Abstract
The aim of the present study was to investigate the evolution of the volatile compounds of aerobically stored sterile pork meat as a consequence of the metabolic activities of inoculated specific spoilage microorganisms. Thus, Pseudomonas fragi, Pseudomonas putida, Lactobacillus sakei and Leuconostoc mesenteroides were inoculated in monocultures, dual cultures and a cocktail culture of all strains on sterile pork meat stored aerobically at 4 and 10 °C. Microbiological and sensory analyses, as well as pH measurements, were performed, along with headspace solid-phase microextraction gas chromatography/mass spectroscopy (headspace SPME–GC/MS) analysis. Data analytics were used to correlate the volatile compounds with the spoilage potential of each stain using multivariate data analysis. The results for the sensory discrimination showed that the volatiles that dominated in spoiled samples consisted mostly of alcohols, ketones and two esters (butyl acetate and ethyl acetate), while at fresh samples, dimethyl sulfide, furans, acetoin and ethyl lactate were detected. On the other hand, 2-butanone, diacetyl and acetaldehyde were among the volatile compounds that were mainly correlated with the inoculated meat during storage. In addition, P. fragi was positively correlated with a higher number of volatiles compared to the other strains, strengthening the hypothesis that volatile compound production is strain-dependent.
Keywords: aerobic storage, sterile pork meat, specific spoilage organisms, solid-phase microextraction, gas chromatography-mass spectrometry, data analytics, metabolomics
1. Introduction
It is well-established that pseudomonads, lactic acid bacteria (LAB), Enterobacteriaceae, Brochothrix thermosphacta and clostridia are the main contributors to meat spoilage, depending on storage conditions [1,2,3,4,5]. Species of the genus pseudomonas are recognized more often as causative agents of spoilage on fresh foods of animal origin stored under aerobic conditions [6]. Pseudomonas fluorescens, along with the psychrotrophs P. fragi, P. ludensis and P. putida, are involved in the spoilage of milk, meat and fish at low storage temperatures, due to their ability to overcome the other groups of microbial consortium by converting glucose to gluconate, a substrate that can be metabolized almost exclusively by this group [7]. On the other hand, LAB and B. thermosphacta have been found to contribute in meat spoilage stored either under aerobic, modified atmosphere packaging (MAP) or vacuum packaging (VP). In this case, the most frequently isolated species are Lactobacillus spp., Carnobacterium spp. and Leuconostoc spp. [1,8]. In addition, Lactobacillus sakei, along with Lactobacillus curvatus, are considered as the predominant Lactobacillus species found on meat [9]. Furthermore, Lb. sakei and Leuconostoc spp. have been associated with spoilt meat stored in VP or MAP at chill temperatures [8]. In both cases, the production of metabolites depends on the dominant small fraction of the initial meat microbiota known as “specific spoilage organisms—SSO” or “ephemeral spoilage organisms—ESO” ascribing the succession of the consortia during storage [3,8,10,11]. These compounds can affect the type (e.g., saccharolytic or proteolytic) and rate of spoilage and, moreover, seem to be the principal precursors of those microbial metabolites that we perceive as spoilage [10]. Indeed, during meat spoilage, a combination of microbial and chemical activity occurs, and by-products are produced, which may be indicative for the rate and the type of spoilage [10,12]. Spoilage rate and type depends on the concentration of substrates on meat (glucose, lactic acid, nitrogenous compounds and free amino acids), which are used as principal precursors of the microbial metabolites associated with spoilage [12]. The aforementioned substrates are consumed by various microbial species, and the derived volatile fraction of the microbial metabolites consists of carbonyl compounds, alcohols, volatile fatty acids, organic acids, sulfur compounds, esters, terpenoids or other molecules that determine the spoilage of meat [4,12]. However, limited studies have been conducted to determine which bacterial species or strains are capable of producing by-products responsible for meat spoilage [4,10,13,14,15,16]. At present, it is important to study the spoilage potential at the strain level, since previous studies showed distinctions among species [13,14,17].
In the current study, sterile pork meat was stored at two different temperatures (4 and 10 °C) in aerobic conditions, after inoculation with spoilage-specific microorganisms (Pseudomonas fragi, P. putida, Lactobacillus sakei and Leuconostoc mesenteroides) in monocultures, dual cultures and a cocktail culture of the four strains. In this context, the current study was designed to monitor the different types of meat spoilage and identify the microbial metabolites produced during the metabolic activity of the strains. The overall objective of the study was the correlation of meat-volatile compounds produced by each strain with the spoilage potential of each strain. For this reason, a headspace solid-phase microextraction gas chromatography/mass spectroscopy (HS-SPME GC/MS) analysis was used for the determination of volatiles, and subsequently, a multivariate analysis (discriminant function analysis—DFA, hierarchical cluster analysis and partial least squares-discriminant analysis—PLS-DA) was performed in order to correlate them with the spoilage profile of mono, dual and mixed cultures of the selected four strains during storage at 4 and 10 °C.
2. Materials and Methods
2.1. Experimental Design and Preparation of Sterile Meat Samples
Fresh pork meat (pH 5.6–5.8) of different batches (originated from different farms) was obtained from the central meat market in Athens, Greece and transported under refrigeration to the laboratory with minimal delay (30–60 min). The raw pork meat used in the current study derived from 4 young male domestic pigs (Sus scrofa f. domesticus). Meat was transported to the laboratory 48 h after slaughter. In detail, 4 different batches of meat were studied, each derived from a different young male domestic pig weighing around 60–70 kg. To reduce the microbial load of the meat, the surface of the pork meat was sprayed with absolute ethanol and ignited with a gas burner. Consequently, the burnt surface tissue of the meat was removed aseptically with the aid of a sterile scalpel in a laminar flow cabinet, and the sterile tissue below was excised and cut into pieces of 45–50 g each, as described elsewhere [18]. Subsequently, the meat was inoculated with different strains of specific spoilage microorganisms, as described in Section 2.2. After inoculation, meat samples were packaged in Styrofoam trays and wrapped manually with air-permeable polyethylene plastic film, ensuring that there was no direct contact of the plastic film with the surface of the meat. All samples were stored at 4 and 10 °C in high-precision (±0.5 °C) incubation chambers (MIR-153, Sanyo Electric Co., Osaka, Japan) for up to 187 h depending on storage temperature, until spoilage was very pronounced (intense discoloration and presence of off-odors). Sampling was performed every 8 h and 12 h for meat stored at 4 °C and 10 °C, respectively. Each sample was divided into two portions of 25 g. One portion was used for microbiological analysis and the other one for GC/MS analysis. At each time point, two packages were withdrawn randomly and analyzed for each storage condition to undergo microbiological and GC/MS analysis, and two packages were employed in sensory evaluation. Overall, 480 samples were analyzed for microbiological, chemical analysis and sensory assessment.
2.2. Preparation of Inoculum and Inoculation of the Sterile Pork Meat
The microorganisms used for the inoculation of sterile meat were: Pseudomonas fragi (CF43, isolated from beef fillet during storage at 0 °C, accession number HQ014889), Pseudomonas putida (CF7, isolated from beef fillet during storage at 20 °C, accession number HQ014881), Lactobacillus sakei (MF41, isolated from beef fillet during storage at 0 °C, accession number HQ014896) and Leuconostoc mesenteroides (MF5, isolated from beef fillet during storage at 20 °C, accession number HQ014894). All the strains were activated from a stock culture stored at −80 °C and were subcultured in 10-mL brain heart infusion broth (BHI; LAB049, LabM, Lancashire, UK) for Pseudomonas spp. and De Man-Rogosa-Sharpe (MRS) broth (094, LabM) for lactic acid bacteria (LAB) incubated at 25 and 30 °C for 24 h, respectively. The cells were harvested by centrifugation (5000× g, 10 min, 4 °C), washed twice with 10-mL quarter-strength Ringer’s solution (Ringer’s solution tablets, 96724-100TAB, Merck, Darmstadt, Germany) and resuspended in 10 mL of the same medium. The concentration of the individual cultures was approximately 8.5–9.0 log CFU/mL, as assessed by pour-plating on MRS agar (MRS ISO; 4017282, Biolife, Milano, Italy) for LAB and spread-plating on Pseudomonas agar base (PAB; CM559 supplemented with Centrimide- Fucidin- Cefalosporin selective supplement CFC SR0103, Oxoid, Basingstoke, UK) for Pseudomonas spp. All strains were then serially diluted with Ringer’s solution to give a final concentration of approximately 2 log CFU/g in the surface of the pork fillet. For the preparation of the dual cultures and the cocktail culture of the 4 strains, the monocultures were mixed in equal volumes. Pork fillets were inoculated with monocultures, dual cultures and a cocktail culture of 4 strains (Table S1). Sterile uninoculated meat samples were also prepared and used as control.
2.3. Microbiological Analyses
Microbiological analyses were carried out until the end of storage at 4 and 10 °C. To estimate the number of viable cells, 25 g of pork meat were aseptically added to 50-mL sterile one-quarter strength Ringer’s solution (to reduce the detection limit of the enumeration method to 1.48 log CFU/g) and homogenized in a stomacher device (Stomacher 400 circulator, Seward Limited, Norfolk, UK) for 60 s at room temperature. The resulting suspensions were serially diluted in the same diluent and 1 or 0.1-mL samples were poured or spread in triplicate on the following agar media: plate count agar (PCA; 4021452, Biolife) for the enumeration of total viable counts (TVC), incubated at 30 °C for 48–72 h; Pseudomonas agar base with CFC-selective supplement (Oxoid) for the enumeration of Pseudomonas spp., incubated at 25 °C for 48–72 h, and MRS agar (Biolife) overlaid with the same medium for the enumeration of LAB, incubated at 30 °C for 48–72 h. In addition, from the resulting suspensions, 1 mL of the first decimal dilution was equally spread on 3 agar plates of each substrate (for spread plating) to ensure that the microbiota was lower than 0.48 log CFU/g during storage of the sterile meat.
In addition to the microbiological analysis, the pH values of the pork meat were also recorded with a digital pH meter (Metrohm pH Lab, Herisau, Switzerland) by immersing the glass electrode in the meat homogenate (stomacher homogenate) after the end of the microbiological analysis.
2.4. Sensory Analysis
The sensory assessment of the pork samples was performed throughout storage as reported elsewhere [19] by a seven-member trained staff from the laboratory, at the same time intervals as for microbiological analyses. In brief, the sensory panel evaluated color, smell and taste (after cooking) of the meat using a three-class evaluation scheme. The first class (fresh) corresponded to acceptable meat quality and the absence of off-flavors (scores 1–2), the second class (semi-fresh) corresponded to the presence of slight off-flavors but not spoiled (still acceptable quality) (scores 3–5) and the third class (spoiled) corresponded to clearly off-flavor development (unacceptable quality) (scores 5–10). Scores ≥5 indicated the end of shelf-life. Semi-fresh was the first indication of meat spoilage (incipient spoilage, i.e., less vivid red color, odor and flavor slightly changed, but still acceptable by the consumer) in which the sample was marginally accepted.
2.5. Determination of Volatile Compounds by Headspace SPME-GC/MS
Headspace SPME–GC/MS analysis was used for the analysis of the volatile compounds of pork meat, as reported elsewhere [20], with slight modifications. In brief, 2.5 g of pork fillet, 5 mL of 25% (w/v) NaCl solution and 10 μL of internal standard (4-methyl-1-pentanol, in vial concentration 1000 μg/L) were homogenized with a glass rod for 2 min into a 20-mL glass vial. The vial was hermetically closed with a Mininert valve (Sigma Aldrich, St. Louis, MO, USA), and the content was stirred at 40 °C for 15 min. Subsequently, the SPME fiber - Divinylbenzene/Carboxen/Polydimethylsiloxane (DVB/CAR/PDMS—50/30 μm, needle length 1 cm, needle size 24 ga; Sigma Aldrich) was inserted to the glass vial and exposed to the headspace for 30 min under the same conditions. Desorption of the volatiles took place in the injector of the GC/MS for 5 min. Before each analysis, the fiber was exposed to the injection port of another GC (270 °C) for 10 min to remove any volatile contaminants. GC/MS analyses were performed on an Agilent 7890A gas chromatograph coupled to an Agilent 5973C mass spectrometer (Agilent Technologies, Santa Clara, CA, USA). The injection port was equipped with a liner (0.75-mm i.d.) suitable for SPME analysis. It was operated in split mode (split ratio 2:1) at 250 °C. The gas chromatograph was equipped with an HP-5MS column (30 m, 0.25-mm i.d., 0.25-μm film thickness; Agilent Technologies), and the carrier gas was helium at a constant flow rate (0.93 mL/min). For the analysis of the volatile compounds, oven temperature was programmed from 40 °C (held for 5 min) to 150 °C at a rate of 4 °C/min and then ramped to 250 °C at a rate of 30 °C/min; it was held there for 5 min. The temperature of the interface, MS source and quadrupole were set at 280, 230 and 150 °C, respectively. The mass spectrometer was operated in electron ionization mode with the electron energy set at 70 eV and mass scan range 29-350 Da (scan rate: 4.37 scans/s, gain factor: 1, resulting EM voltage: 1188 V).
The compounds were identified by comparing: (i) the retention indices (RI) based on a homologous series of even-numbered n-alkanes (C8–C24; Niles, IL, USA) with those of standard compounds and by comparison with the literature data and (ii) MS data with those of the reference compounds and by MS data obtained from the National Institute of Standards and Technology (NIST) library (NIST/EPA/NIH Mass Spectral Library with Search Program, data version NIST 05, software version 2.0d) and Wiley Registry of Mass Spectral Data 7th edition. Amdis software (version 2.62, http://chemdata.nist.gov/dokuwiki/doku.php?id=chemdata:amdis) was used for the deconvolution of mass spectra and identification of target components.
The volatile compounds were semi-quantified by dividing the peak areas of the compounds of interest by the peak area of the internal standard (IS) and multiplying this ratio by the concentration of the IS (expressed as μg/L). The peak areas were measured by selecting single ions.
In total, 208 samples were analyzed for both temperatures. The samples for the volatile compound analysis were selected in order to cover a wide range of samples representative for the inoculated meat with the specific spoilage microorganism cases and the sterile (control) pork meat for both storage conditions, according to the microbial counts and the sensory scores.
2.6. Estimation of Growth Kinetic Parameters and Data Analysis
The primary model of Baranyi and Roberts [21] was applied to the growth data from plate counts (log transformed) using the online program DMFit (available at www.combase.cc) to determine the kinetic parameters of microbial growth, namely the maximum specific growth rate (μmax) and the lag phase duration (λ).
Data mining and interpretation were performed using multivariate statistical methods. The dataset was divided in groups depending on (i) the different strains used for the inoculation of sterile pork meat and ii) the different sensory classes (F, SF and S). The applied data matrix contained the volatile compounds (X variables) and the meat samples (Y variables). Data were transformed by scaling (autoscale) as a column-wise normalization step in order to make each variable comparable to each other [22]. The formed data matrix was uploaded in the online platform “MetaboAnalyst v3.0” (McGill University-Xia Lab, Montreal, QC, Canada), which is a web-based metabolomic data-processing tool (www.metaboanalyst.ca) [23]. Data analytics (partial least squares-discriminant analysis (PLS-DA)), hierarchical cluster analysis (dendrogram and heatmaps) and univariate analysis (one-way analysis of variance (one-way ANOVA) and t-test) were performed in the applied datasets.
Additionally, discriminant function analysis (DFA) was applied using the XLStat software ver. 2006.06 (Addinsoft, Paris, France), a built-in statistical software package of Excel. DFA is a supervised classification method, in which the decision boundary between different classes is calculated so that the variance between the classes is maximized and the variance within the individual classes is minimized [24]. DFA was used to explore the relationship between variables (volatile compounds) and sterile and inoculated pork samples during storage and discriminate the meat samples in the predefined classes. Data were randomly diversified into train (65 samples) and test data (36 samples). The classification accuracy was determined by the number of classified samples in each class divided by the total number of samples in the class.
3. Results and Discussion
3.1. Development of Microbial Association and Sensory Analysis
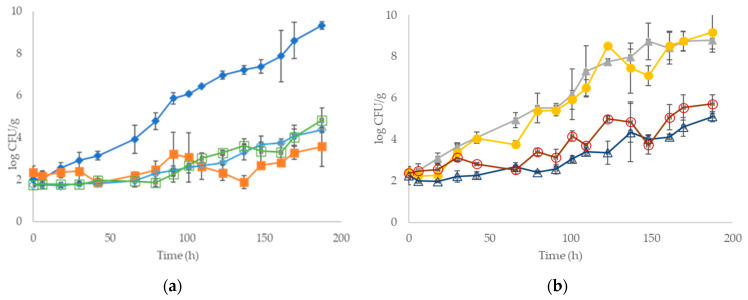
The population dynamics of the inoculated pork fillet samples with different strains during storage at 4 and 10 °C is presented in Figure 1. The microbiota after the inoculation of sterile pork fillets was approximately 2 log CFU/g and consisted of monocultures, dual cultures and a cocktail culture of the four strains of P. fragi, P. putida, Lb. sakei and Ln. mesenteroides, depending on each case (Table S1). According to the results, the population of all microorganisms in the pork samples increased during storage at both storage temperatures.
Figure 1.
Population dynamics of the inoculated strains during the storage of pork fillets at 4 °C (a) and (b) and at 10 °C (c) and (d). (♦): Pseudomonas fragi, (■): P. putida, (▲): co-culture of P. fragi and P. putida, (●): population of P. fragi and P. putida in the cocktail culture of the four strains, (◊): Lactobacillus sakei, (□) Leuconostoc mesenteroides, (∆): co-culture of Lb. sakei and Ln. mesenteroides and (◯): population of Lb. sakei and Ln. mesenteroides in the cocktail culture of the 4 strains. Datapoints represent mean values ± standard deviation of two replicated samples.
In detail, regarding the pork samples inoculated with monocultures of the strains, results showed that at 4 °C P. fragi reached the highest population level at the end of storage (9.33 ± 0.18 log CFU/g), while the lag phase of this strain was the shortest compared to the other monocultures (Table 1). Ln. mesenteroides followed with a final population level of approximately 4.82 ± 0.59 log CFU/g, while the population levels of P. putida and Lb. sakei were found lower and with similar counts (3.56 ± 0.93 and 4.37 ± 0.10 log CFU/g, respectively). Accordingly, for the inoculated meat samples with dual and cocktail cultures at the same temperature, the population of P. fragi and P. putida was ≥ 8.79 log CFU/g in all cases (8.79 ± 0.42 and 9.13 ± 0.97 log CFU/g for dual and cocktail cultures, respectively), while the LAB populations remained at lower levels (5.10 ± 0.25 and 5.72 ± 0.43 log CFU/g for the dual and cocktail culture, respectively).
Table 1.
Estimated kinetic parameters of different microbial groups in sterile pork meat stored at 4 °C.
| Inoculation | Lag Phase (h) 1 |
y0 (log10CFU/g) 2 |
yend (log10CFU/g) 3 |
μmax (h−1) 4 |
R2 |
|---|---|---|---|---|---|
| Monoculture | |||||
| Pseudomonas fragi (F) | 9.31 ± 10.04 | 1.95 ± 0.22 | 10.51 ± 2.38 | 0.04 ± 0.01 | 0.99 |
| Pseudomonas putida (P) | 148.23 ± 15.43 | 2.26 ± 0.21 | - 5 | 0.01 ± 0.01 | - |
| Leuconostoc mesenteroides (M) | 30.98 ± 14.01 | 1.75 ± 0.20 | - | 0.02 ± 0.01 | 0.86 |
| Lactobacillus sakei (S) | 50.67 ± 16.45 | 30.98 ± 14.01 | - | 0.02 ± 0.00 | 0.96 |
| Dual Culture | |||||
| F + P (CF) | 7.39 ± 18.55 | 2.26 ± 0.32 | 8.86 ± 0.28 | 0.05 ± 0.01 | 0.98 |
| M + S (MF) | 50.67 ± 16.45 | 2.03 ± 0.19 | - | 0.02 ± 0.00 | 0.91 |
| Cocktail | |||||
| CF (cocktail) | 68.31 ± 17.44 | 2.07 ± 0.55 | 9.00 ± 0.57 | 0.04 ± 0.01 | 0.94 |
| MF (cocktail) | 17.80 ± 33.98 | 2.19 ± 0.42 | 6.82 ± 10.46 | 0.02 ± 0.01 | 0.84 |
1: lag phase, 2y0: initial microbial population, 3yend: final microbial population, 4μmax: maximum specific growth rate and 5: not determined. CF: dual cultures of P. fragi and P. putida, MF: dual cultures of Ln. mesenteroides and Lb. sakei. The description of coded treatments is referred to in Table S1.
In storage at 10 °C, all samples exhibited similar or higher population levels compared to 4 °C (Figure 1c,d). Specifically, P. fragi and P. putida exceeded 9.0 log CFU/g in all cases, whereas, for the samples inoculated with the monoculture of Ln. mesenteroides, dual and cocktail cultures of LAB, population levels exceeded 8.0 log CFU/g (8.06 ± 0.05, 8.37 ± 0.40 and 8.27 ± 0.40 log CFU/g, respectively) (Figure 1c,d). On the contrary, the inoculated meat with the monoculture of Lb. sakei displayed the lowest population (6.50 log CFU/g) during storage at 10 °C.
The changes in the population of these groups and their contribution to the final microbiota were influenced by storage temperature, as illustrated by the population dynamics on the specific growth media. Growth kinetic parameters, such as lag phase duration (λ) and maximum specific growth rate (μmax) of the different inoculation treatments, are presented in Table 1; Table 2. In general, during cold storage, the growth rate of P. putida was lower compared to P. fragi (Table 1), which could be attributed to the fact that the former strain had been previously isolated from beef during storage at 20 °C, whereas the latter (P. fragi) was isolated from beef at refrigerated storage (0 °C). The same holds for Ln. mesenteroides, which was isolated from beef during storage at 20 °C. However, despite the fact that Lb. sakei was also isolated from beef during storage at 0 °C and its potential psychrotrophic nature [8], it was not able to grow well at 4 °C as a monoculture.
Table 2.
Estimated kinetic parameters of different microbial groups in sterile pork meat stored at 10 °C.
| Inoculation | Lag Phase (h) 1 |
y0 (log10CFU/g) 2 |
yend (log10CFU/g) 3 |
μmax (h−1) 4 |
R2 |
|---|---|---|---|---|---|
| Monoculture | |||||
| P. fragi (F) | 1.24 ± 3.01 | 1.99 ± 0.14 | 10.18 ± 0.17 | 0.09 ± 0.00 | 1.00 |
| P. putida (P) | 13.87 ± 9.63 | 2.80 ± 0.39 | 9.34 ± 0.54 | 0.07 ± 0.01 | 0.95 |
| Ln. mesenteroides (M) | 8.82 ± 7.92 | 1.86 ± 0.33 | 7.93 ± 0.37 | 0.08 ± 0.01 | 0.96 |
| Lb. sakei (S) | 24.72 ± 6.32 | 2.02 ± 0.19 | 7.49 ± 0.31 | 0.06 ± 0.01 | 0.97 |
| Dual Culture | |||||
| F + P (CF) | 2.22 ± 3.3 | 2.35 ± 0.15 | 9.52 ± 0.14 | 0.08 ± 0.00 | 0.99 |
| M + S (MF) | 26.77 ± 6.17 | 2.36 ± 0.26 | 7.91 ± 0.31 | 0.10 ± 0.01 | 0.95 |
| Cocktail | |||||
| CF (cocktail) | 17.80± 5.09 | 1.73± 0.23 | 9.27± 0.29 | 0.09± 0.01 | 0.99 |
| MF (cocktail) | 10.31± 6.24 | 2.04± 0.22 | 8.28± 0.28 | 0.06± 0.01 | 0.98 |
1: lag phase, 2y0: initial microbial population, 3yend: final microbial population, and 4μmax: maximum specific growth rate. CF: dual cultures of P. fragi and P. putida, MF: dual cultures of Ln. mesenteroides and Lb. sakei. The description of coded treatments is referred to in Table S1.
Prolonged lag phases were evident for inoculated meat with LAB, as well as for inoculated meat with a monoculture of P. putida at 4 °C, the duration of which was greatly reduced at the higher storage temperature in all cases (Table 1; Table 2). Additionally, a progressive increase of the maximum specific growth rate (μmax) was observed for all inoculated strains with increasing storage temperature. In general, aerobic storage at 10 °C accelerated spoilage of the samples inoculated with dual culture of P. fragi and P. putida and the cocktail culture of the four strains, due to the fast growth of P. fragi and P. putida that became the dominant species. Finally, it has to be noted that no microorganisms were found during storage of the sterile meat samples at both temperature conditions.
Ercolini et al. [13] used three bacterial strains, namely P. fragi, Serratia proteamaculans and Carnobacterium maltaromaticum, as single or mixed cultures to inoculate sterile beef packaged under vacuum and stored at 7 °C for a month. It was reported that the populations of the inoculated samples increased during storage for all strains used compared with uninoculated meat, where lower counts were found. In detail, Carnobacterium maltaromaticum showed the highest increase when it was inoculated as a single culture, whereas P. fragi displayed the highest increase in the inoculated meat when applied in a mixed culture. In a similar study [14], beef meat was inoculated with 18 strains of P. fragi as a monoculture (4.0 log CFU/g initial inoculum level) and stored at 4 °C for seven days. After one week of storage, the load of the inoculated strains increased to 9.0 log CFU/g, indicating that P. fragi had a significant role in the microbial ecology of meat, and thus, meat could be considered an ecological niche for this species.
The pH values of fresh pork were found within the normal range of fresh pork (pH 5.8 ± 0.4). During the aerobic storage of meat, the pH values fluctuated (prior to spoilage) within the normal pH range of fresh meat. When meat was characterized as spoilt, pH increased or decreased depending on the inoculation treatment. More specifically, pH increased in all spoiled samples inoculated with P. fragi and P. putida (mono cultures, dual cultures and in mixed strain cultures) (p < 0.05), where the highest increase was observed in the case of meat inoculated with a monoculture of P. fragi at both temperatures (4 and 10 °C), reaching values of 6.5–6.8. However, for samples inoculated with LAB (mono and dual cultures), a slight pH decrease was evident in spoiled meat, depending on storage temperature. At 10 °C, pH of spoiled meat was found slightly lower (pH 5.6 ± 0.3) in contrast to cold storage, where the pH values were approximately 5.7 ± 0.2. Those observations are in-line with previous works in aerobic stored meat when Pseudomonas spp. become the dominant microbiota, and it is attributed to the by-products produced, which can increase the pH values of the meat [10,20]. On the other hand, LAB are able to produce lactic acid during the spoilage of meat and, thus, to lower the pH of the meat [10].
According to the sensory analysis (Table S2), the shelf life of pork fillets differed depending on storage temperature and the specific microorganism used for inoculation. More specifically, the sensory panel considered a sample as spoiled in a shorter time when inoculated with P. fragi than with P. putida. In detail, meat inoculated with P. fragi or the mixed species culture were characterized as semi-fresh after 54-h storage at 4 °C, while for P. putida and the dual culture of P. fragi and P. putida, the corresponding time was 68 h at the same storage temperature. The time of sensory rejection (meat characterized as spoiled) was 116 h when the meat was inoculated with P. fragi, and the population level was 7.09 log CFU/g, while for the dual culture of P. fragi and P. putida or the mixed species cocktail, the corresponding time was 106 h, and the final population was 6.70 log CFU/g. In this case, the odor of the meat was intense, and slime had formed on the surface of the fillets. This coincides with the glucose depletion, where a number of genes, e.g., PP5337, Asp, purE and aruF, are overexpressed and have been correlated with spoilage [25].
In contrast for meat inoculated with LAB strains, sensory rejection was reported by the panel after 124.5 and 140 h for mono and dual LAB cultures, respectively, indicating that spoilage from LAB is milder and mostly characterized by muscle souring [10,26]. At 10 °C, the time needed for sample rejection was reduced compared to 4 °C, while the population of the microorganisms was higher (Table S2).
In a recent study [14], 18 strains of P. fragi were used as inocula to spoil meat during aerobic storage at 4 °C. In this work, a sensory panel assessed the different meat odors present in the samples during storage, and the results showed that the different strains affected significantly the meat odor. Thus, it was concluded that the sensory profiles of inoculated meat were strain-dependent.
3.2. Discrimination of Sterile From Inoculated Meat Using Volatile Compounds
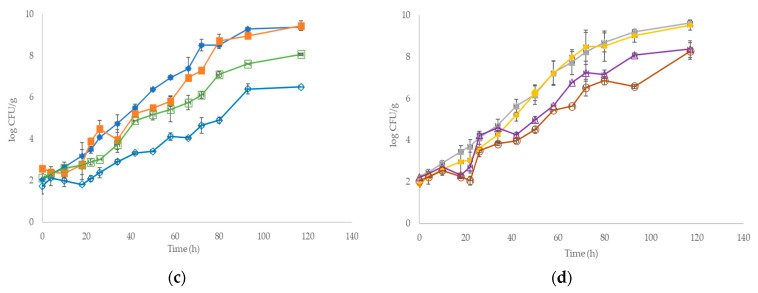
Fifty-one (51) volatile compounds were detected during the storage of pork fillets at both temperatures. The majority of them consisted of alcohols, aldehydes, ketones, esters, terpenoids and sulfur compounds. Table S3 presents the 51 volatile compounds detected for all meat cases (sterile and inoculated). Accordingly, the 51 volatile compounds were selected and used for quantitative estimation and discrimination of the meat samples based on the different inocula employed, as well as in sensory classification. Many of the selected compounds, such as 1-octen-3-ol, diacetyl, acetoin, 2-butanone and ethyl acetate, have already been identified to be involved in meat spoilage [4,12,20,27,28]. The selected data were uploaded to the online platform MetaboAnalyst v3.0 (www.metaboanalyst.ca). Significant differences between the sterile meat and each case of monocultures of inoculated meat were observed. Compounds with significant effects (p < 0.05) during aerobic storage after t-test analysis were found to be 2-butanone, acetoin, diacetyl, hexanal, 6-methyl-5-hepten-2-one, 3-methyl-1-butanol, heptanol, 2-butanol, ethanol and 3-carene (data not shown). Subsequently, PLS-DA was applied in order to extract the important features (volatile compounds) for each case of monocultures and dual cultures in relation to the sterile meat. PLS-DA analysis has some advantages, like discriminating samples between known classes and/or predicting unknown samples, but also, it can associate metabolite data with each class [29]. Regarding the results obtained from the treatment of sterile meat versus inoculated meat with P. fragi, it was shown that most of the compounds were highly correlated with the inoculated meat (Figure 2a).
Figure 2.
Important features identified by partial least squares-discriminant analysis (PLS-DA) for sterile meat and meat inoculated with: (a) P. fragi (monoculture) and (b) Ln. mesenteroides (monoculture) stored at 4 and 10 °C. The color scale on the right represents the scaled abundance of each variable, with red color boxes indicating high abundance and green color boxes indicating low abundance.
Specifically, P. fragi was positively correlated with 2-butanone, 3-carene, diacetyl and acetaldehyde, while acetoin and hexanal were correlated with sterile meat (Figure 2a). As regards Ln. mesenteroides, PLS-DA showed that the inoculated meat was mostly correlated with 2-butanone, diacetyl, alcohols and carbonyls with seven or eight carbon atoms, 2-pentyl-furan and 2-ethyl-furan, while sterile meat was positively correlated with acetoin, hexanal, 2-butanol and 3-methyl-1-butanol (Figure 2b). At the other two inoculation cases with P. putida and Lb. sakei, 2-butanone had the highest VIP score (≥2.2) for the inoculated meat (Figure S1).
According to hierarchical cluster analysis, a clear discrimination between the sterile meat and that inoculated with P. fragi and P. putida (mono and dual cultures) stored at 4 and 10 °C was shown. One major group corresponded to the inoculated meat with P. fragi and P. putida stored at 10 °C, and a second major group was divided into two subgroups: one relevant to sterile meat and another relevant to samples inoculated with P. fragi and P. putida (including all samples stored at 4 °C and two of the samples stored at 10 °C) (Figure S2).
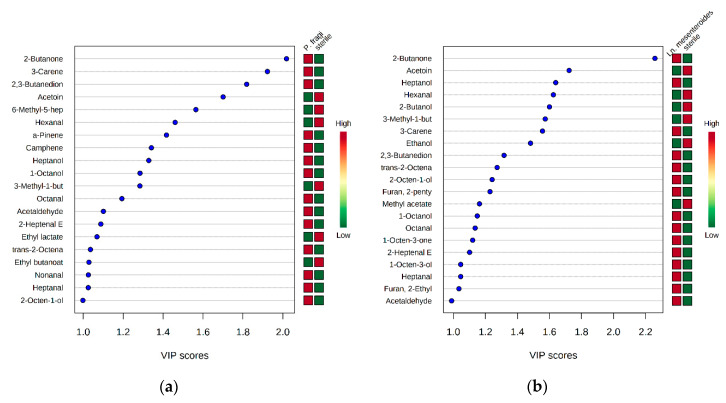
Figure 3 presents the heatmap obtained from the treatment of a combination sterile meat versus meat inoculated with all strains. It can be observed that inoculated meat at 4 °C differentiated from the other cases, and the volatiles positively correlated with that included mainly alcohols, acetaldehyde diacetyl, 2-nonanone, butyl and ethyl acetate and 2 monoterpenes.
Figure 3.
Hierarchical clustering result shown as a heatmap of volatiles associated with the group of sterile meat (green) and meat inoculated with the cocktail of all cultures (red) during storage at 4 and 10 °C. Ward-linkage clustering was based on the Euclidean correlation coefficients of the 51 volatiles identified in the different meat samples. The color scale represents the scaled abundance of each variable, with red indicating high abundance and blue indicating low abundance.
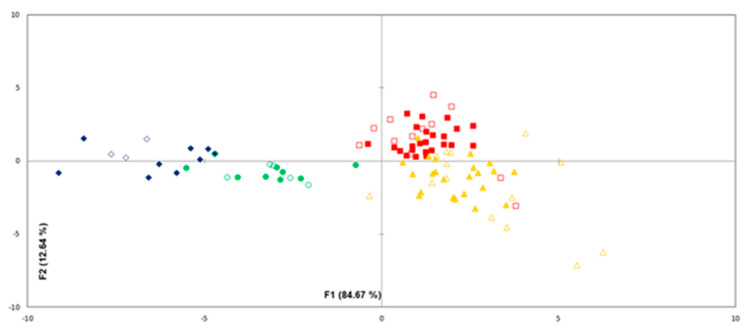
Discriminant function analysis (DFA) was used in order to discriminate meat samples based on the different inocula. As illustrated in Figure 4, when data were combined in a single dataset, sterile meat samples, cultures of P. fragi and P. putida (monocultures and dual cultures), cultures of LAB (monocultures and dual cultures) and mixed cultures (four strain cultures) constituted four separate clusters (Figure 4). However, 15% of the samples were misclassified. The correct classification rate for the validation of the DFA model was 77.0% for the LAB samples, 71.4% for the P. fragi and P. putida samples, 83.3% for the mixed cultures and 100% for the sterile meat samples, with an overall accuracy performance of 77.8%. These results revealed a satisfactory correlation between the inoculated meat samples and the sterile meat, along with the dynamic changes of the amounts of the volatile compounds. As it is evident, sterile meat and inoculated meat treatments formed separate clusters, indicating that data analytics in tandem with GC/MS could determine differences between the volatile compounds produced by the various microorganisms used in the current study.
Figure 4.
Discriminant analysis similarity map for the different microbial groups (♦): sterile meat, (▲): meat inoculated with microbial species of pseudomonads, (■): meat inoculated with microbial species of LAB and (●): meat inoculated with a cocktail culture of 4 strains stored at 4 and 10 °C, as determined by the first two discriminant functions. Empty and filled symbols indicate the validation and training samples, respectively.
According to the previous results, 2-butanone, diacetyl and acetaldehyde were among the volatile compounds that were mainly correlated with the inoculated meat. Previous studies have shown that diacetyl and acetaldehyde could derive from acetoin breakdown through an acetoin dehydrogenase enzyme system, a mechanism that works widely in bacteria, according to the review of Xiao and Xu [30]. Huang et al. [31] recognized that P. putida belongs to the acetoin-utilizing microorganisms. In detail, 2,3-butanediol is metabolized by oxidation to acetoin, and then, acetoin is metabolized to acetaldehyde and acetyl-CoA by the acetoin-cleaving system [31]. In the current study, 2,3-butanediol was not detected in every case of both sterile and inoculated meat. However, acetoin, which was detected in all samples, decreased during storage of the inoculated samples only, and this was more evident in the samples inoculated with P. fragi. Acetoin concentration was the highest in the case of sterile meat and presented a small increase in meat inoculated with P. putida and with the cocktail cultures of four strains, while it presented a decreasing trend in the inoculated meat with Ln. mesenteroides, dual species of pseudomonads and LAB at 4 °C. Regarding the inoculated meat with the monocultures of P. fragi and Lb. sakei, acetoin was found in low levels during storage at 4 °C and 10 °C. Yet, it has to be pointed out that an acetoin concentration was found in all cases of inoculated meat at very low levels in comparison with the sterile meat, where its concentration was tenfold higher. As regards diacetyl, it increased or fluctuated in the cases of inoculated meat, whereas in sterile meat, it was detected at low levels at both temperatures during storage. On the other hand, hexanal showed the highest concentration in all samples in the beginning of storage, and this could be attributed to the oxidation of oleic and arachidonic acids usually found on pork meat [13,32]. Nurjuliana et al. [33] observed that 2-butanone, acetoin and diacetyl were among the main volatiles of fresh pork meat. Ferrocino et al. [27] reported that acetoin was produced in the highest quantity under a vacuum storage of meat at 1 °C [27], while Casaburi et al. [17] observed that the aforenoted compound was one of the most frequent volatiles found in the headspace of meat samples stored under aerobic and vacuum packaged. It is well-documented that ketones derive from the oxidation of fatty acids due to chemical autoxidation or enzymatic a- and b-oxidation [13,34] or are associated with the presence of Gram-negative (i.e., Pseudomonas, Shewanella and Moraxella) or Gram-positive bacteria (Carnobacterium spp.), which form ketones from other metabolic pathways, such as alkane degradation or dehydrogenation of alcohols [4,6,13]. Casaburi et al. [12] reported that P. fragi and P. fluorescence were responsible for the production of a great number of aldehydes, such as 2-methyl-butanal, 3-methyl-butanal, hexanal, heptanal, octanal, nonanal and decanal, some of which were also identified in the present study. However, Casaburi et al. [17] and Ercolini et al. [4,14] reported that, among the carbonyl compounds, nonanal and acetoin could be used as meat-spoilage tracers, since they are often found in spoilt meat, results that are not in-line with the current study.
3.3. Correlation of Meat Sensory Classes with Volatile Compounds
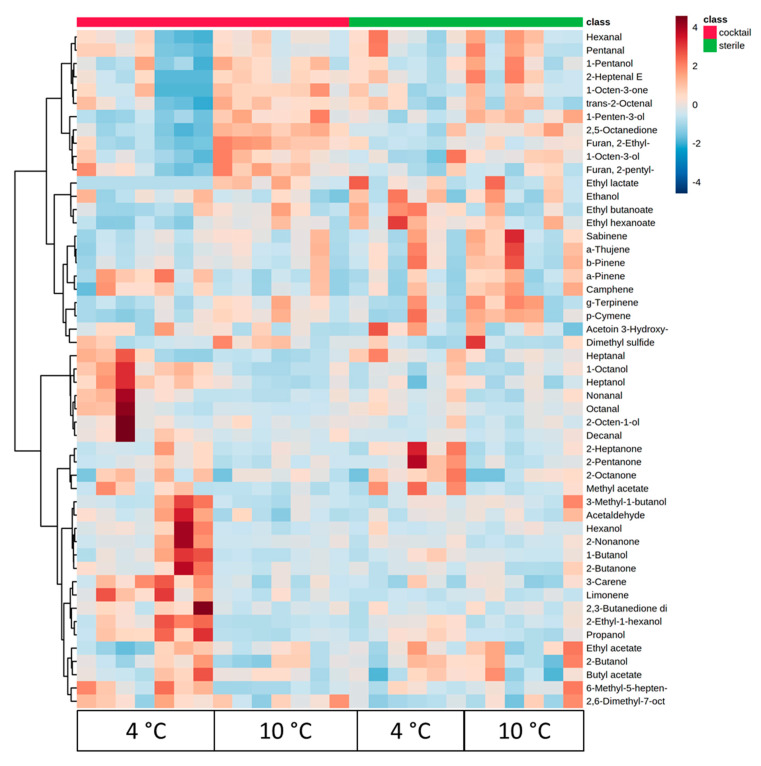
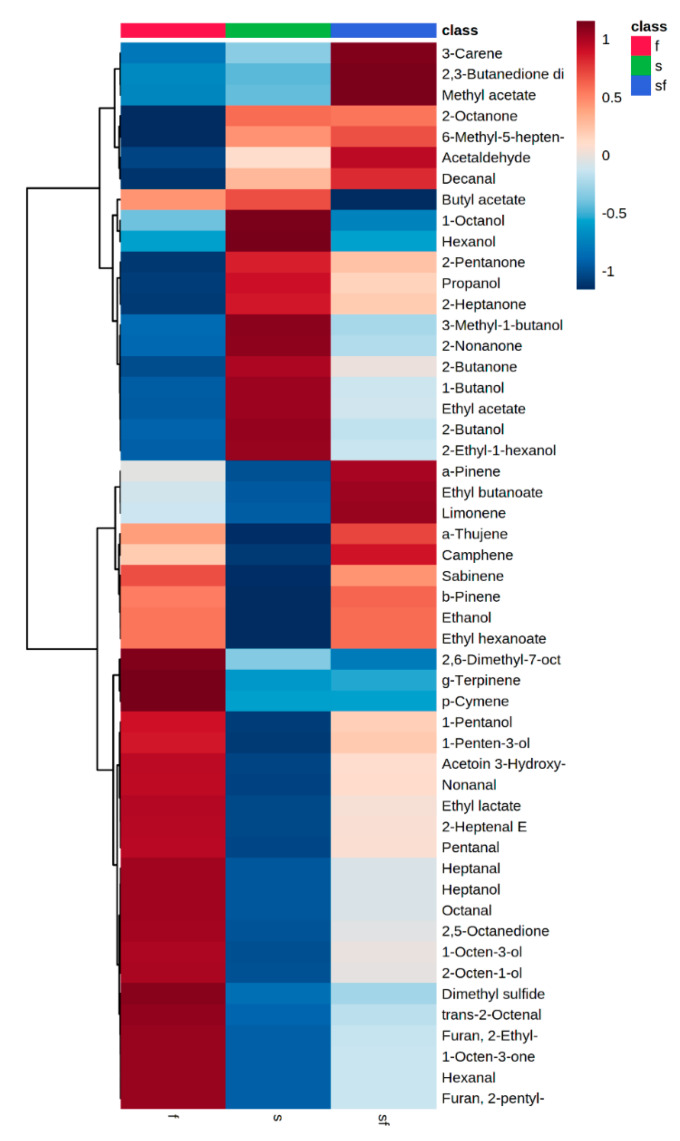
In the case of discrimination between the sensory classes, the selected data (51 volatile compounds) were uploaded to the online platform MetaboAnalyst v3.0 (www.metaboanalyst.ca). From one-way ANOVA, 20 compounds were found significant (p < 0.05) during aerobic storage (data not shown). The differences in the volatile compounds between the sensory classes of aerobically stored meat are clearly distinct in the clustering result shown as a heatmap (Figure 5).
Figure 5.
Heatmap of the volatiles clustered into 3 sensory classes of the sterile and inoculated meat samples during storage at 4 and 10 °C. Ward-linkage clustering was based on the Euclidean correlation coefficients of the 51 volatiles identified in the different sensory classes. The color scale represents the scaled abundance of each variable, with red indicating high abundance and blue indicating low abundance. Columns are colored according to the sensory class: fresh (red), semi-fresh (blue) and spoiled (green).
The volatile compounds of fresh samples were dominated by three terpenoids, dimethyl sulfide, furans, eight aldehydes, alcohols with seven or eight carbon atoms, unsaturated ketones with eight carbon atoms, acetoin and ethyl lactate. Subsequently, semi-fresh samples were correlated with seven terpenoids, ethanol, acetaldehyde, decanal, diacetyl, methyl acetate, ethyl butanoate and ethyl hexanoate. Finally, the volatiles that dominated in spoiled samples consisted mostly of alcohols, ketones and two esters (butyl acetate and ethyl acetate).
According to Figure 5, fresh samples were correlated with alcohols such as 1-pentanol, 1-penten-3-ol, 1-octen-3-ol, and 2-octen-1-ol, whereas 2-ethyl-1-hexanol was positively correlated with spoiled samples. Some of the identified alcohols of this study are commonly found in fresh meat during storage in aerobic conditions, according to a recent review [1]. Insausti et al. [32] reported that, in beef meat stored under MAP, four alcohols were detected: ethanol, 1-pentanol, 1-penten-3-ol and 1-octen-3-ol and contributed to the butter, sweet, acidic or mushroom odors [32]. In other studies, efforts have been made to correlate volatiles as spoilage indicators. In that sense, Ferrocino et al. [27] characterized two alcohols, i.e., 2-ethyl-1-hexanol and 1-octen-3-ol, as possible meat-spoilage indicators. The former compound (2-ethyl-1-hexanol) is related to resin, flowers and green odors [1], while the latter (1-octen-3-ol) is a key odorant in cheeses [17]. On the contrary, in the work of Jääskeläinen et al. [35], where meat was stored under high-oxygen MAP packaging, it was observed that 1-octen-3-ol originated from chemical reactions rather than bacterial metabolic activity. However, in the current study, the production of different alcohols seemed to be strain-dependent. Alcohol production could be attributed to many metabolic pathways, where Gram-negative bacteria such as Pseudomonas spp. and Gram-positive bacteria such as Carnobacterium spp. play a major role [12]. Glucose catabolism, proteolytic activity, amino acid metabolism, oxidation of fatty acids and methyl ketone reduction, along with the reduction of aldehydes, are the main metabolic pathways involved in the biosynthesis of volatile alcohols [10,12,25].
Among the ketones detected, 2-butanone, 2-pentanone, 2-heptanone and 2-nonanone were correlated with spoiled samples, while acetoin was correlated with the fresh samples (Figure 5). On the other hand, aldehydes such pentanal, hexanal, heptanal, octanal, trans-2-octenal and nonanal were associated mostly with fresh samples. Argyri et al. (2015) reported that, when minced beef was stored under aerobic and MAP conditions, 2-pentanone, 2-heptanone, 2-octanone, 3-octanone, acetoin and diacetyl increased at both storage conditions, while 2-butanone decreased during the storage of minced beef. Moreover, these researchers observed an association between fresh and semi-fresh minced beef samples stored under different packaging conditions with the volatile compounds 2-butanone, 2,3-pentanedione, 2,5-octanedione, pentanal, hexanal, trans-2-heptanal and trans-2-octenal, while the compounds positively correlated with the spoiled samples were found to be 2-pentanone, 2-nonanone, 2-and 3-methyl-1-butanol, ethyl hexanoate, ethyl propanoate, ethyl lactate, ethyl acetate, ethanol, 2-heptanone, 3-octanone, diacetyl and acetoin. Many of the volatile compounds reported by Argyri et al. [20] were not found in accordance with the current study; however, their study differentiated from the present work in terms of meat type, storage temperatures and packaging, factors which could affect the produced volatiles. In air-packaged beef inoculated with strains of P. fragi, the most common detected molecules were ethyl hexanoate, ethyl octanoate and ethyl decanoate, and according to the sensory analysis, fruity off-odors of meat were associated with the ester release [14]. Those results are in-line with the present study, since the detected esters were mostly associated with the semi-fresh and spoiled samples.
Saraiva et al. [36] used data analytics in tandem with GC/MS to indicate the volatile compounds as beef spoilage indicators in meat stored under MAP and vacuum-packaged at 4 °C. They demonstrated that compounds such as 2- and 3-methylbutanal, 2- and 3-methylbutanol, 1-pentanol, 1-hexanol, 2,3-octanedione, 3,5-octanedione, octanal and nonanal can be used as meat-spoilage tracers, while 3-methylpentane can be used as an indicator of the early stages of beef spoilage. Accordingly, Insausti et al. [32] discriminated meat quality in terms of storage time and observed that dimethyl sulfide was correlated with fresh meat, whereas acetone and ethanol were associated with meat stored under MAP for longer periods. Resconi et al. [37] proposed pentanoic, hexanoic and heptanoic acid, 1-hexanol, 2-undecenal, ethyl octanoate, 2-heptanone and 2-pentylfuran as shelf-life markers of raw beef in high-oxygen MAP. The concentration of esters in all the inoculated cases started to decline after 50 h of storage until the end of aerobic storage. Insausti et al. [32] reported that esters derive from the esterification of several alcohols with carboxylic acids present on bovine meat. In addition, Ercolini et al. [13] concluded that microbial esterases are responsible for the formation of ethyl esters due to the esterification of alcohols with carboxylic acids. In another study [6], Pseudomonas, Shewanella and Moraxella were found capable of producing methyl- and ethyl-esters. Finally, the monoterpenes 3-carene, a-pinene, limonene and camphene, which were mostly correlated with the inoculated cases of meat, are known to derive from bacteria [38]. It is obvious that contradictory results are found in the literature as regards the volatiles identified in meat during storage. However, the majority of the aforenoted studies dealt with meat stored under MAP and/or vacuum packages, a factor that is known to affect the microbial association on the type of the produced metabolites and the type of spoilage [10].
The results obtained in the present study demonstrated that different microbial groups and/or different strains resulted in different volatile profiles during spoilage. The latter could be considered as fingerprints containing valuable information for each case of inoculated meat. Compounds such as hexanal, 2-butanone, diacetyl and/or acetoin were found to be statistically significant in all cases examined. However, hexanal is known to be produced from the oxidation of unsaturated ω-6 fatty acids or the degradation of amino acids [13,39]. Accordingly, hexanal was found in both sterile and inoculated meat, and therefore, it cannot be used as an indicator of microbial spoilage, since the concentration of this compound depended also on nonmicrobial factors. Pseudomonas are capable of metabolizing acetoin, whereas LAB are capable of producing it [1,31,40]. However, in the present study, acetoin and diacetyl were found in low levels throughout the storage of meat inoculated with a monoculture of Lb. sakei. Furthermore, the inoculation of meat with P. fragi resulted in higher amounts of volatiles as compared to meat inoculated with P. putida. These observations strengthened the hypothesis that the volatile production is strain-dependent [16]. To sum up, the results of the present work supported the idea that microorganisms in meat perform differently when co-cultured in dual or in mixed culture species than when added as monocultures in sterile meat. It is already known that bacterial interactions play a key role in the nutrient’s contribution (which are used as principal precursors of the microbial metabolites associated to spoilage), either with synergistic/syntrophic behavior or with competitive behavior [10,12].
4. Conclusions
In the current study, it was found that different strains shared a different volatilome when meat was inoculated with monocultures, dual or mixed cultures. Among the detected volatile compounds, 2-butanone, diacetyl and acetaldehyde were the compounds mostly associated with the inoculated meat, while acetoin and hexanal were associated with the sterile meat. P. fragi exhibited the highest spoilage potential at all cases and was associated with the highest number of volatiles. In addition, Ln. mesenteroides shared a more abundant volatile profile in contrast to Lb. sakei; however, the main volatiles associated with the LAB strains were alcohols and carbonyls with seven or eight carbon atoms. Terpenoids, dimethyl sulfide and aldehydes indicated fresh meat, whereas 2-ethyl-1-hexanol and esters were more prominent in spoiled samples. However, the interaction of strains that do not contribute directly to spoilage (causing unpleasant changes) may contribute in the generation of associated spoilage compounds or interact with the produced molecules, leading to a complicated profile of sensory spoilage. The above observations could be fundamental in understanding the complexity of spoilage bacteria and their spoilage potential in terms of the produced metabolites and the possible impact on meat quality.
Acknowledgments
The authors acknowledge the Symbiosis-EU (https://cordis.europa.eu/project/id/211638) project (no 211638) financed by the European Commission under the 7th Framework Programme for RTD.
Supplementary Materials
The following are available online at https://www.mdpi.com/2304-8158/9/5/633/s1: Table S1: Coded names for sterile meat and the inoculated meat samples with the monocultures, dual and mixed cultures of the 4 examined strains at 4 and 10 °C. Table S2: Sensory analysis of pork meat in correlation to storage time and population level, stored aerobically at 4 and 10 °C. Table S3: Fifty-one volatile compounds identified in sterile and inoculated pork samples that were further used in statistical analysis and association with the discrimination process. Figure S1: Important features identintified by PLS-DA for sterile meat and meat inoculated with: (a) P. putida (monoculture) and (b) Lb. sakei (monoculture) stored at 4 and 10 °C. The color scale represents the scaled abundance of each variable, with red indicating high abundance and green indicating low abundance. Figure S2: Dendrogram obtained after Ward-linkage clustering based on the Euclidean correlation coefficients of the 51 volatiles identified for the sterile meat and meat inoculated with mono and dual cultures of pseudomonads. The description of the coded treatments is referred to in Table S1.
Author Contributions
Conceptualization, G.-J.E.N., methodology, O.S.P., N.C. and A.M.; software, O.S.P. and E.Z.P.; validation, O.S.P., V.I. and N.C.; formal analysis, V.I. and A.M.; investigation, O.S.P. and E.Z.P.; resources, C.C.T.; data curation, O.S.P., A.M. and N.C.; writing—original draft preparation, O.S.P. and A.M.; writing—review and editing, E.Z.P., G.-J.E.N., O.S.P., A.M. and C.C.T.; visualization, O.S.P. and A.M.; supervision, N.C., C.C.T. and E.Z.P.; project administration, G.-J.E.N. and funding acquisition, G.-J.E.N. and C.C.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Symbiosis-EU project no 211638 financed by the European Commission under the 7th Framework Programme for RTD. The APC was funded by the Agricultural University of Athens.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Casaburi A., Piombino P., Nychas G.-J., Villani F., Ercolini D. Bacterial populations and the volatilome associated to meat spoilage. Int. J. Food Microbiol. 2015;45:83–102. doi: 10.1016/j.fm.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 2.Doulgeraki A.I., Nychas G.-J.E. Monitoring the succession of the biota grown on a selective medium for pseudomonads during storage of minced beef with molecular-based methods. Int. J. Food Microbiol. 2013;34:62–69. doi: 10.1016/j.fm.2012.11.017. [DOI] [PubMed] [Google Scholar]
- 3.Ercolini D., Russo F., Torrieri E., Masi P., Villani F. Changes in the spoilage-related microbiota of beef during refrigerated storage under different packaging conditions. Appl. Environ. Microbiol. 2006;72:4663–4671. doi: 10.1128/AEM.00468-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ercolini D., Ferrocino I., Nasi A., Ndagijimana M., Vernocchi P., La Storia A., Laghi L., Mauriello G., Guerzoni E.M., Villani F. Monitoring of Microbial Metabolites and Bacterial Diversity in Beef Stored under Different Packaging Conditions. Appl. Environ. Microbiol. 2011;77:7372–7381. doi: 10.1128/AEM.05521-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Odeyemi O.A., Alegbeleye O.O., Strateva M., Stratev D. Understanding spoilage microbial community and spoilage mechanisms in foods of animal origin. Compr. Rev. Food Sci Food Saf. 2020;19:311–331. doi: 10.1111/1541-4337.12526. [DOI] [PubMed] [Google Scholar]
- 6.Nychas G.-J.E., Marshall D.L., Sofos J.N. In: Meat, Poultry, and Seafood. Microbiology: Fundamentals and Frontiers. Doyle M.P., Beuchat L.R., editors. ASM Press; Washington, DC, USA: 2007. pp. 105–140. [Google Scholar]
- 7.Nychas G., Dillon V., Board R. Glucose, the Key Substrate in the Microbiological Changes Occurring in Meat and Certain Meat Products. Biotechnol. Appl. Biochem. 1988;10:203–231. [PubMed] [Google Scholar]
- 8.Doulgeraki A.I., Paramithiotis S., Kagkli D.M., Nychas G.-J.E. Lactic acid bacteria population dynamics during minced beef storage under aerobic or modified atmosphere packaging conditions. Int. J. Food Microbiol. 2010;27:1028–1034. doi: 10.1016/j.fm.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 9.Freiding S., Gutsche K.A., Ehrmann M.A., Vogel R.F. Genetic screening of Lactobacillus sakei and Lactobacillus curvatus strains for their peptidolytic system and amino acid metabolism, and comparison of their volatilomes in a model system. Syst. Appl. Microbiol. 2011;34:311–320. doi: 10.1016/j.syapm.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 10.Nychas G.-J.E., Skandamis P.N., Tassou C.C., Koutsoumanis K.P. Meat spoilage during distribution. Meat Sci. 2008;78:77–89. doi: 10.1016/j.meatsci.2007.06.020. [DOI] [PubMed] [Google Scholar]
- 11.Vasilopoulos C., De Maere G., De May E., Paelink H., De Vust L., Leroy F. Technology induced of selection towards the spoilage microbiota of artisan-type cooked ham packed udner modified atmosphere. Food Microbiol. 2010;27:77–84. doi: 10.1016/j.fm.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 12.Casaburi A., De Filippis F., Villani F., Ercolini D. Activities of strains of Brochothrix thermosphacta in vitro and in meat. Food Res. Int. 2014;62:366–374. doi: 10.1016/j.foodres.2014.03.019. [DOI] [Google Scholar]
- 13.Ercolini D., Russo F., Nasi A., Ferranti P., Villani F. Mesophilic and psychrotrophic bacteria from meat and their spoilage potential in vitro and in beef. Appl. Environ. Microbiol. 2009;75:1990–2001. doi: 10.1128/AEM.02762-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ercolini D., Ferrocino I., La Storia A., Mauriello G., Gigli S., Masi P., Villani F. Development of spoilage microbiota in beef stored in nisin activated packaging. Int. J. Food Microbiol. 2010;27:137–143. doi: 10.1016/j.fm.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 15.Nieminen T.T., Vihavainen E., Paloranta A., Lehto J., Paulin L., Auvinen P., Solisma M., Björkroth K.J. Characterization of psychrotrophic bacterial communities in modified atmosphere-packed meat with terminal restriction fragment length polymorphism. Int. J. Food Microbiol. 2011;144:360–366. doi: 10.1016/j.ijfoodmicro.2010.10.018. [DOI] [PubMed] [Google Scholar]
- 16.Pothakos V., Devlieghere F., Villani F., Björkroth J., Ercolini D. Lactic acid bacteria and their controversial role in fresh meat spoilage. Meat Sci. 2015;109:66–74. doi: 10.1016/j.meatsci.2015.04.014. [DOI] [PubMed] [Google Scholar]
- 17.Casaburi A., Nasi A., Ferrocino I., Di Monaco R., Mauriello G., Villani F., Ercolini D. Spoilage-related activity of Carnobacterium maltaromaticum strains in air-stored and vacuum-packed meat. Appl. Environ. Microbiol. 2011;77:382–7393. doi: 10.1128/AEM.05304-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsigarida E., Nychas G.-J.E. Ecophysiological attributes of a Lactobacillus sp. and a Pseudomonas sp. on sterile beef fillets in relation to storage temperature and film permeability. J. Appl. Microbiol. 2001;90:696–705. doi: 10.1046/j.1365-2672.2001.01292.x. [DOI] [PubMed] [Google Scholar]
- 19.Papadopoulou O., Panagou E.Z., Mohareb F.R., Nychas G.-J.E. Sensory and microbiological quality assessment of beef fillets using a portable electronic nose in tandem with support vector machine analysis. Food Res. Int. 2013;50:241–249. doi: 10.1016/j.foodres.2012.10.020. [DOI] [Google Scholar]
- 20.Argyri A.A., Mallouchos A., Panagou E.Z., Nychas G.-J.E. The dynamics of the HS/SPME-GC/MS as a tool to assess the spoilage of beef stored under different packaging and temperature conditions. Int. J. Food Microbiol. 2015;193:51–58. doi: 10.1016/j.ijfoodmicro.2014.09.020. [DOI] [PubMed] [Google Scholar]
- 21.Baranyi J., Roberts T.A. A dynamic approach to predicting bacterial growth in food. Int. J. Food Microbiol. 1994;23:277–294. doi: 10.1016/0168-1605(94)90157-0. [DOI] [PubMed] [Google Scholar]
- 22.Pavlidis D.E., Mallouchos A., Ercolini D., Panagou E.Z., Nychas G.-J.E. A volatilomics approach for off-line discrimination of minced beef and pork meat and their admixture using HS-SPME GC/MS in tandem with multivariate data analysis. Meat Sci. 2019;151:43–53. doi: 10.1016/j.meatsci.2019.01.003. [DOI] [PubMed] [Google Scholar]
- 23.Xia J., Sinelnikov I.V., Han B., Wishart D.S. Metaboanalyst 3.0—Making metabolomics more meaningful. Nucleic Acids Res. 2015;43:W251–W257. doi: 10.1093/nar/gkv380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Otto M. Chemometrics: Statistics and Computer Application in Analytical Chemistry. 3rd ed. Wiley-VCH; Weinheim, Germany: 2007. pp. 184–200. [Google Scholar]
- 25.Mohareb F., Iriondo M., Doulgeraki A.I., Van Hoek A., Aarts H., Cauchia M., Nychas G.-J.E. Identification of meat spoilage gene biomarkers in Pseudomonas putida using gene profiling. Food Control. 2015;57:152–160. doi: 10.1016/j.foodcont.2015.04.007. [DOI] [Google Scholar]
- 26.Argyri A.A., Doulgeraki A.I., Blana V.A., Panagou E.Z., Nychas G.-J.E. Potential of a simple HPLC-based approach for the identification of the spoilage status of minced beef stored at various temperatures and packaging systems. Int. J. Food Microbiol. 2011;150:25–33. doi: 10.1016/j.ijfoodmicro.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 27.Ferrocino I., La Storia A., Torrieri E., Musso S.S., Mauriello G., Villani F., Ercolini D. Antimicrobial packaging to retard the growth of spoilage bacteria and to reduce the release of volatile metabolites in meat stored under vacuum at 1 °C. J. Food Prot. 2013;76:52–58. doi: 10.4315/0362-028X.JFP-12-257. [DOI] [PubMed] [Google Scholar]
- 28.Hernandez-Macedo M.L., Contreras-Castillo C.J., Tsai S.M., Da Cruz S.H., Sarantopoulos C.I.G.L., Padula M., Dias C.T.S. Gases and volatile compounds associated with microorganisms in blown pack spoilage of Brazilian vacuum-packed beef. Lett. Appl. Microbiol. 2012;55:467–475. doi: 10.1111/lam.12004. [DOI] [PubMed] [Google Scholar]
- 29.Cubero-Leon E., Peñalver R., Maquet A. Review on metabolomics for food authentication. Food Res. Int. 2014;60:95–107. doi: 10.1016/j.foodres.2013.11.041. [DOI] [Google Scholar]
- 30.Xiao Z., Xu P. Acetoin Metabolism in Bacteria. Crit. Rev. Microbiol. 2007;33:127–140. doi: 10.1080/10408410701364604. [DOI] [PubMed] [Google Scholar]
- 31.Huang M., Oppermann F.B., Steinbiichel A. Molecular characterization of the Pseudomonas putida 2,3-butanediol catabolic pathway. FEMS Microbiol. Lett. 1994;124:141–150. doi: 10.1111/j.1574-6968.1994.tb07276.x. [DOI] [PubMed] [Google Scholar]
- 32.Insausti H.K., Beriain M.J., Gorraiz C., Purroy A. Volatile Compounds of Raw Beef from 5 Local Spanish Cattle Breeds Stored Under Modified Atmosphere. J. Food Sci. 2002;67:1580–1589. doi: 10.1111/j.1365-2621.2002.tb10325.x. [DOI] [Google Scholar]
- 33.Nurjuliana M., Che Man Y.B., Mat Hashim D., Mohamed A.K.S. Rapid identification of pork for halal authentication using the electronic nose and gas chromatography mass spectrometer with headspace analyzer. Meat Sci. 2011;88:638–644. doi: 10.1016/j.meatsci.2011.02.022. [DOI] [PubMed] [Google Scholar]
- 34.Leroy F., Vasilopoulos C., Van Hemelryck S., Falony G., De Vuyst L. Volatile analysis of spoiled, artisan-type, modified-atmosphere-packaged cooked ham stored under different temperatures. Int. J. Food Microbiol. 2009;26:94–102. doi: 10.1016/j.fm.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 35.Jääskeläinen E., Hultman J., Parshintsev J., Riekkola M.L., Björkroth J. Development of spoilage bacterial community and volatile compounds in chilled beef under vacuum or high oxygen atmospheres. Int. J. Food Microbiol. 2016;223:25–32. doi: 10.1016/j.ijfoodmicro.2016.01.022. [DOI] [PubMed] [Google Scholar]
- 36.Saraiva C., Oliveira I., Silva J.A., Martins C., Ventanas J., García C. Implementation of multivariate techniques for the selection of volatile compounds as indicators of sensory quality of raw beef. J. Food Sci. Technol. 2015;52:3887–3898. doi: 10.1007/s13197-014-1447-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Resconi V.C., Bueno M., Escudero A., Magalhaes D., Ferreira V., Mar Campo M. Ageing and retail display time in raw beef odour according to the degree of lipid oxidation. Food Chem. 2018;242:288–300. doi: 10.1016/j.foodchem.2017.09.036. [DOI] [PubMed] [Google Scholar]
- 38.Schulz S., Dickschat J.S. Bacterial volatiles: The smell of small organisms. Nat. Prod. Rep. 2007;24:814–842. doi: 10.1039/b507392h. [DOI] [PubMed] [Google Scholar]
- 39.Montel M.C., Masson F., Talon R. Bacterial role in flavor development. Meat Sci. 1998;49:111–123. doi: 10.1016/S0309-1740(98)90042-0. [DOI] [PubMed] [Google Scholar]
- 40.Dainty R.H., Edwards R.A., Hibbard C.M., Marnewick J.J. Volatile compound associated with microbial growth on normal and high pH beef stored at chill temperatures. J. Appl. Bacteriol. 1989;66:281–289. doi: 10.1111/j.1365-2672.1989.tb02480.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.