Abstract
This study focused, for the first time, on the evaluation of the seasonal effect on the chemical composition and biological activities of essential oils hydrodistillated from leaves, trunk bark and fruits of Zanthoxylum leprieurii (Z. leprieurii), a traditional medicinal wild plant growing in Côte d’Ivoire. The essential oils were obtained by hydrodistillation from fresh organs of Z. leprieurii growing on the same site over several months using a Clevenger-type apparatus and analyzed by gas chromatography-mass spectrometry (GC/MS). Leaf essential oils were dominated by tridecan-2-one (9.00 ± 0.02–36.80 ± 0.06%), (E)-β-ocimene (1.30 ± 0.50–23.57 ± 0.47%), β-caryophyllene (7.00 ± 1.02–19.85 ± 0.48%), dendrolasin (1.79 ± 0.08–16.40 ± 0.85%) and undecan-2-one (1.20 ± 0.03–8.51 ± 0.35%). Fruit essential oils were rich in β-myrcene (16.40 ± 0.91–48.27 ± 0.26%), citronellol (1.90 ± 0.02–28.24 ± 0.10%) and geranial (5.30 ± 0.53–12.50 ± 0.47%). Tridecan-2-one (45.26 ± 0.96–78.80 ± 0.55%), β-caryophyllene (1.80 ± 0.23–13.20 ± 0.33%), α-humulene (4.30 ± 1.09–12.73 ± 1.41%) and tridecan-2-ol (2.23 ± 0.17–10.10 ± 0.61%) were identified as major components of trunk bark oils. Statistical analyses of essential oil compositions showed that the variability mainly comes from the organs. Indeed, principal component analysis (PCA) and hierarchical cluster analysis (HCA) allowed us to cluster the samples into three groups, each one consisting of one different Z. leprieurii organ, showing that essential oils hydrodistillated from the different organs do not display the same chemical composition. However, significant differences in essential oil compositions for the same organ were highlighted during the studied period, showing the impact of the seasonal effect on essential oil compositions. Biological activities of the produced essential oils were also investigated. Essential oils exhibited high insecticidal activities against Sitophilus granarius, as well as antioxidant, anti-inflammatory and moderate anti-plasmodial properties.
Keywords: Zanthoxylum leprieurii, essential oils, Sitophilus granarius, tridecan-2-one, β-myrcene, (E)-β-ocimene, dendrolasin, antioxidant, anti-inflammatory, insecticidal, anti-plasmodial, Côte d’Ivoire
1. Introduction
Zanthoxylum leprieurii Guill and Perr. (syn. Fagara leprieurii Engl and Fagara Angolensis) is a plant species belonging to the Genus Zanthoxylum of the Rutaceae family, which contains approximatively 150 Genus and 900 species. Z. leprieurii is distributed in rain forests and wooded savannahs in Africa, from Senegal (Western Africa), Ethiopia (Eastern Africa), to Angola, Zimbabwe and Mozambique (Southern Africa) [1]. Known as a multipurpose species, Z. leprieurii has a wide spectrum of applications, as leaves, trunk bark and roots are used in traditional medicine to cure rheumatism and for the treatment of tuberculosis and generalized body pains in Central and Western Africa [2,3,4]. Roots are used as chewing sticks to clean the mouth [5]. Moreover, this plant is also used for canoes, boxes, plywood, general carpentry, domestic utensils, beehives and water pots; the pale yellow wood is tough, medium coarse-grained and light [6]. Dried fruits of Z. leprieurii are used as spices by local populations in many regions of Africa [7]. The plant has shown antioxidant, antimicrobial, anticancer, cytotoxic, schistosomidal and antibacterial properties [8,9,10,11,12]. From a chemical point of view, a large variety of compounds from different chemical classes were reported in Z. leprieurii solvent extracts: acridone alkaloids, benzophenanthridine [13], aliphatic amide [14], coumarins [15] and kaurane diterpenes [11]. Essential oils produced from Z. leprieurii revealed that the main constituents in fruit essential oils were (E)-β-ocimene (29.40%) and β-citronellol (17.37%) [15,16,17]. Our previous study showed the predominance of tridecan-2-one (47.50%) in leaf essential oils from Côte d’Ivoire [18]. Limonene (94.90%) and terpinolene (50.00%) were described as major components in essential oils from Nigeria and Cameroon, respectively [19,20]. The composition of trunk bark essential oils was predominated by sesquiterpenoids in Nigeria and methyl ketones in Côte d’Ivoire [18,19].
All these studies highlighted significant differences in the composition of essential oils extracted from different organs of Z. leprieurii from the same growing site, as well as significant differences in the composition of essential oils extracted from Z. leprieurii growing in different places. The latter can be explained by the fact that many factors affect essential oil amounts as well as their chemical compositions [21,22,23]. These include plant genetic differences, as well as environmental factors such as temperature, soil, precipitation, wind speed, rainfall and pests [24].
The first aim of this study was to explore climate-related variations of the composition of essential oils hydrodistillated from different Z. leprieurii organs (leaves, trunk bark and fruits). The variation over time of essential oil chemical compositions was then studied at one site in Côte d’Ivoire. GC/MS was used to identify essential oil chemical profiles and statistical analyses were performed on the different chemical compositions. In addition, this study also aimed to evaluate the impact of Z. leprieurii essential oil composition variations on their in vitro biological activities, such as antioxidant, anti-inflammatory, insecticidal and anti-plasmodial activities. To our knowledge, such studies have not yet been carried out on this species in Côte d′Ivoire.
2. Materials and Methods
2.1. Plant Material and Hydrodistillation Procedure
Z. leprieurii organs were collected at Adzope (6°06′25” N, 3°51′36” W), in south-eastern Côte d’Ivoire, between May and November 2017 for leaves and trunk bark, respectively, and between July and November 2017 for fruits. At each harvest, leaf, fruit and trunk bark samples were taken from 15 randomly selected trees, in the geographical area described before, by taking the same amount of plant material from each tree and pooling it before distillation. The total amount of plant material was between 700 and 1500 g. The same tree was only sampled once to prevent the previous sampling from influencing the next one (e.g., trunk injury). Plants were identified by the Centre Suisse of Research (Adiopodoumé, Abidjan, Côte d’Ivoire) and by the National Flora Center (CNF; Abidjan, Côte d’Ivoire). The vouchers of the specimen (UCJ016132) have been deposited at the CNF Herbarium. Fresh organ material was hydrodistillated for 3 h using a Clevenger-type apparatus. The pale yellow essential oils were treated with anhydrous sodium sulphate (Na2SO4) as a drying agent, stored in sealed amber vials, and conserved at 4 °C before analysis. The essential oil yields (w/w) were calculated as the rapport between the mass of essential oils obtained compared to the mass of fresh organs.
2.2. GC/MS Chemical Analysis of Essential Oils
Essential oils hydrodistillated from Z. leprieurii organs were analyzed by GC/MS. An Agilent GC system 7890B (Agilent, Santa Clara, CA, USA) equipped with a split/splitless injector and an Agilent MSD 5977B detector was used. The experience was repeated three times for each essential oil. One µL of essential oil dilutions (0.01% in hexane; w/v) was injected in splitless mode at 300 °C on an HP-5MS capillary column (30 m × 0.25 mm, df = 0.25µm). The temperature was maintained one min at 50 °C, and then increased at a rate of 5 °C/min until 300 °C. The final temperature was maintained for 5 min. The sources and quadrupole temperatures were fixed at 230 °C and 150 °C, respectively. The scan range was 40–400 m/z, and the carrier gas was helium at a flow rate of 1.2 mL/min. The component identification was performed on the basis of chromatographic retention indices (RI) and by comparison of the recorded spectra with a computed data library (Pal 600K®) [25,26,27]. RI values were measured on an HP-5MS column (Agilent, Santa Clara, CA, USA). RI calculations were performed in temperature program mode according to a mixture of homologues n-alkanes (C7–C30), which were analyzed under the same chromatographic conditions. The main components were confirmed by comparison of their retention and MS spectrum data with co-injected pure references (Sigma, Darmstadt, Germany) when commercially available.
2.3. Biological Activities
2.3.1. Antioxidant Assay
2,2-Diphenyl-1-Picrylhydrazyl (DPPH) Radical Scavenging Capacity
The hydrogen atom- or electron-donating ability of essential oils and Trolox was determined from the bleaching of the purple-colored methanol DPPH solution. Briefly, the samples were tested at 25, 50, 75 and 100 µg/mL. Ten microliters of various concentrations (1 to 5 mg/mL) of each essential oil in methanol were added to 1990 μL of a 10 mg/mL DPPH methanol solution (0.06 mM). Free radical scavenging activities of leaf, trunk bark and fruit essential oils hydrodistillated from Z. leprieurii were determined spectrophotometrically [28]. The mixture was vortexed for about 1 min and then incubated at room temperature in the dark for 30 min; absorbance was measured at 517 nm with an Ultrospec UV-visible, dual beam spectrophotometer (GE Healthcare, Cambridge, UK). The same sample procedure was followed for Trolox (Sigma, Darmstadt, Germany) used as standard; methanol (Sigma, Darmstadt, Germany) with DPPH was used as control; and all the samples were tested in triplicate. The optical density was recorded, and the inhibition percentage was calculated using the formula given below:
| Inhibition percentage of DPPH activity (%) = (Abs Blank-Abs sample)/(Abs blank) × 100 | (1) |
Abs Blank = absorbance of the blank sample, Abs sample = absorbance of the test sample
Ferric-Reducing Power Assay
The ferric-reducing antioxidant power (FRAP) of essential oils hydrodistillated from Z. leprieurii was determined here. Briefly, four dilutions of essential oils and Trolox were prepared in methanol (25, 50, 75 and 100 µg/mL). Trolox was used as the standard reference. One mL of those methanol solutions were melded with one mL of a phosphate buffer (0.2 M, pH = 6.6) and with one mL of a potassium ferricyanide solution (1%; K3Fe(CN)6). After 20 min at 50 °C and the addition of one mL of trichloroacetic acid (TCA; 10% v/v), the solution was centrifuged at 3000 rpm for 10 min [27,28,29]. Next, 1.5 mL of the upper phase was recovered and melded with 1.5 mL of distillated water and 150 µL of FeCl3 (0.1% v/v). Each concentration was realized as triplicated. Finally, the absorbance of the prepared sample was measured at 700 nm. In comparison with the blank, a higher absorbance shows a high reducing power. For all concentrations, absorbance due to essential oil samples were removed from each measurement.
2.3.2. Anti-Inflammatory Activity
Inhibition Lipoxygenase Assay
The anti-inflammatory activities of Z. leprieurii essential oils were determined by the method previously described by Nikhila [30]. In brief, the reaction mixture containing essential oils in various concentrations (100, 75, 50 and 25 µg/mL of methanol) (in triplicate for each concentration), lipoxygenase (Sigma, Darmstadt, Germany) and 35 µL (0.1 mg/mL) of a 0.2 M borate buffer solution (pH = 9.0) was incubated for 15 min at 25 °C. The reaction was then initiated by the addition of 35 µL of a substrate solution (linoleic acid 250 µM), and the absorbance was measured at 234 nm. Quercetin (Sigma, Darmstadt, Germany) was used as a standard inhibitor at the same concentration as the essential oils. The inhibition percentage of lipoxygenase activity was calculated as follows:
| Inhibition percentage % = (Abs Blank-Abs sample)/(Abs blank) × 100 | (2) |
where Abs blank is the Absorbance (Abs) of the reaction media without the essential oil, and Absorbance sample is the Abs of the reaction media with the essential oil minus the Abs value of the diluted essential oil (to compensate for absorbance due to the essential oils themselves).
Inhibition of Albumin Denaturation Assay
This test was conducted as described by Kar [31] with some slight modifications. The reaction mixture consisted of 1 mL essential oil samples and diclofenac (standard) at 100, 75, 50 and 25 µg/mL in methanol, 0.5 mL bovine serum albumin (BSA) at 2% in water and 2.5 mL phosphate-buffered saline adjusted with hydrochloric acid (HCl) to pH 6.3. The tubes were incubated for 20 min at room temperature, then heated to 70 °C for 5 min and subsequently cooled for 10 min [32]. The absorbance of these solutions was determined using a spectrophotometer at 660 nm. The experiment was performed in triplicate. The inhibition percentage of albumin denaturation was calculated on a percentage basis relative to the control using the formula:
| Inhibition percentage of denaturation % = (Abs Blank-Abs sample)/(Abs blank) × 100 | (3) |
where Abs blank corresponds to the Absorbance (Abs) of the reaction media without the addition of essential oil. Absorbance sample is the Abs of the reaction media with addition of essential oil, subtracted by the Abs value of the diluted essential oil (to compensate for absorbance due to the essential oils themselves).
IC50 (half inhibitory concentration), which corresponds to the essential oil concentration needed to inhibit 50% of the activity, was used to express antioxidant and anti-inflammatory properties of essential oils.
2.3.3. Insecticidal Activity
Determination of Mortality Values
Essential oil dilutions (10, 14, 18, 22, 26 and 30 µL/mL) were prepared in acetone. Talisma UL (Biosix, Hermalle-sous-Huy, Belgium), a classical chemical insecticide used for the protection of stored grains against insects, was also used at the same concentrations. For each test, 500 µL of essential oil or standard solution were homogeneously dispersed in tubes containing 20 g of organic wheat grains. The solvent was allowed to evaporate from grains for 20 min before infesting them by 12 adult insects. The granary weevil, Sitophilus granarius, was chosen for this study because it is one of the most damaging cereal pests in the world. Moreover, this insect is a primary pest, which means it is able to drill holes in grains, laying its eggs inside them and allowing secondary pests to develop in the grains [33,34]. Acetone was used as a negative control. Six replicates were created for all treatments and controls, and they were incubated at 30 °C. The mortality was recorded after 24 h of incubation. Results from all replicates were subjected to Probit Analysis using Python 3.7 program to determine LC50, LC90 and LC95 values.
Repulsive Assay
This test has been conducted to evaluate the repulsive effect of essential oils against insects (Sitophilus granarius). This experiment was carried out by cutting an 8 cm diameter filter paper in half. The six concentrations (10, 14, 18, 22, 26 and 30 µL/mL) of essential oils were prepared in acetone. Each half disk was treated with 100 µL of the solution, and the other half with acetone. After evaporation of acetone, the two treated parts were joined together by an adhesive tape and placed in a petri dish. Ten insects were placed in the center of each petri dish and were incubated at 30 °C. After two hours of incubation, the number of insects present in the part treated with essential oil and the number of insects present in the part treated only with acetone were counted, as described by Mc Donald [35].
The percentage of repulsively was calculated as follows:
| Pr = (Nc-NT)/(Nc+NT) × 100 | (4) |
NC: Number of insects present on the disc part treated with acetone; NT: Number of insects present on the part of the disc treated with the essential oil dilution in acetone
2.3.4. Anti-Plasmodial Activity
Ledoux et al. method [36] was used to determine anti-plasmodial activity. To do so, asexual erythrocyte stages of P. falciparum, chloroquine-sensitive strain 3D7 were continuously cultivated in vitro using the procedure of Trager and Jensen. The erythrocyte had been initially obtained from a patient from Schipol in the Netherlands (BEI Reagent Search) [37]. ATCC, Bei Ressources provides us with the strains. Red blood cells of A+ group were used as human host cell. The culture medium was RPMI 1640 from Gibco, Fisher Scientific (Loughborough, UK) composed of NaHCO3 (32 mM), HEPES (25 mM) and L-glutamine. Glucose (1.76 g/L) from Sigma-Aldrich (Machelen, Belgium), hypoxanthine (44 mg/mL) from Sigma-Aldrich (Machelen, Belgium), gentamin (100 mg/mL) from Gibco Fisher Scientific (Loughborough, UK) and human pooled serum from A+ group (10%) were added to the medium according to [36,38]. DMSO solutions of essential oils were directly diluted in the medium. The dilutions were performed in triplicate by successive two-fold dilutions in a 96-well plate. The essential oil concentrations are expressed in term of µg/mL of essential oil. As interaction between volatile compounds between samples could occur, we decided to alternate one test line with two lines filled with culture media. The growth of the parasite was recorded after 48 h of incubation using lactate dehydrogenase (pLDH) activity as parameter according to Makker method [39]. A positive control was used in all the repetitions. This positive control was composed of Artemisinin from Sigma-Aldrich (Machelen, Belgium) at a concentration of 100 µg/mL. Sigmoidal curves allowed the determination of half inhibitory concentration (IC50).
2.4. Statistical Analysis
2.4.1. Data Analysis
Hierarchical cluster analysis (HCA) and principal component analysis (PCA) (Ward’s method) were used to investigate the seasonal effect on essential oil composition. Analyses on the 19 samples collected during a specific period were performed with Xlstat (Adinsoft, Paris, France).
2.4.2. Biological Activities Analysis
Data were analyzed using IBM SPSS version 20. Results were presented in terms of means. Multiple comparisons of mean values were set up using one-way parametric analysis of variance (ANOVA). The DUNCAN test was used to appreciate the differences between the means at p-value ˂ 0.05. The relationship between the different parameters was studied using Pearson correlation.
3. Results and Discussion
3.1. Chemical Composition of Essential oils and Yields
Z. leprieurii organs were collected over a period of seven months for the leaves and trunk barks and five months for the fruits within one single year. Meteorological data were recorded during the collection period (Table 1). The highest rainfall values were recorded in May, June, October and November, with 164.50 mm, 205.70 mm, 310.80 mm and 206.40 mm. In July and August, the rainfall was moderate, with 71.30 mm and 61.70 mm, respectively. The same trend was observed for the temperature. However, temperature variations were low during the collection period, with temperature ranging from 28.40 °C to 25.00 °C. The trends observed for these two variables in addition to those of relative humidity and daylight confirm that the months of May, June, October and November represent rainy season months; while those of July, August and September were dry season months. Results (Table 2, Table 3 and Table 4, in bold) showed that essential oil yields obtained in this study (0.02 to 0.04% (w/w) for leaves, 0.86 to 1.20% (w/w) for trunk bark and 1.13 to 1.51% (w/w) for fruits) were consistent with those found in the literature [18,40]. Essential oil yields seem dependent on meteorological variations, as, for each organ, the highest yields were observed in July and August, the collecting moment when the lowest precipitations and temperatures were recorded. When precipitations increased and temperatures were higher, the lowest essential oil yields were obtained. However, as temperature and yields variations were low in this study, those results should to be confirmed with a longer experiment. Results obtained here, though, are supported by previous studies showing that lower precipitation induces higher essential oil yields [41].
Table 1.
Meteorological parameters recorded during the collection period of Zanthoxylum leprieurii organs.
| Months | Rainfall (mm) | Relative Humidity (%) | Daylight (h) | Temperature (°C) |
|---|---|---|---|---|
| May | 164.50 | 80.00 | 196.30 | 28.40 |
| June | 205.70 | 85.00 | 101.60 | 26.80 |
| July | 71.30 | 84.00 | 121.00 | 25.80 |
| August | 61.70 | 84.00 | 134.00 | 25.00 |
| September | 102.40 | 81.00 | 124.20 | 25.90 |
| October | 310.80 | 84.00 | 180.40 | 27.10 |
| November | 206.40 | 78.00 | 214.40 | 27.50 |
Source: SODEXAM (Société d’Exploitation et de Développement Aéronautique, Aéroportuaire et Météorologique), 2017.
Table 2.
Chemical composition of essential oils hydrodistillated from Zanthoxylum leprieurii leaves. Each analysis was realized in triplicate.
| Leaves | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N° | Compounds | Cas Number | Identification | Ria | Rib | May | June | July | Aug | Sep | Oct | Nov |
| 1 | α-pinene | 80-56-8 | MS, RI, STD | 931 | 929 | 2.48 ± 0.13 | 8.45 ± 0.57 | 0.30 ± 0.11 | 0.60 ± 0.35 | 0.70 ± 0.15 | 0.50 ± 0.27 | 0.30 ± 0.17 |
| 2 | ß-myrcene | 123-35-3 | MS, RI, STD | 987 | 988 | 0.71 ± 0.03 | - | 5.20 ± 0.21 | 0.90 ± 0.15 | 3.40 ± 0.37 | 0.4 ± 0.31 | 0.70 ± 0.09 |
| 3 | p-cymene | 25155-15-1 | MS, RI, STD | 1022 | 1022 | 1.48 ± 0.03 | - | 0.10 ± 0.01 | 0.30 ± 0.03 | - | 0.40 ± 0.57 | - |
| 4 | limonene | 138-86-3 | MS, RI, STD | 1023 | 1027 | - | 0.90 ± 0.29 | 0.40 ± 0.28 | - | - | - | - |
| 5 | (E)-ß-ocimene | 13877-91-3 | MS, RI, STD | 1041 | 1046 | 23.57 ± 047 | 2.60 ± 0.99 | 3.90 ± 0.84 | 2.50 ± 0.04 | 1.30 ± 0.50 | 2.30 ± 1.91 | - |
| 6 | γ-terpinene | 99-85-4 | MS, RI, STD | 1060 | 1057 | 0.28 ± 0.03 | - | - | - | - | - | - |
| 7 | linalool | 78-70-6 | MS, RI, STD | 1094 | 1098 | 6.44 ± 0.13 | 0.50 ± 0.34 | 1.50 ± 0.55 | 8.50 ± 0.09 | 1.70 ± 0.23 | 4.30 ± 0.95 | 3.20 ± 0.09 |
| 8 | alloocimene | 673-84-7 | MS, RI | 1128 | 1129 | 1.22 ± 0.04 | 0.40 ± 0.13 | 0.20 ± 0.10 | - | - | - | - |
| 9 | terpineol | 98-55-5 | MS, RI, STD | 1190 | 1191 | - | - | 0.20 ± 0.03 | - | - | - | - |
| 10 | decanal | 112-31-2 | MS, RI, STD | 1191 | 1204 | - | - | 1.50 ± 0.52 | - | - | - | - |
| 11 | citronellol | 106-22-9 | MS, RI, STD | 1225 | 1227 | - | - | 2.00 ± 0.48 | - | 1.50 ± 0.27 | - | 0.30 ± 0.02 |
| 12 | geraniol | 106-24-1 | MS, RI, STD | 1250 | 1253 | - | - | 1.40 ± 0.53 | - | 1.00 ± 0.74 | - | - |
| 13 | decyl alcohol | 112-30-1 | MS, RI, STD | 1262 | 1263 | - | - | 1.80 ± 0.92 | - | - | - | - |
| 14 | thymol | 89-83-8 | MS, RI, STD | 1286 | 1291 | - | 2.20 ± 0.96 | 3.10 ± 0.43 | 13.30 ± 0.31 | 4.10 ± 0.22 | - | - |
| 15 | undecan-2-one | 112-12-9 | MS, RI, STD | 1288 | 1293 | 8.51 ± 0.35 | 1.90 ± 0.50 | 1.20 ± 0.03 | 8.40 ± 0.12 | 2.70 ± 0.56 | 3.20 ± 1.06 | 2.10 ± 0.98 |
| 16 | undecan-2-ol | 1653-30-1 | MS, RI, STD | 1298 | 1300 | - | - | 0.20 ± 0.04 | 0.40 ± 0.04 | - | - | - |
| 17 | methyl nerate | 1862-61-9 | MS, RI, STD | 1319 | 1323 | - | - | 2.20 ± 0.74 | - | 0.80 ± 0.44 | - | - |
| 18 | δ-elemene | 20307-84-0 | MS, RI | 1334 | 1339 | - | 1.20 ± 0.69 | 0.20 ± 0.00 | - | - | - | - |
| 19 | α-copaene | 3856-25-5 | MS, RI, STD | 1376 | 1378 | - | 0.60 ± 0.23 | 0.30 ± 0.08 | - | 0.60 ± 0.11 | 0.20 ± 0.07 | 0.40 ± 0.04 |
| 20 | ß-elemene | 515-13-9 | MS, RI | 1388 | 1394 | 3.92 ± 0.06 | - | 2.70 ± 0.83 | 4.20 ± 0.07 | 5.90 ± 0.26 | 2.30 ± 0.10 | 2.90 ± 0.20 |
| 21 | β-caryophyllene | 87-44-5 | MS, RI, STD | 1419 | 1423 | 13.51 ± 0.11 | 18.90 ± 0.48 | 15.60 ± 0.54 | 13.70 ± 0.1 | 19.85 ± 0.82 | 7.00 ± 1.02 | 8.60 ± 0.62 |
| 22 | cadina-4(14),5-diene | 54324-03-7 | MS, RI | 1430 | 1433 | 1.83 ± 0.02 | 0.90 ± 0.26 | 0.70 ± 0.16 | 0.30 ± 0.02 | 2.40 ± 0.81 | 1.70 ± 0.26 | 1.50 ± 0.19 |
| 23 | γ-elemene | 3242-08-8 | MS, RI | 1432 | 1435 | 1.19 ± 0.01 | 4.40 ± 0.45 | 1.10 ± 0.21 | 1.10 ± 0.04 | 3.10 ± 0.06 | 0.8 ± 0.12 | 1.10 ± 0.01 |
| 24 | α-humulene | 6753-98-6 | MS, RI, STD | 1456 | 1457 | 3.92 ± 0.02 | 6.70 ± 0.62 | 4.50 ± 0.98 | 3.60 ± 0.04 | 6.10 ± 0.27 | 4.10 ± 0.58 | 4.20 ± 0.32 |
| 25 | alloaromadendrene | 25246-27-9 | MS, RI | 1457 | 1465 | - | 0.50 ± 0.21 | 0.30 ± 0.06 | - | 0.50 ± 0.11 | 0.40 ± 0.06 | 0.20 ± 0.01 |
| 26 | germacrene D | 23986-74-5 | MS, RI, STD | 1482 | 1485 | 1.96 ± 0.17 | 0.90 ± 0.50 | 0.40 ± 0.10 | - | 1.60 ± 0.40 | 1.40 ± 0.21 | 1.00 ± 0.14 |
| 27 | ß-ionone | 14901-07-6 | MS, RI, STD | 1482 | 1488 | - | - | 0.40 ± 0.12 | - | - | - | 0.20 ± 0.05 |
| 28 | ß-selinene | 17066-67-0 | MS, RI | 1483 | 1490 | 0.32 ± 0.01 | 1.10 ± 0.13 | 0.70 ± 0.14 | 0.80 ± 0.01 | 1.60 ± 0.30 | 0.20 ± 0.21 | 0.50 ± 0.05 |
| 29 | tridecan-2-one | 593-08-8 | MS, RI, STD | 1487 | 1495 | 18.74 ± 0.57 | 9.00 ± 0.02 | 22.50 ± 0.98 | 30.20 ± 0.39 | 15.80 ± 0.84 | 36.80 ± 0.06 | 33.70 ± 0.36 |
| 30 | selina-4(14),7(11)-diene | 515-17-3 | MS, RI | 1495 | 1498 | - | 1.30 ± 0.31 | 0.80 ± 0.15 | 1.50 ± 0.90 | 2.90 ± 0.86 | - | - |
| 31 | tridecan-2-ol | 1653-31-2 | MS, RI, STD | 1495 | 1501 | - | 2.00 ± 0.82 | 1.70 ± 1.17 | - | 2.30 ± 0.29 | 2.40 ± 0.80 | 3.20 ± 0.33 |
| 32 | (3E,6E)-α-farnesene | 502-61-4 | MS, RI | 1499 | 1509 | 3.22 ± 0.29 | 0.60 ± 0.21 | 1.20 ± 0.34 | 0.90 ± 0.07 | 2.50 ± 0.97 | 9.10 ± 0.82 | 4.60 ± 0.55 |
| 33 | γ-cadinene | 39029-4-9 | MS, RI | 1513 | 1517 | 0.42 ± 0.06 | 1.10 ± 0.36 | 0.60 ± 0.08 | 0.20 ± 0.04 | 1.00 ± 0.21 | 1.20 ± 0.26 | 1.30 ± 0.02 |
| 34 | δ-cadinene | 483-76-1 | MS, RI | 1524 | 1526 | 2.25 ± 0.09 | 4.20 ± 1.01 | 2.20 ± 0.09 | 1.00 ± 0.02 | 4.30 ± 0.13 | 3.40 ± 0.49 | 4.30 ± 0.33 |
| 35 | elemol | 639-99-6 | MS, RI, STD | 1547 | 1552 | 0.28 ± 0.02 | 0.60 ± 0.22 | 0.20 ± 0.01 | 0.30 ± 0.01 | 0.30 ± 0.05 | 0.40 ± 0.03 | 1.20 ± 0.09 |
| 36 | nerolidol | 7212-44-4 | MS, RI, STD | 1557 | 1564 | - | 1.50 ± 0.30 | 1.10 ± 0.39 | 1.00 ± 0.03 | 1.60 ± 0.49 | 4.80 ± 0.22 | 3.40 ± 0.32 |
| 37 | dendrolasin | 23262-34-2 | MS, RI | 1576 | 1580 | 1.79 ± 0.08 | 8.60 ± 0.95 | 9.40 ± 0.90 | 4.00 ± 0.06 | 7.60 ± 0.09 | 10.60 ± 0.44 | 16.40 ± 0.85 |
| 38 | spathulenol | 6750-60-3 | MS, RI | 1578 | 1582 | - | 1.50 ± 0.46 | 1.10 ± 0.03 | - | - | - | 0.40 ± 0.00 |
| 39 | caryophyllene oxide | 1139-30-6 | MS, RI, STD | 1583 | 1588 | - | 6.00 ± 0.83 | 5.70 ± 0.79 | - | - | - | - |
| 40 | ζ-cadinol | 5937-11-1 | MS, RI | 1639 | 1645 | - | 1.10 ± 0.08 | 0.60 ± 0.03 | - | 0.70 ± 0.21 | 0.60 ± 0.15 | 0.80 ± 0.04 |
| 41 | α-cadinol | 481-34-5 | MS, RI | 1650 | 1659 | 0.28 ± 0.03 | 1.30 ± 0.11 | 0.70 ± 0.01 | - | 1.10 ± 0.44 | 0.80 ± 0.23 | 0.90 ± 0.09 |
| 42 | pentadecanal | 2765-11-9 | MS, RI | 1713 | 1715 | - | 2.10 ± 0.28 | - | - | - | - | 1.60 ± 0.79 |
| Monoterpene hydrocarbons (%) | 29.74 | 12.85 | 10.10 | 4.30 | 5.40 | 3.60 | 1.00 | |||||
| Oxygenated monoterpene (%) | 6.44 | 2.70 | 11.50 | 21.80 | 8.20 | 4.30 | 3.50 | |||||
| Sesquiterpene hydrocarbons (%) | 32.54 | 48.10 | 30.90 | 27.30 | 52.35 | 31.80 | 30.60 | |||||
| Oxygenated sesquiterpenes (%) | 2.35 | 22.70 | 18.10 | 5.30 | 12.10 | 17.20 | 25.40 | |||||
| Others (%) | 27.25 | 13.10 | 28.90 | 39.00 | 20.80 | 42.40 | 38.50 | |||||
| Identified compounds (%) | 98.32 | 99.45 | 99.50 | 97.70 | 98.85 | 99.30 | 99.00 | |||||
| Yield (%) | 0.02 | 0.02 | 0.03 | 0.04 | 0.02 | 0.02 | 0.02 | |||||
Identification methods: RIa, theoretical kovats indices (Pubchem and NIST); RIb, calculated kovats indices; MS, mass spectra comparison with PAL 600®libraries; STD, retention time and mass spectra comparison with commercially available standards; RI, retention index comparison with the literature; CAS number; -, Under perception threshold.
Table 3.
Chemical composition of essential oils hydrodistillated from Zanthoxylum leprieurii trunk bark. Each analysis was realized in triplicate.
| Trunk Bark | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N° | Compounds | Cas Number | Identification | Ria | Rib | May | June | July | Aug | Sep | Oct | Nov |
| 1 | α-pinene | 80-56-8 | MS, RI, STD | 931 | 929 | 0.52 ± 0.03 | - | - | - | - | - | - |
| 2 | ß-myrcene | 123-35-3 | MS, RI, STD | 987 | 988 | - | - | - | - | 1.00 ± 0.14 | - | - |
| 3 | citronellal | 106-23-0 | MS, RI, STD | 1153 | 1153 | - | - | - | - | 1.50 ± 0.08 | - | - |
| 4 | citronellol | 106-22-9 | MS, RI, STD | 1225 | 1227 | - | - | - | - | 1.60 ± 0.08 | - | - |
| 5 | geraniol | 106-24-1 | MS, RI, STD | 1250 | 1253 | - | - | - | - | 1.50 ± 0.14 | - | - |
| 6 | thymol | 89-83-8 | MS, RI, STD | 1286 | 1291 | - | 5.10 ± 0.36 | 0.50 ± 0.08 | - | 1.30 ± 0.05 | - | 4.10 ± 0.50 |
| 7 | ß-elemene | 515-13-9 | MS, RI | 1388 | 1394 | - | 0.60 ± 0.15 | - | - | 1.30 ± 0.03 | - | - |
| 8 | α-bergamotene | 17699-05-7 | MS, RI | 1415 | 1417 | 4.43 ± 0.10 | 0.50 ± 0.27 | 3.20 ± 0.69 | 1.60 ± 1.12 | 0.70 ± 0.01 | 1.50 ± 0.56 | 4.80 ± 0.60 |
| 9 | β-caryophyllene | 87-44-5 | MS, RI, STD | 1419 | 1423 | 9.51 ± 0.37 | 13.20 ± 0.33 | 8.50 ± 0.35 | 2.10 ± 0.13 | 8.10 ± 0.08 | 4.10 ± 0.79 | 1.80 ± 0.23 |
| 10 | γ-elemene | 3242-08-8 | MS, RI | 1432 | 1435 | - | 0.60 ± 0.21 | 0.10 ± 0.01 | - | - | - | - |
| 11 | geranylacetone | 3796-70-1 | MS, RI | 1455 | 1453 | - | 1.10 ± 0.51 | 0.50 ± 0.19 | - | 0.40 ± 0.02 | 0.30 ± 0.06 | - |
| 12 | α-humulene | 6753-98-6 | MS, RI, STD | 1456 | 1457 | 12.73 ± 1.41 | 4.30 ± 1.09 | 7.70 ± 0.62 | 6.20 ± 0.94 | 7.40 ± 0.11 | 8.10 ± 1.01 | 6.30 ± 0.03 |
| 13 | α-curcumene | 644-30-4 | MS, RI | 1482 | 1484 | 0.83 ± 0.18 | - | 0.70 ± 0.10 | 0.30 ± 0.11 | - | 0.40 ± 0.13 | 1.30 ± 0.08 |
| 14 | ß-selinene | 17066-67-0 | MS, RI | 1483 | 1490 | - | 0.30 ± 0.14 | 0.10 ± 0.01 | - | 0.30 ± 0.01 | - | 0.25 ± 0.33 |
| 15 | tridecan-2-one | 593-08-8 | MS, RI, STD | 1487 | 1495 | 45.26 ± 0.96 | 56.30 ± 0.31 | 51.4 ± 1.15 | 78.80 ± 0.55 | 54.40 ± 0.56 | 70.2 ± 0.95 | 71.36 ± 0.70 |
| 16 | tridecan-2-ol | 1653-31-2 | MS, RI, STD | 1495 | 1501 | 2.23 ± 0.17 | 10.10 ± 0.61 | 6.25 ± 0.17 | 4.30 ± 1.43 | 5.70 ± 0.20 | 6.40 ± 0.04 | 4.20 ± 0.40 |
| 17 | (3E,6E)-α-farnesene | 502-61-4 | MS, RI | 1503 | 1509 | 3.07 ± 0.28 | 1.20 ± 0.03 | 1.70 ± 0.04 | 2.70 ± 0.12 | 2.50 ± 0.09 | 2.30 ± 0.21 | 1.70 ± 0.03 |
| 18 | ß-bisabolene | 495-61-4 | MS, RI | 1505 | 1511 | 1.30 ± 0.05 | - | 1.20 ± 0.24 | 0.60 ± 0.37 | - | 0.50 ± 0.06 | 1.37 ± 0.20 |
| 19 | γ-cadinene | 39029-4-9 | MS, RI | 1513 | 1517 | - | - | 0.20 ± 0.02 | - | 0.60 ± 0.08 | - | 0.2 ± 0.08 |
| 20 | δ-cadinene | 483-76-1 | MS, RI | 1524 | 1526 | - | 0.20 ± 0.04 | 0.30 ± 0.04 | 0.10 ± 0.03 | 1.20 ± 0.05 | - | 0.38 ± 0.23 |
| 21 | elemol | 639-99-6 | MS, RI, STD | 1547 | 1552 | - | 0.30 ± 0.15 | - | - | 2.90 ± 0.07 | - | 0.31 ± 0.30 |
| 22 | nerolidol | 7212-44-4 | MS, RI, STD | 1557 | 1564 | 3.13 ± 0.27 | 0.40 ± 0.16 | 1.80 ± 0.63 | 0.40 ± 0.11 | 0.70 ± 0.03 | 1.20 ± 0.35 | 0.60 ± 0.03 |
| 23 | caryophyllene oxide | 1139-30-6 | MS, RI, STD | 1583 | 1588 | - | 0.20 ± 0.11 | 0.30 ± 0.00 | - | - | - | - |
| 24 | ζ-cadinol | 5937-11-1 | MS, RI | 1639 | 1645 | - | - | - | - | 0.30 ± 0.02 | - | - |
| 25 | α-cadinol | 481-34-5 | MS, RI | 1650 | 1659 | - | - | - | - | 0.50 ± 0.03 | - | - |
| 26 | pentadecan-2-one | 2345-28-0 | MS, RI, STD | 1696 | 1697 | 0.48 ± 0.02 | 0.50 ± 0.23 | 0.70 ± 0.02 | - | - | - | - |
| 27 | (E,E)-farnesol | 106-28-5 | MS, RI | 1722 | 1723 | 12.5 ± 0.85 | 3.00 ± 0.96 | 11.1 ± 0.15 | 1.40 ± 0.90 | 2.20 ± 0.45 | 1.90 ± 0.28 | - |
| 28 | farnesal | 19317-11-4 | MS, RI | 1738 | 1744 | 1.36 ± 0.02 | 0.40 ± 0.15 | 1.20 ± 0.05 | - | 0.30 ± 0.07 | 0.20 ± 0.04 | - |
| 29 | methyl farnesoate | 3675-00-1 | MS, RI | 1779 | 1785 | 1.90 ± 0.10 | 1.00 ± 0.35 | 1.60 ± 0.17 | - | 0.90 ± 0.08 | 0.70 ± 0.14 | - |
| Monoterpene hydrocarbons (%) | 0.52 | 0.00 | 0.00 | 0.00 | 1.00 | 0.00 | 0.00 | |||||
| Oxygenated monoterpene (%) | 0.00 | 5.30 | 0.50 | 0.00 | 5.90 | 0.00 | 4.10 | |||||
| Sesquiterpene hydrocarbons (%) | 31.87 | 21.80 | 24.20 | 13.60 | 22.20 | 16.90 | 18.20 | |||||
| Oxygenated sesquiterpenes (%) | 19.37 | 5.80 | 16.70 | 1.80 | 8.20 | 4.30 | 1.10 | |||||
| Others (%) | 47.46 | 66.40 | 57.65 | 83.10 | 62.10 | 77.10 | 75.60 | |||||
| Identified compounds (%) | 99.22 | 99.30 | 99.05 | 98.50 | 99.40 | 98.30 | 99.00 | |||||
| Yield (%) | 0.88 | 0.91 | 1.18 | 1.20 | 0.86 | 0.89 | 0.86 | |||||
Identification methods: RIa, theoretical kovats indices (Pubchem and NIST); RIb, calculated kovats indices; MS, mass spectra comparison with PAL 600®libraries; STD, retention time and mass spectra comparison with commercially available standards; RI, retention index comparison with the literature; CAS number; -, Under perception threshold.
Table 4.
Chemical composition of essential oils hydrodistillated from Zanthoxylum leprieurii fruits. Each analysis was realized in triplicate.
| Fruits | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N° | Compounds | Cas Number | Identification | Ria | Rib | July | Aug | Sep | Oct | Nov |
| 1 | α-pinene | 80-56-8 | MS, RI, STD | 931 | 929 | 2.30 ± 0.90 | 0.90 ± 0.12 | 0.80 ± 0.04 | 1.90 ± 0.66 | 0.60 ± 0.13 |
| 2 | ß-myrcene | 123-35-3 | MS, RI, STD | 987 | 988 | 16.40 ± 0.91 | 44.80 ± 0.13 | 46.30 ± 0.10 | 48.27 ± 0.26 | 24.7 ± 0.97 |
| 3 | α-terpinene | 99-86-5 | MS, RI, STD | 1008 | 1017 | - | - | - | - | 0.30 ± 0.06 |
| 4 | p-cymene | 25155-15-1 | MS, RI, STD | 1022 | 1022 | 0.20 ± 0.08 | 0.70 ± 0.09 | 0.40 ± 0.04 | 0.30 ± 0.05 | 0.80 ± 0.04 |
| 5 | limonene | 138-86-3 | MS, RI, STD | 1023 | 1027 | 1.10 ± 0.21 | 2.60 ± 0.29 | 2.00 ± 0.07 | 2.20 ± 0.16 | 6.00 ± 0.42 |
| 6 | (E)-ß-ocimene | 13877-91-3 | MS, RI, STD | 1041 | 1046 | 8.30 ± 0.29 | 2.00 ± 0.12 | 1.50 ± 0.08 | 1.50 ± 0.06 | 2.0 ± 0.15 |
| 7 | γ-terpinene | 99-85-4 | MS, RI, STD | 1060 | 1057 | - | - | - | - | 0.20 ± 0.01 |
| 8 | linalool | 78-70-6 | MS, RI, STD | 1094 | 1098 | - | 2.50 ± 0.27 | 3.40 ± 0.12 | 2.10 ± 0.10 | 4.20 ± 0.6 |
| 9 | perillene | 539-52-6 | MS, RI, STD | 1094 | 1100 | 6.50 ± 0.12 | - | - | - | - |
| 10 | alloocimene | 673-84-7 | MS, RI | 1128 | 1129 | 0.50 ± 0.07 | 0.50 ± 0.05 | 0.50 ± 0.04 | 0.40 ± 0.05 | 0.30 ± 0.02 |
| 11 | isopulegol | 7786-67-6 | MS, RI | 1140 | 1145 | - | - | - | - | 0.60 ± 0.15 |
| 12 | citronellal | 106-23-0 | MS, RI, STD | 1153 | 1153 | 0.20 ± 0.01 | 1.00 ± 0.11 | 1.40 ± 0.13 | 0.90 ± 0.12 | 5.70 ± 0.43 |
| 13 | limonene oxide | 1195-92-2 | MS, RI | 1175 | 1182 | - | - | 0.90 ± 0.01 | - | 0.90 ± 0.24 |
| 14 | terpineol | 98-55-5 | MS, RI, STD | 1190 | 1191 | - | - | - | - | 0.20 ± 0.05 |
| 15 | decanal | 112-31-2 | MS, RI, STD | 1202 | 1204 | 8.30 ± 0.17 | 1.80 ± 0.18 | 2.20 ± 0.02 | 1.60 ± 0.14 | 0.70 ± 0.24 |
| 16 | citronellol | 106-22-9 | MS, RI, STD | 1225 | 1227 | 1.90 ± 0.02 | 6.20 ± 0.55 | 6.60 ± 0.14 | 6.30 ± 0.63 | 28.24 ± 0.10 |
| 17 | geraniol | 106-24-1 | MS, RI, STD | 1250 | 1253 | 1.20 ± 0.03 | 5.50 ± 0.59 | 6.20 ± 0.22 | 6.30 ± 0.51 | 2.33 ± 0.74 |
| 18 | decyl alcohol | 112-30-1 | MS, RI, STD | 1262 | 1263 | 4.50 ± 0.18 | - | - | - | - |
| 19 | geranial | 141-27-5 | MS, RI, STD | 1268 | 1270 | - | 5.30 ± 0.53 | 7.60 ± 0.12 | 5.30 ± 0.31 | 12.50 ± 0.47 |
| 20 | undecan-2-one | 112-12-9 | MS, RI, STD | 1288 | 1293 | 0.20 ±0.03 | - | - | - | - |
| 21 | undecanal | 112-44-7 | MS, RI, STD | 1305 | 1306 | 0.20 ± 0.02 | - | - | - | - |
| 22 | methyl nerate | 1862-61-9 | MS, RI, STD | 1319 | 1323 | 6.70 ± 0.23 | 5.7 ± 0.6 | 4.40 ± 0.15 | 5.30 ± 0.13 | 6.10 ± 0.50 |
| 23 | δ-elemene | 20307-84-0 | MS, RI | 1334 | 1339 | - | - | - | 0.10 ± 0.02 | - |
| 24 | α-cubebene | 17699-14-8 | MS, RI, STD | 1349 | 1351 | - | 0.20 ± 0.01 | 0.10 ± 0.00 | 0.10 ± 0.01 | - |
| 25 | α-copaene | 3856-25-5 | MS, RI, STD | 1376 | 1378 | 0.60 ± 0.01 | 0.20 ± 0.02 | 0.20 ± 0.01 | 0.20 ± 0.02 | - |
| 26 | ß-elemene | 515-13-9 | MS, RI | 1388 | 1394 | 1.20 ± 0.04 | 2.10 ± 0.21 | 1.50 ± 0.01 | 1.80 ± 0.07 | 0.20 ± 0.1 |
| 27 | β-caryophyllene | 87-44-5 | MS, RI, STD | 1419 | 1423 | 5.80 ± 0.18 | 4.90 ± 0.55 | 4.10 ± 0.03 | 3.50 ± 0.06 | 0.50 ± 0.23 |
| 28 | cadina-4(14),5-diene | 54324-03-7 | MS, RI | 1430 | 1433 | 0.80 ± 0.06 | 1.80 ± 0.20 | 1.60 ± 0.03 | 1.30 ± 0.07 | - |
| 29 | γ-elemene | 3242-08-8 | MS, RI | 1432 | 1435 | 0.40 ± 0.02 | 1.10 ± 0.11 | 0.90 ± 0.01 | 0.90 ± 0.05 | - |
| 30 | α-humulene | 6753-98-6 | MS, RI, STD | 1456 | 1457 | 3.70 ± 0.21 | 1.90 ± 0.21 | 1.60 ± 0.01 | 1.50 ± 0.07 | 0.20 ± 0.09 |
| 31 | alloaromadendrene | 25246-27-9 | MS, RI | 1457 | 1465 | 0.90 ± 0.04 | 0.30 ± 0.03 | 0.10 ± 0.01 | 0.20 ± 0.08 | - |
| 32 | germacrene D | 23986-74-5 | MS, RI, STD | 1482 | 1485 | 0.50 ± 0.04 | 0.80 ± 0.10 | 1.10 ± 0.12 | 1.40 ± 0.18 | 0.20 ± 0.1 |
| 33 | ß-selinene | 17066-67-0 | MS, RI | 1483 | 1490 | - | - | 0.10 ± 0.02 | 0.10 ± 0.04 | - |
| 34 | α-selinene | 473-13-2 | MS, RI | 1488 | 1498 | - | - | 0.40 ± 0.01 | 0.40 ± 0.09 | - |
| 35 | γ-cadinene | 39029-4-9 | MS, RI | 1513 | 1517 | 0.90 ± 0.06 | 1.10 ± 0.08 | 0.70 ± 0.01 | 0.80 ± 0.05 | 0.20 ± 0.07 |
| 36 | δ-cadinene | 483-76-1 | MS, RI | 1524 | 1526 | 2.70 ± 0.14 | 3.30 ± 0.35 | 2.20 ± 0.02 | 2.70 ± 0.05 | 0.60 ± 0.15 |
| 37 | α-calacorene | 21391-99-1 | MS, RI | 1542 | 1547 | 0.50 ± 0.01 | - | - | - | - |
| 38 | elemol | 639-99-6 | MS, RI, STD | 1547 | 1552 | 0.10 ± 0.02 | 0.40 ± 0.04 | 0.20 ± 0.02 | - | - |
| 39 | spathulenol | 6750-60-3 | MS, RI | 1578 | 1582 | 5.20 ± 0.06 | - | - | - | - |
| 40 | caryophyllene oxide | 1139-30-6 | MS, RI, STD | 1583 | 1588 | 9.60 ± 0.29 | - | - | - | - |
| 41 | ζ-cadinol | 5937-11-1 | MS, RI | 1639 | 1645 | 1.20 ± 0.31 | 0.30 ± 0.06 | 0.10 ± 0.01 | 0.40 ± 0.04 | - |
| 42 | α-cadinol | 481-34-5 | MS, RI | 1650 | 1659 | 1.80 ± 0.24 | 0.50 ± 0.05 | - | 0.70 ± 0.08 | - |
| 43 | cadalene | 483-78-3 | MS, RI | 1678 | 1679 | - | - | - | 0.10 ± 0.02 | - |
| Monoterpene hydrocarbons (%) | 28.80 | 51.50 | 51.50 | 54.17 | 34.90 | |||||
| Oxygenated monoterpene (%) | 9.80 | 20.50 | 26.10 | 21.30 | 54.67 | |||||
| Sesquiterpene hydrocarbons (%) | 18.10 | 17.70 | 14.60 | 15.10 | 1.90 | |||||
| Oxygenated sesquiterpenes (%) | 25.10 | 6.90 | 4.70 | 6.90 | 6.10 | |||||
| Others (%) | 17.50 | 1.80 | 2.20 | 1.60 | 0.70 | |||||
| Identified compounds (%) | 99.30 | 98.40 | 99.10 | 99.07 | 98.27 | |||||
| Yield (%) | 1.42 | 1.51 | 1.22 | 1.13 | 1.14 | |||||
Identification methods: RIa, theoretical kovats indices (Pubchem and NIST); RIb, calculated kovats indices; MS, mass spectra comparison with PAL 600®libraries; STD, retention time and mass spectra comparison with commercially available standards; RI, retention index comparison with the literature; CAS number; -, Under perception threshold.
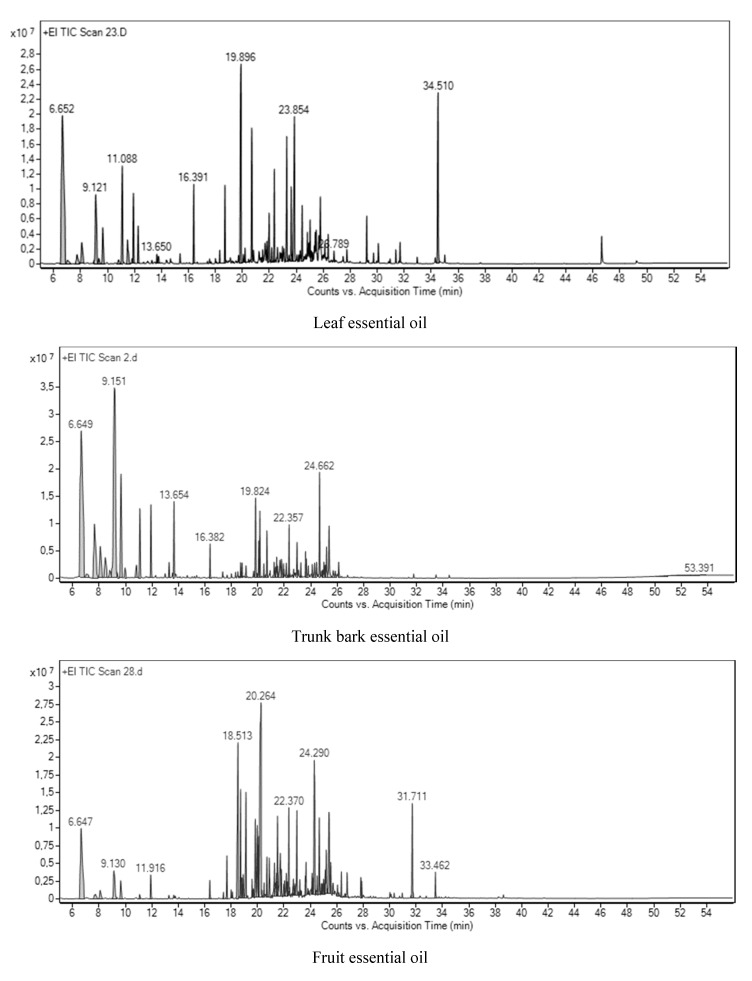
Essential oils hydrodistillated from Z. leprieurii organs were analyzed by GC/MS. Representative chromatograms for essential oils hydrodistillated from each organ are presented in Figure 1. Compounds accounting for 97.70–99.50% of global essential oil compositions were identified in the samples. Essential oils hydrodistillated from leaves were dominated by hydrocarbon sesquiterpenes, while methyl ketones were mainly present in trunk bark essential oils. Oxygenated and hydrocarbon monoterpenes were dominant in fruit essential oils. The major compounds identified in these oils were tridecan-2-one and β-caryophyllene in the leaf oils, tridecan-2-one in the trunk bark oils and β-myrcene in the fruit oils.
Figure 1.
Representative chromatograms for essential oils hydrodistillated from each Z. leprieurii organ.
3.1.1. Leaf Essential Oils
The analysis of essential oils hydrodistillated from leaves allowed for the identification of 42 compounds ranging from 97.70% to 99.50% of the total composition (Table 2). Sesquiterpenes (34.89–70.8%), methylketones (13.10–42.40%) and monoterpenes (4.5–36.18%) were the main components of these essential oils, which were dominated by tridecan-2-one (9.00% to 36.80%) and β-caryophyllene (7.00% to 19.85%). However, the composition of leaf essential oils was not constant over the collecting period, as some compounds that were present in only a minority in some samples were found in higher quantities during certain months. As a first example, there is a drop in tridean-2-one production in June, which is tricky to explain. This is also the case with (E)-β-ocimene, whose content was less than 4% from June to November, while in May, it was found to be at 23.57%. Caryophyllene oxide, which represented 5.7–6% of the total oil compositions from June to July, was only found in trace amounts during the other months. Undecan-2-one was also exceptionally present at 8% in May and August. Dendrolasin was present in significant amounts (4–16.4%) in all months except in May. This last molecule has well-known antimicrobial and antibacterial properties, and is also used in the treatment of cancer [42,43,44]. We also noticed the presence of thymol, an oxygenated monoterpene, at 13.30% in August. The chemical compositions of essential oils previously reported from two different Côte d’Ivoire locations [18] collected in February and November 2016 were different to those described in this study. Z. leprieurii leave essential oils thus exhibiting various chemotypes: for example, we describe here a chemotype with high proportions of dendrolasin. Moreover, the essential oil composition reported from Nigeria and Cameroon was dominated by limonene (94.90%) [19] and ocimene (91.5%) [40], showing that environmental or genetic factors impact essential oil compositions. Most of the major compounds that were detected in leaf essential oils are already known for their beneficial biological activities, such as insecticidal, antioxidant and anti-inflammatory activities. For example, the β-caryophyllene is a molecule characterized by high antioxidant and anti-inflammatory activities [45].
3.1.2. Trunk Bark Essential Oils
In total, 29 compounds were identified in the seven trunk bark oil samples, accounting for 98.30–99.40% of the whole composition (Table 3). Essential oils hydrodistillated from trunk bark were dominated by tridecan-2-one (45.26–78.80%) and α-humulene, which was also present in a significant content (4.3–12.73%). Hydrocarbon monoterpenes were only present as traces. As for leaf essential oils, the composition of essential oils hydrodistillated from the trunk bark was not consistent during the studied period. Indeed, some sesquiterpenes were only present in high a content during a given period: β-caryophyllene (8.1–13.20%) from May to July and September; tridecan-2-ol was found up to 10.10% in June; and (E,E)-farnesol (12.5% and 11.1%) in May and July, respectively. This chemical profile shows differences with those reported during our previous work in Côte d’Ivoire. Those differences may be due to the harvesting season and to the harvesting sites, which were not the same in those studies [18]. Moreover, these described compositions are different from those described in Nigeria, in which caryophyllene oxide (23.00%) and humulenol (17.50%) were the major components of trunk bark essential oil [18], showing that the essential oil composition is largely dependent on the plant localization.
In view of the use of tridecan-2-one in the food, pharmaceutical and cosmetic industry [46], trunk bark essential oils of Ivorian Z. leprieurii has a high potential. In addition to the major compounds, other minor molecules such as β-caryophyllene and α-humulene were also found in this essential oil, those having interesting antioxidant, anti-inflammatory, antibacterial and insecticidal effects, enhancing the potential use of this essential oil in the pharmaceutical industry [47,48].
3.1.3. Fruit Essential Oils
GC/MS analysis resulted in the identification of 43 constituents of the essential oils hydrodistillated from Z. leprieurii fruits (Table 4), accounting for 98.27–99.30% of the total essential oil compositions. This oil was dominated by β-myrcene (16.4–48.27%) but methyl nerate was also present in significant amounts (4.4–6.7%). Moreover, some minor compounds of certain months were present in high quantities in other samples. In particular, citronellol was present at 28.24% in November, but was lower than 6.6% the other months. Furthermore, geranial, which was present in traces in July, saw its content increase in the other months (5.3–6.10%). Some compounds were present in remarkable contents in July: (E)-β-ocimene (8.3%); perillene (6.5%); decanal (8.3%); spathulenol (5.2%); and caryophyllene oxide (9.6%).
The essential oils hydrodistillated from Z. leprieurii fruits during different months mainly contained monoterpenes hydrocarbons, which is in agreement with the chemical composition of fruit essential oils of the same species studied in Cameroon. Indeed, two different studies conducted in two distinct Cameroon sites showed citronellol (29.90% [20]; 17.37% [17]) and (E)-β-ocimene (44% [40]; 90.30% [49]) as the major compounds. Nevertheless, the chemical compositions characterized here are different from those already described. As essential oils obtained here and in previous studies were not hydrodistillated from plants growing in the same place and during the same period, differences in chemical compositions can be explained by climatic and environmental factors depending on each country, while one also cannot exclude a genetic influence that would combine with the other factors of variability.
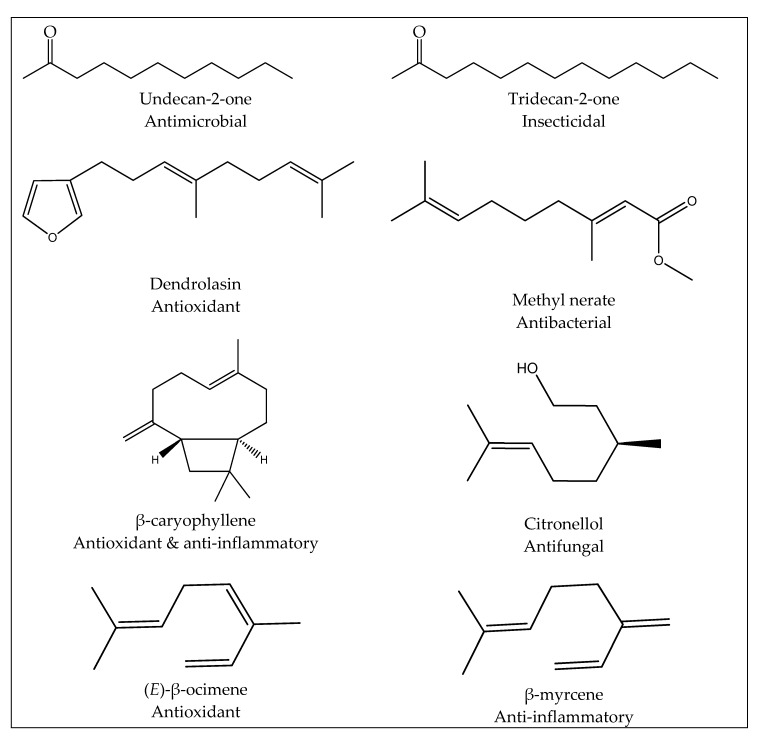
The chemical analysis of essential oils hydrodistillated from the different organs of Z. leprieurii from Côte d’Ivoire highlighted the presence of a wide range of compounds, most of them already known for their different interesting biological activities. The presence of those molecules can explain the various uses of Z. leprieurii in traditional medicine for the treatment of many different affections, as mentioned above. The main molecules found in Ivorian Z. leprieurii essential oils and their known biological properties are presented in Figure 2.
Figure 2.
Some major seasonal compounds present in the leaf, trunk bark and fruit essential oils of Z. leprieurii from Côte d’Ivoire and their known biological activities [45,50,51,52].
3.2. Seasonal Effect on Essential Oil Composition
HCA and PCA analysis were performed to investigate the seasonal effect on essential oil compositions.
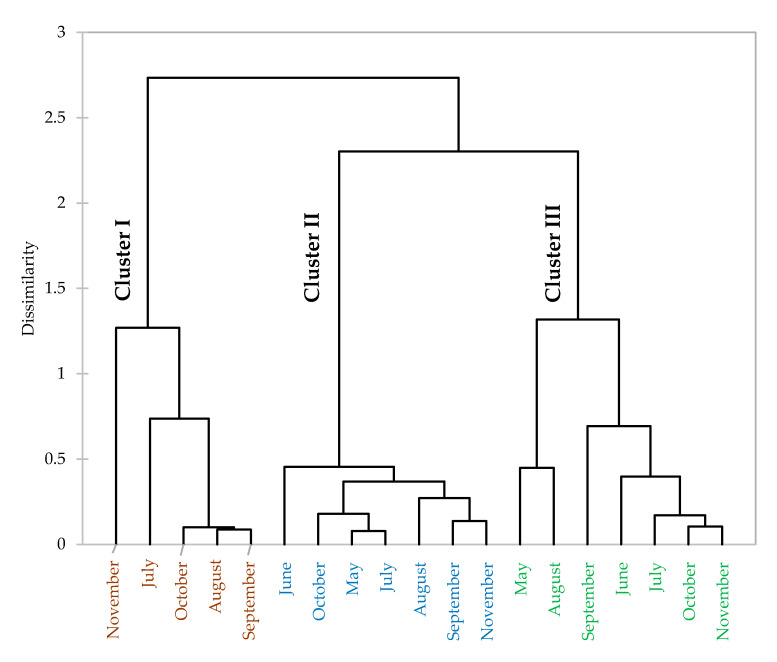
The HCA dendrogram (Figure 3), based on the Euclidean distance between collected samples, showed three distinct clusters, each one specific to one plant organ: (i) cluster I for fruits; (ii) cluster II for trunk bark; and (iii) cluster III for leaves. This shows that there is a significant difference in the composition of essential oils hydrodistillated from different Z. leprieurii organs. In addition, a seasonal effect was observed among each group, showing variation in the compositions of essential oils hydrodistillated from the same organ during the collection period. This seasonal effect was higher for the leaf essential oils, as the intra-class variance for those samples (2.50) was higher than for the fruit (1.77) and for the trunk bark (0.35) samples. It is possible that this higher variance for leaf essential oil samples is related to the fact that new leaves are produced all year long, while fruits are only produced at certain times of the year and the trunk bark develops very slowly over a period of several months or years. Leaves are then more susceptible to seasonal variations, such as levels of light exposure, state of maturity and water stress, than fruits and the trunk bark. Trunk bark essential oils have a lower chemical variability, probably due to the fact that the trunk bark formation is slow, and thus less impacted by environmental factors [53]. Results are supported by the fact that seasonal differences in the chemical composition of essential oils from fruits, trunk bark and leaves have already been highlighted for other Zanthoxylum species [21,23]. Indeed, while the chemical compositions of essential oils are genetically determined, it can be considerably modified by factors such as temperature, light, seasonality, water availability and nutrition. Biosynthesis of different compounds can be induced by environmental stimuli, which can change metabolic pathways [54,55].
Figure 3.
Dendrogram representing Zanthoxylum leprieurii essential oil samples. Cluster I: fruits; cluster II: trunk bark; and cluster III: leaves.
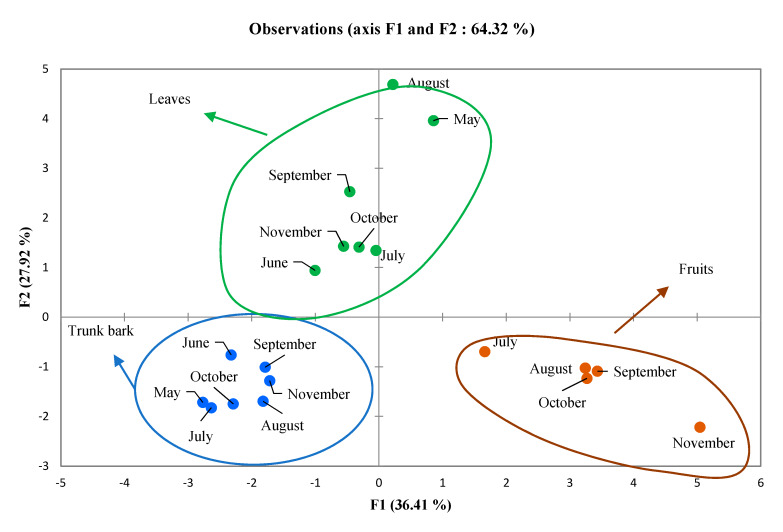
For the PCA analysis, the chemical composition data were projected through linear combinations of the 15 variables that were identified in all samples. Results showed that the first two axes (F1 and F2) explained 64.32% of the total variance (F1: 36.41% and F2: 27.92%). PCA results (Figure 4) showed three different specific clusters, each one being represented by one plant organ. Fruit essential oil samples in cluster I were mainly composed of β-myrcene (36.78 ± 14.67%), citronellol (9.98 ± 10.75%), geraniol (6.14 ± 4.42%), methyl nerate (5.64 ± 0.86%) and geraniol (4.36 ± 2.32%). Cluster II included trunk bark essential oil samples dominated by methylketones with tridecan-2-one (61.56 ± 12.65%) as the principal component. However, α- humulene (7.67 ± 2.71%), β-caryophyllene (6.82 ± 4.25%) and tridecan-2-ol (5.95 ± 2.58%) were also present in significant amounts. Cluster III included the leaf oil samples that were mainly composed of tridecan-2-one (24.11 ± 10.47%), β-caryophyllene (13.97 ± 4.94 %), dendrolasin (8.34 ± 4.72) and α-humulene (4.82 ± 1.29%). This group also showed high levels of (E)-β-ocimene (5.35 ± 8.56%), undecan-2-one (4.20 ± 3.34%), linalool (3.73 ± 2.89%), thymol (3.31 ± 4.91%), α-farnesene (3.16 ± 2.98%) and β-elemene (3.13 ± 1.83%).
Figure 4.
Principal component analysis of the chemical composition of essential oils hydrodistillated from Zanthoxylum leprieurii leaves, trunk bark and fruits from Côte d’Ivoire; described according to months and major compounds.
3.3. Essential oil Biological Activities
As mentioned previously, Z. leprieurii is widely used in traditional medicine for the treatment of different afflictions. Several authors have already supported those uses by reporting interesting biological properties of essential oils and solvent extracts obtained from this species growing in different places. However, it was shown here that Z. leprieurii essential oil chemical composition varies widely depending on the organ of the plant used and depending on the collection month. It is thus important to evaluate the biological activities of the essential oil hydrodistillated in this study, with regards to their compositions. Antioxidant, anti-inflammatory, insecticidal and anti-malarial properties of essential oils hydrodistillated from Z. leprieurii growing in Côte d’Ivoire were then evaluated. Essential oils used for the biological activity tests were selected based on their chemical composition. The August-selected leaf essential oil sample was characterized by high amounts of tridecan-2-one (30.20%), β-caryophyllene (13.70%) and thymol (13.30%). The major compounds of the chosen July trunk bark sample were tridecan-2-one (51.40%), (E,E)-farnesol (11.10%) and β-caryophyllene (8.50%). Finally, the July fruit sample was characterized by high proportions of β-myrcene (16.40%), caryophyllene oxide (9.60%), (E)-β-ocimene (8.30%) and decanal (8.30%).
3.3.1. Antioxidant Activity
DPPH Free Radical Scavenging Assay
According to Rice-Evans [56], the antioxidant activity of a compound corresponds to its ability to resist oxidation. The free radical scavenging ability of selected essential oils from Z. leprieurii leaves, trunk bark and fruits were determined using DPPH with Trolox as a positive control.
The results (Table 5) showed that all essential oil samples were able to reduce the stable DPPH radical to yellow diphenylpicrylhydrazine, with the scavenging effects increasing with higher essential oil concentrations (p-value ˂ 0.05). Leaf essential oil had the highest antioxidant activity (IC50: 33.12 ± 0.07 µg/mL), followed by trunk bark oil (IC50: 65.68 ± 0.12 µg/mL) and fruit oil (IC50: 103,55 ± 0.35 µg/mL). The comparison with the Trolox standard (29.13 ± 0.04 µg/mL) showed that the selected leaf essential oil sample has a high antioxidant activity. This activity could be due to the high contents in β-caryophyllene and thymol of this essential oil, as both of those molecules are already known for their antioxidant properties [57]. Those molecules were either present in lower quantities, or absent in the other tested essential oil samples (trunk bark and fruit).
Table 5.
Biological properties of essential oils hydrodistillated from different Z. leprieurii organs. DPPH: 2,2-diphenyl-1-picrylhydrazyl, LOX: lipoxygenase, BSA: bovine serum albumin.
| Organs and Standards | Biological Activities IC50 (µL/mL) | |||
|---|---|---|---|---|
| DPPH | LOX Denaturation | BSA Denaturation | Anti-Plasmodial | |
| Leaves | 33.12 ± 0.07 | 26.26 ± 0.04 | 26.08 ± 0.12 | 62.3 ± 3.4 |
| Trunk Bark | 65.68 ± 0.12 | 28.40 ± 0.02 | 35.07 ± 0.15 | 36.29 ± 4.2 |
| Fruits | 103.55 ± 0.35 | 32.42 ± 0.15 | 26.68 ± 0.09 | >100 |
| Trolox | 28.13 ± 0.04 | |||
| Quercetin | 21.57 ± 0.10 | |||
| Diclofenac | 21.90 ± 0.08 | |||
| Artemisinin | 0.004 ± 0.001 | |||
High DPPH free radical scavenging activity was also described in leaf essential oils from other Zanthoxylum species; with an IC50 value of 27.00 ± 0.1 µg/mL for Indian samples [58]. However, our results strongly differed to those of Tchabong [40], who obtained IC50 values of 770 µg/mL and 1800 µg/mL for Z. leprieurii fruit and leaf oils from Cameroon, respectively. Those differences in antioxidant properties of essential oil samples from the same species and families collected at different sites and at different periods are probably due to differences in their chemical compositions. Those differences may come from the studied organ, as we showed that essential oil composition variability mainly comes from the chosen organ, but also from genetic factors and/or environmental factors.
Ferric-Reducing Antioxidant Power
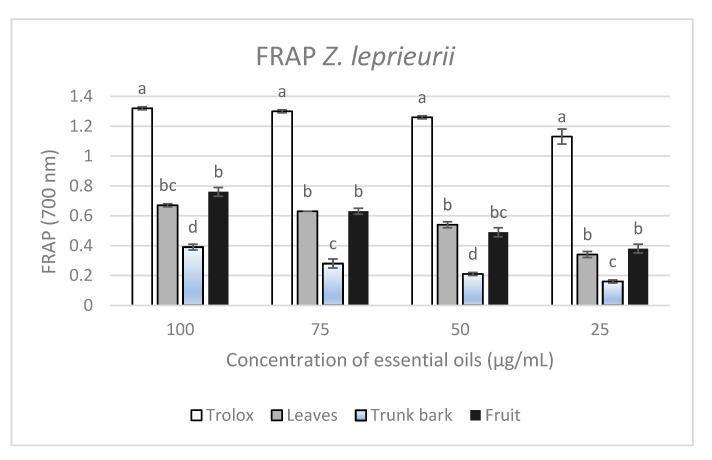
The ferric-reducing antioxidant power (FRAP) of essential oils extracted from leaves, trunk bark and fruits of Z. leprieurii was studied here for the first time. Results (Figure 5) showed that essential oils exhibited strong antioxidant activities, which were higher with increasing oil concentrations (p-value ˂ 0.05) [59]. Fruit and leaf essential oils exhibited higher FRAP activity than trunk bark oils. All these organs were compared to the Trolox, which represented the standard.
Figure 5.
The ferric-reducing power of essential oils hydrodistillated from leaves, trunk bark and fruits of Z. leprieurii. Mean values and standard deviation values were presented (n = 3). For a same concentration, data with the same letter were not significantly different from each other according to Duncan’s test (p-value ˂ 0.05).
Two different assays, DPPH and FRAP, were conducted in this study to evaluate the antioxidant potential of essential oils hydrodistillated from Z. leprieurii leaves, trunk bark and fruits. The two different tests resulted in dissimilar results, as leaf and fruit oils gave the highest and the lowest antioxidant activities with the DPPH free radical scavenging assay, respectively; while in the ferric-reducing antioxidant power assay, the highest antioxidant activities were obtained with leaf and fruit oils. Variations in the antioxidant activities of essential oils evaluated by DPPH and FRAP methods are probably due to the differences in reagents used by each method [60]. Indeed, the DPPH assay evaluates the ability of essential oils to scavenge free radicals, while the FRAP method assesses essential oils’ reducing power. The results obtained here showed that essential oils hydrodistillated from different Z. leprieurii organs have interesting antioxidant properties, which originate from two different modes of action: free radical scavenging and reducing abilities. The various compounds in essential oils hydrodistillated from Z. leprieurii organs are probably the origin of those different antioxidant activities [61,62]. For example, quantities of (E)-β-ocimene, perillene and caryophyllene oxide, which are known for their antioxidant properties [63,64], were found in the fruit oil sample, which were present in much lower proportions or completely absent in other essential oils.
3.3.2. Anti-Inflammatory Activity
In order to assess the anti-inflammatory potential of Z. leprieurii essential oils, their lipoxygenase inhibitory activity was evaluated, and the anti-denaturation method of bovine albumin serum (BSA) was also used.
Lipoxygenase Denaturation Inhibition Activity
The tested essential oils showed high to moderate lipoxygenase inhibitory activity (IC50: 26.26 ± 0.04 µg/mL, 28.40 ± 0.02 µg/mL and 32.42 ± 0.15 µg/mL for leaf, trunk bark and fruit oils, respectively) when compared to standard Quercetin (21.57 ± 0.10 µg/mL) (Table 5). These results show that Z. leprieurii essential oils have anti-inflammatory properties, as has also been previously described with Z. leprieurii growing in different places [65,66].
Inhibition of Albumin Denaturation
In vitro anti-inflammatory properties of Z. leprieurii trunk bark, leaf and fruit essential oils were evaluated by the anti-denaturation method of bovine albumin serum (BSA) for the first time, in comparison with the control Diclofenac (IC50: 21.90 ± 0.08 µg/mL). The results (Table 5) showed that Z. leprieurii leaf, fruit and trunk bark essential oils have high-to-moderate anti-inflammatory activities, with IC50 values of 26.08 ± 0.12 µg/mL, 26.68 ± 0.09 µg/mL and 35.07 ± 0.15 µg/mL, respectively. Moreover, the percentage of BSA protection was dependent on essential oil concentrations (p-value ˂ 0.05). The origin of these high lipoxygenase inhibitory activities could be the difference in organ content of monoterpenes, methylketones and sesquiterpenes, which are known for their anti-inflammatory activities [67,68,69].
3.3.3. Insecticidal Activity
Losses due to insect infestation during grain storage are a serious problem around the world, and more acutely in developing countries. Consumption of grains is not the only loss caused by insects, as a high level of pest detritus also leads to grains being unfit for human consumption in terms of quality. It could be estimated that one third of the world’s food production is destroyed by insects every year, which represents more than $100 billion. The highest losses occur in developing countries (43%), such as Côte d’Ivoire [70]. In the tropical zone, average losses range from 20% to 30%, while in the temperate zones, losses are from 5% to 10% [71]. Moreover, the trend to use natural insecticides to avoid chemical residues in food is growing.
The insecticidal activities of Z. leprieurii trunk bark, leaf and fruit essential oils were evaluated against Sitophilus granarius, one of the most damaging pests of stored cereals in the world. This insect is a primary pest, as it is able to drill holes in grains, laying its eggs inside them and allowing secondary pests to develop [33].
Results showed that all essential oils were efficient to kill insects in 24 h, with trunk bark oil showing the highest insecticidal activity (LC50 = 8.87 µL/mL) in comparison with leaf and fruit essential oils (LC50 = 15.77 µL/mL and 11.26 µL/mL, respectively); those activities were slightly lower than those of the chemical insecticide Talisma UL (LC50 = 3.44 µL/mL). Moreover, in comparison with cinnamon (Cinnamomum zeylanicum) and clove (Syzygium aromaticum) essential oils, generally described as exhibiting high insecticidal activities [72], LC50 of Z. leprieurii essential oils is lower, showing better insecticidal activities of the latter and thus promising prospects for application in the protection of stored foodstuffs. Concerning LC90 and LC95, results showed that the chemical insecticide (LC90 = 27.83 µL/mL, LC95 = 56.66 µL/mL) was less effective than leaf essential oil (LC90 = 26.27 µL/mL, LC95 = 31.26 µL/mL) and trunk bark essential oil (LC90 = 23.69 µL/mL, LC95 = 33.10 µL/mL), but more effective than fruit essential oils (LC90 = 93.20 µL/mL, LC95 = 191.20 µL/mL). These data indicate that the insecticidal effect of essential oils varies depending on the chemical composition and synergistic effects occurring between the compounds [73]. In this study, essential oils hydrodistillated from Z. leprieurii organs had an interesting effect on Sitophilus granarius adults, as insecticidal activities were better than those of Z. fagara and Z. monoplyllum (LC50 of 153.9 µL/mL and 140.1 µL/mL, respectively) [74]; and the LC50 was better than those reported on larvicidal activity [75] with Z. leprieurii extracts and Z. avicennae essential oil [76]. It should be noted that chemical composition of essential oils is different among these Zanthoxylum species and, according to the author [77], mortality evolution showed that toxicity depends on aspects such as the chemical composition and the target insect sensitivity.
The repulsive effect of Z. leprieurii trunk bark, leaf and fruit essential oils and chemical insecticides were also evaluated by the McDonald method. The results (Table 6) showed a high repulsive effect for the trunk bark essential oil (88.83%), followed by leaf essential oil (76.66%) and fruit essential oil (61.00%), in comparison with the low repulsive effect of the chemical insecticide Talisma UL (24.78%). Furthermore, repellent properties were dose–response correlated and high when compared to other essential oils considered to be highly repulsive [78].
Table 6.
Repulsion percentage of Sitophilus granarius after 2 h of treatment with essential oils and Talisma UL. Effect of substance tested [35].
| Tested Substances | Average Repulsion (%) | Class | Effect of Substance Tested |
|---|---|---|---|
| Leaf essential oil | 76.66 | IV | Repulsive |
| Trunk bark essential oil | 88.83 | V | Highly repulsive |
| Fruit essential oil | 61.00 | III | Mildly repulsive |
| Talisma UL | 24.78 | II | Weakly repulsive |
3.3.4. Anti-Plasmodial Activity
The anti-plasmodial activity of Z. leprieurii trunk bark, leaf and fruit essential oils was evaluated here for the first time. The results (Table 5) showed that trunk bark essential oil has a moderate anti-plasmodial activity (IC50: 37.49 ± 4.2 µg/mL), and leaf essential oil has a low activity (IC50: 59.30 ± 3.4 µg/mL), in comparison with the artemisinin standard (IC50: 0.004 ± 0.001 µg/mL). No significant anti-plasmodial activity was highlighted for the fruit essential oil (IC50 > 100). The moderate trunk bark anti-plasmodial activity may be due to methylketones, as tridecan-2-one is the dominant compound in this oil. However, no studies have yet shown the anti-plasmodial activity of this molecule. Moreover, it is possible that the highlighted activity comes from the presence of minor compounds, as well as from the synergy between different molecules. Nevertheless, studies were carried out on Z. leprieurii and other species of Z. chalybeum and Z. zanthoxyloides plant extracts, showing high anti-plasmodial activities [79,80,81,82], all of which supports the effective use of Zanthoxylum species in traditional medicine for the treatment of malaria.
4. Conclusions
In this study, the variability in the chemical composition of leaf, trunk bark and fruit essential oils hydrodistillated from Ivorian Z. leprieurii was studied for the first time over seven months for leaves and trunk bark, and five months for fruits. Results showed that essential oils were mainly dominated by sesquiterpenes (β-caryophyllene, dendrolasin and thymol), methylketones (tridecan-2-one and undecane-2 one) and monoterpenes (β-myrcene, (E)-β-ocinene and perillene) in leaf, trunk bark and fruit samples, respectively. Statistical PCA and HCA analysis showed that the variability in essential oil compositions mainly comes from the organ, as all samples were clustered in three groups, each one corresponding to one organ. However, differences in essential oil compositions inside each cluster were highlighted, showing the probable impact of the seasonal effect on essential oil compositions. Those differences in essential oil compositions may be due to different seasonal parameters, as it was shown here that the temperature, precipitations and humidity were not constant during the plant collecting period. However, it is also known that biotic factors, such as pest attacks, widely impact essential oil chemical compositions. Those were not recorded during this study, but may also be at the origin of essential oil variability. As a perspective, it would be interesting to study their impact on Z. leprieurii essential oil variability. Moreover, the study was conducted with plants growing on the same site. The comparison of the present results with those of the existing literature considering Z. leprieurii plants growing in other countries showed totally different essential oil compositions, showing that genetic differences might also induce dissimilar essential oil compositions, resulting in distinct essential oil chemotypes.
Z. leprieurii is widely used in traditional medicine for the treatment of different diseases, such as rheumatism, tuberculosis, urinary infections and generalized body pains. In order to explain those uses, in-vitro biological activities of hydrodistillated essential oils were studied here. Results obtained in this study showed strong antioxidant, anti-inflammatory and moderate anti-plasmodial activities. Moreover, expected results also showed high differences in the biological activities of essential oils linked with their differences in chemical composition, which should be taken into account in future research on Z. leprieurii essential oil biological activities, but also to find the proper plant harvesting moment for a use in traditional medicine. However, while those results are promising and confirm the relevance of the traditional uses of these plants, in-vitro experiments should be supported by in-vivo tests, as differences can be observed between in-vitro and in-vivo test results.
Grain storage is particularly problematic, as pests cause large losses. Ivoirian essential oils from Z. leprieurii demonstrated an interesting repellent effect and contact toxicity properties against Sitophilus granarius with the essential oils extracted from the three organs tested (trunk bark, leaves and fruits). All these essential oils are promising candidates for developing new plant insecticides to protect stored products. Moreover, according to De Lucas and colleagues [83], parts of the plant could also be used in silos directly without the extraction step of the essential oils to control pest losses. Indeed, this practice is widely used in Africa because plant material is readily available and usable without any transformation. Moreover, it is easy to separate the plant material added to the silo from the grain for use as food or feed. It would then be interesting to study the insecticidal properties of Z. leprieurii organs in that way.
In conclusion, Z. leprieurii from Côte d’Ivoire as a medicinal and aromatic plant provides interesting sources of biologically active compounds, such as antioxidants, anti-inflammatory agents and natural insecticides. The results obtained here support the current uses of this plant in traditional medicine, but also highlight the importance of the location and the season on chemical composition and thus biological properties.
Acknowledgments
We would like to thank the members of the Laboratory of Biological Organic Chemistry of the University Felix Houphouet Boigny of Cocody (Côte d’Ivoire) and all the laboratory staff of Chemistry of Natural Molecules, University of Liege (Gembloux Agro-Bio Tech) for their scientific contribution particularly Danny Trisman, Saskia Sergeant, Thomas Bertrand, Franck Michels, Marie Davin, Laurie Josselin, Pierre-Yves Werrie and Clement Burgeon. We are also grateful to Felicien for his support.
Author Contributions
Conceptualization, E.A.T., M.-L.F. and Z.F.T.; methodology, E.A.T.; software, E.A.T., G.B.B., E.L.W. and H.M.; validation, M.-L.F. and Z.F.T.; formal analysis, E.A.T.; investigation, E.A.T. and F.N.; resources, E.A.T. and M.-L.F.; data curation, E.A.T., M.G., H.M., G.B.B., E.L.W. and A.L.; writing—original draft preparation, E.A.T., M.G. and H.M.; writing—review and editing, M.G., M.-L.F. and Z.F.T.; visualization, E.A.T. and M.G.; supervision, M.-L.F., M.F. and Z.F.T.; project administration, M.-L.F. and Z.F.T.; funding acquisition, M.-L.F. and Z.F.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Education, Audiovisual and Culture Executive Agency (EACEA) trough EOHUB project 600873-EPP-1-2018-1ES-EPPKA2-KA.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Gonçalves M.A.M. Ph.D. Thesis. Universidade de Lisboa; Lisboa, Portugal: 2018. Micromorphology and in vitro Antibacterial Evaluation of Zanthoxylum and Hymenocardia Species. [Google Scholar]
- 2.Dongmo P.M.J., Tchoumbougnang F., Sonwa E.T., Kenfack S.M., Zollo P.H.A., Menut C. Antioxidant and anti-inflammatory potential of essential oils of some Zanthoxylum (Rutaceae) of Cameroon. Int. J. Essent. Oil. 2008;2:82–88. [Google Scholar]
- 3.Sriwichai T., Sookwong P., Siddiqui M.W., Sommano S.R. Aromatic profiling of Zanthoxylum myriacanthum (makwhaen) essential oils from dried fruits using different initial drying techniques. Ind. Crops Prod. 2019;133:284–291. doi: 10.1016/j.indcrop.2019.03.031. [DOI] [Google Scholar]
- 4.Bunalema L., Obakiro S., Tabuti J.R., Waako P. Knowledge on plants used traditionally in the treatment of tuberculosis in Uganda. J. Ethnopharmacol. 2014;151:999–1004. doi: 10.1016/j.jep.2013.12.020. [DOI] [PubMed] [Google Scholar]
- 5.Burkil H.M. The Useful Plants of West Tropical Africa. Volume 2 Royal Botanic Gardens; London, UK: 1994. Royal Botanic Gardens; Kew Year. [Google Scholar]
- 6.Tine Y., Renucci F., Costa J., Wélé A., Paolini J. A Method for LC-MS/MS profiling of coumarins in Zanthoxylum xanthoxyloides (Lam.) B. Zepernich and Timler extracts and essential oils. Molecules. 2017;22:174. doi: 10.3390/molecules22010174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mester I. In: Chemistry and Chemical Taxonomy of the Rutales. Waterman P.G., Grundon M.F., editors. Academic Press; London, UK: 1983. pp. 31–96. [Google Scholar]
- 8.Kpomah E.D., Uwakwe A.A., Abbey B.W. Aphrodisiac studies of diherbal mixture of Zanthoxylum leprieurii Guill. & Perr. And Piper guineense Schumach. & Thonn. on male wistar rats. GJRMI. 2012;1:381. [Google Scholar]
- 9.Agyare C., Kisseih E., Kyere I.Y., Ossei P.P.S. Medicinal plants used in wound care: Assessment of wound healing and antimicrobial properties of Zanthoxylum Leprieurii. IBSPR. 2014;2:81–89. [Google Scholar]
- 10.Bunalema L., Fotso G.W., Waako P., Tabuti J., Yeboah S.O. Potential of Zanthoxylum leprieurii as a source of active compounds against drug resistant Mycobacterium tuberculosis. BMC Complement. Altern. Med. 2017;17:89. doi: 10.1186/s12906-017-1602-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guetchueng S.T., Nahar L., Ritchie K.J., Ismail F., Wansi J.D., Evans A.R., Sarker S.D. Kaurane diterpenes from the fruits of Zanthoxylum leprieurii (Rutaceae) Rec. Nat. Prod. 2017;11:304–309. [Google Scholar]
- 12.Zondegoumba E.N.T., Dibahteu W.L., de Araujo A., Vidari G., Liu Y., Luo S., Li S., Junior F.J.B.M., Scotti L., Tullius M. Cytotoxic and Schistosomidal Activities of Extract, Fractions and Isolated Compounds from Zanthoxylum Leprieurii (Rutaceae) IJSBAR. 2019;44:209–222. [Google Scholar]
- 13.Ngoumfo R.M., Jouda J.B., Mouafo F.T., Komguem J., Mbazoa C.D., Shiao T.C., Choudhary M.I., Laatsch H., Legault J., Pichette A., et al. In vitro cytotoxic activity of isolated acridones alkaloids from Zanthoxylum leprieurii Guill. et Perr. Bioorg. Med. Chem. 2010;18:3601–3605. doi: 10.1016/j.bmc.2010.03.040. [DOI] [PubMed] [Google Scholar]
- 14.Adesina S.K. The Nigerian Zanthoxylum: Chemical and biological values. AJTCAM. 2005;2:282–301. doi: 10.4314/ajtcam.v2i3.31128. [DOI] [Google Scholar]
- 15.Misra L.N., Wouatsa N.V., Kumar S., Kumar R.V., Tchoumbougnang F. Antibacterial, cytotoxic activities and chemical composition of fruits of two Cameroonian Zanthoxylum species. J. Ethnopharmacol. 2013;148:74–80. doi: 10.1016/j.jep.2013.03.069. [DOI] [PubMed] [Google Scholar]
- 16.Tatsadjieu L.N., Ngang J.E., Ngassoum M.B., Etoa F.X. Antibacterial and antifungal activity of Xylopia aethiopica, Monodora myristica, Zanthoxylum xanthoxyloıdes and Zanthoxylum leprieurii from Cameroon. Fitoterapia. 2003;74:469–472. doi: 10.1016/S0367-326X(03)00067-4. [DOI] [PubMed] [Google Scholar]
- 17.Gardini F., Belletti N., Ndagijimana M., Guerzoni M.E., Tchoumbougnang F., Zollo P.H.A., Micci C., Lanciotti R., Kamdem S.L.S. Composition of four essential oils obtained from plants from Cameroon, and their bactericidal and bacteriostatic activity against Listeria monocytogenes, Salmonella enteritidis and Staphylococcus aureus. Afr. J. Microbiol. Res. 2009;3:264–271. [Google Scholar]
- 18.Tanoh E.A., Nea F., Yapi T.A., Boué G.B., Jean-Brice B., Tomi F., Tonzibo Z.F. Essential Oil of Zanthoxylum lepreurii Guill. & Perr. Rich in Undecan-2-One and Tridecan-2-One. J. Essent. Oil-Bear. Plants. 2018;21:1397–1402. [Google Scholar]
- 19.Oyedeji A.O., Lawal O.A., Adeniyi B.A., Alaka S.A., Tetede E. Essential oil composition of three Zanthoxylum species. J. Essent. Oil Res. 2008;20:69–71. doi: 10.1080/10412905.2008.9699425. [DOI] [Google Scholar]
- 20.Fogang H.P., Tapondjou L.A., Womeni H.M., Quassinti L., Bramucci M., Vitali L.A., Petrelli D., Lupidi G., Maggi F., Papa F., et al. Characterization and biological activity of essential oils from fruits of Zanthoxylum xanthoxyloides Lam. and Z. leprieurii Guill. & Perr., two culinary plants from Cameroon. Flavour Fragr. J. 2012;27:171–179. [Google Scholar]
- 21.Eiter L.C., Fadamiro H., Setzer W.N. Seasonal variation in the leaf essential oil composition of Zanthoxylum clava-herculis growing in Huntsville, Alabama. Nat. Prod. Commun. 2010;5:457–460. doi: 10.1177/1934578X1000500323. [DOI] [PubMed] [Google Scholar]
- 22.Kim J.H. Seasonal variations in the content and composition of essential oil from Zanthoxylum Piperitum. J. Ecol. Field Biol. 2012;35:195–201. doi: 10.5141/JEFB.2012.024. [DOI] [Google Scholar]
- 23.Bhatt V., Sharma S., Kumar N., Sharma U., Singh B. Chemical Composition of Essential Oil among Seven Populations of Zanthoxylum armatum from Himachal Pradesh: Chemotypic and Seasonal Variation. Nat. Prod. Commun. 2017;12:1643–1646. [Google Scholar]
- 24.Benini C., Ringuet M., Wathelet J.P., Lognay G., du Jardin P., Fauconnier M.L. Variations in the essential oils from ylang-ylang (Cananga odorata [Lam.] Hook f. & Thomson forma genuina) in the Western Indian Ocean islands. Flavour Fragr. J. 2012;27:356–366. [Google Scholar]
- 25.Bettaieb Rebey I., Bourgou S., Aidi Wannes W., Hamrouni Selami I., Saidani Tounsi M., Marzouk B., Fauconnier M.L., Ksouri R. Comparative assessment of phytochemical profiles and antioxidant properties of Tunisian and Egyptian anise (Pimpinella anisum L.) seeds. Plant Biosyst. 2018;152:971–978. doi: 10.1080/11263504.2017.1403394. [DOI] [Google Scholar]
- 26.Lins L., Dal Maso S., Foncoux B., Kamili A., Laurin Y., Genva M., Jijakli M.H., De Clerck C., Fauconnier M.-L., Deleu M. Insights into the relationships between herbicide activities, molecular structure and membrane interaction of cinnamon and citronella essential oils components. Int. J. Mol. Sci. 2019;20:4007. doi: 10.3390/ijms20164007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tanoh E.A., Nea F., Kenne Kemene T., Genva M., Saive M., Tonzibo F.Z., Fauconnier M.L. Antioxidant and lipoxygenase inhibitory activities of essential oils from endemic plants of Côte d’Ivoire: Zanthoxylum mezoneurispinosum Ake Assi and Zanthoxylum psammophilum Ake Assi. Molecules. 2019;24:2445. doi: 10.3390/molecules24132445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang B., Qu J., Feng S., Chen T., Yuan M., Huang Y., Ding C. Seasonal Variations in the Chemical Composition of Liangshan Olive Leaves and Their Antioxidant and Anticancer Activities. Foods. 2019;8:657. doi: 10.3390/foods8120657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee J.H., Kang B.S., Hwang K.H., Kim G.H. Evaluation for anti-inflammatory effects of Siegesbeckia glabrescens extract in vitro. Food Agric. Immunol. 2011;22:145–160. doi: 10.1080/09540105.2010.549210. [DOI] [Google Scholar]
- 30.Nikhila G.S., Sangeetha G. Anti-inflammatory properties of the root tubers of Gloriosa superba and its conservation through micropropagation. J. Med. Plants Res. 2015;9:1–7. doi: 10.5897/JMPR2014.5512. [DOI] [Google Scholar]
- 31.Kar B., Kumar R.S., Karmakar I., Dola N., Bala A., Mazumder U.K., Hadar P.K. Antioxidant and in vitro anti-inflammatory activities of Mimusops elengi leaves. Asian Pac. J. Trop. Biomed. 2012;2:S976–S980. doi: 10.1016/S2221-1691(12)60346-3. [DOI] [Google Scholar]
- 32.Nea F., Tanoh E.A., Wognin E.L., Kemene T.K., Genva M., Saive M., Tonzibo Z.F., Fauconnier M.L. A new chemotype of Lantana rhodesiensis Moldenke essential oil from Côte d’Ivoire: Chemical composition and biological activities. Ind. Crops Prod. 2019;141:111766. doi: 10.1016/j.indcrop.2019.111766. [DOI] [Google Scholar]
- 33.Fleurat-Lessard F. Gestion intégrée de la protection des stocks de céréales contre les insectes sans traitement insecticide rémanent. Phytoma. 2018;716:32–40. [Google Scholar]
- 34.Darwish Y.A., Omar Y.M., Hassan R.E., Mahmoud M.A. Repellent effects of certain plant essential oil, plant extracts and inorganic salts to granary weevil, Sitophilus granarius (L.) Arch. Arch. Phytopathol. Pflanzenschutz. 2013;46:1949–1957. doi: 10.1080/03235408.2013.781343. [DOI] [Google Scholar]
- 35.Mc Donald L.L., Guyr H., Speire R.R. Preliminary evaluation of new candidate materials as toxicants, repellents, and attractants against stored-product insects. Mark. Res. Rep. 1970;882:189. [Google Scholar]
- 36.Ledoux A., St-Gelais A., Cieckiewicz E., Jansen O., Bordignon A., Illien B., Di Giovanni N., Marvilliers A., Hoareau F., Pendeville H., et al. Antimalarial activities of alkyl cyclohexenone derivatives isolated from the leaves of Poupartia borbonica. J. Nat. Prod. 2017;80:1750–1757. doi: 10.1021/acs.jnatprod.6b01019. [DOI] [PubMed] [Google Scholar]
- 37.BEI Reagent Search. [(accessed on 27 July 2017)]; Available online: https://www.beiresources.org/Catalog/BEIParasiticProtozoa/MRA-102.aspx.
- 38.Bordignon A., Frédérich M., Ledoux A., Campos P.E., Clerc P., Hermann T., Quetin-Leclercq J., Cieckiewicz E. In vitro antiplasmodial and cytotoxic activities of sesquiterpene lactones from Vernonia fimbrillifera Less. (Asteraceae) Nat. Prod. Res. 2018;32:1463–1466. doi: 10.1080/14786419.2017.1350665. [DOI] [PubMed] [Google Scholar]
- 39.Makler M.T., Ries J.M., Williams J.A., Bancroft J.E., Piper R.C., Gibbins B.L., Hinrichs D.J. Parasite lactate dehydrogenase as an assay for Plasmodium falciparum drug sensitivity. Am. J. Trop. Med. Hyg. 1993;48:739–741. doi: 10.4269/ajtmh.1993.48.739. [DOI] [PubMed] [Google Scholar]
- 40.Tchabong F., Sameza S.R., Tchameni M.L., Mounbain N.S., Mouelle F., Jazet S.A., Menut D.P.M., Tchoumbougnang C. Chemical composition, free radical scavenging and antifungal activity of Zanthoxylum leprieurii essential oils against Epidermophyton floccosum and Microsporum gypseum, two most prevalent cutaneous Mycosis. J. Pharm. 2018;8:13–19. [Google Scholar]
- 41.Shams M., Esfahan S.Z., Esfahan E.Z., Dashtaki H.N., Dursun A., Yildirim E. Effects of climatic factors on the quantity of essential oil and dry matter yield of coriander (Coriandrum sativum L.) INDJSRT. 2016;9:1–4. doi: 10.17485/ijst/2016/v9i6/61301. [DOI] [Google Scholar]
- 42.Azzaz Nabil A.E., El-Khateeb Ayman Y., Farag Ayman A. Chemical composition and biological activity of the essential oil of Cyperus articulatus. Int. J. Acad. Res. 2014;6:265–269. [Google Scholar]
- 43.Choucry M.A. Chemical composition and anticancer activity of Achillea fragrantissima (Forssk.) Sch. Bip. (Asteraceae) essential oil from Egypt. J. Pharm. Phytother. 2017;9:1–5. [Google Scholar]
- 44.Azizan N., Mohd Said S., Zainal Abidin Z., Jantan I. Composition and antibacterial activity of the essential oils of Orthosiphon stamineus Benth and Ficus deltoidea Jack against Pathogenic Oral Bacteria. Molecules. 2017;22:2135. doi: 10.3390/molecules22122135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dahham S.S., Tabana Y.M., Iqbal M.A., Ahamed M.B., Ezzat M.O., Majid A.S., Majid A.M. The anticancer, antioxidant and antimicrobial properties of the sesquiterpene β-caryophyllene from the essential oil of Aquilaria crassna. Molecules. 2015;20:11808–11829. doi: 10.3390/molecules200711808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Park J., Rodríguez-Moyá M., Li M., Pichersky E., San K.Y., Gonzalez R. Synthesis of methyl ketones by metabolically engineered Escherichia coli. J. Ind. Microbiol. Biotechnol. 2012;39:1703–1712. doi: 10.1007/s10295-012-1178-x. [DOI] [PubMed] [Google Scholar]
- 47.Pareja M., Qvarfordt E., Webster B., Mayon P., Pickett J., Birkett M., Glinwood R. Herbivory by a phloem-feeding insect inhibits floral volatile production. PLoS ONE. 2012;7:e31971. doi: 10.1371/journal.pone.0031971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Saini M., Wang Z.W., Chiang C.J., Chao Y.P. Metabolic engineering of Escherichia coli for production of butyric acid. J. Agric. Food Chem. 2014;62:4342–4348. doi: 10.1021/jf500355p. [DOI] [PubMed] [Google Scholar]
- 49.Lamaty G., Menut C., Bessiere J.M., Aknin M. Aromatic plants of tropical Central Africa. II. A comparative study of the volatile constituents of Zanthoxylum leprieurii (Guill. et Perr.) Engl. and Zanthoxylum tessmannii Engl. leaves and fruit pericarps from the Cameroon. Flavour Fragr. J. 1989;4:203–205. doi: 10.1002/ffj.2730040411. [DOI] [Google Scholar]
- 50.Pereira F.D., Mendes J.M., Lima I.O., Mota K.S., Oliveira W.A., Lima E.D. Antifungal activity of geraniol and citronellol, two monoterpenes alcohols, against Trichophyton rubrum involves inhibition of ergosterol biosynthesis. Pharm. Biol. 2015;53:228–234. doi: 10.3109/13880209.2014.913299. [DOI] [PubMed] [Google Scholar]
- 51.Watson A.D. Lipidomics: A global approach to lipid analysis in biological systems. J. Lipid Res. 2006;47:2101–2111. doi: 10.1194/jlr.R600022-JLR200. [DOI] [PubMed] [Google Scholar]
- 52.Mirghaed A.T., Yasari M., Mirzargar S.S., Hoseini S.M. Rainbow trout (Oncorhynchus mykiss) anesthesia with myrcene: Efficacy and physiological responses in comparison with eugenol. Fish Physiol. Biochem. 2018;44:919–926. doi: 10.1007/s10695-018-0481-5. [DOI] [PubMed] [Google Scholar]
- 53.Geng S., Cui Z., Huang X., Chen Y., Xu D., Xiong P. Variations in essential oil yield and composition during Cinnamomum cassia bark growth. Ind. Crops Prod. 2011;33:248–252. doi: 10.1016/j.indcrop.2010.10.018. [DOI] [Google Scholar]
- 54.Gobbo-Neto L., Lopes N.P. Plantas medicinais: Fatores de influência no conteúdo de metabólitos secundários. Quim. Nova. 2007;30:374–381. doi: 10.1590/S0100-40422007000200026. [DOI] [Google Scholar]
- 55.Lima H.R.P., Kaplan M.A.C., Cruz A.V.D.M. Influência dos fatores abióticos na produção e variabilidade de terpenóides em plantas. Flor. Am. 2012;10:71–77. [Google Scholar]
- 56.Rice-evans C.A., Miller N.J., Bolwell P.G., Bramley P.M., Pridham J.B. The relative antioxidant activities of plant-derived polyphenolic flavonoids. Free Radic. Res. 1995;22:375–383. doi: 10.3109/10715769509145649. [DOI] [PubMed] [Google Scholar]
- 57.Torres-Martínez R., García-Rodríguez Y.M., Ríos-Chávez P., Saavedra-Molina A., López-Meza J.E., Ochoa-Zarzosa A., Garciglia R.S. Antioxidant activity of the essential oil and its major terpenes of Satureja macrostema (Moc. and Sessé ex Benth.) Briq. Pharmacogn. Mag. 2017;13:S875–S880. doi: 10.4103/pm.pm_316_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Negi J.S., Bisht V.K., Bhandari A.K., Bisht R., Kandari Negi S. Major constituents, antioxidant and antibacterial activities of Zanthoxylum armatum DC. essential oil. IJPT. 2012;11:68–72. [Google Scholar]
- 59.Gogoi R., Loying R., Sarma N., Munda S., Pandey S.K., Lal M. A comparative study on antioxidant, anti-inflammatory, genotoxicity, anti-microbial activities and chemical composition of fruit and leaf essential oils of Litsea cubeba Pers from North-east India. Ind. Crops Prod. 2018;125:131–139. doi: 10.1016/j.indcrop.2018.08.052. [DOI] [Google Scholar]
- 60.Ouedraogo R.A., Koala M., Dabire C., Hema A., Bazie V.B.E.J.T., Outtara L.P., Nebie R.H. Teneur en phénols totaux et activité antioxydante des extraits des trois principales variétés d’oignons (Allium cepa L.) cultivées dans la région du Centre-Nord du Burkina Faso. IJBCS. 2015;9:281–291. doi: 10.4314/ijbcs.v9i1.25. [DOI] [Google Scholar]
- 61.Huang D., Ou B., et Prior R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005;53:1841–1856. doi: 10.1021/jf030723c. [DOI] [PubMed] [Google Scholar]
- 62.Foti M.C., Daquino C., Geraci C. Electron-transfer reaction of cinnamic acids and their methyl esters with the DPPH• radical in alcoholic solutions. J. Org. Chem. 2004;69:2309–2314. doi: 10.1021/jo035758q. [DOI] [PubMed] [Google Scholar]
- 63.Ghasemi P.A., Izadi A., Malek P.F., Hamedi B. Chemical composition, antioxidant and antibacterial activities of essential oils from Ferulago angulata. Pharm. Biol. 2016;54:2515–2520. doi: 10.3109/13880209.2016.1162816. [DOI] [PubMed] [Google Scholar]
- 64.Kunwar G., Prakash O., Chandra M., Pant A.K. Chemical composition, antifungal and antioxidant activities of Perilla frutescens (L.) syn. P. ocimoides L. collected from different regions of Indian Himalaya. AJTM. 2013;8:88–98. [Google Scholar]
- 65.Negi J.S., Bisht V.K., Bhandari A.K., Singh P., Sundriyal R.C. Chemical constituents and biological activities of the genus Zanthoxylum: A review. AJPAC. 2011;5:412–416. [Google Scholar]
- 66.Adebayo S.A., Dzoyem J.P., Shai L.J., Eloff J.N. The anti-inflammatory and antioxidant activity of 25 plant species used traditionally to treat pain in southern African. BMC Complement. Altern. Med. 2015;15:159. doi: 10.1186/s12906-015-0669-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Da Silva K.A.B.S., Klein-Junior L.C., Cruz S.M., Cáceres A., Quintão N.L.M., Delle Monache F., Cechinel-Filho V. Anti-inflammatory and anti-hyperalgesic evaluation of the condiment laurel (Litsea guatemalensis Mez.) and its chemical composition. Food Chem. 2012;132:1980–1986. doi: 10.1016/j.foodchem.2011.12.036. [DOI] [Google Scholar]
- 68.Chen J., Wang W., Shi C., Fang J. A comparative study of sodium houttuyfonate and 2-undecanone for their in vitro and in vivo anti-inflammatory activities and stabilities. Int. J. Mol. Sci. 2014;15:22978–22994. doi: 10.3390/ijms151222978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kim D.S., Lee H.J., Jeon Y.D., Han Y.H., Kee J.Y., Kim H.J., Shin H.J., Kang J., Lee B.S., Kim S.H., et al. Alpha-pinene exhibits anti-inflammatory activity through the suppression of MAPKs and the NF-κB pathway in mouse peritoneal macrophages. Am. J. Chin. Med. 2015;43:731–742. doi: 10.1142/S0192415X15500457. [DOI] [PubMed] [Google Scholar]
- 70.Johnson F., Oussou K.R., Kanko C., Tonzibo Z.F., Foua-Bi K., Tano Y. Bioefficacité des Huiles Essentielles de Trois Espèces végétales (Ocimum gratissimum, Ocimum canum et Hyptis suaveolens), de la Famille des Labiées dans la lutte contre Sitophilus zeamais. Eur. J. Sci. Res. 2017;150:273–284. [Google Scholar]
- 71.Yallappa R., Nandagopal B., Thimmappa S. Botanicals as grain protectants. Psyche J. Entomol. 2012;2012:646740. [Google Scholar]
- 72.Plata-Rueda A., Campos J.M., da Silva Rolim G., Martínez L.C., Dos Santos M.H., Fernandes F.L., Serrão J.E., Zanuncio J.C. Terpenoid constituents of cinnamon and clove essential oils cause toxic effects and behavior repellency response on granary weevil, Sitophilus granarius. Ecotoxicol. Environ. Saf. 2018;156:263–270. doi: 10.1016/j.ecoenv.2018.03.033. [DOI] [PubMed] [Google Scholar]
- 73.Mansour S.A., El-Sharkawy A.Z., Abdel-Hamid N.A. Toxicity of essential plant oils, in comparison with conventional insecticides, against the desert locust, Schistocerca gregaria (Forskål) Ind. Crops Prod. 2015;63:92–99. doi: 10.1016/j.indcrop.2014.10.038. [DOI] [Google Scholar]
- 74.Prieto J.A., Patiño O.J., Delgado W.A., Moreno J.P., Cuca L.E. Chemical composition, insecticidal, and antifungal activities of fruit essential oils of three Colombian Zanthoxylum species. Chil. J. Agric. Res. 2011;71:73–82. doi: 10.4067/S0718-58392011000100009. [DOI] [Google Scholar]
- 75.Talontsi F.M., Matasyoh J.C., Ngoumfo R.M., Chepkorir R. Mosquito larvicidal activity of alkaloids from Zanthoxylum lemairei against the malaria vector Anopheles gambiae. Pestic. Biochem. Physiol. 2011;99:82–85. doi: 10.1016/j.pestbp.2010.11.003. [DOI] [Google Scholar]
- 76.Liu X.C., Liu Q.Y., Zhou L., Liu Q.R., Liu Z.L. Chemical composition of Zanthoxylum avicennae essential oil and its larvicidal activity on Aedes albopictus Skuse. Trop. J. Pharm. Res. 2014;13:399–404. doi: 10.4314/tjpr.v13i3.13. [DOI] [Google Scholar]
- 77.Koba K.W.P., Poutouli C., Raynaud P., Yaka P. Propriétés insecticides de l’huile essentielle d’Aeollanthus pubescens Benth sur les chenilles de deux Lépidoptères: Selepa docilsi butler (noctuidae) et Scrobipalpa ergassima mayr. (geleduidae) J. Rech. Sci. Univ. Lomé. 2007;9:19–25. [Google Scholar]
- 78.Toudert-Taleb K., Hedjal-Chebheb M., Hami H., Debras J.F., Kellouche A. Composition of essential oils extracted from six aromatic plants of Kabylian origin (Algeria) and evaluation of their bioactivity on Callosobruchus maculatus (Fabricius, 1775) (Coleoptera: Bruchidae) Afr. Entomol. 2014;22:417–427. doi: 10.4001/003.022.0220. [DOI] [Google Scholar]
- 79.Tchinda A.T., Fuendjiep V., Sajjad A., Matchawe C., Wafo P., Khan S., Tane P., Choudhary M.I. Bioactive compounds from the fruits of Zanthoxylum leprieurii. PharmacolOnline. 2009;1:406–415. [Google Scholar]
- 80.Goodman C.D., Hoang A.T., Diallo D., Malterud K.E., McFadden G.I., Wangensteen H. Anti-plasmodial Effects of Zanthoxylum zanthoxyloides. Planta Med. 2019;85:1073–1079. doi: 10.1055/a-0973-0067. [DOI] [PubMed] [Google Scholar]
- 81.Muganga R., Angenot L., Tits M., Frederich M. Antiplasmodial and cytotoxic activities of Rwandan medicinal plants used in the treatment of malaria. J. Ethnopharmacol. 2010;128:52–57. doi: 10.1016/j.jep.2009.12.023. [DOI] [PubMed] [Google Scholar]
- 82.Adebayo J.O., Krettli A.U. Potential antimalarials from Nigerian plants: A review. J. Ethnopharmacol. 2011;133:289–302. doi: 10.1016/j.jep.2010.11.024. [DOI] [PubMed] [Google Scholar]
- 83.DE Luca Y. Ingrédients naturels de preservation des grains stockes dans les pays en voie de developpement. J. D’agriculture Tradit. et de Bot. Appliquée. 1979;26:20–52. doi: 10.3406/jatba.1979.3782. [DOI] [Google Scholar]