The Tsp1 protein from Trichoderma virens is a novel protein with no homologous structures available in the Protein Data Bank. Recombinant Tsp1 protein was expressed and purified for the first time, its size and secondary structure were characterized and X-ray diffraction data were collected from crystals of the protein.
Keywords: Trichoderma virens, effector, secreted cysteine-rich proteins, protein crystallization
Abstract
Small secreted cysteine-rich proteins (SSCPs) from fungi play an important role in fungi–host interactions. The plant-beneficial fungi Trichoderma spp. are in use worldwide as biocontrol agents and protect the host plant from soil-borne as well as foliar pathogens. Recently, a novel SSCP, Tsp1, has been identified in the secreted protein pool of T. virens and is overinduced upon its interaction with the roots of the maize plant. The protein was observed to be well conserved in the Ascomycota division of fungi, and its homologs are present in many plant-pathogenic fungi such as Fusarium oxysporum and Magnaporthe oryzae. However, none of these homologs have yet been characterized. Recombinant Tsp1 protein has been expressed and purified using an Escherichia coli expression system. The protein, with four conserved cysteines, forms a dimer in solution as observed by size-exclusion chromatography. The dimerization, however, does not involve disulfide bonds. Circular-dichroism data suggested that the protein has a β-strand-rich secondary structure that matched well with the secondary structure predicted using bioinformatics methods. The protein was crystallized using sodium malonate as a precipitant. The crystals diffracted X-rays to 1.7 Å resolution and belonged to the orthorhombic space group P212121 (R meas = 5.4%), with unit-cell parameters a = 46.3, b = 67.0, c = 173.2 Å. The Matthews coefficient (V M) of the crystal is 2.32 Å3 Da−1, which corresponds to nearly 47% solvent content with four subunits of Tsp1 protein in the asymmetric unit. This is the first report of the structural study of any homolog of the novel Tsp1 protein. These structural studies will help in understanding the classification and function of the protein.
1. Introduction
Small secreted cysteine-rich proteins (SSCPs) constitute a large class of fungal proteins (Rep, 2005 ▸). These secreted proteins play a very important role in the interactions between fungi and their hosts (Stergiopoulos & de Wit, 2009 ▸; Lu & Edwards, 2016 ▸; Mendoza-Mendoza et al., 2018 ▸) and are stabilized by intra-chain and inter-chain disulfide bonds. Many protein families belonging to this class act as microbial signalling effector/elicitor molecules (Baccelli, 2015 ▸; de Wit, 2016 ▸). Trichoderma spp. are widely used in agriculture as biofungicides and plant-growth promoters (Brotman et al., 2010 ▸; Mukherjee et al., 2012 ▸). Trichoderma is able to penetrate the root tissues and colonize the epidermis and outer cortex of the roots of host plants (Benítez et al., 2004 ▸). These fungi control soil-borne pathogens through mycoparasitism, antibiosis and induced defence (Elad, 2000 ▸). A large number of SSCPs have been identified in various species of Trichoderma (Kubicek et al., 2011 ▸, 2019 ▸). The well studied SSCPs Sm1, Sm2 and Epl1 (belonging to the cerato-platanin family) have been reported to elicit systemic resistance in the host plants (Djonović et al., 2006 ▸; Salas-Marina et al., 2015 ▸; Gaderer et al., 2015 ▸). In a recent study of the proteomic response of T. virens to the presence of maize roots, Lamdan et al. (2015 ▸) detected the presence of 29 SSCPs, of which 13 were downregulated and 15 had no change in abundance in the presence of roots. Gene-knockout experiments with four of these genes showed that the downregulated SSCPs induce susceptibility in plants (Lamdan et al., 2015 ▸). It is possible that these proteins act to suppress the plant defence during initial colonization by Trichoderma (Mendoza-Mendoza et al., 2018 ▸). In the same study, only one SSCP (Protein ID 215947), which we have named Tsp1 (Trichoderma small secreted protein 1) here, was observed to be upregulated in the secretome of T. virens co-cultured with maize roots. This protein is well conserved in the Ascomycota division of fungi; however, none of its homologs have yet been characterized. Structural studies would help in the classification of this novel protein and in assigning its function. Here, we report the expression, purification, crystallization and preliminary X-ray diffraction of the Tsp1 protein.
2. Materials and methods
2.1. Macromolecule production
The partial coding sequence for the tsp1 gene (bp 55–441) was PCR-amplified using the primers ATACCATGGCGGCTCCTACTCCTGC and ATACTCGAGTGGCAAGTTGGCGGGGGCATA from the genomic DNA of T. virens (the gene is intron-free and hence genomic DNA was used as a template; GenBank Accession No EHK24054.1) and cloned into the NcoI and XhoI sites of the pET-28a vector (Novagen) such that it encodes Tsp1 without the secretion-signal peptide and with a C-terminal 6×His tag. The recombinant expression vector (pET-28a-tsp1) was confirmed by DNA sequencing (Table 1 ▸).
Table 1. Macromolecule-production information.
| Source organism | T. virens |
| DNA source | Genomic DNA |
| Forward primer | ATACCATGGCGGCTCCTACTCCTGCG |
| Reverse primer | ATACTCGAGTGGCAAGTTGGCGGGGGCATA |
| Cloning vector | pET-28a(+) |
| Expression vector | pET-28a(+) |
| Expression host | E. coli Rosetta (DE3) |
| Complete amino-acid sequence of the construct produced | MAAPTPADKSMMAAVPEWTITNLKRVCNAGNTSCTWTFGVDTHLATATSCTYVVKANANASQASGGPVTCGPYTITSSWSGQFGPNNGFTTFAVTDFSKKLIVWPAYTDVQVQAGKVVSPNQSYAPANLPLEHHHHHH |
The pET-28a-tsp1 construct was transformed into the E. coli Rosetta (DE3) strain (Novagen). The cells were grown in LB medium at 37°C until the late log phase (an OD600 of ∼0.8). The cells were induced with 0.5 mM isopropyl β-d-1-thiogalactopyranoside for protein expression and were maintained at 18°C overnight under shaking conditions. The cells were harvested by centrifugation at 8000 rev min−1 for 10 min. The cell pellet was resuspended in lysis buffer [50 mM Tris–HCl pH 8.0, 150 mM NaCl, 0.5 mM dithiothreitol (DTT), 1 mM phenylmethylsulfonyl fluoride (PMSF), 5% glycerol]. Lysozyme (0.3 mg per millilitre of cell lysate) was added for enzymatic lysis and the mixture was incubated on ice for 30 min. The cells were further lysed by ultrasonication in pulse mode. The cellular debris was separated by high-speed centrifugation (24 000g) for 30 min. The major fraction of the expressed Tsp1 protein was observed in the soluble fraction of the cell lysate. The 6×His-tagged Tsp1 protein was purified by metal-chelating affinity chromatography using Ni–IDA matrix (GE Healthcare). The bound protein was eluted using a linear gradient of 25–500 mM imidazole in 25 mM Tris–HCl pH 8.0, 100 mM NaCl over ten column volumes using a BioLogic LP chromatography system (Bio-Rad). The protein was further purified using a pre-packed High S cation-exchange column (GE Healthcare) in binding buffer (25 mM sodium HEPES pH 6.5, 25 mM NaCl) and was eluted using a 25–500 mM linear gradient of NaCl. Protein purification was monitored using 15% SDS–PAGE. The purified protein was concentrated to 10 mg ml−1 using a membrane-based ultrafiltration method and was stored in storage buffer (20 mM Tris–HCl pH 8.0, 20 mM NaCl) at 4°C. The protein concentration was determined by Lowry’s method using BSA as a standard. To evaluate whether the protein forms disulfide-bonded oligomers, we performed SDS–PAGE under reducing (using 5 mM DTT) and nonreducing conditions.
2.2. Bioinformatics analysis and characterization of the Tsp1 protein
Homologous sequences to that of the Tsp1 protein were retrieved from the NCBI nonredundant sequence database using BlastP. The sequences were aligned with the Clustal Omega multiple sequence-alignment tool (http://www.ebi.ac.uk/Tools/msa/clustalo/; Madeira et al., 2019 ▸). The sequence-alignment figure was prepared with ESPript (Robert & Gouet, 2014 ▸) and the secondary structure was predicted using PSIPRED (Jones, 1999 ▸). The protein fold was predicted using the Phyre2 web server (Kelley et al., 2015 ▸).
The molecular weight and oligomeric state of the purified Tsp1 protein were determined based on the elution profile from gel-filtration chromatography under nonreducing and reducing conditions using a Superdex 75 10/300 GL column. The column was pre-equilibrated with 20 mM Tris–HCl pH 8.0, 100 mM NaCl in the presence or absence of 0.1% β-mercaptoethanol (β-ME). The column was calibrated with markers of known molecular weight (BSA, 66 kDa; ovalbumin, 45 kDa; carbonic anhydrase, 29 kDa; cytochrome c, 12.4 kDa).
Far-UV circular-dichroism (CD) spectra of the purified Tsp1 protein (at 0.2 mg ml−1) were recorded in the wavelength range 190–260 nm at 20°C using a JASCO spectropolarimeter (model J-810) in a 0.1 cm path-length quartz cuvette. The spectrum was the average of three scans. The recorded data were deconvoluted using the K2D3 server to estimate the secondary-structure content of the protein (Louis-Jeune et al., 2012 ▸). The melting curve of the Tsp1 protein was obtained by monitoring its unfolding as a function of temperature. The protein was heated from 25 to 70°C at a rate of 2°C min−1 under reducing [2 mM tris(2-carboxyethyl)phosphine (TCEP)] and nonreducing conditions, and CD spectra were recorded at a wavelength of 216 nm at 1°C intervals. A plot of the derivative of ellipticity against temperature was used to estimate the melting temperature.
2.3. Crystallization, data collection and processing
The Tsp1 protein (at a concentration of 10 mg ml−1) was screened for crystallization by the sitting-drop vapour-diffusion method at 16°C using commercially available crystallization screens. All drops were prepared by mixing 1 µl Tsp1 protein with an equal volume of reservoir solution and were equilibrated against 100 µl reservoir solution. The initial lead conditions were further optimized using the hanging-drop vapour-diffusion method in a 24-well plate using laboratory-made reservoir solutions (Table 2 ▸).
Table 2. Crystallization.
| Method | Hanging-drop vapour diffusion |
| Plate type | 24-well crystallization plate |
| Temperature (K) | 289 |
| Protein concentration (mg ml−1) | 10 |
| Buffer composition of protein solution | 20 mM Tris–HCl pH 8.0, 20 mM NaCl |
| Composition of reservoir solution | 1.9 M sodium malonate pH 6.5, 5% glycerol |
| Volume and ratio of drop | 5 µl, 1:1 (protein:reservoir solution ratio) |
| Volume of reservoir (µl) | 500 |
Crystals with dimensions of 200 × 200 × 100 µm were used for diffraction experiments. The crystals were cryoprotected by rapid soaking for a few seconds in mother liquor supplemented with 20%(v/v) glycerol; they were further soaked into Paratone-N oil (Hampton Research) to remove excess reservoir solution and immediately stored in liquid nitrogen. The diffraction data were collected on the protein crystallography beamline (PXBL) at the Indus-2 synchrotron source at 100 K. The diffraction images were recorded on a MAR CCD detector (MAR Research) at 0.9795 Å. The data were processed using XDS (Kabsch, 2010 ▸) and merged and scaled using AIMLESS in CCP4 (Winn et al., 2011 ▸).
3. Results and discussion
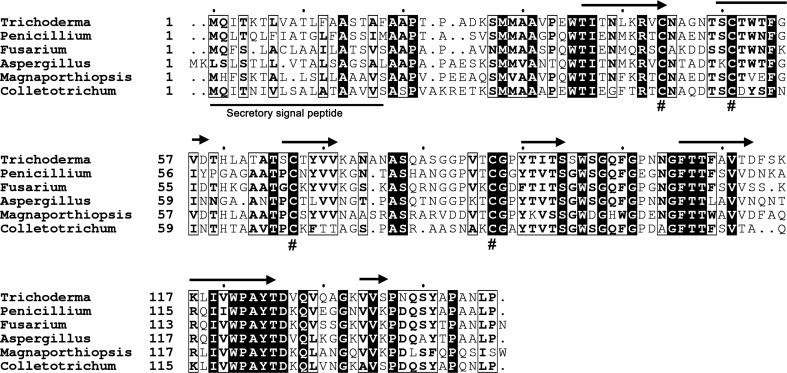
Trichoderma species are important biocontrol agents in agriculture. These fungi colonize roots, promote plant growth and provide protection of host plants against other phytopathogens (Mukherjee et al., 2013 ▸). Even though the direct interaction of Trichoderma spp. with roots has been known for decades, the exact mechanism of how Trichoderma perceive the signal to colonize roots, and how the plants stop ingression leading to an induced defence response, is rather poorly understood. The secreted protein pool is central to any fungus–host interaction (Mendoza-Mendoza et al., 2018 ▸). Tsp1 was the lone SSCP to be upregulated in the secretome of T. virens on interaction with maize roots, while as many as 13 were downregulated (Lamdan et al., 2015 ▸). The 147-residue Tsp1 is secreted into the extracellular space and contains a 17-residue signal peptide (Fig. 1 ▸). The protein is only found in Ascomycota (both pathogenic and nonpathogenic) as observed using sequence-based search methods. Tsp1 contains four conserved cysteines and was predicted to have a β-rich secondary structure using the PSIPRED web server (Fig. 1 ▸). The best model predicted using the Phyre2 server has only 35% sequence coverage, with only 13% sequence identity and a very low confidence value.
Figure 1.
Sequence alignment of Tsp1 homologs. The Tsp1 homologs from T. virens (GenBank Accession No. EHK24054.1), Penicillium arisonense (OGE55327.1), Fusarium fujikuroi (QGI66307.1), Aspergillus calidoustus (CEN62012.1), Magnaporthiopsis poae (KLU92212.1) and Colletotrichum sidae (TEA19135.1) were aligned using Clustal Omega. The N-terminal secretory signal peptide is labelled and underlined. The four conserved cysteine residues are marked with ‘#’. The predicted secondary structure (arrows representing β-strands) of Trichoderma Tsp1 is displayed above the alignment.
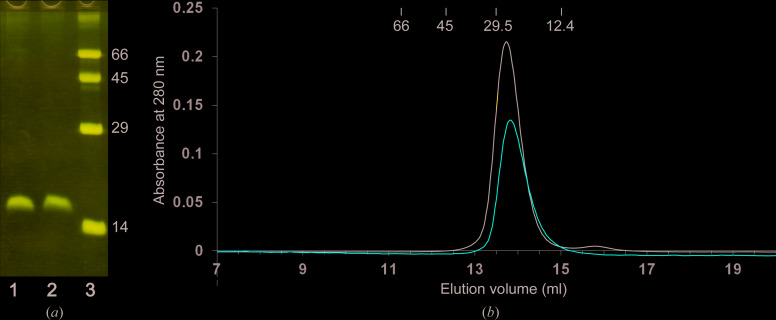
The recombinant Tsp1 protein (residues 18–147, without the N-terminal signal peptide) was expressed with a C-terminal 6×His tag using an E. coli expression system. The protein was purified to homogeneity using two-stage purification: metal-chelating affinity chromatography followed by High-S cation-exchange chromatography. The Tsp1 protein was observed to form a dimer (∼29 kDa) as determined by gel-filtration chromatography. The gel-filtration elution profile of Tsp1 was similar under reducing and nonreducing conditions (Fig. 2 ▸), suggesting that protein dimerization and protein stability are not affected by reducing conditions. The protein migration on SDS–PAGE was also similar under reducing and nonreducing conditions (Fig. 2 ▸) and corresponded to the mass of the Tsp1 peptide (∼15 kDa), suggesting that the protein dimer is not linked through a disulfide bond.
Figure 2.
Purification and size estimation of Tsp1. (a) SDS–PAGE migration of purified Tsp1. Lane 1, purified protein under reducing conditions (5 mM DTT); lane 2, purified Tsp1 under nonreducing conditions; lane 3, molecular-weight markers (labelled in kDa). (b) Elution profile of Tsp1 on Superdex 75 gel filtration under nonreducing (black line) and reducing (red line; buffer supplemented with 0.1% β-ME) conditions. The elution volumes of the standard protein markers used for calibration are also marked with their molecular weights in kDa.
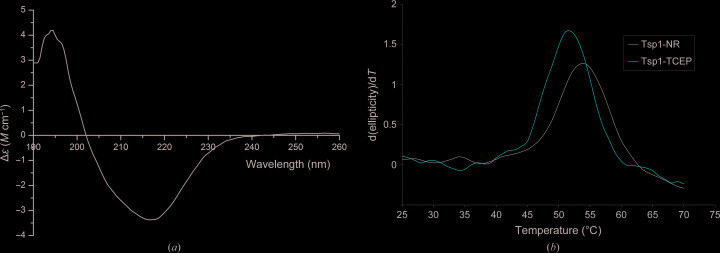
The secondary structure of the purified Tsp1 protein was estimated from CD spectra measured in the 190–260 nm wavelength range. The CD spectra have a negative peak at 216 nm, which is a signature peak for β-strand-rich proteins (Fig. 3 ▸ a). The percentage composition of the secondary structure is nearly ∼40% β-strand, as estimated by deconvoluting the CD spectra using K2D3. The melting of the secondary structure of the protein was estimated by measuring the change in ellipticity at a wavelength of 216 nm. The melting temperature calculated from the peak of the derivative CD spectra was estimated to be ∼54°C under nonreducing conditions and 52°C under reducing conditions (Fig. 3 ▸ b). The crystal structure will reveal whether the difference in the melting temperature is owing to an intramolecular disulfide bridge.
Figure 3.
Circular-dichroism (CD) analysis. (a) Graph showing the change in differential molar extinction coefficient recorded in the wavelength range 190–260 nm. (b) Melting curve of Tsp1 under nonreducing (Tsp1-NR) and reducing conditions including 2 mM TCEP (Tsp1-TCEP) as estimated by recording the change in ellipticity with increasing temperature at 216 nm wavelength. The derivative of ellipticity is plotted against temperature. The melting temperature of Tsp1 was estimated to be nearly 54°C under nonreducing conditions and 52°C under reducing conditions.
Diffraction-quality crystals were obtained with a laboratory-prepared reservoir solution (1.9 M sodium malonate pH 6.5, 5% glycerol) at 16°C using the hanging-drop vapour-diffusion method (Fig. 4 ▸). Diffraction-intensity data were collected on PXBL at the Indus-2 synchrotron, India. The crystals diffracted to 1.7 Å resolution (Fig. 5 ▸). The Tsp1 crystals belonged to the orthorhombic space group P212121, with unit-cell parameters a = 46.3, b = 67.0, c = 173.2 Å. The data-merging statistics were good, with an R meas of 0.054 (Table 3 ▸). The Matthews coefficient (V M) of the crystal is 2.32 Å3 Da−1, which corresponds to nearly 47% solvent content with four protomers of the Tsp1 protein in the asymmetric unit. As no homologous protein structure is available in the Protein Data Bank, experimental phasing methods will be required for further structural studies. The structural studies may help to reveal the role of this novel SSCP protein in fungi–plant interactions.
Figure 4.
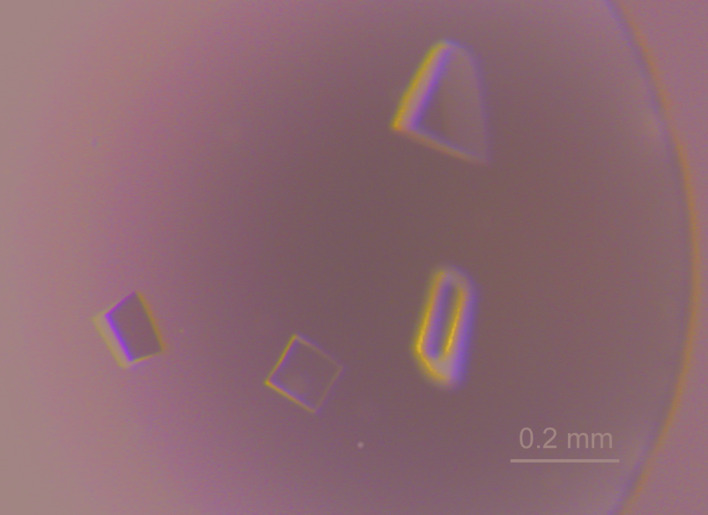
The crystals of Tsp1 used in diffraction experiments. These crystals were grown using the hanging-drop vapour-diffusion method.
Figure 5.
X-ray diffraction image of Tsp1 crystals collected on PXBL at the Indus-2 synchrotron, India. The data were processed to 1.7 Å resolution (the green circle shows the resolution limit used in data processing). The inset shows a magnified image of the highest resolution bin.
Table 3. Data collection and processing.
Values in parentheses are for the outer shell.
| Diffraction source | PXBL, Indus-2 |
| Wavelength (Å) | 0.9795 |
| Temperature (K) | 100 |
| Detector | MAR CCD |
| Crystal-to-detector distance (mm) | 155 |
| Rotation range per image (°) | 0.3 |
| Space group | P212121 |
| a, b, c (Å) | 46.25, 67.02, 173.19 |
| α, β, γ (°) | 90.0, 90.0, 90.0 |
| Mosaicity (°) | 0.29 |
| Resolution range (Å) | 45–1.7 (1.73–1.70) |
| Total No. of reflections | 410302 (21731) |
| No. of unique reflections | 60317 (3173) |
| Completeness (%) | 100 (99.9) |
| Multiplicity | 6.8 (6.8) |
| 〈I/σ(I)〉 | 21.5 (3.7) |
| R meas | 0.054 (0.486) |
| Overall B factor from Wilson plot (Å2) | 17 |
Acknowledgments
We thank Dr Vinay Kumar, ex-Head of the Radiation Biology and Health Sciences Division at Bhabha Atomic Research Center, and Dr P. Suprasanna, Head of the Nuclear Agriculture and Biotechnology Division at Bhabha Atomic Research Center, for their encouragement and support. We thank Dr Ravindra D. Makde of the High Pressure and Synchrotron Radiation Physics Division for his help in data collection on PXBL at Indus-2, Indore, India. Author contributions are as follows: RB cloned the tsp1 gene in the E. coli expression vector under the guidance of PKM. GDG optimized the protein expression, purified and crystallized the protein and performed the biophysical characterization. HM performed the gel-filtration studies. GDG and PKM analysed the results. GDG wrote the manuscript.
Funding Statement
This work was funded by Department of Atomic Energy, Government of India grant .
References
- Baccelli, I. (2015). Front. Plant Sci. 5, 769. [DOI] [PMC free article] [PubMed]
- Benítez, T., Rincón, A. M., Limón, M. C. & Codón, A. C. (2004). Int. Microbiol. 7, 249–260. [PubMed]
- Brotman, Y., Kapuganti, J. G. & Viterbo, A. (2010). Curr. Biol. 20, R390–R391. [DOI] [PubMed]
- De Wit, P. J. G. M. (2016). New Phytol. 212, 805–813. [DOI] [PubMed]
- Djonović, S., Pozo, M. J., Dangott, L. J., Howell, C. R. & Kenerley, C. M. (2006). Mol. Plant Microbe Interact. 19, 838–853. [DOI] [PubMed]
- Elad, Y. (2000). Crop Prot. 19, 709–714.
- Gaderer, R., Lamdan, N. L., Frischmann, A., Sulyok, M., Krska, R., Horwitz, B. A. & Seidl-Seiboth, V. (2015). BMC Microbiol. 15, 2. [DOI] [PMC free article] [PubMed]
- Jones, D. T. (1999). J. Mol. Biol. 292, 195–202. [DOI] [PubMed]
- Kabsch, W. (2010). Acta Cryst. D66, 125–132. [DOI] [PMC free article] [PubMed]
- Kelley, L. A., Mezulis, S., Yates, C. M., Wass, M. N. & Sternberg, M. J. E. (2015). Nat. Protoc. 10, 845–858. [DOI] [PMC free article] [PubMed]
- Kubicek, C. P., Herrera-Estrella, A., Seidl-Seiboth, V., Martinez, D. A., Druzhinina, I. S., Thon, M., Zeilinger, S., Casas-Flores, S., Horwitz, B. A., Mukherjee, P. K., Mukherjee, M., Kredics, L., Alcaraz, L. D., Aerts, A., Antal, Z., Atanasova, L., Cervantes-Badillo, M. G., Challacombe, J., Chertkov, O., McCluskey, K., Coulpier, F., Deshpande, N., von Döhren, H., Ebbole, D. J., Esquivel-Naranjo, E. U., Fekete, E., Flipphi, M., Glaser, F., Gómez-Rodríguez, E. Y., Gruber, S., Han, C., Henrissat, B., Hermosa, R., Hernández-Oñate, M., Karaffa, L., Kosti, I., Le Crom, S., Lindquist, E., Lucas, S., Lübeck, M., Lübeck, P. S., Margeot, A., Metz, B., Misra, M., Nevalainen, H., Omann, M., Packer, N., Perrone, G., Uresti-Rivera, E. E., Salamov, A., Schmoll, M., Seiboth, B., Shapiro, H., Sukno, S., Tamayo-Ramos, J. A., Tisch, D., Wiest, A., Wilkinson, H. H., Zhang, M., Coutinho, P. M., Kenerley, C. M., Monte, E., Baker, S. E. & Grigoriev, I. V. (2011). Genome Biol. 12, R40.
- Kubicek, C. P., Steindorff, A. S., Chenthamara, K., Manganiello, G., Henrissat, B., Zhang, J., Cai, F., Kopchinskiy, A. G., Kubicek, E. M., Kuo, A., Baroncelli, R., Sarrocco, S., Noronha, E. F., Vannacci, G., Shen, Q., Grigoriev, I. V. & Druzhinina, I. S. (2019). BMC Genomics, 20, 485. [DOI] [PMC free article] [PubMed]
- Lamdan, N. L., Shalaby, S., Ziv, T., Kenerley, C. M. & Horwitz, B. A. (2015). Mol. Cell. Proteomics, 14, 1054–1063. [DOI] [PMC free article] [PubMed]
- Louis-Jeune, C., Andrade-Navarro, M. A. & Perez-Iratxeta, C. (2012). Proteins, 80, 374–381. [DOI] [PubMed]
- Lu, S. & Edwards, M. C. (2016). Phytopathology, 106, 166–176. [DOI] [PubMed]
- Madeira, F., Park, Y. M., Lee, J., Buso, N., Gur, T., Madhusoodanan, N., Basutkar, P., Tivey, A. R. N., Potter, S. C., Finn, R. D. & Lopez, R. (2019). Nucleic Acids Res. 47, W636–W641. [DOI] [PMC free article] [PubMed]
- Mendoza-Mendoza, A., Zaid, R., Lawry, R., Hermosa, R., Monte, E., Horwitz, B. A. & Mukherjee, P. K. (2018). Fungal Biol. Rev. 32, 62–85.
- Mukherjee, M., Mukherjee, P. K., Horwitz, B. A., Zachow, C., Berg, G. & Zeilinger, S. (2012). Indian J. Microbiol. 52, 522–529. [DOI] [PMC free article] [PubMed]
- Mukherjee, P. K., Horwitz, B. A., Herrera-Estrella, A., Schmoll, M. & Kenerley, C. M. (2013). Annu. Rev. Phytopathol. 51, 105–129. [DOI] [PubMed]
- Rep, M. (2005). FEMS Microbiol. Lett. 253, 19–27. [DOI] [PubMed]
- Robert, X. & Gouet, P. (2014). Nucleic Acids Res. 42, W320–W324. [DOI] [PMC free article] [PubMed]
- Salas-Marina, M. A., Isordia-Jasso, M. I., Islas-Osuna, M. A., Delgado-Sánchez, P., Jimínez-Bremont, J. F., Rodríguez-Kessler, M., Rosales-Saavedra, M. T., Herrera-Estrella, A. & Casas-Flores, S. (2015). Front. Plant. Sci. 6, 77. [DOI] [PMC free article] [PubMed]
- Stergiopoulos, I. & de Wit, P. J. (2009). Annu. Rev. Phytopathol. 47, 233–263. [DOI] [PubMed]
- Winn, M. D., Ballard, C. C., Cowtan, K. D., Dodson, E. J., Emsley, P., Evans, P. R., Keegan, R. M., Krissinel, E. B., Leslie, A. G. W., McCoy, A., McNicholas, S. J., Murshudov, G. N., Pannu, N. S., Potterton, E. A., Powell, H. R., Read, R. J., Vagin, A. & Wilson, K. S. (2011). Acta Cryst. D67, 235–242. [DOI] [PMC free article] [PubMed]