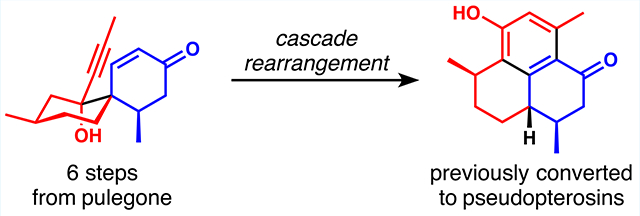
Abstract
An anionic oxy-Cope/transannular Michael addition cascade converts a spirocyclic architecture—readily available by Diels–Alder cycloaddition—into the hydrophenalene carbon skeleton of the pseudopterosin aglycones. Oxidation of the resulting cyclohexenone ring to the catechol that is characteristic of the targets completes a short formal synthesis.
Graphical Abstarct
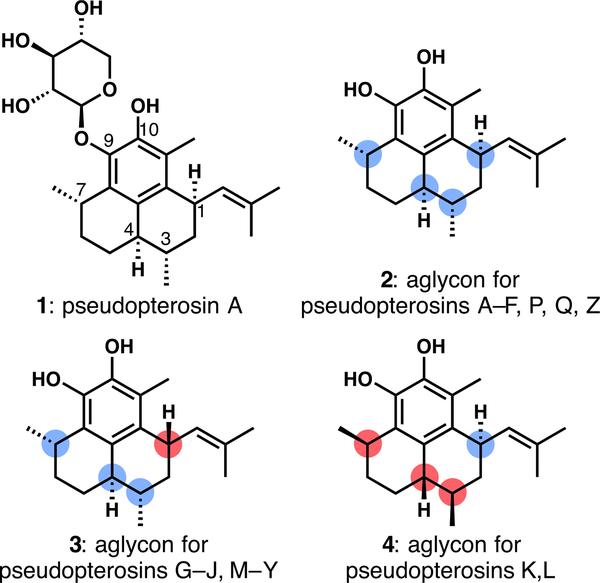
T he pseudopterosin family of diterpenoid glycosides includes over 30 members.1,2 Their diversity arises from only three stereoisomeric aglycons and the identity of the sugar attached via the C9 or C10 phenolic oxygen (Figure 1). These secondary metabolites have been of significant interest to the scientific community since the initial discovery of pseudopter-osins A–D in 1986,3 due in part to their structures and especially to their wide range of biological activities. Members of the pseudopterosin family show anticancer,4 antimalarial,5 antibacterial,5 and anti-inflammatory6 properties. For example, pseudopterosin A (1) is a significantly more potent anti-inflammatory and analgesic agent than the clinically used indomethacin.3,6 These natural products are still under active investigation, and recent studies have found new anticancer7,8 and neuroprotective properties,9,10 while an extract containing pseudopterosins is a key component in Resilience, Estee Lauder’s cosmetic skin care product.11
Figure 1.
Structures of pseudopterosin A and the three stereoisomeric pseudopterosin aglycons.
The pseudopterosins have been targeted by many synthetic chemists over the past 30 years, resulting in a large body of work on their total synthesis and the preparation of their aglycons, starting with the first synthesis of pseudopterosin A by Broka in 1988.12 As clearly articulated by Sherburn in the 2015 report of his group’s outstanding synthesis,13 all of the syntheses that preceded theirs were based on “structure-goal strategies”.14 These syntheses start from commercially available terpene or aromatic building blocks that overlap with a portion of the tricyclic scaffold; the target is synthesized by sequential chain elongations and ring closures.2 On the other hand, the synthesis by Sherburn13 and a later one by Luo15 are transformation-based, focusing on the Diels–Alder reaction and the Cope rearrangement, respectively. These approaches allow for a quick increase in molecular complexity, in both cases ultimately arriving at the target aglycons in fewer than a dozen steps. In this communication, we report a formal synthesis of the pseudopterosins that starts from the chiralpool precursor pulegone, but whose carbocyclic ring is not featured in the final product.
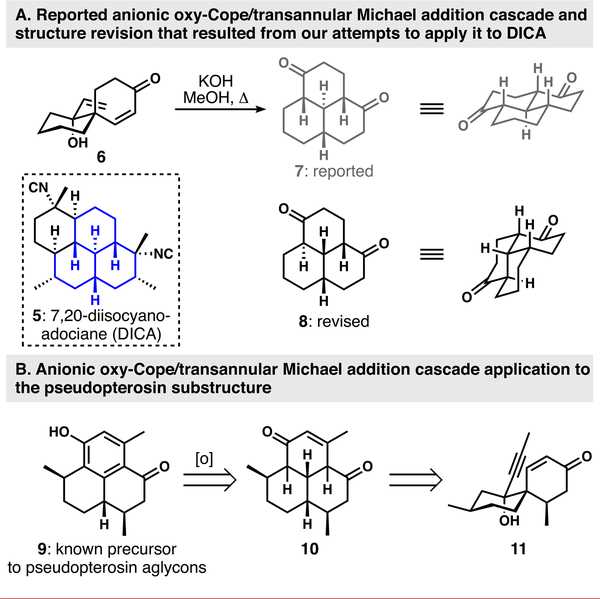
Our research group has been involved in the synthesis of isocyanoterpenes for several years.16–19 One of our early, but unsuccessful, approaches to the flagship member 7,20-diisocyanoadociane (DICA, 5) was predicated on the cascade reaction shown in Scheme 1A.16 anionic oxy-Cope/transannular Michael addition sequence was reported by Swaminathan and co-workers, who indicated that this reaction type provided the all-trans, all-chair arrangement of hydro-phenalene-type products (6 to 7).20,21 During our efforts to apply this interesting spiro-to-fused scaffold interchange to DICA, we noted that the stereochemical outcome was not as originally reported, and we offered a stereochemical revision of the product of this otherwise highly productive rearrangement to diastereomer 8.16,22
Scheme 1.
(A) Anionic Oxy-Cope/Transannular Michael Addition Cascade as Initially Reported by Swaminathan20 and the Revised Structure; (B) Strategic Application of the Cascade Reaction for a Formal Synthesis of the Pseudopterosins
While of no service to a synthesis of DICA, we noted that the hydrophenalene scaffold that results from the Swaminathan rearrangement is well-represented in terpenoid natural products and considered featuring this chemistry in an approach to the pseudopterosins (Scheme 1B). Phenol 9 and its enantiomer are known precursors of the pseudopterosin aglycons.23 9 can be generated by oxidation of cyclohexenone 10,24 which is the projected product of the anionic oxy-Cope/transannular Michael addition cascade of spirocyclic yne/enone 11. The attractiveness of this route rests in the rapid access to spirocycle 11. In this communication, we demonstrate the feasibility of this approach with a short formal synthesis of the pseudopterosins starting from pulegone.
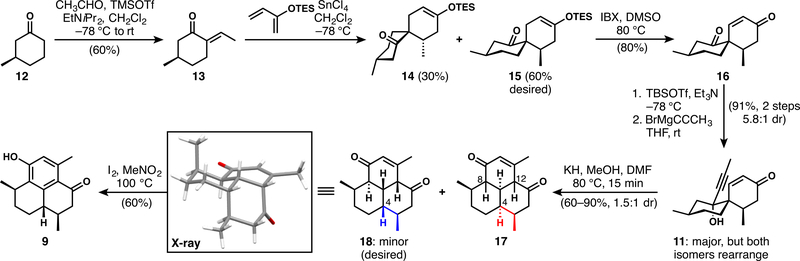
Scheme 2 describes the conversion of 3-methylcyclohexanone to the late-stage pseudopterosin aglycon intermediate 9 in only seven steps. Aldol condensation of 12 with acetaldehyde under carefully optimized conditions25 provides 13, the dienophile for the subsequent Diels–Alder reaction. SnCl4-catalyzed cycloaddition with 2-triethylsilyloxybutadiene forms the spirocyclic products in high yields, favoring the desired diasteromer 15 (2:1 dr). Significant attempts were made to bias the diastereoselectivity further; while it was possible to increase the 15:14 ratio with the application of chiral Lewis acids, the efficiency of these reactions was low. Fortunately, the diastereomers can easily be separated at this stage. Oxidation of the desired silyl enol ether to enone 16 proceeds smoothly. Attempts to selectively add propynyl organometallic reagents to the ketone over the enone were unsuccessful, so the enone was transiently protected as its cross-conjugated silyl enol ether. Propynylmagnesium bromide then adds cleanly to provide 11 with a diastereoselectivity of 5.8:1 (major isomer shown, minor isomer characterized by X-ray crystallography) after the silyl enol ether is removed in the workup.26 In our previous efforts toward DICA, we showed that both diastereomers at the alcohol undergo the oxy-Cope rearrangement to give the same product,16 and that proved true in this case as well.
Scheme 2.
Formal Synthesis of the Pseudopterosins
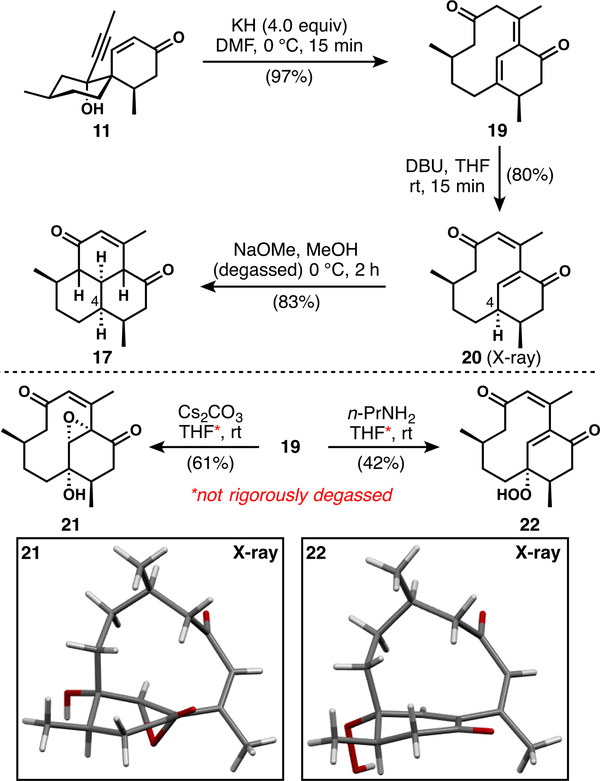
With propargylic alcohol 11 in hand, we investigated the critical anionic oxy-Cope/transannular Michael cascade. Initial attempts to effect a one-pot transformation of 11 to tricyclic products via the full cascade resulted in decomposition. Therefore, we examined a stepwise version of the process (Scheme 3). Anionic oxy-Cope rearrangement of the diastereomeric mixture of alcohols 11 induced with KH at low temperature gives the bicyclo[7.3.1] system 19 in nearly quantitative yield as a single diastereomer.27 Investigation of the double-bond isomerization and transannular Michael addition steps eventually led to clean isomerization using DBU in carefully degassed THF to give 20, which unfortunately has the undesired configuration at C4. Without careful removal of oxygen, oxygenated products such as 21 and 22 were observed during attempted transannular bond formation; they presumably arise in part from strain-induced reactivity of the various conjugate base forms of 19. Treatment of 20 with methanolic base led to efficient transannular ring closure to give 17; however, this product retains the undesired configuration at C4.28,29 Adapting the knowledge gained from the stepwise rearrangement studies, we were able to induce formation of the desired compound 18 in a one-pot process by treating alcohol 11 with potassium methoxide at 85 °C in DMF for a short time (Scheme 2). This procedure affords the desired tricyclic core in good yield, slightly favoring the undesired diastereomer (17 again) at C4 (1:1.5 dr). Further attempts to bias the selectivity toward the desired stereoisomer were unsuccessful; however, the reaction is noteworthy for forming only two diastereomers of a possible 16.30 The cascade reaction is subject to fluctuations in the isolated product yield because competitive decomposition of the products occurs under the reaction conditions. Finally, oxidation of the enone to the phenol completes a very brief formal synthesis of the pseudopterosins.24 This approach shortens the synthesis of phenol 9 to eight steps (from 13 steps by Corey31). Conversion of the phenol to the pseudopterosin aglycon can be achieved in seven further steps, and therefore, a total synthesis using this approach would be only slightly longer than those reported by Sherburn13 and Luo.15
Scheme 3.
Results of Attempted Stepwise Protocols for the Anionic Oxy-Cope/Transannular Michael Process
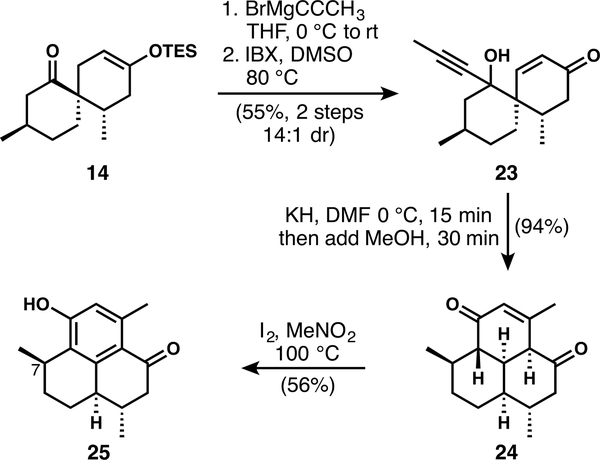
We also investigated the reactivity of 14, the minor diastereomeric product of the Diels–Alder reaction (Scheme 4). Addition of propynylmagnesium bromide followed by IBX oxidation provided the cascade precursor 23.32 In contrast to the reaction of the desired diastereomer 11, the one-pot anionic oxy-Cope/transannular Michael cascade of 23 proceeds quickly and in nearly quantitative yield at 0 °C, giving a single diastereomer of tricyclic cascade product 24, which bears the desired C3/C4 vicinal relationship. Oxidation of 24 affords phenol 25. In principle, the C7 stereogenic center might be equilibrated to the natural pseudopterosin configuration via o-quinone methide intermediates, though we have not investigated this possibility by experiment.
Scheme 4.
Reactivity of Diels–Alder Side Product 14
In conclusion, we have developed a concise formal synthesis of the pseudopterosins using a transformation-based approach. We have demonstrated that the key anionic oxy-Cope/transannular Michael cascade can be both efficient and remarkably selective and therefore might prove useful in the stereocontrolled synthesis of other polycyclic natural products. Although selectivity in the key step was not quite in our favor, the related case of substrate 23 is highly efficient and perfectly stereoselective. These results frame the need for further investigations into the impact of the structural features and reaction conditions on the diastereoselectivity of this cascade rearrangement. Overall, as a result of the rapid synthesis of the spirocyclic cascade precursor and the efficiency of the key step, our synthesis competes favorably with much of the work on the pseudopterosins that has gone before.
Supplementary Material
■ ACKNOWLEDGMENTS
This work was funded by a DFG fellowship (RA 3139/1–1; 1–2 to V.R.) and the NIH (AI-138139 to C.D.V.). We thank Dr. Joe Ziller for expert assistance with X-ray crystallographic structure determination.
Footnotes
The authors declare no competing financial interest.
■ Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.orglett.0c00486.
Experimental protocols for the preparation of, and characterization data for, all new compounds (PDF)
Accession Codes
CCDC 1982473–1982478 contain the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif, or by emailing data_request@ccdc.cam.ac.uk, or by contacting The Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, U.K.; fax: +44 1223 336033.
Contributor Information
Vincenzo Ramella, Department of Chemistry, University of California, Irvine, California 92697-2025, United States.
Philipp C. Roosen, Department of Chemistry, University of California, Irvine, California 92697-2025, United States
Christopher D. Vanderwal, Department of Chemistry and Department of Pharmaceutical Sciences, University of California, Irvine, California 92697-2025, United States
■ REFERENCES
- (1).Berrué F; McCulloch MWB; Kerr RG Marine Diterpene Glycosides. Bioorg. Med. Chem. 2011, 19, 6702–6719. [DOI] [PubMed] [Google Scholar]
- (2).Newton CG; Sherburn MS Total Synthesis of the Pseudopterosin Aglycones. Nat. Prod. Rep. 2015, 32, 865–876. [DOI] [PubMed] [Google Scholar]
- (3).Look SA; Fenical W; Matsumoto GK; Clardy J The Pseudopterosins: A New Class of Antiinflammatory and Analgesic Diterpene Pentosides from the Marine Sea Whip Pseudopterogorgia Elisabethae (Octocorallia). J. Org. Chem. 1986, 51, 5140–5145. [Google Scholar]
- (4).Rodríguez II; Shi Y-P; García OJ; Rodríguez AD; Mayer AMS; Sánchez JA; Ortega-Barria E; González J New Pseudopterosin and Seco-Pseudopterosin Diterpene Glycosides from Two Colombian Isolates of Pseudopterogorgia Elisabethae and Their Diverse Biological Activities. J. Nat. Prod. 2004, 67, 1672–1680. [DOI] [PubMed] [Google Scholar]
- (5).Ata A; Win HY; Holt D; Holloway P; Segstro EP; Jayatilake GS New Antibacterial Diterpenes from Pseudopter- ogorgia Elisabethae. Helv. Chim. Acta 2004, 87, 1090–1098. [Google Scholar]
- (6).Look SA; Fenical W; Jacobs RS; Clardy J The Pseudopterosins: Anti-Inflammatory and Analgesic Natural Products from the Sea Whip Pseudopterogorgia Elisabethae. Proc. Natl. Acad. Sci. U. S. A. 1986, 83, 6238–6240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Sperlich J; Teusch N Pseudopterosin Inhibits Proliferation and 3D Invasion in Triple-Negative Breast Cancer by Agonizing Glucocorticoid Receptor Alpha. Molecules 2018, 23, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Sperlich J; Kerr R; Teusch N The Marine Natural Product Pseudopterosin Blocks Cytokine Release of Triple-Negative Breast Cancer and Monocytic Leukemia Cells by Inhibiting NF-κB Signaling. Mar. Drugs 2017, 15, 262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Niu X; Yu C; Jiang G; Wu J; Tan X; Zhang W; Hou L Pseudopterosin A Ameliorates Ischaemia-Induced Brain Injury by Acting on Akt Signalling Pathway. Folia Neuropathol. 2018, 56, 104–111. [DOI] [PubMed] [Google Scholar]
- (10).Caplan LS; Zheng B; Dawson-Scully K; White AC; West ML Pseudopterosin A: Protection of Synaptic Function and Potential as a Neuromodulatory Agent. Mar. Drugs 2016, 14, 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Kijjoa A; Sawangwong P Drugs and Cosmetics from the Sea. Mar. Drugs 2004, 2, 73–82. [Google Scholar]
- (12).Broka CA; Chan S; Peterson B Total Synthesis of (−)-Pseudopterosin A. J. Org. Chem. 1988, 53, 1584–1586. [Google Scholar]
- (13).Newton CG; Drew SL; Lawrence AL; Willis AC; Paddon-Row MN; Sherburn MS Pseudopterosin Synthesis from a Chiral Cross-Conjugated Hydrocarbon through a Series of Cycloadditions. Nat. Chem. 2015, 7, 82–86. [DOI] [PubMed] [Google Scholar]
- (14).Corey EJ Retrosynthetic Thinking—Essentials and Examples. Chem. Soc. Rev. 1988, 17, 111–133. [Google Scholar]
- (15).Yu X; Su F; Liu C; Yuan H; Zhao S; Zhou Z; Quan T; Luo T Enantioselective Total Syntheses of Various Amphilectane and Serrulatane Diterpenoids via Cope Rearrangements. J. Am. Chem. Soc. 2016, 138, 6261–6270. [DOI] [PubMed] [Google Scholar]
- (16).Roosen PC; Vanderwal CD Investigations into an Anionic Oxy-Cope/Transannular Conjugate Addition Approach to 7,20-Diisocyanoadociane. Org. Lett. 2014, 16, 4368–4371. [DOI] [PubMed] [Google Scholar]
- (17).Daub ME; Prudhomme J; Le Roch K; Vanderwal CD Synthesis and Potent Antimalarial Activity of Kalihinol B. J. Am. Chem. Soc. 2015, 137, 4912–4915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Roosen PC; Vanderwal CD A Formal Enantiospecific Synthesis of 7,20-Diisocyanoadociane. Angew. Chem., Int. Ed. 2016, 55, 7180–7183. [DOI] [PubMed] [Google Scholar]
- (19).Karns AS; Ellis BD; Roosen PC; Chahine Z; Le Roch KG; Vanderwal CD Concise Synthesis of the Antiplasmodial Isocyanoterpene 7,20-Diisocyanoadociane. Angew. Chem., Int. Ed. 2019, 58, 13749–13752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Rao CSS; Kumar G; Rajagopalan K; Swaminathan S Base Catalysed Rearrangement of a Spiro Oxy-Cope System and Related Studies: A New Entry into Perhydrophenalene and Perhydroacenaphthylene Systems. Tetrahedron 1982, 38, 2195–2199. [Google Scholar]
- (21).Ravikumar VT; Rajagopalan K; Swaminathan S Anionic Oxycope Rearrangements in Acetylenic Spiro Systems. Tetrahedron Lett. 1985, 26, 6137–6138. [Google Scholar]
- (22).Although 8 was the major diastereomer of this reaction, a minor stereoisomer was also observed, but no evidence of 7 was seen.
- (23).Corey EJ; Carpino P Enantiospecific Total Synthesis of Pseudopterosins A and E. J. Am. Chem. Soc. 1989, 111, 5472–5474. [Google Scholar]
- (24).Yadav JS; Bhasker EV; Srihari P Synthesis of a key intermediate for the total synthesis of pseudopteroxazole. Tetrahedron 2010, 66, 1997–2004. [Google Scholar]
- (25).See the Supporting Information for details.
- (26).The more direct CeCl3-mediated addition of Grignard reagent to 15 was successful, although after oxidation with IBX, the overall yield of this two-step protocol is significantly lower. Intriguingly, in that situation the diastereoselectivity for the 1,2-addition switches, favoring the other diastereomer than that formed via the three-step sequence shown.
- (27).(a) Shanmugam P; Devan B; Srinivasan R; Rajagopalan K Studies in bromoacetylenic oxy-Cope rearrangement: Synthesis of functionalised medium-size, bicyclic and angularly fused tricyclic compounds. Tetrahedron 1997, 53, 12637–12650. [Google Scholar]; (b) Thornton PD; Cameron TS; Burnell DJ Vinylogous anionic processes in the formation and interconversion of tetracyclic ring systems. Org. Biomol. Chem. 2011, 9, 3447–3456. [DOI] [PubMed] [Google Scholar]
- (28).The relative configuration of 17 could not be ascertained; however, the configuration at C4 was determined by structural analysis of its oxidation product to a phenol that was epimeric to 9 at C4.
- (29).Of course, the transannular Michael addition of 20 could equally be characterized as a 6π -electrocyclic ring closure.
- (30).In principle, the undesired diastereomer 17 could be salvaged for a synthesis of the target molecules. Oxidation to the phenol and epimerization of C4, which is activated by the o-ketone, could provide the desired relative configuration. Initial attempts to do so were unsuccessful, likely because deprotonation of the phenol greatly reduces the acidity of the C4 proton.
- (31).Corey E; Carpino P A New Enantiospecific Route to the Pseudopterosins. Tetrahedron Lett. 1990, 31, 3857–3858. [Google Scholar]
- (32).The configuration of the propargylic carbinol stereogenic center could not be ascertained spectroscopically.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.