Abstract
Background and Objective
There is a pressing need for evidence-based interventions to address the devastating clinical and public health effects of the coronavirus disease 2019 (COVID-19) pandemic. The number of registered trials related to COVID-19 is increasing by the day. The objective of this study was to describe the characteristics of the currently registered interventional clinical trials related to COVID-19.
Methods
We searched the World Health Organization's International Clinical Trials Registry Platform on May 15th, 2020. We included any entry that is related to COVID-19. We abstracted and then descriptively analyzed the following characteristics of the registered trials: study design, status, phase, primary endpoints, experimental interventions, and geographic location among other qualifiers.
Results
We identified 1,308 eligible registered trials. Most trials were registered with ClinicalTrials.gov (n = 703; 53.7%) and the Chinese Clinical Trial Registry (n = 291; 22.2%). The number of participants to be enrolled across these trials was 734,657, with a median of 110 participants per trial. The most commonly studied intervention category was pharmacologic (n = 763; 58.3%), with antiparasitic medications being the most common subcategory. Although over half of the trials were already recruiting, we identified published peer-reviewed results for only 8 of those trials.
Conclusion
There is a relatively large number of registered trials but with very few results published so far. Although our findings suggest an appropriate initial response by the research community, the real challenge will be to get these trials completed, published, and translated into practice and policy.
Keywords: Novel coronavirus disease, COVID-19, Clinical trials, Trial registration, Trial characteristics
What is new?
Key findings
-
•
Thousand three hundred and eight eligible trials related to COVID-19 were registered up to May 15th, 2020.
-
•
Trials were planned in 71 countries, with most in Europe.
-
•
Although more than half of the trials were already recruiting, eight had peer-reviewed publications.
What this adds to what was known?
-
•
The research community has shown a good response to the pandemic in terms of initiating trials.
What is the implication and what should change now?
-
•
The ultimate test will be whether the research community will be able to generate the needed evidence to guide the management of the pandemic.
-
•
Efforts should focus on completing the trials and publishing them in a timely fashion.
1. Introduction
In December 2019, the Chinese city of Wuhan witnessed the outbreak of a pneumonia of unknown origin [1]. The outbreak was traced back to Wuhan's Seafood Market [2], and characterized by a strong person-to-person transmission [3]. Subsequently, scientists identified a new strain of coronavirus, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), as the source of the outbreak [4]. SARS-CoV-2 is a novel member of the beta coronavirus family, which includes SARS-CoV, source of an outbreak in 2002, and MERS-CoV, the origin of an outbreak in Saudi Arabia in 2012 [5].
On 11th of February 2020, the World Health Organization (WHO) announced coronavirus disease 2019 (COVID-19) as the name of this new disease [6]. Within 3 months, COVID-19 outbreak had already affected six continents [7], and the WHO upgraded its status from epidemic to pandemic on March 11, 2020 [8]. As of May 30th, 2020, there have been 6,052,315 confirmed cases and 367,288 deaths [9].
Patients infected with COVID-19 present with a wide spectrum of clinical presentations [10], ranging from no symptoms to acute respiratory distress syndrome and death [11]. In parallel, the high infectivity rates of the virus have led to the overstraining of health care systems [[12], [13], [14]]. Supportive management remain the pivot of treatment protocols in the absence of evidence on efficacious antiviral or anti-inflammatory medications [15].
To date, most recommendations on preventing disease transmission and treating infected patients are based on anecdotal evidence and experts’ opinions. Randomized control trials (RCTs) are needed to provide unbiased evidence to guide the clinical care and public health practices aimed to control the COVID-19 outbreak [16]. Analyzing the status of clinical trials is a way of describing the current status of research in a scientific field, assessing the direction and magnitude of progress, and identifying potential gaps in interventional research [17]. Thus, this study aimed to describe the characteristics of the currently registered clinical trials related to COVID-19.
2. Materials and methods
2.1. Search strategy
We used the WHO International Clinical Trials Registry Platform (ICTRP) database [18] to identify all COVID-19 clinical trials and retrieve related information. The ICTRP is a network of international clinical trial registries which ensures single-point access and unambiguous identification of trials [18] The source registries included as of May 15th were Chinese Clinical Trial Registry (ChiCTR), ClinicalTrials.gov (NCT), European Union Clinical trials Register (EUCTR), Iranian Registry of Clinical Trials (IRCT), Japan Primary Registries Network (JPRN), International Standard Randomized Controlled Trial Number (ISRCTN), Australian New Zealand Clinical Trials Registry(ANZCTR), Clinical Trial Registry—India (CTRI), German Clinical Trials Registry (DRKS), Pan African Clinical Trial Registry (PACTR), Thai Clinical Trials Registry (TCTR), Brazilian Clinical Trials Registry (RBR), Cuban Public Registry of Clinical Trials (RPCEC), Netherlands Trial Register (NL) and Clinical Trials Peruvian Registry (PER). The ICTRP is making available a downloadable csv-type file accessible via Microsoft Excel, that includes all COVID-19 registered trials and is updating it on a weekly basis, URL: https://www.who.int/ictrp/en/. We downloaded the COVID-19 file on May 15th 2020.
2.2. Study selection
We included all records retrieved on May 15th, 2020, from the ICTRP that were labeled as one of the following study types: interventional, screening, prevention, treatment. We excluded trials not directly related to treating or preventing COVID-19 disease, non-interventional trials, and the following types of trials: basic science, diagnostic test, epidemiological research, expanded access, health-services, observational, and prognosis.
2.3. Data collection
We exported for each record all the variables reported by ICTRP (See Appendix A). We included the following variables for our analysis: study ID, source registry unique identifier, original registry, public title, primary sponsor, location (country and region), recruitment status, age range, gender, target size, study design, phase, publication (yes/no, count, and URL), intervention (category, subcategory, and name), primary outcomes, registration date, enrollment date, retrospective label, and trial URL.
Using the source registry unique identifier numbers, we verified the data exported from the ICTRP data file and collected any missing data. Then, two investigators (A.A.N. and H.H.K.) categorized in duplicate and independently the intervention variables into detailed subcategories, as shown in Appendix B. Similarly, they categorized outcomes into the following types: mortality, morbidity, patient-reported, surrogate, composite, and other.
In addition, we searched for publications related to the eligible trials. We used the source registry unique identifier to search for peer-reviewed publications related to the eligible trials (on PubMed, Medline, Embase, and Scopus), and for preprint articles (on medRxiv and OSF) [19,20]. Two investigators (S.H.F. and Z.A.N.) reviewed potentially relevant peer-reviewed publications and preprint articles independently to confirm their relatedness to the eligible trials.
3. Results
3.1. Results of the search
The complete COVID-19 file retrieved from the ICTRP database included a total of 2,487 records. We excluded 1,179 records for the following reasons: non-interventional trials (n = 1,050); canceled/withdrawn/suspended/terminated/retracted trials (n = 44), not directly related to COVID-19 (n = 67), duplicate records (n = 13), not found in source registry (n = 5).
As a result, 1,308 records met our eligibility criteria. Fig. 1 (the cumulative number of confirmed cases of COVID-19 is retrieved from https://ourworldindata.org/) shows the time distributions of the cumulative number of registered trials with the cumulative number of confirmed cases of COVID-19 [21]. While the former follows an exponential growth pattern, the latter follows an arithmetic growth pattern.
Fig. 1.
The time distributions of the cumulative number of registered trials (blue curve) and of the cumulative number of confirmed cases of COVID-19 (orange curve); January 1st, 2020, to May 15th, 2020. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
3.2. Characteristics of registered trials
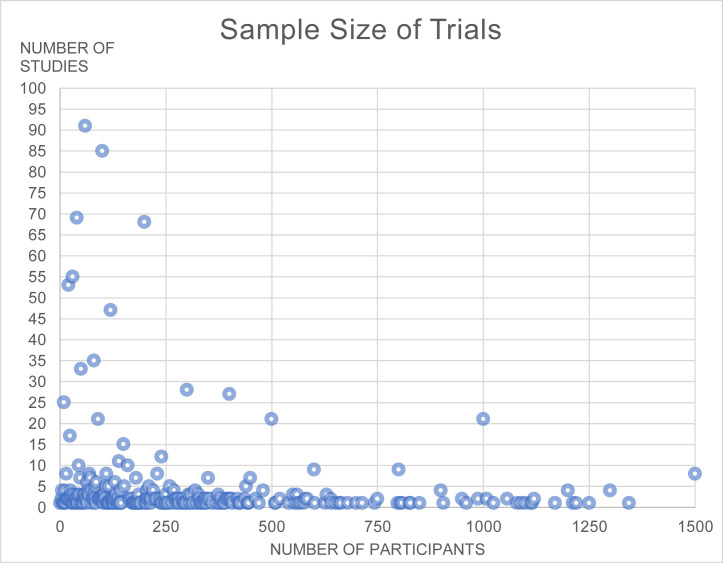
Fig. 2 (eight trials registered between 2015 and 2019 changed their selection criteria to include COVID-19 patients, they have not been included in this figure) shows the distribution of trials by planned date of first enrollment. The earliest trial registration date was January 10th, 2020, 34 days after the official reporting of the first COVID19 case [1]. It is worth mentioning that seven trials started between the years of 2015 and 2019, respectively, and adjusted their protocols and eligibility criteria to include COVID-19 patients. Fig. 3 (only trials with sample sizes at or below 1,500 participants are shown in the figure [n = 1,240]) shows the distribution of trials by number of participants. A total of 734,657 participants are to be enrolled across the registered trials, with a median of 110 participants per trial (interquartile range = 50 to 300).
Fig. 2.
Distribution of studies by date of registration in source registries (N = 1,301).
Fig. 3.
Distribution of studies by the number of participants (N = 1,240).
Table 1 shows the characteristics of 1,308 trials stratified by phase, and across phases. Only 129 trials (10%) are in phase 4. These records originated mostly from ClinicalTrials.gov (n = 703; 53.7%) and the ChiCTR (n = 291; 22.2%). Trials are planned to be conducted in 71 countries, with 36 (2.8%) planned as multicountry trials. The five countries with the largest number of registered trials are People's Republic of China (n = 360, 27.5%), the United States (n = 227, 17.4%), Iran (n = 125, 9,6%), France (n = 91, 7.0%), and Spain (n = 86, 6.6%), More than half of the trials are described as recruiting/ongoing (143 trials (10.9%) registered in the EU Clinical Trials Register were classified as “ongoing” without indicating their respective recruiting status) (n = 761; 58.0%), whereas only 48 trials (3.7%) are described as completed. The vast majority of trials are in either adults only (n = 1,152, 88.1%) or adults and pediatric age (n = 74, 5.7%).
Table 1.
Characteristics of registered trials stratified by phase, and across phases (N = 1,308)
| Trial characteristic | Phase |
Unknown | Total | Percentage | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 1,2 | 2 | 2,3 | 3 | 4 | N/A | ||||
| Number of Studies | 137 | 57 | 50 | 309 | 95 | 264 | 129 | 259 | 8 | 1,308 | - |
| Percent of Studies | 11 | 4 | 4 | 24 | 7 | 20 | 10 | 20 | 1 | 100 | - |
| Clinical Trial Registry | |||||||||||
| ClinicalTrials.gov | 3 | 46 | 39 | 215 | 65 | 146 | 46 | 143 | 0 | 703 | 54 |
| ChiCTR | 132 | 7 | 2 | 6 | 2 | 2 | 56 | 84 | 0 | 291 | 22 |
| EU CTR | 0 | 0 | 2 | 64 | 4 | 48 | 24 | 1 | 0 | 143 | 11 |
| IRCT | 0 | 1 | 5 | 16 | 21 | 56 | 1 | 11 | 0 | 111 | 9 |
| ANZCTR | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 17 | 1 |
| JPRN | 0 | 0 | 0 | 4 | 0 | 2 | 0 | 0 | 0 | 6 | <1 |
| ISRCTN | 0 | 0 | 1 | 1 | 3 | 0 | 3 | 0 | 0 | 8 | <1 |
| Other | 1 | 3 | 1 | 2 | 1 | 5 | 1 | 7 | 8 | 29 | 2 |
| Geographic Location | |||||||||||
| Europe | 1 | 5 | 12 | 121 | 32 | 96 | 35 | 71 | 8 | 381 | 29 |
| People's Republic of China | 133 | 14 | 11 | 17 | 8 | 9 | 64 | 103 | 0 | 359 | 27 |
| North America (US, Canada) | 0 | 24 | 15 | 133 | 15 | 45 | 9 | 39 | 0 | 260 | 20 |
| MENA | 0 | 9 | 7 | 23 | 30 | 67 | 7 | 21 | 0 | 164 | 13 |
| Multicountry | 1 | 1 | 0 | 7 | 2 | 17 | 6 | 2 | 0 | 36 | 3 |
| Central and South America | 1 | 3 | 4 | 12 | 4 | 12 | 2 | 7 | 0 | 45 | 3 |
| Asia | 0 | 0 | 0 | 15 | 2 | 12 | 5 | 8 | 0 | 42 | 3 |
| Oceania | 1 | 1 | 1 | 1 | 1 | 2 | 1 | 8 | 0 | 16 | 1 |
| Africa | 0 | 0 | 0 | 0 | 1 | 4 | 0 | 0 | 0 | 5 | <1 |
| Status | |||||||||||
| Recruiting | 71 | 30 | 27 | 130 | 49 | 114 | 66 | 128 | 3 | 618 | 47 |
| Not recruiting | 66 | 26 | 18 | 109 | 34 | 81 | 36 | 124 | 5 | 499 | 38 |
| Ongoing | 0 | 0 | 2 | 64 | 4 | 48 | 24 | 1 | 0 | 143 | 11 |
| Completed | 0 | 1 | 3 | 6 | 8 | 21 | 3 | 6 | 0 | 48 | 4 |
| Unknown | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Publications | |||||||||||
| Peer-reviewed | 0 | 1 | 0 | 1 | 0 | 2 | 1 | 3 | 0 | 8 | <1 |
| Preprint | 4 | 0 | 1 | 1 | 0 | 2 | 6 | 0 | 0 | 14 | 1 |
| Age group | |||||||||||
| Adult Only | 109 | 54 | 43 | 289 | 80 | 229 | 125 | 215 | 8 | 1,152 | 88 |
| Adult and Pediatric | 8 | 0 | 1 | 11 | 10 | 17 | 2 | 25 | 0 | 74 | 6 |
| Pediatric Only | 20 | 3 | 6 | 9 | 5 | 18 | 2 | 19 | 0 | 82 | 6 |
| Unknown | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Gender | |||||||||||
| Both | 136 | 54 | 50 | 307 | 94 | 263 | 129 | 257 | 8 | 1,298 | 99 |
| Male | 1 | 3 | 0 | 1 | 1 | 0 | 0 | 2 | 0 | 8 | <1 |
| Female | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 2 | <1 |
Abbreviations: ANZCTR, Australian New Zealand Clinical Trials Registry; ChiCTR, Chinese Clinical Trial Registry; IRCT, Iranian Registry of Clinical Trials; ISRCTN, International Standard Randomized Controlled Trial Number; JPRN, Japan Primary Registries Network.
3.3. Characteristics of interventions
Table 2 shows the characteristics of assessed interventions across phases (see Appendix C for stratification by phase). Of the 1,308 eligible trials, 765 (58.4%) investigated pharmacologic interventions. The top three subcategories of studied pharmacologic interventions were antiparasitics (n = 195; 25.8%), immunomodulators (n = 138; 18.0%), and antivirals (n = 129; 16.8%). Of these studies, 206 trials (27.0%) were in phase 3 and 172 trials (22.5%) were in phase 2; 94 trials (12.3%) were phase nonapplicable.
Table 2.
Characteristics of assessed interventions stratified across phases (N = 1,308)
| Intervention categories |
N = 1,308 (% of total) |
Intervention subcategories | (% of category) |
||
|---|---|---|---|---|---|
| n= | % | n= | % | ||
| Pharmacologic | 765 | 58 | Antiparasitics (± other) Immunomodulators (± other) Antivirals (± other) Traditional Chinese Medicine Medicinal Herbs Gas Anticoagulant Antihypertensive Other |
195 138 129 113 31 26 21 20 53 |
26 18 17 15 4 3 3 3 7 |
| Biological | 305 | 23 | Monoclonal antibodies (± other) Plasma or IVIG Stem cells (± other) Protein Other |
88 83 55 47 32 |
29 27 18 17 10 |
| Devices | 72 | 6 | Extracorporeal filtration Ventilation Detection Others |
18 17 15 22 |
25 24 21 31 |
| Vaccine | 37 | 3 | COVID-19 Other |
17 20 |
46 54 |
| Psychological | 25 | 2 | |||
| Physical Therapies | 28 | 2 | |||
| Protocol | 30 | 2 | Management Other |
27 3 |
90 10 |
| Nutritional | 41 | 3 | Supplements Enteral feeding |
37 4 |
90 10 |
| Education | 5 | <1 | |||
The second most studied category was biological interventions (including monoclonal antibodies and stem cells) (n = 305; 23.3%). 126 of these trials (41.3%) were in phase 2, and 32 trials (10.5%) were in phase 3. Thirty-seven of 1,308 trials were investigating COVID-19 vaccines (2.8%). 20 trials (1.5%) studied the potential uses of non-COVID vaccines on COVID-19 patients.
3.4. Characteristics of studied outcomes
Table 3 provides a recategorization of the types of primary outcomes assessed in the eligible trials. Overall, most trials planned to include morbidity outcomes as primary outcomes (n = 704; 53.8%). The next types by order of frequency were surrogate outcomes (n = 611; 46.7%), mortality (n = 319; 24.4%), and composite outcomes (n = 129; 9.9%). Of these trials, 346 (26.5%) had surrogate only outcomes, 351 (26.8%) had morbidity-only outcomes, whereas 147 trials (11.2%) had both. Only one study did not report any primary outcomes.
Table 3.
Types of primary outcomes in the eligible trials stratified by phase
| Outcome | Phase |
Total | Percent | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 1,2 | 2 | 2,3 | 3 | 4 | N/A | Unknown | |||
| Morbidity | 68 | 34 | 35 | 167 | 56 | 159 | 78 | 105 | 2 | 704 | 54 |
| Surrogate | 79 | 28 | 19 | 137 | 52 | 104 | 67 | 121 | 4 | 611 | 47 |
| Mortality | 20 | 8 | 10 | 96 | 26 | 93 | 24 | 40 | 2 | 319 | 24 |
| Composite | 9 | 4 | 5 | 40 | 11 | 25 | 12 | 22 | 1 | 129 | 10 |
| Patient Reported | 16 | 2 | 0 | 2 | 1 | 3 | 1 | 33 | 0 | 58 | 4 |
| Other | 4 | 3 | 2 | 3 | 1 | 8 | 3 | 18 | 0 | 42 | 3 |
4. Discussion
4.1. Summary of findings
We have described the characteristics of 1,308 currently registered clinical trials related to COVID-19. The trials were planned in 71 countries, with the majority in China. The median number of participants per trial was 110, with only 10% of trials being in phase 4. Fifty-eight percent of the trials were recruiting or ongoing, and 3.7% were completed. We only found 8 peer-reviewed original articles reporting results for the eligible trials. We also found 14 preprints of trial results. Pharmacologic interventions were the most studied category, with antiparasitic drugs, alone or in combination, being the most studied subcategory. Most trials included morbidity outcomes as primary outcomes.
4.2. Strengths and limitations
Our study was based on trials registered in the WHO ICTRP, which allows single-point access and unambiguous identification of trials [18]. In addition, we used duplicate and independent approach to categorizing interventions and outcomes. A major limitation of our study is that the pool of COVID-19 registered trials is rapidly growing; hence, the data would need to be periodically updated. Moreover,some registered trials may have incorrect, missing, or outdated information [22].
4.3. Interpretations of findings
We had carried out an interim search of the registries on April 1, 2020 (See Appendix D; Tables D1–D3, reproduce Table 1, Table 2, Table 3), but with a comparison of the results of the interim search on April 1st and of the current search on May 15th. Over the 6-week period between the two searches, the number of eligible trials increased by more than three-fold(395 to 1,308). Two of most notable differences relate to the trial geographical location and intervention category. While most of the trials captured by the interim search were in China (27.4%), most of the trials captured by the current search were in Europe (29%), China (27%), and North America (20%). This shift in geographical location reflects the change of the geographical focus of the pandemic itself to Europe and United States. The number of trials also remarkably increased in Iran, which is now the third largest country of origin for registered trials (9.6%). The previous focus of trials was on traditional Chinese medicine, which is somehow expected given that the pandemic started in China [23]. The current focus is on antiparasitic drugs, immunomodulators, and antivirals.
During the COVID-19 pandemic, breakthrough news from Chinese hospitals regarding the effectiveness of hydroxychloroquine in COVID-19 patients drew significant attention to the drug [24]. It has especially made the subject of public attention after a cohort study in France found the use of hydroxychloroquine with azithromycin effective and free of side effects in patients if used early after diagnosis [25]. Chloroquine and its reportedly less-toxic derivative, hydroxychloroquine [26], used alone or in combination with azithromycin, are now part of clinical practice for the management of COVID-19 in more than 10 countries including China, Iran, and Italy [27]. On March 28, 2020, the US Food and Drug Administration (FDA) also approved hydroxychloroquine for emergency use authorization in treating COVID-19 patients [28]. The increasingly widespread use of the drug brings important considerations regarding evidence supporting its use.
As of May 31, 2020, the results of four clinical trials and one prospective observational study on the use of hydroxychloroquine in COVID-19 were published. Three found it superior to conventional treatment [25,29,30], whereas the others did not observe significant difference between groups [31,32]. One target trial emulation of 181 patients did not find evidence supporting the use of hydroxychloroquine for COVID-19-related hypoxic pneumonia [33]. With no long-term follow-up, small sample sizes, multiple methodological flaws, and conflicting results, these published trials do not offer enough high-quality evidence to adequately support guideline recommendations [34].
We found 235 registered trials investigating the use of hydroxychloroquine in COVID-19 patients for either treatment or prophylaxis. Most are either in phase 3 (n = 93) or phase 2 (n = 53), and most are conducted in Europe (n = 89). Only three of these trials had published results [29,30,32]. With the known side-effect profile of the drug, including cardiomyopathy and arrhythmias [34] and its suggested ability to induce renal and liver impairment [35], the inclusion of hydroxychloroquine in clinical practice remains questionable until strong and convincing evidence can be generated.
Other therapeutic agents under study included antivirals, immunomodulators, and biological agents. These include many drugs previously used for the treatment of other infectious pathogens. One example is umifenovir (brand name Arbidol), an antiviral agent used in Russia and China for treating influenza infection, but not approved by the US FDA [36]. Another example is oseltamivir, an FDA-approved drug for the treatment of influenza A and B [37].
Remdesivir is an antiviral agent that has recently received considerable attention. The Adaptive COVID-19 Treatment Trial (ACTT), a phase 3 trial involving 1,063 participants lead by the National Institute of Allergy and Infectious Diseases, found that patients treated with remdesivir had significantly quicker recovery and lower mortality compared with placebo [38]. In light of the optimistic results, remdesivir was announced as the new standard of care for COVID-19 patients in the United States on April 19, 2020 [39]. Available results by another, although smaller, clinical trial lead by Gilead Sciences did not find a significant difference in outcomes between 5 and 10 days of treatment with remdesivir [40].
Ongoing trials investigating vaccines for COVID-19 are very important, as vaccination is the sustainable solution for counteracting this public health threat [41]. Traditionally, vaccine development is a lengthy process faced by multiple challenges including unknown virus immunogenic profile, vaccine safety, and participants recruitment/adherence [42]. In times of pandemics, additional challenges appear, such as difficulty randomizing populations in high mortality situations, overburdening ethics and regulatory authorities, as well as the absence of large-scale manufacturing for any novel platform technology [43]. Although promising results are available for one trial investigating the adenovirus type 5 vectored COVID-19 vaccine [44], and most of the 37 identified trials on COVID-19 vaccines are in phase 1 and/or 2, the much-awaited vaccine is not expected to be available before at least 1 year to 18 months [14].
The selection of primary outcomes reflects how researchers define meaningful evidence for the success of an intervention. However, the selection of outcomes has to insure adequate validity of their measurements and their generalizability for translation in clinical practice or health policies [45]. We found that pharmacologic and biological interventional trials addressed mainly morbidity and surrogate outcomes more frequently than composite, mortality, or patient-reported outcomes. Trials targeting psychological interventions or physical therapy measured patient-related outcomes, an expected finding. We found that 27% of registered trials addressed surrogate outcomes exclusively. In general, it is unclear to what degree surrogate outcomes correlate with clinically meaningful effects, like those targeted by clinical outcomes [31]. However, surrogate outcomes are used in accelerated approval pathways during epidemics or increases in life-threatening diseases, as they allow the measurement of intervention effects with smaller sample sizes and shorter trial durations [46].
We identified no completed trials, and only 8 peer-reviewed articles were available at the time of our search. The most likely cause is the recency of the pandemic, which triggered the initiation of these interventional trials. Although peer review can be a lengthy process, peer-review platforms are making changes to optimize their assessments, such as directly posting their reviews to preprint servers [47].
4.4. Implications for practice
There is no available evidence to date that appropriately guides recommendations for the prevention and treatment of COVID-19. Those guiding health policies and clinical practice may therefore have to rely on a limited number of trial results, or indirect evidence derived from other diseases. There is a need for evidence-based interventions to mitigate the global humanitarian and economic sequelae of the pandemic. Registration of ongoing trials is essential for researchers to coordinate efforts, but more importantly to optimize the methods of those trials and ensure the transparency of their methods. The case of hydroxychloroquine illustrates very well how the medical community adopted a promising but still unproven intervention, in spite of the supporting evidence being inadequate, and that trials are still ongoing. This could be explained by the lack of effective medications in the face of high mortality rate. Still, the medication comes with significant side effects and harms. Therefore, it is imperative to have a living process to keep the evidence up to date as the results of trials start coming out [48], and then feeding them into living recommendations [49]. Tracking registered trials would improve the efficiency of those living processes.
4.5. Implications for future research
Future research can focus on improving clinical trial recruitment processes and generating results in the most expeditious ways possible. In the face of ongoing challenges, collaborative international efforts may be the key to success. There is also a need to explore how to make trial outcomes available to decision makers, including guideline developers, in a timely fashion. Future research on trial registration mechanisms will need to explore its dynamics over time, given the rapidly increasing rate of trial registration. It would be interesting to explore whether or how the geographic distribution of clinical trials is affected by the pandemic's geographic evolution over time. Similarly, it will be important to explore how the intervention categories and the studied outcomes would change with time and with the generation of new evidence.
CRediT authorship contribution statement
Ali A. Nasrallah: Conceptualization, Methodology, Funding acquisition, Data curation, Formal analysis, Writing - original draft, Writing - review & editing. Sarah H. Farran: Funding acquisition, Formal analysis, Writing - original draft, Writing - review & editing. Zainab A. Nasrallah: Funding acquisition, Formal analysis, Writing - original draft, Writing - review & editing. Mohamad A. Chahrour: Conceptualization, Methodology, Formal analysis, Writing - original draft, Writing - review & editing. Hamza A. Salhab: Data curation, Writing - review & editing. Mohamad Y. Fares: Data curation, Writing - review & editing. Hussein H. Khachfe: Conceptualization, Methodology, Funding acquisition, Writing - review & editing. Elie A. Akl: Conceptualization, Methodology, Formal analysis, Writing - original draft, Writing - review & editing.
Acknowledgments
Authors' contributions: A.A.N., M.A.C., E.A.A., and H.H.K. were responsible for the concept and design of the study; A.A.N., S.H.F., Z.A.N., and H.H.K. data acquisition; A.A.N., S.H.F., and M.A.C. statistical analysis; A.A.N., H.A.S., and M.Y.F. interpretation of results; A.A.N., S.A.F., Z.A.N., M.A.C., and E.A.A. analyzed the data and drafted the manuscript. All authors critically revised the manuscript, approved the final version to be published, and agree to be accountable for all aspects of the work.
Footnotes
Funding: None.
Conflicts of interest: None to declare.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jclinepi.2020.06.005.
Supplementary data
References
- 1.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Millán-Oñate J., Rodriguez-Morales A., Camacho-Moreno G., Mendoza-Ramírez H., Rodríguez-Sabogal I., Álvarez-Moreno C. A new emerging zoonotic virus of concern: the 2019 novel Coronavirus (COVID-19) Infection. 2020;24:187–192. [Google Scholar]
- 3.Li Q., Guan X., Wu P., Wang X., Zhou L., Tong Y. Early transmission dynamics in Wuhan, China, of novel coronavirus–infected pneumonia. N Engl J Med. 2020;382(13):1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gorbalenya A.E., Baker S.C., Baric R.S., de Groot R.J., Drosten C., Gulyaeva A.A. bioRxiv; Cold Spring Harbor; NY: 2020. Severe acute respiratory syndrome-related coronavirus: The species and its viruses – a statement of the Coronavirus Study Group. 2020.02.07.937862. [Google Scholar]
- 5.Evans S.R. Clinical trial structures. J Exp Stroke Transl Med. 2010;3(1):8–18. doi: 10.6030/1939-067x-3.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Organization, W.H. Naming the coronavirus disease (COVID-19) and the virus that causes it. 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/naming-the-coronavirus-disease-(covid-2019)-and-the-virus-that-causes-it Available at.
- 7.Chahrour M., Assi S., Bejjani M., Nasrallah A.N., Salhab H.A., Fares M.Y. A bibliometric analysis of COVID-19 research activity: a call for increased output. Cureus. 2020;12:e7357. doi: 10.7759/cureus.7357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Organization, W.H. Coronavirus disease (COVID-2019) situation reports. World Health Organization; Geneva, Switzerland: 2020. Coronavirus disease 2019 (COVID-19) situation report – 51. [Google Scholar]
- 9.Dong E., Du H., Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020;12:533–534. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guan W.-J., Ni Z.-Y., Hu Y., Liang W.-H., Ou C.-Q., He J.-X. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spina S., Marrazzo F., Migliari M., Stucchi R., Sforza A., Fumagalli R. The response of Milan’s emergency medical system to the COVID-19 outbreak in Italy. Lancet. 2020;395:e49–e50. doi: 10.1016/S0140-6736(20)30493-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grasselli G., Pesenti A., Cecconi M. Critical care utilization for the COVID-19 outbreak in Lombardy, Italy: early experience and Forecast during an emergency response. JAMA. 2020 doi: 10.1001/jama.2020.4031. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 14.Anderson R.M., Heesterbeek H., Klinkenberg D., Hollingsworth T.D. How will country-based mitigation measures influence the course of the COVID-19 epidemic? Lancet. 2020;395(10228):931–934. doi: 10.1016/S0140-6736(20)30567-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guo Y.R., Cao Q.D., Hong Z.S., Tan Y.Y., Chen S.D., Jin H.J. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak - an update on the status. Mil Med Res. 2020;7(1):11. doi: 10.1186/s40779-020-00240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kabisch M., Ruckes C., Seibert-Grafe M., Blettner M. Randomized controlled trials: part 17 of a series on evaluation of scientific publications. Dtsch Arztebl Int. 2011;108(39):663–668. doi: 10.3238/arztebl.2011.0663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pasquali S.K., Lam W.K., Chiswell K., Kemper A.R., Li J.S. Status of the pediatric clinical trials enterprise: an analysis of the US ClinicalTrials.gov registry. Pediatrics. 2012;130(5):e1269–e1277. doi: 10.1542/peds.2011-3565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.WHO WHO International Clinical Trials Registry Platform (ICTRP) 2020. https://www.who.int/ictrp/en/ Available at.
- 19.medRxiv. 2020. https://www.medrxiv.org/ Available at.
- 20.OSF. 2020. https://osf.io/ Available at.
- 21.Lab, G.C.D. Our World in Data. https://ourworldindata.org/grapher/total-cases-covid-19?time=2020-01-23..2020-04-01 Available at.
- 22.Clement C., Edwards S., Rapport F., Russell I., Hutchings H. Exploring qualitative methods reported in registered trials and their yields (EQUITY): Systematic review. Trials. 2018;19:589. doi: 10.1186/s13063-018-2983-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang Y., Islam M.S., Wang J., Li Y., Chen X. Traditional Chinese medicine in the treatment of patients infected with 2019-new coronavirus (SARS-CoV-2): a review and perspective. Int J Biol Sci. 2020;16(10):1708–1717. doi: 10.7150/ijbs.45538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gao J., Tian Z., Yang X. Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci Trends. 2020;14(1):72–73. doi: 10.5582/bst.2020.01047. [DOI] [PubMed] [Google Scholar]
- 25.Gautret P., Lagier J.C., Parola P., Hoang V.T., Meddeb L., Doudier B. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020:105949. doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 26.Liu J., Cao R., Xu M., Wang X., Zhang H., Hu H. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov. 2020;6(1):16. doi: 10.1038/s41421-020-0156-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Infection, M Coronavirus: countries where hydroxychloroquine is recommended. 2020. https://www.mediterranee-infection.com/coronavirus-pays-ou-lhydroxychloroquine-est-recommandee/ Available at.
- 28.Administration, U.S. FDA . U.S. Food and Drug Administration; Maryland, United States: 2020. Coronavirus (COVID-19) Update: Daily Roundup March 30, 2020. [Google Scholar]
- 29.Chen Z., Hu J., Zhang Z., Han S., Yan D., Zhuang R. medRxiv; Cold Spring Harbor, NY, United States: 2020. Efficacy of hydroxychloroquine in patients with COVID-19: results of a randomized clinical trial. 2020.03.22.20040758. [Google Scholar]
- 30.Huang M., Li M., Xiao F., Liang J., Pang P., Tang T. medRxiv; Cold Spring Harbor, NY, United States: 2020. Preliminary evidence from a multicenter prospective observational study of the safety and efficacy of chloroquine for the treatment of COVID-19. 2020.04.26.20081059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen Jun L.D., Liu L., Liu P., Xu Q., Xia L., Ling Y. A pilot study of hydroxychloroquine in treatment of patients with common coronavirus disease-19 (COVID-19) J Zhejiang Univ. 2020;49(1):215–219. doi: 10.3785/j.issn.1008-9292.2020.03.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tang W., Cao Z., Han M., Wang Z., Chen J., Sun W. medRxiv; Cold Spring Harbor, NY, United States: 2020. Hydroxychloroquine in patients mainly with mild to moderate COVID-19: an open-label, randomized, controlled trial. 2020.04.10.20060558. [Google Scholar]
- 33.Mahevas M., Tran V.-T., Roumier M., Chabrol A., Paule R., Guillaud C. medRxiv; Cold Spring Harbor, NY, United States: 2020. No evidence of clinical efficacy of hydroxychloroquine in patients hospitalized for COVID-19 infection with oxygen requirement: results of a study using routinely collected data to emulate a target trial. 2020.04.10.20060699. [Google Scholar]
- 34.Gbinigie K., Frie K. Should chloroquine and hydroxychloroquine be used to treat COVID-19? A rapid review. BJGP Open. 2020;4(2):1–7. doi: 10.3399/bjgpopen20X101069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rismanbaf A., Zarei S. Liver and kidney injuries in COVID-19 and their effects on drug therapy; a letter to editor. Arch Acad Emerg Med. 2020;8(1):e17. [PMC free article] [PubMed] [Google Scholar]
- 36.Blaising J., Polyak S.J., Pécheur E.I. Arbidol as a broad-spectrum antiviral: an update. Antivir Res. 2014;107:84–94. doi: 10.1016/j.antiviral.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Esposito S., Principi N. Oseltamivir for influenza infection in children: risks and benefits. Expert Rev Respir Med. 2016;10(1):79–87. doi: 10.1586/17476348.2016.1126182. [DOI] [PubMed] [Google Scholar]
- 38.Beigel J.H., Tomashek K.M., Dodd L.E., Mehta A.K., Zingman B.S., Kalil A.C. Remdesivir for the treatment of Covid-19 — preliminary report. N Engl J Med. 2020 doi: 10.1056/NEJMc2022236. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 39.Wu J., McCaffrey S. Emory helps lead research on drug to treat COVID-19 patients. 2020. https://news.emory.edu/stories/2020/04/coronavirus_emory_helps_lead_research_on_remdesivir/index.html Available at.
- 40.Goldman J.D., Lye D.C.B., Hui D.S., Marks K.M., Bruno R., Montejano R. Remdesivir for 5 or 10 Days in patients with severe Covid-19. N Engl J Med. 2020 doi: 10.1056/NEJMoa2015301. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Prem K., Liu Y., Russell T.W., Kucharski A.J., Eggo R.M., Davies N. The effect of control strategies to reduce social mixing on outcomes of the COVID-19 epidemic in Wuhan, China: a modelling study. Lancet Public Health. 2020;5:e261–e270. doi: 10.1016/S2468-2667(20)30073-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oyston P., Robinson K. The current challenges for vaccine development. J Med Microbiol. 2012;61(Pt 7):889–894. doi: 10.1099/jmm.0.039180-0. [DOI] [PubMed] [Google Scholar]
- 43.Lurie N., Saville M., Hatchett R., Halton J. Developing Covid-19 vaccines at pandemic speed. N Engl J Med. 2020;382:1969–1973. doi: 10.1056/NEJMp2005630. [DOI] [PubMed] [Google Scholar]
- 44.Zhu F.-C., Li Y.-H., Guan X.-H., Hou L.-H., Wang W.-J., Li J.-X. Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: a dose-escalation, open-label, non-randomised, first-in-human trial. Lancet. 2020;395:1845–1854. doi: 10.1016/S0140-6736(20)31208-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McLeod C., Norman R., Litton E., Saville B.R., Webb S., Snelling T.L. Choosing primary endpoints for clinical trials of health care interventions. Contemp Clin Trials Commun. 2019;16:100486. doi: 10.1016/j.conctc.2019.100486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ciani O., Buyse M., Drummond M., Rasi G., Saad E.D., Taylor R.S. Time to review the role of surrogate end points in health policy: state of the art and the way Forward. Value Health. 2017;20(3):487–495. doi: 10.1016/j.jval.2016.10.011. [DOI] [PubMed] [Google Scholar]
- 47.Eisen M.B., Akhmanova A., Behrens T.E., Weigel D. Publishing in the time of COVID-19. Elife. 2020;9:1–3. doi: 10.7554/eLife.57162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Elliott J.H., Synnot A., Turner T., Simmonds M., Akl E.A., McDonald S. Living systematic review: 1. Introduction-the why, what, when, and how. J Clin Epidemiol. 2017;91:23–30. doi: 10.1016/j.jclinepi.2017.08.010. [DOI] [PubMed] [Google Scholar]
- 49.Akl E.A., Meerpohl J.J., Elliott J.J., Kahale L.A., Schünemann H.J. Living systematic reviews: 4. Living guideline recommendations. J Clin Epidemiol. 2017;91:47–53. doi: 10.1016/j.jclinepi.2017.08.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.