Dear Editor,
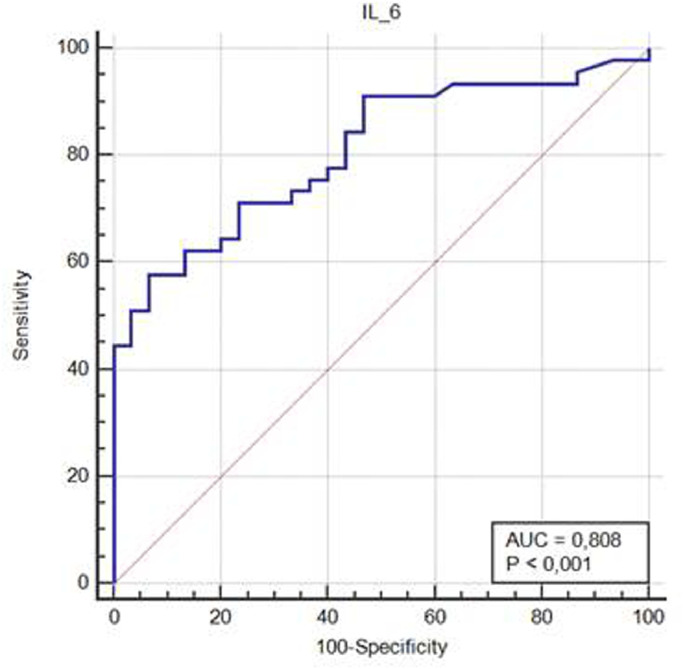
identifying risk factors for early progression toward severe disease and/or mortality is fundamental for the practical management of COVID-19 patients. Evidence shows that pro-inflammatory cytokines play a pivotal role in the pathophysiology of lung damage in patients affected by coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Therefore we read with much interest the recent article published in Your Journal by Ye Q. et al. who describe the “cytokine storm” in COVID patients.1 A lot of patients affected by COVID-19 develop a fulminant and damaging immune reaction sustained by cytokines leading to alveolar infiltration by macrophages and monocytes.1 Interleukin-6 (IL-6) is one of the main mediators of inflammatory and immune response initiated by infection or injury and increased levels of IL-6 are found in more than one half of patients with COVID-19.2 Levels of IL-6 seem to be associated with inflammatory response, respiratory failure, needing for mechanical ventilation and/or intubation and mortality in COVID-19 patients.3 , 4 In a meta-analysis including nine studies (total 1426 patients) reporting on IL-6 and outcome in COVID-19, mean IL-6 levels were more than three times higher in patients with complicated COVID-19 compared with those with non complicated disease, and IL-6 levels were associated with mortality risk.4 However, whether IL-6 could be a better prognosticator than clinical and laboratory variables remains unclear. Therefore, we tested the role of IL-6 as risk factor for negative outcome compared with other demographic and clinical variables or biomarkers collected at hospital admission. Age over 60 years, presence of at least one co-morbidity among arterial hypertension, diabetes, cardiovascular disease, asthma, chronic lung disease, chronic kidney disease, liver disease, HIV infections, and malignancy for at least 6 months, lymphocyte count under 1.0 × 109/L, lactate dehydrogenase (LDH) over 500 U/L, CALL score > 9 points (C=presence of co-morbidity, A=age over 60 years, L=lymphocyte count under 1.0 × 109/L, L=LDH over 250 U/L or 500 U/L)5, D-Dimer over 500 microg/L, and IL-6 over 25 pg/mL were the analyzed variables. Quantitative determination of IL-6 levels was performed by using an immunoenzymatic chemiluminescent assay (Access Immunoassay System, Beckman Coulter, USA, lowest limit of detection 0.5 pg/mL). After exclusion of patients requiring immediate intensive care unit (ICU) admission, we analyzed risk factors for the combined endpoint progression to severe COVID-19 syndrome and/or in-hospital mortality in an Italian COVID-19 population admitted to a non intensive ward from March 12 to April 20, 2020. Progression toward clinical worsening was defined as respiratory rate ≥ 30 breaths/min, resting SatO2 ≤ 93%, paO2/FiO2 ratio ≤ 300 or requiring of mechanical ventilation, such as in previous studies.5 The study population consisted of 77 patients, 44 males (57.1%), with mean age 64 ± 17 years. Of them, 45 patients (58.4%) met criteria for the combined endpoint. Six patients (7.8%) died. CALL score > 9 points (55.3% vs 26.6%, p = 0.0099) and IL-6 > 25 pg/mL (65.9% vs 23.3%, p = 0.0004) were significantly more frequent in patients with the combined endpoint. At logistic regression analysis IL-6 over 25 pg/mL (OR 11.6, 95% CI 2.8–48,2) was found independent risk factor for the combined endpoint (Table 1 ). Mean levels of IL-6 in patients who met criteria for the combined endpoint were significantly higher compared with those of patients who did not (134.3 ± 19.5 vs 15.6 ± 14.8 pg/mL, p < 0.001). The area under the receiver operating characteristic (ROC) curve (AUC) for IL-6 as predictor of the combined endpoint was 0.80 (95% CI 0.70–0.89) (Fig. 1 ). The AUC for IL-6 as predictor of in-hospital mortality was 0.90 (95% CI 0.81–0.95), while it was 0.75 (95% CI 0.64–0.84) for IL-6 as predictor of progression to severe COVID-19.
Table 1.
Risk factors for the combined endpoint progression to severe COVID-19 and/or in-hospital mortality. Logistic regression analysis.
| Variable | Odds ratio | 95% CI |
|---|---|---|
| Age over 60 years | 1,4882 | 0,3663–6,0466 |
| CALL score > 9 points | 4,5577 | 0,7383–28,1352 |
| Co-morbidity | 0,3150 | 0,0634–1,1561 |
| D-Dimer > 500 microg/L | 0,9882 | 0,2638–3,7009 |
| IL-6 > 25 pg/mL | 11,6460 | 2,8123–48,2277 |
| LDH > 500 U/L | 0,5033 | 0,1061–2,3888 |
| Lymphocyte count< 1.0 x 109 | 0,6145 | 0,1473–2,5638 |
CI: confidence interval; CALL score: C=presence of co-morbidity, A=age over 60 years, L=lymphocyte count under 1.0 x 109/L, L=LDH over 250 U/L or 500 U/L; IL-6: Interleukin-6; LDH: lactate dehydrogenase.
Fig. 1.
Receiver operating characteristic (ROC) curve showing the predictive power of IL-6 for predicting progression to severe COVID-19 and/or in-hospital mortality.
In conclusion, in our COVID-19 population, IL-6 levels at hospital admission seem to be a good prognosticator for the combined endpoint progression to severe disease and/or in-hospital mortality, and it seems to be the best prognosticator for negative outcome. Therefore, our study supports the hypothesis that targeting the cytokine storm induced by SARS-CoV-2 by using anti-IL-6 drugs could be a valid therapeutic option, together with supportive care strategies, for improving outcomes in COVID-19 patients.6
References
- 1.Ye Q., Wang B., Mao J. The pathogenesis and treatment of the “Cytokine Storm” in COVID-19. J Infect. 2020;80:607–613. doi: 10.1016/j.jinf.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang Z.L., Hou Y.L., Li D.T., Li F.Z. Laboratory findings of COVID-19: a systematic review and meta-analysis [published online ahead of print, 2020 May 23] Scand J Clin Lab Investig. 2020:1‐7. doi: 10.1080/00365513.2020.1768587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Herold T., Jurinovic V., Arnreich C. Elevated levels of interleukin-6 and CRP predict the need for mechanical ventilation in COVID-19 [published online ahead of print, 2020 May 18] J Allergy Clin Immunol. 2020 doi: 10.1016/j.jaci.2020.05.008. 10.1016/j.jaci.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aziz M., Fatima R., Assaly R. Elevated interleukin-6 and Severe COVID-19: a meta-analysis. J Med Virol. 2020 doi: 10.1002/jmv.25948. 10.1002/jmv.25948Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ji D., Zhang D., Xu J. Prediction for progression risk in patients with COVID-19 pneumonia: the CALL score. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa414. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Magro G. SARS-CoV-2 and COVID-19: is interleukin-6 (IL-6) the 'culprit lesion' of ARDS onset? What is there besides Tocilizumab? SGP130Fc. Cytokine X. 2020 doi: 10.1016/j.cytox.2020.100029. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]