Abstract
Background
Rhinitis is the most common clinical manifestation of allergy, affecting more than 400 million people around the world. Rhinitis increases the risk of developing bronchial hyper-responsiveness and asthma. Previous studies have shown that rhinitis is closely related with the physiology, pathology, and pathogenesis of asthma. We analyzed co-expressed genes to explore the relationships between rhinitis and asthma and to find biomarkers of comorbid rhinitis and asthma.
Material/Methods
Asthma- and rhinitis-related differentially-expressed genes (DEGs) were identified by bioinformatic analysis of GSE104468 and GSE46171 datasets from the Gene Expression Omnibus (GEO) database. After assessment of Gene Ontology (GO) terms and pathway enrichment for DEGs, a protein–protein interaction (PPI) network was conducted via comprehensive target prediction and network analyses. We also evaluated co-expressed DEGs and corresponding predicted miRNAs involved in the developing process of rhinitis and asthma.
Results
We identified 687 and 1001 DEGs in bronchial and nasal epithelia samples of asthma patients, respectively. For patients with rhinitis, we found 245 DEGs. The hub-genes of PAX6, NMU, NTS, NMUR1, PMCH, and KRT6A may be associated with rhinitis, while CPA3, CTSG, POSTN, CLCA1, HDC, and MUC5B may be involved in asthma. The co-expressed DEGs of BPIFA1, CCL26, CPA3, and CST1, together with corresponding predicted miRNAs (e.g., miR-195-5p and miR-125a-3p) were found to be significantly correlated with rhinitis and asthma.
Conclusions
Rhinitis and asthma are related, and there are significant correlations of BPIFA1, CCL26, CPA3, and CST1 genes with novel biomarkers involved in the comorbidity of rhinitis and asthma.
MeSH Keywords: Asthma, Biological Markers, Genetic Association Studies, Rhinitis
Background
Rhinitis is a common inflammatory response to allergens; it affects more than 400 million people worldwide. It is characterized by people of all ages exhibiting various symptoms, such as repetitive sneezing, nasal itching, rhinorrhea, as well as nasal obstruction [1–3]. While it is mostly associated with discomfort, rhinitis also increases the risk of developing bronchial hyper-responsiveness and asthma [4,5]. Similarly, the prevalence of asthma has been growing steadily in China and globally [6]. These 2 common allergic diseases of the respiratory system are thought to result from complex genetic and environmental factors. Key players in the barrier system, such as airway epithelia, secret allergic mediators in response to allergen stimulation. The micro-environment, which is composed of innate immune cells, including innate lymphoid cells (ILC), and various immune effector molecules, modulates cytokines/chemokines production, which affects cells involved in adaptive immunity [7]. Furthermore, a mounting body of evidence implicates epigenetics and the microbiota in allergic diseases [8].
Asthma and rhinitis are common allergic conditions of the respiratory system. Epidemiological reports show that the incidence of asthma and rhinitis is rising, negatively affecting patients’ health and quality of life [9]. Rhinitis has been shown to be closely correlated with the physiology, pathology, and pathogenesis of asthma [10–12]. It is estimated that 40–50% of people with rhinitis will develop asthma and 74–90% of people with allergic asthma also have rhinitis [7,9,13]. These observations gave rise to the theory of “one airway, one disease” that posits a close relationship between asthma and rhinitis [14].
Rhinitis is a complex disorder thought to result from the interaction between over 100 genetic loci and complex environmental factors [15]. However, there is no satisfactory treatment for allergic diseases, and a strategy combining the treatment of both compartments appears to be optimal. Therefore, there is an urgent need to better understand the pathogenesis and genetic modulators of allergic diseases to develop effective therapies. In this study, we identified genes that are co-differentially-expressed (co-DEGs) between persistent rhinitis and asthma. We then investigated the molecular mechanisms through which the rhinitis-related DEGs and asthma-related DEGs drive pathogenesis. Finally, using bioinformatic analysis of the DEGs, we predicted microRNAs that may be involved in the process of rhinitis patients’ developing asthma.
Material and Methods
GSE104468 and GSE46171 datasets were downloaded from the GEO database (http://www.ncbi.nlm.nih.gov/geo/) [16] and expression profiling arrays were generated using GPL21185 Agilent-072363 SurePrint G3 Human GE v3 8×60K Microarray (Agilent, Santa Clara, CA) and GPL6480 Agilent-014850 Whole Human Genome Microarray 4×44K G4112F (Agilent, Santa Clara, CA), respectively. Additionally, the GSE104468 dataset, including collected nasal epithelia and bronchial epithelia sample from 12 subjects with allergic asthma and 12 control subjects, was used to identify differentially-expressed genes and molecular mechanisms of asthma [17]. In this study, the nasal epithelia and bronchial epithelia expression profiles were used to explore the comorbidity rate of rhinitis and asthma. Nasal epithelia samples of GSE46171 were collected from adults with asthma, allergic rhinitis, or no underlying respiratory disease. Nasal mucosa sampling was taken on day 2 and day 6 of symptomatic illness, and an asymptomatic BL sample was taken at least 29 days later [18]. Traditionally, general research about asthma has always focused on bronchial epithelia. In order to conduct joint research with rhinitis, we found target genes on the nasal epithelia of asthma patients at the same time, allowing us to analyze common target genes of rhinitis and asthma. Common target genes were found in 2 different tissues of asthma patients, then the correlation between asthma and rhinitis was analyzed, and underlying biomarkers and therapeutic targets of comorbid rhinitis and asthma were revealed.
Data processing
The Bioconductor R packages “limma” [19], was applied to analyze GSE104468 and GSE46171 RAW datasets. Original p-values were corrected using the Benjamini-Hochberg method. The following gene expression thresholds were applied to identify DEGs: fold-change >1.5 or <0.6667. Co-DEGs were visualized by plotting the respective co-DEGs for rhinitis and asthma on Venn diagrams.
Finally, an online prediction tool utilizing microRNA data integration portal (mirDIP) was used [20] to predict potential microRNA targeting. mirDIP was then used to predict which of the identified miRNAs target co-DEGs and to select the top 5 candidate miRNAs.
Identification of protein–protein interaction (PPI) networks of DEGs
The Search Tool for the Retrieval of Interacting Genes (STRING database, V11; http://string-db.org/) was used to create a PPI network of rhinitis and asthma DEGs to predict protein–protein interactions and the functions of the DEGs [21]. Subsequently, Cytoscape software (V3.5.2; http://cytoscape.org/) was used to visualize and analyze biological networks and node degrees based on a confidence score >0.4 [22].
GO and KEGG functional enrichment analysis
Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analyses of rhinitis and asthma DEGs were performed using Bioconductor’s “clusterProfiler” package in R [23]. GO terms of biological processes, cellular components, and molecular functions associated with a p-value <0.05 were considered to be significantly enriched.
Identification of co-DEGs associated with respiratory diseases
To generate expanded networks and predict novel associations, the comparative toxicogenomics database (http://ctdbase.org/) was used to identify integrated chemical-gene, chemical-disease, and gene-disease interactions [24,25]. These data were analyzed for relationships between genes and respiratory disease like rhinitis and asthma, and we identified relationships between co-DEGs and diseases and association or an implied association.
Results
Identification of DEGs
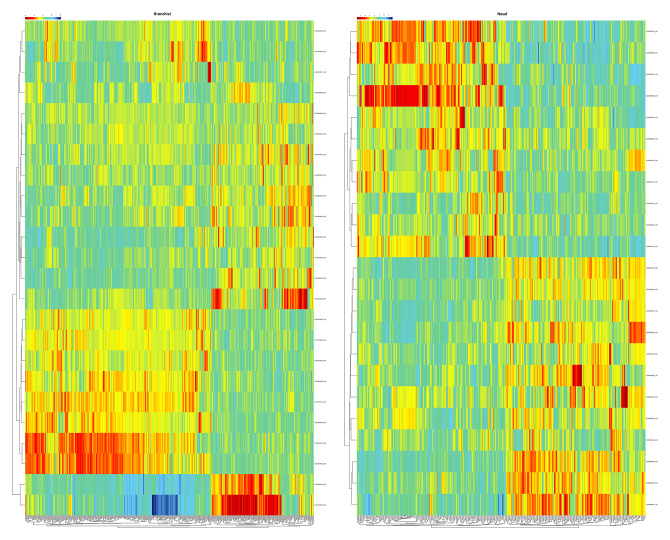
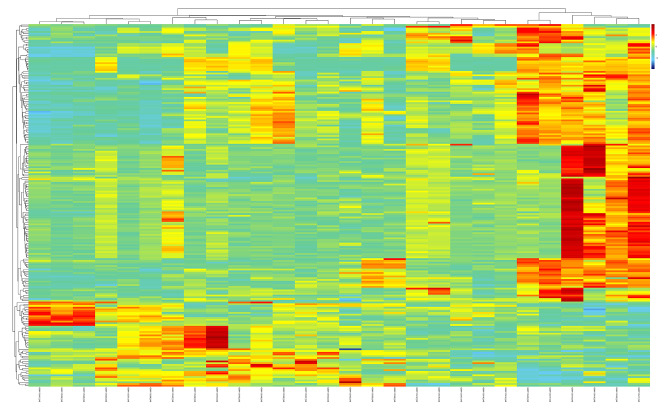
We identified 58 201 probes in GSE104468 dataset and confirmed 687 genes as DEGs in bronchial epithelia specimens, and 1353 probes corresponding to 1001 DEGs were identified in nasal epithelia samples (Figure 1). In the GSE58294 dataset, we defined 245 rhinitis DEGs (Figure 2). Except for the inconsistent upregulation and downregulation of the ADTRP gene in the bronchial epithelia and nasal epithelia dataset, 6 co-DEGs emerged: BPIFA1, CCL26, CPA3, CST1, CST2, and FETUB.
Figure 1.
Heatmap of clustering analysis for asthma-related differentially-expressed genes. Left panel shows the heatmap of differentially-expressed genes in bronchial epithelia sample, while right panel shows the heatmap of differentially-expressed genes in nasal epithelia sample.
Figure 2.
Hierarchical clustering analysis and the heatmap of rhinitis-related differentially-expressed genes. Red – greater expression. Green – less expression.
Functional enrichment in co-DEGs
Intriguingly, 6 co-expressed DEGs – BPI fold containing family A member 1 (BPIFA1), C-C motif chemokine ligand 26 (CCL26), carboxypeptidase A3 (CPA3), cystatin SN (CST1), cystatin SA (CST2), and fetuin B (FETUB) – were observed.
Next, we used the AmiGO database to confirm GO term enrichment related to the biological processes, cellular components, and molecular functions, and found that the co-DEGs were associated with miscellaneous processes (Table 1).
Table 1.
The Gene Ontology (GO) terms enrichment for the co-expressed genes of rhinitis and asthma.
| Gene/product | GO class (direct) | Evidence | Reference |
|---|---|---|---|
| BPIFA1 | Protein binding | IPI | PMID: 25416956 |
| Extracellular region | IDA | PMID: 11425234 | |
| Extracellular space | IDA | PMID: 21805676 | |
| Lipid binding | IEA | GO_REF: 0000037 | |
| Antimicrobial humoral response | TAS | Reactome: R-HSA-6803157 | |
| Antibacterial humoral response | IDA | PMID: 23499554 | |
| Innate immune response | IDA | PMID: 23499554 | |
| Regulation of liquid surface tension | IDA | PMID: 23499554 | |
| Multicellular organismal water homeostasis | IDA | PMID: 24124190 | |
| Defense response to virus | IEP | PMID: 21805676 | |
| Antimicrobial humoral immune response mediated by antimicrobial peptide | IEP | PMID: 21805676 | |
| Negative regulation of single-species biofilm formation in or on host organism | IMP | PMID: 23499554 | |
| Regulation of sodium ion transmembrane transport | IDA | PMID: 24124190 | |
| CCL26 | Positive regulation of endothelial cell proliferation | IDA | PMID: 19525930 |
| Monocyte chemotaxis | IDA | PMID: 10373330 | |
| Protein binding | IPI | PMID: 28381538 | |
| Extracellular space | IDA | PMID: 10373330 | |
| Chemotaxis | TAS | PMID: 10373330 | |
| Signal transduction | NAS | PMID: 10373330 | |
| Cell–cell signaling | TAS | PMID: 10373330 | |
| Chemokine activity | IDA | PMID: 10373330 | |
| T cell chemotaxis | IDA | PMID: 10373330 | |
| Positive regulation of cell migration | IDA | PMID: 19525930 | |
| Positive regulation of actin filament polymerization | IDA | PMID: 19525930 | |
| CCR3 chemokine receptor binding | IDA | PMID: 11425309 | |
| Positive regulation of GTPase activity | IDA | PMID: 19525930 | |
| Receptor ligand activity | IDA | PMID: 11425309 | |
| Positive regulation of chemotaxis | IDA | PMID: 10373330 | |
| Chemokine-mediated signaling pathway | IDA | PMID: 10373330 | |
| CCR chemokine receptor binding | IBA | PMID: 21873635 | |
| Positive regulation of GTPase activity | IBA | PMID: 21873635 | |
| Lymphocyte chemotaxis | IBA | PMID: 21873635 | |
| Chemokine activity | IBA | PMID: 21873635 | |
| Monocyte chemotaxis | IBA | PMID: 21873635 | |
| Cellular response to tumor necrosis factor | IBA | PMID: 21873635 | |
| Extracellular space | IBA | PMID: 21873635 | |
| Inflammatory response | IBA | PMID: 21873635 | |
| Chemokine-mediated signaling pathway | IBA | PMID: 21873635 | |
| G protein-coupled receptor signaling pathway | IBA | PMID: 21873635 | |
| Cellular response to interleukin-1 | IBA | PMID: 21873635 | |
| Neutrophil chemotaxis | IBA | PMID: 21873635 | |
| Positive regulation of ERK1 and ERK2 cascade | IBA | PMID: 21873635 | |
| Cellular response to interferon-gamma | IBA | PMID: 21873635 | |
| CPA3 | Angiotensin maturation | TAS | Reactome: R-HSA-2028294 |
| Metallocarboxypeptidase activity | TAS | PMID: 1629626 | |
| Extracellular region | TAS | Reactome: R-HSA-2028294 | |
| Proteolysis | TAS | PMID: 2708524 | |
| Zinc ion binding | IEA | GO_REF: 0000002 | |
| Transport vesicle | IEA | GO_REF: 0000039 | |
| Secretory granule | NAS | PMID: 2594780 | |
| Collagen-containing extracellular matrix | HDA | PMID: 27559042 | |
| Metallocarboxypeptidase activity | IBA | PMID: 21873635 | |
| Proteolysis | IBA | PMID: 21873635 | |
| Extracellular space | IBA | PMID: 21873635 | |
| CST1 | Detection of chemical stimulus involved in sensory perception of bitter taste | IDA | PMID: 24248522 |
| Cysteine-type endopeptidase inhibitor activity | IEA | GO_REF: 0000037 | |
| Protein binding | IPI | PMID: 25416956 | |
| Extracellular space | HDA | PMID: 22664934 | |
| Negative regulation of endopeptidase activity | IEA | GO_REF: 0000108 | |
| Extracellular space | IBA | PMID: 21873635 | |
| CST2 | Detection of chemical stimulus involved in sensory perception of bitter taste | IDA | PMID: 24248522 |
| Cysteine-type endopeptidase inhibitor activity | IEA | GO_REF: 0000037 | |
| Protein binding | IPI | PMID: 25416956 | |
| Extracellular space | HDA | PMID: 22664934 | |
| Negative regulation of endopeptidase activity | IEA | GO_REF: 0000108 | |
| Extracellular space | IBA | PMID: 21873635 | |
| FETUB | Cysteine-type endopeptidase inhibitor activity | IEA | GO_REF: 0000002 |
| Single fertilization | ISS | GO_REF: 0000024 | |
| Binding of sperm to zona pellucida | ISS | GO_REF: 0000024 | |
| Metalloendopeptidase inhibitor activity | ISS | GO_REF: 0000024 | |
| Negative regulation of endopeptidase activity | ISS | GO_REF: 0000024 | |
| Extracellular exosome | HDA | PMID: 23533145 | |
| Binding of sperm to zona pellucida | IBA | PMID: 21873635 | |
| Negative regulation of endopeptidase activity | IBA | PMID: 21873635 | |
| Metalloendopeptidase inhibitor activity | IBA | PMID: 21873635 | |
| Extracellular region | IBA | PMID: 21873635 | |
| Endopeptidase inhibitor activity | IBA | PMID: 21873635 |
Functional GO terms and pathway enrichment analyses and PPI network analysis
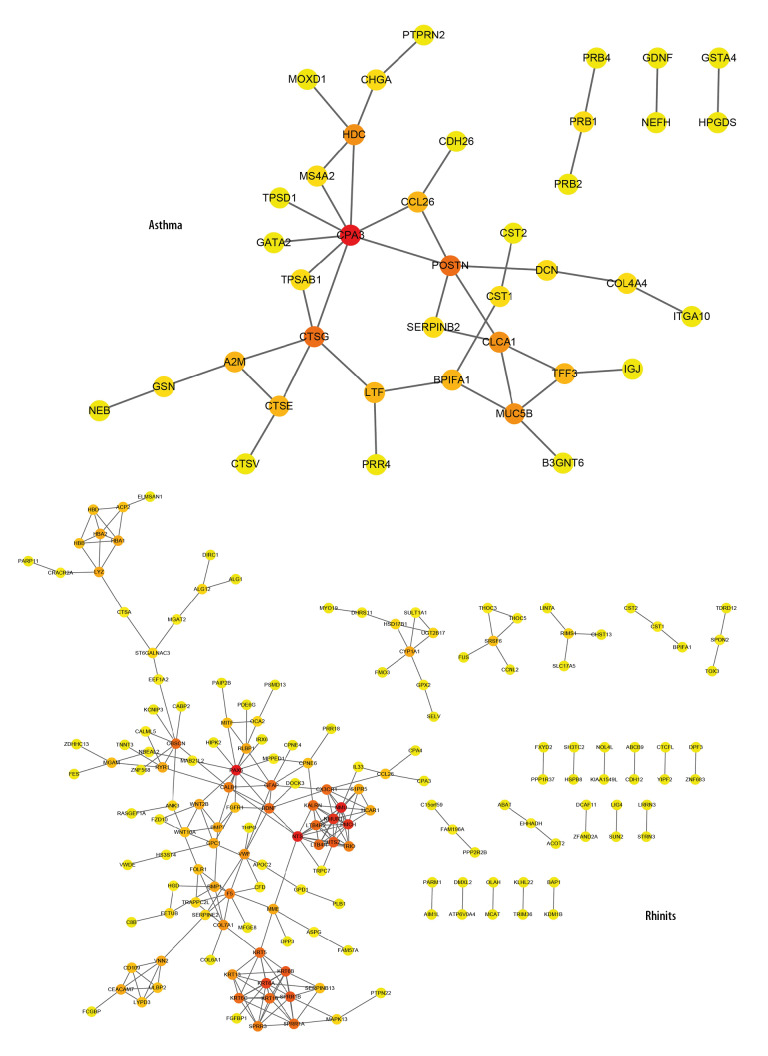
Analysis of PPI networks of rhinitis DEGs and asthma DEGs revealed 263 and 42 nodes, respectively (Figure 3). Paired box 6 (PAX6; degree=13), neuromedin U (NMU; degree=12), neurotensin (NTS; degree=12), neuromedin U receptor 1 (NMUR1; degree=11), pro-melanin concentrating hormone (PMCH; degree=11), and keratin 6A (KRT6A; degree=10) are considered hub-genes related to rhinitis. However, the hub-genes involved in carboxypeptidase A3 (CPA3; degree=8), cathepsin G (CTSG; degree=5), periostin (POSTN; degree=5), chloride channel accessory 1 (CLCA1; degree=4), and histidine decarboxylase (HDC; degree=4) are demonstrated in asthma DEGs at relatively higher degree.
Figure 3.
PPI network of asthma- and rhinitis-related DEGs. PPI networks from asthma and rhinitis constructed using STRING database for DEGs (threshold >0.4). Red, greater degree. Yellow, lesser degree.
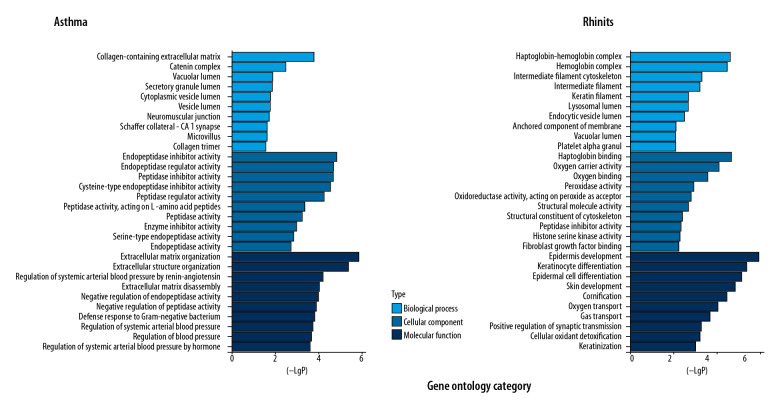
GO term analysis for biological processes indicated that these genes are significantly associated with epidermis development (p-value: 1.91E-07), keratinocyte differentiation (p-value: 8.57E-07), epidermal cell differentiation (p-value: 1.53E-06), skin development (p-value: 3.09E-06), and cornification (p-value: 7.97E-06). In the cellular components, DEGs were significantly correlated with haptoglobin-hemoglobin complex (p-value: 5.58E-06), hemoglobin complex (p-value: 8.3E-06), and intermediate filament cytoskeleton (p-value: 1.85E-04). In the molecular function component, DEGs were mainly involved in haptoglobin binding (p-value: 4.94E-06), oxygen carrier activity (p-value: 2.26E-05), and oxygen binding (p-value: 8.51E-05). Analysis of the relationship between asthma DEGs and biological processes indicated they are significantly associated with regulation of extracellular matrix organization (p-value: 1.36E-06), extracellular structure organization (p-value: 4.18E-06), regulation of systemic arterial blood pressure by renin-angiotensin (p-value: 6.33E-05), and extracellular matrix disassembly (p-value: 9.69E-05). There are significant correlations in collagen-containing extracellular matrix (p-value: 1.62E-04), catenin complex (p-value: 0.0032), and vacuolar lumen (p-value: 0.014) in relation to cellular components. Similarly, the terms of endopeptidase inhibitor activity (p-value: 1.5E-05), peptidase inhibitor activity (p-value: 1.87E-05), and endopeptidase regulator activity (p-value: p-value: 1.87E-05) related to molecular functions were primarily enriched (Figure 4).
Figure 4.
Gene Ontology categories of asthma- and rhinitis-related DEGs.
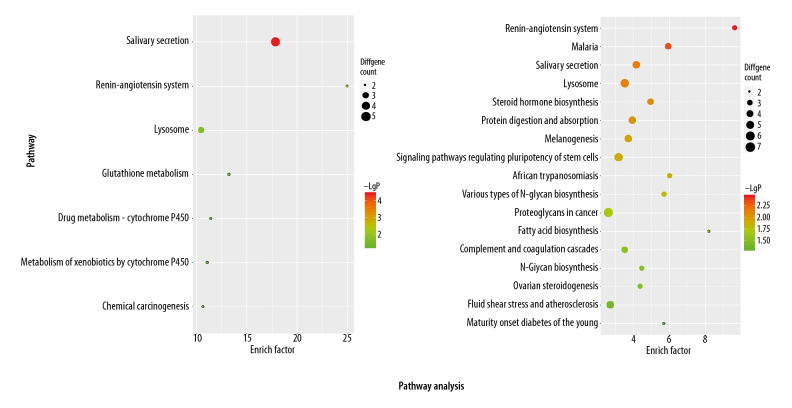
KEGG pathway analysis data are shown in Figure 5. KEGG pathway analysis indicated that rhinitis DEGs are mainly enriched for pathways of renin-angiotensin system (p-value: 0.0035), malaria (p-value: 0.0046), salivary secretion (p-value: 0.0075), and lysosome (p-value: 0.0078). However, these KEGG terms, including salivary secretion (p-value: 3.96E-05), renin-angiotensin system (p-value: 0.0044), and lysosome (p-value: 0.018) are also enriched in asthma DEGs.
Figure 5.
KEGG pathway enrichment of asthma- and rhinitis-related DEGs.
Identification of functional and pathway enrichment among predicted miRNAs and co-DEGs
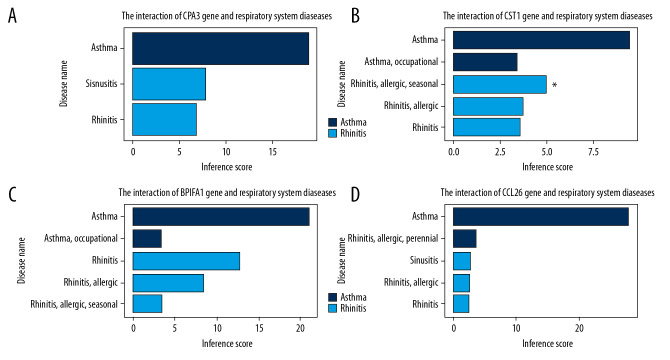
The CTD database revealed that co-DEGs targeted various nasal sinus and respiratory system diseases (Figure 6, Supplementary Table 1). By setting an inference score filter at >5, we found that BPIFA1, CCL26, CPA3, and CST1 are associated with asthma and rhinitis. Next, we mirDIP analysis was done to predict microRNAs that may regulate the 4 genes and the top 5 predicted microRNAs for each gene, along with related pathway enrichment selected (Table 2). These analyses provided insight into the mechanisms by which the predicted miRNAs influence rhinitis-asthma comorbidity.
Figure 6.
(A–D) Relationship to respiratory system diseases related to co-expressed genes based on the CTD database.
Table 2.
The Gene Ontology (GO) terms and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways enrichment among predicted miRNAs and co-DEGs.
| Genes | Predicted miRNAs | Category | P value | |
|---|---|---|---|---|
| BPIFA1 | hsa-miR-195-5p | KEGG pathway | Fatty acid biosynthesis | 1.04E-07 |
| hsa-miR-16-5p | Adherens junction | 1.70E-07 | ||
| hsa-miR-424-5p | TGF-beta signaling pathway | 3.28E-05 | ||
| hsa-miR-497-5p | GO terms | Neurotrophin TRK receptor signaling pathway | 1.79E-31 | |
| hsa-miR-15b-5p | cell death | 1.41E-28 | ||
| Response to stress | 1.41E-28 | |||
| Blood coagulation | 1.84E-25 | |||
| Fc-epsilon receptor signaling pathway | 5.45E-19 | |||
| Immune system process | 3.87E-15 | |||
| Activation of signaling protein activity involved in unfolded protein response | 3.77E-14 | |||
| Toll-like receptor 10 signaling pathway | 1.22E-13 | |||
| Epidermal growth factor receptor signaling pathway | 1.82E-13 | |||
| Toll-like receptor TLR1: TLR2 signaling pathway | 1.06E-12 | |||
| Toll-like receptor TLR6: TLR2 signaling pathway | 1.06E-12 | |||
| CCL26 | hsa-miR-326 | KEGG pathway | Steroid biosynthesis | 8.04E-10 |
| hsa-miR-615-5p | ECM-receptor interaction | 3.39E-08 | ||
| hsa-miR-559 | GO terms | Neurotrophin TRK receptor signaling pathway | 5.30E-26 | |
| hsa-miR-335-5p | Small molecule metabolic process | 5.89E-21 | ||
| hsa-miR-548d-5p | Blood coagulation | 8.06E-18 | ||
| Cellular protein modification process | 4.43E-15 | |||
| Fc-epsilon receptor signaling pathway | 3.94E-13 | |||
| Cellular nitrogen compound metabolic process | 3.94E-13 | |||
| Immune system process | 7.59E-11 | |||
| CPA3 | hsa-miR-125a-3p | KEGG pathway | Adherens junction | 2.60E-07 |
| hsa-miR-155-5p | Hippo signaling pathway | 1.21E-05 | ||
| hsa-miR-196a-5p | TGF-beta signaling pathway | 1.96E-05 | ||
| hsa-miR-196b-5p | Lysine degradation | 4.43E-05 | ||
| GO terms | cellular nitrogen compound metabolic process | 6.05E-108 | ||
| gene expression | 7.55E-68 | |||
| biosynthetic process | 8.95E-64 | |||
| cellular protein modification process | 8.70E-52 | |||
| CST1 | hsa-miR-452-5p | KEGG pathway | ECM-receptor interaction | 0.000132 |
| hsa-miR-608 | Hippo signaling pathway | 0.00019 | ||
| hsa-miR-138-5p | Adherens junction | 0.00019 | ||
| hsa-miR-4685-5p | Apoptosis | 0.005037 | ||
| hsa-miR-1321 | Focal adhesion | 0.005037 | ||
| GO terms | Cellular nitrogen compound metabolic process | 2.81E-42 | ||
| Gene expression | 2.91E-40 | |||
| Biosynthetic process | 7.87E-34 | |||
| mRNA metabolic process | 8.00E-21 | |||
| Response to stress | 8.00E-21 | |||
| RNA metabolic process | 1.38E-17 | |||
| Symbiosis, encompassing mutualism through parasitism | 1.97E-17 | |||
| Cellular component assembly | 2.11E-17 |
Discussion
Rhinitis, a chronic inflammatory cascade in nasal mucosa mediated by allergen-specific IgE, is clinically characterized by pruritus, sneezing, and rhinorrhea [26]. Studies of the nose-bronchi functional links have indicated that allergy is not a disease localized to a specific organ, but is rather a disorder of the entire respiratory tract, exhibiting a wide range of symptoms [27]. Rhinitis has been identified as an independent risk factor for asthma development. Therefore, development of effective treatments for rhinitis effectively prevent or delay asthma onset [28]. Endotype-driven treatments of upper-airway disease are effective against asthma. Four categories of asthma are recognized based and can be managed by specific treatments [29]. Knowledge about type 2 inflammation much more advanced relative to other endotypes. Type 2 targeted treatments with monoclonal antibodies against IgE, IL5, and IL4Rα have been proven to be effective in the management of chronic upper-airway diseases. Neurogenic inflammation has been shown to cause nasal hyperreactivity and it can be effectively managed with capsaicin [30]. Endotype-driven treatment can be used as a reference for rhinitis management since treatment options for rhinitis are still in their infancy due to the lack of suitable classification indexes. Accumulating evidence has demonstrated the roles of microRNAs and long non-coding RNAs in the modulation of disease pathology [31,32]. However, factors that negatively impact nasal conditioning during rhinitis have not been systematically evaluated. Therefore, the identification of biomarkers for the association between rhinitis and asthma are of great interest and could facilitate therapeutic strategies. Here, we identified several rhinitis-asthma co-DEGs that directly or indirectly regulate the respiratory system.
Bacterial permeability family member A1 (BPIFA1), also known as the short palate, lung, and nasal epithelium clone 1 (SPLUNC1), is an epithelium-secreted protein involved in innate immunity and anti-inflammatory responses. It is one of the most abundant proteins in respiratory secretions and has been implicated with increasing frequency in pulmonary disease. Reduced BPIFA1 expression may contribute to the persistent nature of bacterial infections in airways, suggesting that BPIFA1 may serve as a host defense protein against bacterial infection [33,34]. Better understanding of the role of BPIFA1 in disease pathogenesis will elucidate its potential as a biomarker and potential drug target against pulmonary disease. Nasal SPLUNC1 expression is inhibited by Th2 cytokines (IL-4 and IL-13) [35,36] but stimulated by Toll-like receptor (TLR) agonists and glucocorticoids [37].
Recent studies have shown that the chemokine CCL26 mediates eosinophilic inflammation diseases by promoting eosinophils infiltration from peripheral blood into affected organs. CCL26 is the important acidophilic granulocyte chemokine for IL-13-induced epithelial cell generation [38–40]. Additionally, eosinophilic and neutrophilic asthma endotypes are defined by epithelium-derived CCL26 and osteopontin, respectively [41].
As a member of the type 2 cystatin (CST) superfamily, CST1 is known to inhibit proteolytic activities of cysteine proteases and is involved in the progression of several human cancers [42]. CST1 expression is elevated in the nasal epithelia of patients with allergic rhinitis [43]. A previous study that constructed an allergic rhinitis-specific transcriptional regulatory network ranked CST1 as the most differentially-expressed gene in AR [44].
We identified miR-195-5p, miR-16-5p, and miR-125a-3p as co-DEGs that may serve as potential biomarkers for rhinitis and asthma. Interestingly, previous studies have reported that miR-195-5p inhibits cell migration and invasion in cervical carcinoma by suppressing ARL2 [45]. Similarly, circulating miR-16-5p and miR-19b-3p have been proposed as novel biomarkers for gastric cancer progression due to their ability to inhibit cell proliferation, invasion, and metastasis [46]. Aberrant expression of miR-125a-3p is associated with fibroblast activation. Regulation of miR-125a-3p levels may be a novel treatment option against proliferative vascular diseases [47].
Conclusions
CPA3, CTSG, POSTN, CLCA1, HDC, and MUC5B may be involved in asthma, and the hub-genes of PAX6, NMU, NTS, NMUR1, PMCH, and KRT6A may be associated with rhinitis. Besides, co-expressed DEGs of BPIFA1, CCL26, CPA3, and CST1 connect rhinitis and asthma. Lastly, the top 5 corresponding predicted miRNAs of each co-DEGs could be underlying biomarkers or therapeutic targets for rhinitis-related asthma, especially miR-195-5p, miR-16-5p, and miR-125a-3p. Therefore, rhinitis and asthma are related, and the co-expression of BPIFA1, CCL26, CPA3, and CST1 genes revealed the comorbidity of rhinitis and asthma.
Supplementary Data
Supplementary Table 1.
The relationship between co-expressed genes and respiratory system diseases based on the CTD database.
| Gene symbol | Gene ID | Disease name | Disease ID | Direct evidence | Inference network | Inference score | Reference count |
|---|---|---|---|---|---|---|---|
| BPIFA1 | 51297 | Rhinitis | MESH: D012220 | Particulate Matter | Tobacco Smoke Pollution | Vehicle Emissions | 12.67 | 3 | |
| BPIFA1 | 51297 | Rhinitis, allergic | MESH: D065631 | Particulate Matter | Soot | 8.45 | 2 | |
| BPIFA1 | 51297 | Rhinitis, allergic, seasonal | MESH: D006255 | Particulate Matter | 3.57 | 1 | |
| BPIFA1 | 51297 | Asthma | MESH: D001249 | Acetaminophen | Arsenic | Particulate Matter | Soot | Tobacco Smoke Pollution | Vehicle Emissions | 21.02 | 29 | |
| BPIFA1 | 51297 | Asthma, occupational | MESH: D059366 | Silicon Dioxide | 3.35 | 1 | |
| BPIFA1 | 51297 | Nose diseases | MESH: D009668 | Propylthiouracil | Tobacco Smoke Pollution | 9.59 | 4 | |
| CCL26 | 10344 | Asthma | MESH: D001249 | Aerosols | Antigens, Dermatophagoides | Arsenic | Cadmium | Dexamethasone | Ozone | Resveratrol | Tobacco Smoke Pollution | Zinc | 27.99 | 28 | |
| CCL26 | 10344 | Rhinitis, allergic, perennial | MESH: D012221 | Ozone | 3.73 | 1 | |
| CCL26 | 10344 | Rhinitis, allergic | MESH: D065631 | Atrazine | 2.83 | 1 | |
| CCL26 | 10344 | Rhinitis | MESH: D012220 | Tobacco Smoke Pollution | 2.6 | 1 | |
| CCL26 | 10344 | Nose diseases | MESH: D009668 | Lipopolysaccharides | Propylthiouracil | Tobacco Smoke Pollution | 14.31 | 5 | |
| CCL26 | 10344 | Sinusitis | MESH: D012852 | Tobacco Smoke Pollution | 2.9 | 1 | |
| CPA3 | 1359 | Rhinitis | MESH: D012220 | Tobacco Smoke Pollution | Vehicle Emissions | 6.86 | 2 | |
| CPA3 | 1359 | Asthma | MESH: D001249 | Acetaminophen | Decitabine | epigallocatechin gallate | Tobacco Smoke Pollution | trimellitic anhydride | Vehicle Emissions | 18.84 | 19 | |
| CPA3 | 1359 | Nose diseases | MESH: D009668 | Tobacco Smoke Pollution | 3.4 | 2 | |
| CPA3 | 1359 | Sinusitis | MESH: D012852 | Acetaminophen | Tobacco Smoke Pollution | 7.83 | 2 | |
| CST1 | 1469 | Rhinitis, allergic, seasonal | MESH: D006255 | Marker/mechanism | 1 | ||
| CST1 | 1469 | Rhinitis, allergic | MESH: D065631 | Air Pollutants | 3.83 | 1 | |
| CST1 | 1469 | Rhinitis | MESH: D012220 | Air Pollutants | 3.6 | 1 | |
| CST1 | 1469 | Asthma | MESH: D001249 | Air Pollutants | Methotrexate | Tretinoin | 9.41 | 14 | |
| CST1 | 1469 | Asthma, occupational | MESH: D059366 | Silicon Dioxide | 3.45 | 1 | |
| CST2 | 1470 | Asthma, occupational | MESH: D059366 | Silicon Dioxide | 3.85 | 1 | |
| FETUB | 26998 | Rhinitis | MESH: D012220 | Tobacco Smoke Pollution | Vehicle Emissions | 5.71 | 2 | |
| FETUB | 26998 | Rhinitis, allergic | MESH: D065631 | Atrazine | 2.66 | 1 | |
| FETUB | 26998 | Nose diseases | MESH: D009668 | cobaltous chloride | Tobacco Smoke Pollution | 7.86 | 3 | |
| FETUB | 26998 | Sinusitis | MESH: D012852 | Acetaminophen | Tobacco Smoke Pollution | 6.66 | 2 | |
Footnotes
Conflicts of interest
None.
Source of support: This research was financially supported by the Beijing Natural Science Foundation (7194292), the National Natural Science Foundation of China (Grants NFSC 81770993), and the Fundamental Research Funds for the Central Universities (2019-JYB-JS-052)
References
- 1.Greisner WA, 3rd, Settipane RJ, Settipane GA. Co-existence of asthma and allergic rhinitis: A 23-year follow-up study of college students. Allergy Asthma Proc. 1998;19(4):185–88. doi: 10.2500/108854198778557836. [DOI] [PubMed] [Google Scholar]
- 2.Leynaert B, Neukirch F, Demoly P, Bousquet J. Epidemiologic evidence for asthma and rhinitis comorbidity. J Allergy Clin Immunol. 2000;106(5 Suppl):S201–5. doi: 10.1067/mai.2000.110151. [DOI] [PubMed] [Google Scholar]
- 3.Pols DH, Wartna JB, van Alphen EI, et al. Interrelationships between atopic disorders in children: A meta-analysis based on ISAAC questionnaires. PLoS One. 2015;10(7):e0131869. doi: 10.1371/journal.pone.0131869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aronsson D, Tufvesson E, Bjermer L. Allergic rhinitis with or without concomitant asthma: Difference in perception of dyspnoea and levels of fractional exhaled nitric oxide. Clin Exp Allergy. 2005;35(11):1457–61. doi: 10.1111/j.1365-2222.2005.02363.x. [DOI] [PubMed] [Google Scholar]
- 5.Aronsson D, Tufvesson E, Ankerst J, et al. Allergic rhinitis with hyper-responsiveness differ from asthma in degree of peripheral obstruction during methacholine challenge test. Clin Physiol Funct Imaging. 2008;28(2):81–85. doi: 10.1111/j.1475-097X.2007.00772.x. [DOI] [PubMed] [Google Scholar]
- 6.Huang K, Yang T, Xu J, et al. Prevalence, risk factors, and management of asthma in China: A national cross-sectional study. Lancet. 2019;394(10196):407–18. doi: 10.1016/S0140-6736(19)31147-X. [DOI] [PubMed] [Google Scholar]
- 7.Wawrzyniak P, Wawrzyniak M, Wanke K, et al. Regulation of bronchial epithelial barrier integrity by type 2 cytokines and histone deacetylases in asthmatic patients. J Allergy Clin Immunol. 2017;139(1):93–103. doi: 10.1016/j.jaci.2016.03.050. [DOI] [PubMed] [Google Scholar]
- 8.Hilty M, Burke C, Pedro H, et al. Disordered microbial communities in asthmatic airways. PLoS One. 2010;5(1):e8578. doi: 10.1371/journal.pone.0008578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leynaert B, Neukirch C, Kony S, et al. Association between asthma and rhinitis according to atopic sensitization in a population-based study. J Allergy Clin Immunol. 2004;113(1):86–93. doi: 10.1016/j.jaci.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 10.van Beijsterveldt CE, Boomsma DI. Genetics of parentally reported asthma, eczema and rhinitis in 5-yr-old twins. Eur Respir J. 2007;29(3):516–21. doi: 10.1183/09031936.00065706. [DOI] [PubMed] [Google Scholar]
- 11.Loh PR, Bhatia G, Gusev A, et al. Contrasting genetic architectures of schizophrenia and other complex diseases using fast variance-components analysis. Nat Genet. 2015;47(12):1385–92. doi: 10.1038/ng.3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferreira MA, Vonk JM, Baurecht H, et al. Shared genetic origin of asthma, hay fever and eczema elucidates allergic disease biology. Nat Genet. 2017;49(12):1752–57. doi: 10.1038/ng.3985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tufvesson E, Aronsson D, Ankerst J, et al. Peripheral nitric oxide is increased in rhinitic patients with asthma compared to bronchial hyperresponsiveness. Respir Med. 2007;101(11):2321–26. doi: 10.1016/j.rmed.2007.06.015. [DOI] [PubMed] [Google Scholar]
- 14.Grossman J. One airway, on disease. Chest. 1997;111(2 Suppl):11S–16S. doi: 10.1378/chest.111.2_supplement.11s. [DOI] [PubMed] [Google Scholar]
- 15.Ideker T, Sharan R. Protein networks in disease. Genome Res. 2008;18(4):644–52. doi: 10.1101/gr.071852.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barrett T, Wilhite SE, Ledoux P, et al. NCBI GEO: Archive for functional genomics datasets – update. Nucleic Acids Res. 2013;41(Database issue):D991–95. doi: 10.1093/nar/gks1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang IV, Richards A, Davidson EJ, et al. The masal methylome: A key to understanding allergic asthma. Am J Respir Crit Care Med. 2017;195(6):829–31. doi: 10.1164/rccm.201608-1558LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McErlean P, Berdnikovs S, Favoreto S, Jr, et al. Asthmatics with exacerbation during acute respiratory illness exhibit unique transcriptional signatures within the nasal mucosa. Genome Med. 2014;6(1):1. doi: 10.1186/gm520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ritchie ME, Phipson B, Wu D, et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43(7):e47. doi: 10.1093/nar/gkv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pio G, Malerba D, D’Elia D, et al. Integrating microRNA target predictions for the discovery of gene regulatory networks: A semi-supervised ensemble learning approach. BMC Bioinformatics. 2014;15(Suppl 1):S4. doi: 10.1186/1471-2105-15-S1-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Szklarczyk D, Gable AL, Lyon D, et al. STRING v11: Protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019;47:D607–13. doi: 10.1093/nar/gky1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shannon P, Markiel A, Ozier O, et al. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13(11):2498–504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu G, Wang L, Han Y, et al. clusterProfiler: An R package for comparing biological themes among gene clusters. OMICS. 2012;16(5):284–87. doi: 10.1089/omi.2011.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davis AP, Grondin CJ, Johnson RJ, et al. The Comparative Toxicogenomics Database: Update 2019. Nucleic Acids Res. 2019;47(D1):D948–54. doi: 10.1093/nar/gky868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carbon S, Ireland A, Mungall CJ, et al. AmiGO: Online access to ontology and annotation data. Bioinformatics. 2009;25(2):288–89. doi: 10.1093/bioinformatics/btn615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vercelli D. Discovering susceptibility genes for asthma and allergy. Nat Rev Immunol. 2008;8:169–82. doi: 10.1038/nri2257. [DOI] [PubMed] [Google Scholar]
- 27.Crimi E, Milanese M, Oddera S, et al. Inflammatory and mechanical factors of allergen-induced bronchoconstriction in mild asthma and rhinitis. J Appl Physiol. 2001;91:1029–34. doi: 10.1152/jappl.2001.91.3.1029. [DOI] [PubMed] [Google Scholar]
- 28.Moller C, Dreborg S, Ferdousi HA, et al. Pollen immunotherapy reduces the development of asthma in children with seasonal rhinoconjunctivitis (the PAT study) J Allergy Clin Immunol. 2002;109:251–56. doi: 10.1067/mai.2002.121317. [DOI] [PubMed] [Google Scholar]
- 29.Agache I, Rogozea L. Asthma biomarkers: Do they bring precision medicine closer to the clinic. Allergy Asthma Immunol Res. 2017;9(6):466–76. doi: 10.4168/aair.2017.9.6.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Greve G, Hellings PW, Fokkens WJ, et al. Endotype-driven treatment in chronic upper airway diseases. Clin Transl Allergy. 2017;7:22. doi: 10.1186/s13601-017-0157-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yu S, Zhang R, Liu G, et al. Microarray analysis of differentially expressed microRNAs in allergic rhinitis. Am J Rhinol Allergy. 2011;25:e242–46. doi: 10.2500/ajra.2011.25.3682. [DOI] [PubMed] [Google Scholar]
- 32.Ma Z, Teng Y, Liu X, et al. Identification and functional profiling of differentially expressed long non-coding RNAs in Nasal Mucosa with allergic rhinitis. Tohoku J Exp Med. 2017;242(2):143–50. doi: 10.1620/tjem.242.143. [DOI] [PubMed] [Google Scholar]
- 33.Lukinskiene L, Liu Y, Reynolds SD, et al. Antimicrobial activity of PLUNC protects against Pseudomonas aeruginosa infection. J Immunol. 2011;187:382–90. doi: 10.4049/jimmunol.1001769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leclair EE. Four BPI (bactericidal/permeability-increasing protein)-like genes expressed in the mouse nasal, oral, airway and digestive epithelia. Biochem Soc Trans. 2003;31:801–5. doi: 10.1042/bst0310801. [DOI] [PubMed] [Google Scholar]
- 35.Wei Y, Han M, Wen W, et al. Differential short palate, lung, and nasal epithelial clone 1 suppression in eosinophilic and noneosinophilic chronic rhinosinusitis with nasal polyps: Implications for pathogenesis and treatment. Curr Opin Allergy Clin Immunol. 2016;16(1):31–38. doi: 10.1097/ACI.0000000000000228. [DOI] [PubMed] [Google Scholar]
- 36.Tsou YA, Lin CD, Chen HC, et al. Interleukin-13 inhibits lipopolysaccharide-induced BPIFA1 expression in nasal epithelial cells. PLoS One. 2015;10(12):e0143484. doi: 10.1371/journal.pone.0143484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wei Y, Xia W, Ye X, et al. The antimicrobial protein short palate, lung, and nasal epithelium clone 1 (SPLUNC1) is differentially modulated in eosinophilic and noneosinophilic chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2014;133(2):420–28. doi: 10.1016/j.jaci.2013.09.052. [DOI] [PubMed] [Google Scholar]
- 38.Min JY, Ocampo CJ, Stevens WW, et al. Proton pump inhibitors decrease eotaxin-3/CCL26 expression in patients with chronic rhinosinusitis with nasal polyps: Possible role of the nongastric H, K-ATPase. J Allergy Clin Immunol. 2017;139(1):130–41.e11. doi: 10.1016/j.jaci.2016.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Larose MC, Chakir J, Archambault AS, et al. Correlation between CCL26 production by human bronchial epithelial cells and airway eosinophils: Involvement in patients with severe eosinophilic asthma. J Allergy Clin Immunol. 2015;136:904–13. doi: 10.1016/j.jaci.2015.02.039. [DOI] [PubMed] [Google Scholar]
- 40.Provost V, Larose MC, Langlois A, et al. CCL26/eotaxin-3 is more effective to induce the migration of eosinophils of asthmatic than CCL11/eotaxin-1 and CCL24/eotaxin-2. J Leukoc Biol. 2013;94:213–22. doi: 10.1189/jlb.0212074. [DOI] [PubMed] [Google Scholar]
- 41.Zissler UM, Esser-von Bieren J, Jakwerth CA, et al. Current and future biomarkers in allergic asthma. Allergy. 2016;71(4):475–94. doi: 10.1111/all.12828. [DOI] [PubMed] [Google Scholar]
- 42.Dai D, Li Y, Chen B, et al. Elevated expression of CST1 promotes breast cancer progression and predicts a poor prognosis. J Mol Med (Berl) 2017;95(8):873–86. doi: 10.1007/s00109-017-1537-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fukuoka A, Matsushita K, Morikawa T, et al. Human cystatin SN is an endogenous protease inhibitor that prevents allergic rhinitis. J Allergy Clin Immunol. 2019;143(3):1153–62.e12. doi: 10.1016/j.jaci.2018.06.035. [DOI] [PubMed] [Google Scholar]
- 44.Lei Y, Guo P, An J, et al. Identification of pathogenic genes and upstream regulators in allergic rhinitis. Int J Pediatr Otorhinolaryngol. 2018;115:97–103. doi: 10.1016/j.ijporl.2018.09.005. [DOI] [PubMed] [Google Scholar]
- 45.Pan S, Zhou H, Yu H, et al. MiR-195-5p inhibits the cell migration and invasion of cervical carcinoma through suppressing ARL2. Eur Rev Med Pharmacol Sci. 2019;23(24):10664–71. doi: 10.26355/eurrev_201912_19764. [DOI] [PubMed] [Google Scholar]
- 46.Zhang H, Yang K, Ren T, et al. miR-16-5p inhibits chordoma cell proliferation, invasion and metastasis by targeting Smad3. Cell Death Dis. 2018;9(6):680. doi: 10.1038/s41419-018-0738-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hu W, Chang G, Zhang M, et al. MicroRNA-125a-3p affects smooth muscle cell function in vascular stenosis. J Mol Cell Cardiol. 2019;136:85–94. doi: 10.1016/j.yjmcc.2019.08.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1.
The relationship between co-expressed genes and respiratory system diseases based on the CTD database.
| Gene symbol | Gene ID | Disease name | Disease ID | Direct evidence | Inference network | Inference score | Reference count |
|---|---|---|---|---|---|---|---|
| BPIFA1 | 51297 | Rhinitis | MESH: D012220 | Particulate Matter | Tobacco Smoke Pollution | Vehicle Emissions | 12.67 | 3 | |
| BPIFA1 | 51297 | Rhinitis, allergic | MESH: D065631 | Particulate Matter | Soot | 8.45 | 2 | |
| BPIFA1 | 51297 | Rhinitis, allergic, seasonal | MESH: D006255 | Particulate Matter | 3.57 | 1 | |
| BPIFA1 | 51297 | Asthma | MESH: D001249 | Acetaminophen | Arsenic | Particulate Matter | Soot | Tobacco Smoke Pollution | Vehicle Emissions | 21.02 | 29 | |
| BPIFA1 | 51297 | Asthma, occupational | MESH: D059366 | Silicon Dioxide | 3.35 | 1 | |
| BPIFA1 | 51297 | Nose diseases | MESH: D009668 | Propylthiouracil | Tobacco Smoke Pollution | 9.59 | 4 | |
| CCL26 | 10344 | Asthma | MESH: D001249 | Aerosols | Antigens, Dermatophagoides | Arsenic | Cadmium | Dexamethasone | Ozone | Resveratrol | Tobacco Smoke Pollution | Zinc | 27.99 | 28 | |
| CCL26 | 10344 | Rhinitis, allergic, perennial | MESH: D012221 | Ozone | 3.73 | 1 | |
| CCL26 | 10344 | Rhinitis, allergic | MESH: D065631 | Atrazine | 2.83 | 1 | |
| CCL26 | 10344 | Rhinitis | MESH: D012220 | Tobacco Smoke Pollution | 2.6 | 1 | |
| CCL26 | 10344 | Nose diseases | MESH: D009668 | Lipopolysaccharides | Propylthiouracil | Tobacco Smoke Pollution | 14.31 | 5 | |
| CCL26 | 10344 | Sinusitis | MESH: D012852 | Tobacco Smoke Pollution | 2.9 | 1 | |
| CPA3 | 1359 | Rhinitis | MESH: D012220 | Tobacco Smoke Pollution | Vehicle Emissions | 6.86 | 2 | |
| CPA3 | 1359 | Asthma | MESH: D001249 | Acetaminophen | Decitabine | epigallocatechin gallate | Tobacco Smoke Pollution | trimellitic anhydride | Vehicle Emissions | 18.84 | 19 | |
| CPA3 | 1359 | Nose diseases | MESH: D009668 | Tobacco Smoke Pollution | 3.4 | 2 | |
| CPA3 | 1359 | Sinusitis | MESH: D012852 | Acetaminophen | Tobacco Smoke Pollution | 7.83 | 2 | |
| CST1 | 1469 | Rhinitis, allergic, seasonal | MESH: D006255 | Marker/mechanism | 1 | ||
| CST1 | 1469 | Rhinitis, allergic | MESH: D065631 | Air Pollutants | 3.83 | 1 | |
| CST1 | 1469 | Rhinitis | MESH: D012220 | Air Pollutants | 3.6 | 1 | |
| CST1 | 1469 | Asthma | MESH: D001249 | Air Pollutants | Methotrexate | Tretinoin | 9.41 | 14 | |
| CST1 | 1469 | Asthma, occupational | MESH: D059366 | Silicon Dioxide | 3.45 | 1 | |
| CST2 | 1470 | Asthma, occupational | MESH: D059366 | Silicon Dioxide | 3.85 | 1 | |
| FETUB | 26998 | Rhinitis | MESH: D012220 | Tobacco Smoke Pollution | Vehicle Emissions | 5.71 | 2 | |
| FETUB | 26998 | Rhinitis, allergic | MESH: D065631 | Atrazine | 2.66 | 1 | |
| FETUB | 26998 | Nose diseases | MESH: D009668 | cobaltous chloride | Tobacco Smoke Pollution | 7.86 | 3 | |
| FETUB | 26998 | Sinusitis | MESH: D012852 | Acetaminophen | Tobacco Smoke Pollution | 6.66 | 2 | |