Abstract
Density functional theory was employed to highlight the antioxidant working mechanism of higenamine in aqueous and lipid-like environments. Different reaction mechanisms were considered for the reaction of higenamine with the •OOH radical. The pH values and the molar fraction at physiological pH were determined in aqueous solution. The results show that the preferred reaction mechanism was the hydrogen atom transfer from the catecholic ring. The computed kinetic constants revealed that, in order to obtain reliable results, it is important to consider all the species present in water solution derived from acid–base equilibria. From the present investigation, it emerges that at physiological pH (7.4), the scavenging activity of higenamine against the •OOH radical is higher than that of Trolox, chosen as a reference antioxidant. Furthermore, higenamine results to be more efficient for that purpose than melatonin and caffeine, whose protective action against oxidative stress is frequently associated with their reactive oxygen species (ROS) scavenging activity.
Keywords: DFT, antioxidant mechanism, kinetic constants, higenamine, acid–base equilibria
1. Introduction
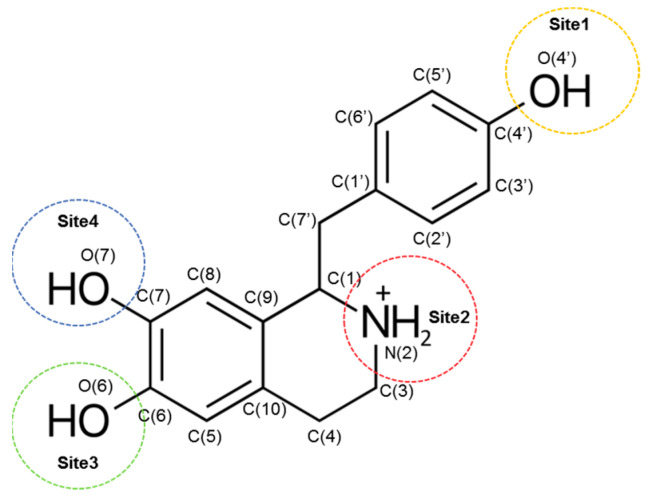
Higenamine [1-(4′-hydroxybenzyl)−6,7-dihydroxy-1,2,3,4-tetrahydroisoquinoline] (Figure 1), also known as norcoclaurine or dl-demethylcoclaurine, is a plant-based alkaloid belonging to the structural class of protoberberines. It is present in many plants such as Aconitum japonicum, Nandina Domestica, Gnetum Parvifolium, Asarum heterotropoides, Nelumbo nucifera, Galium divaricatum, Annona squamosal, and Aconitum carmichaelii [1,2].
Figure 1.
The 2D structure of higenamine.
For 40 years, higenamine has been known as a cardiotonic due to its beta-agonist activity and inotropic and chronotropic properties [3]. More recently, a revived interest in this compound has been motivated by its possible applications in many other therapeutic fields. Indeed, several studies have highlighted that higenamine exerts a hypotensive effect (it is a α1-adrenergic receptor antagonist) [4] and a protective effect on ischemia/reperfusion injuries (it activates the Phosphatidylinositol-3-kinase–Protein kinase B also known as Akt (PI3K/AKT) pathway) [5] and, more recently, it has been proposed as pharmacological stress agent for myocardial perfusion imaging. In addition to these properties, higenamine exhibits pharmacological activity towards other diseases, such as sepsis, heart failure, breathing difficulties, erectile dysfunction (ED), bradyarrhythmia, arthritis, and disseminated intravascular coagulation [1]. This multi-target activity led researchers to focus on the mechanisms and on the pathways implicated in higenamine action in different diseases. Recent literature [6,7,8,9,10] indicates that the common denominator could be its antioxidant activity against reactive oxygen species (ROS). These highly reactive species may originate endogenously and exogenously, such as from metabolic pathways and external influences (e.g., smoking, radiation, drugs, and other environmental contaminants). Although under normal conditions the organism is able to maintain a good balance between production and removal of free radicals, their overproduction leads to oxidative stress, necrosis, apoptosis, and damage to biological macromolecules and compromises homeostasis and cellular function [11,12,13].
Antioxidant compounds act by detoxifying and reducing ROS and controlling oxidative stress through different reaction mechanisms, such as electron transfer (ET), proton transfer (PT), sequential proton loss electron transfer (SPLET), hydrogen atom transfer (HAT) and radical adduct formation (RAF). Considering that natural products offer a wide range of antioxidant compounds, the identification of the specific role of each chemical portion of these compounds and the associated reaction mechanism is paramount in order to overcome oxidative stress in a targeted manner [14,15].
The presence of an OH group with phenolic nature proves to be essential for these properties, particularly, in the alkoxyl, carboxyl, ester, and carbonyl groups in which the O atom provides acidity or neutrality. Uncommonly, alkaline phenolic compounds are studied for their antioxidant activity [16,17,18].
N-containing compounds, such as alkaloids, are abundantly present in natural products. It has been shown that these alkaloids exhibit protective action against free radicals due to the elimination of the cation radical 2,2′-azinobis-(3-ethylbenzthiazoline-6-sulfonic acid) (ABTS•+) [19,20] as well as to their scavenging activity against the 1,1-diphenyl-2-picryl-hydrazyl (•DPPH) radical and their inhibitory effects on hydrogen peroxide radical [21].
Recently, among the phenolic alkaloids, Xie et al. [22] studied higenamine as an attractive scaffold to test its antioxidant effect. In particular, as shown in Figure 1, the peculiarity of higenamine structure is the presence of a protonated N-atom (site 2) which provides it with a strong electron-withdrawing capacity, thus resulting in an electron density change and suggesting an effect of pH on the antioxidant power of higenamine. For all these reasons and in order to rationalize the existing experimental results [22], we decided to carefully investigate the antioxidant properties of higenamine by employing a density functional theory (DFT)-based computational protocol previously and successfully used for a series of natural antioxidants [23,24,25]. The hydroperoxyl radical (•OOH) was chosen because its half-life allows the best interception by chemical scavengers [26,27]. Several reaction mechanisms (HAT, single-electron transfer (SET), and RAF) were considered, and the overall kinetic behaviour was evaluated.
2. Materials and Methods
All the calculations were carried out with the Gaussian 09 package of programs [28]. Full geometry optimizations and frequency calculations were done by using the DFT. The M06-2X functional coupled with the extended 6-311+G(d) basis set was chosen because of the good performance of this level of theory for kinetic calculations [29] and was successfully used in previous works for modelling chemical reactions between antioxidant species and free radicals [30,31,32,33,34,35,36]. Unrestricted calculations were used for open-shell systems. The solvent effects, in water and pentylethanoate (PE) environments, were taken into account by using the Solvent Model based on Density SMD [37], which has been proven to estimate the solvation free energies for charged or uncharged solutes with relatively low errors. Local minima and transition states (TS) were identified by the number of imaginary frequencies (0 or 1, respectively). Intrinsic reaction coordinate calculations (IRC) were performed to verify if the located TS properly connected the relative minima along the reaction coordinate [38]. Thermodynamic corrections at 298.15 K were included in the calculation of relative energies. The used computational protocol is in line with the quantum mechanics-based test for overall free-radical scavenging activity (QM-ORSA), [39,40] which was validated by comparison with experimental results. Spin density computations were performed for the most stable open-shell species. Natural bond orbital (NBO) analysis [41,42,43], as implemented in the Gaussian 09 package, was to evaluate net charges, bond order, and conjugation.
3. Results and Discussion
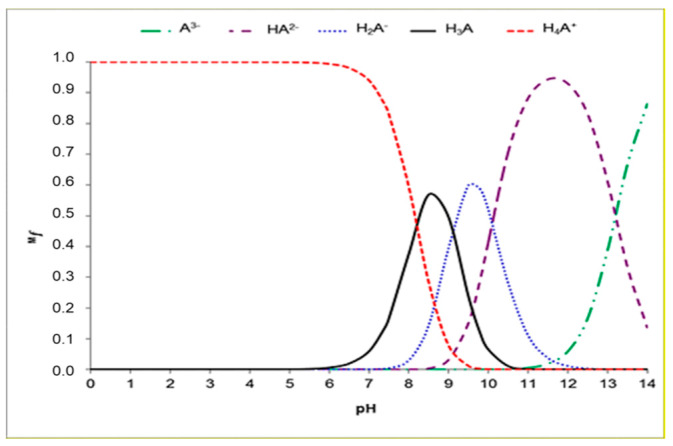
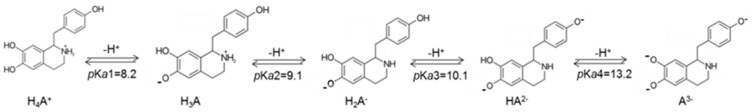
As it is well known, in the aqueous phase, knowledge of the acid−base equilibrium is crucial for the individuation of the chemical species present in physiological conditions. For this reason, our preliminary calculations were devoted to the computation of the acid dissociation constants (pKas) of the investigated compound, using the parameter fitting method [44], and to the quantification of the relative molar fractions at pH 7.4 (see Figure 2). Considering all the possible deprotonation paths (see Figure 3), our results indicated that the first deprotonation occurred at the OH in position C6 (Figure 1), and the relative pKa1 value was 8.2, in agreement with experimental results [45]. The second deprotonation at pKa2 = 9.1 (see Figure 3) involved the NH2 group, giving rise to the H2A− species. Furthermore, the loss of H+ from the OH in position C4 generated the HA2− anion (pKa3 = 10.1), while the last deprotonation (pKa4 = 13.2) generated the A3− species. Looking at Table 1, it is possible to evidence that at physiological pH, in addition to the dominant H4A+ species, also the H3A and H2A− species are present in aqueous solution, in molar fractions of 0.136 and 0.002, respectively.
Figure 2.
Distribution diagram of higenamine as a function of pH.
Figure 3.
pKa values of the relative deprotonation paths of higenamine at physiological pH.
Table 1.
Molar fractions (M f) of the different acid–base species of higenamine at physiological pH.
| H4A+ | H3A | H2A− | HA2− | A3− |
|---|---|---|---|---|
| 0.861 | 0.136 | 0.002 | 0.000 | 0.000 |
These results indicated that for higenamine, it is necessary to take into account the species produced by the first two acid–base equilibria for the determination of the antioxidant power of higenamine toward the •OOH radical in an aqueous environment.
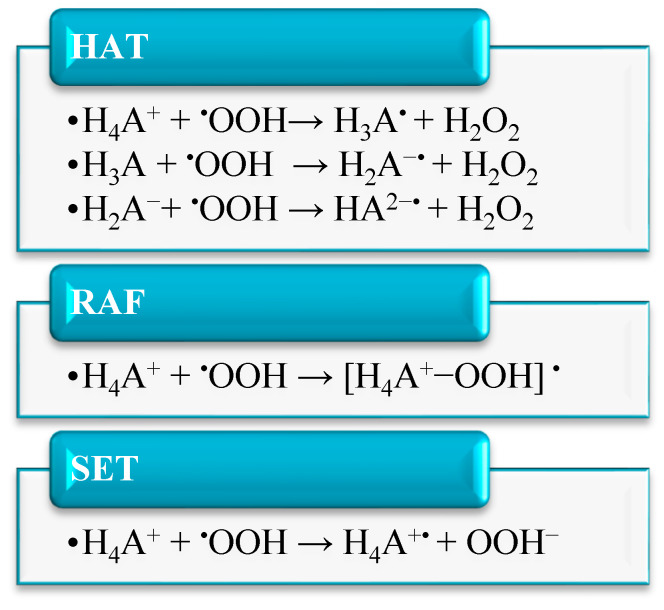
The investigated reaction mechanisms are summarized in Scheme 1:
Scheme 1.
Schematic representation of the considered mechanisms for higenamine and its acid–base forms. Gibbs free energies of reaction (ΔG) and activation (ΔG⧧), for all populated species in aqueous and pentylethanoate (PE) solvents, involved in the studied mechanisms are summarized in Table 2. HAT, hydrogen atom transfer, RAF, radical adduct formation, SET, single-electron transfer.
Concerning the HAT mechanism, in the H4A+ form, only the •OOH attack on hydrogens at site 3 (O6) and site 4 (O7) of the catechol moiety results to be exergonic by 3.62 and 2.58 kcal mol−1, respectively, while at the H3A sites, 1 (O4′) and 4 (O7) are thermodynamically favoured. In the H2A− species, the Gibbs energies for the reaction at sites 1 and 4 are −3.81 and −15.63 kcal mol−1, respectively. In addition, for the catecholic moiety in higenamine going from the neutral form to the anionic one, the trend of the obtained ΔG values well reproduces that observed for catechol [46].
The most exergonic reaction path is HAT from site 4 of the catechol moiety, for both H2A− and H3A species.
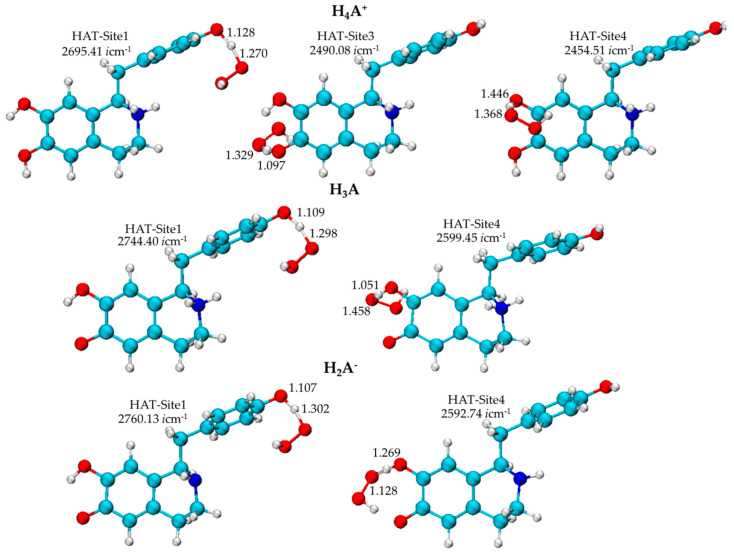
The optimized geometries of the transition states are illustrated in Figure 4.
Figure 4.
Geometries of the transition states of the H4A+, H3A, and H2A− forms obtained in water as a result of hydrogen atom transfer mechanism at the M062X/6-311+G(d) level of theory.
From Table 2, it is possible to note that the activation energy for the reaction of H4A+ species for which the TS have been characterized, assumes values ranging from about 20 to 28 kcal mol−1, whereas for the reaction of H3A and H2A− species, the ΔG⧧ values range from 1 to 21 kcal mol−1.
Table 2.
Gibbs free energies of reaction (ΔG) and activation (ΔG⧧), expressed in kcal mol−1, at 298.15 K. in aqueous solution involved in HAT and SET. The ΔG values calculated in PE medium are reported in parenthesis.
| H4A+ | H3A | H2A− | ||||
|---|---|---|---|---|---|---|
| ΔG | ΔG⧧ | ΔG | ΔG⧧ | ΔG | ΔG⧧ | |
| HAT-O4′ | 1.82 | 23.35 | −0.26 | 21.80 | −3.81 | 21.36 |
| HAT-O6 | −3.62 | 19.99 | ||||
| HAT-O7 | −2.58 | 20.93 | −13.34 | 8.49 | −15.63 | 1.33 |
| HAT-C1 | 2.43 | −0.85 | 13.39 | |||
| HAT-C3 | 16.46 | 15.58 | ||||
| HAT-C4 | 3.19 | 2.55 | ||||
| HAT-C7 | 6.33 | 7.57 | ||||
| SET | 31.19 (91.53) | 4.42 (36.60) | −1.03 (15.11) | |||
| RAF-C7 | 15.58 | 23.89 | ||||
| RAF-C8 | 19.14 | 21.61 | ||||
| RAF-C9 | 22.73 | 24.11 | ||||
| RAF-C10 | 15.93 | 20.17 | ||||
| RAF-C5 | 19.14 | 21.61 | ||||
| RAF-C6 | 15.65 | 24.37 | ||||
| RAF-C1′ | 19.84 | 26.42 | ||||
| RAF-C6′ | 22.21 | 28.95 | ||||
| RAF-C2′ | 18.17 | 28.09 | ||||
| RAF-C5′ | 18.65 | 24.36 | ||||
In pentylethanoate solvent, that mimics the lipid environment and in which only the H4A+ form is present, the obtained ΔG for the •OOH attack at the different sites had positive values (0.50, 6.43, 0.58 kcal mol−1 for O4′, O6, O7, respectively), indicating that the HAT process can hardly take place.
Concerning the RAF mechanism for the most abundant species H4A+, all •OOH addition channels were found to be endergonic (Table 2), with ΔG values larger than 20 kcal mol−1. Generally, the kinetic calculations for endergonic channels are excluded because, although they might occur at significant rates, the reaction is reversible, and no products are observed. Instead, if the products are able to further react quickly producing a driving force and barriers are small, these processes also need to be considered [44]. For this reason, they were considered in our kinetic computation.
In a water environment, SET reactions for all the investigated species, with the exception of the anionic form, gave also endergonic ΔG (see Table 2). The degree of deprotonation contributed to increasing the thermochemical viability of the SET process, thus pointing out the role of all the existing species at physiological pH in the determination of the antioxidant power of a chemical species. In PE medium, the SET mechanism probably does not contribute to the overall reactivity of higenamine towards •OOH, since such an environment does not provide the necessary solvation of the intermediate ionic species yielded by this mechanism. Accordingly, the calculated ΔG for the SET mechanism were found to be largely endergonic.
The computed rate constants in aqueous solution for all the considered mechanisms are shown in Table 3, which also reports the overall rate coefficient calculated as the sum of the rate constants of each path.
Table 3.
Rate constants (M−1 s−1) and branching ratios (Γ) computed at the M062x level of theory at 298.15 K.
| H4A+ | H3A | H2A− | ||||
|---|---|---|---|---|---|---|
| k | Γ(%) | k | Γ(%) | k | Γ(%) | |
| HAT-O4′ | 1.62 × 101 | 11.02 | 3.11 × 101 | ~0.00 | 6.85 × 101 | ~0.00 |
| HAT-O6 | 1.01 × 102 | 68.67 | ~ | ~ | ~ | ~ |
| HAT-O7 | 2.12 × 101 | 14.41 | 5.03 × 108 | 65.58 | 2.79 × 109 | 29.25 |
| HAT-C1 | ~ | ~ | 1.25 × 105 | 0.02 | ~ | ~ |
| SET | 1.04 × 10−13 | ~0.00 | 2.64 × 108 | 34.40 | 6.75 × 109 | 70.75 |
| RAF-C7 | 1.53 × 10−2 | 0.01 | ||||
| RAF-C8 | 4.07 × 10−3 | ~0.00 | ||||
| RAF-C9 | 9.68 × 10−3 | 0.01 | ||||
| RAF-C10 | 7.85 × 101 | 5.34 | ||||
| RAF-C5 | 7.22 × 10−1 | 0.49 | ||||
| RAF-C6 | 7.15 × 10−3 | ~0.00 | ||||
| RAF-C1′ | 2.07 × 10−4 | ~0.00 | ||||
| RAF-C6′ | 1.46 × 10−5 | ~0.00 | ||||
| RAF-C2′ | 3.43 × 10−6 | ~0.00 | ||||
| RAF-C5′ | 5.69 × 10−2 | 0.04 | ||||
| RAF-C3′ | 7.37 × 10−3 | 0.01 | ||||
| RAF-C4′ | 2.10 × 10−5 | ~0.00 | ||||
| Overall | 1.47 × 102 | 7.67 × 108 | 9.54 × 109 | |||
The rate constants showed that for the H4A+ species, the HAT mechanism was favoured with respect to SET and RAF. In particular, the rate constants for H abstraction from site 3 of catechol were 6.23 and 4.76 times higher than those for site 1 and site 4, respectively. For the H3A species, the rate constant associated with the SET mechanism was feasible, with a value equal to 2.64 x 108 L mol−1 s−1. In general, the results indicated that the rate constant increased in the presence of the anionic form of higenamine.
In order to assert the substantial contribution of each species, the calculated overall rate coefficients were corrected by considering the population of each acid–base form at physiological pH. The derived sum of the corrected-by-fraction total rate coefficients are reported in Table 4.
Table 4.
Molar fractions (𝑓), total rate constants (ktot, M−1 s−1), and corrected-by-fraction total rate coefficients (𝑓ktot, M−1 s−1) at 298.15 K, in aqueous solution at pH 7.4.
| f | ktot | fktot | |
|---|---|---|---|
| H4A+ | 0.861 | 1.47 × 102 | 1.26 × 102 |
| H3A | 0.136 | 7.67 × 108 | 1.04 × 108 |
| H2A− | 0.002 | 9.54 × 109 | 1.91 × 107 |
From Table 4, it is evident that the overall reactivity of higenamine against the •OOH radical is almost entirely due to the presence in aqueous solution of the H3A form. Furthermore, even if the total rate coefficient of the anionic form is higher than those of the other two forms, H2A− is present in very small quantities and cannot be considered the driving force of the antioxidant activity. On the other hand, at pH 7.4, the most abundant species remains H4A+, with a total rate constant of 1.26 × 102 M−1 s−1.
To put into perspective the potential role of higenamine as an antioxidant, the overall rate coefficient of its reaction with •HOO, in aqueous solution at physiological pH, was compared with those obtained for known antioxidants using a similar methodology. According to the estimated data, higenamine (koverall = 1.23 × 108 M−1 s−1, this work) is more efficient for scavenging hydroperoxyl radicals than Trolox (8.96 × 104 M−1 s−1)[47], which is frequently used as a reference antioxidant. It is also more efficient for that purpose than melatonin (2.0 × 101 M−1 s−1) and caffeine (3.3 × 10−1 M−1 s−1) [48,49], whose protective action against oxidative stress is frequently associated with their ROS scavenging activity [50,51,52].
The results underline that the antioxidant power of higenamine is strongly influenced by pH. Higher pH values increase the protective effects against oxidative stress due to the increase of the H3A and H2A− forms. In particular, at more basic pHs, SET and SPLET become the favored mechanisms.
4. Conclusions
A systematic study of the reactivity of higenamine toward •OOH was carried out in aqueous and lipid environments considering hydrogen transfer, single-electron transfer, and radical adduct formation. In aqueous solution, the species coming from acid–base equilibria were taken into account. It was found that the hydrogen atom transfer from the catecholic ring was the main reaction channel for the H4A+ species. In contrast, the H3A and H2A− forms, •OOH scavenging activity took place almost exclusively via SET. Our computed kinetic constants revealed that in water solution it is important to consider all the species derived from acid–base equilibria to obtain reliable results. Comparisons with other species considered good antioxidants revealed that the •OOH scavenging activity of higenamine is higher than that of Trolox. In addition, higenamine resulted to be more efficient than melatonin and caffeine for that purpose.
Acknowledgments
I.R.: A.P., N.R., T.M. gratefully acknowledge the Dipartimento di Chimica e Tecnologie Chimiche dell’Università della Calabria and thank the Ministero degli Affari Esteri e della Cooperazione Internazionale-Italia. A.G. and J.R.A.-I. gratefully acknowledge the Laboratorio de Visualizacion y Computo Paralelo at Universidad Autonoma Metropolitana Iztapalapa.
Author Contributions
T.M. and N.R. conceived the presented idea and supervised the project. I.R. and A.P. performed the computations. I.R. and T.M. verified and analyzed the data. I.R. wrote the manuscript with support from N.R., A.G., T.M. and J.R.A.-I. contributed to the final version of the manuscript, drafted the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Zhang N., Lian Z., Peng X., Li Z., Zhu H. Applications of Higenamine in pharmacology and medicine. J. Ethnopharmacol. 2017;196:242–252. doi: 10.1016/j.jep.2016.12.033. [DOI] [PubMed] [Google Scholar]
- 2.Bai J., Mao J., Yang H., Khan A., Fan A., Liu S., Zhang J., Wang D., Gao H., Zhang J. Sucrose non-ferment 1 related protein kinase 2 (SnRK2) genes could mediate the stress responses in potato (Solanum tuberosum L.) BMC Genet. 2017;18:41. doi: 10.1186/s12863-017-0506-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kosuge T., Yokota M. Studies on cardiac principle of aconite root. Chem. Pharm. Bull. 1976;24:176–178. doi: 10.1248/cpb.24.176. [DOI] [PubMed] [Google Scholar]
- 4.Zhang N., Qu K., Wang M., Yin Q., Wang W., Xue L., Fu H., Zhu H., Li Z. Identification of higenamine as a novel α1 -adrenergic receptor antagonist. Phytother. Res. 2019;33:708–717. doi: 10.1002/ptr.6261. [DOI] [PubMed] [Google Scholar]
- 5.Lee Y.S., Kang Y.J., Kim H.J., Park M.K., Seo H.G., Lee J.H., Yun-Choi H.S., Chang K.C. Higenamine reduces apoptotic cell death by induction of heme oxygenase-1 in rat myocardial ischemia-reperfusion injury. Apoptosis. 2006;11:1091–1100. doi: 10.1007/s10495-006-7110-y. [DOI] [PubMed] [Google Scholar]
- 6.Ramalakshmi K., Kubra I.R., Rao L.J.M. Antioxidant potential of low-grade coffee beans. Food Res. Int. 2008;41:96–103. doi: 10.1016/j.foodres.2007.10.003. [DOI] [Google Scholar]
- 7.Dandlen S.A., Lima A.S., Mendes M.D.D.S., Miguel M.G., Faleiro M., Sousa M.J., Pedro L.G., Barroso J.G., Figueiredo A.C. Antioxidant activity of six Portuguese thyme species essential oils. Flavour Fragr. J. 2010;25:150–155. doi: 10.1002/ffj.1972. [DOI] [Google Scholar]
- 8.Lobo V., Patil A., Phatak A., Chandra N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010;4:118–126. doi: 10.4103/0973-7847.70902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pandey K.B., Rizvi S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxidative Med. Cell. Longev. 2009;2:270–278. doi: 10.4161/oxim.2.5.9498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duthie G.G., Duthie S., Kyle J.A.M. Plant polyphenols in cancer and heart disease: Implications as nutritional antioxidants. Nutr. Res. Rev. 2000;13:79–106. doi: 10.1079/095442200108729016. [DOI] [PubMed] [Google Scholar]
- 11.Betteridge D.J. What is oxidative stress? Metab. Clin. Exp. 2000;49:3–8. doi: 10.1016/S0026-0495(00)80077-3. [DOI] [PubMed] [Google Scholar]
- 12.Pham-Huy L.A., He H., Pham-Huy C. Free Radicals, Antioxidants in Disease and Health. Int. J. Biomed. Sci. 2008;4:89–96. [PMC free article] [PubMed] [Google Scholar]
- 13.Seifried H.E., Anderson D.E., Fisher E.I., Milner J.A. A review of the interaction among dietary antioxidants and reactive oxygen species. J. Nutr. Biochem. 2007;18:567–579. doi: 10.1016/j.jnutbio.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 14.Ichikawa K., Sasada R., Chiba K., Gotoh H. Effect of Side Chain Functional Groups on the DPPH Radical Scavenging Activity of Bisabolane-Type Phenols. Antioxidants. 2019;8:65. doi: 10.3390/antiox8030065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Devasagayam T.P., Tilak J.C., Boloor K.K., Sane K.S., Ghaskadbi S.S., Lele R.D. Free radicals and antioxidants in human health: Current status and future prospects. J. Assoc. Phys. India. 2004;52:4. [PubMed] [Google Scholar]
- 16.Galano A., Alvarez-Idaboy J.R. Computational strategies for predicting free radical scavengers’ protection against oxidative stress: Where are we and what might follow? Int. J. Quantum Chem. 2018;119:e25665. doi: 10.1002/qua.25665. [DOI] [Google Scholar]
- 17.Lee S.E., Hwang H.J., Ha J.-S., Jeong H.-S., Kim J.H. Screening of medicinal plant extracts for antioxidant activity. Life Sci. 2003;73:167–179. doi: 10.1016/S0024-3205(03)00259-5. [DOI] [PubMed] [Google Scholar]
- 18.Li X., Lin J., Chen B., Xie H., Chen D. Antioxidant and Cytoprotective Effects of Kukoamines A and B: Comparison and Positional Isomeric Effect. Molecules. 2018;23:973. doi: 10.3390/molecules23040973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herraiz T., Galisteo J. Tetrahydro-β-carboline Alkaloids Occur in Fruits and Fruit Juices. Activity as Antioxidants and Radical Scavengers. J. Agric. Food Chem. 2003;51:7156–7161. doi: 10.1021/jf030324h. [DOI] [PubMed] [Google Scholar]
- 20.Herraiz T., Galisteo J., Chamorro C. l-Tryptophan Reacts with Naturally Occurring and Food-Occurring Phenolic Aldehydes To Give Phenolic Tetrahydro-β-carboline Alkaloids: Activity as Antioxidants and Free Radical Scavengers. J. Agric. Food Chem. 2003;51:2168–2173. doi: 10.1021/jf0210066. [DOI] [PubMed] [Google Scholar]
- 21.Yang Z., Liu C., Xiang L., Zheng Y. Phenolic alkaloids as a new class of antioxidants inPortulaca oleracea. Phytother. Res. 2009;23:1032–1035. doi: 10.1002/ptr.2742. [DOI] [PubMed] [Google Scholar]
- 22.Xie Y., Li X., Chen J., Deng Y., Lu W., Chen D. pH Effect and Chemical Mechanisms of Antioxidant Higenamine. Molecules. 2018;23:2176. doi: 10.3390/molecules23092176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marino T., Russo N., Galano A. A deeper insight on the radical scavenger activity of two simple coumarins toward OOH radical. Comput. Theor. Chem. 2016;1077:133–138. doi: 10.1016/j.comptc.2015.12.010. [DOI] [Google Scholar]
- 24.Galano A., Mazzone G., Alvarez-Diduk R., Marino T., Alvarez-Idaboy J.R., Russo N. Food Antioxidants: Chemical Insights at the Molecular Level. Annu. Rev. Food Sci. Technol. 2016;7:335–352. doi: 10.1146/annurev-food-041715-033206. [DOI] [PubMed] [Google Scholar]
- 25.Leopoldini M., Russo N., Toscano M. The molecular basis of working mechanism of natural polyphenolic antioxidants. Food Chem. 2011;125:288–306. doi: 10.1016/j.foodchem.2010.08.012. [DOI] [Google Scholar]
- 26.Terpinc P., Abramovič H. A kinetic approach for evaluation of the antioxidant activity of selected phenolic acids. Food Chem. 2010;121:366–371. doi: 10.1016/j.foodchem.2009.12.037. [DOI] [Google Scholar]
- 27.Sies H. Oxidative stress: Oxidants and antioxidants. Exp. Physiol. 1997;82:291–295. doi: 10.1113/expphysiol.1997.sp004024. [DOI] [PubMed] [Google Scholar]
- 28.Frisch M. J., Trucks G. W., Schlegel H. B., Scuseria G. E., Robb M. A., Cheeseman J. R., Scalmani G., Barone V., Petersson G. A., Nakatsuji H., et al. Gaussian 09, revision B. 01, Gaussian, Inc., Wallingford CT, 2009 Search PubMed;(b) NM O’Boyle, AL Tenderholt and KM Langner. J. Comput. Chem. 2008;29:839. [Google Scholar]
- 29.Zhao Y., Truhlar D. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06 functionals and 12 other functionals. Theor. Chem. Acc. 2008;119:525. doi: 10.1007/s00214-007-0401-8. [DOI] [Google Scholar]
- 30.Kumar M., Busch D.H., Subramaniam B., Thompson W.H. Role of Tunable Acid Catalysis in Decomposition of α-Hydroxyalkyl Hydroperoxides and Mechanistic Implications for Tropospheric Chemistry. J. Phys. Chem. A. 2014;118:9701–9711. doi: 10.1021/jp505100x. [DOI] [PubMed] [Google Scholar]
- 31.Alarcón P., Bohn B., Zetzsch C., Rayez M.-T. Reversible addition of the OH radical to p -cymene in the gas phase: Multiple adduct formation. Part 2. Phys. Chem. Chem. Phys. 2014;16:17315–17326. doi: 10.1039/C4CP02073A. [DOI] [PubMed] [Google Scholar]
- 32.Denegri B., Matić M., Kronja O. A DFT-based model for calculating solvolytic reactivity. The nucleofugality of aliphatic carboxylates in terms of N f parameters. Org. Biomol. Chem. 2014;12:5698. doi: 10.1039/C4OB00563E. [DOI] [PubMed] [Google Scholar]
- 33.Parkhomenko D., Edeleva M.V., Kiselev V., Bagryanskaya E.G. pH-Sensitive C–ON Bond Homolysis of Alkoxyamines of Imidazoline Series: A Theoretical Study. J. Phys. Chem. B. 2014;118:5542–5550. doi: 10.1021/jp5024372. [DOI] [PubMed] [Google Scholar]
- 34.Da Silva G. Reaction of Methacrolein with the Hydroxyl Radical in Air: Incorporation of Secondary O2 Addition into the MACR + OH Master Equation. J. Phys. Chem. A. 2012;116:5317–5324. doi: 10.1021/jp303806w. [DOI] [PubMed] [Google Scholar]
- 35.Zhao Y., Truhlar D.G. How Well Can New-Generation Density Functionals Describe the Energetics of Bond-Dissociation Reactions Producing Radicals? J. Phys. Chem. A. 2008;112:1095–1099. doi: 10.1021/jp7109127. [DOI] [PubMed] [Google Scholar]
- 36.Galano A., Alvarez-Idaboy J.R. Kinetics of radical-molecule reactions in aqueous solution: A benchmark study of the performance of density functional methods. J. Comput. Chem. 2014;35:2019–2026. doi: 10.1002/jcc.23715. [DOI] [PubMed] [Google Scholar]
- 37.Marenich A.V., Cramer C.J., Truhlar D. Universal Solvation Model Based on Solute Electron Density and on a Continuum Model of the Solvent Defined by the Bulk Dielectric Constant and Atomic Surface Tensions. J. Phys. Chem. B. 2009;113:6378–6396. doi: 10.1021/jp810292n. [DOI] [PubMed] [Google Scholar]
- 38.Maeda S., Harabuchi Y., Ono Y., Taketsugu T., Morokuma K. Intrinsic reaction coordinate: Calculation, bifurcation, and automated search. Int. J. Quantum Chem. 2014;115:258–269. doi: 10.1002/qua.24757. [DOI] [Google Scholar]
- 39.Galano A., Medina M.E., Tan D.X., Reiter R.J. Melatonin and its metabolites as copper chelating agents and their role in inhibiting oxidative stress: A physicochemical analysis. J. Pineal Res. 2014;58:107–116. doi: 10.1111/jpi.12196. [DOI] [PubMed] [Google Scholar]
- 40.Galano A., Alvarez-Idaboy J.R. A computational methodology for accurate predictions of rate constants in solution: Application to the assessment of primary antioxidant activity. J. Comput. Chem. 2013;34:2430–2445. doi: 10.1002/jcc.23409. [DOI] [PubMed] [Google Scholar]
- 41.Foster J.P., Weinhold F. Natural hybrid orbitals. J. Am. Chem. Soc. 1980;102:7211–7218. doi: 10.1021/ja00544a007. [DOI] [Google Scholar]
- 42.Reed A.E., Weinhold F. Natural bond orbital analysis of near-Hartree–Fock water dimer. J. Chem. Phys. 1983;78:4066–4073. doi: 10.1063/1.445134. [DOI] [Google Scholar]
- 43.Reed A.E., Weinstock R.B., Weinhold F. Natural population analysis. J. Chem. Phys. 1985;83:735–746. doi: 10.1063/1.449486. [DOI] [Google Scholar]
- 44.Galano A., Perez-Gonzalez A., Castañeda-Arriaga R., Muñoz-Rugeles L., Mendoza-Sarmiento G., Romero-Silva A., Ibarra-Escutia A., Rebollar-Zepeda A.M., Leon-Carmona J.R., Hernández-Olivares M.A., et al. Empirically Fitted Parameters for Calculating pKaValues with Small Deviations from Experiments Using a Simple Computational Strategy. J. Chem. Inf. Model. 2016;56:1714–1724. doi: 10.1021/acs.jcim.6b00310. [DOI] [PubMed] [Google Scholar]
- 45.Grunewald G.L., Seim M.R., Lu J., Makboul M., Criscione K.R. Application of the Goldilocks Effect to the Design of Potent and Selective Inhibitors of PhenylethanolamineN-Methyltransferase: Balancing pKaand Steric Effects in the Optimization of 3-Methyl-1,2,3,4-tetrahydroisoquinoline Inhibitors by β-Fluorination1. J. Med. Chem. 2006;49:2939–2952. doi: 10.1021/jm051262k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Castañeda-Arriaga R., Perez-Gonzalez A., Reina M., Alvarez-Idaboy J.R., Galano A. Comprehensive Investigation of the Antioxidant and Pro-oxidant Effects of Phenolic Compounds: A Double-Edged Sword in the Context of Oxidative Stress? J. Phys. Chem. B. 2018;122:6198–6214. doi: 10.1021/acs.jpcb.8b03500. [DOI] [PubMed] [Google Scholar]
- 47.Alberto M.E., Grand A., Russo N., Galano A. A physicochemical examination of the free radical scavenging activity of Trolox: Mechanism, kinetics and influence of the environment. Phys. Chem. Chem. Phys. 2013;15:4642. doi: 10.1039/c3cp43319f. [DOI] [PubMed] [Google Scholar]
- 48.Leon-Carmona J.R., Galano A. Is Caffeine a Good Scavenger of Oxygenated Free Radicals? J. Phys. Chem. B. 2011;115:4538–4546. doi: 10.1021/jp201383y. [DOI] [PubMed] [Google Scholar]
- 49.Galano A. On the direct scavenging activity of melatonin towards hydroxyl and a series of peroxyl radicals. Phys. Chem. Chem. Phys. 2011;13:7178. doi: 10.1039/c0cp02801k. [DOI] [PubMed] [Google Scholar]
- 50.Carrillo-Vico A., Guerrero J., Lardone P.J., Reiter R.J. A Review of the Multiple Actions of Melatonin on the Immune System. Endocrine. 2005;27:189–200. doi: 10.1385/ENDO:27:2:189. [DOI] [PubMed] [Google Scholar]
- 51.Tan D.X., Chen L.D., Poeggeler B., Manchester L.C., Reiter R.J., Poeggler B. Melatonin a potent endogenous hydroxyl radical scavenger. Endocr. J. 1993;1:57–60. [Google Scholar]
- 52.Shi X., Dalal N., Jain A. Antioxidant behaviour of caffeine: Efficient scavenging of hydroxyl radicals. Food Chem. Toxicol. 1991;29:1–6. doi: 10.1016/0278-6915(91)90056-D. [DOI] [PubMed] [Google Scholar]