Abstract
Chemotherapy is a life‐saving treatment for cancer patients, but also causes long‐term cognitive impairment, or “chemobrain”, in survivors. However, several challenges, including imprecise diagnosis criteria, multiple confounding factors, and unclear and heterogeneous molecular mechanisms, impede effective investigation of preventions and treatments for chemobrain. With the rapid increase in the number of cancer survivors, chemobrain is an urgent but unmet clinical need. Here, we leverage the extensive knowledge in various fields of neuroscience to gain insights into the mechanisms for chemobrain. We start by outlining why the post‐mitotic adult brain is particularly vulnerable to chemotherapy. Next, through drawing comparisons with normal aging, Alzheimer's disease, and traumatic brain injury, we identify universal cellular mechanisms that may underlie the cognitive deficits in chemobrain. We further identify existing neurological drugs targeting these cellular mechanisms that can be repurposed as treatments for chemobrain, some of which were already shown to be effective in animal models. Finally, we briefly describe future steps to further advance our understanding of chemobrain and facilitate the development of effective preventions and treatments.
Keywords: aging, chemotherapy, cognitive impairment, neurodegenerative diseases, traumatic brain injury
Subject Categories: Cancer, Neuroscience, Chemical Biology
Timely and important review on chemobrain, from the existing consensus to new potential mechanisms based on Alzheimer's disease or traumatic brain injury pathologies, suggesting chemobrain as a persistent neurological disorder.
Glossary
- Alzheimer's disease
Disease characterized by a pathological deposition of amyloid‐ß protein and fibrillary tau tangles, resulting in progressive synaptic and neuronal loss. AD initially manifests as mild short‐term memory loss, followed by severe loss of memory, speech, and executive functions.
- Astrocytes
Supply neurons with oxygen, growth factors, and nutrients, and recycle ions and neurotransmitters to maintain the homeostasis of the microenvironment of the brain.
- Blood–brain barrier
Forms a selective boundary between the peripheral and the central nervous systems, also blocks the entry of most drugs into the brain but can become compromised following neuroinflammation.
- Chemobrain
A constellation of symptoms reflecting cognitive decline, either reversible or irreversible, that a subset of adult, non‐CNS cancer patients experience as a direct effect of chemotherapy. These effects persist even after controlling for factors such as treatment regimen, emotional status, and cancer burden.
- Chemotherapy
The use of drugs to target rapidly dividing cancer cells include the alkylating agents, antimetabolites, antibiotics, topoisomerase inhibitors, and mitotic inhibitors.
- Dendrites
Branching structures of neurons that receive input from other neurons.
- Frontal cortex
Region important for working memory and higher cognitive functions such as planning, attention, and decision making. Loss of neurons, spines, or dendrites results in the thinning of the cortex.
- Hippocampus
Structure essential for the consolidation of short‐term memory to long‐term memory, and for spatial memory.
- Microglia
Resident macrophages that continuously survey the CNS for injuries and abnormalities. In response to an insult, microglia become highly motile, migrate to, and isolate the lesion site, and secrete inflammatory cytokines and phagocytose cell debris or damaged neurons.
- Myelination
Formation of the insulating myelin sheath around axons, occurs rapidly during early childhood, then continues through adolescence and into adulthood.
- Neurogenesis and gliogenesis
The process through which new neurons and glial cells are produced from neural precursor cells in niche regions in the brain.
- Neuroinflammation
Activation of the brain's innate immunity mechanisms in response to harmful stimuli such as trauma, pathogens, and toxic metabolites.
- Neurotransmitters
Chemical messengers that facilitate communications between neurons and between various brain regions.
- Normal aging
Process characterized by a progressive accumulation of changes and errors at the molecular, cellular, and systemic levels, resulting in a slow deterioration of mental faculties and increased susceptibility to diseases and death.
- Oligodendrocytes
Form the myelin sheath surrounding the axons of neurons, allow for faster propagation of electrical signals.
- Quiescence
Cellular state characterized by reversible proliferation arrest. Dormant cells can rapidly re‐enter the cell cycle in response to various stimuli such as tissue injury.
- Senescence
Cellular state characterized by irreversible proliferation arrest and increased secretion of inflammatory cytokines, growth factors, and proteases to contribute to age‐related inflammation.
- Spines
Small membranous protrusions on dendrites that are specialized for making synaptic contacts.
- Traumatic brain injury
Occurs when an external force injures the brain, resulting in acute symptoms such as loss of consciousness, followed by chronic symptoms
Introduction
Cancer survival rates have significantly improved due to advances in awareness, screening, prevention, diagnosis, and treatment. For example, the average 5‐year survival rates for breast cancer increased from 75% in the 1975–1977 cohort to 91% in the 2008–2014 cohort (Noone et al, 2018). However, most treatments, including conventional chemotherapeutics and newer therapies such as immunotherapy, are associated with severe, sometimes long‐lasting or irreversible, side effects. With an estimate of 16.9 million cancer survivors in the United States alone in 2019 (Miller et al, 2019), it is clear that alleviating these side effects is an urgent clinical need.
Since the discovery of antifolates for treating acute lymphoblastic leukemia in the 1940s (Farber & Diamond, 1948), chemotherapy remains a mainstream treatment for many types of cancer (Noone et al, 2018), and is essential in later stages where metastasis renders local surgery insufficient. In 1978, concerns were raised about the impacts of chemotherapy on the emotional and cognitive status of cancer patients, and how they were severely underreported by clinicians and patients (Levine et al, 1978). However, it was not until the early 2000s that a series of epidemiological and imaging studies conclusively supported that cognitive decline in breast cancer patients had real physiological bases (Ahles & Saykin, 2001, 2002; Ahles et al, 2002; Saykin et al, 2003). Still, the underlying mechanism remains poorly understood.
We focus on the effects of chemotherapy on the central nervous system (CNS) in adults, resulting in symptoms colloquially known as chemobrain. Here, we compare chemobrain with aging, Alzheimer's disease (AD), and traumatic brain injury (TBI). We aim to leverage knowledge from more extensively studied disciplines to address the more recently acknowledged topic of chemobrain. Although disorders affecting cognitive capabilities are complex in terms of mechanisms, symptoms, risks, onsets, and anatomical loci affected, they share similarities. This review will facilitate cross‐disciplinary thinking and enable laboratories to share expertise to address chemobrain.
Symptoms, epidemiology, and findings from imaging studies
Cognitive complaints are common among cancer patients during and after chemotherapy. Cross‐sectional and longitudinal studies suggest that short‐term memory, working memory, and verbal ability are most frequently affected, followed by visuospatial memory, executive functions, and attention span (for meta‐analyses, see Stewart et al, 2006; Jim et al, 2012; Lindner et al, 2014). These deficits tend to be subtle, such that cancer survivors with chemobrain perform at the lower end of the normal range, but not yet in the pathological range (Nelson & Suls, 2013). This subtlety, together with the reliance on tests designed to detect more severe, localized deficits such as TBI, strokes, and AD, means that these cognitive changes are often undetected or underestimated by clinicians (Horowitz et al, 2018). The difficulties with objectively defining and measuring chemobrain result in vast differences in estimating the percentage of cancer survivors with chemobrain, which range from 17 to 75% (Wefel & Schagen, 2012). Notably, the percentage of cancer survivors diagnosed with chemobrain tends to be inversely correlated with time after treatment, suggesting that some recovery occurs. Nevertheless, deficits could be detected up to 10 years after treatment, suggesting that they are permanent in some cancer survivors (Ahles et al, 2002).
Furthermore, structural studies reveal decreased gray matter density in several brain regions, including the frontal and temporal cortices, the cerebellum, and the right thalamus immediately after chemotherapy, with only partial recovery a year later (McDonald et al, 2010, 2013). Functional magnetic resonance imaging (fMRI) studies also found decreased activation during cognitive tasks in similar regions (Kesler et al, 2011; de Ruiter et al, 2011; Lopez Zunini et al, 2013). However, other studies found increased activation in the same regions, and propose that this is a compensatory mechanism as cancer survivors need to utilize more mental resources for the same tasks. These mental resources then become more quickly depleted for complex tasks (Ferguson et al, 2007; Menning et al, 2017). Nevertheless, together, these studies provide concrete evidence that the symptoms of chemobrain have biological bases, rather than being purely psychological.
Chemotherapy and the post‐mitotic adult brain
At first glance, cancer and neurodegeneration appear to lie on opposite ends of the disease mechanism spectrum (Plun‐Favreau et al, 2010). Cancer involves an abnormal resistance, whereas neurodegeneration involves an abnormal susceptibility, to cell death. Moreover, chemotherapeutic drugs are designed to selectively target rapidly dividing cells, but most neurons are non‐dividing, post‐mitotic cells, except for those in niche regions in the brain. Although several studies focus on diminished cell division, other intrinsic properties of the adult brain likely contribute to its vulnerability to chemotherapy (Fig 1A).
Figure 1. Molecular mechanisms for chemobrain are highly complex and heterogeneous.
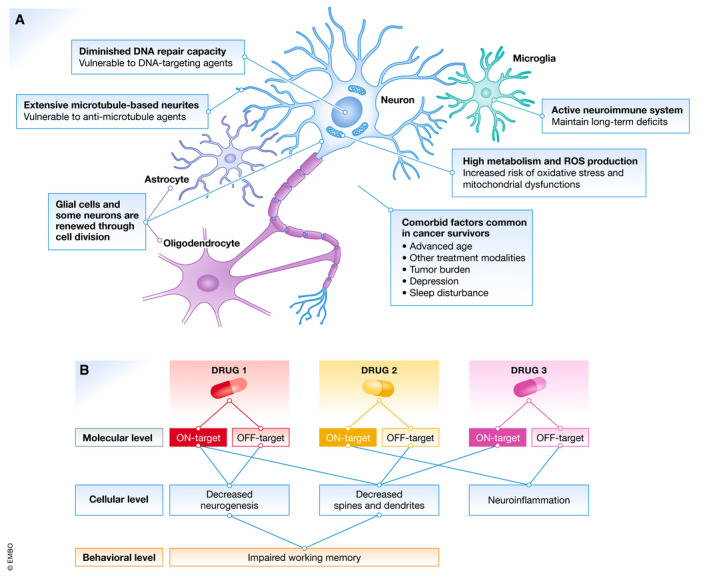
(A) The central nervous system (CNS) is intrinsically vulnerable to the on‐target effects of chemotherapeutic drugs and possesses low recovery capacity. First, as most neurons are non‐dividing cells, they lack several DNA repair mechanisms that make them susceptible to DNA‐targeting agents. Second, neurons rely on an extensive microtubule‐based network for proper functions and communication, making them vulnerable to microtubule‐targeting agents. Third, chemotherapy can reduce neurogenesis and gliogenesis, which are crucial processes required for maintaining the health and plasticity of the CNS. Fourth, glial cells contribute to the vigilant neuroimmune system, and can be damaging when hyperactivated. Lastly, high metabolism, high production of reactive oxidative species (ROS), and comorbid factors common in cancer survivors make the CNS particularly vulnerable to external insults. (B) A model illustrating the complexity and heterogeneity of mechanisms for chemobrain. We propose that focusing on the cellular consequences is currently the most feasible approach for the development of treatments and preventions for chemobrain.
First, chemotherapeutics drugs, especially the DNA‐targeting agents, can cause DNA damage in post‐mitotic neurons, which accelerates senescence and eventual cell death (Hoeijmakers, 2009; Maynard et al, 2015). The post‐mitotic brain also exhibits diminished DNA repair capacity. Some DNA repair pathways, including mismatch repair, homologous recombination, and non‐homologous end joining, are associated with replication and therefore attenuated in non‐dividing neurons (Maynard et al, 2015). Thus, the accumulation of DNA damage caused by chemotherapy can accelerate neuronal dysfunction and death.
Second, many chemotherapeutic drugs target the microtubule network critical for segregating chromosomes during mitosis (Mihlon et al, 2010). These drugs disrupt microtubule dynamics either through hyperstabilizing (paclitaxel, docetaxel, and ixabepilone) or destabilizing (vincristine and vinblastine) microtubule formation. The microtubule network is essential for regulating neuronal polarity and morphology, axonal transport, and scaffolding signaling hubs (Dubey et al, 2015). Therefore, either excessive microtubule stabilization or destabilization can dysregulate neuronal morphology, functions, and communication.
Third, non‐neuronal cells, including astrocytes, oligodendrocytes, and microglia, play essential roles in maintaining the health and normal functions of the CNS. The lifelong proliferation and turnover of glial cells make them vulnerable to chemotherapy. In addition, damage to neurons or glial cells can activate microglia and astrocytes, leading to neuroinflammation that maintains chronic deficits.
Fourth, the post‐mitotic brain accounts for ~ 2% of the bodyweight, but consumes ~ 20% of glucose‐derived energy (Patel, 2016), resulting in a high production of reactive oxygen species (ROS)—a major source of DNA damage. Furthermore, a majority of cancer survivors are older, with 50% of new cases diagnosed in patients aged 55–74 (Miller et al, 2019). Additional stress sources, including the tumor itself and psychiatric comorbidity such as depression, contribute to a highly vulnerable brain environment. This suggests that a small insult can “tip the scale”, triggering a cascade of events resulting in chemobrain.
In addition, chemotherapeutic drugs may also have off‐target effects independent of their anticancer mechanisms. For example, our laboratory studies how paclitaxel also dysregulates calcium signaling (Boehmerle et al, 2006; Mo et al, 2012). Moreover, a typical patient receives a cocktail of drugs during chemotherapy. In this case, the molecular mechanisms for chemobrain will be a combination of (i) each drug's on‐target effects, (ii) each drug's off‐target effects, and (iii) the synergistic effects of (i) and (ii) (Fig 1B). With such complexity and heterogeneity in molecular mechanisms, potential convergent downstream cellular consequences present more readily available targets for treatments or preventions.
Cellular mechanisms
In the following sections, we will draw extensive comparisons to aging, AD, and TBI due to several reasons (Fig 2). First, these conditions share similar symptoms with chemobrain, particularly impairments to memory and higher cognitive functions. Second, there is an extensive field of literature describing mechanisms and intervention strategies. Third, they represent different aspects of cognitive decline, in terms of both onset and specificity of loci affected. Similar to normal aging, chemobrain involves a subtle loss of cognitive functions, such that chemobrain has been proposed to mimic accelerated aging (Ahles et al, 2012), and is comparable with early stages of AD. Similar to TBI, chemobrain has a known onset, an acute phase, followed by a recovery period. Insights from aging, AD, and TBI will improve our understanding of chemobrain and facilitate the discovery of effective therapies.
Figure 2. Proposed trajectory of chemobrain in comparison with normal aging, Alzheimer's disease, and traumatic brain injury.
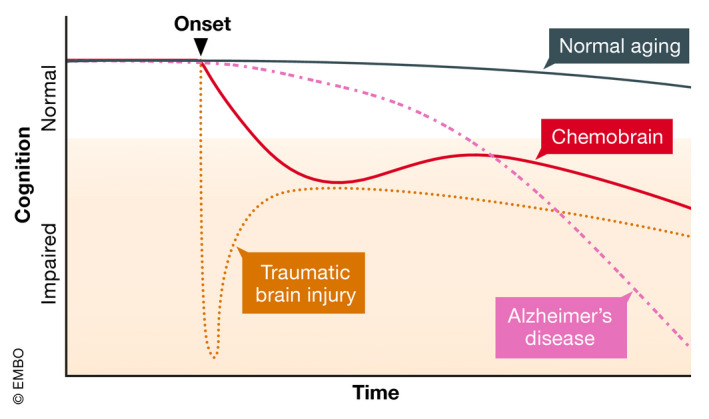
Normal aging displays a slow and gradual reduction in cognitive capability over time, which, however, remains above the threshold for normal cognitive performance. Similar to Alzheimer's disease, chemobrain involves an accelerated decline in cognitive impairment, though less severe. Similar to traumatic brain injury, the onset of chemobrain is known, and the initial decline is followed by a period of recovery, which, however, may not return cognitive capability to the normal level.
Reduced neurogenesis
After rapid cell division and maturation during the embryonic and postnatal periods, most neurons in the adult brain are fully differentiated, non‐dividing cells. Adult neurogenesis occurs primarily in niche regions: the subgranular zone (SGZ) of the dentate gyrus of the hippocampus, the subventricular zone (SVZ) lining the lateral ventricles (Ming & Song, 2011), and the striatum (Ernst et al, 2014). In the SGZ, neural precursor cells (NPCs) undergo cell division for self‐renewal or to give rise to immature cells that can differentiate into neurons and glial cells. At baseline, neurogenesis provides a buffer for restoring neurons lost due to daily wear and tear (Choi & Goldstein, 2018). In cases of acute insults, such as a stroke, neurogenesis after injuries is crucial for the recovery of cognitive functions (Richardson et al, 2007). For example, migration of cells born in the SVZ can be rerouted to injured areas such as the cerebral cortex (Sundholm‐Peters et al, 2005) and the striatum (Yamashita et al, 2006).
Reduced neurogenesis is a common factor in aging and neurodegenerative diseases. Neurogenesis declines with age, primarily through a reduction in NPCs, quiescence of the remaining NPCs, and an extracellular environment hostile to cell division (Shruster et al, 2010; Dubey et al, 2015). Neurogenesis is diminished in several AD mouse models (Hollands et al, 2016). Such reduced neurogenesis also increases the risk of acquiring new cognitive impairment or exacerbating existing impairment.
Because memory problems are common symptoms of chemobrain, it is not surprising that reduced neurogenesis is the most commonly studied mechanism for chemobrain (Table 1 and Fig 3). Intraperitoneal injection (IP) or intravenous injections (IV) of various drugs, ranging from methotrexate, 5‐fluorouracil, cyclophosphamide, doxorubicin, docetaxel, paclitaxel, cisplatin, and thioTEPA, were observed to lead to impairment of memory from a few days to up to 20 weeks after injection (Table 1). Correspondingly, various protein markers of neurogenesis were reduced, though not in all studies. These markers include BrdU and Ki‐67, which label proliferating cells; doublecortin (DCX), which label NPCs and immature new neurons; and NeuN, which label mature neurons (Shruster et al, 2010). For example, several studies found a reduction in the number of BrdU‐ or Ki‐67‐positive cells, suggesting that proliferating precursor cells were directly affected (Seigers et al, 2008; ElBeltagy et al, 2010; Briones & Woods, 2011; Nokia et al, 2012). In contrast, 5‐fluorouracil administration did not change the number of Ki‐67‐positive cells, but caused a decrease in DCX‐positive cells (Mustafa et al, 2008), suggesting that the early maturation phase was affected. Similarly, cyclophosphamide or doxorubicin treatment did not change the number of BrdU‐positive cells, but reduced the number of DCX‐positive and doubly labeled BrdU/NeuN cells (Christie et al, 2012), suggesting that both the early and late maturation phases were affected. Future studies will benefit from assessing a range of protein markers to determine which phases of neurogenesis are affected.
Table 1.
Summary of studies of mechanisms for development of chemobrain
| Drugs and known mechanism of actions | Neurogenesis | Spines/dendrites | Neurotransmitter | Inflammation/blood–brain barrier | Glial cells |
|---|---|---|---|---|---|
| Antimetabolites | |||||
| Methotrexate: folate derivative, inhibits nucleotide synthesis | Seigers et al (2008), Lyons et al (2011b), Yang et al (2012), Wu et al (2017) | Wu et al (2017) | Yang et al (2012) | Seigers et al (2010), Geraghty et al (2019), Gibson et al (2019) | |
| Cytarabine: pyrimidine analog, inhibits nucleotide synthesis | Dietrich et al (2006) | Dietrich et al (2006) | |||
| 5‐Fluorouracil: pyrimidine analog, inhibits nucleotide synthesis | Han et al (2008), Mustafa et al (2008), ElBeltagy et al (2010), Lyons et al (2012) | Groves et al (2017) | Mustafa et al (2008), Kaplan et al (2016), Park et al (2018), Jarmolowicz et al (2019) | Groves et al (2017) | Han et al (2008) |
| Alkylating agents | |||||
| Cyclophosphamide: facilitates DNA crosslinks | Yang et al (2010), Lyons et al (2011a), Christie et al (2012) | Acharya et al (2015) | Christie et al (2012) | ||
| Cisplatin: facilitates DNA crosslinks and adducts | Dietrich et al (2006), Manohar et al (2014) | Andres et al (2014), Zhou et al (2016) | Dietrich et al (2006) | ||
| Carboplatin: facilitates DNA crosslinks and adducts | Kaplan et al (2016) | ||||
| ThioTEPA: facilitates DNA crosslinks | Mondie et al (2010) | ||||
| Temozolomide: methylates DNA to cause damage | Nokia et al (2012) | ||||
| Mitotic inhibitors | |||||
| Paclitaxel: binds tubulin to stabilize microtubule polymerization | Huehnchen et al (2017), Lee et al (2017) | ||||
| Docetaxel: binds tubulin to stabilize microtubule polymerization | Fardell et al (2014) | ||||
| Vinblastine: binds tubulin to block microtubule polymerization | Parsania et al (2014) | ||||
| Topoisomerase inhibitors | |||||
| Doxorubicin: intercalates between DNA bases to inhibit progression of topoisomerases | Christie et al (2012), Park et al (2018) | Thomas et al (2017), El‐Agamy et al (2018), Keeney et al (2018) | El‐Agamy et al (2018), Keeney et al (2018) | El‐Agamy et al (2018) | |
| Combination | |||||
| CMF (cyclophosphamide + methotrexate + 5‐fluorouracil) | Briones and Woods (2011), Rendeiro et al (2016) | ||||
| MF (methotrexate + 5‐fluorouracil) | Winocur et al (2014, 2016), Jiang et al (2018) | ||||
| MC (methotrexate + cytarabine) | Alexander et al (2018) | ||||
| AC (doxorubicin + cyclophosphamide) | Kang et al (2018) | Kang et al (2018) | |||
| DAC (docetaxel + doxorubicin + cyclophosphamide) | Shi et al (2019) | Shi et al (2018, 2019) | |||
“A” refers to Adriamycin, which is the trade name for doxorubicin.
Figure 3. Convergent cellular mechanisms for chemobrain and how they lead to cognitive deficits.
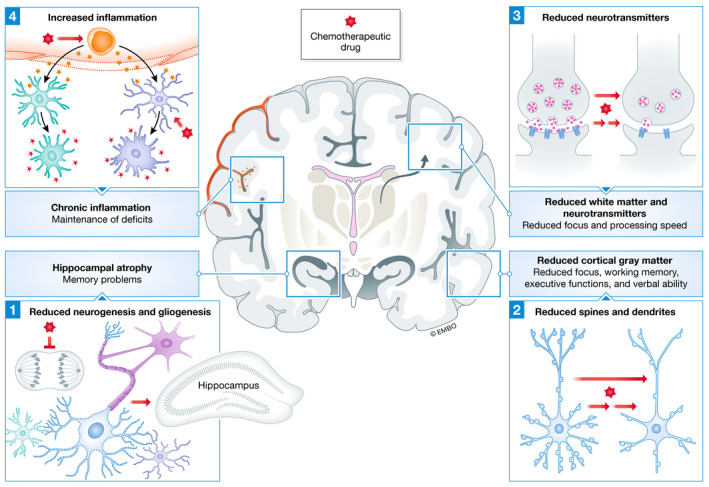
The red hexagon represents a chemotherapeutic drug. First, as most drugs are designed to stop cell division, they can block neurogenesis and gliogenesis, particularly in the hippocampus. This, in turn, leads to hippocampal atrophy and memory problems. Second, chemotherapeutic drugs can lead to a decrease in cortical spines and dendrites. The subsequent loss of cortical gray matter results in impaired cortex‐based task performance, including attention, working memory, and executive functions. Third, reduced white matter due to reduced gliogenesis and alterations of neurotransmitter balance can lead to decreased focus, arousal, and processing speed. Fourth, chemotherapeutic drugs can induce peripheral or central inflammation, which hyperactivates astrocytes and microglia, resulting in chronic central inflammation that can maintain deficits for years after treatments cease.
Additionally, brain‐derived neurotrophic factor (BDNF), a member of the neurotrophin family of growth factors, is secreted into the extracellular environment to promote neurogenesis. Low serum BDNF levels were associated with cognitive impairment in cancer patients (Jehn et al, 2015; Zimmer et al, 2015). In a rodent model, BDNF levels in the hippocampus decreased following injections of 5‐fluorouracil (Mustafa et al, 2008) or doxorubicin (Park et al, 2018). The mechanisms for loss of BDNF, and how this affects neurogenesis, remain unclear. However, methotrexate treatment was recently reported to deplete cortical Bdnf mRNA and protein expression (Geraghty et al, 2019), suggesting that transcriptional regulation of BDNF is an underlying factor.
While most studies focus on neurogenesis in the hippocampus, other neurogenic regions may also be vulnerable. Systemic exposure to cisplatin, cytarabine, or 5‐fluorouracil was found to decrease cell division in the SGZ, the SVZ, and the corpus callosum (Dietrich et al, 2006; Han et al, 2008). Reduced neurogenesis in multiple regions may result in symptoms beyond memory lapses. For example, in AD, olfactory dysfunction due to reduced SVZ neurogenesis is an early symptom preceding the onset of frank dementia (Zou et al, 2016). Furthermore, neurogenesis can be subtly affected such that no visible symptoms are observable, but survivors may still have increased risk of cognitive impairment later in life. Notably, some studies found that chemotherapy increased the risk of dementia later in life (Heck et al, 2008; Kesler et al, 2017), whereas others found no association (Baxter et al, 2009; Raji et al, 2009). Future epidemiology studies should explore these potential increased risks of neurodegenerative diseases in the population of cancer survivors compared to the control population.
Loss of spines and dendritic arborization
Most neurons are highly polarized cells with complex morphology that are critical for their interactions and functions (Barnes & Polleux, 2009). Spines and dendrites regulate the synaptic plasticity essential for learning, memory, and executive functions (Forrest et al, 2018). Spines and dendrites proliferate during early development, followed by controlled pruning in childhood and adolescence, and then stabilize in adulthood. Nevertheless, both structures, particularly spines, remain dynamic in mature neurons, thereby facilitating the plasticity required for learning and adapting to new experiences (Forrest et al, 2018). Spines and dendrites are often reduced due to several factors, including glutamate toxicity, reduced presynaptic neurotransmitter release, protein oligomers such as amyloid‐β oligomers, unregulated calcium flux, disruption of the cytoskeleton, and disruption of the ubiquitin–proteasome system (Forrest et al, 2018). A gradual loss of spines and dendrites also occurs in aging (Dickstein et al, 2013), AD (Dorostkar et al, 2015), and TBI (Gao et al, 2011; Przekwas et al, 2016). These losses result in the thinning of the cortex, which may account for the reduction in gray matter in the brains of cancer survivors after chemotherapy treatment.
Several studies have observed a reduction in dendritic and spinal complexity following the administration of chemotherapeutic drugs in rodent models. Reduction in the number of spines and dendritic branching in granule cells, and CA1 and CA3 pyramidal neurons in the hippocampus, was observed following the administration of cisplatin (Andres et al, 2014), fluorouracil (Groves et al, 2017), doxorubicin, and cyclophosphamide (Acharya et al, 2015; Kang et al, 2018). In addition, reduced spine number and dendritic branching in the cingulate cortex, an integral part of the limbic system involved in emotion, learning, and memory, were observed (Zhou et al, 2016). Interestingly, little research has been done regarding the effect of microtubule agents, considering that the microtubule network is vital for the formation and stabilization of spines, dendrites, and axons. We are aware of only two studies linking the microtubule‐stabilizing effect of paclitaxel to impaired memory acquisition in rodent models (Atarod et al, 2015; You et al, 2018), although both studies did not further examine possible effects on neuronal morphology. There have been proposals to use these drugs to counter spine instability, specifically in AD (Brunden et al, 2014), supporting that the effects of microtubule agents in the CNS need to be further investigated.
Current studies are limited primarily to the hippocampus and associated regions. Future studies will benefit from examining other brain regions to determine whether the aversive effects are general or specific to particular regions. For instance, behavioral tasks to measure cortical‐based performance in chemobrain animal models, such as attention and executive functions, are lacking. We found only one study employing the 5‐choice serial reaction time task to examine prefrontal cortex impairment caused by cisplatin (Huo et al, 2018). Cortical‐based tasks have been developed, continuously updated, and utilized in mouse models of psychiatric disorders such as schizophrenia and bipolar disorder (Powell & Miyakawa, 2006). Similar tasks should be used to study cognitive deficits in chemobrain.
Decreased neurotransmitter release
Neurotransmitter dysregulation, often a reduction in availability, is observed in most neurological disorders. For AD, a decrease in acetylcholine is frequently observed, which explains why three out of four FDA‐approved drugs for treating AD are acetylcholinesterase inhibitors (Graham et al, 2017). In aging, a loss of dopaminergic neurons, approximately 5–10% per decade, was reported (Naoi & Maruyama, 1999). The secondary injury phase of TBI is initiated by an excess of glutamate, leading to calcium overload (Walker & Tesco, 2013). Notably, a majority of neurological drugs act through modulating neurotransmitters. Prominent examples include the cholinergic system for AD, the dopaminergic system for Parkinson's disease, and the serotonergic system for depression.
Supporting evidence for the involvement of neurotransmitters comes from studies correlating variants of catechol‐O‐methyltransferase (COMT) with differential risks of developing chemobrain in cancer survivors. COMT regulates dopamine, epinephrine, and norepinephrine metabolism (Sheldrick et al, 2008). Particularly, for the COMT Val158Met polymorphism (rs4680), the Val allele is associated with higher COMT enzymatic activity, and hence lower cortical dopamine availability (Small et al, 2011). Consequently, cancer survivors carrying at least one Val allele are at higher risk of developing chemobrain (Small et al, 2011), presumably due to their smaller dopamine reservoir. Another COMT variant, rs165599 G/G, also increases the risk of chemobrain in breast cancer patients (Cheng et al, 2016).
Work investigating neurotransmitter alterations in chemobrain remains sparse. Mice receiving a single injection of doxorubicin had slower glutamate uptake into cells in both the cortex and the dentate gyrus (Thomas et al, 2017). Similarly, a reduction in dopamine release in the striatum following injections of carboplatin (Kaplan et al, 2016) or 5‐fluorouracil (Jarmolowicz et al, 2019) was reported. Serotonin release was also reduced in the raphe nucleus after carboplatin injection (Kaplan et al, 2016). The underlying mechanisms remain largely unclear, although reduced glutamate transporter expression (Thomas et al, 2017) and impaired exocytosis (Kaplan et al, 2016) were implicated. In addition, increased acetylcholine esterase activity was observed in the hippocampus of rats treated with doxorubicin (El‐Agamy et al, 2018). A reduction in choline content, the precursor for acetylcholine, was also observed after doxorubicin treatment (Keeney et al, 2018), suggesting that reduced cholinergic activity may be a factor in chemobrain.
Although existing research is promising, it remains unclear whether specific neurotransmitters are affected, or whether all systems are affected. As many neurological drugs target neurotransmitter systems, further studies focusing on neurotransmitters will be particularly helpful in informing therapeutic options.
Glial cells
Glial cells are non‐neuronal cells that provide crucial support and protection for neurons, allowing neurons to perform their functions (Jessen, 2004). Similar to neurogenesis, reduced gliogenesis in the SVZ and SGZ can lead to fewer new astrocytes and oligodendrocytes. As astrocytes and oligodendrocytes modulate memory encoding and consolidation (Fields et al, 2014), this reduction can impair memory acquisition. Proper axonal myelination is required for fast conduction speed and enhanced cognitive processing both in rodents (McDougall et al, 2018) and in healthy young and elderly adults (Bendlin et al, 2010; Lu et al, 2011). Generation of new oligodendrocytes is also critical for complex motor learning (McKenzie et al, 2014) and spatial memory consolidation (Steadman et al, 2020). Imaging studies on human cancer survivors reveal a reduction in several white matter tracts (Deprez et al, 2011, 2012; Chen et al, 2020), suggesting reduced myelination. Supporting these observations, several studies reported that oligodendrocyte precursor cells (OPCs) and non‐dividing mature oligodendrocytes are especially vulnerable to chemotherapy as compared to neurons and astrocytes (Dietrich et al, 2006; Han et al, 2008; Hyrien et al, 2010). In addition to depleting OPCs and mature oligodendrocytes, various chemotherapeutics also alter OPC differentiation, which may further impair proper myelination (Hyrien et al, 2010; Gibson et al, 2019).
Other studies examining glial cells in neurodegenerative diseases focus on reactive gliosis, a series of events that starts with the migration of activated microglia to the site of injury, followed by activated astrocytes and oligodendrocytes, often ending with the formation of a glial scar (Burda & Sofroniew, 2014). Gliosis is the universal response to acute brain injury including TBI, ischemia, and stroke. Similarly, activated microglia and astrocytes are observed in many mouse models of AD, often predating the onset of cognitive abnormalities (Newcombe et al, 2018). In aging, glial cells also become activated (Lynch et al, 2010). These hyperactivation states and their maintenance may contribute to long‐term cognitive deficits.
Specifically, microglia activation in chemobrain occurred one week and three weeks after a single injection of methotrexate, a DNA crosslinker (Seigers et al, 2010). Two additional studies showed that microglia, astrocytes, and oligodendrocytes are all dysregulated following methotrexate treatment (Geraghty et al, 2019; Gibson et al, 2019). Methotrexate activates microglia, which in turn activates astrocytes, further leading to depletion of OPCs, reduced myelination, and reduced cortical BDNF levels. Astrocyte activation was observed after docetaxel injection (Fardell et al, 2014), and microglia activation was observed after cyclophosphamide (Christie et al, 2012).
The involvement of glial cells, either hypoactivation or hyperactivation, requires more investigation. As discussed in the context of other diseases such as AD, these investigations will benefit from recognizing the heterogeneity, including morphological, functional, and regional specificity, of glial cells, and whether they reduce or enhance the detrimental effects of chemotherapeutic drugs (Alibhai et al, 2018).
Inflammation and breakdown of the blood–brain barrier
There is a common consensus that chronic neuroinflammation is responsible for maintaining long‐term cognitive dysfunctions in aging and neurodegenerative diseases (Glass et al, 2010; Michaud et al, 2013). Cytokines are small proteins secreted by cells of the immune system, including B cells, T cells, macrophages, mast cells, neutrophils, basophils, and eosinophils, and microglia and astrocytes (Wang et al, 2015a). Activated microglia and astrocytes can produce cytokines directly in CNS. However, peripherally released cytokines can also access the brain to invoke the local release of cytokines. Cytokines can also compromise the protective blood–brain barrier, thereby enabling the entrance of more cytokines and chemotherapeutic drugs (Ren et al, 2017). Of translational significance, peripheral and central cytokine levels can be routinely measured from the serum or the cerebrospinal fluid, making them convenient as potential biomarkers (Reale et al, 2009).
In aging, the gradual deterioration of the immune system, termed immunosenescence, is believed to underlie a chronic state of low‐grade inflammation (Sparkman & Johnson, 2008; Di Benedetto et al, 2017). AD and TBI are also associated with elevated levels of pro‐inflammatory cytokines (Remarque et al, 2001; Swardfager et al, 2010; Kumar et al, 2015; Schimmel et al, 2017). In all conditions, higher levels of inflammatory cytokines are correlated with worse cognitive performance, as well as higher morbidity and mortality (Guerreiro et al, 2007; Gorska‐Ciebiada et al, 2015; Chen et al, 2018). Interestingly, elevated peripheral cytokines were also observed in cancer survivors receiving various regimens of chemotherapeutic drugs (Wang et al, 2015a).
Few studies using animal models have examined the direct release of cytokines by activated microglia and astrocytes in the CNS. 5‐Fluorouracil and a combination of docetaxel, doxorubicin, and cyclophosphamide upregulated cytokines in the hippocampus (Groves et al, 2017; Shi et al, 2019). Methotrexate activated microglia, but no changes in CNS cytokine levels were observed (Seigers et al, 2010). In contrast, several chemotherapeutic drugs, including methotrexate, cisplatin, oxaliplatin, paclitaxel, and vincristine, elevated peripheral inflammatory cytokines to induce chronic pain (Brandolini et al, 2019). Elevation of peripheral cytokines may also penetrate the blood–brain barrier to directly act on the CNS, and to activate microglia and astrocytes to secrete further cytokines. However, the correlation between elevated peripheral cytokines and their effect on CNS inflammation remains poorly understood.
Therapeutic avenues: current status, challenges, and repurposing existing drugs
Despite significant advances in our understanding of chemobrain, both at the clinical level and at the cellular–molecular basis, several challenges persist. First, despite increased awareness, there are currently no validated or approved tests for the diagnosis of chemobrain. Indeed, many studies find that cancer survivors score within, albeit often at the lower end of, the normal range of the population (Horowitz et al, 2018). This limitation is likely due to the lack of sensitivity of assessment tools used (Horowitz et al, 2018). Second, chemobrain is highly heterogeneous, with many confounding factors including genetic variability, treatment regimen, and comorbidity with other neurological conditions. Third, there are no clear disease mechanisms for chemobrain. Each chemotherapy drug is expected to exert a range of effects, which further vary when combined with other drugs and treatment modalities. Owing to the complexity and unclear mechanisms, the current clinical approach is to refer cancer survivors to psychiatrists who can prescribe cognitive rehabilitation, changes to lifestyle, mind‐training exercises, cognitive–behavioral therapy, and general coping strategies (Ferguson et al, 2012; Kesler et al, 2013; Henneghan & Harrison, 2015). Additionally, neuropsychiatric drugs may be prescribed to alleviate symptoms (Vance et al, 2017).
Considering the complexity of discovering, fine‐tuning, and approving new therapeutic compounds for the CNS (Pangalos et al, 2007), we propose that repurposing existing drugs is a feasible approach to successfully treating chemobrain in the near future. Despite the heterogeneity of molecular mechanisms, there are convergent cellular mechanisms that can be targeted (Table 2 and Fig 4A). This, of course, is not to discount the importance of studies that continue to examine the specific molecular mechanisms of each chemotherapeutic drug. With sufficient knowledge of the consequence of chemotherapy at all levels—molecular, cellular, and behavioral—better prevention or treatment options can be developed. Eventually, the more efficient therapies will not only treat the symptoms but also directly modify the trajectory of chemobrain (Fig 4B), either through alleviating the initial toxic effects or through enhancing recovery after chemotherapy.
Table 2.
Therapeutic strategies for preventing or alleviating chemobrain
| Cellular mechanism | Potential therapeutic options | |
|---|---|---|
| Tested in models of chemobrain | Tested in models of aging and neurodegenerative diseases | |
| Reduction in neurogenesis and gliogenesis | Exercise (Fardell et al, 2012; Winocur et al, 2014; Park et al, 2018) | Neurotrophic factors: BDNF, GDNF, NGF, VEGF, IGF‐1 |
| Environmental enrichment (Winocur et al, 2016) | Transcranial magnetic stimulation | |
| Lithium (Huehnchen et al, 2017) | ||
| SSRIs: fluoxetine (ElBeltagy et al, 2010; Lyons et al, 2011b, 2012) | ||
| Stem cell transplantation (Acharya et al, 2015) | ||
| Loss of spines and dendritic structure | Metformin (Zhou et al, 2016) | Neurotrophic factors |
| PDEIs: rolipram (Callaghan & O'Mara, 2015), ibudilast (Johnston et al, 2017) | Other PDEIs: sildenafil, roflumilast, milrinone, cilostazol, tadalafil | |
| Reduction in neurotransmitter release | ACheIs: donepezil (Winocur et al, 2011; Lim et al, 2016) and astaxanthin (El‐Agamy et al, 2018) | Other ACheIs: tacrine, rivastigmine, galantamine |
| NMDAR antagonists: dextromethorphan (Vijayanathan et al, 2011) and memantine (Cheng et al, 2017) | Dopamine and norepinephrine modulators: amphetamines, atomoxetine, methylphenidate, bupropion | |
| Glutamate modulators: riluzole, ketamine | ||
| Glial cells | Microglia inhibitor/depletion: PLX5622 (Gibson et al, 2019) | Microglia inhibitors: minocycline |
| Rescue oligodendrocyte and myelination: LM22A‐4 (Geraghty et al, 2019) | Oligodendrocyte precursor cell transplantation | |
| Inflammation and blood–brain barrier breakdown | NSAIDs: aspirin, ibuprofen | |
| Immunosuppressant drugs: copaxone, rituximab, and cladribine | ||
| Monoclonal antibodies: anti‐TNF, anti‐IL‐1, anti‐IL‐6 | ||
ACheIs, acetylcholinesterase inhibitors; BDNF, brain‐derived neurotrophic factor; GDNF, glia‐derived neurotrophic factor; IGF‐1, insulin‐like growth factor 1; IL, interleukin; NGF, nerve growth factor; NMDAR, N‐methyl‐d‐aspartate receptor; NSAIDs, non‐steroidal anti‐inflammatory drugs; PDEIs, phosphodiesterase inhibitors; SSRIs, selective serotonin reuptake inhibitors; TNF, tumor necrosis factor; VEGF, vascular endothelial growth factor.
Figure 4. Re‐purposing of existing approved drugs to treat chemobrain.
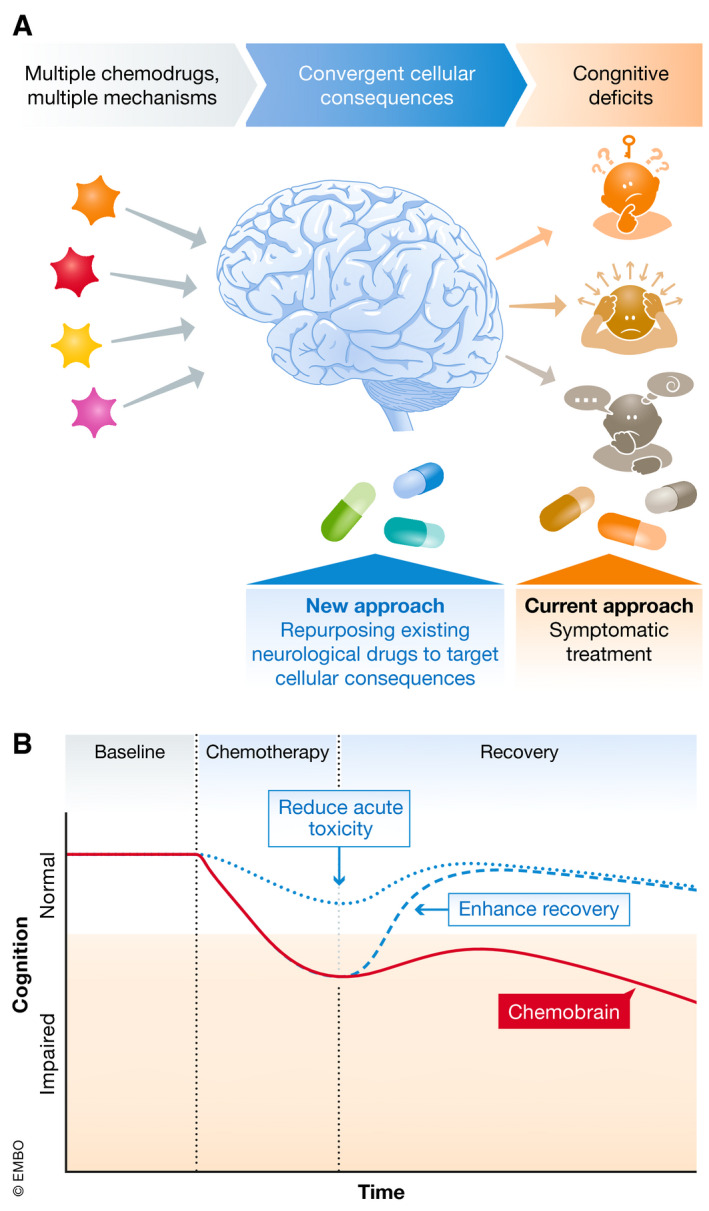
(A) Although the current clinical approach is to prescribe interventions to treat the behavioral symptoms of chemobrain, a more targeted approach is to prescribe interventions that address likely convergent cellular consequences such as those discussed in “Cellular mechanisms”. (B) With sufficient knowledge of both cellular and molecular mechanisms, we can aim to directly modify the trajectory of chemobrain, either through reducing acute toxicity during chemotherapy, or enhancing recovery after chemotherapy, to return cognitive capability to the normal level.
Targeting neurogenesis
The adult hippocampus remains plastic and sensitive to environmental changes, and is therefore highly amenable to treatments (Ming & Song, 2011). Physical exercise and environmental enrichment enhance neurogenesis and alleviate symptoms in rodent models of aging (Voss et al, 2013), AD (Lazarov et al, 2010), TBI (Bondi et al, 2014), depression (Samuels & Hen, 2011), and chemobrain (Fardell et al, 2012; Winocur et al, 2014, 2016; Park et al, 2018). BDNF secretion is also essential for maintaining proper spines and dendrites, and for promoting neurogenesis in the hippocampus. BDNF secretion was increased by exercise in animal models (Lima Giacobbo et al, 2019). Additionally, electroconvulsive shock treatment and deep brain stimulation, often used for treating major depression, are also effective through enhancing neurogenesis (Schoenfeld & Cameron, 2015). However, studies examining the efficiency of these electrical treatments for ameliorating symptoms of chemobrain are lacking.
Many classical antidepressant drugs, including fluoxetine, reboxetine, and tranylcypromine, increase neurogenesis in the adult hippocampus (Schoenfeld & Cameron, 2015; Shohayeb et al, 2018). Lithium, a mood stabilizer used to treat bipolar disorder and complement treatments for depression, also improves neurogenesis (Young, 2009). Notably, fluoxetine and lithium reduce cognitive impairment in rodent models of chemobrain (ElBeltagy et al, 2010; Lyons et al, 2011b, 2012; Huehnchen et al, 2017). Therefore, antidepressant drugs may be useful in addressing both the cellular deficits and the behavioral manifestations of chemobrain.
Transplant of neural stem cells into various brain regions, including the hippocampus, frontal cortex, and striatum, is currently intensively studied as an approach to replace lost neurons in neurodegenerative diseases (Lindvall & Kokaia, 2010). Cells, either of human or of rodent origins, injected into rodent models, successfully survive, integrate, and differentiate into neurons and glia in the recipient's brain, and alleviate cognitive impairment (Wang et al, 2015b). One study found that transplantation of human neural stem cells into the hippocampus of rats rescued behavioral and cellular deficits caused by cyclophosphamide (Acharya et al, 2015), suggesting that this is a promising, albeit very invasive, approach.
Targeting spines and dendrites
Spine formation and stabilization also remain highly dynamic and sensitive to environmental changes in adulthood (Forrest et al, 2018). The glutamate receptors, particularly the N‐methyl‐d‐aspartate receptors (NMDARs) and α‐amino‐3‐hydroxy‐5‐methyl‐4‐isoxazolepropionic acid receptors (AMPARs), play critical roles in spine formation and stabilization. For example, memantine, an NMDAR inhibitor, and dextromethorphan, a non‐competitive NMDAR antagonist, rescued cognitive impairment induced by cisplatin and methotrexate, respectively (Vijayanathan et al, 2011; Cheng et al, 2017). Other regulators of the NDMARs and AMPARs, including ketamine and the benzamides, can induce spine formation or remodel the dendritic arborization to reverse symptoms of depression and alleviate cognitive impairment (Partin, 2015; Phoumthipphavong et al, 2016; Duman, 2018). Although reversing a reduction in spines would be the desired outcome in the context of chemobrain, glutamate modulators will need careful investigation. Special attention is warranted because glutamate overload, as often is the case in TBI, can damage neurons, and reduce spinal and dendritic complexity.
Calcium signaling is also important for the proper maintenance of spines and dendrites (Higley & Sabatini, 2012). For example, dysregulated calcium/cyclic adenosine monophosphate (cAMP) signaling, such as during stress, can lead to spine destabilization and loss (Arnsten, 2015). Interestingly, phosphodiesterase inhibitors, which regulate cAMP levels, including rolipram and ibudilast, rescued cognitive impairment induced by docetaxel and oxaliplatin, respectively (Callaghan & O'Mara, 2015; Johnston et al, 2017). Calcium can also activate several protein kinase C isoforms, which in turn phosphorylate and activate myristoylated alanine‐rich C‐kinase substrate (MARCKS), an important regulator of spinal and dendritic complexity. Hyperactivation of protein kinase C underlies the reduction in dendritic complexity and cognitive impairment in aging and chronic psychological stress (Hains et al, 2009; Brudvig & Weimer, 2015), suggesting that inhibition of protein kinase C is a promising therapeutic strategy.
Targeting neurotransmitters
Because the homeostasis of various neurotransmitters is required for normal cognitive performance (Noudoost & Moore, 2011), a majority of drugs approved for treating neurological disorders are regulators of neurotransmitters. These drugs include the selective serotonin reuptake inhibitors (SSRIs) and the acetylcholinesterase inhibitors (tacrine, donepezil, rivastigmine, and galantamine), which have been approved to treat depression and AD, respectively. Donepezil alleviated cognitive problems in two studies of chemobrain (Winocur et al, 2011; Lim et al, 2016).
Catecholaminergic drugs, which are used to treat ADHD, may also help with the attention deficits associated with chemobrain. Examples include bupropion, a dopamine reuptake inhibitor; atomoxetine, a norepinephrine reuptake inhibitor; and amphetamine and methylphenidate, which enhance both dopamine and norepinephrine availability (Heal et al, 2012). Bupropion and methylphenidate reduced cancer‐related (including chemotherapy) fatigue (Cullum et al, 2004; Gong et al, 2014), although methylphenidate had no effect on depression and cognition. Animal studies would provide mechanisms to complement the results of findings in human patients.
Targeting neuroinflammation and glial cells
Neuroinflammation remains a significant risk factor for neurodegeneration and can be targeted at both the peripheral and central levels. Several large‐scale studies have examined the effects of over‐the‐counter non‐steroidal anti‐inflammatory drugs (NSAIDs) such as aspirin, ibuprofen, and naproxen on preventing or treating AD. However, the results are highly mixed, ranging from beneficial, to neutral, to harmful (Ozben & Ozben, 2019). Drugs approved for treating multiple sclerosis, a disease characterized by excessive inflammation and blood–brain barrier disruption, work through actively suppressing the immune system. Examples include copaxone, rituximab, and cladribine, which target T cells and B cells, and natalizumab, which blocks the migration of immune cells across the blood–brain barrier (Gholamzad et al, 2019). Because these drugs are associated with serious side effects including systemic immunosuppression and liver damage, the risks may outweigh the benefits of reducing mild cognitive impairment in chemobrain.
Neuroinflammation can also be targeted by directly targeting astrocytes and microglia. Recent evidence shows that PLX5622, a colony‐stimulating factor 1 receptor (CSF1R) inhibitor that specifically eliminates microglia, could block methotrexate‐induced memory deficits (Gibson et al, 2019). PLX5622 also reduced inflammation and rescued cognitive deficits in a mouse model of AD (Dagher et al, 2015). Minocycline is a common antibiotic drug that also inhibits microglial activation (Kobayashi et al, 2013). However, findings about minocycline's effects in animal models of AD and TBI have been mixed, ranging from beneficial to harmful (Garwood et al, 2010; Ferretti et al, 2012; Yang et al, 2012; Scott et al, 2018). Minocycline also did not delay the progression of cognitive impairment in people with mild AD over a 2‐year period (Howard et al, 2019). These results suggest that more specific targets of microglia or astrocytes are required to alleviate cognitive impairment without also triggering side effects.
As white matter tracts are often compromised following chemotherapy (Matsos et al, 2017), improving oligodendrogenesis and myelination is another therapeutic strategy. LM22A‐4, a TrkB agonist that promotes OPC proliferation and oligodendrogenesis, was found to rescue methotrexate‐induced myelin loss and cognitive impairment (Geraghty et al, 2019). In addition, OPC transplantation has long been investigated as a treatment for demyelinating diseases such as multiple sclerosis (Ben‐Hur, 2011) and, more recently, for spinal cord injury (Assinck et al, 2017), and can be repurposed for treating chemobrain.
Future directions
In recent years, chemobrain has gained attention as a serious side effect of chemotherapy, and several studies have advanced our understanding of the underlying mechanisms and factors. Going forward, addressing chemobrain will require concerted efforts on multiple fronts, informed by similar efforts made for aging, AD, and TBI (Langa & Levine, 2014). On the clinical front, efforts are needed to raise awareness about the risk of chemobrain among clinicians, chemotherapy patients, and their caretakers, thereby enabling more vigilant lookout for subtle deficits such as memory lapses that may otherwise be overlooked. Improvement in sensitivity of diagnostic tools to detect mild cognitive impairment, as well as utilization of neuroimaging techniques, such as structural brain MRI for possible hippocampal atrophy, and positron‐emission tomography (PET) imaging for hypometabolism, will improve the sensitivity and reliability of chemobrain diagnoses. In addition, epidemiology studies will continue to determine whether genetic risk factors for neurodegenerative diseases, for example, variations in apolipoprotein E (APOE) (Ahles et al, 2003; Mandelblatt et al, 2018) or COMT, can predict risks of developing chemobrain in cancer survivors. Conversely, cancer survivors who do not show symptoms of chemobrain immediately after treatment may also be at increased risks of developing neurodegenerative diseases later in life. On the basic science front, efforts are needed to establish animal models that better mimic the complexity and subtlety of chemobrain in humans. Examples include utilizing animal models that are aged or carry tumors and that receive common combination of drugs instead of a single drug. In addition to tasks measuring memory acquisition, tasks that measure working memory, attention, and executive functions are also needed in studying chemobrain. Additionally, determination of whether specific cognitive modalities, anatomical regions, or cell populations are more vulnerable will further aid efforts to develop therapeutic compounds. With these focused approaches, the future for improved therapies is promising.
Conflict of interest
B.E.E is a founder of Osmol Therapeutics, a company that is targeting neuronal calcium sensor 1 for therapeutic purposes, including chemotherapy‐induced neuropathy.
Pending issues
-
(1)
Refinement of diagnosis criteria for chemobrain, including utilization of diagnostic imaging tools.
-
(2)
Investigation of genetic risks and biomarkers for chemobrain, and whether cancer survivors are at increased risk of neurodegenerative diseases later in life.
-
(3)
Development of animal models that better capture the complexity of chemobrain, including animals of single and combinatorial chemotherapy drugs and potential rescue with antichemobrain drugs.
-
(4)
Determination of whether specific cognitive modalities, anatomical regions, and cell populations are more vulnerable to chemotherapy.
For more information
https://www.cancer.gov/about-cancer/treatment/research/understanding-chemobrain
https://www.mayoclinic.org/diseases-conditions/chemo-brain/symptoms-causes/syc-20351060
Acknowledgements
The authors acknowledge Tom T. Fischer for helpful discussion and edits.
EMBO Mol Med (2020) 12: e12075
See the Glossary for abbreviations used in this article.
References
- Acharya MM, Martirosian V, Chmielewski NN, Hanna N, Tran KK, Liao AC, Christie LA, Parihar VK, Limoli CL (2015) Stem cell transplantation reverses chemotherapy‐induced cognitive dysfunction. Cancer Res 75: 676–686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahles TA, Saykin A (2001) Cognitive effects of standard‐dose chemotherapy in patients with cancer. Cancer Invest 19: 812–820 [DOI] [PubMed] [Google Scholar]
- Ahles TA, Saykin AJ (2002) Breast cancer chemotherapy‐related cognitive dysfunction. Clin Breast Cancer 3(Suppl 3): S84–S90 [DOI] [PubMed] [Google Scholar]
- Ahles TA, Saykin AJ, Furstenberg CT, Cole B, Mott LA, Skalla K, Whedon MB, Bivens S, Mitchell T, Greenberg ER et al (2002) Neuropsychologic impact of standard‐dose systemic chemotherapy in long‐term survivors of breast cancer and lymphoma. J Clin Oncol 20: 485–493 [DOI] [PubMed] [Google Scholar]
- Ahles TA, Saykin AJ, Noll WW, Furstenberg CT, Guerin S, Cole B, Mott LA (2003) The relationship of APOE genotype to neuropsychological performance in long‐term cancer survivors treated with standard dose chemotherapy. Psychooncology 12: 612–619 [DOI] [PubMed] [Google Scholar]
- Ahles TA, Root JC, Ryan EL (2012) Cancer‐ and cancer treatment‐associated cognitive change: an update on the state of the science. J Clin Oncol 30: 3675–3686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander TC, Simecka CM, Kiffer F, Groves T, Anderson J, Carr H, Wang J, Carter G, Allen AR (2018) Changes in cognition and dendritic complexity following intrathecal methotrexate and cytarabine treatment in a juvenile murine model. Behav Brain Res 346: 21–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alibhai JD, Diack AB, Manson JC (2018) Unravelling the glial response in the pathogenesis of Alzheimer's disease. FASEB J 32: 5766–5777 [DOI] [PubMed] [Google Scholar]
- Andres AL, Gong X, Di K, Bota DA (2014) Low‐doses of cisplatin injure hippocampal synapses: a mechanism for ‘chemo’ brain? Exp Neurol 255: 137–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AF (2015) Stress weakens prefrontal networks: molecular insults to higher cognition. Nat Neurosci 18: 1376–1385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assinck P, Duncan GJ, Hilton BJ, Plemel JR, Tetzlaff W (2017) Cell transplantation therapy for spinal cord injury. Nat Neurosci 20: 637–647 [DOI] [PubMed] [Google Scholar]
- Atarod D, Eskandari‐Sedighi G, Pazhoohi F, Karimian SM, Khajeloo M, Riazi GH (2015) Microtubule dynamicity is more important than stability in memory formation: an in vivo study. J Mol Neurosci 56: 313–319 [DOI] [PubMed] [Google Scholar]
- Barnes AP, Polleux F (2009) Establishment of axon‐dendrite polarity in developing neurons. Annu Rev Neurosci 32: 347–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter NN, Durham SB, Phillips KA, Habermann EB, Virning BA (2009) Risk of dementia in older breast cancer survivors: a population‐based cohort study of the association with adjuvant chemotherapy. J Am Geriatr Soc 57: 403–411 [DOI] [PubMed] [Google Scholar]
- Bendlin BB, Fitzgerald ME, Ries ML, Xu G, Kastman EK, Thiel BW, Rowley HA, Lazar M, Alexander AL, Johnson SC (2010) White matter in aging and cognition: a cross‐sectional study of microstructure in adults aged eighteen to eighty‐three. Dev Neuropsychol 35: 257–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben‐Hur T (2011) Cell therapy for multiple sclerosis. Neurotherapeutics 8: 625–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehmerle W, Splittgerber U, Lazarus MB, McKenzie KM, Johnston DG, Austin DJ, Ehrlich BE (2006) Paclitaxel induces calcium oscillations via an inositol 1,4,5‐trisphosphate receptor and neuronal calcium sensor 1‐dependent mechanism. Proc Natl Acad Sci USA 103: 18356–18361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondi CO, Klitsch KC, Leary JB, Kline AE (2014) Environmental enrichment as a viable neurorehabilitation strategy for experimental traumatic brain injury. J Neurotrauma 31: 873–888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandolini L, d'Angelo M, Antonosante A, Allegretti M, Cimini A (2019) Chemokine signaling in chemotherapy‐induced neuropathic pain. Int J Mol Sci 20: 2904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briones TL, Woods J (2011) Chemotherapy‐induced cognitive impairment is associated with decreases in cell proliferation and histone modifications. BMC Neurosci 12: 124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brudvig JJ, Weimer JM (2015) X MARCKS the spot: myristoylated alanine‐rich C kinase substrate in neuronal function and disease. Front Cell Neurosci 9: 407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunden KR, Trojanowski JQ, Smith AB 3rd, Lee VM, Ballatore C (2014) Microtubule‐stabilizing agents as potential therapeutics for neurodegenerative disease. Bioorg Med Chem 22: 5040–5049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burda JE, Sofroniew MV (2014) Reactive gliosis and the multicellular response to CNS damage and disease. Neuron 81: 229–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaghan CK, O'Mara SM (2015) Long‐term cognitive dysfunction in the rat following docetaxel treatment is ameliorated by the phosphodiesterase‐4 inhibitor, rolipram. Behav Brain Res 290: 84–89 [DOI] [PubMed] [Google Scholar]
- Chen X, Hu Y, Cao Z, Liu Q, Cheng Y (2018) Cerebrospinal fluid inflammatory cytokine aberrations in Alzheimer's disease, Parkinson's disease and amyotrophic lateral sclerosis: a systematic review and meta‐analysis. Front Immunol 9: 2122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen BT, Ye N, Wong CW, Patel SK, Jin T, Sun CL, Rockne RC, Kim H, Root JC, Saykin AJ et al (2020) Effects of chemotherapy on aging white matter microstructure: a longitudinal diffusion tensor imaging study. J Geriatr Oncol 11: 290–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H, Li W, Gan C, Zhang B, Jia Q, Wang K (2016) The COMT (rs165599) gene polymorphism contributes to chemotherapy‐induced cognitive impairment in breast cancer patients. Am J Transl Res 8: 5087–5097 [PMC free article] [PubMed] [Google Scholar]
- Cheng J, Liu X, Cao L, Zhang T, Li H, Lin W (2017) Neo‐adjuvant chemotherapy with cisplatin induces low expression of NMDA receptors and postoperative cognitive impairment. Neurosci Lett 637: 168–174 [DOI] [PubMed] [Google Scholar]
- Choi R, Goldstein BJ (2018) Olfactory epithelium: cells, clinical disorders, and insights from an adult stem cell niche. Laryngoscope Investig Otolaryngol 3: 35–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie LA, Acharya MM, Parihar VK, Nguyen A, Martirosian V, Limoli CL (2012) Impaired cognitive function and hippocampal neurogenesis following cancer chemotherapy. Clin Cancer Res 18: 1954–1965 [DOI] [PubMed] [Google Scholar]
- Cullum JL, Wojciechowski AE, Pelletier G, Simpson JS (2004) Bupropion sustained release treatment reduces fatigue in cancer patients. Can J Psychiatry 49: 139–144 [DOI] [PubMed] [Google Scholar]
- Dagher NN, Najafi AR, Kayala KM, Elmore MR, White TE, Medeiros R, West BL, Green KN (2015) Colony‐stimulating factor 1 receptor inhibition prevents microglial plaque association and improves cognition in 3xTg‐AD mice. J Neuroinflammation 12: 139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deprez S, Amant F, Yigit R, Porke K, Verhoeven J, Van den Stock J, Smeets A, Christiaens MR, Leemans A, Van Hecke W et al (2011) Chemotherapy‐induced structural changes in cerebral white matter and its correlation with impaired cognitive functioning in breast cancer patients. Hum Brain Mapp 32: 480–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deprez S, Amant F, Smeets A, Peeters R, Leemans A, Van Hecke W, Verhoeven JS, Christiaens MR, Vandenberghe J, Vandenbulcke M et al (2012) Longitudinal assessment of chemotherapy‐induced structural changes in cerebral white matter and its correlation with impaired cognitive functioning. J Clin Oncol 30: 274–281 [DOI] [PubMed] [Google Scholar]
- Di Benedetto S, Muller L, Wenger E, Duzel S, Pawelec G (2017) Contribution of neuroinflammation and immunity to brain aging and the mitigating effects of physical and cognitive interventions. Neurosci Biobehav Rev 75: 114–128 [DOI] [PubMed] [Google Scholar]
- Dickstein DL, Weaver CM, Luebke JI, Hof PR (2013) Dendritic spine changes associated with normal aging. Neuroscience 251: 21–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich J, Han R, Yang Y, Mayer‐Proschel M, Noble M (2006) CNS progenitor cells and oligodendrocytes are targets of chemotherapeutic agents in vitro and in vivo . J Biol 5: 22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorostkar MM, Zou C, Blazquez‐Llorca L, Herms J (2015) Analyzing dendritic spine pathology in Alzheimer's disease: problems and opportunities. Acta Neuropathol 130: 1–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey J, Ratnakaran N, Koushika SP (2015) Neurodegeneration and microtubule dynamics: death by a thousand cuts. Front Cell Neurosci 9: 343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS (2018) Ketamine and rapid‐acting antidepressants: a new era in the battle against depression and suicide. F1000Res 7: 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El‐Agamy SE, Abdel‐Aziz AK, Wahdan S, Esmat A, Azab SS (2018) Astaxanthin ameliorates doxorubicin‐induced cognitive impairment (Chemobrain) in experimental rat model: impact on oxidative, inflammatory, and apoptotic machineries. Mol Neurobiol 55: 5727–5740 [DOI] [PubMed] [Google Scholar]
- ElBeltagy M, Mustafa S, Umka J, Lyons L, Salman A, Chur‐yoe GT, Bhalla N, Bennett G, Wigmore PM (2010) Fluoxetine improves the memory deficits caused by the chemotherapy agent 5‐fluorouracil. Behav Brain Res 208: 112–117 [DOI] [PubMed] [Google Scholar]
- Ernst A, Alkass K, Bernard S, Salehpour M, Perl S, Tisdale J, Possnert G, Druid H, Frisen J (2014) Neurogenesis in the striatum of the adult human brain. Cell 156: 1072–1083 [DOI] [PubMed] [Google Scholar]
- Farber S, Diamond LK (1948) Temporary remissions in acute leukemia in children produced by folic acid antagonist, 4‐aminopteroyl‐glutamic acid. N Engl J Med 238: 787–793 [DOI] [PubMed] [Google Scholar]
- Fardell JE, Vardy J, Shah JD, Johnston IN (2012) Cognitive impairments caused by oxaliplatin and 5‐fluorouracil chemotherapy are ameliorated by physical activity. Psychopharmacology 220: 183–193 [DOI] [PubMed] [Google Scholar]
- Fardell JE, Zhang J, De Souza R, Vardy J, Johnston I, Allen C, Henderson J, Piquette‐Miller M (2014) The impact of sustained and intermittent docetaxel chemotherapy regimens on cognition and neural morphology in healthy mice. Psychopharmacology 231: 841–852 [DOI] [PubMed] [Google Scholar]
- Ferguson RJ, McDonald BC, Saykin AJ, Ahles TA (2007) Brain structure and function differences in monozygotic twins: possible effects of breast cancer chemotherapy. J Clin Oncol 25: 3866–3870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson RJ, McDonald BC, Rocque MA, Furstenberg CT, Horrigan S, Ahles TA, Saykin AJ (2012) Development of CBT for chemotherapy‐related cognitive change: results of a waitlist control trial. Psychooncology 21: 176–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferretti MT, Allard S, Partridge V, Ducatenzeiler A, Cuello AC (2012) Minocycline corrects early, pre‐plaque neuroinflammation and inhibits BACE‐1 in a transgenic model of Alzheimer's disease‐like amyloid pathology. J Neuroinflammation 9: 62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields RD, Araque A, Johansen‐Berg H, Lim SS, Lynch G, Nave KA, Nedergaard M, Perez R, Sejnowski T, Wake H (2014) Glial biology in learning and cognition. Neuroscientist 20: 426–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrest MP, Parnell E, Penzes P (2018) Dendritic structural plasticity and neuropsychiatric disease. Nat Rev Neurosci 19: 215–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X, Deng P, Xu ZC, Chen J (2011) Moderate traumatic brain injury causes acute dendritic and synaptic degeneration in the hippocampal dentate gyrus. PLoS ONE 6: e24566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garwood CJ, Cooper JD, Hanger DP, Noble W (2010) Anti‐inflammatory impact of minocycline in a mouse model of tauopathy. Front Psychiatry 1: 136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geraghty AC, Gibson EM, Ghanem RA, Greene JJ, Ocampo A, Goldstein AK, Ni L, Yang T, Marton RM, Pasca SP et al (2019) Loss of adaptive myelination contributes to methotrexate chemotherapy‐related cognitive impairment. Neuron 103: 250–265.e258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gholamzad M, Ebtekar M, Ardestani MS, Azimi M, Mahmodi Z, Mousavi MJ, Aslani S (2019) A comprehensive review on the treatment approaches of multiple sclerosis: currently and in the future. Inflamm Res 68: 25–38 [DOI] [PubMed] [Google Scholar]
- Gibson EM, Nagaraja S, Ocampo A, Tam LT, Wood LS, Pallegar PN, Greene JJ, Geraghty AC, Goldstein AK, Ni L et al (2019) Methotrexate chemotherapy induces persistent tri‐glial dysregulation that underlies chemotherapy‐related cognitive impairment. Cell 176: 43–55.e13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH (2010) Mechanisms underlying inflammation in neurodegeneration. Cell 140: 918–934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong S, Sheng P, Jin H, He H, Qi E, Chen W, Dong Y, Hou L (2014) Effect of methylphenidate in patients with cancer‐related fatigue: a systematic review and meta‐analysis. PLoS ONE 9: e84391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorska‐Ciebiada M, Saryusz‐Wolska M, Borkowska A, Ciebiada M, Loba J (2015) Serum levels of inflammatory markers in depressed elderly patients with diabetes and mild cognitive impairment. PLoS ONE 10: e0120433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham WV, Bonito‐Oliva A, Sakmar TP (2017) Update on Alzheimer's disease therapy and prevention strategies. Annu Rev Med 68: 413–430 [DOI] [PubMed] [Google Scholar]
- Groves TR, Farris R, Anderson JE, Alexander TC, Kiffer F, Carter G, Wang J, Boerma M, Allen AR (2017) 5‐Fluorouracil chemotherapy upregulates cytokines and alters hippocampal dendritic complexity in aged mice. Behav Brain Res 316: 215–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerreiro RJ, Santana I, Bras JM, Santiago B, Paiva A, Oliveira C (2007) Peripheral inflammatory cytokines as biomarkers in Alzheimer's disease and mild cognitive impairment. Neurodegener Dis 4: 406–412 [DOI] [PubMed] [Google Scholar]
- Hains AB, Vu MA, Maciejewski PK, van Dyck CH, Gottron M, Arnsten AF (2009) Inhibition of protein kinase C signaling protects prefrontal cortex dendritic spines and cognition from the effects of chronic stress. Proc Natl Acad Sci USA 106: 17957–17962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han R, Yang YM, Dietrich J, Luebke A, Mayer‐Proschel M, Noble M (2008) Systemic 5‐fluorouracil treatment causes a syndrome of delayed myelin destruction in the central nervous system. J Biol 7: 12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heal DJ, Smith SL, Findling RL (2012) ADHD: current and future therapeutics In Behavioral Neuroscience of Attention Deficit Hyperactivity Disorder and Its Treatment, Stanford C, Tannock R. (eds), pp 361–390. Berlin, Heidelberg: Springer Berlin Heidelberg; [DOI] [PubMed] [Google Scholar]
- Heck JE, Albert SM, Franco R, Gorin SS (2008) Patterns of dementia diagnosis in surveillance, epidemiology, and end results breast cancer survivors who use chemotherapy. J Am Geriatr Soc 56: 1687–1692 [DOI] [PubMed] [Google Scholar]
- Henneghan AM, Harrison T (2015) Complementary and alternative medicine therapies as symptom management strategies for the late effects of breast cancer treatment. J Holist Nurs 33: 84–97 [DOI] [PubMed] [Google Scholar]
- Higley MJ, Sabatini BL (2012) Calcium signaling in dendritic spines. Cold Spring Harb Perspect Biol 4: a005686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeijmakers JH (2009) DNA damage, aging, and cancer. N Engl J Med 361: 1475–1485 [DOI] [PubMed] [Google Scholar]
- Hollands C, Bartolotti N, Lazarov O (2016) Alzheimer's disease and hippocampal adult neurogenesis; exploring shared mechanisms. Front Neurosci 10: 178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz TS, Suls J, Trevino M (2018) A call for a neuroscience approach to cancer‐related cognitive impairment. Trends Neurosci 41: 493–496 [DOI] [PubMed] [Google Scholar]
- Howard R, Zubko O, Bradley R, Harper E, Pank L, O'Brien J, Fox C, Tabet N, Livingston G, Bentham P et al (2019) Minocycline at 2 different dosages vs placebo for patients with mild Alzheimer disease: a randomized clinical trial. JAMA Neurol 20: 164–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huehnchen P, Boehmerle W, Springer A, Freyer D, Endres M (2017) A novel preventive therapy for paclitaxel‐induced cognitive deficits: preclinical evidence from C57BL/6 mice. Transl Psychiatry 7: e1185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huo X, Reyes TM, Heijnen CJ, Kavelaars A (2018) Cisplatin treatment induces attention deficits and impairs synaptic integrity in the prefrontal cortex in mice. Sci Rep 8: 17400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyrien O, Dietrich J, Noble M (2010) Mathematical and experimental approaches to identify and predict the effects of chemotherapy on neuroglial precursors. Cancer Res 70: 10051–10059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarmolowicz DP, Gehringer R, Lemley SM, Sofis MJ, Kaplan S, Johnson MA (2019) 5‐Fluorouracil impairs attention and dopamine release in rats. Behav Brain Res 362: 319–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jehn CF, Becker B, Flath B, Nogai H, Vuong L, Schmid P, Luftner D (2015) Neurocognitive function, brain‐derived neurotrophic factor (BDNF) and IL‐6 levels in cancer patients with depression. J Neuroimmunol 287: 88–92 [DOI] [PubMed] [Google Scholar]
- Jessen KR (2004) Glial cells. Int J Biochem Cell Biol 36: 1861–1867 [DOI] [PubMed] [Google Scholar]
- Jiang ZG, Winocur G, Wojtowicz JM, Shevtsova O, Fuller S, Ghanbari HA (2018) PAN‐811 prevents chemotherapy‐induced cognitive impairment and preserves neurogenesis in the hippocampus of adult rats. PLoS ONE 13: e0191866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jim HS, Phillips KM, Chait S, Faul LA, Popa MA, Lee YH, Hussin MG, Jacobsen PB, Small BJ (2012) Meta‐analysis of cognitive functioning in breast cancer survivors previously treated with standard‐dose chemotherapy. J Clin Oncol 30: 3578–3587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston IN, Tan M, Cao J, Matsos A, Forrest DRL, Si E, Fardell JE, Hutchinson MR (2017) Ibudilast reduces oxaliplatin‐induced tactile allodynia and cognitive impairments in rats. Behav Brain Res 334: 109–118 [DOI] [PubMed] [Google Scholar]
- Kang S, Lee S, Kim J, Kim JC, Kim SH, Son Y, Shin T, Youn B, Kim JS, Wang H et al (2018) Chronic treatment with combined chemotherapeutic agents affects hippocampal micromorphometry and function in mice, independently of neuroinflammation. Exp Neurobiol 27: 419–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan SV, Limbocker RA, Gehringer RC, Divis JL, Osterhaus GL, Newby MD, Sofis MJ, Jarmolowicz DP, Newman BD, Mathews TA et al (2016) Impaired brain dopamine and serotonin release and uptake in Wistar rats following treatment with carboplatin. ACS Chem Neurosci 7: 689–699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeney JTR, Ren X, Warrier G, Noel T, Powell DK, Brelsfoard JM, Sultana R, Saatman KE, Clair DKS, Butterfield DA (2018) Doxorubicin‐induced elevated oxidative stress and neurochemical alterations in brain and cognitive decline: protection by MESNA and insights into mechanisms of chemotherapy‐induced cognitive impairment (“chemobrain”). Oncotarget 9: 30324–30339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesler SR, Kent JS, O'Hara R (2011) Prefrontal cortex and executive function impairments in primary breast cancer. Arch Neurol 68: 1447–1453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesler S, Hadi Hosseini SM, Heckler C, Janelsins M, Palesh O, Mustian K, Morrow G (2013) Cognitive training for improving executive function in chemotherapy‐treated breast cancer survivors. Clin Breast Cancer 13: 299–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesler SR, Rao V, Ray WJ, Rao A; Alzheimer's Disease Neuroimaging Initiative (2017) Probability of Alzheimer's disease in breast cancer survivors based on gray‐matter structural network efficiency. Alzheimers Dement (Amst) 9: 67–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K, Imagama S, Ohgomori T, Hirano K, Uchimura K, Sakamoto K, Hirakawa A, Takeuchi H, Suzumura A, Ishiguro N et al (2013) Minocycline selectively inhibits M1 polarization of microglia. Cell Death Dis 4: e525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar RG, Boles JA, Wagner AK (2015) Chronic inflammation after severe traumatic brain injury: characterization and associations with outcome at 6 and 12 months postinjury. J Head Trauma Rehabil 30: 369–381 [DOI] [PubMed] [Google Scholar]
- Langa KM, Levine DA (2014) The diagnosis and management of mild cognitive impairment: a clinical review. JAMA 312: 2551–2561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarov O, Mattson MP, Peterson DA, Pimplikar SW, van Praag H (2010) When neurogenesis encounters aging and disease. Trends Neurosci 33: 569–579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BE, Choi BY, Hong DK, Kim JH, Lee SH, Kho AR, Kim H, Choi HC, Suh SW (2017) The cancer chemotherapeutic agent paclitaxel (Taxol) reduces hippocampal neurogenesis via down‐regulation of vesicular zinc. Sci Rep 7: 11667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine PM, Silberfarb PM, Lipowski ZJ (1978) Mental disorders in cancer patients: a study of 100 psychiatric referrals. Cancer 42: 1385–1391 [DOI] [PubMed] [Google Scholar]
- Lim I, Joung HY, Yu AR, Shim I, Kim JS (2016) PET evidence of the effect of donepezil on cognitive performance in an animal model of chemobrain. Biomed Res Int 2016: 6945415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima Giacobbo B, Doorduin J, Klein HC, Dierckx R, Bromberg E, de Vries EFJ (2019) Brain‐derived neurotrophic factor in brain disorders: focus on neuroinflammation. Mol Neurobiol 56: 3295–3312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindner OC, Phillips B, McCabe MG, Mayes A, Wearden A, Varese F, Talmi D (2014) A meta‐analysis of cognitive impairment following adult cancer chemotherapy. Neuropsychology 28: 726–740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindvall O, Kokaia Z (2010) Stem cells in human neurodegenerative disorders–time for clinical translation? J Clin Invest 120: 29–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez Zunini RA, Scherling C, Wallis N, Collins B, MacKenzie J, Bielajew C, Smith AM (2013) Differences in verbal memory retrieval in breast cancer chemotherapy patients compared to healthy controls: a prospective fMRI study. Brain Imaging Behav 7: 460–477 [DOI] [PubMed] [Google Scholar]
- Lu PH, Lee GJ, Raven EP, Tingus K, Khoo T, Thompson PM, Bartzokis G (2011) Age‐related slowing in cognitive processing speed is associated with myelin integrity in a very healthy elderly sample. J Clin Exp Neuropsychol 33: 1059–1068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch AM, Murphy KJ, Deighan BF, O'Reilly JA, Gun'ko YK, Cowley TR, Gonzalez‐Reyes RE Lynch MA (2010) The impact of glial activation in the aging brain. Aging Dis 1: 262–278 [PMC free article] [PubMed] [Google Scholar]
- Lyons L, Elbeltagy M, Bennett G, Wigmore P (2011a) The effects of cyclophosphamide on hippocampal cell proliferation and spatial working memory in rat. PLoS ONE 6: e21445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons L, ElBeltagy M, Umka J, Markwick R, Startin C, Bennett G, Wigmore P (2011b) Fluoxetine reverses the memory impairment and reduction in proliferation and survival of hippocampal cells caused by methotrexate chemotherapy. Psychopharmacology 215: 105–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons L, ElBeltagy M, Bennett G, Wigmore P (2012) Fluoxetine counteracts the cognitive and cellular effects of 5‐fluorouracil in the rat hippocampus by a mechanism of prevention rather than recovery. PLoS ONE 7: e30010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandelblatt JS, Small BJ, Luta G, Hurria A, Jim H, McDonald BC, Graham D, Zhou X, Clapp J, Zhai W et al (2018) Cancer‐related cognitive outcomes among older breast cancer survivors in the thinking and living with cancer study. J Clin Oncol 36: 3211–3222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manohar S, Jamesdaniel S, Salvi R (2014) Cisplatin inhibits hippocampal cell proliferation and alters the expression of apoptotic genes. Neurotox Res 25: 369–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsos A, Loomes M, Zhou I, Macmillan E, Sabel I, Rotziokos E, Beckwith W, Johnston IN (2017) Chemotherapy‐induced cognitive impairments: white matter pathologies. Cancer Treat Rev 61: 6–14 [DOI] [PubMed] [Google Scholar]
- Maynard S, Fang EF, Scheibye‐Knudsen M, Croteau DL, Bohr VA (2015) DNA damage, DNA repair, aging, and neurodegeneration. Cold Spring Harb Perspect Med 5: a025130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald BC, Conroy SK, Ahles TA, West JD, Saykin AJ (2010) Gray matter reduction associated with systemic chemotherapy for breast cancer: a prospective MRI study. Breast Cancer Res Treat 123: 819–828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald BC, Conroy SK, Smith DJ, West JD, Saykin AJ (2013) Frontal gray matter reduction after breast cancer chemotherapy and association with executive symptoms: a replication and extension study. Brain Behav Immun 30(Suppl): S117–S125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougall S, Vargas Riad W, Silva‐Gotay A, Tavares ER, Harpalani D, Li GL, Richardson HN (2018) Myelination of axons corresponds with faster transmission speed in the prefrontal cortex of developing male rats. eNeuro 5: ENEURO.0203–18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie IA, Ohayon D, Li H, de Faria JP, Emery B, Tohyama K, Richardson WD (2014) Motor skill learning requires active central myelination. Science 346: 318–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menning S, de Ruiter MB, Veltman DJ, Boogerd W, Oldenburg HS, Reneman L, Schagen SB (2017) Changes in brain activation in breast cancer patients depend on cognitive domain and treatment type. PLoS ONE 12: e0171724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaud M, Balardy L, Moulis G, Gaudin C, Peyrot C, Vellas B, Cesari M, Nourhashemi F (2013) Proinflammatory cytokines, aging, and age‐related diseases. J Am Med Dir Assoc 14: 877–882 [DOI] [PubMed] [Google Scholar]
- Mihlon FT, Ray CE Jr, Messersmith W (2010) Chemotherapy agents: a primer for the interventional radiologist. Semin Intervent Radiol 27: 384–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KD, Nogueira L, Mariotto AB, Rowland JH, Yabroff KR, Alfano CM, Jemal A, Kramer JL, Siegel RL (2019) Cancer treatment and survivorship statistics, 2019. CA Cancer J Clin 69: 363–385 [DOI] [PubMed] [Google Scholar]
- Ming GL, Song H (2011) Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron 70: 687–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo M, Erdelyi I, Szigeti‐Buck K, Benbow JH, Ehrlich BE (2012) Prevention of paclitaxel‐induced peripheral neuropathy by lithium pretreatment. FASEB J 26: 4696–4709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondie CM, Vandergrift KA, Wilson CL, Gulinello ME, Weber ET (2010) The chemotherapy agent, thioTEPA, yields long‐term impairment of hippocampal cell proliferation and memory deficits but not depression‐related behaviors in mice. Behav Brain Res 209: 66–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustafa S, Walker A, Bennett G, Wigmore PM (2008) 5‐Fluorouracil chemotherapy affects spatial working memory and newborn neurons in the adult rat hippocampus. Eur J Neurosci 28: 323–330 [DOI] [PubMed] [Google Scholar]
- Naoi M, Maruyama W (1999) Cell death of dopamine neurons in aging and Parkinson's disease. Mech Ageing Dev 111: 175–188 [DOI] [PubMed] [Google Scholar]
- Nelson WL, Suls J (2013) New approaches to understand cognitive changes associated with chemotherapy for non‐central nervous system tumors. J Pain Symptom Manage 46: 707–721 [DOI] [PubMed] [Google Scholar]
- Newcombe EA, Camats‐Perna J, Silva ML, Valmas N, Huat TJ, Medeiros R (2018) Inflammation: the link between comorbidities, genetics, and Alzheimer's disease. J Neuroinflammation 15: 276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nokia MS, Anderson ML, Shors TJ (2012) Chemotherapy disrupts learning, neurogenesis and theta activity in the adult brain. Eur J Neurosci 36: 3521–3530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noone AM, Howlader N, Krapcho M, Miller D, Brest A, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR et al (eds) (2018) National Cancer Institute. Bethesda, MD: https://seer.cancer.gov/csr/1975_2015/, based on November 2017 SEER data submission, posted to the SEER web site, April 2018. [Google Scholar]
- Noudoost B, Moore T (2011) The role of neuromodulators in selective attention. Trends Cogn Sci 15: 585–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozben T, Ozben S (2019) Neuro‐inflammation and anti‐inflammatory treatment options for Alzheimer's disease. Clin Biochem 72: 87–89 [DOI] [PubMed] [Google Scholar]
- Pangalos MN, Schechter LE, Hurko O (2007) Drug development for CNS disorders: strategies for balancing risk and reducing attrition. Nat Rev Drug Discov 6: 521–532 [DOI] [PubMed] [Google Scholar]
- Park HS, Kim CJ, Kwak HB, No MH, Heo JW, Kim TW (2018) Physical exercise prevents cognitive impairment by enhancing hippocampal neuroplasticity and mitochondrial function in doxorubicin‐induced chemobrain. Neuropharmacology 133: 451–461 [DOI] [PubMed] [Google Scholar]
- Parsania S, Shabani M, Moazzami K, Razavinasab M, Larizadeh MH, Nazeri M, Asadi‐Shekaari M, Kermani M (2014) Gender difference in motor impairments induced by chronic administration of vinblastine. Iran J Basic Med Sci 17: 433–440 [PMC free article] [PubMed] [Google Scholar]
- Partin KM (2015) AMPA receptor potentiators: from drug design to cognitive enhancement. Curr Opin Pharmacol 20: 46–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel M (2016) Targeting oxidative stress in central nervous system disorders. Trends Pharmacol Sci 37: 768–778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phoumthipphavong V, Barthas F, Hassett S, Kwan AC (2016) Longitudinal effects of ketamine on dendritic architecture in vivo in the mouse medial frontal. Cortex eNeuro: 3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plun‐Favreau H, Lewis PA, Hardy J, Martins LM, Wood NW (2010) Cancer and neurodegeneration: between the devil and the deep blue sea. PLoS Genet 6: e1001257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell CM, Miyakawa T (2006) Schizophrenia‐relevant behavioral testing in rodent models: a uniquely human disorder? Biol Psychiatry 59: 1198–1207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przekwas A, Somayaji MR, Gupta RK (2016) Synaptic mechanisms of blast‐induced brain injury. Front Neurol 7: 2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raji MA, Tamborello LP, Kuo YF, Ju H, Freeman JL, Zhang DD, Giordano SH, Goodwin JS (2009) Risk of subsequent dementia diagnoses does not vary by types of adjuvant chemotherapy in older women with breast cancer. Med Oncol 26: 452–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reale M, Greig NH, Kamal MA (2009) Peripheral chemo‐cytokine profiles in Alzheimer's and Parkinson's diseases. Mini Rev Med Chem 9: 1229–1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remarque EJ, Bollen EL, Weverling‐Rijnsburger AW, Laterveer JC, Blauw GJ, Westendorp RG (2001) Patients with Alzheimer's disease display a pro‐inflammatory phenotype. Exp Gerontol 36: 171–176 [DOI] [PubMed] [Google Scholar]
- Ren X, St Clair DK, Butterfield DA (2017) Dysregulation of cytokine mediated chemotherapy induced cognitive impairment. Pharmacol Res 117: 267–273 [DOI] [PubMed] [Google Scholar]
- Rendeiro C, Sheriff A, Bhattacharya TK, Gogola JV, Baxter JH, Chen H, Helferich WG, Roy EJ, Rhodes JS (2016) Long‐lasting impairments in adult neurogenesis, spatial learning and memory from a standard chemotherapy regimen used to treat breast cancer. Behav Brain Res 315: 10–22 [DOI] [PubMed] [Google Scholar]
- Richardson RM, Sun D, Bullock MR (2007) Neurogenesis after traumatic brain injury. Neurosurg Clin N Am 18: 169–181, xi [DOI] [PubMed] [Google Scholar]
- de Ruiter MB, Reneman L, Boogerd W, Veltman DJ, van Dam FS, Nederveen AJ, Boven E, Schagen SB (2011) Cerebral hyporesponsiveness and cognitive impairment 10 years after chemotherapy for breast cancer. Hum Brain Mapp 32: 1206–1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels BA, Hen R (2011) Neurogenesis and affective disorders. Eur J Neurosci 33: 1152–1159 [DOI] [PubMed] [Google Scholar]
- Saykin AJ, Ahles TA, McDonald BC (2003) Mechanisms of chemotherapy‐induced cognitive disorders: neuropsychological, pathophysiological, and neuroimaging perspectives. Semin Clin Neuropsychiatry 8: 201–216 [PubMed] [Google Scholar]
- Schimmel SJ, Acosta S, Lozano D (2017) Neuroinflammation in traumatic brain injury: a chronic response to an acute injury. Brain Circ 3: 135–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenfeld TJ, Cameron HA (2015) Adult neurogenesis and mental illness. Neuropsychopharmacology 40: 113–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott G, Zetterberg H, Jolly A, Cole JH, De Simoni S, Jenkins PO, Feeney C, Owen DR, Lingford‐Hughes A, Howes O et al (2018) Minocycline reduces chronic microglial activation after brain trauma but increases neurodegeneration. Brain 141: 459–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seigers R, Schagen SB, Beerling W, Boogerd W, van Tellingen O, van Dam FS, Koolhaas JM, Buwalda B (2008) Long‐lasting suppression of hippocampal cell proliferation and impaired cognitive performance by methotrexate in the rat. Behav Brain Res 186: 168–175 [DOI] [PubMed] [Google Scholar]
- Seigers R, Timmermans J, van der Horn HJ, de Vries EF, Dierckx RA, Visser L, Schagen SB, van Dam FS, Koolhaas JM, Buwalda B (2010) Methotrexate reduces hippocampal blood vessel density and activates microglia in rats but does not elevate central cytokine release. Behav Brain Res 207: 265–272 [DOI] [PubMed] [Google Scholar]
- Sheldrick AJ, Krug A, Markov V, Leube D, Michel TM, Zerres K, Eggermann T, Kircher T (2008) Effect of COMT val158met genotype on cognition and personality. Eur Psychiatry 23: 385–389 [DOI] [PubMed] [Google Scholar]
- Shi DD, Dong CM, Ho LC, Lam CTW, Zhou XD, Wu EX, Zhou ZJ, Wang XM, Zhang ZJ (2018) Resveratrol, a natural polyphenol, prevents chemotherapy‐induced cognitive impairment: involvement of cytokine modulation and neuroprotection. Neurobiol Dis 114: 164–173 [DOI] [PubMed] [Google Scholar]
- Shi DD, Huang YH, Lai CSW, Dong CM, Ho LC, Wu EX, Li Q, Wang XM, Chung SK, Sham PC et al (2019) Chemotherapy‐induced cognitive impairment is associated with cytokine dysregulation and disruptions in neuroplasticity. Mol Neurobiol 56: 2234–2243 [DOI] [PubMed] [Google Scholar]
- Shohayeb B, Diab M, Ahmed M, Ng DCH (2018) Factors that influence adult neurogenesis as potential therapy. Transl Neurodegener 7: 4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shruster A, Melamed E, Offen D (2010) Neurogenesis in the aged and neurodegenerative brain. Apoptosis 15: 1415–1421 [DOI] [PubMed] [Google Scholar]
- Small BJ, Rawson KS, Walsh E, Jim HS, Hughes TF, Iser L, Andrykowski MA, Jacobsen PB (2011) Catechol‐O‐methyltransferase genotype modulates cancer treatment‐related cognitive deficits in breast cancer survivors. Cancer 117: 1369–1376 [DOI] [PubMed] [Google Scholar]
- Sparkman NL, Johnson RW (2008) Neuroinflammation associated with aging sensitizes the brain to the effects of infection or stress. NeuroImmunoModulation 15: 323–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steadman PE, Xia F, Ahmed M, Mocle AJ, Penning ARA, Geraghty AC, Steenland HW, Monje M, Josselyn SA, Frankland PW (2020) Disruption of oligodendrogenesis impairs memory consolidation in adult mice. Neuron 105: 150–164.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart A, Bielajew C, Collins B, Parkinson M, Tomiak E (2006) A meta‐analysis of the neuropsychological effects of adjuvant chemotherapy treatment in women treated for breast cancer. Clin Neuropsychol 20: 76–89 [DOI] [PubMed] [Google Scholar]
- Sundholm‐Peters NL, Yang HK, Goings GE, Walker AS, Szele FG (2005) Subventricular zone neuroblasts emigrate toward cortical lesions. J Neuropathol Exp Neurol 64: 1089–1100 [DOI] [PubMed] [Google Scholar]
- Swardfager W, Lanctot K, Rothenburg L, Wong A, Cappell J, Herrmann N (2010) A meta‐analysis of cytokines in Alzheimer's disease. Biol Psychiatry 68: 930–941 [DOI] [PubMed] [Google Scholar]
- Thomas TC, Beitchman JA, Pomerleau F, Noel T, Jungsuwadee P, Butterfield DA, Clair DKS, Vore M, Gerhardt GA (2017) Acute treatment with doxorubicin affects glutamate neurotransmission in the mouse frontal cortex and hippocampus. Brain Res 1672: 10–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance DE, Frank JS, Bail J, Triebel KL, Niccolai LM, Gerstenecker A, Meneses K (2017) Interventions for cognitive deficits in breast cancer survivors treated with chemotherapy. Cancer Nurs 40: E11–E27 [DOI] [PubMed] [Google Scholar]
- Vijayanathan V, Gulinello M, Ali N, Cole PD (2011) Persistent cognitive deficits, induced by intrathecal methotrexate, are associated with elevated CSF concentrations of excitotoxic glutamate analogs and can be reversed by an NMDA antagonist. Behav Brain Res 225: 491–497 [DOI] [PubMed] [Google Scholar]
- Voss MW, Vivar C, Kramer AF, van Praag H (2013) Bridging animal and human models of exercise‐induced brain plasticity. Trends Cogn Sci 17: 525–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker KR, Tesco G (2013) Molecular mechanisms of cognitive dysfunction following traumatic brain injury. Front Aging Neurosci 5: 29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XM, Walitt B, Saligan L, Tiwari AF, Cheung CW, Zhang ZJ (2015a) Chemobrain: a critical review and causal hypothesis of link between cytokines and epigenetic reprogramming associated with chemotherapy. Cytokine 72: 86–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Peng W, Zhang C, Sheng C, Huang W, Wang Y, Fan R (2015b) Effects of stem cell transplantation on cognitive decline in animal models of Alzheimer's disease: a systematic review and meta‐analysis. Sci Rep 5: 12134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wefel JS, Schagen SB (2012) Chemotherapy‐related cognitive dysfunction. Curr Neurol Neurosci Rep 12: 267–275 [DOI] [PubMed] [Google Scholar]
- Winocur G, Binns MA, Tannock I (2011) Donepezil reduces cognitive impairment associated with anti‐cancer drugs in a mouse model. Neuropharmacology 61: 1222–1228 [DOI] [PubMed] [Google Scholar]
- Winocur G, Wojtowicz JM, Huang J, Tannock IF (2014) Physical exercise prevents suppression of hippocampal neurogenesis and reduces cognitive impairment in chemotherapy‐treated rats. Psychopharmacology 231: 2311–2320 [DOI] [PubMed] [Google Scholar]
- Winocur G, Wojtowicz JM, Merkley CM, Tannock IF (2016) Environmental enrichment protects against cognitive impairment following chemotherapy in an animal model. Behav Neurosci 130: 428–436 [DOI] [PubMed] [Google Scholar]
- Wu L, Guo D, Liu Q, Gao F, Wang X, Song X, Wang F, Zhan RZ (2017) Abnormal development of dendrites in adult‐born rat hippocampal granule cells induced by cyclophosphamide. Front Cell Neurosci 11: 171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita T, Ninomiya M, Hernandez Acosta P, Garcia‐Verdugo JM, Sunabori T, Sakaguchi M, Adachi K, Kojima T, Hirota Y, Kawase T et al (2006) Subventricular zone‐derived neuroblasts migrate and differentiate into mature neurons in the post‐stroke adult striatum. J Neurosci 26: 6627–6636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Kim JS, Song MS, Kim SH, Kang SS, Bae CS, Kim JC, Wang H, Shin T, Moon C (2010) Cyclophosphamide impairs hippocampus‐dependent learning and memory in adult mice: possible involvement of hippocampal neurogenesis in chemotherapy‐induced memory deficits. Neurobiol Learn Mem 93: 487–494 [DOI] [PubMed] [Google Scholar]
- Yang M, Kim JS, Kim J, Jang S, Kim SH, Kim JC, Shin T, Wang H, Moon C (2012) Acute treatment with methotrexate induces hippocampal dysfunction in a mouse model of breast cancer. Brain Res Bull 89: 50–56 [DOI] [PubMed] [Google Scholar]
- You Z, Zhang S, Shen S, Yang J, Ding W, Yang L, Lim G, Doheny JT, Tate S, Chen L et al (2018) Cognitive impairment in a rat model of neuropathic pain: role of hippocampal microtubule stability. Pain 159: 1518–1528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young W (2009) Review of lithium effects on brain and blood. Cell Transplant 18: 951–975 [DOI] [PubMed] [Google Scholar]
- Zhou W, Kavelaars A, Heijnen CJ (2016) Metformin prevents cisplatin‐induced cognitive impairment and brain damage in mice. PLoS ONE 11: e0151890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer P, Mierau A, Bloch W, Struder HK, Hulsdunker T, Schenk A, Fiebig L, Baumann FT, Hahn M, Reinart N et al (2015) Post‐chemotherapy cognitive impairment in patients with B‐cell non‐Hodgkin lymphoma: a first comprehensive approach to determine cognitive impairments after treatment with rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone or rituximab and bendamustine. Leuk Lymphoma 56: 347–352 [DOI] [PubMed] [Google Scholar]
- Zou YM, Lu D, Liu LP, Zhang HH, Zhou YY (2016) Olfactory dysfunction in Alzheimer's disease. Neuropsychiatr Dis Treat 12: 869–875 [DOI] [PMC free article] [PubMed] [Google Scholar]


