Abstract
Aquaporin-3 (AQP3) is a water and glycerol channel expressed in epidermal keratinocytes. Despite many studies, controversy remains about the role of AQP3 in keratinocyte differentiation. Previously, our laboratory has shown co-localization of AQP3 and phospholipase D2 (PLD2) in caveolin-rich membrane microdomains. We hypothesized that AQP3 transports glycerol and “funnels” this primary alcohol to PLD2 to form a pro-differentiative signal, such that the action of AQP3 to induce differentiation should require PLD2. To test this idea, we re-expressed AQP3 in mouse keratinocytes derived from AQP3-knockout mice. The re-expression of AQP3, which increased [3H]glycerol uptake, also induced mRNA and protein expression of epidermal differentiation markers such as keratin 1, keratin 10 and loricrin, with or without the induction of differentiation by an elevated extracellular calcium concentration. Re-expression of AQP3 had no effect on the expression of the proliferation markers keratin 5 and cyclin D1. Furthermore, a selective inhibitor of PLD2, CAY10594, and a lipase-dead PLD2 mutant, but not a lipase-dead PLD1 mutant, significantly inhibited AQP3 re-expression-induced differentiation marker expression with calcium elevation, suggesting a role for PLD2 in this process. Thus, our results indicate that AQP3 has a pro-differentiative role in epidermal keratinocytes and that PLD2 activity is necessary for this effect.
Keywords: adenovirus, aquaporin 3 (AQP3), calcium, differentiation, epidermis, glycerol, keratin 1 (K1), keratin 10 (K10), keratinocytes, knockout, phospholipase D1 (PLD1), phospholipase D2 (PLD2), proliferation, skin
INTRODUCTION
The outer layer of the skin, the epidermis, is a stratified squamous epithelium that predominantly consists of keratin-producing cells, the keratinocytes. These keratinocytes form different layers of the epidermis depending on their proliferation and differentiation status. In the basal layer, keratinocytes proliferate and express markers of a proliferative status, such as keratin 5 and keratin 14. These keratinocytes move upward and form the differentiating spinous, granular and cornified layers. The keratinocytes in the spinous layer express markers of early differentiation such as keratin 1 (K1) and keratin 10 (K10). These spinous keratinocytes later express markers of intermediate differentiation such as involucrin, while the keratinocytes that move up into the granular layer express markers of late differentiation such as loricrin (LOR) and filaggrin. The transition of granular keratinocytes into cornified keratinocytes, forming the outermost cornified layer, is marked by destruction of organelles and maturation of the cornified envelope into an insoluble, highly resistant structure surrounding the keratin-filaggrin complex and linked to the extracellular lipid milieu (Bikle and Pillai, 1993). Thus, the cornified layer provides a protective skin barrier to allow retention of water and other important metabolites. These programmed and balanced processes of proliferation and differentiation are therefore important in maintaining skin health. Although there have been many studies performed to elucidate the regulation of these proliferation and differentiation processes, the exact regulators and signaling mechanism(s) underlying these events are still unclear. One of the well-known regulators of epidermal differentiation is the extracellular calcium concentration, with lower concentrations maintaining a proliferative state and elevated levels inducing differentiation (Tu and Bikle, 2013).
Aquaporins are a family of transmembrane proteins that facilitate the transport of water, and in some cases small solutes, across cell membranes (Verkman, 2008). Thirteen aquaporins have been identified to date and the expression of aquaporins 3, 9, and 10 has been reported in the epidermis (Boury-Jamot et al., 2006; Ma et al., 2002; Sougrat et al., 2002; Sugiyama et al., 2001). Aquaporin 3 (AQP3) not only transports water but also allows transport of small solutes like glycerol across the plasma membrane (Hara-Chikuma and Verkman, 2005; Hara and Verkman, 2003). In mammalian skin, AQP3 is expressed in the basal layer as well as in the suprabasal layers of the epidermis (Guo et al., 2013; Hara-Chikuma and Verkman, 2008; Lee et al., 2012; Verkman and Mitra, 2000), while its presence in the stratum corneum is controversial (Jungersted et al., 2013; Sougrat et al., 2002). Studies characterizing AQP3 knockout mice suggest that this aquaglyceroporin is required for maintaining normal stratum corneum hydration, skin elasticity, wound healing, epidermal biosynthesis and barrier recovery (Hara-Chikuma and Verkman, 2008; Ma et al., 2002). AQP3 has been shown to be involved in keratinocyte proliferation (Hara-Chikuma et al., 2009; Nakahigashi et al., 2011; Serna et al., 2014) as well as in keratinocyte differentiation (Bollag et al., 2007; Dumas et al., 2002; Kim and Lee, 2010; Zheng and Bollag, 2003). The epidermal phenotype of AQP3 knockout mice is linked to decreased epidermal glycerol content and can be corrected by treatment with glycerol, but not with other humectants (Ma et al., 2002). These reports highlight the importance of glycerol and its transport channel in keratinocyte physiology.
The phospholipase D (PLD) isoforms PLD1 and PLD2 are lipolytic enzymes that have been implicated in a variety of cellular processes, including cell proliferation and differentiation (Banno, 2002; Di Fulvio et al., 2012; Jenkins and Frohman, 2005). PLD2 can hydrolyze phosphatidylcholine to produce choline and phosphatidic acid, or, in the presence of a primary alcohol, catalyzes a transphosphatidylation reaction to produce phosphatidylalcohols. Our laboratory has previously demonstrated that PLD2 can use glycerol in the transphosphatidylation reaction to form phosphatidylglycerol (Zheng et al., 2003), a largely unrecognized lipid signal, and that this signaling module/lipid shows pro-differentiative effects in keratinocytes [(Bollag et al., 2007) and reviewed in (Qin et al., 2011)].
Considering the importance of regulators of proliferation and differentiation processes in maintaining the normal function of the skin, there is a need to further define the role of AQP3 in keratinocytes and to identify the mechanisms underlying its effects in the epidermis. Here, we have re-expressed AQP3 in AQP3-knockout keratinocytes and determined the effect of this manipulation on proliferative and differentiative processes. We hypothesized that AQP3 increases glycerol transport and “funnels” this primary alcohol to PLD2 such that its effects on differentiation are mediated by PLD2.
RESULTS
Validation of expression and activity of re-expressed AQP3 in AQP3-knockout keratinocytes
To better understand the role of AQP3 in keratinocytes, we utilized an approach that involved re-expression of wild-type AQP3 in keratinocytes derived from AQP3-knockout mice. We first confirmed the re-expression of AQP3 protein upon adenoviral infection. There was significant re-expression of AQP3 protein in keratinocytes infected with adenovirus expressing AQP3 as compared to vector-infected keratinocytes (Figure 1a). The diffuse band around 35-40 kDa represents glycosylated-AQP3 (Boury-Jamot et al., 2006), which suggests that the re-expressed AQP3 was able to localize to the plasma membrane and function as a transporter. Since AQP3 has been shown to be important for transporting glycerol into keratinocytes, the functionality of re-expressed AQP3 was further confirmed by measuring [3H]glycerol uptake by these cells. As shown in Figure 1b, cells re-expressing AQP3 exhibited significantly increased glycerol uptake. These results confirmed the validity of our re-expression approach.
Figure 1. Validation of expression and activity of re-expressed AQP3 in AQP3-knockout keratinocytes.
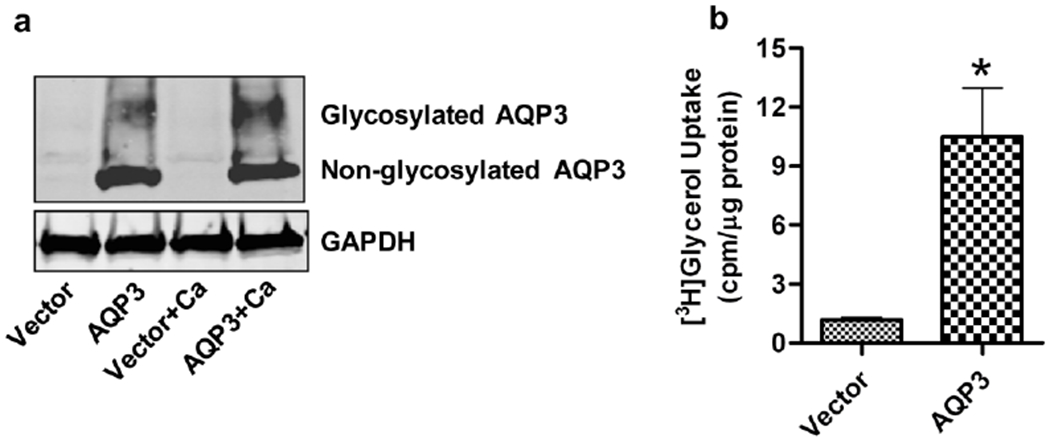
Primary cultures of AQP3-knockout keratinocytes were allowed to reach approximately 70-80% confluence and then infected with adenoviruses expressing either wild-type AQP3 or vector alone using a MOI of 25 for 24 hours. Fresh control medium (50μM calcium) or medium containing 125μM calcium was added for another 24 hours. (a) Total cell lysates were analyzed by Western blotting using antibodies against AQP3 and the GAPDH loading control. AQP3 is seen as a non-glycosylated, 28 kDa band and a diffuse band at 35-40 kDa, representing the glycosylated form. The figure shown is representative of three independent experiments. (b) [3H]glycerol uptake by the cells is shown as cpm/μg protein. The data represent the means ± SEM from three independent experiments. *p<0.05 versus vector-infected keratinocytes.
AQP3 re-expression increases expression of differentiation markers in AQP3-knockout keratinocytes
Once we confirmed the re-expression and activity of AQP3 in AQP3-knockout keratinocytes, we then investigated the role/effect of re-expressed AQP3 in these cells. To better understand the effect of the re-expressed AQP3 on various differentiation and proliferation markers, we treated one set of cells with a moderately increased calcium concentration to stimulate keratinocyte differentiation. The mRNA expression of the early differentiation marker, keratin 1, was significantly increased in AQP3-re-expressing keratinocytes (Figure 2a). Under differentiating conditions (treatment with 125μM calcium), mRNA levels of both early (keratin 1 and keratin 10) and late differentiation markers (loricrin) were significantly increased in AQP3 re-expressing keratinocytes compared to AQP3-null keratinocytes (Figure 2a–c). Consistent with the changes in mRNA expression, the protein levels of keratin 10 and loricrin were also significantly increased upon AQP3 re-expression (Figure 2d–e). These results clearly suggested a pro-differentiative role for AQP3 in mouse keratinocytes.
Figure 2. AQP3 re-expression increases expression of differentiation markers in AQP3-knockout keratinocytes.
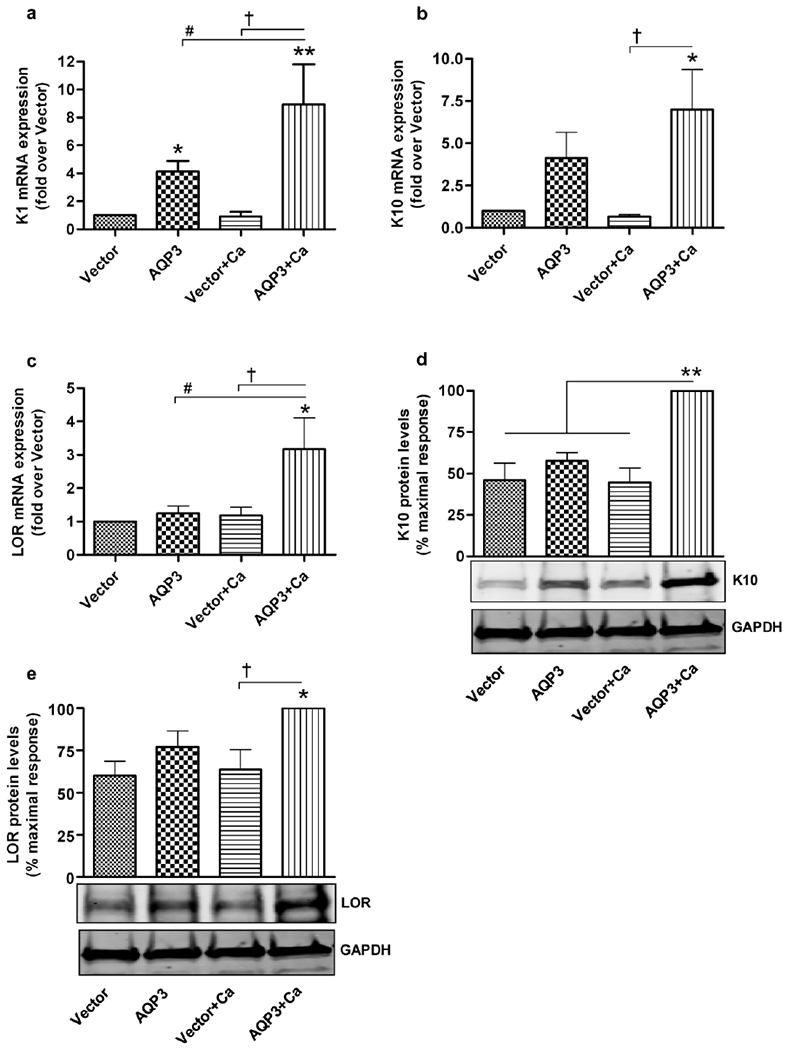
Keratinocytes from AQP3-knockout neonatal mice were treated as described in Figure 1. Cells were harvested for quantitative RT-PCR (qRT-PCR) analysis of the expression of (a) keratin 1 (K1), (b) keratin 10 (K10) and (c) loricrin (LOR). Expression was analyzed with the ΔΔCt method using an average value of GAPDH and RPLP0 as endogenous controls, and values are shown as the fold over the vector-infected group. (d and e) Cells were harvested for Western analysis using antibodies against (d) keratin 10 (K10) and (e) loricrin (LOR) with GAPDH as the loading control. Quantitation of the values from three independent experiments is shown, with values expressed as the percent (%) maximal response. *p<0.05, **p<0.01 compared to the vector-infected group and the other indicated groups (in panel d); † or #p<0.05 versus the indicated groups.
AQP3 re-expression has no effect on expression of markers of keratinocyte proliferation
Since earlier reports suggested a pro-proliferative role for AQP3 in keratinocytes (Hara-Chikuma et al., 2009; Nakahigashi et al., 2011; Serna et al., 2014), we investigated the effect of re-expressed AQP3 on proliferation markers. As shown in Figure 3, the presence or absence of AQP3 had no significant effect on the mRNA expression of the proliferation markers keratin 5 and cyclin D1. These results suggest that AQP3 does not play a significant role in the proliferation of keratinocytes, at least under these conditions.
Figure 3. AQP3 re-expression had no effect on the expression of markers of keratinocyte proliferation.
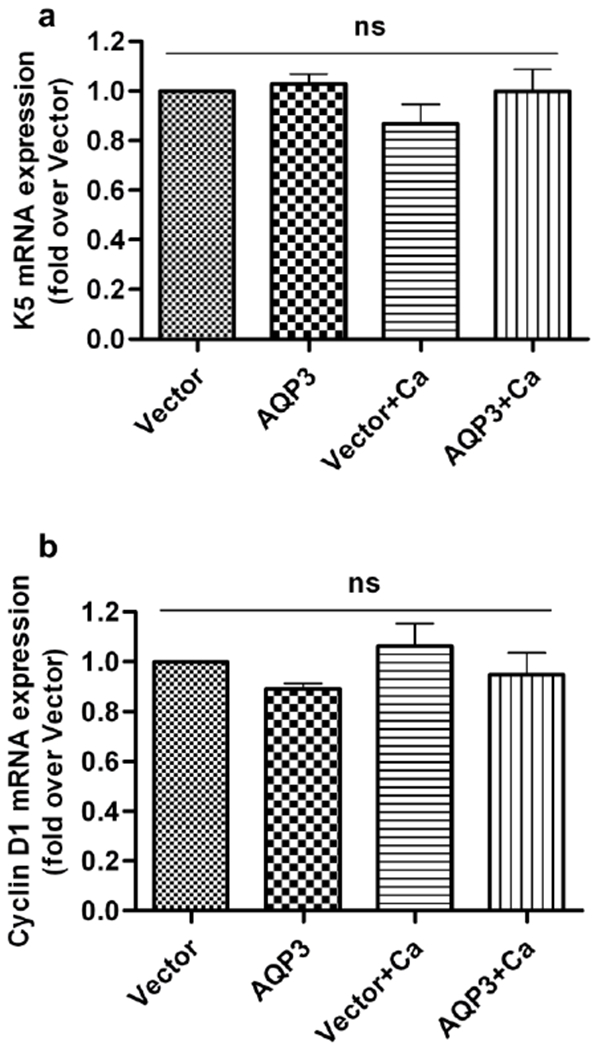
Keratinocytes from AQP3-knockout neonatal mice were treated as described in Figure 1. Cells were harvested for qRT-PCR analysis, and the mRNA expression of (a) keratin 5 (K5) and (b) cyclin D1 was analyzed using the ΔΔCt method with the average of GAPDH and RPLP0 expression as the endogenous control. These results are shown as the fold over the vector-infected group and were derived from the analysis of at least three independent experiments; ns: non-significant.
The PLD2 inhibitor, CAY10594, inhibits the AQP3 re-expression-induced expression of markers of keratinocyte differentiation
Since our results demonstrated a pro-differentiative role for re-expressed AQP3 in keratinocytes, we sought to identify the possible downstream signaling pathway involved. Earlier reports from our laboratory have shown that the PLD2/AQP3 module is activated by a moderately elevated calcium concentration and suggested the involvement of this module in promoting early keratinocyte differentiation (Zheng et al., 2003; Bollag et al., 2007). Therefore, we investigated the role of PLD2 in AQP3 re-expression-induced keratinocyte differentiation. We used the PLD2-selective inhibitor, CAY10594, at a dose previously shown to selectively inhibit PLD2 in intact cells (Scott et al., 2009). In an earlier report we demonstrated the effectiveness of CAY10594 in inhibiting PLD activity in keratinocytes, and in the same study we showed that treatment with this concentration of CAY10594 has no cytotoxic effect on keratinocytes (Arun et al., 2013). Treatment of keratinocytes with this PLD2 inhibitor, together with stimulation of differentiation with an elevated calcium concentration, resulted in a significant inhibition of the AQP3-re-expression-induced expression of the early differentiation markers, keratin 1 and keratin 10 (Figure 4a–b). The protein levels of keratin 10 also correlated with the mRNA results (Figure 5a). We did not observe statistically significant differences in the mRNA expression of loricrin (Figure 4c). However, western analysis showed that the increased loricrin protein levels were significantly reduced by CAY10594 treatment in AQP3-re-expressing keratinocytes under conditions of an elevated extracellular calcium concentration (Figure 5b). These results suggest that the ability of AQP3 re-expression to induce differentiation depends upon PLD2 activity.
Figure 4. The PLD2 inhibitor, CAY10594, inhibited the AQP3 re-expression-induced increase in mRNA levels of markers of keratinocyte differentiation.
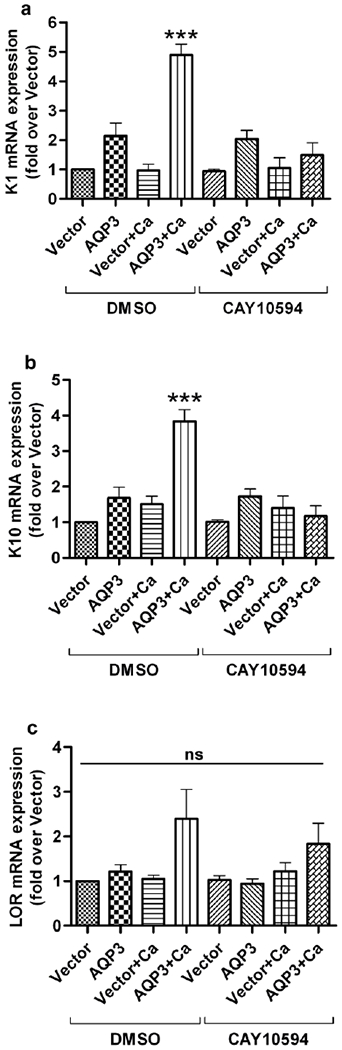
Primary AQP3-knockout mouse keratinocytes were allowed to reach approximately 70-80% confluence and then infected with adenoviruses expressing either wild-type AQP3 or vector alone using an MOI of 25 for 24 hours. Medium containing the PLD2-selective inhibitor, CAY10594 (1 μM) alone or in combination with an elevated calcium concentration (125μM) was added to the keratinocytes for an additional 24 hours. (a) The cells were harvested for qRT-PCR analysis, and mRNA expression of (a) keratin 1 (K1), (b) keratin 10 (K10) and (c) loricrin (LOR) was determined using the ΔΔCt method with GAPDH as the endogenous control. The results are shown as the fold over the vector-infected group and were derived from at least three independent experiments. *** p<0.001 compared to all other groups; ns, non-significant.
Figure 5. The PLD2 specific inhibitor, CAY10594, inhibited the AQP3 re-expression-induced increase in protein levels of markers of keratinocyte differentiation.
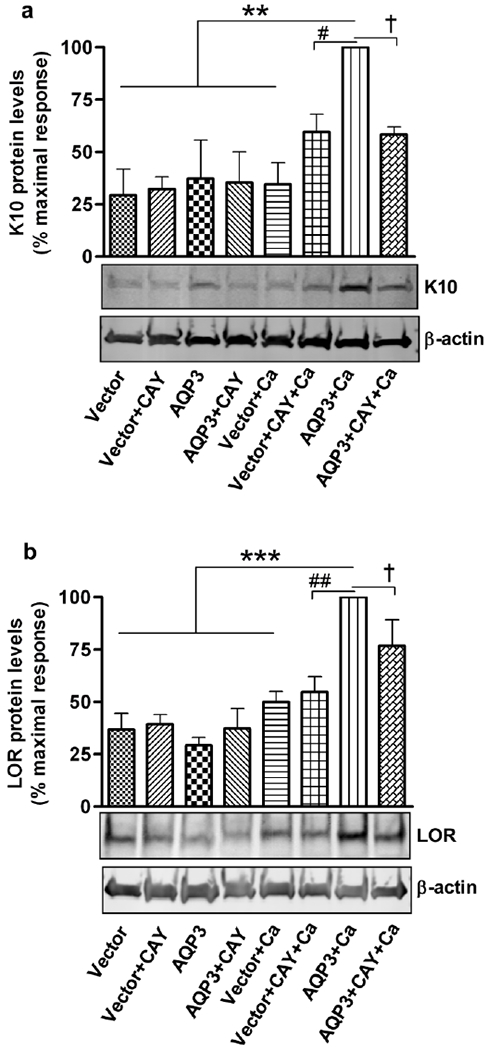
Experiments were performed as described in Figure 4 and cells were harvested for Western analysis using antibodies against (a) keratin 10 (K10) and (b) loricrin (LOR). β-actin served as the loading control. Quantitation of the values from three independent experiments is shown, with values expressed as the percent (%) maximal response. **p<0.01, ***p<0.001 versus the vector-infected control and the other indicated groups; † or #p<0.05, ##p<0.01 versus the indicated groups.
Lipase-dead PLD2, but not lipase-dead PLD1, inhibits the AQP3 re-expression-induced increase in markers of keratinocyte differentiation
To further confirm this PLD2 requirement, we utilized lipase-dead (LD) PLD2 mutant-expressing adenoviruses to inhibit endogenous PLD2. Lipase-dead PLD2 mutant expression inhibited AQP3 re-expression-induced mRNA expression of K1, K10 and loricrin under elevated calcium conditions (Figure 6a–c). Even under basal conditions, AQP3-induced K10 expression was significantly inhibited by over-expression of the lipase-dead PLD2 mutant (Figure 6b). However, over-expression of the lipase-dead PLD1 mutant did not inhibit AQP3 re-expression-mediated induction of these differentiation markers (Figure 6d–f). These results suggested the involvement of PLD2, but not PLD1, in AQP3-mediated keratinocyte differentiation.
Figure 6. The lipase-dead PLD2 mutant, but not lipase-dead PLD1, inhibited the AQP3 re-expression-induced increase in markers of keratinocyte differentiation.
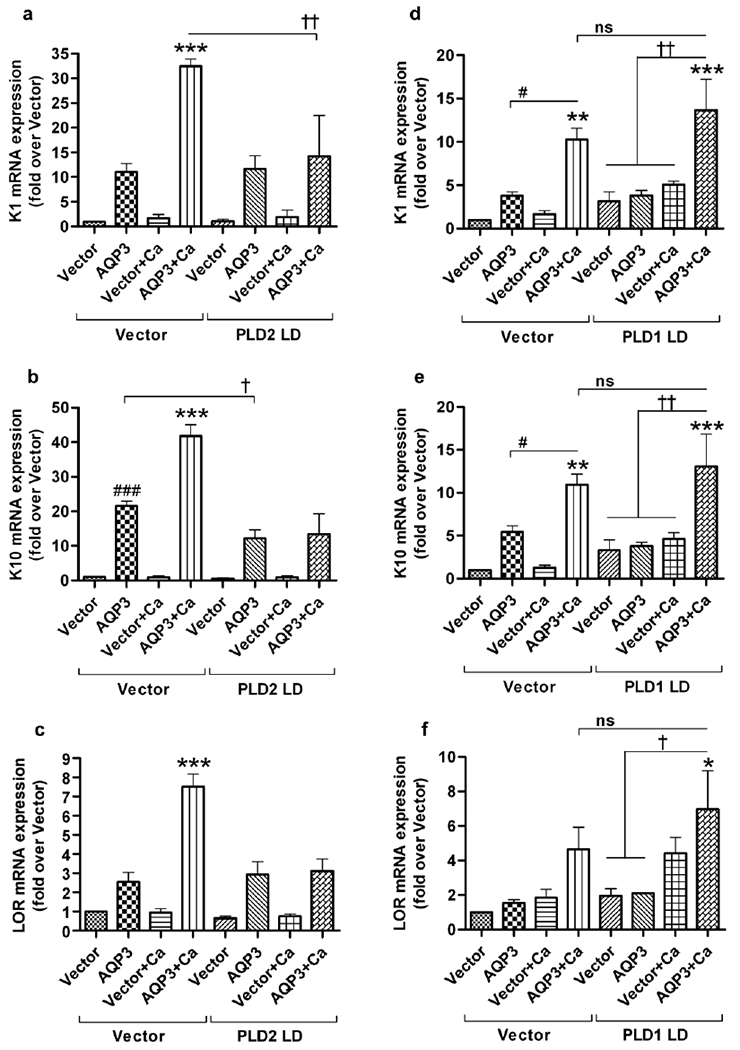
(a,b,c) AQP3 knockout primary keratinocytes at approximately 70-80% confluence were infected with adenovirus expressing either wild-type AQP3 or vector using a MOI of 25 in two groups. One group received additional adenovirus (25 MOI) expressing the lipase-dead PLD2 mutant (PLD2 LD) while the other group received adenovirus expressing vector. After 24 hours fresh medium with basal or an elevated calcium concentration (125μM) was added to the keratinocytes for an additional 24 hours. The cells were harvested for qRT-PCR analysis and mRNA expression of (a) keratin 1 (K1), (b) keratin 10 (K10) and (c) loricrin (LOR) was determined using the ΔΔCt method with GAPDH as the endogenous control. These results are shown as the fold over the vector/vector-infected group and were derived from at least three independent experiments. *** p<0.001 versus all other groups unless indicated otherwise; ###p<0.001 versus vector/vector; †p<0.05, ††p<0.01 as indicated. (d,e,f) AQP3 knockout primary keratinocytes were infected as above except that an MOI of 12.5 was used both for adenovirus expressing AQP3 or vector and the additional adenovirus expressing lipase-dead PLD1 mutant (PLD1 LD) or vector. mRNA expression of (d) keratin 1 (K1), (e) keratin 10 (K10) and (f) loricrin (LOR) was determined as above. ***p<0.001 versus all other groups unless otherwise indicated; **p<0.01 and *p<0.05 versus vector/vector; †p<0.05, ††p<0.01 as indicated. ns: non-significant. Similar results were obtained in another experiment (repeated once for n=2) using an MOI of 25 for both viruses.
DISCUSSION
In this study we determined the role of AQP3 in keratinocyte differentiation and identified PLD2 as a mediator of an AQP3-induced differentiative effect. Although previous studies have suggested a role for AQP3 in keratinocytes, the results have been controversial with both pro-differentiative (Dumas et al., 2002; Kim and Lee, 2010; Matsuo and Kawano, 2014; Zheng and Bollag, 2003) and pro-proliferative effects described (Hara-Chikuma et al., 2009; Nakahigashi et al., 2011; Serna et al., 2014). Here we have attempted to address this question using a strategy that involved the re-expression of AQP3 in primary cultures of keratinocytes derived from AQP3-knockout mice using adenovirus expressing wild-type AQP3. First, we verified the re-expression of AQP3 by Western analysis (Figure 1a). The glycosylation of aquaporins helps them to localize to the plasma membrane (Hendriks et al., 2004), where they function to transport water and glycerol. Thus, the presence of glycosylated AQP3 (Figure 1a) upon adenoviral transduction of keratinocytes suggested the functioning of the re-expressed AQP3. The functionality was confirmed by the demonstration of increased [3H]glycerol uptake into keratinocytes expressing AQP3 as compared to those lacking AQP3 (Figure 1b). Similar to these results, Jiang et al. (Jiang et al., 2011) also demonstrated a significant increase in glycerol uptake upon an increase in AQP3 levels following stimulation with agonists of peroxisome proliferator-activated receptors.
Once our approach was validated, we investigated the effect of the re-expressed AQP3 on keratinocyte differentiation and proliferation. However, we suspected that the presence or absence of AQP3 alone may not be sufficient for observing changes in various differentiation markers, particularly late markers, under basal growth conditions. Therefore, to maximize the effect of manipulating AQP3 levels, differentiation markers were examined under basal conditions (50μM calcium) as well as with a moderately increased extracellular calcium concentration (125μM) to stimulate keratinocyte differentiation. Previous reports have suggested the importance of extracellular calcium levels in inducing differentiation, as an increasing concentration gradient of calcium from the proliferating basal layer to the differentiated granular layer is observed in the epidermis in situ (Elias et al., 2002; Lee et al., 1992; Menon et al., 1992). Indeed, low medium calcium levels promote a basal-like state of the keratinocyte, and raising the medium calcium concentration triggers keratinocyte differentiation (Pillai et al., 1990). Moreover, a calcium concentration in the range of 90 to 120μM has been reported to be optimal for inducing mouse keratinocyte differentiation (Li et al., 1995; Yuspa et al., 1989). Using a comparable moderately elevated extracellular calcium concentration, we demonstrated that re-expression of AQP3 in AQP3-knockout keratinocytes not only induced a significant increase in the expression of the early differentiation marker K1 but also enhanced the effect of this calcium concentration on the expression of the early differentiation marker, K10, as well as the late differentiation marker, loricrin (Figure 2). The lack of induction of differentiation markers in the vector-infected, calcium-treated keratinocytes is likely attributable to the absence of AQP3 in these keratinocytes. Kim and Lee (2010) also have reported attenuation of the calcium-induced increase in the expression of differentiation markers such as K10, involucrin and loricrin upon siRNA-mediated AQP3 knockdown in normal human keratinocytes, again suggesting a requirement for AQP3 in the induction of differentiation. Another group showed that urea not only increases AQP3 expression but also the mRNA expression of various differentiation markers (Grether-Beck et al., 2012), also consistent with the results of this study.
Although several studies support the role of AQP3 in the proliferation of keratinocytes (Hara-Chikuma et al., 2009; Nakahigashi et al., 2011; Serna et al., 2014), we did not observe a significant effect of the presence or absence of AQP3 on markers of proliferation such as keratin 5 and cyclin D1 (Figure 3). These results suggest that the AQP3 is a pro-differentiative signaling protein with no significant role in proliferation of keratinocytes, at least under the examined conditions.
We and others have shown that AQP3-mediated transport of glycerol plays a biological role in epidermal differentiation (Bellemere et al., 2008; Qin and Bollag, 2013; Qin et al., 2011; Bollag et al., 2007). Therefore, we were interested in identifying/confirming the downstream signaling factors involved in the keratinocyte differentiation induced by the glycerol transported by AQP3. Previously, our laboratory has shown that AQP3 and PLD2 co-localize in caveolin-rich membrane microdomains (Zheng and Bollag, 2003). We have also demonstrated the involvement of this signaling module in keratinocyte differentiation (Bollag et al., 2007) and have shown that PLD2 can utilize glycerol in vitro in the transphosphatidylation reaction to generate phosphatidylglycerol (Zheng et al., 2003). Recently we have shown that an agent that decreases phosphatidylglycerol levels (a membrane-permeant caveolin-1 scaffolding domain peptide) also inhibits calcium-induced differentiation of mouse keratinocytes (Qin and Bollag, 2013), suggesting a role for phosphatidylglycerol in this process. Therefore, we hypothesized that the effect of re-expression of AQP3 on keratinocyte differentiation might be mediated by PLD2. Utilizing CAY10594, a PLD2-selective inhibitor, and a lipase-dead PLD2 mutant, we demonstrated inhibition of the AQP3 re-expression-induced increase in the expression of differentiation markers upon stimulation of differentiation with a moderately elevated calcium concentration (Figures 4, 5 and 6). However, no inhibitory effect of CAY10594 on AQP3-induced expression of differentiation markers was observed under basal, low calcium conditions. This result is likely due to the fact that, during these experiments, AQP3 was re-expressed for 24 hours before inhibiting PLD2 (by treating with CAY10594). Thus, 24 hours seems to be sufficient not only for re-expressing AQP3 but also for the re-expressed protein to exert its effect on differentiation. However, in experiments involving the lipase-dead PLD2 mutant, the AQP3 re-expression-induced increase in K10 expression was inhibited by the PLD mutant under basal conditions (Figure 5b). In these experiments adenovirus expressing lipase-dead PLD2 and wild-type AQP3 were simultaneously infected and thus lipase-dead PLD2 was able to inhibit AQP3-induced K10 expression. This result is consistent with our findings of an association between AQP3 and PLD2 in epidermal keratinocytes (Zheng et al., 2003; Zheng and Bollag, 2003). On the other hand, lipase-dead PLD1 had no significant inhibitory effect on the mRNA levels of differentiation markers (Figure 6d–f) in response to AQP3 re-expression, suggesting that PLD1 is not involved in AQP3-induced differentiation of keratinocytes. Thus, the present results confirmed the involvement of PLD2 in mediating the role of AQP3 in inducing keratinocyte differentiation.
In summary, we identified a signaling pathway for keratinocyte differentiation. AQP3, as a glycerol transporter, allows glycerol to enter the cell and provide this primary alcohol to associated PLD2. Although we have not eliminated the possibility of a role for PLD2-generated phosphatidic acid or other lipid signal in AQP3 re-expression-induced differentiation, we hypothesize that PLD2 through its transphosphatidylation reaction converts the glycerol to phosphatidylglycerol, and phosphatidylglycerol then exerts a differentiating effect (Bollag et al., 2007). This hypothesis will be tested in future studies. Finally, the identification of a differentiative role of AQP3 in keratinocytes suggests the possibility of targeting this signal to develop treatments for hyperproliferative skin diseases such as non-melanoma skin cancers and psoriasis, in which the AQP3 levels and/or localization are abnormal (Lee et al., 2012; Voss et al., 2011) and the differentiation process is impaired (Kim and Lee, 2010; Qin et al., 2011; Sugimoto et al., 2013).
MATERIALS AND METHODS
Materials
Antibodies against proteins of interest were obtained from the following suppliers: AQP3 (#B8185; LifeSpan Biosciences, Inc., Seattle, WA); β-actin (#A5441; Sigma-Aldrich, St. Louis, MO); keratin 10 (#PRB-159P) and loricrin (#PRB-140C) (Covance Inc., Denver, PA); and glyceraldehyde-3-phosphate dehydrogenase (GAPDH; #CB1001; EMD Millipore, Billerica, MA). AlexaFluor IR680- and IR800-conjugated secondary antibodies were from LiCor Biosciences (Lincoln, NE). PVDF membrane was from Millipore (Billerica, MA), and iScript cDNA synthesis kits were purchased from Bio-Rad (Hercules, CA.). The keratinocyte serum-free media (K-SFM) containing human recombinant EGF and bovine pituitary extract (added fresh) was purchased from Life Technologies (Carlsbad, CA; Cat. No. 7010022). The AQP3 knockout mice were generously provided by Dr. Alan Verkman (University of California, San Francisco, USA).
Adenoviral constructs amplification
The adenovirus pAdTrack-CMV shuttle vectors were acquired from Dr. Bert Vogelstein (Howard Hughes Medical Institute, MD), and adenovirus expressing wild-type AQP3 (Zheng and Bollag, 2003) was generated using the AdEasy system as described in (He et al., 1998; Shapiro et al., 2010). The lipase-dead PLD2 and PLD1 mutants were generated as in (Sung et al., 1997). Amplification of viruses was performed as previously described (Arun et al., 2013).
Cell culture and experimental design
All animal studies were approved by the Georgia Regents University Institutional Animal Care and Use Committee. Primary epidermal mouse keratinocytes were prepared from 1-3 day old neonatal AQP3 knockout mice (Ma et al., 2002) and seeded in plating medium as described previously (Jung et al., 1999). After attachment the medium was replaced the next day with keratinocyte serum-free medium (K-SFM) containing 50μM CaCl2. When the keratinocytes reached 70-80% confluence they were infected with adenoviruses expressing either vector or wild-type AQP3 at a multiplicity of infection (MOI) of 25 in K-SFM. In the experiments involving the PLD2 lipase-dead mutant, a total of 50 MOI was used in all the infected groups with 25 MOI of vector or AQP3-expressing adenovirus and 25 MOI of vector or lipase-dead PLD2 adenovirus. For PLD1 experiments, an adenovirus concentration of 12.5 MOI each (for a total of 25 MOI) was used. The virus-containing medium was removed 24 hours post-infection and replaced with K-SFM containing control (50μM) or an elevated calcium concentration (125μM) to stimulate keratinocyte differentiation (Li et al., 1995; Yuspa et al., 1989). After an additional 24 hours, the cells were harvested for further analysis. For studies involving the PLD2 inhibitor, the cells were infected with virus for 24 hours and then treated with CAY10594 in the presence or absence of an elevated calcium concentration for 24 hours prior to quantitative real-time RT-PCR (qRT-PCR) and Western blot analyses.
Western blot analysis
At the end of the treatment period, primary keratinocytes were harvested using hot lysis buffer [0.1875M Tris-HCl (pH 8.5), 3% SDS, and 1.5mM EDTA]. The solubilized cells were scraped and homogenized by repeated pipetting, and 3x sample buffer (30% glycerol, 15% beta-mercaptoethanol, 1% bromophenol blue, 54% water) was added prior to boiling the samples. Equal sample volumes were subjected to SDS-PAGE and then transferred to PVDF membranes using a Trans-Blot Turbo Transfer system (Bio-Rad, Hercules, CA). Membranes were blocked with 5% non-fat dry milk for at least 30 minutes before incubating with the appropriate antibodies. Immunoreactivity was visualized and quantified using infrared imaging on an Odyssey imaging system (LI-COR Biosciences, Lincoln, NE).
Quantitative real-time RT-PCR analysis
Total RNA was extracted using a PerfectPure RNA tissue kit (5 PRIME, Inc, Gaithersburg, MD, USA) as per the manufacturer’s protocol. The quality and quantity of total RNA were assayed using a Nanodrop instrument (NanoDrop Technologies, Wilmington, DE). An iScript cDNA synthesis kit (Bio-Rad Laboratories, Hercules, CA, USA) was used to reverse transcribe equal quantities of total RNA (1μg) following the manufacturer’s instructions. The cDNA was diluted five times with DNase-free water and an equal amount of diluted cDNA was used in qRT-PCR reactions. Taqman probes were purchased from Applied Biosystems (Life Technologies, Grand Island, NY). qRT-PCR reactions were performed using the Fast Reagent PCR Master Mix (Applied Biosystems) and the StepOnePlus Real-Time PCR System (Applied Biosystems) as per the manufacturer’s protocol. Relative gene expression was calculated by the delta-delta cycle threshold (ΔΔCt) method using GAPDH and/or ribosomal protein, large, P0 (Rplp0) as endogenous control genes with normalization to the vector (or vector/vector) control group.
[3H]Glycerol uptake assay
To quantify the function of re-expressed AQP3, we performed a radiolabeled glycerol uptake assay as described in (Zheng et al., 2003). Briefly, after viral infection for 24 hours, the medium was aspirated and replaced with pre-equilibrated K-SFM containing 20mM HEPES and 1μCi/mL [3H]glycerol for exactly 5 minutes. The reactions were terminated and the excess [3H]glycerol was removed with three washes of ice-cold PBS lacking divalent cations. Keratinocytes were solubilized in 0.3M NaOH, and aliquots were subjected to liquid scintillation counting.
Statistical Analysis
Data from at least three independent experiments are presented as the means ± SEM. An unpaired, 2 tailed t-test was used to analyze differences between two groups. For more than two groups, we compared group mean values using one-way analysis of variance with a Newman-Keuls multiple comparison post-hoc test (GraphPad Prism, La Jolla, CA).
ACKNOWLEDGEMENTS
We thank Purnima Merai for her excellent technical assistance in the preparation of primary mouse keratinocytes. This work was supported in part by a Veterans Affairs Merit Review (#I01CX000590) to WBB. WBB is also supported by a VA Research Career Scientist Award. The contents of this article do not represent the views of the Department of Veterans Affairs or the United States Government.
Abbreviations:
- AQP3
aquaporin 3
- PLD
phospholipase D
- K1
keratin 1
- K10
keratin 10
- LOR
loricrin
- K5
keratin 5
- LD
lipase-dead
- K-SFM
keratinocyte serum-free media
- MOI
multiplicity of infection
- ΔΔCt
delta-delta cycle threshold
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- qRT-PCR
quantitative real-time PCR
- RPLP0
ribosomal protein, large, P0
Footnotes
CONFLICT OF INTEREST
The authors state no conflict of interest.
REFERENCES
- Arun SN, Xie D, Howard AC , et al. (2013) Cell wounding activates phospholipase D in primary mouse keratinocytes. J Lipid Res 54:581–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banno Y (2002) Regulation and possible role of mammalian phospholipase D in cellular functions. J Biochem 131:301–306. [DOI] [PubMed] [Google Scholar]
- Bellemere G, Von Stetten O, Oddos T (2008) Retinoic acid increases aquaporin 3 expression in normal human skin. J Invest Dermatol 128:542–548. [DOI] [PubMed] [Google Scholar]
- Bikle DD, Pillai S (1993) Vitamin D, calcium, and epidermal differentiation. Endocr Rev 14:3–19. [DOI] [PubMed] [Google Scholar]
- Bollag WB, Xie D, Zheng X, et al. (2007) A potential role for the phospholipase D2-aquaporin-3 signaling module in early keratinocyte differentiation: production of a phosphatidylglycerol signaling lipid. J Invest Dermatol 127:2823–2831. [DOI] [PubMed] [Google Scholar]
- Boury-Jamot M, Sougrat R, Tailhardat M, et al. (2006) Expression and function of aquaporins in human skin: Is aquaporin-3 just a glycerol transporter? Biochim Biophys Acta 1758:1034–1042. [DOI] [PubMed] [Google Scholar]
- Di Fulvio M, Frondorf K, Henkels KM, et al. (2012) Phospholipase D2 (PLD2) shortens the time required for myeloid leukemic cell differentiation: mechanism of action. J Biol Chem 287:393–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumas M, Gondran C, Barre P, et al. (2002) Effect of an Ajuga turkestanica extract on aquaporin 3 expression, water flux, differentiation and barrier parameters of the human epidermis. Eur J Dermatol 12:XXV–XXVI. [PubMed] [Google Scholar]
- Elias P, Ahn S, Brown B, et al. (2002) Origin of the epidermal calcium gradient: regulation by barrier status and role of active vs passive mechanisms. J Invest Dermatol 119:1269–1274. [DOI] [PubMed] [Google Scholar]
- Grether-Beck S, Felsner I, Brenden H, et al. (2012) Urea uptake enhances barrier function and antimicrobial defense in humans by regulating epidermal gene expression. J Invest Dermatol 132:1561–1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L, Chen H, Li Y, et al. (2013) An aquaporin 3-notch1 axis in keratinocyte differentiation and inflammation. PLoS One 8:e80179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara-Chikuma M, Takahashi K, Chikuma S, et al. (2009) The expression of differentiation markers in aquaporin-3 deficient epidermis. Arch Dermatol Res 301:245–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara-Chikuma M, Verkman AS (2005) Aquaporin-3 functions as a glycerol transporter in mammalian skin. Biol Cell 97:479–486. [DOI] [PubMed] [Google Scholar]
- Hara-Chikuma M, Verkman AS (2008) Aquaporin-3 facilitates epidermal cell migration and proliferation during wound healing. J Mol Med (Berl) 86:221–231. [DOI] [PubMed] [Google Scholar]
- Hara M, Verkman AS (2003) Glycerol replacement corrects defective skin hydration, elasticity, and barrier function in aquaporin-3-deficient mice. Proc Natl Acad Sci U S A 100:7360–7365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He TC, Zhou S, da Costa LT, et al. (1998) A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci U S A 95:2509–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendriks G, Koudijs M, van Balkom BW, et al. (2004) Glycosylation is important for cell surface expression of the water channel aquaporin-2 but is not essential for tetramerization in the endoplasmic reticulum. J Biol Chem 279:2975–2983. [DOI] [PubMed] [Google Scholar]
- Jenkins GM, Frohman MA (2005) Phospholipase D: a lipid centric review. Cell Mol Life Sci 62:2305–2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang YJ, Kim P, Lu YF, et al. (2011) PPARgamma activators stimulate aquaporin 3 expression in keratinocytes/epidermis. Exp Dermatol 20:595–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung EM, Betancourt-Calle S, Mann-Blakeney R, et al. (1999) Sustained phospholipase D activation is associated with keratinocyte differentiation. Carcinogenesis 20:569–576. [DOI] [PubMed] [Google Scholar]
- Jungersted JM, Bomholt J, Bajraktari N, et al. (2013) In vivo studies of aquaporins 3 and 10 in human stratum corneum. Arch Dermatol Res 305:699–704. [DOI] [PubMed] [Google Scholar]
- Kim NH, Lee AY (2010) Reduced aquaporin3 expression and survival of keratinocytes in the depigmented epidermis of vitiligo. J Invest Dermatol 130:2231–2239. [DOI] [PubMed] [Google Scholar]
- Lee SH, Elias PM, Proksch E, et al. (1992) Calcium and potassium are important regulators of barrier homeostasis in murine epidermis. J Clin Invest 89:530–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Je YJ, Lee SS, et al. (2012) Changes in transepidermal water loss and skin hydration according to expression of aquaporin-3 in psoriasis. Ann Dermatol 24:168–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Tucker RW, Hennings H, et al. (1995) Chelation of intracellular Ca2+ inhibits murine keratinocyte differentiation in vitro. J Cell Physiol 163:105–114. [DOI] [PubMed] [Google Scholar]
- Ma T, Hara M, Sougrat R, et al. (2002) Impaired stratum corneum hydration in mice lacking epidermal water channel aquaporin-3. J Biol Chem 277:17147–17153. [DOI] [PubMed] [Google Scholar]
- Matsuo K, Kawano K (2014) Immunohistochemical distribution and morphometric analysis of aquaporin-3 in oral squamous cell carcinoma. Int J Oral Maxillofac Surg 43:13–21. [DOI] [PubMed] [Google Scholar]
- Menon GK, Elias PM, Lee SH, et al. (1992) Localization of calcium in murine epidermis following disruption and repair of the permeability barrier. Cell Tissue Res 270:503–512. [DOI] [PubMed] [Google Scholar]
- Nakahigashi K, Kabashima K, Ikoma A, et al. (2011) Upregulation of aquaporin-3 is involved in keratinocyte proliferation and epidermal hyperplasia. J Invest Dermatol 131:865–873. [DOI] [PubMed] [Google Scholar]
- Pillai S, Bikle DD, Mancianti ML, et al. (1990) Calcium regulation of growth and differentiation of normal human keratinocytes: modulation of differentiation competence by stages of growth and extracellular calcium. J Cell Physiol 143:294–302. [DOI] [PubMed] [Google Scholar]
- Qin H, Bollag WB (2013) The caveolin-1 scaffolding domain peptide decreases phosphatidylglycerol levels and inhibits calcium-induced differentiation in mouse keratinocytes. PLoS One 8:e80946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin H, Zheng X, Zhong X, et al. (2011) Aquaporin-3 in keratinocytes and skin: its role and interaction with phospholipase D2. Arch Biochem Biophys 508:138–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott SA, Selvy PE, Buck JR, et al. (2009) Design of isoform-selective phospholipase D inhibitors that modulate cancer cell invasiveness. Nat Chem Biol 5:108–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serna A, Galan-Cobo A, Rodrigues C, et al. (2014) Functional Inhibition of Aquaporin-3 With a Gold-Based Compound Induces Blockage of Cell Proliferation. J Cell Physiol. 229:1787–1801. [DOI] [PubMed] [Google Scholar]
- Shapiro BA, Olala L, Arun SN, et al. (2010) Angiotensin II-activated protein kinase D mediates acute aldosterone secretion. Mol Cell Endocrinol 317:99–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sougrat R, Morand M, Gondran C, et al. (2002) Functional expression of AQP3 in human skin epidermis and reconstructed epidermis. J Invest Dermatol 118:678–685. [DOI] [PubMed] [Google Scholar]
- Sugimoto T, Huang L, Minematsu T, et al. (2013) Impaired aquaporin 3 expression in reepithelialization of cutaneous wound healing in the diabetic rat. Biol Res Nurs 15:347–355. [DOI] [PubMed] [Google Scholar]
- Sugiyama Y, Ota Y, Hara M, et al. (2001) Osmotic stress up-regulates aquaporin-3 gene expression in cultured human keratinocytes. Biochim Biophys Acta 1522:82–88. [DOI] [PubMed] [Google Scholar]
- Sung TC, Roper RL, Zhang Y, et al. (1997) Mutagenesis of phospholipase D defines a superfamily including a trans-Golgi viral protein required for poxvirus pathogenicity. EMBO J 16:4519–4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu CL, Bikle DD (2013) Role of the calcium-sensing receptor in calcium regulation of epidermal differentiation and function. Best Pract Res Clin Endocrinol Metab 27:415–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkman AS (2008) A cautionary note on cosmetics containing ingredients that increase aquaporin-3 expression. Exp Dermatol 17:871–872. [DOI] [PubMed] [Google Scholar]
- Verkman AS, Mitra AK (2000) Structure and function of aquaporin water channels. Am J Physiol Renal Physiol 278:F13–F28. [DOI] [PubMed] [Google Scholar]
- Voss KE, Bollag RJ, Fussell N, et al. (2011) Abnormal aquaporin-3 protein expression in hyperproliferative skin disorders. Arch Dermatol Res 303:591–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuspa SH, Kilkenny AE, Steinert PM, et al. (1989) Expression of murine epidermal differentiation markers is tightly regulated by restricted extracellular calcium concentrations in vitro. The Journal of cell biology 109:1207–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X, Bollag WB (2003) Aquaporin 3 colocates with phospholipase d2 in caveolin-rich membrane microdomains and is downregulated upon keratinocyte differentiation. J Invest Dermatol 121:1487–1495. [DOI] [PubMed] [Google Scholar]
- Zheng X, Ray S, Bollag WB (2003) Modulation of phospholipase D-mediated phosphatidylglycerol formation by differentiating agents in primary mouse epidermal keratinocytes. Biochim Biophys Acta 1643:25–36. [DOI] [PubMed] [Google Scholar]


