Abstract
Nicotinamide adenine dinucleotide (NAD+) is an essential pyridine nucleotide that has garnered considerable interest in the last century due to its critical role in cellular processes associated with energy production, cellular protection against stress and longevity. Research in NAD+ has been reinvigorated by recent findings that components of NAD+ metabolism and NAD-dependent enzymes can influence major signalling processes associated with the neurobiology of addiction. These studies implicate raising intracellular NAD+ levels as a potential target for managing and treating addictive behaviour and reducing cravings and withdrawal symptoms in patients with food addiction and/or substance abuse. Since clinical studies showing the use of NAD+ for the treatment of addiction are limited, this review provides literature evidence that NAD+ can influence the neurobiology of addiction and may have benefits as an anti-addiction intervention.
Keywords: NAD+, cocaine, addiction, alcohol, cellular energetics
1. Addiction: Today’s Most Common Modern Disease
Addiction represents the most common untreatable disorder in the 21st century. Addiction has a profound effect on individuals, their family and carers, and represents a major socio-economic burden on today’s healthcare system. Some epidemiological studies have suggested that addiction may be endemic in some populations [1]. The most common forms of addiction are alcoholism and smoking, both of which have been associated with increased risk of developing age-related disorders which manifest in the cardiovascular and central nervous system [2,3,4,5]. Tobacco smoking is the most preventable cause of morbidity and mortality worldwide. It has been estimated that of the 38 million people in the United States of America alone who attempt to quit smoking, more than 85% relapse within the first few weeks following withdrawal interventions. Abstinent smokers who successfully withdraw in the first few months are susceptible to relapse after six months or even years later [6]. Alcoholism is the third leading cause of death after cardiovascular disease and cancer and is associated with over 2.3 million deaths worldwide every year. Alcoholism is the ninth major contributor to global disease burden as measured by the disability-adjusted life years (DALYs) [2,3,4,5]. As with other forms of addiction, relapse occurs in response to exposure to environmental stimuli that are linked to the rewarding effects of the stimulant. Moreover, substance abuse (e.g., cocaine and heroin), internet addiction and excessive gambling are growing problems among the younger generation [7].
Food addiction has been associated with obesity, a global complex health problem that is dependent on multidisciplinary treatment and various health practitioners including experts in mental health, medicine and surgery [1]. Obesity has been associated with addictive behaviour that has a profound effect on morbidity and mortality leading to a reduction in the overall quality of life. The World Health Organization estimates that by the year 2030, over 57.8% of the world’s population will be obese. Obesity represents the second cause of death in the United States alone, affecting nearly 35 million people alone. Obesity has a negative effect on metabolic and endocrine processes that can lead to multiple organ disorders, malignant diseases such as cancers, mechanical impairments, surgical complications, and psychosocial deficits [8,9,10,11]. Obesity is also associated with genetic predisposition, impaired hormonal and metabolic processes, as well as confounding psychological and lifestyle factors [12,13,14,15]. This suggests that obesity is not a single disease, but rather a manifestation of several pathological, physiological and psychological processes leading to endocrine-metabolic dysfunction.
Substantial research into the pathobiology and mechanisms of addiction, and addiction therapy are yet to yield effective responses. The most successful addiction programs demonstrate a relapse-response rate of 56% in subjects [16,17,18,19,20]. Given the importance of improving the prevention and successful treatment of addiction, there is a growing need to investigate new factors that may be associated with addictive behaviour. Various experimental approaches suggest that replenishment or augmentation of cellular levels of the essential pyridine nucleotide, nicotinamide adenine dinucleotide (NAD+) can provide ameliorative benefits and significantly lower relapse in addiction.
2. Neurobiology of Addiction
A wealth of evidence has identified several similarities between individuals who demonstrate addictive food behaviour and those that are diagnosed as addicted to substances. DiLeone et al. showed that individuals that are addicted to food describe similar processes (e.g., rewarding properties, withdrawal symptoms and overeating) to those addicted to drug and alcohol addicts [21,22]. Food in today’s contemporary society is rich in calories, saturated fats, sugars, synthetic additives (e.g., colours) and preservatives which are of low nutritional value and widely accessible. Compulsive over-consumption of foods rich in salt and additives or saturated with sugars and lots of calories has the greatest potential for addiction [23]. These foods are termed ‘hyper palatable’, and can induce hedonistic behaviours and inhibit negative thoughts similar to substance abuse [24,25]. This can induce excessive preoccupation of food and periodic eating of large amounts of food within a very short time frame. These events may occur either once a day or once a week, leading to feelings of culpability, ignominy and depression, triggering further over-consumption of foods due to increased emotional stress [26,27]. Like substance abuse and sex addiction, ‘over-eating’ represents an in-effective mechanism to overcome negative thoughts or mediate ‘self-control’ [1]. There are also various similarities between the molecular basis of food and drug addiction which are detailed below.
2.1. Hyper-Activation of the Glutaminergic System
Overactivation of the glutaminergic system has been shown to play an important role in substance abuse and obese conditions. In particular, it has been previously shown that treatment with N-methyl-D-aspartate receptor (NMDAR) antagonists can attenuate drug cue associations, and reduce cue-induced drug seeking and relapse-like behaviour [28]. Moreover, intracerebroventricular and lateral hypothalamic injections of glutamate, or its excitatory amino acid agonists, kainic acid, AMPA, and N-methyl-D-aspartate (NMDA), enhance food appetite in murine models, while the mGluR5 receptor antagonist, (R,S)-2-chloro-5-hydroxyphenylglycine, suppresses appetite [29]. However, alternative therapies are warranted since NMDAR antagonism can induce psycho-mimetic adverse effects (e.g., hallucinations, paranoia, cognitive deficits) due to NMDAR antagonism [30].
2.2. Impaired Mitochondria Function
There is also substantial evidence indicating that brain energy homeostasis is perturbed in substance abuse and metabolic syndrome. This includes impairments in glucose metabolism, increased oxidative stress and reduced cellular respiration [31,32,33,34,35]. However, very few studies are available in the literature that investigate mitochondrial changes in selected brain regions associated with the central reward pathway for substance abuse and food addiction. Recent studies have reported evidence of mitochondrial dysfunction in impaired motivation states such as depression, anxiety and stress [36,37]. For example, BCL-2, which regulates mitochondrial Ca2+ homeostasis, has been associated with impairments in mood, and increased BCL-2 has been reported to stabilise mood [38]. In addition, mutations in several mitochondrial genes have been associated with increased anxiety in several animal models [39]. Transcriptomic profiling of the nucleus accumbens (NAc)—an important brain reward nucleus—identified enrichment in gene ontology of mitochondria-related transcripts following repeated cocaine exposure [40]. Exposure to substances also impaired the activity and function of mitochondrial complex I, oxidative phosphorylation and ATP production, and mitochondrial membrane potential [31].
Furthermore, studies using functional magnetic resonance imaging (fMRI) reported increased activity in regions of the brain associated with motivation and reward when a sample of 48 women were drinking a chocolate milkshake or exposed to photographs of milkshake cups getting filled. Higher activity was observed in women with greater addiction scores, and less activation was reported in regions of the brain associated with inhibition of certain behaviours [25,26]. This suggests that these women were less able to control their behaviours when exposed to milkshakes as stimuli. Another study showed that food addiction was correlated with increased activation in the anterior cingulate cortex, medial orbitofrontal cortex and amygdala in response to eating. Greater activation was reported dorsolaterally in the prefrontal cortex and caudate nucleus, and less activation in the lateral orbitofrontal cortex, similar to drug addicts [25,26].
2.3. Increased Neuroinflammation and Kynurenine Pathway Activation
Numerous studies have shown that neuroinflammation can induce depressive symptoms and increase the likelihood of developing addiction [41,42,43,44,45,46]. Activation of the kynurenine pathway (KP) of tryptophan (TRYP) degradation has been thought to play a major role in drug-related conditioned behaviours [47]. Several products of the KP are neuroreactive. For example, quinolinic acid (QUIN) is an NMDAR agonist and its accumulation can lead to excitotoxicity and impaired glutaminergic transmission [48,49,50]. Kynurenic acid (KYNA) is an NMDAR antagonist that exerts neuroprotective effects on the brain and can counteract the cytotoxic effects of QUIN [51]. We and others have hypothesised that the QUIN/KYNA ratio represents a balance between neurodegeneration and neuroprotection in the brain which may be associated with immune activation [49,52,53]. Inhibition of kynurenine-3-monooxygenase (KMO) by Ro61-8048 and its prodrug JM6 has been previously reported to induce a metabolic shift in the KP towards KYNA, thus reducing glutaminergic/NMDAR activity [47]. KMO inhibition also abolished relapse-like alcohol drinking and alcohol and cocaine-seeking behaviours [47]. However, given that nicotinamide adenine dinucleotide (NAD+) represents the final end product of the KP, it is unclear whether NAD+ levels can be effectively maintained when KMO is inhibited [54].
2.4. Alterations in the Mesolimbic-Fronto Cortical Dopamine Pathway
The mesolimbic-fronto cortical dopamine (DA) system (composed of the mesolimbic and mesocortical DA systems) is an important pathway in brain reward [55]. The neurotransmitter, DA, is a key component in the brain reward system. DA has been associated with both drug and food addiction. Alcohol consumption has been reported to directly stimulate DA release and increase DA levels [56]. In addition, the behavioural rewards of nicotine are associated with DA release in the mesolimbic pathway [57], and lesions in the mesolimbic DA pathway have been reported to lead to a reduction in self-administration of nicotine [55]. Cannabinoid receptors have also been identified in regions of the brain associated with reward. The active component of cannabis, A’-tetrahydrocannabinol (A9-THC) has been shown to increase DA levels [58].
The brain reward system also plays a crucial role in food addiction, promoting adaptive behaviours (e.g., consuming palatable nutrients by linking them to pleasurable thoughts). Release of gastrointestinal (GI) hormones in response to nutrients in the GI tract, limit food intake through activation of the reward system by influencing motivational behaviour and its reward value [59]. For instance, reduced GI production of the fat specific satiety factor oleoylethanolamide (OEA) can induce high-fat-diet induced obesity in mice [60]. Negative feedback mechanisms also exist between the brain and the gut for sugars, consumption of which is dependent on taste and post-ingestive factors which have a direct effect on the brain reward system or mediating the production of endocrine factors that influence sucrose satiety and reward [61].
2.5. Dysregulation of Endocrine Factors
The neuropeptide and hormone oxytocin (OXT) has been thought to play a role in reward, stress, social and cognitive processes which are affected by addiction [62]. OXT is a nonapeptide that is secreted by the brain from oxytocinergic neurons in the paraventricular and supraoptic nuclei of the hypothalamus [63]. The association between OXT and addiction stems from similarities between behaviours reported in human addiction and social behaviours [64]. Increased OXT levels following exogenous OXT via intranasal administration has been shown to boost trust, generosity and social recognition in humans without adverse effects [65]. However, these effects are context or situation dependent. There is also evidence of a common neurobiology between social interaction and addition as demonstrated in prairie voles and rodents [66]. Several preclinical studies have also shown that OXT can reverse the neuroadaptations occurring with chronic drug and alcohol use [67].
The liver is thought to be involved in the production of endocrine factors that influence addiction [68]. Fibroblast growth factor 21 (FGF21) is a liver-derived hormone that has various physiological and pharmacological effects. FGF21 has been reported to normalise blood glucose levels in diabetic animals, promote fatty acid oxidation, prevent β cell dysfunction and reduce weight gain in the high-fat-diet induced mice models [69,70,71,72,73,74,75]. FGF21 has also been reported to reduce sweet consumption in mice and humans whereas FGF21 knockout increases sugar consumption in murine models [76,77,78]. Additionally, overproduction of FGF21 signals to the brain to regulate food intake, energy expenditure and fertility, and prevent age-related increases in weight gain and insulin resistance [68,76,77,78]. FGF21 gene therapy has been demonstrated to be a potential therapy for obesity and type 2 diabetes [79]. Recently, FGF21 has also been reported to inhibit alcohol consumption in mice [80]. The levels of FGF21 are increased in human plasma following acute alcohol consumption and sustained binge drinking [80]. Taken together, these studies suggest that FGF21 is an endocrine inhibitor of alcohol and sugar consumption in humans.
2.6. Importance of Circadian Rhythms
The underlying pathobiology of addiction and DA release may be regulated diurnally by interplay between circadian rhythms and metabolism. Circadian disruption has been reported to influence behavioural responses to substance abuse [81,82,83,84,85]. For example, mutations in circadian genes in mice mediate differential locomotor sensitisation and conditioned preference to cocaine [86]. It is thought that these circadian rhythms are controlled transcriptional-translational feedback loops and selected protein coding genes governed by the molecular clock. In particular, the main circadian transcription factors CLOCK and BMAL1 have been reported to couple with various intracellular metabolic signalling pathways [87]. Circadian rhythms can regulate DA synthesis and release, which are dependent on the availability and activity of tyrosine hydroxylase (TH), whose transcriptional regulation is dependent on CLOCK/BMAL1 [88].
2.7. Role of Endogenous Opiates
Additionally, substance abuse and/or food have been shown to activate endogenous opiates, which represent a group of peptides that are produced in several organs and the brain and pituitary glands in particular [89]. The endogenous opioid system influences the mesolimbic DA and the cortisol stress response, which are both implicated in addiction reward [90]. The opioid antagonist naltrexone has been shown to inhibit the desire for sweet, salty and fatty foods, and alcohol and drugs [91].
3. Historical Background of NAD+
Pellagra is a debilitating disorder caused by the deficiency of niacin and/or its precursor tryptophan. Symptoms of pellagra were first documented in 18th century by the Spanish doctor Gasper Casal, who described a disorder attributed to a diet deficient in meat [92]. Pellagra was epidemic in malnourished regions of Europe and the southern states of the United States of America. Pellagra has been characterised as a ‘disease with four D’s’—dermatitis, diarrhoea, dementia and death [93]. However, these classic symptoms rarely occur together or follow a predictable pattern, and are modified by environmental factors such as sun exposure, other concomitant vitamin deficiencies and disease progression [94]. Excessive alcohol consumption represents a major risk factor for pellagra and chronic alcohol use can induce niacin deficiency [95]. It has been reported that more than 100,000 Americans died from pellagra between 1907 and 1940. In 1914, Sir Joseph Goldberger linked pellagra to a nutrient deficiency due to a corn-rich diet. He suggested that dried yeast could be a cheap and effective therapeutic strategy to prevent pellagra [96]. However, it was not until 1937 that Dr. Conrad Elvehjem demonstrated that nicotinic acid (NA) and nicotinamide (NAM) cured pellagra [97].
The metabolic significance of nicotinamide adenine dinucleotide (NAD+) was first identified by Sir Arthur Harden in 1906, who demonstrated that boiling and filtering yeast extract enhanced alcoholic fermentation in unboiled yeast extract. The active component was termed extract conferment or cozymase [98]. In 1923, von Euler-Chelpin showed that cozymase was composed of a nucleoside sugar phosphate [99]. Subsequently, the role of NAD+ as a hydrogen carrier in anaerobic and aerobic oxidation was identified by Sir Otto Warburg in 1933 [100]. The pathways involved in NAD+ anabolism were fully characterised by Sir Arthur Kornberg, and the work of Priess and Handler in the 1940s and 1950s, respectively [101]. The redox roles of NAD+ in glycolysis, the tricarboxylic acid cycle, oxidative metabolism and energy production were elucidated by several scientists including Krebs. The non-redox roles of NAD+ as a substrate for ADP-ribosylation reactions and histone deacetylase activities have been elucidated in the last few decades [101]. Remarkably, maintaining NAD+ homeostasis is not only essential for the treatment of pellagra, but may also be associated with addiction, cardiovascular and neurodegenerative diseases and metabolic syndrome [54].
The significance of NAD+ in addictive disorders stems from the work of Dr. Paul O’ Hollaren (1961) who claimed to have successfully utilised IV NAD+ for the prevention and treatment of over 104 cases of addiction to alcohol and other drugs of abuse, including heroin, opium extract, morphine, dihydromorphine, meperidine, codeine, cocaine, amphetamines, barbiturates and tranquilisers [102]. In his retrospective case series, IV NAD+ was administered at a dose of 500–1000 mg added to 300 cc normal saline daily for 4 days, twice per week for a month, followed by a maintenance dose twice per month until addiction was ameliorated, with limited toxic effects [102]. NAD+ is likely to represent a cheap and useful holistic approach for the estimated millions of addicts worldwide, and may be an effective adjunct to psychotherapy, by ameliorating symptoms of physical addiction through a variety of mechanisms.
4. Summary of NAD+ Dependent Processes
There is a growing consensus that NAD+ levels decline at the cellular, tissue and organismal levels during ageing and progression of age-related degenerative diseases. NAD+ and its closely related phosphate NADP are cofactors in several anabolic and catabolic processes, such as fatty acid and cholesterol synthesis [103]. NAD+ and NADP, and their reduced forms, NADH and NADPH, are important cofactors in more than 400 enzymatic reactions including dehydrogenases, hydroxylases and reductases [104]. Recently, NAD+ has recently been reported to be an important reductant and hydride donor in biological oxidation of carbohydrate pathways [105]. NAD+ is essential for the dehydrogenation of acetylaldehyde, which is essential for alcohol metabolism. Impaired oxidative phosphorylation in rat brain cortical slices following exposure to acetylaldehyde was attenuated by the addition of NAD+ [106]. Although the reduced and phosphorylated forms can interconvert, they do not alter the levels of NAD+. The activity of several NAD-dependent enzymes or NAD+ ‘consumers’ are also affected by the decline in NAD+, influencing multiple processes and age-associated pathophysiologies.
4.1. Poly(ADP-Ribose)Polymerases (PARPs)
Poly(ADP-ribose)polymerases (PARPs) are a family of DNA ‘nick’ sensors that detect DNA strand breaks through its N-terminal zinc-finger domain [107]. Poly(ADP)ribosylation breaks down NAD+ to NAM and an ADP-ribosyl product [108]. Oxidative damage and neuroinflammation have been reported parallel to DNA damage. Therefore, NAD+ depletion due to hyperactivation of PARP1 and 2 in the nucleus may play an important role in the pathology of central nervous system (CNS) disorders [109,110,111,112,113]. PARPs also regulate the tumour suppressor protein, p53. For instance, inhibition of poly(ADP)ribosylation and inactivation of p53 following treatment with etoposide was reported in PARP-deficient cell lines derived from Chinese hamster V79 cells [114]. PARP has also been reported to activate DNA-dependent protein kinases which influence p53 activity via phosphorylation [115]. Therefore, PARP activity is essential for the maintenance of DNA repair and genomic stability.
4.2. CD38/NAD+ Glycohydrolase
CD38 is a major NADase in mammalian cells, and another NAD+-consuming enzyme [116]. CD38 and CD157 hydrolyse NAD+ to generate ADP ribose (ADPR) and NAM. CD38 also produces the secondary messenger signalling molecule, cyclic-ADP-ribose (cADPR) which induces transient intracellular calcium waves. It has been estimated that about 100 molecules of NAD+ are necessary for hydrolysis to generate one molecular of cADPR. CD38 can also use the NAD+ precursors, nicotinamide mononucleotide (NMN) and nicotinamide riboside (NR) to regulate intracellular NAD+ levels [117]. It can also catalyse a base exchange between NADP and NA, generating nicotinic acid adenine dinucleotide phosphate (NAADP). CD38 has various immunomodulatory roles and increases in the levels of CD38 protein have been reported in several tissues over time, thus contributing to age-related NAD+ decline. CD38/cADPR also regulate oxytocin release, which is associated with social behaviours, and impaired CD38 function may induce several forms of neurological deficits [118].
4.3. Sirtuins
Silent information regulators of gene transcription, or sirtuins, are a family of class III NAD+ dependent histone deacetylases enzymes that translate changes in NAD+ levels to the regulation of several proteins associated with cellular metabolism, cellular stress responses, circadian rhythms and endocrine functions. The deacetylation reaction involves acetyl group transfer to ADPR to form a novel compound called 2′-O-acetyl-ADPR or acetylated ADP-ribose [119]. Cleavage of the glycosidic bonds in NAD+, leads to production of NAM as a by-product [120]. Mammalian cells have seven classes of sirtuins (SIRT1–7) located in various cellular organelles, and control a variety of important biological processes [121]. SIRT1 is a nuclear protein that is found mainly in the nucleus but can translocate to the cytoplasm, and is involved in promoting cellular longevity [122] by influencing the acetylation/deacetylation status of several important transcription factors, including the metabolic regulator, peroxisome proliferator-activated receptor-γ (PPARγ), tumour suppressor protein (p53) and the cell growth linked FOXO forkhead family of transcription factors [123]. SIRT2 is found in the cytoplasm but can also be found in the nucleus where it regulates gene expression and the cytoskeletal structure [124]. SIRT3 [125], SIRT4 [126] and SIRT5 [127] are found in mitochondrial compartments where they are involved in maintenance of mitochondrial redox status. SIRT6, another nuclear sirtuin, is involved in mediating an age-resistant phenotype [128]. Finally, SIRT7 is found in the nucleolus of mammalian cells where it mediates growth and metabolism [129]. Importantly, the beneficial effects of sirtuin activity are dependent on optimal NAD+ levels.
4.4. Sterile Alpha and Toll/Interleukin-1 Receptor Motif-Containing 1 (SARM1)
Sterile alpha and Toll/interleukin-1 receptor motif-containing 1 (SARM1) is a recently discovered NAD+ hydrolase. Axonal degeneration is associated with NAD+ depletion and inhibition of SARM1 function delays axonal degeneration. The Toll/interleukin-1 receptor (TIR) domain of SARM1 is dependent on NAD+ for NAD+ hydrolase activity and enhances axonal degeneration [130,131,132,133,134,135].
4.5. Interactions between NAD+ Consumers
We and others have previously demonstrated that the activity of PARP and SIRT1 is regulated by CD38, by potentially limiting the availability of NAD+ to its target enzymes [136]. NAM, which is also produced by the catalytic activity of CD38, is also an endogenous inhibitor of SIRT1 and CD38. Therefore, CD38 represents an important regulator of SIRT1 activity and SIRT1 functions, including maintenance of cellular bioenergetics, obesity and senescence, mainly because it modulates the availability of NAD+ to the SIRT1 enzyme [137]. There also exists a relationship between PARP1/2 and sirtuins, through their common substrate, NAD+. For example, PARP1/2 knockout enhances SIRT1 activity leading to increased mitochondrial function, fatty acid oxidation and protection against obesity [138,139]. However, while PARP1 knockout promotes NAD+ availability, PARP2 knockout promotes SIRT1 expression [140,141]. Therefore, chronic accumulation of oxidative stress and increased PARP activity plays a causal role in the decline in NAD+ and SIRT1 activity.
5. Overview of NAD+ Biosynthetic Pathways
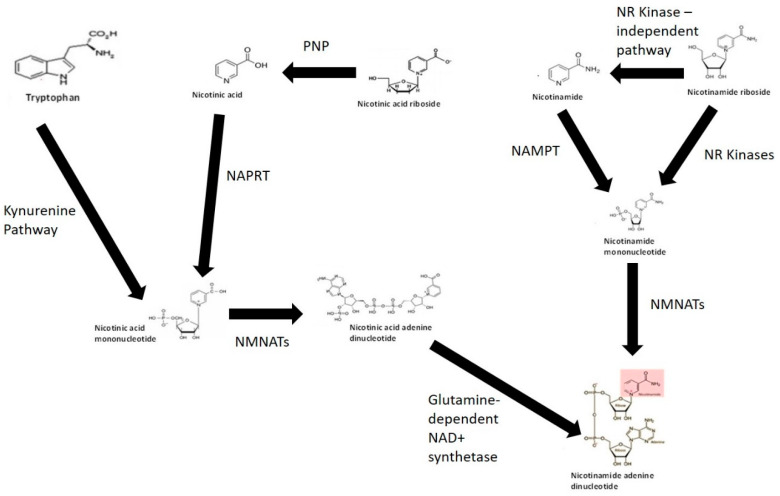
Given the importance of NAD+ in cellular bioenergetics and the need to maintain intracellular NAD+ pools in cells, several pathways are involved in the synthesis of NAD+. These include but may not be limited to: (1) de novo NAD+ synthesis from the amino acid TRYP via the KP; (2) NAD+ salvage pathway from vitamin B3 derivatives NA, NAM, NR and nicotinic acid riboside (NAR); and (3) major recycling pathway through NAM (Figure 1) (reviewed in [54]). Evidence suggests that the entire intracellular NAD+ pool may be consumed several times a day [142]. NAD+ anabolism is dependent on the availability of potential precursors, and not all of these precursors are bioequivalent [143].
Figure 1.
NAD+ biosynthesis pathways. The kynurenine pathway represents the de novo pathway of NAD+ is synthesis from catabolism of the amino acid tryptophan. NAD+ can also be synthesised via salvage of nicotinamide, nicotinic acid, nicotinic acid riboside and nicotinamide riboside form of vitamin B3. Abbreviations: NR kinase, nicotinamide riboside kinase; NAMPT, nicotinamide phosphoribosyltransferase; NMNAT, nicotinamide mononucleotide adenyltransferase; NAPRT, nicotinic acid phosphoribosyltransferase; PNP, purine nucleoside phosphorylase.
5.1. NAD+ from Tryptophan
TRYP is converted to NAD+ via an eight-step process known as the KP. The conversion of TRYP to NAD+ occurs when the substrate supply is greater than the enzymatic capacity of 2-amino-3-carboxymuconate semialdehyde (ACMSD), an enzyme required for the synthesis of the NMDAR antagonist and endogenous metal chelator, picolinic acid (PIC) [54]. The NAD+ equivalence of 60 mg TRYP to 1 mg niacin may be partially explained by the fact that most of TRYP is used by the cell for protein synthesis. The daily recommend TRYP intake is 4 mg/kg body weight for adults [101]. Studies have shown that the levels of NAM have little effect on the de novo biosynthesis rate of NAD+ from TRYP. Oral supplementation with TRYP at 15 g/day has been reported to induce some adverse effects including drowsiness and headache [144]. Overconsumption of TRYP is capable of increasing the levels of QUIN which has been associated with neurodegenerative disorders, anxiety and seizures [48]. Therefore, TRYP is unlikely to represent an ideal pharmaceutical source to raise NAD+ levels.
5.2. NAD+ from Nicotinic Acid
NA is converted to nicotinic acid mononucleotide (NAMN) by the ATP-dependent enzyme nicotinic acid phosphoribosyl transferase (NAPRT) (EC 6.3.4.21) using PRPP as a co-substrate. As QUIN is converted to NAMN by the enzyme QPRT, the sequence of events leading to NAD+ production is identical to NAD+ from TRYP after NAMN formation [145]. NAPRT appears to be expressed in several catabolic tissues including the colon, heart, kidney and liver [146]. Adenylation of NAMN is dependent on the catalytic activity of NAD pyrophosphorylase or nicotinamide mononucleotide adenylyltransferase (NMNAT) (EC 2.7.7.1) in the presence of ATP to produce desamido NAD [147,148]. Three NMNAT isoforms are NMNAT-1 (nucleus), NMNAT-2 (Golgi complex) and NMNAT-3 (mitochondria) [149]. This suggests an organelle-specific function for enzymes, and specific nuclear, mitochondrial and Golgi-specific NAD+ anabolic pathways. NMNAT-2 and -3 can also form NADH directly from reduced nicotinamide mononucleotide (NMN) [150]. NMNAT activity (and predominantly NMNAT-1) is high and non-rate-limiting in catabolic tissue, but not in blood [151].
The process involved in the synthesis of NAD+ from NA is known as the Priess–Handler process. NA has been shown to increase intracellular NAD+ levels in a kidney cell line [152]. Oral supplementation of 1–3 g of NA daily lowers blood triglyceride levels and low-density lipoproteins (LDLs), and increases the levels of high-density lipoproteins (HDLs) [153,154]. Exogenous NA also increases intracellular NAD+ levels in brain cells [155]. However, NA therapy induces significant skin flushing in most individuals, thus limiting its clinical use as an NAD+ precursor. A mild skin flush has been reported in patients exposed to doses as low as 50 mg oral NA [156]. The lipid lowering effects and adverse effects of NA are thought to be mediated by interaction of NA to the cell surface of a G-protein coupled receptor known as HM74A or GPR109A, which enhances the conversion of the omega-6 metabolite arachidonic acid (AA) into prostaglandin E2, stimulating vasodilation of skin capillaries, causing unwanted skin flush [156].
5.3. NAD+ from Nicotinamide Recycling
Data in the literature suggest that half-lives of most enzymes involved in NAD+ consumption are 4–10 h [142]. This suggests that 200–600 µmol/kg of NAM needs to be recycled back to NAD+ per day per tissue in rats. This is equivalent to consumption of 3 g of NAM several times a day in a 75 kg adult human [142]. These levels are considerably lower than the recommended doses of NAM from diet (i.e., 1 lb tuna is required for 100 mg NAM, 1 lb beef generates 30 mg NAM while four cups of broccoli retain 4 mg NAM). Therefore, effective NAM recycling pathways are necessary to protect against NAD+ deficiency in humans. Recycling of NAM to NAD+ is dependent on the enzyme nicotinamide phosphoribosyl transferase (NAMPT) (EC 2.4.2.12) using PRPP as a co-substrate which converts NAM to NMN, and then to NAD+ by the action of NAD pyrophosphorylases in the presence of ATP [157]. The rate of NAM recycling is highest in the liver and kidney, and lowest in blood [151]. The recommended dose of NAM is 14–16 mg per day and is suggestive of a net loss not exceeding 0.5% total NAD+ per day to maintain NAD+ homeostasis. Considering that the intracellular NAD+ pools may be replaced up to four times per day, this reflects a total loss of 0.1–0.2% NAM per cycle [158]. NAM is methylated by the enzyme nicotinamide N-methyltransferase (NNMT) (EC 2.1.1.1) to N-methylnicotinamide (MeNAM). MeNAM plays important roles in several metabolic and epigenetic processes and can influence neurodegeneration and ageing. Therefore, while supplementation with NAM can raise NAD+ and does not cause flushing, it is not considered an ideal supplement due to its enzyme inhibiting (e.g., PARPs, sirtuins, CD38) and methyl depleting potential.
5.4. NAD+ from Nicotinamide Riboside and Nicotinic Acid Riboside
NR or NAR represent newly identified precursors that can be used to synthesise NAD+ via the NR kinase (NRK) (EC 2.7.1.173) pathway [159]. Two NRK enzymes have been identified, NRK1 and NRK2, although, their exact physiological roles remain unclear. Dephosphorylation of NMN into NR, which is required to produce NAD+ in yeast, represents a crucial step for increasing intracellular NAD+ in mammalian cells [160]. NR can also be catabolised into a ribosyl product and NAM via an NRK-independent pathway, which can then be further recycled to yield NAD+. Purine nucleoside phosphorylase (PNP) (EC 2.4.2.1) can convert NAR to NA, which is then converted to NAMN by the activity of NAPRT [161]. NR appears to be safe and orally bioavailable in mice and humans with very few minor side effects reported [162]. NR is currently a lead candidate in several preclinical and human clinical trials to evaluate whether it can be used for the treatment of age-related degenerative disorders [163], given that NAD+ decline and/or increased NAD+ consumption may be a major risk factor in these debilitating disorders.
6. NAD+ Metabolism: Cellular Energy, Secondary Messenger Signalling and Manipulation for Addiction
Given the global effects of NAD+ and SIRT1 on human physiology and function, NAD+ levels can influence anxiety, exploratory and depressive behaviour, and the brain reward system linked to addiction. NAD+ is likely to represent a molecular link between metabolism and psychiatric conditions [164]. It has been well established that substance abuse and food addiction can impair metabolism, alter the cellular redox status, disrupt circadian rhythms and promote oxidative stress and neuroinflammation [165]. NAD+ regulates intracellular calcium levels during DA release via increased CD38 activity, therefore reducing NAD+ availability [166]. There is also evidence of a functional relationship between NAD+ and DA levels in the brain which is likely to be critical to brain function in physiological and pathological settings [101,167,168]. Mechanisms by which NAD+ and its related processes can influence the neurobiology of addiction are described below.
6.1. SIRT1 Regulates Behavioural Responses Associated with Drug Addiction
The crucial role for SIRT1 and SIRT2 in modulating behavioural responses to cocaine and morphine in the NAc has been previously studied. One study reported increases in SIRT1 and SIRT2 expression in the mouse NAc following chronic cocaine administration, while only SIRT1 expression was increased following chronic morphine administration [169]. Interestingly, cocaine and morphine had no effect on the expression of other sirtuin family members. Increased expression of SIRT1 and SIRT2 following exposure to drugs of abuse is partly attributed to the drug-induced transcription factor ΔFosB. In addition, the rewarding effects of cocaine and morphine were enhanced in mice following viral-mediated overexpression of SIRT1 or SIRT2 in the NAc and localised knockdown of SIRT1 in the NAc reduced drug reward [169]. Therefore, sirtuins and SIRT1 and SIRT2, play key roles in mediating alterations in molecular and cellular plasticity induced by drugs of abuse in NAc, and may regulate behavioural adaptations following exposure to substance abuse.
The exact mechanisms by which SIRT1 can mediate cocaine-induced plasticity in NAc remain unclear. One study used chromatin immunoprecipitation after repeated cocaine (20 mg/kg) or saline injections, to characterise the SIRT1 binding genome-wide in mice NAc [170]. The study reported three major findings. Firstly, chronic cocaine causes depletion of SIRT1 from most affected gene promoters and increases in H4K16ac (a deacetylation substrate of SIRT1). Secondly, cocaine-induced SIRT1 induction in the NAc promotes deacetylation and activation of FOXO3a and upregulation of various FOXO3a gene targets in other systems. Thirdly, overexpression of FOXO3a in NAc promotes cocaine place conditioning [170]. Taken together, these findings suggest that SIRT1, an NAD-dependent enzyme, can influence molecular adaptations associated with cocaine addiction.
6.2. NAD+ and SIRT1 Regulate Diurnal Rhythms Associated with Addiction Behaviour
The circadian transcription factors CLOCK and BMAL1 form heterodimers bind to enhancer E-box promoter elements to directly regulate gene transcription (Figure 2) [171]. Owing to the location of E-box promoter elements to the transcriptional start site and the cAMP response element (CRE), direct transcriptional regulation of TH is mediated by CLOCK/BMAL1 [172]. TH is the enzyme responsible for catalysing the synthesis of L-3,4-dihydroxyphenylalanine (L-DOPA) from the amino acid L-tyrosine, using molecular oxygen (O2), iron (Fe2+) and tetrahydrobiopterin as cofactors [173]. L-DOPA is a precursor for DA, which is essential for the synthesis of the vital neurotransmitters norepinephrine (noradrenaline) and epinephrine (adrenaline) [173]. TH enzyme represents the rate limiting step in the synthesis of catecholamines and is encoded by the TH gene. The TH gene is expressed in the CNS, peripheral sympathetic neurons and the adrenal medulla [173].
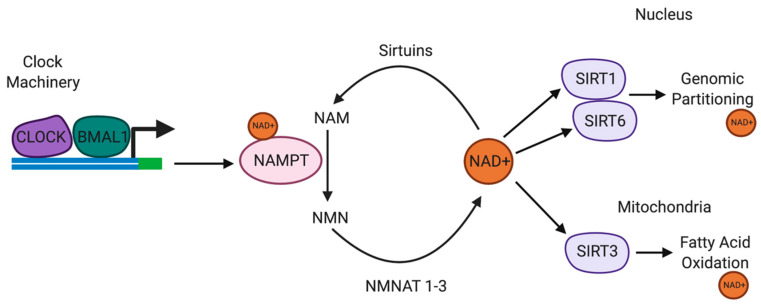
Figure 2.
Circadian rhythms are regulated by the availability of NAD+. NAMPT is a SIRT1/CLOCK/BMAL1-regulated circadian gene. SIRT1 and NAMPT and NMNAT form part of a circadian regulatory feedback loop, regulating the availability of NAD+. NAD+ regulates SIRT1, SIRT3 and SIRT6 activities. SIRT1 also regulates CLOCK/BMAL1 expression in the suprachiasmatic nucleus. SIRT6 regulates chromatin recruitment of CLOCK/BMAL1. SIRT3 regulates oxidative phosphorylation and fatty acid oxidation in the mitochondria via circadian deacetylation of mitochondrial enzymes. Abbreviations: NAD+, nicotinamide adenine dinucleotide; NAMPT, nicotinamide phosphoribosyltransferase; NMNAT, nicotinamide mononucleotide adenyltransferase; SIRT, sirtuins.
CLOCK is disrupted in substance abuse and mice exhibiting mutations in CLOCK are hyperdopaminergic and more susceptible to drug addiction [174]. TH expression is upregulated in the mesolimbic DA system in mice harbouring the CLOCKΔ19 mutation, providing evidence of the regulatory role of CLOCK on the transcriptional activity of TH [175]. Recent evidence suggests that the interaction between CLOCK and BMAL1 is regulated by the NAD-dependent histone deacetylase enzyme SIRT1 to repress CLOCK-mediated transcription [176]. A recent study showed that CLOCK and NAD-dependent SIRT1 antagonise CREB-mediated transcription during the day, which is attenuated during the night, thus facilitating CREB-induced TH transcription at night. Impaired CLOCK in CLOCKΔ19 mice induces CREB-induced TH transcription during the day [176].
Nicotinamide phosphoribosyltransferase (NAMPT) is a rate-limiting enzyme in NAD+ biosynthesis pathway. NAMPT catalyses the conversion of NAM into NMN, which is subsequently converted to NAD+. One study recently demonstrated that NAMPT expression was significantly upregulated in the ventral tegmental area (VTA) of cocaine-conditioned mice [177]. Intraperitoneal or intra-VTA injection of the NAMPT inhibitor FK866, attenuated cocaine reward, although the effect was repressed by increasing intra-VTA expression of NAMPT or supplementation with NMN [177]. The levels of NAD+ and NMN, and SIRT1 expression were elevated in the VTA in cocaine-conditioned mice. However, the inhibitory effect of FK866 on cocaine reward was markedly reduced in SIRT1 midbrain conditional knockout mice [177]. These results suggest that NAMPT-mediated NAD+ biosynthesis may influence cocaine behavioural effects via SIRT1.
NMN, an NAD+ precursor, also reduced CREB-mediated transcription, providing support for the importance of maintaining NAD+ levels as a key regulator of SIRT1-mediated transcriptional repression. Interestingly, the recycling of NAM to NMN is regulated by the CLOCK target gene, NAMPT [176]. NAMPT and SIRT1 expression are highest during the diurnal phases and are opposite to the levels of NAD+ and SIRT1 protein, suggestive of NAD-dependent SIRT1 mediated repression of TH expression during the inactive phase. Daily changes in NAD+ levels regulate the CLOCK/BMAL1/SIRT1 heterodimer complexes [178]. More specifically, CLOCK/SIRT1 preferentially binds to the TH promoter during the day when the levels of NAD+ are highest and the expression of TH is lowest.
Upregulation of SIRT1 and SIRT2 activities appears to be associated with increased excitability of NAc neurons following repeated cocaine exposure [179]. This raises the possibility for the application of SIRT1 and SIRT2 inhibitors as potential agents to treat cocaine addiction.
6.3. NAD+ Increases Adenosine Levels Which Counteract the Effects of Dopamine
The modulatory effects of DA are mediated by postsynaptic D1 and D2 receptors. The neuromodulator, adenosine, has been reported to attenuate DA signalling via modulation of adenosine A1 and A2A receptors. Thus adenosine receptors serve as endogenous ‘brakes’ on overactivated DA receptors and can attenuate drug-induced changes to neuronal function and cognitive disorders [180]. Adenosine A1 receptors are found in the presynapsis where they compete with A2A receptors by receptor–receptor mediated interactions to inhibit glutamate release. Adenosine A1 and DA D1 receptors are expressed in the post synapsis on dynorphin-containing neurons while adenosine A2A receptors are expressed with dopamine D2 receptors in encephalin containing neurons [181]. These receptors mediate opposing effects including allosteric receptor-receptor interactions and intracellular signalling cascades. Drugs of abuse have been reported to alter the expression of adenosine and DA receptors in the mesolimbic DA system, this limiting adenosine receptor activity in favour of increased DA stimulation and promoting addictive behaviour although the exact mechanism(s) remain unclear [182].
Treatment with exogenous NAD+ has been shown to increase adenosine levels which are likely to activate adenosine receptors to counteract the degenerative effects of DA on neuronal cells [183]. Endogenous adenosine levels have been reported to be in the nanomolar range [184], and the potential beneficial effects of NAD+ in addiction may be partially mediated by increased adenosine levels and activation of adenosine receptors. A recent study showed that treatment with exogenous NAD+ increased intracellular adenosine levels in BV2 microglial cells which could enter cells through equilibrative nucleoside transporters [185]. Increases in the intracellular adenylate pool can be converted to AMP by the catalytic activity of adenosine kinase, which increases intracellular ATP levels by enhancing AMP kinase (AMPK) activity and increasing ADP to promote mitochondrial ATP synthase activity [186]. Therefore, extracellular NAD+ may have beneficial effects in addiction therapy through its degradation into adenosine, attenuation of DA receptor activity and the activities of adenosine kinase and AMPK.
6.4. NAD+ and SIRT1 Regulate Monoamine Oxidase A
It has been reported that SIRT1 regulates anxiety and addictive behaviour, although the exact mechanism remains unclear [187,188,189,190,191]. It has been recently reported that the NAD-dependent SIRT1 deacetylates the brain-specific transcription factor NHLH2 on lysine 49, which leads to increases in the activity of the monoamine oxidase A (MAO-A) promoter, thus activating MAO-transcription [190]. Since MAO-A is responsible for the degradation of serotonin, increased MAO-A leads to reduced serotonin levels, thus increasing anxiety and depression. MAO-A inhibitors have been reported to normalise anxiety differences in murine models exhibiting altered brain SIRT1 levels. Genetic analysis of unbiased human cohorts reported that the role of sirtuins in regulating anxiety and behavioural disorders is conserved. These studies provide evidence for the role of sirtuins and the essential substrate NAD+ in mediating psychological effects on stress-response pathways [190].
6.5. NAD+ Regulates FGF21 and Oxytocin Signalling via SIRT1 and CD38
NAD-dependent SIRT1 plays an important role in regulating cellular energy homeostasis. In peripheral tissue, SIRT1 promotes the use of fat as a substrate [192,193,194,195,196]. On the other hand, brain SIRT1 facilitates homeostatic feeding control enhancing hormone sensing. Recently, SIRT1 has been reported to be a regulator of macronutrient preferences and regulates macronutrient preference via FGF21-Nrf2-OXT signalling [197]. FGF21 enhances OXT-mediated neuronal activation via ERK signalling and regulates OXT expression via AKT signalling. SIRT1 promotes FGF21 sensitivity in OXT neurons and enhances negative feedback processes that regulate simple sugar preference [197].
Importantly, obesity is associated with FGF21 resistance in peripheral tissue and the CNS due to reduced FGF21 receptor expression. FGF21 resistance in the CNS may play a critical role in obesity and metabolic syndrome [198]. Hepatic SIRT1 is a key regulator of FGF21 transcription. Increased systemic SIRT1 activity due to increased NAD+ levels can enhance FGF21 signalling by raising the levels of FGF21 from the liver and increasing FGF21 signalling in OXT neurons [199]. Interestingly, the circulating levels of OXT are reduced in obesity, and intranasal treatment with OXT led to a metabolic shift from using carbohydrates to fat [197,200]. Given the brain FGF21 stimulates OXT neurons and inhibits simple sugar preference, increasing FGF21 levels may also improve food selection and improve health and promote stress resistance.
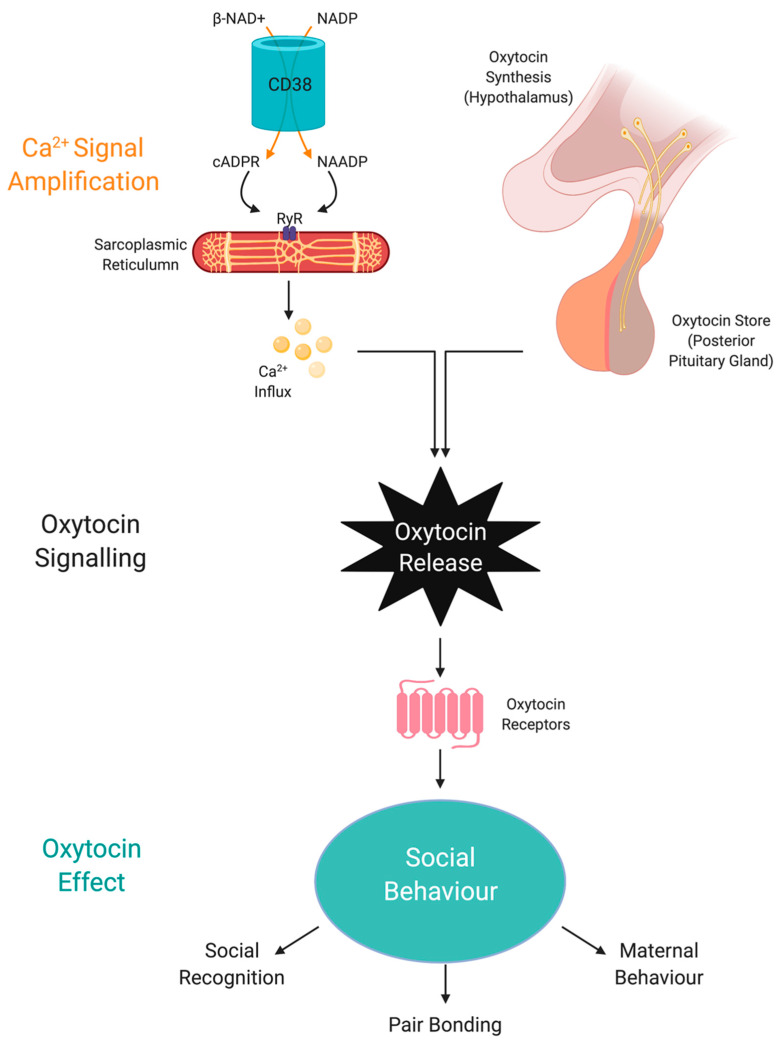
Apart from SIRT1, NAD+ can also regulate OXT through CD38 [201]. Effective social behaviour is dependent of optimal CD38 activity to regulate OXT secretion from the hypothalamus and pituitary [202] (Figure 3). Social amnesia may be induced by reducing OXT secretion from the hypothalamus when CD38 activity is reduced due to gene manipulation or reduced availability of NAD+ [203].
Figure 3.
NAD+ regulates oxytocin activity via CD38 and cADPR. Social behaviour is regulated by the amount of central OXT released due to NAD-dependent calcium release by CD38 activity and cADPR generation. Abbreviations: NAD+, nicotinamide adenine dinucleotide; NADP, nicotinamide adenine dinucleotide phosphate; CD38, NAD+ glycohydrolase; cADPR, cyclic adenosine diphosphoribose; OXT, oxytocin; NAADP, nicotinic acid adenine dinucleotide phosphate; Ryr, ryanodine receptor.
6.6. NAD+ and SIRT1 Regulate Drp1 and Mitochondrial Fission to Potentiate Drug Seeking
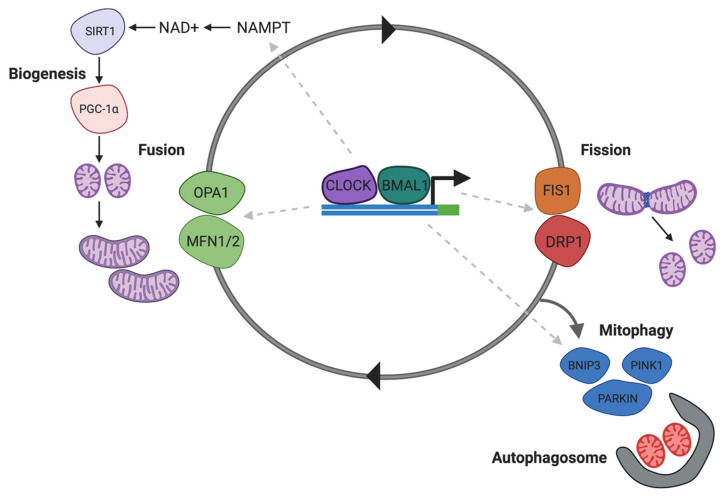
Impaired energy metabolism has been reported in the brains of subjects addicted to substances, and cocaine, although the exact mechanism remains unclear. It has been recently demonstrated that the dynamin-related protein-1 (Drp1), which mediated mitochondrial fission, is increased in the NAc following chronic cocaine exposure [204]. A Drp1 inhibitor, Mdivi-1 inhibited cocaine seeking behaviour, and antagonised c-Fos induction and excitation onto D1 DA receptors [204]. Since Drp1 expression and mitochondrial fission are regulated by SIRT1 [205] and NAD+ (Figure 4) by deacetylating p53, reduced SIRT1 activity and NAD+ availability can alter brain energy homeostasis affecting behavioural and cellular plasticity during addiction.
Figure 4.
NAD+ regulates Drp1 associated in mitochondrial homeostasis. CLOCK/BMAL1 modulated mitochondrial biogenesis and mitophagy via NAD-dependent SIRT1. Deacetylation of the transcription factor PGC1A by SIRT1 regulates mitochondrial biogenesis. Drp1-mediated mitochondrial fission precedes mitophagy leading to formation of fragmented mitochondria that are taken up by autophagosomes. Abbreviations: NAD+, nicotinamide adenine dinucleotide; NAMPT, nicotinamide phosphoribosyltransferase; SIRT1, sirtuin-1; OPA1, mitochondrial dynamin like GTPase; MFN1/2, mitofusin-1/2; FIS1, mitochondrial fission 1 protein; DRP1, dynamin-related protein-1; PINK1, PTEN-induced kinase 1; BNIP1, BCL-2 interacting protein 1; PARKIN, E3 ubiquitin-protein ligase parkin.
7. Is There Room for NAD+ in Addiction Therapy?
While NAD+ precursors (e.g., NAM, NMN and NR) have been shown to increase NAD+ levels after treatment, IV NAD+ remains the most direct method of raising NAD+ levels, although it is yet to gain U.S. Food and Drug Administration (FDA) approval. While data from experimental studies are limited, significant clinical benefit of IV NAD+ infusion in alcohol and opioid withdrawal has been previously reported [102]. These authors found significant improvements in the removal of cravings and withdrawal symptoms. The benefits of NAD+ are likely to be extended to improving emotional disorders in patients with psychological disorders. Whilst there has been growing enthusiasm for the benefits of IV NAD+ therapy, the pharmacokinetics and pharmacodynamics of IV NAD+ remain nascent in the current literature.
A recent study documented changes in levels of NAD+ and key metabolites in the NAD+ metabolome (henceforth the NADome) in both plasma and urine over 8 h using a typical clinical dosing regimen of 750 mg NAD+ administered IV over a 6 h period [206]. The study was the first to report that: (a) the level of NAD+ remained constant in the plasma for at least the first 2 h when administered at a flow rate of 3 μmole/min; (b) increases in the levels of metabolic bi-products analysed were consistent with the activity of NAD+ consumers (e.g., PARPs, CD38 and sirtuins); and (c) by-products of NAD+ metabolism and unbound NAD+ itself can be excreted in urine [206]. However, further research is necessary to fully understand the complex metabolic fate of NAD+ following treatment.
Importantly, no adverse effect was reported following IV NAD+ infusion when administered at an appropriate rate [102,206]. However, reduced plasma activities of enzymes associated with hepatic stress such as the intrahepatic LD and AST and the post-hepatic (bile duct) enzyme GGT suggest that NAD+ is essential for the maintenance of structural and functional integrity of both intra-hepatic and post-hepatic tissue. Increases in bilirubin, a red cell degradation product, after 8 h may be indicative of a minor increase in red cell turnover, possibly due to infusion-induced haemolysis, or reduced heme metabolism. Since these increases are in a low magnitude of change, it was not considered to be of clinical relevance [206]. IV NAD+ therapy may provide significant improvement in addictive disorders likely due to increased NAD+ availability. Further longitudinal and follow-up studies are necessary to cement the role of IV NAD+ in addiction therapy. In addition, the effect of oral administration of the NAD+ precursors, NMN and NR, as potential therapeutic agents for raising NAD+ and improving addictive symptoms warrants further clinical investigation.
8. Conclusions
The central role of NAD+ in the maintenance and regulation of cellular bioenergetics, and modulation of numerous secondary messenger signalling pathways suggest that NAD+ is essential for promoting cellular regeneration and repair in neuropsychiatric conditions such as addiction, Alzheimer’s disease and other neurodegenerative dementias. A growing body of evidence suggests that NAD+ levels decline with age and an imbalance in NAD+ anabolism and NAD+ consumption can lead to a significant derailment of fundamental molecular processes leading to accelerated degeneration and ageing [163,164,207]. This review attempts to provide renewed insight into current knowledge of the NADome, and mechanisms by which NAD+ can interact with various processes in cells and tissues to attenuate addictive behaviour and reduce the addictive phenotype in the clinic. If NAD+ anabolism is impaired and/or if NAD+ consumption is increased during addiction, this is likely to reduce intracellular NAD+ pools which contribute to functional decline. NAD+ consumers such as PARPs, CD38, sirtuins and the more recently identified SARM1, may be affected not only in the brain but in other cells and tissues leading to a loss in NAD+ homeostasis that plays a contributory, if not causal, role in deficits in basic physiological processes associated with the neurobiology of addiction.
For several decades, IV NAD+ has been used as a holistic ‘underground’ approach for the treatment of various forms of addiction. IV NAD+ showed complete withdrawal of addictive drugs without subjects experiencing ‘agony of withdrawal’ symptoms [102,206]. It also provides a cheap and direct means for physicians to treat addiction without the necessity of using synthetic therapeutic agents that are more costly, pose greater risk of adverse effects and induce addiction or regimens that require ‘trafficking’ of addictive drugs for gradual withdrawal. However, it should be clarified that the use of NAD+ does not provide allowance for continued use of addicted drugs. Rather, NAD+ therapy is aimed at improving ‘productive ageing’ by restoring health processes, removing cravings and withdrawal symptoms and assisting in abstinence. Emerging studies using IV NAD+ in addiction set the stage for deeper investigations into the mechanisms by which addiction is sensitive to NAD+ status, and how it can improve, if not attenuate, addiction in the clinic.
Apart from the historical benefits of niacin and IV NAD+ in addiction therapy, the identification of NR, NAR and NMN as NAD+ precursors provide ‘newer’ alternatives for raising NAD+ levels and to possibly alleviate biochemical processes associated with addiction. These NAD+ precursors have already demonstrated protective effects in several animal models of disease including neurodegenerative disorders, cardiovascular disease, metabolic syndrome, cancer and ageing [54,163]. Given recent advances in quantifying the NADome in various biological specimens using specific biosensors and mass spectrometry techniques [208], we are at an exciting time when we can evaluate the importance of NAD+ metabolism not only for prevention and management of ageing and age-related disorders, but in addictive behaviour as well.
Author Contributions
Conceptualization N.B. and S.v.E.—original draft preparation, N.B.; writing—review and editing, N.B., M.D.V. and S.v.E.; supervision, N.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Dimitrijević I., Popović N., Sabljak V., Škodrić-Trifunović V., Dimitrijević N. Food addiction-diagnosis and treatment. Psychiatr. Danub. 2015;27:101–106. [PubMed] [Google Scholar]
- 2.Axley P.D., Richardson C.T., Singal A.K. Epidemiology of Alcohol Consumption and Societal Burden of Alcoholism and Alcoholic Liver Disease. Clin. Liver Dis. 2019;23:39–50. doi: 10.1016/j.cld.2018.09.011. [DOI] [PubMed] [Google Scholar]
- 3.Fama R., Hardcastle C., Sassoon S.A., Pfefferbaum A., Sullivan E.V., Zahr N.M., Le Berre A.-P. Neurological, nutritional and alcohol consumption factors underlie cognitive and motor deficits in chronic alcoholism. Addict. Boil. 2019;24:290–302. doi: 10.1111/adb.12584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ouellette L., Tenbrink W., Gier C., Shepherd S., Mitten S., Steinberger M., Riley B., Sutliffe C., Jones J. Alcoholism in elderly patients: Characteristics of patients and impact on the emergency department. Am. J. Emerg. Med. 2019;37:776–777. doi: 10.1016/j.ajem.2018.08.061. [DOI] [PubMed] [Google Scholar]
- 5.Pucci M., Di Bonaventura M.V.M., Wille-Bille A., Fernández M.S., Maccarrone M., Pautassi R.M., Cifani C., D’Addario C. Environmental stressors and alcoholism development: Focus on molecular targets and their epigenetic regulation. Neurosci. Biobehav. Rev. 2019;106:165–181. doi: 10.1016/j.neubiorev.2018.07.004. [DOI] [PubMed] [Google Scholar]
- 6.Santoro A., Tomino C., Prinzi G., Lamonaca P., Cardaci V., Fini M., Russo P. Tobacco Smoking: Risk to Develop Addiction, Chronic Obstructive Pulmonary Disease, and Lung Cancer. Recent Pat. Anti-Cancer Drug Discov. 2019;14:39–52. doi: 10.2174/1574892814666190102122848. [DOI] [PubMed] [Google Scholar]
- 7.Jamir L., Duggal M., Nehra R., Singh P., Grover S. Epidemiology of technology addiction among school students in rural India. Asian J. Psychiatry. 2019;40:30–38. doi: 10.1016/j.ajp.2019.01.009. [DOI] [PubMed] [Google Scholar]
- 8.Armoon B., Karimy M. Epidemiology of childhood overweight, obesity and their related factors in a sample of preschool children from Central Iran. BMC Pediatr. 2019;19:159. doi: 10.1186/s12887-019-1540-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bluher M. Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019;15:288–298. doi: 10.1038/s41574-019-0176-8. [DOI] [PubMed] [Google Scholar]
- 10.Chooi Y.C., Ding C., Magkos F. The epidemiology of obesity. Metabolism. 2019;92:6–10. doi: 10.1016/j.metabol.2018.09.005. [DOI] [PubMed] [Google Scholar]
- 11.Nansseu J.R., Noubiap J.J., Bigna J.J. Epidemiology of Overweight and Obesity in Adults Living in Cameroon: A Systematic Review and Meta-Analysis. Obesity. 2019;27:1682–1692. doi: 10.1002/oby.22566. [DOI] [PubMed] [Google Scholar]
- 12.Giusti V. [Obesity: Epidemiology, socio-political implications and conventional management] Ther. Umsch. 2019;76:117–121. doi: 10.1024/0040-5930/a001071. [DOI] [PubMed] [Google Scholar]
- 13.Imam T., Coleman K.J. Obesity and Mortality in End-Stage Renal Disease. Is It Time to Reverse the “Reverse Epidemiology”-at Least in Peritoneal Dialysis? J. Ren. Nutr. 2019;29:269–275. doi: 10.1053/j.jrn.2018.09.005. [DOI] [PubMed] [Google Scholar]
- 14.So E., Choi S.K., Joung H. Impact of dietary protein intake and obesity on lean mass in middle-aged individuals after a 12-year follow-up: The Korean Genome and Epidemiology Study (KoGES) Br. J. Nutr. 2019;122:322–330. doi: 10.1017/S000711451900117X. [DOI] [PubMed] [Google Scholar]
- 15.Vyas V., Lambiase P., Centre S.B.H.B.H. Obesity and Atrial Fibrillation: Epidemiology, Pathophysiology and Novel Therapeutic Opportunities. Arrhythmia Electrophysiol. Rev. 2019;8:28–36. doi: 10.15420/aer.2018.76.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guenzel N., McChargue D. Addiction Relapse Prevention. [(accessed on 8 December 2019)]; Available online: https://www.ncbi.nlm.nih.gov/books/NBK551500/
- 17.Heinz A., Beck A., Mir J., Grüsser S., Grace A., Wrase J. Alcohol Craving and Relapse Prediction: Imaging Studies. In: Kuhn C.M., Koob G.F., editors. Advances in the Neuroscience of Addiction. 2nd ed. CRC Press; Boca Raton, FL, USA: 2010. [PubMed] [Google Scholar]
- 18.Marshall S.W., Albery I.P., Frings D. Who stays in addiction treatment groups? Anxiety and avoidant attachment styles predict treatment retention and relapse. Clin. Psychol. Psychother. 2018;25:525–531. doi: 10.1002/cpp.2187. [DOI] [PubMed] [Google Scholar]
- 19.Mcgonigle J., Murphy A., Paterson L., Reed L.J., Nestor L., Nash J., Elliott R., Ersche K.D., Flechais R.S., Newbould R., et al. The ICCAM platform study: An experimental medicine platform for evaluating new drugs for relapse prevention in addiction. Part B: fMRI description. J. Psychopharmacol. 2017;31:3–16. doi: 10.1177/0269881116668592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vo H.T., Robbins E., Westwood M., Lezama D., Fishman M. Relapse prevention medications in community treatment for young adults with opioid addiction. Subst. Abus. 2016;37:392–397. doi: 10.1080/08897077.2016.1143435. [DOI] [PubMed] [Google Scholar]
- 21.DiLeone R.J. Neuroscience gets nutrition. Nat. Neurosci. 2011;14:271–272. doi: 10.1038/nn0311-271. [DOI] [PubMed] [Google Scholar]
- 22.Gearhardt A., Grilo C.M., Dileone R.J., Brownell K.D., Potenza M.N. Can food be addictive? Public health and policy implications. Addiction. 2011;106:1208–1212. doi: 10.1111/j.1360-0443.2010.03301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schulte E.M., Avena N.M., Gearhardt A.N. Which foods may be addictive? The roles of processing, fat content, and glycemic load. PLoS ONE. 2015;10:e0117959. doi: 10.1371/journal.pone.0117959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gearhardt A.N., Brownell K.D. Can food and addiction change the game? Biol. Psychiatry. 2013;73:802–803. doi: 10.1016/j.biopsych.2012.07.024. [DOI] [PubMed] [Google Scholar]
- 25.Gearhardt A.N., Corbin W.R., Brownell K.D. Food addiction: An examination of the diagnostic criteria for dependence. J. Addict. Med. 2009;3:1–7. doi: 10.1097/ADM.0b013e318193c993. [DOI] [PubMed] [Google Scholar]
- 26.Gearhardt A.N., Corbin W.R. The role of food addiction in clinical research. Curr. Pharm. Des. 2011;17:1140–1142. doi: 10.2174/138161211795656800. [DOI] [PubMed] [Google Scholar]
- 27.Gearhardt A., Yokum S., Stice E., Harris J.L., Brownell K.D. Relation of obesity to neural activation in response to food commercials. Soc. Cogn. Affect. Neurosci. 2013;9:932–938. doi: 10.1093/scan/nst059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alaghband Y., Marshall J.F. Common influences of non-competitive NMDA receptor antagonists on the consolidation and reconsolidation of cocaine-cue memory. Psychopharmacology. 2013;226:707–719. doi: 10.1007/s00213-012-2793-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stanley B.G., Ha L.H., Spears L.C., Dee M.G. Lateral hypothalamic injections of glutamate, kainic acid,d,l-α-amino-3-hydroxy-5-methyl-isoxazole propionic acid or N-methyl-d-aspartic acid rapidly elicit intense transient eating in rats. Brain Res. 1993;613:88–95. doi: 10.1016/0006-8993(93)90458-Y. [DOI] [PubMed] [Google Scholar]
- 30.Holmes A., Spanagel R., Krystal J.H. Glutamatergic targets for new alcohol medications. Psychopharmacology. 2013;229:539–554. doi: 10.1007/s00213-013-3226-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cunha-Oliveira T., Silva L., Silva A.M., Moreno A.J., Oliveira C., Santos M. Acute effects of cocaine, morphine and their combination on bioenergetic function and susceptibility to oxidative stress of rat liver mitochondria. Life Sci. 2013;92:1157–1164. doi: 10.1016/j.lfs.2013.04.016. [DOI] [PubMed] [Google Scholar]
- 32.Cunha-Oliveira T., Silva L., Silva A.M., Moreno A.J., Oliveira C., Santos M. Mitochondrial complex I dysfunction induced by cocaine and cocaine plus morphine in brain and liver mitochondria. Toxicol. Lett. 2013;219:298–306. doi: 10.1016/j.toxlet.2013.03.025. [DOI] [PubMed] [Google Scholar]
- 33.Pomierny B., Starek A., Krzyżanowska W., Starek-Swiechowicz B., Smaga I., Pomierny-Chamiolo L., Regulska M., Budziszewska B. Potential neurotoxic effect of ethylene glycol ethers mixtures. Pharmacol. Rep. 2013;65:1415–1421. doi: 10.1016/S1734-1140(13)71501-9. [DOI] [PubMed] [Google Scholar]
- 34.Pomierny-Chamiolo L., Moniczewski A., Wydra K., Suder A., Filip M. Oxidative Stress Biomarkers in Some Rat Brain Structures and Peripheral Organs Underwent Cocaine. Neurotox. Res. 2013;23:92–102. doi: 10.1007/s12640-012-9335-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pomierny-Chamiolo L., Rup K., Pomierny B., Niedzielska-Andres E., Kalivas P.W., Filip M. Metabotropic glutamatergic receptors and their ligands in drug addiction. Pharmacol. Ther. 2014;142:281–305. doi: 10.1016/j.pharmthera.2013.12.012. [DOI] [PubMed] [Google Scholar]
- 36.Cai N., Li Y., Chang S., Liang J., Lin C., Zhang X., Liang L., Hu J., Chan W., Kendler K.S., et al. Genetic Control over mtDNA and Its Relationship to Major Depressive Disorder. Curr. Boil. 2015;25:3170–3177. doi: 10.1016/j.cub.2015.10.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hollis F., Van Der Kooij M.A., Zanoletti O., Lozano L., Cantó C., Sandi C. Mitochondrial function in the brain links anxiety with social subordination. Proc. Natl. Acad. Sci. USA. 2015;112:15486–15491. doi: 10.1073/pnas.1512653112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Manji H.K., Kato T., Di Prospero N.A., Ness S., Beal M.F., Krams M., Chen G. Impaired mitochondrial function in psychiatric disorders. Nat. Rev. Neurosci. 2012;13:293–307. doi: 10.1038/nrn3229. [DOI] [PubMed] [Google Scholar]
- 39.Picard M., McManus M.J., Gray J.D., Nasca C., Moffat C., Kopinski P.K., Seifert E.L., McEwen B.S., Wallace D.C. Mitochondrial functions modulate neuroendocrine, metabolic, inflammatory, and transcriptional responses to acute psychological stress. Proc. Natl. Acad. Sci. USA. 2015;112:E6614–E6623. doi: 10.1073/pnas.1515733112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Feng J., Wilkinson M., Liu X., Purushothaman I., Ferguson D., Vialou V., Maze I., Shao N.-Y., Kennedy P., Koo J., et al. Chronic cocaine-regulated epigenomic changes in mouse nucleus accumbens. Genome Boil. 2014;15:R65. doi: 10.1186/gb-2014-15-4-r65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bergmans R.S., Kelly K.M., Mezuk B. Inflammation as a unique marker of suicide ideation distinct from depression syndrome among U.S. adults. J. Affect. Disord. 2019;245:1052–1060. doi: 10.1016/j.jad.2018.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Felger J.C. Role of Inflammation in Depression and Treatment Implications. Pharmacol. Ther. Cough. 2019;250:255–286. doi: 10.1007/164_2018_166. [DOI] [PubMed] [Google Scholar]
- 43.Francija E., Petrovic Z., Brkic Z., Mitic M., Radulovic J., Adzic M. Disruption of the NMDA receptor GluN2A subunit abolishes inflammation-induced depression. Behav. Brain Res. 2019;359:550–559. doi: 10.1016/j.bbr.2018.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.González-Parra S., Daudén E. Psoriasis and Depression: The Role of Inflammation. Actas Dermo Sifiliogr. 2019;110:12–19. doi: 10.1016/j.ad.2018.05.009. [DOI] [PubMed] [Google Scholar]
- 45.Konsman J.P. Inflammation and Depression: A Nervous Plea for Psychiatry to Not Become Immune to Interpretation. Pharmaceuticals. 2019;12:29. doi: 10.3390/ph12010029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mac Giollabhui N., Swistun D., Murray S., Moriarity D., Kautz M.M., Ellman L.M., Olino T.M., Coe C.L., Abramson L.Y., Alloy L.B. Executive dysfunction in depression in adolescence: The role of inflammation and higher body mass. Psychol. Med. 2019;50:683–691. doi: 10.1017/S0033291719000564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vengeliene V., Cannella N., Takahashi T., Spanagel R. Metabolic shift of the kynurenine pathway impairs alcohol and cocaine seeking and relapse. Psychopharmacology. 2016;233:3449–3459. doi: 10.1007/s00213-016-4384-9. [DOI] [PubMed] [Google Scholar]
- 48.Braidy N., Grant R., Adams S., Brew B.J., Guillemin G.J. Mechanism for quinolinic acid cytotoxicity in human astrocytes and neurons. Neurotox. Res. 2009;16:77–86. doi: 10.1007/s12640-009-9051-z. [DOI] [PubMed] [Google Scholar]
- 49.Rahman A., Ting K., Cullen K.M., Braidy N., Brew B.J., Guillemin G.J. The Excitotoxin Quinolinic Acid Induces Tau Phosphorylation in Human Neurons. PLoS ONE. 2009;4:e6344. doi: 10.1371/journal.pone.0006344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ting K.K., Brew B.J., Guillemin G.J. Effect of quinolinic acid on human astrocytes morphology and functions: Implications in Alzheimer’s disease. J. Neuroinflammation. 2009;6:36. doi: 10.1186/1742-2094-6-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hodgkins P.S., Schwarcz R. Interference with cellular energy metabolism reduces kynurenic acid formation in rat brain slices: Reversal by lactate and pyruvate. Eur. J. Neurosci. 1998;10:1986–1994. doi: 10.1046/j.1460-9568.1998.00208.x. [DOI] [PubMed] [Google Scholar]
- 52.Guillemin G.J., Kerr S.J., Pemberton L.A., Smith D.G., Smythe G.A., Armati P.J., Brew B.J. IFN-beta1b induces kynurenine pathway metabolism in human macrophages: Potential implications for multiple sclerosis treatment. J. Interf. Cytokine Res. 2001;21:1097–1101. doi: 10.1089/107999001317205231. [DOI] [PubMed] [Google Scholar]
- 53.Wu W., Nicolazzo J.A., Wen L., Chung R., Stankovic R., Bao S.S., Lim C.K., Brew B.J., Cullen K.M., Guillemin G.J. Expression of Tryptophan 2,3-Dioxygenase and Production of Kynurenine Pathway Metabolites in Triple Transgenic Mice and Human Alzheimer’s Disease Brain. PLoS ONE. 2013;8:e59749. doi: 10.1371/journal.pone.0059749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Braidy N., Berg J., Clement J., Poljak A., Grant R., Sachdev P. Role of Nicotinamide Adenine Dinucleotide and Related Precursors as Therapeutic Targets for Age-Related Degenerative Diseases: Rationale, Biochemistry, Pharmacokinetics, and Outcomes. Antioxid. Redox Signal. 2019;30:251–294. doi: 10.1089/ars.2017.7269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Adinoff B. Neurobiologic processes in drug reward and addiction. Harv. Rev. Psychiatry. 2004;12:305–320. doi: 10.1080/10673220490910844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ma H., Zhu G. The dopamine system and alcohol dependence. Shanghai Arch. Psychiatry. 2014;26:61–68. doi: 10.3969/j.issn.1002-0829.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.De Biasi M., Dani J.A. Reward, addiction, withdrawal to nicotine. Annu. Rev. Neurosci. 2011;34:105–130. doi: 10.1146/annurev-neuro-061010-113734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bloomfield M.A., Ashok A.H., Volkow N.D., Howes O.D. The effects of Delta(9)-tetrahydrocannabinol on the dopamine system. Nature. 2016;539:369–377. doi: 10.1038/nature20153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lv Y., Liang T., Wang G., Li Z. Ghrelin, a gastrointestinal hormone, regulates energy balance and lipid metabolism. Biosci. Rep. 2018;38 doi: 10.1042/BSR20181061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Igarashi M., DiPatrizio N.V., Narayanaswami V., Piomelli D. Feeding-induced oleoylethanolamide mobilization is disrupted in the gut of diet-induced obese rodents. Biochim. Biophys. Acta. 2015;1851:1218–1226. doi: 10.1016/j.bbalip.2015.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ochoa M., Lallès J.-P., Malbert C.-H., Val-Laillet D. Dietary sugars: Their detection by the gut-brain axis and their peripheral and central effects in health and diseases. Eur. J. Nutr. 2015;54:1–24. doi: 10.1007/s00394-014-0776-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Leong K.-C., Cox S., King C., Becker H., Reichel C.M. Oxytocin and Rodent Models of Addiction. Int. Rev. Neurobiol. 2018;140:201–247. doi: 10.1016/bs.irn.2018.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yang H.-P., Wang L., Han L., Wang S.C. Nonsocial functions of hypothalamic oxytocin. ISRN Neurosci. 2013;2013:179272. doi: 10.1155/2013/179272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Buisman-Pijlman F.T.A., Sumracki N.M., Gordon J.J., Hull P.R., Carter C.S., Tops M. Individual differences underlying susceptibility to addiction: Role for the endogenous oxytocin system. Pharmacol. Biochem. Behav. 2014;119:22–38. doi: 10.1016/j.pbb.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 65.Churchland P.S., Winkielman P. Modulating social behavior with oxytocin: How does it work? What does it mean? Horm. Behav. 2012;61:392–399. doi: 10.1016/j.yhbeh.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tabbaa M., Paedae B., Liu Y., Wang Z. Neuropeptide Regulation of Social Attachment: The Prairie Vole Model. Compr. Physiol. 2016;7:81–104. doi: 10.1002/cphy.c150055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Volkow N.D., Boyle M. Neuroscience of Addiction: Relevance to Prevention and Treatment. Am. J. Psychiatry. 2018;175:729–740. doi: 10.1176/appi.ajp.2018.17101174. [DOI] [PubMed] [Google Scholar]
- 68.Greenhill C. Liver: FGF21—The cause of having a ‘sweet tooth’? Nat. Rev. Endocrinol. 2017;13:378. doi: 10.1038/nrendo.2017.62. [DOI] [PubMed] [Google Scholar]
- 69.Crooks D.R., Natarajan T.G., Jeong S.Y., Chen C., Park S.Y., Huang H., Ghosh M.C., Tong W.-H., Haller R.G., Wu C., et al. Elevated FGF21 secretion, PGC-1alpha and ketogenic enzyme expression are hallmarks of iron-sulfur cluster depletion in human skeletal muscle. Hum. Mol. Genet. 2014;23:24–39. doi: 10.1093/hmg/ddt393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Domingo P., Gallego-Escuredo J.M., Domingo J.C., Gutierrez M.D.M., Mateo M.G., Fernandez I., Vidal F., Giralt M., Villarroya F. Serum FGF21 levels are elevated in association with lipodystrophy, insulin resistance and biomarkers of liver injury in HIV-1-infected patients. AIDS. 2010;24:2629–2637. doi: 10.1097/QAD.0b013e3283400088. [DOI] [PubMed] [Google Scholar]
- 71.Guasti L., Silvennoinen S., Bulstrode N., Ferretti P., Sankilampi U., Dunkel L. Elevated FGF21 leads to attenuated postnatal linear growth in preterm infants through GH resistance in chondrocytes. J. Clin. Endocrinol. Metab. 2014;99:E2198–E2206. doi: 10.1210/jc.2014-1566. [DOI] [PubMed] [Google Scholar]
- 72.Hao L., Huang N.K.-H., Ito K., Sae-Tan S., Lambert J.D., Ross A.C. Fibroblast Growth Factor 21 (Fgf21) Gene Expression Is Elevated in the Liver of Mice Fed a High-Carbohydrate Liquid Diet and Attenuated by a Lipid Emulsion but Is Not Upregulated in the Liver of Mice Fed a High-Fat Obesogenic Diet. J. Nutr. 2016;146:184–190. doi: 10.3945/jn.115.216572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Morrice N., McIlroy G.D., Tammireddy S.R., Reekie J., Shearer K.D., Doherty M., Delibegović M., Whitfield P.D., Mody N. Elevated Fibroblast growth factor 21 (FGF21) in obese, insulin resistant states is normalised by the synthetic retinoid Fenretinide in mice. Sci. Rep. 2017;7:43782. doi: 10.1038/srep43782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Murakami T., Ueba Y., Shinoto Y., Koga Y., Kaneda D., Hatoko T., Kato T., Yonemitsu S., Muro S., Oki S. Successful Glycemic Control Decreases the Elevated Serum FGF21 Level without Affecting Normal Serum GDF15 Levels in a Patient with Mitochondrial Diabetes. Tohoku J. Exp. Med. 2016;239:89–94. doi: 10.1620/tjem.239.89. [DOI] [PubMed] [Google Scholar]
- 75.Schoenberg K.M., Giesy S.L., Harvatine K.J., Waldron M.R., Cheng C., Kharitonenkov A., Boisclair Y.R. Plasma FGF21 is elevated by the intense lipid mobilization of lactation. Endocrinology. 2011;152:4652–4661. doi: 10.1210/en.2011-1425. [DOI] [PubMed] [Google Scholar]
- 76.Søberg S., Sandholt C.H., Jespersen N., Toft U., Madsen A.L., Von Holstein-Rathlou S., Grevengoed T.J., Christensen K.B., Bredie W.L., Potthoff M.J., et al. FGF21 Is a Sugar-Induced Hormone Associated with Sweet Intake and Preference in Humans. Cell Metab. 2017;25:1045–1053. doi: 10.1016/j.cmet.2017.04.009. [DOI] [PubMed] [Google Scholar]
- 77.Talukdar S., Owen B.M., Song P., Hernandez G., Zhang Y., Zhou Y., Scott W.T., Paratala B.S., Turner T., Smith A., et al. FGF21 Regulates Sweet and Alcohol Preference. Cell Metab. 2016;23:344–349. doi: 10.1016/j.cmet.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Von Holstein-Rathlou S., BonDurant L.D., Peltekian L., Naber M.C., Yin T.C., Claflin K.E., Urizar A.I., Madsen A.N., Ratner C., Holst B., et al. FGF21 Mediates Endocrine Control of Simple Sugar Intake and Sweet Taste Preference by the Liver. Cell Metab. 2016;23:335–343. doi: 10.1016/j.cmet.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jimenez V., Jambrina C., Casana E., Sacristan V., Muñoz S., Darriba S., Rodó J., Mallol C., Garcia M., León X., et al. FGF21 gene therapy as treatment for obesity and insulin resistance. EMBO Mol. Med. 2018;10:e8791. doi: 10.15252/emmm.201708791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Søberg S., Andersen E.S., Dalsgaard N.B., Jarlhelt I., Hansen N.L., Hoffmann N., Vilsbøll T., Chenchar A., Jensen M., Grevengoed T.J., et al. FGF21, a liver hormone that inhibits alcohol intake in mice, increases in human circulation after acute alcohol ingestion and sustained binge drinking at Oktoberfest. Mol. Metab. 2018;11:96–103. doi: 10.1016/j.molmet.2018.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Freyberg Z., Logan R.W. The Intertwined Roles of Circadian Rhythms and Neuronal Metabolism Fueling Drug Reward and Addiction. Curr. Opin. Physiol. 2018;5:80–89. doi: 10.1016/j.cophys.2018.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Logan R.W., Hasler B.P., Forbes E.E., Franzen P.L., Torregrossa M.M., Huang Y.H., Buysse D.J., Clark D.B., McClung C.A. Impact of Sleep and Circadian Rhythms on Addiction Vulnerability in Adolescents. Biol. Psychiatry. 2018;83:987–996. doi: 10.1016/j.biopsych.2017.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Logan R.W., Williams W.P., McClung C.A. Circadian rhythms and addiction: Mechanistic insights and future directions. Behav. Neurosci. 2014;128:387–412. doi: 10.1037/a0036268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.McClung C.A. Circadian rhythms, the mesolimbic dopaminergic circuit, and drug addiction. Sci. World J. 2007;7:194–202. doi: 10.1100/tsw.2007.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Webb I.C. Circadian Rhythms and Substance Abuse: Chronobiological Considerations for the Treatment of Addiction. Curr. Psychiatry Rep. 2017;19 doi: 10.1007/s11920-017-0764-z. [DOI] [PubMed] [Google Scholar]
- 86.Abarca C., Albrecht U., Spanagel R. Cocaine sensitization and reward are under the influence of circadian genes and rhythm. Proc. Natl. Acad. Sci. USA. 2002;99:9026–9030. doi: 10.1073/pnas.142039099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Asher G., Schibler U. Crosstalk between components of circadian and metabolic cycles in mammals. Cell Metab. 2011;13:125–137. doi: 10.1016/j.cmet.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 88.Korshunov K.S., Blakemore L.J., Trombley P. Dopamine: A Modulator of Circadian Rhythms in the Central Nervous System. Front. Cell. Neurosci. 2017;11:91. doi: 10.3389/fncel.2017.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zagon I.S., McLaughlin P.J. Endogenous Opioids in the Etiology and Treatment of Multiple Sclerosis. In: Zagon I.S., McLaughlin P.J., editors. Multiple Sclerosis: Perspectives in Treatment and Pathogenesis. Codon Publications; Brisbane, Australia: 2017. [PubMed] [Google Scholar]
- 90.Stephens M.A.C., Wand G. Stress and the HPA axis: Role of glucocorticoids in alcohol dependence. Alcohol Res. 2012;34:468–483. [PMC free article] [PubMed] [Google Scholar]
- 91.Mysels D.J., Sullivan M.A. The relationship between opioid and sugar intake: Review of evidence and clinical applications. J. Opioid Manag. 2010;6:445–452. doi: 10.5055/jom.2010.0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rajakumar K. Pellagra in the United States: A historical perspective. South. Med. J. 2000;93:272–277. doi: 10.1097/00007611-200093030-00005. [DOI] [PubMed] [Google Scholar]
- 93.Bryan C.S., Mull S.R. Pellagra Pre-Goldberger: Rupert Blue, Fleming Sandwith, and the “Vitamine Hypothesis”. Trans. Am. Clin. Clim. Assoc. 2015;126:20–45. [PMC free article] [PubMed] [Google Scholar]
- 94.Kirkland J.L. Translating advances from the basic biology of aging into clinical application. Exp. Gerontol. 2013;48:1–5. doi: 10.1016/j.exger.2012.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Oldham M.A., Ivkovic A. Pellagrous encephalopathy presenting as alcohol withdrawal delirium: A case series and literature review. Addict. Sci. Clin. Pract. 2012;7:12. doi: 10.1186/1940-0640-7-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jayakumar K.L., Micheletti R.G. Joseph Goldberger-Public Health Champion and Investigator of Pellagra. JAMA Dermatol. 2017;153:1262. doi: 10.1001/jamadermatol.2017.4044. [DOI] [PubMed] [Google Scholar]
- 97.Elvehjem C.A., Madden R.J., Strong F.M., Woolley D.W. The isolation and identification of the anti-black tongue factor. 1937. J. Biol. Chem. 2002;277:e22. [PubMed] [Google Scholar]
- 98.Kohler R.E., Jr. The background to Arthur Harden’s discovery of cozymase. Bull. Hist. Med. 1974;48:22–40. [PubMed] [Google Scholar]
- 99.Myrback K. In memoriam. Hans von Euler-Chelpin. Enzymologia. 1965;29:105–107. [PubMed] [Google Scholar]
- 100.Otto A.M. Warburg effect(s)-a biographical sketch of Otto Warburg and his impacts on tumor metabolism. Cancer Metab. 2016;4:5. doi: 10.1186/s40170-016-0145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yang Y., Sauve A.A. NAD(+) metabolism: Bioenergetics, signaling and manipulation for therapy. Biochim. Biophys. Acta. 2016;1864:1787–1800. doi: 10.1016/j.bbapap.2016.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.O’Hollaren P. Diphosphopyridine nucleotide in the prevention, diagnosis and treatment of drug addiction. A preliminary report. West. J. Surg. Obstet. Gynecol. 1961;69:213–215. [PubMed] [Google Scholar]
- 103.Spaans S.K., Weusthuis R.A., Van Der Oost J., Kengen S.W.M., Kengen S. NADPH-generating systems in bacteria and archaea. Front. Microbiol. 2015;6:742. doi: 10.3389/fmicb.2015.00742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Marohnic C.C., Bewley M.C., Barber M.J. Engineering and characterization of a NADPH-utilizing cytochrome b5 reductase. Biochemistry. 2003;42:11170–11182. doi: 10.1021/bi034819b. [DOI] [PubMed] [Google Scholar]
- 105.Htet Y., Tennyson A.G. NAD(+) as a Hydride Donor and Reductant. J. Am. Chem. Soc. 2016;138:15833–15836. doi: 10.1021/jacs.6b10451. [DOI] [PubMed] [Google Scholar]
- 106.Lamanna J.C., Younts B., Rosenthal M. The cerebral oxidative metabolic response to acute ethanol administration in rats and cats. Neuropharmacology. 1977;16:283–288. doi: 10.1016/0028-3908(77)90108-3. [DOI] [PubMed] [Google Scholar]
- 107.Song J., Keppler B.K., Wise R.R., Bent A. PARP2 Is the Predominant Poly(ADP-Ribose) Polymerase in Arabidopsis DNA Damage and Immune Responses. PLoS Genet. 2015;11:e1005200. doi: 10.1371/journal.pgen.1005200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hassa P.O., Haenni S.S., Elser M., Hottiger M.O. Nuclear ADP-ribosylation reactions in mammalian cells: Where are we today and where are we going? Microbiol. Mol. Boil. Rev. 2006;70:789–829. doi: 10.1128/MMBR.00040-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Abeti R., Duchen M.R. Activation of PARP by oxidative stress induced by beta-amyloid: Implications for Alzheimer’s disease. Neurochem. Res. 2012;37:2589–2596. doi: 10.1007/s11064-012-0895-x. [DOI] [PubMed] [Google Scholar]
- 110.Martire S., Fuso A., Mosca L., Forte E., Correani V., Fontana M., Scarpa S., Maras B., D’Erme M. Bioenergetic Impairment in Animal and Cellular Models of Alzheimer’s Disease: PARP-1 Inhibition Rescues Metabolic Dysfunctions. J. Alzheimer’s Dis. 2016;54:307–324. doi: 10.3233/JAD-151040. [DOI] [PubMed] [Google Scholar]
- 111.Martire S., Mosca L., D’Erme M. PARP-1 involvement in neurodegeneration: A focus on Alzheimer’s and Parkinson’s diseases. Mech. Ageing Dev. 2015;146:53–64. doi: 10.1016/j.mad.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 112.Turunc Bayrakdar E., Uyanikgil Y., Kanit L., Koylu E., Yalcin A. Nicotinamide treatment reduces the levels of oxidative stress, apoptosis, and PARP-1 activity in Abeta(1-42)-induced rat model of Alzheimer’s disease. Free Radic. Res. 2014;48:146–158. doi: 10.3109/10715762.2013.857018. [DOI] [PubMed] [Google Scholar]
- 113.Zeng J., Libien J., Shaik F., Wolk J., Hernández A.I. Nucleolar PARP-1 Expression Is Decreased in Alzheimer’s Disease: Consequences for Epigenetic Regulation of rDNA and Cognition. Neural Plast. 2016;2016:8987928. doi: 10.1155/2016/8987928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Whitacre C.M., Hashimoto H., Tsai M.L., Chatterjee S., Berger S.J., Berger N.A. Involvement of NAD-poly(ADP-ribose) metabolism in p53 regulation and its consequences. Cancer Res. 1995;55:3697–3701. [PubMed] [Google Scholar]
- 115.Sun S., Cheng S., Zhu Y., Zhang P., Liu N., Xu T., Sun C., Lv Y. Identification of PRKDC (Protein Kinase, DNA-Activated, Catalytic Polypeptide) as an essential gene for colorectal cancer (CRCs) cells. Gene. 2016;584:90–96. doi: 10.1016/j.gene.2016.03.020. [DOI] [PubMed] [Google Scholar]
- 116.Horenstein A., Sizzano F., Lusso R., Besso F.G., Ferrero E., Deaglio S., Corno F., Malavasi F. CD38 and CD157 ectoenzymes mark cell subsets in the human corneal limbus. Mol. Med. 2009;15:76–84. doi: 10.2119/molmed.2008.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Camacho-Pereira J., Tarragó M.G., Chini C.C.S., Nin V., Escande C., Warner G.M., Puranik A.S., Schoon R.A., Reid J.M., Galina A., et al. CD38 Dictates Age-Related NAD Decline and Mitochondrial Dysfunction through an SIRT3-Dependent Mechanism. Fac. Opin. 2016;23:1127–1139. doi: 10.1016/j.cmet.2016.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Guerreiro S., Privat A.-L., Bressac L., Toulorge D. CD38 in Neurodegeneration and Neuroinflammation. Cells. 2020;9:471. doi: 10.3390/cells9020471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Vaquero A., Sternglanz R., Reinberg D. NAD+-dependent deacetylation of H4 lysine 16 by class III HDACs. Oncogene. 2007;26:5505–5520. doi: 10.1038/sj.onc.1210617. [DOI] [PubMed] [Google Scholar]
- 120.Fan J., Krautkramer K., Feldman J.L., Denu J.M. Metabolic Regulation of Histone Post-Translational Modifications. ACS Chem. Boil. 2015;10:95–108. doi: 10.1021/cb500846u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Braidy N., Poljak A., Grant R., Jayasena T., Mansour H., Chan-Ling T., Smythe G., Sachdev P.S., Guillemin G.J. Differential expression of sirtuins in the aging rat brain. Front. Cell. Neurosci. 2015;9:167. doi: 10.3389/fncel.2015.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Satoh A., Brace C.S., Rensing N., Cliften P., Wozniak D.F., Herzog E.D., Yamada K.A., Imai S.-I., Clifton P. Sirt1 extends life span and delays aging in mice through the regulation of Nk2 homeobox 1 in the DMH and LH. Cell Metab. 2013;18:416–430. doi: 10.1016/j.cmet.2013.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lan F. SIRT1 modulation of the acetylation status, cytosolic localization, and activity of LKB1. Possible role in AMP-activated protein kinase activation. J. Biol. Chem. 2008;283:27628–27635. doi: 10.1074/jbc.M805711200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Rothgiesser K.M. SIRT2 regulates NF-kappaB dependent gene expression through deacetylation of p65 Lys310. J. Cell Sci. 2010;123:4251–4258. doi: 10.1242/jcs.073783. [DOI] [PubMed] [Google Scholar]
- 125.Nogueiras R., Habegger K.M., Chaudhary N., Finan B., Banks A., Dietrich M., Horvath T.L., Sinclair D., Pfluger P.T., Tschöp M.H., et al. Sirtuin 1 and sirtuin 3: Physiological modulators of metabolism. Physiol. Rev. 2012;92:1479–1514. doi: 10.1152/physrev.00022.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Li T., Li Y., Liu T., Hu B., Li J., Liu C., Liu T., Li F. Mitochondrial PAK6 inhibits prostate cancer cell apoptosis via the PAK6-SIRT4-ANT2 complex. Theranostics. 2020;10:2571–2586. doi: 10.7150/thno.42874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Li W., Yang Y., Li Y., Zhao Y., Jiang H. Sirt5 Attenuates Cisplatin-Induced Acute Kidney Injury through Regulation of Nrf2/HO-1 and Bcl-2. BioMed Res. Int. 2019;2019 doi: 10.1155/2019/4745132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sharma A., Diecke S., Zhang W.Y., Lan F., He C., Mordwinkin N.M., Chua K.F., Wu J.C. The role of SIRT6 protein in aging and reprogramming of human induced pluripotent stem cells. J. Biol. Chem. 2013;288:18439–18447. doi: 10.1074/jbc.M112.405928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Vazquez B.N., Thackray J., Serrano L. Sirtuins and DNA damage repair: SIRT7 comes to play. Nucleus. 2017;8:107–115. doi: 10.1080/19491034.2016.1264552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Essuman K., Summers D.W., Sasaki Y., Mao X., DiAntonio A., Milbrandt J. The SARM1 Toll/Interleukin-1 Receptor (TIR) Domain Possesses Intrinsic NAD+ Cleavage Activity that Promotes Pathological Axonal Degeneration. Neuron. 2017;93:1334–1343. doi: 10.1016/j.neuron.2017.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gerdts J., Brace E.J., Sasaki Y., DiAntonio A., Milbrandt J. SARM1 activation triggers axon degeneration locally via NAD(+) destruction. Science. 2015;348:453–457. doi: 10.1126/science.1258366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Gerdts J., Summers D.W., Milbrandt J., DiAntonio A. Axon Self-Destruction: New Links among SARM1, MAPKs, and NAD+ Metabolism. Neuron. 2016;89:449–460. doi: 10.1016/j.neuron.2015.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Murata H., Khine C.C., Nishikawa A., Yamamoto K.-I., Kinoshita R., Sakaguchi M. c-Jun N-terminal kinase (JNK)-mediated phosphorylation of SARM1 regulates NAD(+) cleavage activity to inhibit mitochondrial respiration. J. Biol. Chem. 2018;293:18933–18943. doi: 10.1074/jbc.RA118.004578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sasaki Y., Nakagawa T., Mao X., DiAntonio A., Milbrandt J. NMNAT1 inhibits axon degeneration via blockade of SARM1-mediated NAD+ depletion. eLife. 2016;5:1010. doi: 10.7554/eLife.19749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Summers D.W., Gibson D.A., DiAntonio A., Milbrandt J. SARM1-specific motifs in the TIR domain enable NAD+ loss and regulate injury-induced SARM1 activation. Proc. Natl. Acad. Sci. USA. 2016;113:E6271–E6280. doi: 10.1073/pnas.1601506113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Verdin E. NAD(+) in aging, metabolism, and neurodegeneration. Science. 2015;350:1208–1213. doi: 10.1126/science.aac4854. [DOI] [PubMed] [Google Scholar]
- 137.Aksoy P., Escande C., White T.A., Thompson M., Soares S., Benech J.C., Chini E.N. Regulation of SIRT 1 mediated NAD dependent deacetylation: A novel role for the multifunctional enzyme CD38. Biochem. Biophys. Res. Commun. 2006;349:353–359. doi: 10.1016/j.bbrc.2006.08.066. [DOI] [PubMed] [Google Scholar]
- 138.Hegedűs C., Boros G., Fidrus E., Kis G., Antal M., Juhász T., Janka E., Jankó L., Paragh G., Emri G., et al. PARP1 Inhibition Augments UVB-Mediated Mitochondrial Changes—Implications for UV-Induced DNA Repair and Photocarcinogenesis. Cancers. 2019;12:5. doi: 10.3390/cancers12010005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Xu S., Bai P., Little P.J., Liu P. Poly(ADP-ribose) Polymerase 1 (PARP1) in Atherosclerosis: From Molecular Mechanisms to Therapeutic Implications. Med. Res. Rev. 2014;34:644–675. doi: 10.1002/med.21300. [DOI] [PubMed] [Google Scholar]
- 140.Bai P., Cantó C., Brunyánszki A., Huber A., Szántó M., Cen Y., Yamamoto H., Houten S.M., Kiss B., Oudart H., et al. PARP-2 Regulates SIRT1 Expression and Whole-Body Energy Expenditure. Cell Metab. 2011;13:450–460. doi: 10.1016/j.cmet.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Bai P., Cantó C., Oudart H., Brunyánszki A., Cen Y., Thomas C., Yamamoto H., Huber A., Kiss B., Houtkooper R.H., et al. PARP-1 inhibition increases mitochondrial metabolism through SIRT1 activation. Cell Metab. 2011;13:461–468. doi: 10.1016/j.cmet.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ijichi H., Ichiyama A., Hayaishi O. Studies on the biosynthesis of nicotinamide adenine dinucleotide. 3. Comparative in vivo studies on nicotinic acid, nicotinamide, and quinolinic acid as precursors of nicotinamide adenine dinucleotide. J. Boil. Chem. 1966;241:3701–3707. [PubMed] [Google Scholar]
- 143.Jackson T.M., Rawling J.M., Roebuck B.D., Kirkland J.B. Large supplements of nicotinic acid and nicotinamide increase tissue NAD+ and poly(ADP-ribose) levels but do not affect diethylnitrosamine-induced altered hepatic foci in Fischer-344 rats. J. Nutr. 1995;125:1455–1461. doi: 10.1093/jn/125.6.1455. [DOI] [PubMed] [Google Scholar]
- 144.Kimura T., Bier D.M., Taylor C.L. Summary of workshop discussions on establishing upper limits for amino acids with specific attention to available data for the essential amino acids leucine and tryptophan. J. Nutr. 2012;142:2245S–2248S. doi: 10.3945/jn.112.160846. [DOI] [PubMed] [Google Scholar]
- 145.Galassi L., Di Stefano M., Brunetti L., Orsomando G., Amici A., Ruggieri S., Magni G. Characterization of human nicotinate phosphoribosyltransferase: Kinetic studies, structure prediction and functional analysis by site-directed mutagenesis. Biochimie. 2012;94:300–309. doi: 10.1016/j.biochi.2011.06.033. [DOI] [PubMed] [Google Scholar]
- 146.Duarte-Pereira S., Pereira-Castro I., Silva S.S., Correia M.G., Neto C., Da Costa L.T., Amorim A., Silva R. Extensive regulation of nicotinate phosphoribosyltransferase (NAPRT) expression in human tissues and tumors. Oncotarget. 2016;7:1973–1983. doi: 10.18632/oncotarget.6538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lau C., Niere M., Ziegler M. The NMN/NaMN adenylyltransferase (NMNAT) protein family. Front. Biosci. 2009;14:410–431. doi: 10.2741/3252. [DOI] [PubMed] [Google Scholar]
- 148.Schweiger M., Hennig K., Lerner F., Niere M., Hirsch-Kauffmann M., Specht T., Weise C., Oei S.L., Ziegler M. Characterization of recombinant human nicotinamide mononucleotide adenylyl transferase (NMNAT), a nuclear enzyme essential for NAD synthesis. FEBS Lett. 2001;492:95–100. doi: 10.1016/S0014-5793(01)02180-9. [DOI] [PubMed] [Google Scholar]
- 149.Berger F., Lau C., Dahlmann M., Ziegler M. Subcellular compartmentation and differential catalytic properties of the three human nicotinamide mononucleotide adenylyltransferase isoforms. J. Biol. Chem. 2005;280:36334–36341. doi: 10.1074/jbc.M508660200. [DOI] [PubMed] [Google Scholar]
- 150.Jayaram H.N., Kusumanchi P., Yalowitz J.A. NMNAT expression and its relation to NAD metabolism. Curr. Med. Chem. 2011;18:1962–1972. doi: 10.2174/092986711795590138. [DOI] [PubMed] [Google Scholar]
- 151.Mori V., Amici A., Mazzola F., Di Stefano M., Conforti L., Magni G., Ruggieri S., Raffaelli N., Orsomando G. Metabolic profiling of alternative NAD biosynthetic routes in mouse tissues. PLoS ONE. 2014;9:e113939. doi: 10.1371/journal.pone.0113939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Hara N., Yamada K., Shibata T., Osago H., Hashimoto T., Tsuchiya M. Elevation of Cellular NAD Levels by Nicotinic Acid and Involvement of Nicotinic Acid Phosphoribosyltransferase in Human Cells. J. Boil. Chem. 2007;282:24574–24582. doi: 10.1074/jbc.M610357200. [DOI] [PubMed] [Google Scholar]
- 153.Guyton J.R. Niacin in cardiovascular prevention: Mechanisms, efficacy, and safety. Curr. Opin. Lipidol. 2007;18:415–420. doi: 10.1097/MOL.0b013e3282364add. [DOI] [PubMed] [Google Scholar]
- 154.Vaccari C.S., Nagamia S., Thoenes M., Oguchi A., Hammoud R., Khan B.V. Efficacy of controlled-release niacin in treatment of metabolic syndrome: Correlation to surrogate markers of atherosclerosis, vascular reactivity, and inflammation. J. Clin. Lipidol. 2007;1:605–613. doi: 10.1016/j.jacl.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 155.Grant R., Kapoor V. Murine glial cells regenerate NAD, after peroxide-induced depletion, using either nicotinic acid, nicotinamide, or quinolinic acid as substrates. J. Neurochem. 1998;70:1759–1763. doi: 10.1046/j.1471-4159.1998.70041759.x. [DOI] [PubMed] [Google Scholar]
- 156.Soudijn W., van Wijngaarden I., Ijzerman A.P. Nicotinic acid receptor subtypes and their ligands. Med. Res. Rev. 2007;27:417–433. doi: 10.1002/med.20102. [DOI] [PubMed] [Google Scholar]
- 157.Wang F., Zhang W.-P. [Research progress on nicotinamide phosphoribosyl transferase involved in aging and age-related diseases] Zhejiang Da Xue Xue Bao Yi Xue Ban. 2011;40:680–684. doi: 10.3785/j.issn.1008-9292.2011.06.018. [DOI] [PubMed] [Google Scholar]
- 158.Bender D.A. Tryptophan and niacin nutrition--is there a problem? Adv. Exp. Med. Biol. 1996;398:565–569. [PubMed] [Google Scholar]
- 159.Bogan K.L., Brenner C. Nicotinic acid, nicotinamide, and nicotinamide riboside: A molecular evaluation of NAD+ precursor vitamins in human nutrition. Annu. Rev. Nutr. 2008;28:115–130. doi: 10.1146/annurev.nutr.28.061807.155443. [DOI] [PubMed] [Google Scholar]
- 160.Ratajczak J., Joffraud M., Trammell S.A., Ras R., Canela N., Boutant M., Kulkarni S.S., Rodrigues M., Redpath P., Migaud M.E., et al. NRK1 controls nicotinamide mononucleotide and nicotinamide riboside metabolism in mammalian cells. Nat. Commun. 2016;7:13103. doi: 10.1038/ncomms13103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Tempel W., Rabeh W.M., Bogan K.L., Belenky P., Wojcik M., Seidle H.F., Nedyalkova L., Yang T., Sauve A., Park H.-W., et al. Nicotinamide riboside kinase structures reveal new pathways to NAD+ PLoS Biol. 2007;5:e263. doi: 10.1371/journal.pbio.0050263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Trammell S.A., Schmidt M.S., Weidemann B.J., Redpath P., Jaksch F., Dellinger R.W., Li Z., Abel E.D., Migaud M.E., Brenner C. Nicotinamide riboside is uniquely and orally bioavailable in mice and humans. Nat. Commun. 2016;7:12948. doi: 10.1038/ncomms12948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Braidy N., Liu Y. NAD+ therapy in age-related degenerative disorders: A benefit/risk analysis. Exp. Gerontol. 2020;132:110831. doi: 10.1016/j.exger.2020.110831. [DOI] [PubMed] [Google Scholar]
- 164.Braidy N., Jayasena T., Poljak A., Sachdev P.S. Sirtuins in cognitive ageing and Alzheimer’s disease. Curr. Opin. Psychiatry. 2012;25:226–230. doi: 10.1097/YCO.0b013e32835112c1. [DOI] [PubMed] [Google Scholar]
- 165.Samodien E., Johnson R., Pheiffer C., Mabasa L., Erasmus M., Louw J., Chellan N. Diet-induced hypothalamic dysfunction and metabolic disease, and the therapeutic potential of polyphenols. Mol. Metab. 2019;27:1–10. doi: 10.1016/j.molmet.2019.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Fricker R., Green E.L., Jenkins S.I., Griffin S.M. The Influence of Nicotinamide on Health and Disease in the Central Nervous System. Int. J. Tryptophan Res. 2018;11:1178646918776658. doi: 10.1177/1178646918776658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Requardt R.P. Ca(2)(+) signals of astrocytes are modulated by the NAD(+)/NADH redox state. J. Neurochem. 2012;120:1014–1025. doi: 10.1111/j.1471-4159.2012.07645.x. [DOI] [PubMed] [Google Scholar]
- 168.Requardt R.P., Wilhelm F., Rillich J., Winkler U., Hirrlinger J. The biphasic NAD(P)H fluorescence response of astrocytes to dopamine reflects the metabolic actions of oxidative phosphorylation and glycolysis. J. Neurochem. 2010;115:483–492. doi: 10.1111/j.1471-4159.2010.06940.x. [DOI] [PubMed] [Google Scholar]
- 169.Ferguson D., Koo J.W., Feng J., Heller E., Rabkin J., Heshmati M., Renthal W., Neve R., Liu X., Shao N.-Y., et al. Essential role of SIRT1 signaling in the nucleus accumbens in cocaine and morphine action. J. Neurosci. 2013;33:16088–16098. doi: 10.1523/JNEUROSCI.1284-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Ferguson D., Shao N.-Y., Heller E.A., Feng J., Neve R., Kim H.-D., Call T., Magazu S., Shen L., Nestler E.J. SIRT1-FOXO3a regulate cocaine actions in the nucleus accumbens. J. Neurosci. 2015;35:3100–3111. doi: 10.1523/JNEUROSCI.4012-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Zhang R., Lahens N.F., Ballance H.I., Hughes M.E., HogenEsch J.B. A circadian gene expression atlas in mammals: Implications for biology and medicine. Proc. Natl. Acad. Sci. USA. 2014;111:16219–16224. doi: 10.1073/pnas.1408886111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Lazaroff M., Patankar S., Yoon S.O., Chikaraishi D.M. The cyclic AMP response element directs tyrosine hydroxylase expression in catecholaminergic central and peripheral nervous system cell lines from transgenic mice. J. Boil. Chem. 1995;270:21579–21589. doi: 10.1074/jbc.270.37.21579. [DOI] [PubMed] [Google Scholar]
- 173.Nagatsu T. Tyrosine hydroxylase: Human isoforms, structure and regulation in physiology and pathology. Essays Biochem. 1995;30:15–35. [PubMed] [Google Scholar]
- 174.Ozburn A.R., Falcon E., Mukherjee S., Gillman A., Arey R., Spencer S., McClung C.A. The role of clock in ethanol-related behaviors. Neuropsychopharmacology. 2013;38:2393–2400. doi: 10.1038/npp.2013.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.McClung C.A., Sidiropoulou K., Vitaterna M., Takahashi J.S., White F.J., Cooper N.C., Nestler E.J. Regulation of dopaminergic transmission and cocaine reward by the Clock gene. Proc. Natl. Acad. Sci. USA. 2005;102:9377–9381. doi: 10.1073/pnas.0503584102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Logan R.W., Parekh P.K., Kaplan G.N., Becker-Krail D., Williams W.P., Yamaguchi S., Yoshino J., Shelton M.A., Zhu X., Zhang H., et al. NAD+ cellular redox and SIRT1 regulate the diurnal rhythms of tyrosine hydroxylase and conditioned cocaine reward. Mol. Psychiatry. 2019;24:1668–1684. doi: 10.1038/s41380-018-0061-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Kong J., Du C., Jiang L., Jiang W., Deng P., Shao X., Zhang B., Li Y., Zhu R., Zhao Q., et al. Nicotinamide phosphoribosyltransferase regulates cocaine reward through Sirtuin 1. Exp. Neurol. 2018;307:52–61. doi: 10.1016/j.expneurol.2018.05.010. [DOI] [PubMed] [Google Scholar]
- 178.Aguilar-Arnal L., Katada S., Orozco-Solis R., Sassone-Corsi P. NAD(+)-SIRT1 control of H3K4 trimethylation through circadian deacetylation of MLL1. Nat. Struct. Mol. Boil. 2015;22:312–318. doi: 10.1038/nsmb.2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Renthal W., Kumar A., Xiao G., Wilkinson M., Covington H.E., Maze I., Sikder D., Robison A., LaPlant Q., Dietz D.M., et al. Genome-wide analysis of chromatin regulation by cocaine reveals a role for sirtuins. Neuron. 2009;62:335–348. doi: 10.1016/j.neuron.2009.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Real J.I., Simões A.P., Rial D., Cunha R.A., Ferreira S.G. Adenosine A2A receptors modulate the dopamine D2 receptor-mediated inhibition of synaptic transmission in the mouse prefrontal cortex. Eur. J. Neurosci. 2018;47:1127–1134. doi: 10.1111/ejn.13912. [DOI] [PubMed] [Google Scholar]
- 181.Sichardt K., Nieber K. Adenosine A1 receptor: Functional receptor-receptor interactions in the brain. Purinergic Signal. 2007;3:285–298. doi: 10.1007/s11302-007-9065-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Filip M., Zaniewska M., Frankowska M., Wydra K., Fuxe K. The importance of the adenosine A(2A) receptor-dopamine D(2) receptor interaction in drug addiction. Curr. Med. Chem. 2012;19:317–355. doi: 10.2174/092986712803414231. [DOI] [PubMed] [Google Scholar]
- 183.Zhang J., Hong Y., Cao W., Yin S., Shi H.-B., Ying W. SIRT2, ERK and Nrf2 Mediate NAD(+) Treatment-Induced Increase in the Antioxidant Capacity of PC12 Cells Under Basal Conditions. Front. Mol. Neurosci. 2019;12:108. doi: 10.3389/fnmol.2019.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Fredholm B.B. Adenosine, an endogenous distress signal, modulates tissue damage and repair. Cell Death Differ. 2007;14:1315–1323. doi: 10.1038/sj.cdd.4402132. [DOI] [PubMed] [Google Scholar]
- 185.Zhang J., Wang C., Shi H.-B., Wu D., Ying W. Extracellular Degradation Into Adenosine and the Activities of Adenosine Kinase and AMPK Mediate Extracellular NAD(+)-Produced Increases in the Adenylate Pool of BV2 Microglia Under Basal Conditions. Front. Cell. Neurosci. 2018;12:343. doi: 10.3389/fncel.2018.00343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Hardie D.G. Keeping the home fires burning: AMP-activated protein kinase. J. R. Soc. Interface. 2018;15:20170774. doi: 10.1098/rsif.2017.0774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Fan J., Guang H., Zhang H., Chen D., Ding L., Fan X., Hu L. SIRT1 Mediates Apelin-13 in Ameliorating Chronic Normobaric Hypoxia-induced Anxiety-like Behavior by Suppressing NF-kappaB Pathway in Mice Hippocampus. Neuroscience. 2018;381:22–34. doi: 10.1016/j.neuroscience.2018.04.013. [DOI] [PubMed] [Google Scholar]
- 188.Jang H.M., Han S.K., Kim J.K., Oh S.J., Jang H.B., Kim D.H. Lactobacillus sakei Alleviates High-Fat-Diet-Induced Obesity and Anxiety in Mice by Inducing AMPK Activation and SIRT1 Expression and Inhibiting Gut Microbiota-Mediated NF-kappaB Activation. Mol. Nutr. Food Res. 2019;63:e1800978. doi: 10.1002/mnfr.201800978. [DOI] [PubMed] [Google Scholar]
- 189.Liang X., Liu Y., Jia S., Xu X., Dong M., Wei Y. SIRT1: The Value of Functional Outcome, Stroke-Related Dementia, Anxiety, and Depression in Patients with Acute Ischemic Stroke. J. Stroke Cerebrovasc. Dis. 2019;28:205–212. doi: 10.1016/j.jstrokecerebrovasdis.2018.09.037. [DOI] [PubMed] [Google Scholar]
- 190.Libert S., Pointer K., Bell E.L., Das A., Cohen D.E., Asara J.M., Kapur K., Bergmann S., Preisig M., Otowa T., et al. SIRT1 activates MAO-A in the brain to mediate anxiety and exploratory drive. Cell. 2011;147:1459–1472. doi: 10.1016/j.cell.2011.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Yu D., Homiack D.R., Sawyer E.J., Schrader L.A. BK channel deacetylation by SIRT1 in dentate gyrus regulates anxiety and response to stress. Commun. Biol. 2018;1:82. doi: 10.1038/s42003-018-0088-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Cantó C., Gerhart-Hines Z., Feige J.N., Lagouge M., Noriega L., Milne J.C., Elliott P.J., Puigserver P., Auwerx J., Noriega L. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature. 2009;458:1056–1060. doi: 10.1038/nature07813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Chau M.D.L., Gao J., Yang Q., Wu Z., Gromada J. Fibroblast growth factor 21 regulates energy metabolism by activating the AMPK-SIRT1-PGC-1alpha pathway. Proc. Natl. Acad. Sci. USA. 2010;107:12553–12558. doi: 10.1073/pnas.1006962107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Li X. SIRT1 and energy metabolism. Acta Biochim. Biophys. Sin. 2013;45:51–60. doi: 10.1093/abbs/gms108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Oliva J., French B.A., Li J., Bardag-Gorce F., Fu P., French S.W. Sirt1 is involved in energy metabolism: The role of chronic ethanol feeding and resveratrol. Exp. Mol. Pathol. 2008;85:155–159. doi: 10.1016/j.yexmp.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Boily G., Seifert E.L., Bevilacqua L., He X.H., Sabourin G., Estey C., Moffat C., Crawford S., Saliba S., Jardine K., et al. SirT1 regulates energy metabolism and response to caloric restriction in mice. PLoS ONE. 2008;3:e1759. doi: 10.1371/journal.pone.0001759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Matsui S., Sasaki T., Kohno D., Yaku K., Inutsuka A., Yokota-Hashimoto H., Kikuchi O., Suga T., Kobayashi M., Yamanaka A., et al. Neuronal SIRT1 regulates macronutrient-based diet selection through FGF21 and oxytocin signalling in mice. Nat. Commun. 2018;9:4604. doi: 10.1038/s41467-018-07033-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Tezze C., Romanello V., Sandri M. FGF21 as Modulator of Metabolism in Health and Disease. Front. Physiol. 2019;10:419. doi: 10.3389/fphys.2019.00419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Martínez-Garza Ú., Torres-Oteros D., Yarritu-Gallego A., Marrero P.F., Haro D., Relat J. Fibroblast Growth Factor 21 and the Adaptive Response to Nutritional Challenges. Int. J. Mol. Sci. 2019;20:4692. doi: 10.3390/ijms20194692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Lawson E.A. The effects of oxytocin on eating behaviour and metabolism in humans. Nat. Rev. Endocrinol. 2017;13:700–709. doi: 10.1038/nrendo.2017.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Chini E.N. CD38 as a regulator of cellular NAD: A novel potential pharmacological target for metabolic conditions. Curr. Pharm. Des. 2009;15:57–63. doi: 10.2174/138161209787185788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Higashida H., Yokoyama S., Kikuchi M., Munesue T. CD38 and its role in oxytocin secretion and social behavior. Horm. Behav. 2012;61:351–358. doi: 10.1016/j.yhbeh.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 203.Higashida H., Yokoyama S., Huang J.-J., Liu L., Ma W.-J., Akther S., Higashida C., Kikuchi M., Minabe Y., Munesue T. Social memory, amnesia, and autism: Brain oxytocin secretion is regulated by NAD+ metabolites and single nucleotide polymorphisms of CD38. Neurochem. Int. 2012;61:828–838. doi: 10.1016/j.neuint.2012.01.030. [DOI] [PubMed] [Google Scholar]
- 204.Chandra R., Engeln M., Schiefer C., Patton M., Martin J.A., Werner C.T., Riggs L.M., Francis T.C., McGlincy M., Evans B., et al. Drp1 Mitochondrial Fission in D1 Neurons Mediates Behavioral and Cellular Plasticity during Early Cocaine Abstinence. Neuron. 2017;96:1327–1341. doi: 10.1016/j.neuron.2017.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Ding M., Feng N., Tang D., Feng J., Li Z., Jia M., Liu Z., Gu X., Wang Y., Fu F., et al. Melatonin prevents Drp1-mediated mitochondrial fission in diabetic hearts through SIRT1-PGC1α pathway. J. Pineal Res. 2018;65:e12491. doi: 10.1111/jpi.12491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Grant R., Berg J., Mestayer R., Braidy N., Bennett J., Broom S., Watson J. A Pilot Study Investigating Changes in the Human Plasma and Urine NAD+ Metabolome during a 6 Hour Intravenous Infusion of NAD. Front. Aging Neurosci. 2019;11:257. doi: 10.3389/fnagi.2019.00257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Braidy N., Guillemin G.J., Mansour H., Chan-Ling T., Poljak A., Grant R. Age related changes in NAD+ metabolism oxidative stress and Sirt1 activity in wistar rats. PLoS ONE. 2011;6:e19194. doi: 10.1371/journal.pone.0019194. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 208.Liu Y., Clement J., Grant R., Sachdev P.S., Braidy N. Quantitation of NAD+: Why do we need to measure it? Biochim. Biophys. Acta Gen. Subj. 2018;1862:2527–2532. doi: 10.1016/j.bbagen.2018.07.023. [DOI] [PubMed] [Google Scholar]