Abstract
Microbial contamination of dairy products with a high fat content (e.g., butter) has been studied insufficiently. No studies using modern molecular methods to investigate microbial communities in butter have been conducted so far. In this work, we used high-throughput sequencing and Sanger sequencing of individual bacterial colonies to analyze microbial content of commercially available butter brands. A total of 21 samples of commercially available butter brands were analyzed. We identified a total of 94 amplicon sequence variants corresponding to different microbial taxa. The most abundant lactic acid bacteria in butter were Lactobacillus kefiri, Lactobacillus parakefiri, Lactococcus taiwanensis and Lactococcus raffinolactis. A large amount of Streptococcus spp. bacteria (87.9% of all identified bacteria) was found in one of the butter samples. Opportunistic pathogens such as Bacillus cereus group, Pseudomonas aeruginosa, Cronobacter spp., Escherichia coli, Listeria innocua, Citrobacter spp., Enterococcus spp., Klebsiella pneumonia were detected. The analyzed butter samples were most strongly contaminated with bacteria from the Bacillus cereus group, and to a lesser extent - with Cronobacter spp. and Enterococcus spp. The plating and Sanger sequencing of individual colonies revealed the presence of Enterobacter cloacae and Staphylococcus epidermidis. The Sanger sequencing also showed the presence of Cronobacter sakazakii in butter which can be dangerous for children under the age of 1 year. We demonstrated that butter is a good growth medium for opportunistic pathogenic bacteria. Our data indicate that despite the fact that butter is a dairy product with a long shelf life, it should be subjected to quality control for the presence of opportunistic bacteria.
Keywords: butter, high-throughput sequencing, Sanger sequencing, microbiota, lactic acid bacteria, opportunistic bacteria
1. Introduction
Contamination of dairy products with pathogenic microorganisms, such as Listeria monocytogenes, Campylobacter jejuni, Staphylococcus aureus and Salmonella spp. is often a cause of serious digestive system disorders [1]. Dairy products have a short shelf life, because they serve as an excellent growth medium for a wide range of microbes. Consumption of dairy products contaminated with Listeria spp., Salmonella spp., Escherichia coli, Campylobacter spp., Shigella spp., and mycotoxin-producing fungi [2] can result in fever, nausea, vomiting, diarrhea, and abdominal pain.
Dairy products are one of the main causes of food poisoning in humans. Thus, 46 cases of gastroenteritis related to the consumption of raw milk have been registered in the USA from 1973 till 1992. In most of these cases, raw milk was contaminated with Campylobacter spp., Salmonella spp., and E. coli 0157:H7 [3]. Other microbial species found in raw milk are Yersinia enterocolitica [4] and L. monocytogenes [5]. Dairy products are often spoiled by Bacillus spp., Pseudomonas spp., Lactococcus spp. [6] and Streptococcus spp. [7]. The gut-associated genera Prevotella, Ruminococcus, Bacteroides, Rikenella and Alistipes were detected in milk samples [8].
Microbial contamination of dairy products with a high fat content (e.g., butter) has been studied insufficiently. Various pathogenic and opportunistic pathogens, such as psychotrophic bacteria, molds, yeast, coliforms, fecal coliforms, E. coli, and S. aureus, have been found in cooking butter [9]. Analysis of butter made of goat milk revealed the presence of lactic acid bacteria, psychotrophic bacteria, molds, yeasts, and lipolytic bacteria (as well as coliforms in one of the samples) [10]. Studies of bacterial communities in petroleum oil in stockpiles in Japan based on 16S rRNA gene sequencing showed the presence of Ochrobactrum anthropi, Burkholderia cepacia, Stenotrophomonas maltophilia, Propionibacterium acnes, and Brevundimonas diminuta [11]. Analysis of microbial content of traditional butters produced in the West Azerbaijan province of Iran identified coliforms, molds, and yeast in the assayed samples. Thus, S. aureus and E. coli were found in 8.3 and 50% of samples, respectively [12]. Identification of microbial contaminants in commercially available butters in Turkey revealed the presence of Enterococcus faecalis followed by Staphylococcus hominis, Micrococcus luteus, Pseudomonas putida, P. fluorescens, Brevibacillus brevis, Streptococcus sanguis, Weissella viridescence, Lactococcus lactis, and Lactobacillus kefir, as well as molds and yeast [13]. In mayonnaise, another high-fat product, the yeast Pichia kudriavzevii was found [14].
It should be noted that these few cited works have used classical microbiological approaches (except the work of Yoshida et al. [11]) to survey butter or oil for microbial contamination. We believe that the use of modern methods of high-throughput sequencing might considerably expand our knowledge on microbial communities present in food with a high fat content. NGS (next generation sequencing) can be used as a new approach in food quality control for identification of microorganisms directly in food samples [15,16].
Studies on the microbial contamination of products with a high fat content are scarce, probably, due to the fact that such products have a relatively long shelf life and the high fat content is commonly misconceived as a factor preventing rapid bacterial growth. Moreover, identification of butter-contaminating microbiota requires preliminary sample processing, which complicates its analysis. No studies using modern molecular methods to investigate microbial communities in butter have been conducted so far. In this work, we used high-throughput sequencing and Sanger sequencing of individual colonies to analyze microbial content of commercially available butter brands.
2. Materials and Methods
2.1. Samples
Different brands of unsalted butter from pasteurized milk cream (both Russian and foreign) available on the Russian market were studied. For analysis, 1 g of butter was used.
2.2. Plating and Microbial Enrichment
To evaluate microbial contamination of the analyzed butter samples, the samples were plated on universal sterile solid media—fish peptone agar (FPA) (State Scientific Center of Applied Microbiology and Biotechnology, Obolensk, Russia) containing 12 g/L fish meal hydrolysate; 12 g/L peptone, 6 g/L NaCl, and 10 g/L agar (pH 7.1–7.5).
For high-throughput sequencing, microbial cultures were enriched by growing in broth sterile medium containing only 2% glucose (Dia-M, Moscow, Russia) and 1% peptone (State Scientific Center of Applied Microbiology and Biotechnology, Obolensk, Russia). Food substrate (1 g of butter) was cut into 5 to 7 mm pieces and added to 9 mL of the medium, and the microorganisms were grown for 24 h at 27 °C.
2.3. DNA Isolation
DNA from microbial colonies for Sanger sequencing or cells grown in the enrichment medium for high-throughput sequencing was isolated using ZymoBIOMICS DNA Kit (Zymo Research, Irvine, CA, USA).
2.4. DNA Barcoding
PCR was performed on individual bacterial colonies using a ScreenMix-HS reaction mixture (Evrogen, Moscow, Russia) in an Eppendorf MasterCycler Personal cycler. Direct and reverse primers for bacterial DNA amplification were 785F (5′-GGATTAGATACCCTGGTA) and 1492R (5′-TACGGYTACCTTGTTACGACTT) [17], respectively. PCR was performed in the following regime: Denaturation at 94 °C for 4 min, 35 cycles of 94 °C for 30 s, 52 °C for 30 s, and 72 °C for 45 s, and final elongation at 72 °C for 10 min. Amplified fragments were isolated from the gel and purified with a Cleanup Standard kit (Evrogen, Moscow, Russia). Purified PCR products were sequenced with an Applied Biosystems 3500 sequencer using a BigDye Terminator v3.1 Cycle Sequencing Kit, and obtained nucleotide sequences were analyzed using the GenBank database. Organisms were assigned to certain taxa if their DNA sequences coincided by no less than 99% with the corresponding sequences already deposited in the databases by at least five different authors.
2.5. High-Throughput Sequencing
The microorganisms were collected from the enrichment broth medium by centrifugation at 10,000× g for 5 min. Bacterial DNA isolated from collected bacteria was amplified with the universal direct 785F forward primer (5′-GGATTAGATACCCTGGTA) and reverse 1100R primer (5′-GGGTTGCGCTCGTTG) [17]. PCR was performed using a ScreenMix-HS Master Mix (Evrogen, Moscow, Russia) in the following regime: 94 °C for 4 min followed by 37 cycles of 94 °C for 30 s, 53 °C for 30 s, and 72 °C for 30 s, with the final elongation at 72 °C for 5 min. PCR products were purified with AMPure XP magnetic beads (Beckman Coulter, Miami, FL, USA) and used for the construction of sequencing libraries using Ion AmpliSeq Library Kit 2.0 (Thermo Fisher Scientific, Waltham, MA, USA) as recommended by the manufacturer. Barcoding was done using the Ion Xpress barcode adapters (Thermo Fisher Scientific, Waltham, MA, USA). Library DNA concentration was determined by qPCR using Library Quantification Kit Ion Torrent Platforms (Kapa Biosystems, Wilmington, MA, USA).
Sequencing was performed with the IonTorrent PGM platform using Ion PGM Hi-Q View Sequencing Kit, Ion OneTouch 2 System, and Ion PGM Hi-Q View OT2 Kit (Thermo Fisher Scientific, Waltham, MA, USA).
The results of sequencing were obtained as binary alignment map (BAM) files that were converted into FASTQ format using the SAMtool v.1.2 software. Demultiplexing and primer stripping was done using the fastq-multx application of the ea-utils v.1.3. program package. The reads were then filtered according to the reading quality based on the number of expected errors [18,19].
Unique sequences were identified using the DADA2 package version 1.8.0. We used the negative homopolymer gap penalty value (parameter HOMOPOLYMER_GAP_PENALTY = −1), which causes homopolymer gaps to be treated as homopolymer sequences, and increased net cumulative number of insertion of one sequence relative to the other (parameter BAND_SIZE = 32).
After that, we constructed an amplicon sequence variant (ASV) table and filtered out chimeric sequences. Taxonomy (with genus-level resolution) was assigned to the sequence variants using DADA2 implementation of the naive Bayesian classifier method [20]. Species level taxonomy was assigned using exact matching (100% identity) with amplicon sequence variants. Both genus and species identification were performed with the SILVA database (https://www.arb-silva.de) version 132 as a reference. We used R version 3.4.4 for all operations related to NGS data analysis and taxonomy assignment.
3. Results
We analyzed 21 samples of commercially available brands. Since isolation of microbial DNA directly from the samples was impossible because of the high fat content of butter, we enriched the samples by incubation in liquid nutritive medium. DNA was amplified by PCR and subjected to high-throughput sequencing followed by bioinformatics analysis. Raw sequencing data is available from NCBI Sequence Read Archive (SRA), unique ID PRJNA526897.
Using DADA2 algorithm—which produces amplicon sequence variants (ASVs) or zero-radius OTUs (operational taxonomic units), instead of OTUs produced by traditional methods—we identified a total of 108 ASVs. After taxonomy assignment, we filtered out 14 ASVs that did not match any records in the reference database. The remaining 94 ASVs were identified up to genus level, and 85 of them were identified up to species level. In this paper, we report species of the microorganism only if there were no ambiguity during taxonomy identification (i.e., all exact matches of the ASV sequence were to the reference sequences of the same species). In all other cases, genus of the microorganism is reported.
The prevalence of particular bacterial species varied in the samples. The reads were unevenly distributed among different bacterial taxa. We estimated the total abundance of all identified taxa based on the number of reads. The most abundant bacteria for all samples are shown in Table 1. See the supplemental materials for the ASVs of “Minor species” for all butter samples.
Table 1.
The most abundant bacteria in butter samples.
| Bacteria | Butter Sample | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | |
| Geobacillus spp. | |||||||||||||||||||||
| Stenotrophomonas spp. | |||||||||||||||||||||
| Thermus thermophilus | |||||||||||||||||||||
| Cedecea davisae | |||||||||||||||||||||
| Tatumella citrea | |||||||||||||||||||||
| Lactobacillus spp. | |||||||||||||||||||||
| Lactococcus spp. | |||||||||||||||||||||
| Streptococcus spp. | |||||||||||||||||||||
| Acinetobacter spp. | |||||||||||||||||||||
| Anoxybacillus spp. | |||||||||||||||||||||
| Xanthomonas bromi | |||||||||||||||||||||
| Pseudoxanthomonas spp. | |||||||||||||||||||||
| Propionibacterium acnes | |||||||||||||||||||||
| Leuconostoc spp. | |||||||||||||||||||||
| Pseudomonas spp. | |||||||||||||||||||||
| Massilia varians | |||||||||||||||||||||
| Ochrobactrum spp. | |||||||||||||||||||||
| Bacillus cereus group | |||||||||||||||||||||
| Chryseobacterium spp. | |||||||||||||||||||||
| Trabulsiella spp. | |||||||||||||||||||||
| Shewanella japonica | |||||||||||||||||||||
| Haemophilus sputorum | |||||||||||||||||||||
| Pantoea spp. | |||||||||||||||||||||
| Gibbsiella spp. | |||||||||||||||||||||
| Brevundimonas spp. | |||||||||||||||||||||
| <1% -no color | 1–10% | 21–30% | 21–30% | 31–40% | 41–50% | 51–60% | >61% | ||||||||||||||
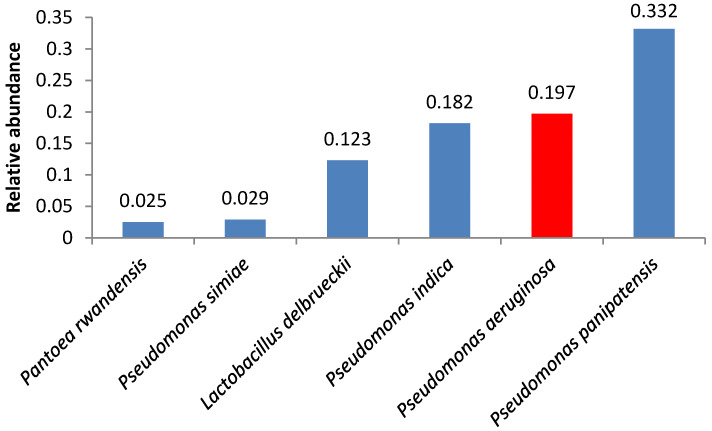
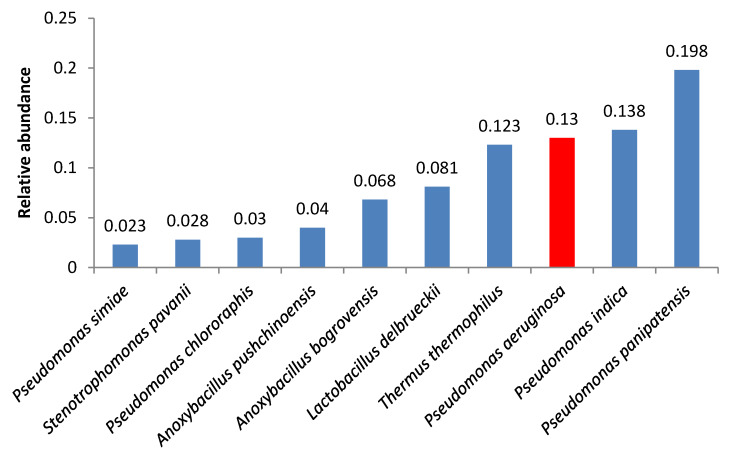
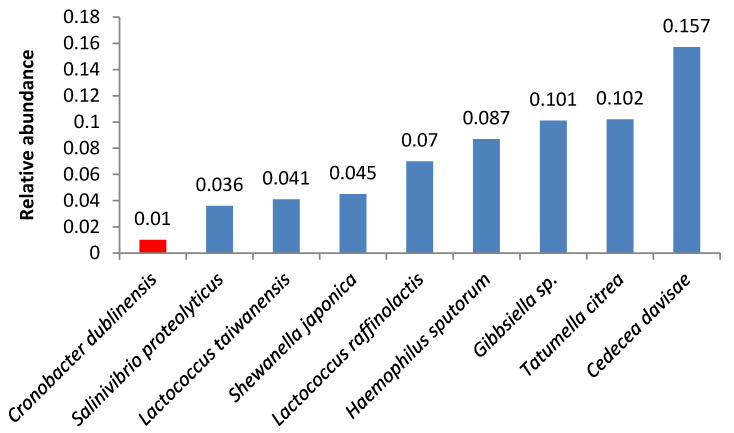
A number of samples contained opportunistic bacteria; in some of these samples, the amount of these bacteria was relatively high. Pseudomonas aeruginosa was the second and the third most abundant microorganism in samples 4 and 2, respectively (Figure 1 and Figure 2).
Figure 1.
Relative abundance of amplicon sequence variants in butter sample no. 4.
Figure 2.
Relative abundance of amplicon sequence variants in butter sample no. 2.
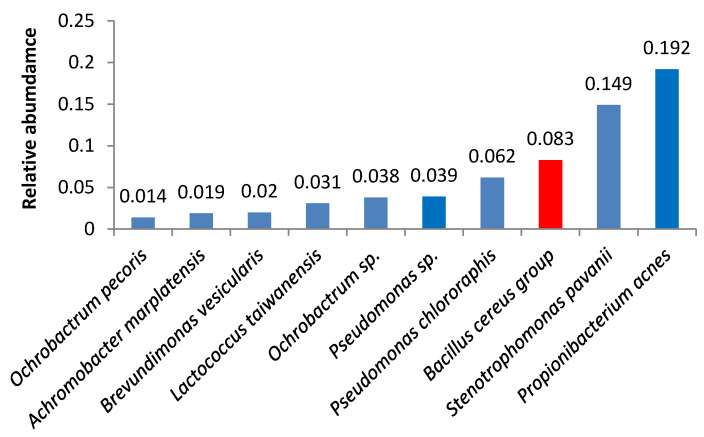
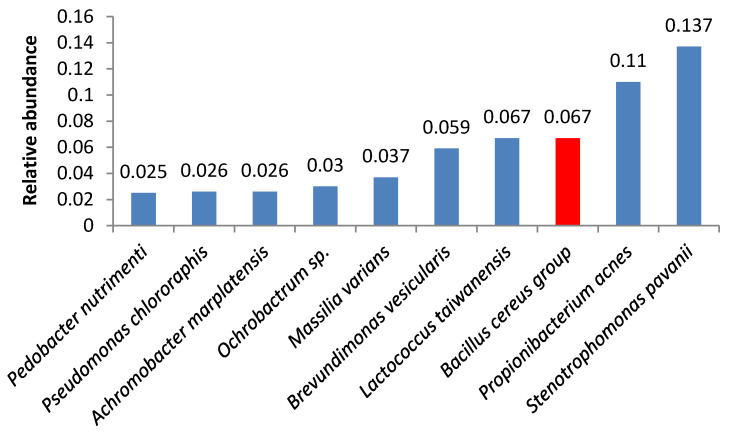
Bacteria of the Bacillus cereus group were the third most abundant microbes in samples 3 and 18 (Figure 3 and Figure 4). Samples 1, 6, 16, 5, 19, and 21 also contained high amounts of Bacillus cereus group bacteria.
Figure 3.
Relative abundance of amplicon sequence variants in butter sample no. 3.
Figure 4.
Relative abundance of amplicon sequence variants in butter sample no. 18.
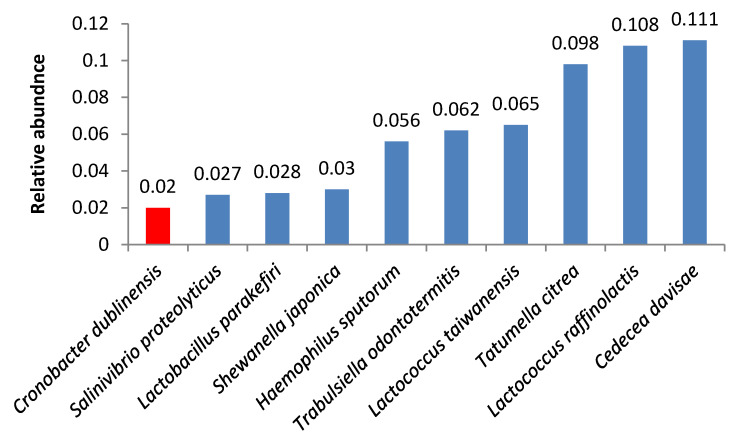
We also found other opportunistic pathogens. Samples 8 and 12 contained high quantities of bacteria from the Cronobacter genus (Figure 5, Figure 6); the same species were identified in smaller amounts in samples 7 and 9.
Figure 5.
Relative abundance of amplicon sequence variants in butter sample no. 8.
Figure 6.
Relative abundance of amplicon sequence variants in butter sample no. 12.
Enterococcus sp. bacteria were found in samples 10, 13, and 19. E. coli and Klebsiella pneumoniae were found in three samples each. Six samples contained bacteria of the Enterobacter genus; one sample (no. 6) was contaminated with Citrobacter sp. bacteria. Sample 12 contained Listeria innocua. Bacteria of the Streptococcus and Staphylococcus genera were identified in almost all analyzed butter samples. All the aforementioned bacteria were considered to be present in the samples when the number of reads per ASV exceeded 500. However, the probability of ASV identification for a particular microorganism varied depending on the threshold value for the number of reads per ASV (Table 2).
Table 2.
The prevalence of opportunistic bacteria at different threshold values for the number of reads per ASV.
| Opportunistic Pathogen |
Found in Samples, % (10–100 Reads Per ASV) | Found in Samples, % (100–500 Reads Per ASV) | Found in Samples, % (>500 Reads Per ASV) |
|---|---|---|---|
| Bacillus cereus group | 100 | 66.7 | 52.4 |
| Pseudomonas aeruginosa | 38.1 | 23.8 | 9.6 |
| Cronobacter spp. | 57.2 | 42.9 | 14.3 |
| Escherichia coli | 61.9 | 28.6 | 14.3 |
| Listeria innocua | 38.1 | 14.3 | 4.8 |
| Citrobacter spp. | 14.3 | 9.5 | 4.8 |
| Enterococcus spp. | 90.5 | 66.7 | 14.3 |
| Klebsiella pneumoniae | 28.6 | 28.6 | 14.3 |
The analyzed butter samples were most strongly contaminated with bacteria from the Bacillus cereus group, and to a lesser extent, with Cronobacter spp. and Enterococcus spp. No dangerous foodborne pathogens, such as Salmonella spp. and L. monocytogenes, were found.
Samples containing dangerous pathogenic bacteria were plated onto universal nutritive medium (see Section 2 Materials and Methods), and the grown colonies were used for Sanger sequencing of the 16S rRNA gene fragment. Results are shown in Table 3.
Table 3.
Bacteria identified by Sanger sequencing of individual colonies.
| Butter Sample | Amount of Bacteria, CFU/g |
Bacteria Identified in the Sample |
|---|---|---|
| 2 | 3.0 × 105 | Bacillus pumilus, Acinetobacter guillouiae, Rothia sp., Rahnella aquatilis, Pseudomonas sp. |
| 3 | 3.0 × 104 | Bacillus thuringiensis, Neisseria sp., Moraxella osloensis |
| 4 | 2.0 × 104 | Enterococcus sp., Zimmermannella faecalis, Pseudomonas sp. |
| 6 | 5.0 × 104 | Lysinibacillus sp., Carnobacterium maltaromaticum, Pseudomonas sp. |
| 12 | 7.0 × 105 | Enterococcus italicus, Pseudomonas sp., Kocuria salsicia, Cronobacter sakazakii, |
| 18 | 1.0 × 106 | Enterobacter cloacae, Staphylococcus epidermidis, Lysinibacillus sp., Micrococcus sp. |
Using the method of sequencing of individual bacterial colonies, opportunistic bacteria were identified in butter samples, such as Cronobacter sakazakii, Bacillus thuringiensis (Bacillus cereus group) and Enterobacter cloacae.
Butter samples also contained a high amount of lactic acid bacteria. Relative abundance of lactic acid bacteria (order Lactobacillales) in all butter samples is presented in Table 4.
Table 4.
Relative abundance of lactic acid bacteria (order Lactobacillales) amplicon sequence variants in all butter samples.
| Bacteria | Butter Sample | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | |
| Lactobacillus kisonensis | |||||||||||||||||||||
| Lactobacillus senioris | |||||||||||||||||||||
| Lactobacillus diolivorans | |||||||||||||||||||||
| Lactobacillus kefiri | |||||||||||||||||||||
| Lactobacillus parakefiri | |||||||||||||||||||||
| Lactobacillus delbrueckii | |||||||||||||||||||||
| Lactococcus taiwanensis | |||||||||||||||||||||
| Lactococcus chungangensis | |||||||||||||||||||||
| Lactococcus plantarum | |||||||||||||||||||||
| Lactococcus raffinolactis | |||||||||||||||||||||
| Streptococcus infantarius | |||||||||||||||||||||
| Streptococcus vestibularis | |||||||||||||||||||||
| Streptococcus porcorum | |||||||||||||||||||||
| Streptococcus hongkongensis | |||||||||||||||||||||
| Leuconostoc pseudomesenteroides | |||||||||||||||||||||
| 0% -no color | <0.1% | 0.1–1% | 2–15% | 16–30% | 31–45% | 46–60% | 61–75% | ||||||||||||||
Samples 11 and 14 contained a large number of bacteria of Lactobacillus genus (Lactobacillus kefiri and Lactobacillus parakefiri)—overall relative abundance, 66.8% and 68.7%, respectively. Samples 16, 17 and 20 contained a large number of bacteria Lactococcus taiwanensis—relative abundance, 14.2%, 71.3% and 34.2%, respectively. In addition, sample 20 contained Lactococcus raffinolactis in high abundance (46.6%). Sample 13 contained a large number of bacteria of Streptococcus genus (overall genus relative abundance, 87.9%). For the relative abundance of lactic acid bacteria ASV (expressed in percentage) in butter samples, see the supplemental materials.
4. Discussion
In this work, we studied bacteria contaminating Russian and foreign brands of butter available on the Russian market. The microbial communities in butter samples were very diverse and included many opportunistic pathogens, as demonstrated by high-throughput sequencing. The advantage of this method, in comparison to classical microbiological and immunological approaches, as well as PCR, is the possibility of simultaneous sequencing of DNA fragments from all the bacteria present in the sample. We used the universal primer pair 785F/1100R (see Section 2 Materials and Methods) to amplify a fragment of the 16S rRNA gene including the V4, V5, and V6 regions that have been demonstrated to be the most efficient hypervariable regions for phylogenetic analysis and taxonomic classification of bacterial species [21].
For over a decade, OTUs with 97% identity threshold were used for the identification of microorganisms using 16S rRNA gene sequencing. Recently, it was proposed that exact sequence variants (also referred to as zero-radius OTUs or OTUs with 100% identity) can be used instead of 97% threshold operational taxonomic units in metagenomic studies [22]. Later, it was shown that ASVs demonstrate higher accuracy and lower error rates in species-level classification compared to OTUs both in mock communities [23] and in research applications [24,25] while decreasing the number of identified taxa. In this study, we used the DADA2 algorithm for the identification of ASVs.
We used naïve Bayesian classifier for the identification of microorganisms up to the genus level. Recently, it was shown that species-level identification is possible only if 100% identity threshold is used for V4 hypervariable region of 16s rRNA gene [26]. As we used the V4–V6 hypervariable region, we decided to use 100% identity for species-level classification. Species-level classification was performed using exact string matching against a reference database (SILVA v. 132).
Since it is almost impossible to isolate DNA directly from bacteria in butter samples because of the high fat content in butter, we first enriched the bacteria in liquid nutritive medium. It is important to remember that enrichment can change the relative content of individual bacterial species in the sample. At the same time, enrichment allows the contamination of the sequencing results with DNA from dead bacteria to be minimized, so long as the number of live bacteria in the sample after enrichment is significantly higher than the number of dead bacteria. Therefore, ASVs corresponding to dead bacteria can be filtered out based on the low relative abundance. This is important, since detection of DNA from dead microbes is a drawback of the use of molecular-genetic methods for bacterial identification. We have decreased this possibility, because cultivation in the nutritive medium resulted in the propagation of only live bacteria that were then collected by centrifugation and used for DNA isolation.
Butter contained a large number of lactic acid bacteria. The most abundant bacteria were Lactobacillus kefiri, Lactobacillus parakefiri, Lactococcus taiwanensis and Lactococcus raffinolactis. A large amount of Streptococcus spp. bacteria (87.9% of all identified bacteria) was found in one of the butter samples (no. 13). However, the number of lactic acid bacteria dominated other bacteria in only 5 of the 21 samples.
We have identified different species of bacteria from the genus Bacillus, Pseudomonas, Lactococcus and Streptococcus. This is consistent with previous data for dairy products [6,7]. However, we did not find the most dangerous representatives of foodborne bacteria, such as Salmonella spp. and L. monocytogenes. Interestingly, some of the bacteria, such as Ochrobactrum sp., Stenotrophomonas sp., Propionibacterium acnes, and Brevundimonas sp., that was found in the commercial butter matched those found in petroleum oil earlier [11].
The analyzed butter samples were contaminated with bacteria of the Bacillus cereus group that includes at least 8 closely related species: Bacillus cereus, B. anthracis, B. pseudomycoides, B. mycoides, B. weihenstephanensis, B. toyonensis, B. cytotoxicus and B. thuringiensis [27,28]. We were not able to distinguish between these three species based on the sequence of the analyzed DNA fragment (unpublished data). Bacteria of the Bacillus cereus group can cause severe food poisoning [29], including diarrhea (due to production of thermolabile enterotoxin during propagation in small intestine) [30] or vomiting (due to secretion of the thermostable peptide toxin cereulide) [31]. The prevalence of these microorganisms in the samples was very high. Thus, the Bacillus cereus group was the third most abundant group of microorganisms in two butter samples. Note that the presence of these bacteria was confirmed by plating the samples onto solid medium, followed by the isolation of the grown colonies and the sequencing of the 16S rRNA gene fragment.
Two samples contained Pseudomonas aeruginosa; in one of them, this bacterium was the second most abundant microorganism. P. aeruginosa possesses an array of virulence factors counteracting host defenses and is one of the most common microbes causing respiratory infections in patients in hospitals [32]. Infections caused by P. aeruginosa are difficult to cure because of this microorganism’s ability to acquire resistance to antibiotics [33].
To our surprise, we identified bacteria of the Cronobacter genus in several butter samples by high-throughput sequencing. Sanger sequencing of bacterial colonies grown after plating of these samples on the universal nutritive medium identified them as Cronobacter sakazakii. C. sakazakii causes serious infections, such as necrotic enterocolitis, meningitis, and sepsis in formula-fed infants under 1 year of age [34]. Neonatal infections caused by C. sakazakii are often epidemiologically linked to the consumption of contaminated powdered infant formula [35]. It is known that C. sakazakii can live in low-moisture foods [36] and has high tolerance to elevated temperatures [37], which explains the presence of Cronobacter bacteria in dried food products. Cronobacter spp. have been found in powdered milk, spices, fresh vegetables, and pasta [38,39]. Here, we demonstrated for the first time, the presence of Cronobacter spp. in butter, which might be explained by the high tolerance of these microorganisms to media with low water content. Also, it may indicate that powdered milk could be used for the production of butter.
Identification of opportunistic microorganisms using high-throughput sequencing requires further steps to improve the methodology. We have shown that the detection of certain species of bacteria depends on the amount of reads on ASV (Table 2). It is necessary to optimize the method for detecting dangerous microorganisms in food to avoid false identification of bacteria that is dangerous to humans.
In conclusion, we demonstrated for the first time, that butter is a good growth medium for opportunistic pathogens. Bacteria from a broad range of taxa were present in butter samples in very high amounts. We were able to identify the contaminating species by both high-throughput sequencing and traditional plating on nutritive medium. Our data indicate that despite the fact that butter is a dairy product with a long shelf life, it should be subjected to quality control for the presence of pathogenic microorganisms. Based on the fact that the bacteria identified by us in the butter partially coincided with the data on the bacterial contaminant of dairy products [6,7], milk powder [35] and oil [11], we suggest that opportunistic bacteria was able to get into the butter, along with the ingredients necessary for its making. The possibility of contamination with C. sakazakii should be taken into account when feeding babies less than 1 year of age with butter-containing food products.
Supplementary Materials
The following are available online at https://www.mdpi.com/2304-8158/9/5/608/s1, File “S1_Minor bacteria species in butter”. File “S2_Relative abundance of lactic acid bacteria (order Lactobacillales) ASV in all butter samples”.
Author Contributions
Conceptualization, E.S.P. and M.Y.S.; methodology and software, S.A.S. and A.V.P.; writing—original draft preparation, M.Y.S. and A.V.K.; writing—review and editing, E.S.P. and V.N.P.; project administration, V.N.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by Russian Science Foundation (project 19-76-10023).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Oliver S.P., Jayarao B.M., Almeida R.A. Foodborne pathogens in milk and the dairy farm environment: Food safety and public health implications. Foodborne Pathog. Dis. 2005;2:115–129. doi: 10.1089/fpd.2005.2.115. [DOI] [PubMed] [Google Scholar]
- 2.Quigley L., O’Sullivan O., Stanton C., Beresford T.P., Ross R.P., Fitzgerald G.F., Cotter P.D. The complex microbiota of raw milk. FEMS Microbiol. Rev. 2013;37:664–698. doi: 10.1111/1574-6976.12030. [DOI] [PubMed] [Google Scholar]
- 3.Headrick M.L., Korangy S., Bean N.H., Angulo F.J., Altekruze S.F., Potter M.E., Klontz K.C. The epidemiology of raw milk-associated foodborne disease outbreaks reported in the United States, 1973 through 1992. Am. J. Public Health. 1998;88:1219–1221. doi: 10.2105/AJPH.88.8.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tibana A., Warnken M.M., Nunes M., Ricciardi I.D., Noleto A.L.S. Occurrence of Yersinia species in raw and pasteurized milk in Rio de Janeiro, Brazil. Int. J. Food Microbiol. 1987;50:580–583. doi: 10.4315/0362-028X-50.7.580. [DOI] [PubMed] [Google Scholar]
- 5.Gitter M., Bradley R., Blampied P.H. Listeria monocytogenes infection in bovine mastitis. Vet. Rec. 1980;107:390–393. doi: 10.1136/vr.107.17.390. [DOI] [PubMed] [Google Scholar]
- 6.Syromyatnikov M.Y., Kokina A.V., Savinkova O.V., Panevina A.V., Solodskikh S.A., Orlova M.V., Grabovich M.Y., Starkov A.A., Popov V.N. Study of the microbiological composition of dairy products and mayonaise using DNA barcoding and metabarcoding. Foods Raw Mater. 2018;6:144–153. doi: 10.21603/2308-4057-2018-1-144-153. [DOI] [Google Scholar]
- 7.Cremonesi P., Ceccarani C., Curone G., Severgnini M., Pollera C., Bronzo V., Riva F., Addis M.F., Filipe J., Amadori M., et al. Milk microbiome diversity and bacterial group prevalence in a comparison between healthy Holstein Friesian and Rendena cows. PLoS ONE. 2018;13:e0205054. doi: 10.1371/journal.pone.0205054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pang M., Xie X., Bao H., Sun L., He T., Zhao H., Zhou Y., Zhang L., Zhang H., Wei R., et al. Insights into the Bovine Milk Microbiota in Dairy Farms with Different Incidence Rates of Subclinical Mastitis. Front. Microbiol. 2018;9:2379. doi: 10.3389/fmicb.2018.02379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meshref A.M. Microbiological quality and safety of cooking butter in Beni-Suef governorate-Egypt. Afr. Health Sci. 2010;10:193–198. [PMC free article] [PubMed] [Google Scholar]
- 10.Idoui T., Rechak H., Zabayou N. Microbial quality, physicochemical characteristics and fatty acid composition of a traditional butter made from goat milk. Ann. Food Sci. Technol. 2013;14:108–114. [Google Scholar]
- 11.Yoshida N., Yagi K., Sato D., Watanabe N., Kuroishi T., Nishimoto K., Yanagida A., Katsuragi T., Kanagawa T., Kurane R., et al. Bacterial communities in petroleum oil in stockpiles. J. Biosci. Bioeng. 2005;99:143–149. doi: 10.1263/jbb.99.143. [DOI] [PubMed] [Google Scholar]
- 12.Ghasemloy Incheh K.H., Hassanzadazar H., Forouzan S.H., Banafshehchin E.I., Mozafarian E.I., Aminzare M., Hashemi M. A survey on the quality of traditional butters produced in West Azerbaijan province, Iran. Int. Food Res. J. 2017;24:327–332. [Google Scholar]
- 13.Yilmaz Ö. Identification of microflora in butter samples from Turkey by using the microbial identification system S. Asian J. Chem. 2009;21:3257–3262. [Google Scholar]
- 14.Syromyatnikov M.Y., Kiryanova S.V., Popov V.N. Development and validation of a TaqMan RT-PCR method for identification of mayonnaise spoilage yeast Pichia kudriavzevii. AMB Express. 2018;8:186. doi: 10.1186/s13568-018-0716-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mayo B. Impact of Next Generation Sequencing Techniques in Food Microbiology. Curr. Genom. 2014;15:293–309. doi: 10.2174/1389202915666140616233211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferrario C., Lugli G.A., Ossiprandi M.C., Turroni F., Milani C., Duranti S., Mancabelli L., Mangifesta M., Alessandri G., Sinderen D., et al. Next generation sequencing-based multigene panel for high throughput detection of food-borne pathogens. Int. J. Food. Microbiol. 2017;256:20–29. doi: 10.1016/j.ijfoodmicro.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 17.Techo S., Shiwa Y., Tanaka N., Fujita N., Miyashita M., Shibata C., Booncharoen A., Tanasupawat S. Enterococcus florum sp. nov., isolated from a cotton flower (Gossypium hirsutum L.) Int. J. Syst. Evol. Microbiol. 2019;69:2506–2513. doi: 10.1099/ijsem.0.003524. [DOI] [PubMed] [Google Scholar]
- 18.Edgar R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics. 2010;26:2460–2461. doi: 10.1093/bioinformatics/btq461. [DOI] [PubMed] [Google Scholar]
- 19.Edgar R.C., Flyvbjerg H. Error filtering, pair assembly and error correction for next-generation sequencing reads. Bioinformatics. 2015;31:3476–3482. doi: 10.1093/bioinformatics/btv401. [DOI] [PubMed] [Google Scholar]
- 20.Wang Q., Garrity G.M., Tiedje J.M., Cole J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007;73:5261–5267. doi: 10.1128/AEM.00062-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yong B., Wang Y., Qian P. Sensitivity and correlation of hypervariable regions in 16S rRNA genes in phylogenetic analysis. BMC Bioinform. 2016;17:135. doi: 10.1186/s12859-016-0992-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Callahan B.J., McMurdie P.J., Holmes S.P. Exact sequence variants should replace operational taxonomic units in marker-gene data analysis. ISME J. 2017;11:2639–2643. doi: 10.1038/ismej.2017.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Callahan B.J., Proctor D., Relman D., Fukuyama J., Holmes S. Reproducible research workflow in r for the analysis of personalized human microbiome data. Pac. Symp. Biocomput. 2016;21:183–194. [PMC free article] [PubMed] [Google Scholar]
- 24.Macheriotou L., Guilini K., Bezerra T.N., Tytgat B., Nguyen D.T., Nguyen T.X.P., Noppe F., Armenteros M., Boufahja F., Rigaux A., et al. Metabarcoding free-living marine nematodes using curated 18S and CO1 reference sequence databases for species-level taxonomic assignments. Ecol. Evol. 2019;9:1211–1226. doi: 10.1002/ece3.4814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Caruso V., Song X., Asquith M., Karstens L. Performance of microbiome sequence inference methods in environments with varying biomass. mSystems. 2019;4:e00163-18. doi: 10.1128/mSystems.00163-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Edgar R.C. Updating the 97% identity threshold for 16S ribosomal RNA OTUs. Bioinformatics. 2018;34:2371–2375. doi: 10.1093/bioinformatics/bty113. [DOI] [PubMed] [Google Scholar]
- 27.Rasko D.A., Altherr M.R., Han C.S., Ravel J. Genomics of the Bacillus cereus group of organisms. FEMS Microbiol. Rev. 2005;29:303–329. doi: 10.1016/j.femsre.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 28.Liu Y., Lai Q., Göker M., Meier-Kolthoff J.P., Wang M., Sun Y., Wang L., Shao Z. Genomic insights into the taxonomic status of the Bacillus cereus group. Sci. Rep. 2015;5:14082. doi: 10.1038/srep14082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guinebretière M.H., Velge P., Couvert O., Carlin F., Debuyser M.L., Nguyen-The C. Ability of Bacillus cereus group strains to cause food poisoning varies according to phylogenetic affiliation (groups I to VII) rather than species affiliation. J. Clin. Microbiol. 2010;48:3388–33891. doi: 10.1128/JCM.00921-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Granum P.E., Lund T. Bacillus cereus and its food poisoning toxins. FEMS Microbiol. Lett. 1994;157:223–228. doi: 10.1111/j.1574-6968.1997.tb12776.x. [DOI] [PubMed] [Google Scholar]
- 31.Agata N., Ohta M., Mori M., Isobe M. A novel dodecadepsipeptide, cereulide, is an emetic toxin of Bacillus cereus. FEMS Microbiol. Lett. 1995;129:17–20. doi: 10.1016/0378-109700119-P. [DOI] [PubMed] [Google Scholar]
- 32.Gellatly S.L., Hancock R.E. Pseudomonas aeruginosa: New insights into pathogenesis and host defenses. Pathog. Dis. 2013;67:159–173. doi: 10.1111/2049-632X.12033. [DOI] [PubMed] [Google Scholar]
- 33.Lambert P.A. Mechanisms of antibiotic resistance in Pseudomonas aeruginosa. J. R. Soc. Med. 2002;41:22–26. [PMC free article] [PubMed] [Google Scholar]
- 34.Yan Q.Q., Condell O., Power K., Butler F., Tall B.D., Fanning S. Cronobacter species (formerly known as Enterobacter sakazakii) in powdered infant formula: A review of our current understanding of the biology of this bacterium. J. Appl. Microbiol. 2012;113:1–15. doi: 10.1111/j.1365-2672.2012.05281.x. [DOI] [PubMed] [Google Scholar]
- 35.McMullan R., Menon V., Beukers A.G., Jensen S.O., van Hal S.J., Davis R. Cronobacter sakazakii Infection from Expressed Breast Milk, Australia. Emerg. Infect. Dis. 2018;24:393–394. doi: 10.3201/eid2402.171411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lang E., Guyot S., Peltier C., Alvarez-Martin P., Perrier-Cornet J.M., Gervais P. Cellular injuries in Cronobacter sakazakii CIP 103183T and Salmonella enteric exposed to drying and subsequent heat treatment in milk powder. Front. Microbiol. 2018;9:475. doi: 10.3389/fmicb.2018.00475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Osaili T.M., Shaker R.R., Al-Haddaq M.S., Al-Nabulsi A.A., Holley R.A. Heat resistance of Cronobacter species (Enterobacter sakazakii) in milk and special feeding formula. J. Appl. Microbiol. 2009;107:928–935. doi: 10.1111/j.1365-2672.2009.04271.x. [DOI] [PubMed] [Google Scholar]
- 38.Ueda S. Occurrence of Cronobacter spp. in Dried Foods, Fresh Vegetables and Soil. Biocontrol. Sci. 2017;22:55–59. doi: 10.4265/bio.22.55. [DOI] [PubMed] [Google Scholar]
- 39.Akineden Ö., Murata K.J., Gross M., Usleber E. Microbiological quality of raw dried pasta from the German market, with special emphasis on Cronobacter species. J. Food. Sci. 2015;80:2860–2867. doi: 10.1111/1750-3841.13117. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.