Abstract
Patients with cancer are at a higher risk of cardiovascular disease, which contributes to significant morbidity and mortality. The rapid progress in the field of oncological treatments has led to a steady increase in long-term cancer survivors. Care for cardiovascular complications is therefore becoming increasingly important. In addition, the establishment of new oncological therapies has resulted in the identification of previously unknown cardiovascular side effects. Oncocardiology aims to detect and treat cardiovascular diseases associated with cancer and cancer therapy. Continuous scientific, clinical, and structural developments are necessary as the basis for the best care of the growing number of affected patients. This review summarizes current developments in the field of oncocardiology with regard to advances in cancer therapy and challenges in clinical oncocardiology work. Cardiovascular side effects by targeted cancer therapies are characterized and recent advances in the field of cardiovascular diagnostics are outlined. Developments to better integrate oncocardiology into the medical care system and perspectives for modern, patient-oriented care are shown. In light of the coronavirus disease 2019 (COVID-19) pandemic, current challenges and opportunities are highlighted. The relevance of profitable further advances in oncocardiology including standardized guidelines and educational programs is delineated as a mandatory requirement for the successful development of oncocardiology.
Keywords: Cancer, Cardiovascular disease, Cardiotoxicity, COVID-19, Imaging
Abstract
Patienten mit einer Krebserkrankung sind einem erhöhten Risiko von Herz-Kreislauf-Erkrankungen ausgesetzt, welche zu einer erheblichen Morbidität und Mortalität beitragen. Der schnelle Fortschritt bei der onkologischen Behandlung führt zu einer stetigen Zunahme von Langzeitüberlebenden nach Krebserkrankung, sodass der Behandlung von kardiovaskulären Komplikationen eine wachsende Bedeutung zukommt. Darüber hinaus wurden mit der Etablierung von neuen onkologischen Therapien neue, bisher unbekannte kardiovaskuläre Nebenwirkungen identifiziert. Ziele der onkologischen Kardiologie sind das Erkennen und Behandeln von Herz-Kreislauf-Erkrankungen im Zusammenhang mit Krebs und Krebstherapien. Eine kontinuierliche wissenschaftliche, klinische und strukturelle Entwicklung ist die Grundlage für die bestmögliche Versorgung der wachsenden Zahl betroffener Patienten. In der vorliegenden Übersichtsarbeit sind aktuelle Entwicklungen im Bereich der onkologischen Kardiologie in Hinblick auf Fortschritte in der modernen Tumortherapie und aktuelle Herausforderungen der klinischen onkokardiologischen Arbeit zusammengefasst. Kardiovaskuläre Nebenwirkungen durch zielgerichtete Krebstherapien und Weiterentwicklungen auf dem Gebiet der kardiovaskulären Diagnostik werden erörtert. Ansätze zur besseren Einbindung der onkologischen Kardiologie in das medizinische Betreuungssystem und Chancen für eine moderne, patientenorientierte Versorgung werden beschrieben. Angesichts der aktuellen Coronavirus-Pandemie (COVID-19) werden aktuelle Herausforderungen und Chancen in der onkokardiologischen Patientenbetreuung dargestellt. Die Relevanz einer profitablen Weiterentwicklung in der onkologischen Kardiologie mit standardisierten Leitlinien und Fortbildungsprogrammen wird als Voraussetzung für eine erfolgreiche Entwicklung in der Kardioonkologie aufgezeigt.
Schlüsselwörter: Krebs, Herz-Kreislauf-Erkrankungen, Kardiotoxizität, COVID-19, Bildgebung
Cardiovascular disease is the most common cause of death in Germany (38.5%) followed by cancer (25%; [1]). Cardiovascular disease and cancer share common risk factors, e.g., smoking, diabetes mellitus, and age. Hence, a significant portion of patients are affected by both diseases. With improvements in cancer therapy and subsequently increased numbers of long-term cancer survivors, cardiovascular side effects related to cancer therapy are a health concern of increasing importance considering their significant impact on morbidity and mortality [2]. Cancer and heart disease are closely related in terms of pathophysiology and influence on each other [3]. While the induction of cardiovascular diseases by cancer and cancer therapy has already been extensively characterized, there are now indications that cancer itself can be promoted by heart diseases [3]. Oncocardiology aims to identify mechanisms that lead to cardiovascular diseases through cancer and cancer therapy, to establish appropriate diagnostic measures, and to identify the best possible therapy to reduce the burden of cardiovascular disease in cancer patients [4].
The novel field of oncocardiology has gained recognition within the medical and scientific community. Scientific effort has raised awareness of this field, particularly among oncologists and cardiologists. During the past 4 years, the number of annual PubMed-listed scientific publications dealing with “oncocardiology” has more than doubled. Specialist societies, including the European Society of Cardiology (ESC) and the German Society of Cardiology (DGK), have established oncocardiology working groups [5]. Importantly, a joint working group was also initialized within the German Society for Hematology and Medical Oncology (DGHO) and the DGK as an effort to improve interdisciplinary cooperation. Specialized oncocardiology units have been embedded at centers with a large cardiologic and oncologic focus [5].
Despite growing recognition and advances in scientific research, significant gaps of knowledge can still be found among specialized personnel involved in this discipline. A recent international survey on healthcare providers’ knowledge on oncocardiology identified in particular profound differences regarding the definition, monitoring, and treatment of cancer therapy-related cardiotoxicity [6]. Particularly, only 45.8% of oncologists interviewed believed that oncocardiology clinics could improve patients’ outcome [6]. In light of the considerable relevance of providing the best possible care for the growing patient population, the results indicate persisting deficits and call for more educational and scientific effort in the field of oncocardiology. Complete integration into the medical care system and evaluation within the current socioeconomic context is mandatory for future progress. In the following, recent advances in oncocardiology are summarized and the necessary scientific effort and structural improvements to facilitate the best-possible oncocardiology care are outlined.
Cardiotoxicity of new substances
Anthracyclines (e.g., doxorubicin, daunorubicin) are widely regarded as the prototype of cardiotoxic cancer therapy [7]. They were identified as one of the first chemotherapeutic agents in 1963 and were first applied for the treatment of leukemia and solid tumors shortly thereafter [8]. Anthracyclines continue to be a cornerstone of modern cancer therapy and represent integral parts of systemic cancer therapy, including the standard form of adjuvant therapy in breast cancer, which is the cancer with the highest incidence in Germany [2]. Anthracyclines are associated with a significant risk of cardiotoxic side effects, with left ventricular (LV) dysfunction and consecutive manifest heart failure as the most important form considering their profound impact on short- and long-term morbidity and mortality [9].
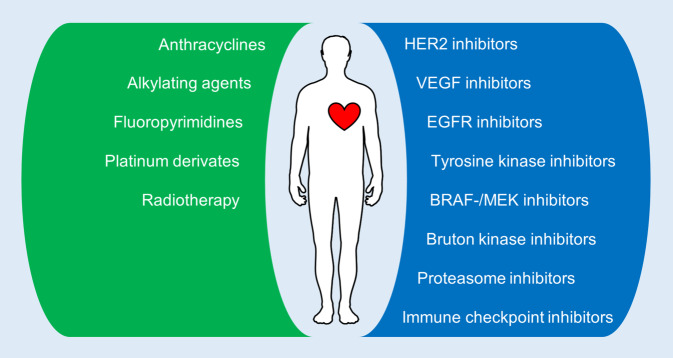
During the past two decades, targeted therapeutics have been developed and are increasingly applied in cancer therapy. By specifically targeting cancer-specific aberrant signaling or biochemical mechanisms, this new form of therapy aims for better anti-cancer efficacy while simultaneously reducing systemic side effects. The first evidence of significant cardiotoxicity from a widely used targeted therapy was found for trastuzumab, an inhibitor of human epidermal growth factor receptor 2 (HER2) that is broadly used in the treatment of HER2-positive breast cancer. Patients receiving trastuzumab are at risk of LV dysfunction in 7–34% of cases, and concomitant use of anthracyclines appears to augment the cardiotoxic potential of both agents [2, 4]. Significant progress in the development of new drugs has led to the increasing use of targeted therapeutics with great improvements in morbidity and mortality, but the increased use has unmasked various forms of side effects, including severe cardiovascular toxicities (Fig. 1; [10]). Considering the broad application and rapid progress in the field of targeted therapeutics together with the growing number of long-term survivors after cancer therapy, in-depth knowledge of the cardiotoxic side effects of specific classes of targeted therapies is essential for the best possible oncocardiology treatment. Identifying relevant cardiovascular toxicities and appropriate preventive measures will be the most important challenge for oncocardiology in the future.
Fig. 1.
Illustration of various cancer therapeutics with known cardiovascular side effects. Green, conventional cancer therapy; blue, targeted cancer therapy. BRAF mutated rapidly accelerated fibrosarcoma kinase B, EGFR endothelial growth factor receptor, HER2 human epidermal growth factor receptor 2, MEK mitogen-activated protein kinase kinase, VEGF vascular endothelial growth factor receptor
Immune checkpoint inhibitors
Immune checkpoint inhibitor (ICI) therapy induces an anti-tumor immune reaction by blocking immune-inhibitory signaling via the programmed death 1 (PD1), or T‑lymphocyte associated protein 4 (CTLA4) pathways [11]. The survival rate of various cancers has been greatly improved thanks to ICI therapy, particularly melanoma and non-small cell lung cancer. However, ICI therapy is associated with the risk of autoimmune-triggered immune-related adverse events (irAEs), including a significant risk of cardiotoxicity. The most recognized form is ICI-related myocarditis, which is found in 1–2% of treated patients with a fatality rate of 27–46% due to the high rate of cardiogenic shock and severe arrhythmia [11]. Increasing use of ICI therapy has unmasked further cardiotoxic side effects, including subclinical LV dysfunction or elevations in cardiac troponin, takotsubo syndrome, and pericardial disease [12]. Cardiac irAEs are commonly treated by immunosuppressive therapy. It is currently unclear whether it is safe to re-initiate a potentially life-saving ICI therapy after the resolution of cardiac adverse events [12]. Future research is needed to determine the underlying pathomechanisms and to identify a specific therapy for cardiovascular side effects without compromising the anti-cancer efficacy.
VEGF inhibitors
Inhibitors of the vascular endothelial growth factor (VEGF) receptor such as bevacizumab are used in various forms of solid cancers, including colorectal carcinoma, non-small cell lung cancer, and renal cell cancer. These inhibitors decrease tumor growth by inhibiting intra-tumoral angiogenesis. They are associated with a dose-dependent risk of arterial adverse events including cardiac ischemia, venous thromboembolism, and arterial hypertension [13]. Cardiotoxic effects are dependent on the type of cancer and the stage of disease. Decreased endothelial generation of nitric oxide is hypothesized as a potential underlying pathomechanism. Interestingly, arterial hypertension may serve as a surrogate parameter for monitoring an effective VEGF inhibition and response to therapy as it is considered a direct, on-target effect on VEGF signaling [14]. Oncocardiology care of patients receiving VEGF inhibitors focuses on optimal control of arterial hypertension to reduce the risk of major adverse events. The potential benefits of thromboprophylaxis remain controversial due to the higher bleeding risk linked to VEGF inhibitors [15].
BRAF and MEK inhibitors
Inhibitors of mutated rapidly accelerated fibrosarcoma kinase B (BRAF), e.g., dabrafenib and vemurafenib, and inhibitors of mitogen-activated protein kinase kinase (MEK), e.g., trametinib and cobimetinib, are used for melanoma therapy. As combination therapy, BRAF and MEK serine/threonine protein kinase inhibitors have improved survival rates in metastatic disease in the presence of the BRAF-V600 mutation [16]. However, both classes of drug are associated with a high risk of cardiovascular adverse events that are more commonly found when they are used as combination therapy. Adverse effects include LV dysfunction, pulmonary embolism, and arterial hypertension [15]. Currently, a combination of BRAF-/MEK-inhibitor therapy and ICI therapy targeting PD1 is under clinical evaluation [17]. It is clearly expected that this form of combination therapy may exhibit a complex pattern of cardiovascular side effects that requires oncocardiologic surveillance.
Proteasome inhibitors
The proteasome inhibitor carfilzomib improved treatment of multiple myeloma but large-scale application has revealed cardiotoxicity in 8.7% of treated patients, particularly with arterial hypertension and cardiac failure in 6–8% of cases [18–20]. Although the underlying pathomechanism has not been elucidated, proteasome inhibitor-associated perturbation of endothelial nitric oxide synthase (eNOS) has been hypothesized to facilitate cardiovascular dysfunction [21]. Additionally, a direct effect on cardiac protein homeostasis has been demonstrated in a preclinical model [22]. Risk factors for cardiotoxicity (e.g., diabetes mellitus, poorly controlled arterial hypertension, known history of heart failure) should be assessed at baseline. Echocardiography is recommended at every two to three cycles. Monitoring of brain natriuretic peptide (BNP) levels is recommended for patients who are at increased cardiac risk. Elevation of BNP (>400 pg/ml) is associated with higher rates of hospitalization for cardiovascular adverse events [21]. Metformin may exhibit a preventive effect, but prospective clinical data are still warranted [22].
Bruton kinase inhibitors
Ibrutinib is an inhibitor of Bruton tyrosine kinase. It is used for treatment of chronic lymphocytic leukemia, mantle cell lymphoma, and Waldenström macroglobulinemia. Patients receiving ibrutinib therapy are at risk of atrial fibrillation (AF) with a cumulative incidence of up to 11%. Additionally, ibrutinib induced new-onset arterial hypertension in 71.6% of ibrutinib users, consequently predisposing them to major cardiovascular events [23]. In a recent phase-III trial on ibrutinib together with the CD20 inhibitor rituximab for chronic lymphatic leukemia, the combination therapy showed a twofold higher incidence of severe cardiovascular events compared with chemoimmunotherapy [24]. Despite this finding, FDA approval was granted in April 2020 [25].
It is hypothesized that inhibition of phosphoinositide 3‑kinase (PI3K) in cardiomyocytes by ibrutinib induces AF [26]. Therefore, ECG is recommended at baseline and every 3 months thereafter or when symptoms occur [27]. Importantly, ibrutinib interacts pharmacologically with several common cardiac drugs via cytochrome P3A4 and the P transporter, e.g., dabigatran, calcium antagonists, amiodarone, and digitoxin [28]. It exhibits a platelet aggregation defect and is associated with an increased incidence of central nervous system hemorrhagic events [28]. Elevated bleeding risk can be further augmented by oral anticoagulation that is introduced following new-onset AF.
New imaging in oncocardiology
Despite a robust body of evidence on anthracycline-related cardiotoxicity, diagnosis often remains challenging due to suboptimal diagnostic tools. Echocardiography is the diagnostic gold standard in oncocardiology. Left ventricular ejection fraction (LVEF) serves as the main parameter for detecting changes in LV function [4]. However, manifest changes in LVEF are found at an advanced stage of myocardial impairment due to cancer therapy, and may not be entirely reversible. It is crucial to detect early, subclinical changes in LV function during cancer therapy so as to initiate cardioprotective measures before manifest heart failure develops [9]. Myocardial strain is thought to meet this requirement by offering a method for detecting early changes before heart function deteriorates, and it has been evaluated in various studies. As demonstrated in a new meta-analysis, aggregate evidence confirms a good prognostic performance of global longitudinal strain for subsequent LV dysfunction from anthracycline therapy with or without trastuzumab [29]. Combining assessment of cardiac troponin and strain analysis further augments the sensitivity of predicting manifest cardiotoxicity [9, 30].
While a robust body of evidence covering LV function during cancer therapy is available, the characteristics and clinical relevance of right ventricular (RV) impairment are poorly understood. Right ventricular dysfunction upon cancer therapy is more common in cancer survivors than LV dysfunction and develops 6–7.5 months after exposure to anthracyclines and/or trastuzumab with a dose-dependent incidence [31]. Three-dimensional assessment of RV volumes including the assessment of RV strain may represent a beneficial tool for the evaluation of chemotherapy-related RV dysfunction and has yielded consistent results, but it is not yet broadly applied in standardized echocardiographic examinations [32]. Future studies are needed to determine appropriate cutoff values and recommendations for the use in oncocardiology [31].
Nuclear cardiology offers a wide array of diagnostic possibilities and may become indispensable as a diagnostic tool in oncocardiology. Fluorodeoxyglucose positron emission tomography computed tomography (18FDG-PET/CT) shows enhanced myocardial 18FDG-uptake in patients exposed to anthracycline chemotherapy as a sign of oxidative stress and alterations in cardiac metabolism [33]. Distinct patterns of 18FDG uptake, particularly including the RV, have been associated with anthracycline cardiotoxicity. Furthermore, 18FDG-PET/CT offers an elegant approach for a simultaneous assessment of cardiac involvement and tumor response to therapy [33]. Cardiac iodine-123 meta-iodobenzylguanidine (123I‑mIBG) scintigraphy is a novel approach for imaging dysregulated presynaptic norepinephrine homeostasis as a prognostic marker in heart failure. Early evidence proposes increasing 123I‑mIBG washout as a marker for myocardial compensation to cardiotoxic injury from anthracycline exposure [34]. Future studies are warranted to assess the promising potential of nuclear imaging in oncocardiology.
Excursus: oncocardiology during COVID-19
The present coronavirus disease 2019 (COVID-19) pandemic requires an evaluation of the overall risk associated with the two largest health burdens, cardiovascular disease and cancer. COVID-19 is caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), a positive-sense single-stranded RNA virus, and induces a variety of symptoms ranging in severity through to acute respiratory distress syndrome (ARDS). Cancer and cancer therapy are important risk contributors for immune deficiency that may be aggravated by cardiovascular risk factors and diseases known to weaken the immune system, such as diabetes mellitus, advanced age, and heart failure. Patients with underlying cardiovascular disease and cancer are at an increased risk of life-threatening complications from COVID-19 due to poor tolerance of a decreased respiratory capacity, and they account for a large proportion of COVID-19-related deaths. Hence, oncocardiology patients are considered as a particularly vulnerable collective (Fig. 2).
Fig. 2.
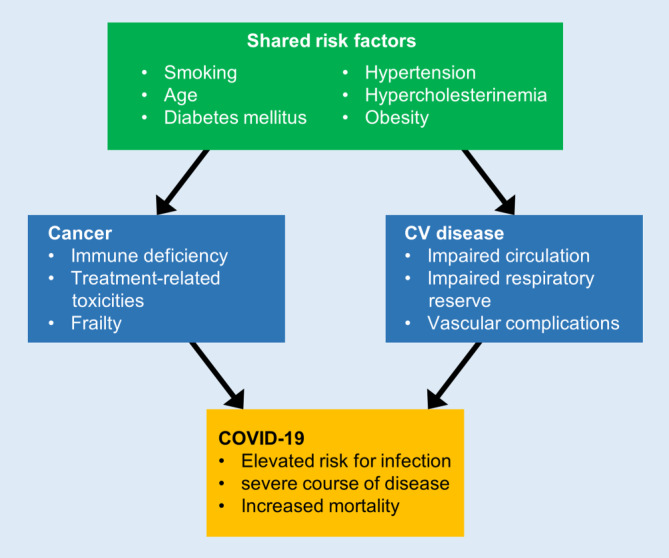
Risk factors for coronavirus 2019 (COVID-19) in oncocardiology. Shared risk factors, cancer-associated risk factors, and risk factors from cardiovascular disease (CV) predisposing to COVID-19 infections with adverse outcomes are depicted
Angiotensin-converting enzyme 2 (ACE2) is a membrane-bound receptor that plays a crucial role in cardiovascular physiology and disease, and has been identified as a functional receptor for SARS-CoV‑2 [35]. Through the use of spike proteins, the virus binds to the ACE2 receptor and enters the cell. The ACE2 receptor can be upregulated in patients with cardiovascular disease, particularly during ACE inhibitor therapy. According to anecdotal evidence, this mechanism can result in a systemic viral vasculitis including the cardiac vasculature with currently unknown consequences [36]. It is under investigation whether this mechanism promotes uptake of virus particles and whether ACE inhibitor therapy affects the disease phenotype [35].
Cardiac involvement during a COVID-19 infection predicts adverse outcome; particularly myocardial injury from COVID-19 with increased levels of high-sensitivity cardiac troponin can be seen in a significant proportion of patients (>12% of the first 41 diagnosed patients from Wuhan; [35]) and predisposes them to a severe course of disease with the need for intensive care treatment. It is furthermore expected that SARS-CoV‑2 can cause viral myocarditis analogous to the reports on Middle East respiratory syndrome (MERS) coronavirus infection [37].
Oncocardiology care is severely affected by the current COVID-19 pandemic. Potential delays in antineoplastic therapy or elective surgery, as well as logistic restrictions for outpatient care, challenge the best possible diagnostic and therapeutic care in oncocardiology. Determining whether certain medical measures are elective or urgent may be difficult in individual cases, particularly when doctor–patient contact is restricted due to measures of infection prevention [38]. This can be aggravated by patients misjudging life-threatening symptoms to avoid a hospital visit in order to prevent COVID-19 infection, as has already been shown for acute myocardial infarction and stroke [39].
The COVID-19 pandemic is an opportunity for the role of telemedicine in oncocardiology [40]. By using digital health technology including virtual patient visits and remote data monitoring, telemedicine provides an opportunity to maintain effective, patient-centered oncocardiology surveillance while avoiding hospital visits. Particularly for routine follow-ups, telemedicine might offer a valuable option during the time of the pandemic and beyond [40].
Future requirements
The growing number of long-term cancer survivors is a challenge, but also an opportunity for healthcare. Rapid progress in the field of oncology research and drug development leading to new substances with improved efficacy but also new cardiovascular side effects requires a high-quality care. The ESC has outlined requirements for oncocardiology in their 2016 Position Paper on cancer treatments and cardiovascular toxicity, but standardized oncocardiology guidelines are not available so far [4]. With the establishment of a consensus paper by the DKG, a significant step toward constant, high-quality oncocardiology care in Germany has been taken.
Persisting deficits regarding recognition and knowledge of oncocardiology in the medical community indicate that training and educational programs are of great importance [6]. Particular in the field of oncocardiology, a profitable interdisciplinary cooperation between cardiologists and oncologists is mandatory for satisfactory patient care.
Technology platforms, electronic consultations, and services via mobile devices can serve to improve follow-up of oncocardiology patients, particularly in the outpatient setting and for routine oncocardiology check-ups. Embedding knowledge from behavioral economics with telemedicine can have a positive effect on medical care and improve healthy behavior [41]. Easy accessibility to digital healthcare services including oncocardiology may serve as an innovative approach for better patient-centered care. Significant technical and structural prerequisites must be addressed in order to promote the great potential of telehealth for oncocardiology [41].
Finally, future scientific efforts to characterize cardiovascular disease in cancer patients and its underlying pathomechanisms, as well as the best possible treatment strategies, are an integral part of successful oncocardiology care. This includes basic science research with appropriate preclinical models, as well as prospective clinical trials to provide evidence for diagnostic and therapeutic approaches. In this context, the standardized recording of cardiovascular side effects in phase I–III studies for new cancer therapeutics is of utmost importance in order to detect and characterize associated cardiovascular toxicity that requires oncocardiology management.
Conclusion
Patients with cancer are at increased risk of cardiovascular disease, which contributes to significant morbidity and mortality. Advances in the field of oncological treatments have led to an ever-growing number of long-term cancer survivors, and care for cardiovascular complications is thus becoming ever more important. Moreover, the establishment of new oncological therapies has resulted in the identification of previously unknown cardiovascular side effects. Oncocardiology aims to detect and treat cardiovascular diseases associated with cancer and cancer therapy. Continuous scientific, clinical, and structural developments are necessary as the basis for the best care of affected patients.
Compliance with ethical guidelines
Conflict of interest
L. Michel, D. Schadendorf and T. Rassaf declare that they have no competing interests.
For this article no studies with human participants or animals were performed by any of the authors. All studies performed were in accordance with the ethical standards indicated in each case.
References
- 1.Destatis (2017) Todesursachen in Deutschland. https://www.destatis.de/DE/Themen/Gesellschaft-Umwelt/Gesundheit/Todesursachen/Publikationen/Downloads-Todesursachen/todesursachen-2120400157004.pdf?__blob=publicationFile. Accessed 1 May 2020
- 2.Totzeck M, Schuler M, Stuschke M, et al. Cardio-oncology—strategies for management of cancer-therapy related cardiovascular disease. Int J Cardiol. 2019;280:163–175. doi: 10.1016/j.ijcard.2019.01.038. [DOI] [PubMed] [Google Scholar]
- 3.Meijers Wouter C, Maglione M, Bakker Stephan JL, et al. Heart failure stimulates tumor growth by circulating factors. Circulation. 2018;138(7):678–691. doi: 10.1161/CIRCULATIONAHA.117.030816. [DOI] [PubMed] [Google Scholar]
- 4.Zamorano JL, Lancellotti P, Rodriguez Muñoz D, et al. 2016 ESC position paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC committee for practice guidelines: the task force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC) Eur Heart J. 2016;37(36):2768–2801. doi: 10.1093/eurheartj/ehw211. [DOI] [PubMed] [Google Scholar]
- 5.Rassaf T, Lehmann L (2020) Cardio-oncology in Germany. https://www.escardio.org/Councils/council-of-cardio-oncology/cardio-oncology-in-germany. Accessed 1 May 2020
- 6.Peng J, Rushton M, Johnson C, et al. An international survey of healthcare providers’ knowledge of cardiac complications of cancer treatments. Cardiooncology. 2019;5:12. doi: 10.1186/s40959-019-0049-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Michel L, Rassaf T, Totzeck M. Biomarkers for the detection of apparent and subclinical cancer therapy-related cardiotoxicity. J Thorac Dis. 2018;10(Suppl 35):S4282–S4295. doi: 10.21037/jtd.2018.08.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McGowan JV, Chung R, Maulik A, et al. Anthracycline chemotherapy and cardiotoxicity. Cardiovasc Drugs Ther. 2017;31(1):63–75. doi: 10.1007/s10557-016-6711-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Michel L, Mincu RI, Mahabadi AA, et al. Troponins and brain natriuretic peptides for the prediction of cardiotoxicity in cancer patients: a meta-analysis. Eur J Heart Fail. 2020;22(2):350–361. doi: 10.1002/ejhf.1631. [DOI] [PubMed] [Google Scholar]
- 10.Michel L, Rassaf T. Cardio-oncology: need for novel structures. Eur J Med Res. 2019;24(1):1. doi: 10.1186/s40001-018-0359-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Michel L, Rassaf T, Totzeck M. Cardiotoxicity from immune checkpoint inhibitors. Int J Cardiol Heart Vasc. 2019;25:100420. doi: 10.1016/j.ijcha.2019.100420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lyon AR, Yousaf N, Battisti NML, et al. Immune checkpoint inhibitors and cardiovascular toxicity. Lancet Oncol. 2018;19(9):e447–e458. doi: 10.1016/S1470-2045(18)30457-1. [DOI] [PubMed] [Google Scholar]
- 13.Totzeck M, Mincu Raluca I, Rassaf T. Cardiovascular adverse events in patients with cancer treated with bevacizumab: a meta-analysis of more than 20 000 patients. J Am Heart Assoc. 2017;6(8):e006278. doi: 10.1161/JAHA.117.006278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robinson ES, Khankin EV, Karumanchi SA, Humphreys BD. Hypertension induced by vascular endothelial growth factor signaling pathway inhibition: mechanisms and potential use as a biomarker. Semin Nephrol. 2010;30(6):591–601. doi: 10.1016/j.semnephrol.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mincu RI, Mahabadi AA, Michel L, et al. Cardiovascular adverse events associated with BRAF and MEK inhibitors: a systematic review and meta-analysis. JAMA Netw Open. 2019;2(8):e198890. doi: 10.1001/jamanetworkopen.2019.8890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Long GV, Stroyakovskiy D, Gogas H, et al. Combined BRAF and MEK inhibition versus BRAF inhibition alone in melanoma. N Engl J Med. 2014;371(20):1877–1888. doi: 10.1056/NEJMoa1406037. [DOI] [PubMed] [Google Scholar]
- 17.Ribas A, Lawrence D, Atkinson V, et al. Combined BRAF and MEK inhibition with PD-1 blockade immunotherapy in BRAF-mutant melanoma. Nat Med. 2019;25(6):936–940. doi: 10.1038/s41591-019-0476-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Waxman AJ, Clasen S, Hwang WT, et al. Carfilzomib-associated cardiovascular adverse events: a systematic review and meta-analysis. JAMA Oncol. 2018;4(3):e174519. doi: 10.1001/jamaoncol.2017.4519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jain T, Narayanasamy H, Mikhael J, et al. Systolic dysfunction associated with carfilzomib use in patients with multiple myeloma. Blood Cancer J. 2017;7(12):642. doi: 10.1038/s41408-017-0026-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shah C, Bishnoi R, Jain A, et al. Cardiotoxicity associated with carfilzomib: systematic review and meta-analysis. Leuk Lymphoma. 2018;59(11):2557–2569. doi: 10.1080/10428194.2018.1437269. [DOI] [PubMed] [Google Scholar]
- 21.Chari A, Hajje D. Case series discussion of cardiac and vascular events following carfilzomib treatment: possible mechanism, screening, and monitoring. BMC Cancer. 2014;14:915. doi: 10.1186/1471-2407-14-915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Efentakis P, Kremastiotis G, Varela A, et al. Molecular mechanisms of carfilzomib-induced cardiotoxicity in mice and the emerging cardioprotective role of metformin. Blood. 2019;133(7):710–723. doi: 10.1182/blood-2018-06-858415. [DOI] [PubMed] [Google Scholar]
- 23.Dickerson T, Wiczer T, Waller A, et al. Hypertension and incident cardiovascular events following ibrutinib initiation. Blood. 2019;134(22):1919–1928. doi: 10.1182/blood.2019000840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shanafelt TD, Wang XV, Kay NE, et al. Ibrutinib-rituximab or chemoimmunotherapy for chronic lymphocytic leukemia. N Engl J Med. 2019;381(5):432–443. doi: 10.1056/NEJMoa1817073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.FDA (2020) FDA approves ibrutinib plus rituximab for chronic lymphocytic leukemia. https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-ibrutinib-plus-rituximab-chronic-lymphocytic-leukemia. Accessed 1 May 2020
- 26.Vrontikis A, Carey J, Gilreath JA, et al. Proposed algorithm for managing ibrutinib-related atrial fibrillation. Oncology. 2016;30(11):970–981. [PubMed] [Google Scholar]
- 27.Thompson PA, Lévy V, Tam CS, et al. Atrial fibrillation in CLL patients treated with ibrutinib. An international retrospective study. Br J Haematol. 2016;175(3):462–466. doi: 10.1111/bjh.14324. [DOI] [PubMed] [Google Scholar]
- 28.Ganatra S, Sharma A, Shah S, et al. Ibrutinib-associated atrial fibrillation. JACC Clin Electrophysiol. 2018;4(12):1491–1500. doi: 10.1016/j.jacep.2018.06.004. [DOI] [PubMed] [Google Scholar]
- 29.Mahjoob MP, Sheikholeslami SA, Dadras M, et al. Prognostic value of cardiac biomarkers assessment in combination with myocardial 2D strain echocardiography for early detection of anthracycline-related cardiac toxicity. Cardiovasc Hematol Disord Drug Targets. 2020;20(1):74–83. doi: 10.2174/1871529X19666190912150942. [DOI] [PubMed] [Google Scholar]
- 30.Kang Y, Xu X, Cheng L, Li L, Sun M, Chen H, Pan C, Shu X. Two-dimensional speckle tracking echocardiography combined with high-sensitive cardiac troponin T in early detection and prediction of cardiotoxicity during epirubicine-based chemotherapy. Eur J Heart Fail. 2014;16(3):300–308. doi: 10.1002/ejhf.8. [DOI] [PubMed] [Google Scholar]
- 31.Keramida K, Farmakis D. Right ventricular involvement in cancer therapy-related cardiotoxicity: the emerging role of strain echocardiography. Heart Fail Rev. 2020 doi: 10.1007/s10741-020-09938-8. [DOI] [PubMed] [Google Scholar]
- 32.Zhao R, Shu F, Zhang C, et al. Early detection and prediction of anthracycline-induced right ventricular cardiotoxicity by 3-dimensional echocardiography. JACC CardioOncol. 2020;2(1):13–22. doi: 10.1016/j.jaccao.2020.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim J, Cho SG, Kang SR, et al. Association between FDG uptake in the right ventricular myocardium and cancer therapy-induced cardiotoxicity. J Nucl Cardiol. 2019 doi: 10.1007/s12350-019-01617-y. [DOI] [PubMed] [Google Scholar]
- 34.Verberne HJ, Verschure DO. Anthracycline-induced cardiotoxicity: Is there a role for myocardial (123)I-mIBG scintigraphy? J Nucl Cardiol. 2019 doi: 10.1007/s12350-018-01584-w. [DOI] [PubMed] [Google Scholar]
- 35.Zheng YY, Ma YT, Zhang JY, Xie X. COVID-19 and the cardiovascular system. Nat Rev Cardiol. 2020;17(5):259–260. doi: 10.1038/s41569-020-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395(10234):1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alhogbani T. Acute myocarditis associated with novel Middle East respiratory syndrome coronavirus. Ann Saudi Med. 2016;36(1):78–80. doi: 10.5144/0256-4947.2016.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ganatra S, Hammond SP, Nohria A. The novel coronavirus disease (COVID-19) threat for patients with cardiovascular disease and cancer. JACC CardioOncol. 2020 doi: 10.1016/j.jaccao.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.CardioSmart (2020) Coronavirus and your heart. https://www.cardiosmart.org/~/media/Images/Infographics/Coronavirus-and-Your-Heart-Dont-Ignore-Heart-Symptoms.ashx. Accessed 1 May 2020
- 40.Parikh A, Kumar AA, Jahangir E. Cardio-oncology care in the time of COVID-19 and the role of telehealth. JACC CardioOncol. 2020 doi: 10.1016/j.jaccao.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Waddell KJ, Shah PD, Adusumalli S, Patel MS. Using behavioral economics and technology to improve outcomes in cardio-oncology. JACC CardioOncol. 2020;2(1):84–96. doi: 10.1016/j.jaccao.2020.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]