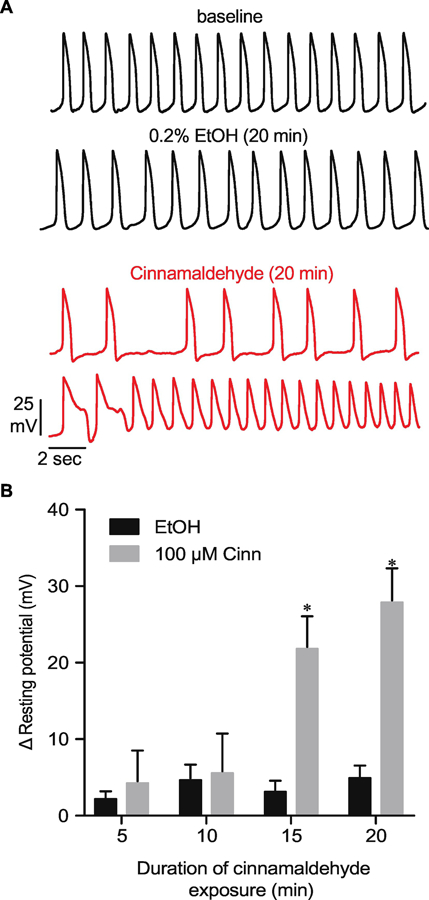
(A) Representative hiPSC-CM AP recordings before (baseline) and after 20 min of perfusion of bath solution containing either ethanol (EtOH, 0.2%) or cinnamaldehyde (100 µM). (B) Summary data showing change in membrane potential compared with baseline (positive value reflects membrane depolarization) values obtained after 5–20 min perfusion of bath solution containing either cinnamaldehyde or EtOH. *P<0.05 vs. EtOH, n = 4 each.
Heating cinnamaldehyde attenuates its acute effects on hiPSC-CMs.
Considering that cinnamaldehyde is commonly heated in e-cigarette devices prior to its inhalation, we next tested whether heating cinnamaldehyde (see Methods for details) at 200 ± 50°C (i.e., “low temperature”) or 700 ± 50°C (i.e., “high temperature”) modifies its cardiotoxicity. Cinnamaldehyde is variably heated in a number of unique e-cigarette devices in combination with humectants (i.e., propylene glycol, vegetable glycerin), nicotine, and variable combinations of other flavorants that may modify aerosol compositions and biological effects. Thus, our primary objective here was to test the hypothesis that the temperature sensitivity of cinnamaldehyde itself may alter its biological effects. Figure 4 shows the results of impedance assays with cinnamaldehyde (100 µM) after heating at low or high temperatures. Consistent with this hypothesis, and in contrast to changes in impedance amplitude, BR, and CI, observed with parent cinnamaldehyde (Figure 1, shown as dashed lines in Figure 4), the degradation products, generated upon heating at low or high temperatures, elicited only modest changes in these parameters. Moreover, when compared with the effects of parent cinnamaldehyde on impedance amplitude, BR and CI (Figure 1), the change in each of these parameters was abolished for both low and high temperature heating products of cinnamaldehyde when applied at concentrations equivalent to 100 μM parent cinnamaldehyde. Taken together these observations suggest that elevated temperatures, such as those achieved in ENDS products containing commercially available e-liquids, may alter the bioactive properties of cinnamaldehyde and may attenuate or abolish any direct cardioactive effects of cinnamaldehyde.
Figure 4: Heating and burning cinnamaldehyde attenuates its adverse effects on hiPSC-CMs.
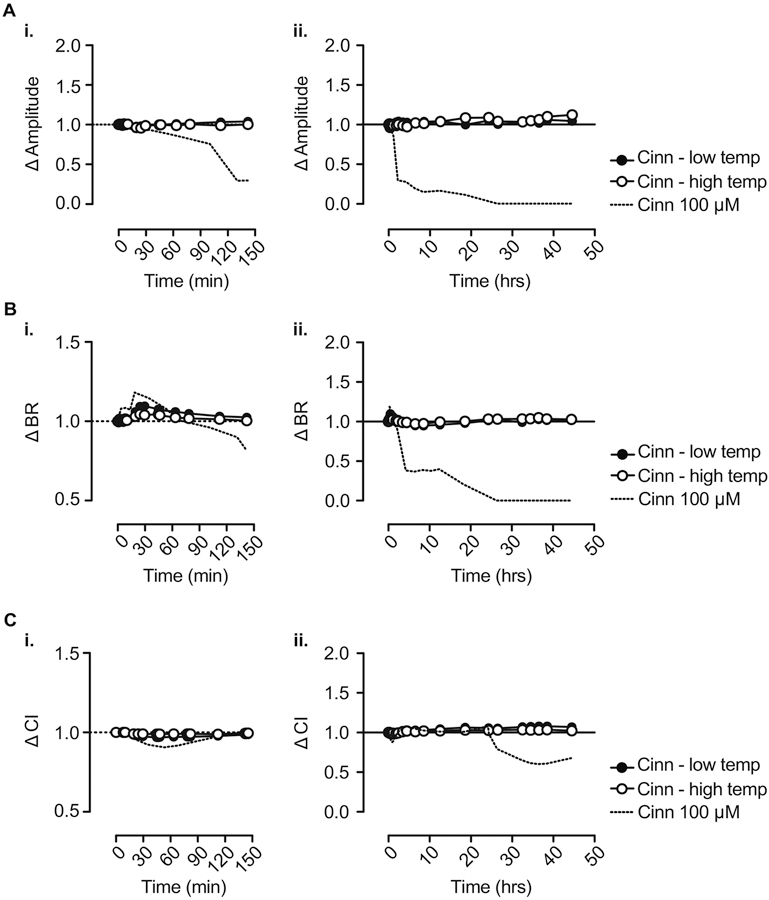
Change in hiPSC-CM impedance signal amplitude (A), BR (B) and CI (C) at 0–2.5 h (i.) and 0–48 h (ii.) following application of thermal degradation products of cinnamaldehyde (100 µM) at low and high temperature. n = 3. Dashed lines show effect of unheated cinnamaldehyde at 100 µM, as in Figure 1.
Discussion
In this study, we provide evidence that the aromatic aldehyde cinnamaldehyde directly and adversely impacts the function of human iPSC-CMs in vitro, yet these effects are largely attenuated after the compound is heated. Consistent with this notion, we report the following novel findings: (1) cinnamaldehyde applied at micromolar concentrations caused a progressive reduction in impedance signal amplitude and beat rate after several hours of exposure; (2) treatment of hiPSC-CMs with cinnamaldehyde significantly altered cell morphology and attachment quality, and caused cessation of spontaneous hiPSC-CM beating activity (at 100 µM); (3) acute application (≤ 2 min) of 1–100 µM cinnamaldehyde did not significantly change the hiPSC-CM action potential waveform, although it caused a modest depolarization of resting membrane potential; (4) prolonged exposure (≥15 min) of hiPSC-CMs to 100 µM cinnamaldehyde impairs membrane potential regulation and leads to arrhythmic action potential firing; and, (5) heating of cinnamaldehyde at 200 ± 50°C and 700 ± 50°C attenuated its impact on hiPSC-CM function. Taken together, these results suggest that cinnamaldehyde in its parent form may adversely impact hiPSC-CM function as a result of direct effects on the electrical activity and contractility of cardiac myocytes, but that these effects may be modified by subjecting the compound to elevated temperatures prior to exposure.
Cinnamaldehyde is classically known for its potent anti-inflammatory activity and is generally thought to be beneficial for cardiovascular health, as it has been found to reduce inflammation in the heart and to induce vasodilation of blood vessels (Kang et al., 2016; Ranasinghe et al., 2017; Yanaga et al., 2006; Yang et al., 2015). However, in contrast, a recent study has shown that high doses of cinnamon extract (2000 mg/kg oral, daily for 2 weeks) increases heart weights in female rats (Yun et al., 2018), although cardiac function was not examined in the study. In our current work, using hiPSC-CMs as an in vitro model for cardiotoxicity screening, we found that prolonged exposure to elevated levels of parent cinnamaldehyde could negatively impact the heart by impairing cellular processes involved in the maintenance of resting membrane potential - changes which could potentially contribute to arrhythmia or electrical remodeling of the heart. However, further investigations are required to elucidate the cellular mechanisms that mediate the functional effects of cinnamaldehyde on hiPSC-CMs and whether these are reproducible in adult human cardiac myocytes.
Acute cinnamaldehyde exposure may impair cardiac myocyte function by changing levels of superoxide and other reactive oxygen species, or by affecting the production of myocyte-derived inflammatory cytokines (e.g., TNFα, IL-6) (Aoyagi and Matsui, 2011; Atefi et al., 2011). Nonetheless, it remains plausible that effects of cinnamaldehyde on other cell types may mitigate long-term effects of exposure on cardiac electrical remodeling and/or fibrosis (Yang et al., 2015). Our current results together with those of previous studies also suggest that these effects may be critically dependent upon: 1) the route of cinnamaldehyde administration, 2) for inhalation, the chemical constituents of e-cigarette liquids containing cinnamaldehyde, and 3) the temperature generated by the device and transfer efficiency of native compound in the inhaled aerosols.
The delayed onset of the effects of cinnamaldehyde on the resting membrane potential, as well as the time course of the effects on impedance parameters in synchronously beating hiPSC-CM monolayers, suggest that the cinnamaldehyde-induced dysfunction may be mediated by its metabolites, or it could be a consequence of other indirect, rather than direct, processes involving the modulation of ion channels involved in the generation and the repolarization of the cardiac action potential. Previous work has shown that cinnamaldehyde is a high affinity ligand for the chemosensory cation channel, TRPA1 in nociceptive neurons (Alpizar et al., 2013) and in isolated superior mesenteric arteries (Jin et al., 2019). In addition, cinnamaldehyde has also been shown to directly inhibit the L-type calcium channel in smooth muscle cells; an effect that has been linked to the well-known vasodilatory effects of cinnamaldehyde (Alvarez-Collazo et al., 2014; Harada and Yano, 1975). However, in view of our data, obtained from current clamp recordings of isolated hiPSC-CMs, it is plausible that the effects of parent cinnamaldehyde observed in the current study may be due to activation of TRPA1, as these channels appear to mediate deleterious effects of acrolein in cardiac tissue (Conklin et al., 2019). Moreover, the inability of cinnamaldehyde to decrease action potential duration suggests little or no significant functional contribution by inhibition of the L-type calcium channel. In contrast, the decrease in maximal diastolic potential (MDP) after treatment with cinnamaldehyde suggests that the aldehyde can inhibit one or several K+ conductances that regulate the resting membrane potential of cardiac myocytes. Although it is possible that K+ currents affected in hiPSC-CMs are not completely reflective of those that mediate repolarization and resting potential in human adult cardiac myocytes (for example, see (Goversen et al., 2018)), our observations provide new insights into the toxicity of this aldehyde and can inform future analyses of its specific effects in the presence of other e-cigarette constituents or products of their heating or metabolism. In particular, our observation that exposure to unheated cinnamaldehyde induced abnormal automaticity of hiPSC-CMs raises the possibility that flavoring chemicals could potentially modulate K+-selective channels.
Cinnamaldehyde has a flash point of 71°C, which is below the “low temperature” used in our experiments used to generate the heating product by drop-wise addition to a heated quartz tube. We predicted that heating may exacerbate its effects on hiPSC-CMs. However, contrary to this expectation, we observed that effects of the parent aldehyde were attenuated by heating. The temperatures used in this study reflect a range at or well above that used in cooking/baking and e-cigarettes, and heating to this temperature nearly abolished the reduction in signal amplitude, decline in beat rate, and the eventual cessation of beating observed when cells were treated at high micromolar levels of the native unheated compound. While the reasons for the loss of the effects of cinnamaldehyde cannot be readily explained, it is likely that heating cinnamaldehyde at these temperatures leads to pyrolysis of the native form of the compound such that a substantially lower concentration of this form is present in the degradation products collected for testing. Consistent with this, a previous report demonstrated that heating (up to 210°C) pure trans-cinnamaldehyde at temperatures resulted in temperature- and time-dependent transformation to benzaldehyde, which has little cytotoxic effects on cells of the cardiovascular system at micromolar concentrations (Conklin et al., 1998; Friedman et al., 2000). Moreover, unsaturated aldehydes, such as cinnamaldehyde, can degrade upon heating due to double bond breakage, leading to the formation of less reactive products such as formaldehyde and acetaldehyde (Zamora et al., 2015). However, full chemical characterization of the thermal degradation products of cinnamaldehyde generated at temperatures relevant to e-cigarette devices requires additional in-depth investigations, especially given the identification of benzene formation from benzaldehyde (cherry flavorant) and benzoic acid constituents of e-liquids (Pankow et al., 2017).
We caution that our current results do not necessarily indicate a reduction in toxicity risk related to the use of e-cigarettes with liquids consisting of cinnamaldehyde. Although we assessed only the potential toxicity of cinnamaldehyde when heated by itself, this compound is currently used as an additive in many unique e-liquid formulations. These formulations are heated in hundreds of distinct device types at varying coil wattages and, perhaps most importantly, in the presence of numerous other compounds such as humectants (i.e., glycerol, propylene glycol), nicotine and other flavorings (i.e., benzaldehyde). The presence of other compounds in the heated solutions could also stabilize the parent form of cinnamaldehyde (Friedman et al., 2000), which we demonstrate in the current study has functional effects on iPSC-CMs. Currently, many cinnamon-flavored e-liquids of varying compositions are available and each could give rise to its own profile of degradation products when heated. Each of these compounds can also react with other compounds present in the e-liquid as well. Thus, it appears likely that a specific e-liquid formulation could display a distinct toxicological profile (Tierney et al., 2016) that would depend upon its constituents, device characteristics and usage conditions. Additionally, the attenuation of cinnamaldehyde-mediated effects on cardiac myocytes by heating described here could be modified by the presence of other constituents, and their combustion products collectively could affect many organ systems, such as the heart or the coronary and peripheral vasculature (Qasim et al., 2017).
For this study, we used hiPSC-CMs from two different commercial sources. It has been shown before that cells from these vendors display distinct ion channel expressions and therefore disparate electrophysiological profiles (Blinova et al., 2017; Huo et al., 2017). While these differences may influence the magnitude of functional effects observed, our findings of effects of cinnamaldehyde on both beating activity in impedance assays and action potential waveform characteristics suggest that these effects likely result via an impact on pathways that are independent of electrophysiological phenotype of the cell. In addition, we applied specific compounds with well-established cardiac effects (e.g., IKr blocker, E-4031) to cells from both sources in an effort to provide a reference for the potential effects of cinnamaldehyde and its thermal degradation products applied in parallel for each assay. Thus, it seems unlikely that the effects observed in the study are specific to a particular preparation of hiPSC-CM. However, we also acknowledge that hiPSC-CMs are an artificial model system, and we cannot rule out any potential differences in effects observed in our study between these cells and native adult human cardiac myocytes.
In summary, the findings of this study suggest that exposure to cinnamaldehyde could have adverse effects on cardiac myocyte function and that subjecting cinnamaldehyde to elevated temperature could alter the compound and consequently its toxicity. The impact of parent cinnamaldehyde, assessed in our study, may be due to modulation of cell metabolism or signaling, which ultimately lead to dysregulation of membrane potential and/or contractility. As these effects were surprisingly dampened upon heating cinnamaldehyde at low or high temperatures, further characterization of cinnamaldehyde and products of its potential reactivity with other compounds present in e-cigarette liquids is needed, especially with respect to cardiovascular toxicity. While acute exposure to cinnamaldehyde may be beneficial for its anti-inflammatory effects, based on our current data, we suggest that repeated exposure to high levels of cinnamaldehyde, when it is aerosolized even by relatively low heat e-cigarette devices, could alter cellular action potential characteristics and lead to progressive electrical remodeling and cardiac dysfunction, especially in susceptible populations at risk for arrhythmia.
Highlights.
Cinnamaldehyde impairs contractility and rhythmicity of human induced pluripotent stem cell-derived cardiac myocytes (hiPSC-CMs).
Exposure of hiPSC-CMs to cinnamaldehyde leads to depolarization of resting membrane potential.
Heating cinnamaldehyde attenuates effects on hiPSC-CM function.
Acknowledgements
This research was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health and Center for Tobacco Products under Award Numbers P50HL120163, U54HL120163, and R01HL122676. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Food and Drug Administration.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alpizar YA, Gees M, Sanchez A, Apetrei A, Voets T, Nilius B, Talavera K, 2013. Bimodal effects of cinnamaldehyde and camphor on mouse TRPA1. Pflugers Arch 465, 853–864. [DOI] [PubMed] [Google Scholar]
- Alvarez-Collazo J, Alonso-Carbajo L, Lopez-Medina AI, Alpizar YA, Tajada S, Nilius B, Voets T, Lopez-Lopez JR, Talavera K, Perez-Garcia MT, Alvarez JL, 2014. Cinnamaldehyde inhibits L-type calcium channels in mouse ventricular cardiomyocytes and vascular smooth muscle cells. Pflugers Arch 466, 2089–2099. [DOI] [PubMed] [Google Scholar]
- Aoyagi T, Matsui T, 2011. The Cardiomyocyte as a Source of Cytokines in Cardiac Injury. J Cell Sci Ther 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atefi G, Zetoune FS, Herron TJ, Jalife J, Bosmann M, Al-Aref R, Sarma JV, Ward PA, 2011. Complement dependency of cardiomyocyte release of mediators during sepsis. FASEB J 25, 2500–2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandell M, Story GM, Hwang SW, Viswanath V, Eid SR, Petrus MJ, Earley TJ, Patapoutian A, 2004. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron 41, 849–857. [DOI] [PubMed] [Google Scholar]
- Behar RZ, Luo W, Lin SC, Wang Y, Valle J, Pankow JF, Talbot P, 2016. Distribution, quantification and toxicity of cinnamaldehyde in electronic cigarette refill fluids and aerosols. Tob Control 25, ii94–ii102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behar RZ, Luo W, McWhirter KJ, Pankow JF, Talbot P, 2018. Analytical and toxicological evaluation of flavor chemicals in electronic cigarette refill fluids. Sci Rep 8, 8288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatnagar A, 2004. Cardiovascular pathophysiology of environmental pollutants. Am J Physiol Heart Circ Physiol 286, H479–485. [DOI] [PubMed] [Google Scholar]
- Blinova K, Stohlman J, Vicente J, Chan D, Johannesen L, Hortigon-Vinagre MP, Zamora V, Smith G, Crumb WJ, Pang L, Lyn-Cook B, Ross J, Brock M, Chvatal S, Millard D, Galeotti L, Stockbridge N, Strauss DG, 2017. Comprehensive Translational Assessment of Human-Induced Pluripotent Stem Cell Derived Cardiomyocytes for Evaluating Drug-Induced Arrhythmias. Toxicol Sci 155, 234–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapp PW, Pawlak EA, Lackey JT, Keating JE, Reeber SL, Glish GL, Jaspers I, 2017. Flavored e-cigarette liquids and cinnamaldehyde impair respiratory innate immune cell function. Am J Physiol Lung Cell Mol Physiol 313, L278–L292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin DJ, Guo Y, Nystoriak MA, Jagatheesan G, Obal D, Kilfoil PJ, Hoetker JD, Guo L, Bolli R, Bhatnagar A, 2019. TRPA1 channel contributes to myocardial ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol 316, H889–H899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin DJ, Langford SD, Boor PJ, 1998. Contribution of serum and cellular semicarbazide-sensitive amine oxidase to amine metabolism and cardiovascular toxicity. Toxicol Sci 46, 386–392. [DOI] [PubMed] [Google Scholar]
- Fetterman JL, Weisbrod RM, Feng B, Bastin R, Tuttle ST, Holbrook M, Baker G, Robertson RM, Conklin DJ, Bhatnagar A, Hamburg NM, 2018. Flavorings in Tobacco Products Induce Endothelial Cell Dysfunction. Arterioscler Thromb Vasc Biol 38, 1607–1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman M, Kozukue N, Harden LA, 2000. Cinnamaldehyde content in foods determined by gas chromatography-mass spectrometry. J Agric Food Chem 48, 5702–5709. [DOI] [PubMed] [Google Scholar]
- Gelinas R, El Khoury N, Chaix MA, Beauchamp C, Alikashani A, Ethier N, Boucher G, Villeneuve L, Robb L, Latour F, Mondesert B, Rivard L, Goyette P, Talajic M, Fiset C, Rioux JD, 2017. Characterization of a Human Induced Pluripotent Stem Cell-Derived Cardiomyocyte Model for the Study of Variant Pathogenicity: Validation of a KCNJ2 Mutation. Circ Cardiovasc Genet 10. [DOI] [PubMed] [Google Scholar]
- Goversen B, van der Heyden MAG, van Veen TAB, de Boer TP, 2018. The immature electrophysiological phenotype of iPSC-CMs still hampers in vitro drug screening: Special focus on IK1. Pharmacol Ther 183, 127–136. [DOI] [PubMed] [Google Scholar]
- Harada M, Yano S, 1975. Pharmacological studies on Chinese cinammon. II. Effects of cinnamaldehyde on the cardiovascular and digestive systems. Chem Pharm Bull (Tokyo) 23, 941–947. [DOI] [PubMed] [Google Scholar]
- Huo J, Kamalakar A, Yang X, Word B, Stockbridge N, Lyn-Cook B, Pang L, 2017. Evaluation of Batch Variations in Induced Pluripotent Stem Cell-Derived Human Cardiomyocytes from 2 Major Suppliers. Toxicol Sci 156, 25–38. [DOI] [PubMed] [Google Scholar]
- Izzotti A, Bagnasco M, D’Agostini F, Cartiglia C, Lubet RA, Kelloff GJ, De Flora S, 1999. Formation and persistence of nucleotide alterations in rats exposed whole-body to environmental cigarette smoke. Carcinogenesis 20, 1499–1505. [DOI] [PubMed] [Google Scholar]
- Jin L, Jagatheesan G, Guo L, Nystoriak M, Malovichko M, Lorkiewicz P, Bhatnagar A, Srivastava S, Conklin DJ, 2019. Formaldehyde Induces Mesenteric Artery Relaxation via a Sensitive Transient Receptor Potential Ankyrin-1 (TRPA1) and Endothelium-Dependent Mechanism: Potential Role in Postprandial Hyperemia. Front Physiol 10, 277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ka H, Park HJ, Jung HJ, Choi JW, Cho KS, Ha J, Lee KT, 2003. Cinnamaldehyde induces apoptosis by ROS-mediated mitochondrial permeability transition in human promyelocytic leukemia HL-60 cells. Cancer Lett 196, 143–152. [DOI] [PubMed] [Google Scholar]
- Kang LL, Zhang DM, Ma CH, Zhang JH, Jia KK, Liu JH, Wang R, Kong LD, 2016. Cinnamaldehyde and allopurinol reduce fructose-induced cardiac inflammation and fibrosis by attenuating CD36-mediated TLR4/6-IRAK4/1 signaling to suppress NLRP3 inflammasome activation. Sci Rep 6, 27460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karakikes I, Ameen M, Termglinchan V, Wu JC, 2015. Human induced pluripotent stem cell-derived cardiomyocytes: insights into molecular, cellular, and functional phenotypes. Circ Res 117, 80–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan JM, Lyon AR, Harding SE, 2013. The case for induced pluripotent stem cell-derived cardiomyocytes in pharmacological screening. Br J Pharmacol 169, 304–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koci B, Luerman G, Duenbostell A, Kettenhofen R, Bohlen H, Coyle L, Knight B, Ku W, Volberg W, Woska JR Jr., Brown MP, 2017. An impedance-based approach using human iPSC-derived cardiomyocytes significantly improves in vitro prediction of in vivo cardiotox liabilities. Toxicol Appl Pharmacol 329, 121–127. [DOI] [PubMed] [Google Scholar]
- Kustermann S, Boess F, Buness A, Schmitz M, Watzele M, Weiser T, Singer T, Suter L, Roth A, 2013. A label-free, impedance-based real time assay to identify drug-induced toxicities and differentiate cytostatic from cytotoxic effects. Toxicol In Vitro 27, 1589–1595. [DOI] [PubMed] [Google Scholar]
- Leipnitz G, Mohsen AW, Karunanidhi A, Seminotti B, Roginskaya VY, Markantone DM, Grings M, Mihalik SJ, Wipf P, Van Houten B, Vockley J, 2018. Evaluation of mitochondrial bioenergetics, dynamics, endoplasmic reticulum-mitochondria crosstalk, and reactive oxygen species in fibroblasts from patients with complex I deficiency. Sci Rep 8, 1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J, Guo L, Fiene SJ, Anson BD, Thomson JA, Kamp TJ, Kolaja KL, Swanson BJ, January CT, 2011. High purity human-induced pluripotent stem cell-derived cardiomyocytes: electrophysiological properties of action potentials and ionic currents. Am J Physiol Heart Circ Physiol 301, H2006–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millard D, Dang Q, Shi H, Zhang X, Strock C, Kraushaar U, Zeng H, Levesque P, Lu HR, Guillon JM, Wu JC, Li Y, Luerman G, Anson B, Guo L, Clements M, Abassi YA, Ross J, Pierson J, Gintant G, 2018. Cross-Site Reliability of Human Induced Pluripotent Stem-Cell Derived Cardiomyocyte Based Safety Assays using Microelectrode Arrays: Results from a Blinded CiPA Pilot Study. Toxicol Sci [DOI] [PMC free article] [PubMed]
- Noh J, Kwon B, Han E, Park M, Yang W, Cho W, Yoo W, Khang G, Lee D, 2015. Amplification of oxidative stress by a dual stimuli-responsive hybrid drug enhances cancer cell death. Nat Commun 6, 6907. [DOI] [PubMed] [Google Scholar]
- Pankow JF, Kim K, McWhirter KJ, Luo W, Escobedo JO, Strongin RM, Duell AK, Peyton DH, 2017. Benzene formation in electronic cigarettes. PLoS One 12, e0173055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ping P, Baines CP, Gu Y, Prabhu SD, Zhang J, Tsai LL, Cardwell E, Zong NC, Vondriska TM, Korge P, Bhatnagar A, Wang GW, 2003. Cardiac toxic effects of trans-2-hexenal are mediated by induction of cardiomyocyte apoptotic pathways. Cardiovasc Toxicol 3, 341–351. [DOI] [PubMed] [Google Scholar]
- Qasim H, Karim ZA, Rivera JO, Khasawneh FT, Alshbool FZ, 2017. Impact of Electronic Cigarettes on the Cardiovascular System. J Am Heart Assoc 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranasinghe P, Jayawardena R, Pigera S, Wathurapatha WS, Weeratunga HD, Premakumara GAS, Katulanda P, Constantine GR, Galappaththy P, 2017. Evaluation of pharmacodynamic properties and safety of Cinnamomum zeylanicum (Ceylon cinnamon) in healthy adults: a phase I clinical trial. BMC Complement Altern Med 17, 550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott CW, Zhang X, Abi-Gerges N, Lamore SD, Abassi YA, Peters MF, 2014. An impedance-based cellular assay using human iPSC-derived cardiomyocytes to quantify modulators of cardiac contractility. Toxicol Sci 142, 331–338. [DOI] [PubMed] [Google Scholar]
- Sharma A, Wu JC, Wu SM, 2013. Induced pluripotent stem cell-derived cardiomyocytes for cardiovascular disease modeling and drug screening. Stem Cell Res Ther 4, 150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tierney PA, Karpinski CD, Brown JE, Luo W, Pankow JF, 2016. Flavour chemicals in electronic cigarette fluids. Tob Control 25, e10–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wani KD, Kadu BS, Mansara P, Gupta P, Deore AV, Chikate RC, Poddar P, Dhole SD, Kaul-Ghanekar R, 2014. Synthesis, characterization and in vitro study of biocompatible cinnamaldehyde functionalized magnetite nanoparticles (CPGF Nps) for hyperthermia and drug delivery applications in breast cancer. PLoS One 9, e107315. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Yanaga A, Goto H, Nakagawa T, Hikiami H, Shibahara N, Shimada Y, 2006. Cinnamaldehyde induces endothelium-dependent and -independent vasorelaxant action on isolated rat aorta. Biol Pharm Bull 29, 2415–2418. [DOI] [PubMed] [Google Scholar]
- Yang L, Wu QQ, Liu Y, Hu ZF, Bian ZY, Tang QZ, 2015. Cinnamaldehyde attenuates pressure overload-induced cardiac hypertrophy. Int J Clin Exp Pathol 8, 14345–14354. [PMC free article] [PubMed] [Google Scholar]
- Yun JW, You JR, Kim YS, Kim SH, Cho EY, Yoon JH, Kwon E, Jang JJ, Park JS, Kim HC, Che JH, Kang BC, 2018. In vitro and in vivo safety studies of cinnamon extract (Cinnamomum cassia) on general and genetic toxicology. Regul Toxicol Pharmacol 95, 115–123. [DOI] [PubMed] [Google Scholar]
- Zamora R, Navarro JL, Aguilar I, Hidalgo FJ, 2015. Lipid-derived aldehyde degradation under thermal conditions. Food Chem 174, 89–96. [DOI] [PubMed] [Google Scholar]
- Zhao H, Xie Y, Yang Q, Cao Y, Tu H, Cao W, Wang S, 2014. Pharmacokinetic study of cinnamaldehyde in rats by GC-MS after oral and intravenous administration. J Pharm Biomed Anal 89, 150–157. [DOI] [PubMed] [Google Scholar]
- Zhao H, Zhang M, Zhou F, Cao W, Bi L, Xie Y, Yang Q, Wang S, 2016. Cinnamaldehyde ameliorates LPS-induced cardiac dysfunction via TLR4-NOX4 pathway: The regulation of autophagy and ROS production. J Mol Cell Cardiol 101, 11–24. [DOI] [PubMed] [Google Scholar]


