Abstract
Background
Dexmedetomidine (Dex), a selective a2-adrenergic receptor agonist, has been previously reported to attenuate intrapulmonary shunt during one-lung ventilation (OLV) and to alleviate bronchoconstriction. However, the therapeutic effects of nebulized Dex on pulmonary shunt and lung mechanics during OLV have not been evaluated. Here we determine whether nebulized dexmedetomidine improved pulmonary shunt and lung mechanics in patients undergoing elective thoracic surgery in a prospective randomized controlled clinical trial.
Methods
One hundred and twenty-eight patients undergoing elective thoracoscopic surgery were included in this study and randomly divided into four groups: 0.9% saline (Placebo group), 0.5 µg/kg (Dex0.5 group), 1 µg/kg (Dex1 group) and 2 µg/kg (Dex2group) dexmedetomidine. After bronchial intubation, patients received different nebulized doses of dexmedetomidine (0.5 µg/kg, 1 µg/kg and 2 µg/kg) or 0.9% saline placebo during two-lung ventilation(TLV). OLV was initiated 15 min after bronchial intubation. Anesthesia was maintained with intravenous infusion of cisatracurium and propofol. Bispectral Index values were maintained within 40–50 by adjusting the infusion of propofol in all groups. Arterial blood gas samples and central venous blood gas samples were taken as follows: 15 min after bronchial intubation during two-lung ventilation (TLV15), after 30 and 60 min of OLV (OLV30and OLV60, respectively) and 15 min after reinstitution of TLV (ReTLV). Dynamic compliance was also calculated at TLV15, OLV30, OLV60 and ReTLV.
Results
Dex decreased the requirement of propofol in a dose-dependent manner(P = 0.000). Heart rate (HR) and mean arterial pressure (MAP) displayed no significant difference among groups (P = 0.397 and 0.863). Compared with the placebo group, Dex administered between 0.5 and 2 µg/kg increased partial pressure of oxygen (PaO2) significantly at OLV30 and OLV60(P = 0.000); however, Dex administered between 1 and 2 µg/kg decreased pulmonary shunt fraction (Qs/Qt) at OLV30 and OLV60(P = 0.000). Compared with the placebo group, there were significant increases with dynamic compliance (Cdyn) after OLV in Dex0.5, Dex1 and Dex2group(P = 0.000). Conclusions. Nebulized dexmedetomidine improved oxygenation not only by decreasing pulmonary shunt but also by improving lung compliance during OLV, which may be effective in managing OLV.
Keywords: Nebulizer, Arterial oxygenation, Intrapulmonary shunt, Lung mechanics, One-lung ventilation, Thoracic surgery, Hypoxic pulmonary vasoconstriction, Anesthesia, Dexmedetomidine
Introduction
Thoracic surgical procedure frequently requires one-lung ventilation (OLV) to improve the operational field of vision and access to the operative space. However, OLV is commonly associated with hypoxemia due to intrapulmonary shunt in the nonventilated collapsed lung. Hypoxemia, defined as a drop in arterial hemoglobin oxygen saturation(SaO2), therefore leads to acute hypoxic pulmonary vasoconstriction (HPV) (Cheng et al., 2017). HPV is an important protective mechanism by which blood flow is diverted from the nonventilated lung toward a better-ventilated region, thereby maintaining adequate arterial oxygenation. However, many anesthetics, such as inhalation anesthetics and propofol, have shown positive evidence of inhibiting HPV and increasing hypoxemia (Lumb & Slinger, 2015).
Dexmedetomidine (Dex)is an a2-adrenoreceptor agonist that has found increasing clinical use—for lung protection, for gentle emergence from anesthesia, as an analgesic, as an adjuvant to local anesthetics during regional anesthesia, and even as a supplemental sedative/anxiolytic (Barends et al., 2017; Nguyen et al., 2017). In the last few years, some studies have shown that nebulized Dex administration may allow minimal systemic effects and rapid drug absorption through the respiratory mucosa (Zanaty & El Metainy, 2015; Abdel-Ghaffar et al., 2018). Although nebulized drug administration may be preferred through pulmonary delivery, there are currently no data describing the nebulized effects of Dex on arterial oxygenation during OLV. This study was designed to test the hypothesis that nebulized Dex may improve arterial oxygenation during OLV. Additionally, this study was meant to explore the feasibility of the application of nebulized Dex in OLV during elective thoracic surgery.
Materials & Methods
Study design and participants
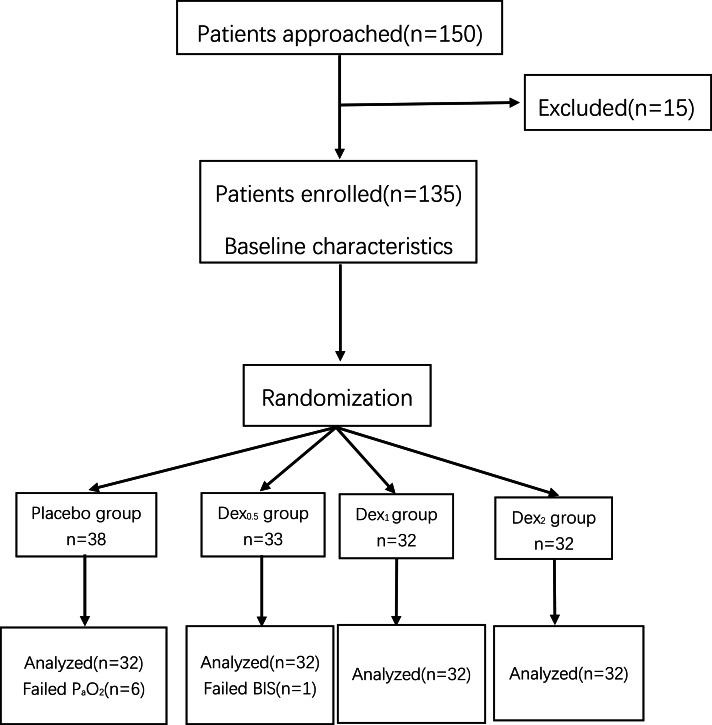
This study was a prospective, randomized controlled clinical trial, performed in Wuxi People’s Hospital in China. These researcher obtained ethical approval for the study protocol from the Medical Ethics Committee of Wuxi People’s Hospital (Ethical Application Ref: KYuKS201816). This study was registered at the Chinese Ethics Committee of Registering Clinical Trials (ChiCTR1800020112). One hundred and fifty patients undergoing elective thoracoscopic surgery were approached and 128 of them completed the study (Fig. 1). Written informed consent was taken from all participants. The inclusion criteria were as follow: an American Society of Anesthesiologists Physical Status rating of I to II ,aged 20–80 years and height 150–180 cm. Patients with the following conditions were excluded from participation: previous allergic reaction to Dex, serious cardiovascular disorders, liver or kidney dysfunction, arrhythmia, hypertensive patients, severe neuropsychiatric disease, long-term alcohol dependence, or other drug addiction. Patients were randomly allocated into four study groups: 0.9% saline (placebo group), 0.5 µg/kg (Dex0.5 group), 1 µg/kg (Dex1 group) or 2 µg/kg (Dex2 group). Randomization was performed by a computer-generated randomization table, with group allocation concealed in sealed opaque envelopes. An anesthesia nurse who was not involved in the research study was tasked with opening the envelopes 1 h prior to induction of anesthesia and preparation of dexmedetomidine (Jiangsu Hengrui Medicine Co., Ltd) or placebo in identical nebulizer with matching randomization codes. Dexmedetomidine (0.5 µg/kg, 1 µg/kg and 2 µg/kg) was diluted with 0.9% saline into 5 ml and an equal volume of 0.9% saline was used as a control in the placebo group. All preoperative and intraoperative management was performed by the same anesthesiologist who was blinded to the study drug. All the data analysis and statistical evaluation was completed by a professional assistant who was blinded to the patient group allocation.
Figure 1. CONSORT flow diagram among four groups.
Placebo group, 0.9% saline. Dex0.5 group, 0.5 µg/kg Dex. Dex1 group, 1 µg/kg Dex. Dex2 group, 2 µg/kg Dex.
Study protocol
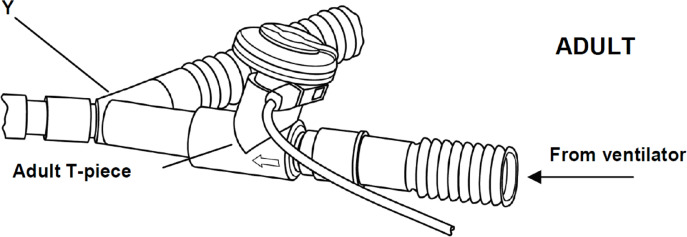
Patients were monitored by standard monitoring devices upon arrival at the operating room. A 22-gauge arterial catheter (Braun Co.) was inserted in the right or left radial artery. A central venous catheter was placed through the right internal jugular vein and therefore the tip would lie near the right atrium. After anesthesia was induced with midazolam(0.05 mg/kg), sufentanil (0.3–0.4 µg/kg), propofol(1.5–2 mg/kg) and cisatracurium (0.15 mg/kg), bronchial intubation was performed with a left-sided double lumen tube (size 37/35 for males and 35/33 for females), then the correct position was confirmed using fiberoptic bronchoscopy. Mechanical ventilation is used in a pressure-controlled mode for protective ventilation during the study. The ventilator parameters were as follow: inspiratory pressure (Pinsp ) 20 cm H2O, respiratory quotient(I:E) 1:2, oxygen concentration (FO2) 100% and respiratory rate adjusted to maintain end-tidal carbon dioxide partial pressure (PEtCO 2) between 30 and 35 mmHg. 5 ml of the study drug was immediately administered via the ventilator circuit (Fig. 2) over 10 min after bronchial intubation during two-lung ventilation. OLV was initiated 15 min after bronchial intubation. During the study, anesthesia was maintained within a bispectral index (BIS) range of 40 to 50 using continuously infused propofol and intermittent administration of cisatracurium. Positive end expiratory pressure (PEEP) to the ventilated lung during OLV was applied in patients who failed to maintain adequate oxygenation (SpO2 >92%) . Patients requiring PEEP or other recruitment maneuvers for oxygenation were excluded from final analysis. Atropine was administered if heart rate(HR) was less than 50, and ephedrine was required to maintain hemodynamic stability if mean arterial pressure (MAP) had more than 20% decrease from baseline.
Figure 2. The ventilator circuit.
The vibrating mesh nebulizer (Aeroneb Solo, Aerogen, Galway, Ireland) was connected to the circuit with adult T-piece. The devices were placed in the inspiratory limb before the Y-piece.
Outcome Measures
Arterial and central venous samples were obtained for blood gas analysis at four time points: 15 min after bronchial intubation during two-lung ventilation (TLV15), 30 min and 60min after OLV (OLV30 and OLV60) and 15 min after reinstitution of TLV (ReTLV). Pulmonary shunt fraction (Qs/Qt) was calculated using the following formula:
Qs/Q t= (CcO2 − CaO2)/(CcO2 −CvO2) ×100%.
Whereby CaO2 (oxygen content of arterial blood) = (PaO2 ×0.0031) + (Hb ×1.34 ×SaO2).
CvO2 (oxygen content of venous blood) = (PvO2 ×0.0031) +(Hb ×1.34 ×SvO2).
CcO2= ([FiO2 × (PB − pH2O) − (PaCO2/RQ)] ×0.0031) + (Hb ×1.34).
PB –Barometric pressure (760 mmHg), pH2O–47 mmHg,
Hb –Hemoglobin, RQ –Respiratory quotient (0.8).
Dynamic compliance(Cdyn) was obtained from the Primus ventilator at TLV15, OLV30, OLV60, and ReTLV. HR and MAP were recorded at these different times.
Statistical analysis
Sample size calculation was based on the difference of PaO2 by 40 mmHg between groups with a power of 90% and two-sided a of 0.05 by repeated measures analysis of variance (Lee et al., 2016). The enumeration data (gender, ASA and operative site) were measured by χ2 test. Data with normal distributions were presented as mean ±standard deviation. Intergroup comparisons were determined by LSD test. Variables with repeated measures such as PaO2, Cdyn, Qs/Q t, HR, MAP and BIS were analyzed using repeated measures analysis of variance. All statistical analyses were performed with SPSS version 23.0(IBM Inc., Armonk, NY, USA) and P < 0.05 was considered statistically significant.
Results
Here we enrolled and completed studies of total 128 patients during this study. The CONSORT flow diagram is shown in Fig. 1.
Demographic and perioperative data are detailed in Table 1. Overall, there were no differences among groups regarding age (P = 0.960), ASA(P = 0.982), gender(P = 0.919), weight(P = 0.204), height(P = 0.259), operative site(P = 0.988), operational time(P = 0.648), crystalloid(P = 0.611), colloid(P = 0.675), urine(P = 0.291), PH(P = 0.978), PaO2 (P = 0.547), PaCO2(P = 0.791), Hb(P = 0.934), FEV1(P = 0.705), FVC(P = 0.417) and FEV1∕FVC(P = 0.809).
Table 1. Demographic and perioperative data (N = 32).
All data are expressed as means ± SD.
| Parameters | Groups | Significance(P) | |||
|---|---|---|---|---|---|
| Placebo | Dex0.5 | Dex1 | Dex2 | ||
| Age(year) | 55.5 ± 11.9 | 55.6 ± 13.0 | 56.9 ± 8.9 | 56.3 ± 12.3 | 0.960 |
| ASA I/II(n) | 27/5 | 26/6 | 27/5 | 27/5 | 0.982 |
| Gender(male/female) | 15/17 | 17/15 | 17/15 | 15/17 | 0.919 |
| Weight(kg) | 62.8 ± 11.4 | 65.0 ± 11.5 | 60.1 ± 9.6 | 60.1 ± 10.4 | 0.204 |
| Height(cm) | 164.4 ± 7.6 | 167.3 ± 7.7 | 165.1 ± 5.6 | 164.4 ± 5.2 | 0.259 |
| Operative site(Right/Left) | 14/18 | 13/19 | 14/18 | 13/19 | 0.988 |
| Operational time(min) | 117.9 ± 25.8 | 116.3 ± 25.9 | 112.3 ± 21.8 | 111.8 ± 19.0 | 0.648 |
| Crystalloid(ml) | 571.9 ± 172.7 | 521.9 ± 131.3 | 562.5 ± 164.1 | 553.1 ± 158.6 | 0.611 |
| Colloid(ml) | 440.6 ± 126.6 | 462.5 ± 100.8 | 435.9 ± 133.3 | 425.6 ± 128.4 | 0.675 |
| Urine(ml) | 168.1 ± 38.1 | 184.1 ± 43.5 | 168.1 ± 53.5 | 163.4 ± 45.6 | 0.291 |
| PH | 7.41 ± 0.03 | 7.41 ± 0.02 | 7.40 ± 0.03 | 7.40 ± 0.02 | 0.978 |
| PaO2(mmHg) | 84.3 ± 5.8 | 83.6 ± 6.3 | 82.9 ± 4.8 | 82.3 ± 6.2 | 0.547 |
| PaCO2(mmHg) | 35.3 ± 1.5 | 35.3 ± 1.5 | 35.2 ± 1.4 | 35.0 ± 1.0 | 0.791 |
| Hb(mg l−1) | 117.2 ± 12.0 | 117.2 ± 11.7 | 118.3 ± 12.5 | 118.8 ± 11.9 | 0.934 |
| FEV1(%) | 80.5 ± 10.3 | 82.0 ± 9.5 | 79.2 ± 10.4 | 80.0 ± 8.5 | 0.705 |
| FVC(% ) | 88.2 ± 8.5 | 88.8 ± 7.8 | 86.1 ± 8.9 | 86.2 ± 6.9 | 0.417 |
| FEV1/FVC(%) | 84.3 ± 8.5 | 85.6 ± 8.2 | 83.7 ± 8.7 | 84.0 ± 7.5 | 0.809 |
Notes.
Compared with Placebo group.
P < 0.05.
- ASA
- American society of anesthesiologists physical status
- PH
- Hydrogen ion concentration
- PaO2
- Arterial oxygen partial pressure;
- PaCO2
- Arterial partial pressure of carbon dioxide
- Hb
- Hemoglobin concentrations
- FEV1
- Forced expiratory volume in one second
- FVC
- Forced vital capacity
The values of HR, MAP and BIS weren’t significantly different among groups (P = 0.397, 0.863 and 0.815, respectively). During the transition from TLV15 to OLV, PaO2 and Cdyn decreased and Qs/Qt increased significantly in the four groups (P = 0.000). During OLV, PaO2 and Cdyn had a significant increase in Dex0.5, Dex1 and Dex2 groups compared with the placebo group (P = 0.000) while Qs/Q t decreased in the Dex1 and Dex2 groups compared with the placebo group (P = 0.000).
Patients in the Dex1 and Dex2 group required significantly less propofol during surgery than patients in the placebo group to maintain BIS values between 40 and 50(P = 0.000) (Tables 2 and 3). However, the requirement of sufentanil, cisatracurium, ephedrine and atropine did not differ significantly among groups(P = 0.728, 0.204, 1.0 and 1.0, respectively) (Table 3).
Table 2. Changes of hemodynamics and respiratory mechanics (N = 32).
All data are expressed as means ± SD.
| Parameters | Groups | TLV15min | OLV30min | OLV60min | ReTLV | P value |
|---|---|---|---|---|---|---|
| PaO2(mmHg) | 0.000b | |||||
| Placebo | 431.8 ± 54.3 | 168.6 ± 43.6 | 178.5 ± 41.3 | 402.1 ± 42.1 | 0.000 | |
| Dex0.5 | 435.8 ± 44.1 | 217.9 ± 43.5* | 255.6 ± 47.0* | 418.7 ± 33.2 | 0.000 | |
| Dex1 | 424.5 ± 38.7 | 242.5 ± 60.8* | 282.1 ± 54.6* | 405.8 ± 37.8 | 0.000 | |
| Dex2 | 423.4 ± 53.3 | 262.7 ± 53.6* | 298.6 ± 38.4* | 409.1 ± 51.6 | 0.000 | |
| P value | 0.691 | 0.000 | 0.000 | 0.429 | 0.000a(P = 0.000c) | |
| Cdyn (ml/cmH2O) | 0.000b | |||||
| Placebo | 43.4 ± 7.1 | 21.0 ± 2.8 | 19.7 ± 2.8 | 32.4 ± 2.7 | 0.000 | |
| Dex0.5 | 42.8 ± 6.0 | 26.7 ± 2.4* | 26.2 ± 2.4* | 38.7 ± 2.6* | 0.000 | |
| Dex1 | 42.2 ± 5.3 | 26.4 ± 2.6* | 25.5 ± 2.6* | 37.8 ± 1.8* | 0.000 | |
| Dex2 | 41.5 ± 4.3 | 26.9 ± 3.2* | 26.2 ± 2.9* | 38.5 ± 2.5* | 0.000 | |
| P value | 0.619 | 0.000 | 0.000 | 0.000 | 0.000a(P = 0.000c) | |
| Qs/Qt (%) | 0.000b | |||||
| Placebo | 9.9 ± 2.2 | 30.4 ± 2.3 | 27.5 ± 1.4 | 9.5 ± 0.5 | 0.000 | |
| Dex0.5 | 9.8 ± 1.2 | 30.0 ± 3.0 | 27.2 ± 2.5 | 9.4 ± 0.7 | 0.000 | |
| Dex1 | 9.4 ± 2.0 | 24.6 ± 2.2* | 22.3 ± 3.6* | 9.4 ± 0.6 | 0.000 | |
| Dex2 | 9.7 ± 2.3 | 22.6 ± 2.5* | 21.6 ± 3.1* | 9.8 ± 0.6 | 0.000 | |
| P value | 0.789 | 0.000 | 0.000 | 0.112 | 0.000a(P = 0.000c) | |
| HR (bpm) | 0.000b | |||||
| Placebo | 68.9 ± 9.3 | 74.6 ± 11.4 | 76.0 ± 10.3 | 79.3 ± 10.9 | 0.002 | |
| Dex0.5 | 70.4 ± 11.9 | 73.6 ± 13.2 | 72.8 ± 12.3 | 74.3 ± 13.1 | 0.640 | |
| Dex1 | 72.1 ± 10.3 | 71.6 ± 11.0 | 71.0 ± 11.5 | 74.9 ± 9.7 | 0.478 | |
| Dex2 | 68.3 ± 8.4 | 73.0 ± 10.0 | 70.3 ± 8.0 | 72.6 ± 9.0 | 0.121 | |
| P value | 0.424 | 0.772 | 0.143 | 0.086 | 0.397a(P = 0.064c) | |
| MAP (mmHg) | 0.000b | |||||
| Placebo | 82.0 ± 8.2 | 84.1 ± 9.5 | 83.0 ± 9.2 | 92.0 ± 10.2 | 0.000 | |
| Dex0.5 | 83.3 ± 10.6 | 81.5 ± 9.8 | 84.4 ± 8.7 | 88.3 ± 9.3 | 0.043 | |
| Dex1 | 85.6 ± 18.1 | 84.2 ± 8.5 | 83.6 ± 9.3 | 90.0 ± 9.1 | 0.132 | |
| Dex2 | 84.7 ± 6.8 | 82.2 ± 11.0 | 83.0 ± 9.3 | 90.7 ± 10.8 | 0.002 | |
| P value | 0.633 | 0.611 | 0.914 | 0.502 | 0.863a(P = 0.580c) | |
| BIS | 0.000b | |||||
| Placebo | 45.6 ± 2.9 | 43.5 ± 2.2 | 44.1 ± 2.0 | 47.5 ± 2.0 | 0.000 | |
| Dex0.5 | 45.4 ± 2.4 | 43.2 ± 2.4 | 43.9 ± 2.3 | 47.2 ± 1.3 | 0.000 | |
| Dex1 | 45.6 ± 2.9 | 43.0 ± 2.4 | 44.8 ± 2.0 | 47.6 ± 2.0 | 0.000 | |
| Dex2 | 45.3 ± 2.8 | 42.9 ± 2.2 | 44.9 ± 2.3 | 47.8 ± 2.5 | 0.000 | |
| P value | 0.974 | 0.727 | 0.171 | 0.759 | 0.815a(P = 0.650c) |
Notes.
Compared with Placebo group.
P < 0.05.
P value of main effect(group).
P value of main effect(time).
P value of crossover effect.
Table 3. Amount of anesthetic and hemodynamic agents administrated during OLV (N = 32).
All data are expressed as means ± SD.
| Parameters | Groups | Significance(P) | ||||
|---|---|---|---|---|---|---|
| Placebo | Dex0.5 | Dex1 | Dex2 | |||
| Propofol(mg) | 491.3 ± 25.6 | 494.5 ± 28.3 | 444.1 ± 20.8* | 388.3 ± 23.7* | 0.000 | |
| Sufentanil(µg) | 48.3 ± 6.4 | 47.5 ± 6.3 | 47.0 ± 5.3 | 46.8 ± 5.2 | 0.728 | |
| Cisatracurium(mg) | 24.4 ± 1.7 | 24.8 ± 1.7 | 24.0 ± 1.4 | 24.0 ± 1.6 | 0.204 | |
| Ephedrine(mg) | 0.3 ± 1.3 | 0.3 ± 1.2 | 0.3 ± 1.2 | 0.3 ± 1.2 | 1.0 | |
| Atropine(mg) | 0.0 ± 0.1 | 0.0 ± 0.1 | 0.0 ± 0.1 | 0.0 ± 0.1 | 1.0 | |
Notes.
Compared with Placebo group.
P < 0.05.
Discussion
Intraoperative hypoxemia can lead to long term complications including end organ failure and increased mortality. One two-center study of a large non-cardiac surgical population estimated that one in fifteen patients experienced hypoxemia for at least 2 min and that one in sixty-four patients experienced hypoxemia for a minimum of 5 min (Ehrenfeld et al., 2010). The decreased arterial oxygen tension (PaO2) may be a reliable indicator of abnormal lung function and predict intraoperative hypoxemia during OLV (Karzai & Schwarzkopf, 2009). Anesthetics may negatively impact the hemodynamic changes that influence PaO2. For instance, isoflurane and halothane reduce HPV by up to 50% at 2 MAC and increase the shunt fraction and risk of hypoxia (Saraswat, 2015). Since a majority of intraoperative hypoxemia occurs during OLV, emphasis remains on the discovery and development of appropriate anesthetic agents.
Dex was applied to produce a level of sedation during the perioperative period and in mechanically ventilated patients (Chen & Shen, 2014; Saraswat, 2015). In a recent meta-analysis of 14 randomized controlled trials, Dex was found to increase oxygenation index in seven studies, decrease intrapulmonary shunt in five studies, decrease perioperative HR and MAP in nine studies, and reduce the concentration of the inflammatory factors TNF-α and IL-6 in four studies during OLV (Huang et al., 2017). Intravenous Dex along with isoflurane inhalation was shown to increase PaO2 and significantly decrease Qs/Qt compared to a saline + isoflurane group in a population of adults undergoing elective thoracic surgery (Xia et al., 2013), indicating its safety and feasibility during OLV.
Dex is commonly delivered intravenously but can also be administered as a nebulized premedication in uncooperative children. Inhalation of nebulized Dex is an alternative method of administration, which is related to high bioavailability of Dex (Anttila et al., 2003; McCormick et al., 2008). Nebulized respiratory administration may results in maximizing the surface area of absorption, less drug loss and increased clinical effectiveness (Wolfe & Braude, 2010). In pediatric populations, it has been demonstrated nebulized Dex (as a premedicant) significantly improved cannulating conditions such as parental separation, face mask acceptance and IV placement (Abdel-Ghaffar et al., 2018), with no hemodynamic side-effects (Zanaty & El Metainy, 2015; Abdel-Ghaffar et al., 2018). However, these studies were often applied to pediatric populations.
In the present study, we evaluated whether nebulized Dex could improve oxygenation in adult patients undergoing thoracic surgery. The major finding of this study was that nebulized Dex increased PaO2 during OLV. This is consistent with the results of previous study done by Kernan et al. (2011) and Xia et al. (2013). We surmise this finding may be associated with the fact that intravenous Dex improved oxygenation and lung mechanics during OLV. Additionally, there was no marked systemic hemodynamic change in our study. This may be due to the tiny systemic effect of nebulized Dex. In contrast, Nguyen et al. (2017) found that intravenous Dex can cause hypotension and bradycardia. Thus, this is especially meaningful as nebulized Dex is superior to standard intravenous route.
We also found that 0.5–2 µg/kg nebulized Dex significantly increased dynamic compliance during OLV. These results were in line with intravenous administration in chronic obstructive pulmonary disease (COPD) patients undergoing lung cancer surgery (Lee et al., 2016). Similar results have been reported in a study of morbidly obese patients with restrictive lung disease undergoing bariatric surgery (Hasanin et al., 2018). However, Groeben has been previously reported that inhalation of Dex caused significant bronchoconstriction in an animal study in contrast to intravenous administration (Groeben, Mitzner & Brown, 2004). This initial bronchoconstriction is because of a variety of factors. Firstly, aerosols of water generated ultrasonically to provoke bronchoconstriction in persons without asthma (Gonzalez et al., 1994; Groeben et al., 2000). Secondly, the initial bronchoconstriction may result from release of inflammatory mediators on airway smooth muscle or activating reflexes mediated via afferent fibers of the vagus nerve (Gonzalez et al., 1994; Bulut, Hirshman & Brown, 1996). Nevertheless, subsequent bronchodilation can be demonstrated after airway was irritated by aerosolized drugs. Thus, it should be likely that Dex may directly improve oxygenation by stimulating bronchodilation to increase dynamic compliance during OLV, though this remains to be substantiated in future studies.
Another finding of this study was that the utilization of nebulized Dex significantly reduced the requirements of anesthetic, specifically propofol. This phenomenon has been previously characterized in intravenous Dex, which decreased the need of isoflurane (Xia et al., 2013) and propofol (Sen et al., 2013) during elective spinal surgery. Although bradycardia and hypotension have been reported in the adult population, patients receiving nebulized Dex did not require more atrophine and ephedrine to maintain adequate hemodynamic stability. Thus it’s likely that nebulized Dex may reduce haemodynamic changes related to propofol (Sherman & Barrick, 2019).
Interestingly, 1–2 µg/kg Dex reduced Qs/Qt and improved oxygenation during OLV. This may be due to a variety of factors. Firstly, the attenuation of local inflammation factors contributing to the hypoxic vasodilator effect of OLV may have been influenced by Dex as has been characterized to diminish the production of these pro-inflammatory factors (Zhang et al., 2018; Meng et al., 2018). Secondly, Dex may have played a direct role in pulmonary artery mechanics, promoting the occurrence of HPV by directly impacting bronchodilation (Lee et al., 2016). Finally, reduction in the requirement of propofol, which has been shown to attenuate HPV in a dose-dependent manner, may have contributed to decreased pulmonary shunt and improved HPV (Huang et al., 2017). All of the above mechanisms are based solely on pathophysiologic speculations and remain to be further ascertained.
Despite the novel findings of the present study, there are several limitations to consider. Firstly, this study used central venous samples instead of mixed venous samples as pulmonary catheter placement isn’t routine in thoracotomy cases at the participating hospital. However, this technique has been validated and used in many earlier studies (Turnaoglu et al., 2001; Ozcan et al., 2007). Secondly, both left and right thoracotomies and multiple lung pathologies were included in the study. In the future, designing a study aimed at patients with shared trauma and sided thoracotomy may bring to light more accurate results. Thirdly, studies with larger sample sizes are warranted to analyze the safety of nebulized Dex.
Conclusion
Nebulized dexmedetomidine improved oxygenation not only by reducing intrapulmonary shunt but also by increasing lung compliance during OLV. This strategy simultaneously decreased the requirement of propofol without hemodynamic instability. Thus, this study demonstrated that nebulized dexmedetomidine can be used as a feasible strategy for improving oxygenation during OLV in patients undergoing thoracotomy. Nonetheless, studies with larger sample sizes are necessary to clarify the effects and the safety of nebulized Dex used.
Supplemental Information
Demographic and perioperative data; changes of hemodynamics and respiratory mechanics; amount of anesthetic and hemodynamic agents administrated during OLV.
Acknowledgments
The authors would like to thank the nurse anesthetists in the operating room of the Wuxi People’s Hospital, Jiangsu, Republic of China, for their involvement and support.
Funding Statement
This work was supported by the Science and Technology Project Wuxi City (NO.WX18IVKN016). There was no additional external funding received for this study. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Contributor Information
Chunxiao Hu, Email: huchunxiao91211@163.com.
Jianping Yang, Email: szjpyang@126.com.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Bo Xu conceived and designed the experiments, performed the experiments, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft.
Hong Gao performed the experiments, prepared figures and/or tables, and approved the final draft.
Dan Li analyzed the data, prepared figures and/or tables, and approved the final draft.
Chunxiao Hu and Jianping Yang conceived and designed the experiments, authored or reviewed drafts of the paper, and approved the final draft.
Human Ethics
The following information was supplied relating to ethical approvals (i.e., approving body and any reference numbers):
The medical ethics committee of Wuxi People’s Hospital granted ethical approval to carry out the study within its facilities (Ethical Application Ref: KYuKS201816).
Data Availability
The following information was supplied regarding data availability:
The raw data are available in Dataset S1.
Clinical Trial Registration
The following information was supplied regarding Clinical Trial registration:
Chinese Clinical Trial Registry:ChiCTR1800020112.
References
- Abdel-Ghaffar et al. (2018).Abdel-Ghaffar HS, Kamal SM, Sherif FAEl, Mohamed SA. Comparison of nebulised dexmedetomidine, ketamine, or midazolam for premedication in preschool children undergoing bone marrow biopsy. British Journal of Anaesthesia. 2018;121(2):445–452. doi: 10.1016/j.bja.2018.03.039. [DOI] [PubMed] [Google Scholar]
- Anttila et al. (2003).Anttila M, Penttilä J, Helminen A, Vuorilehto L, Scheinin H. Bioavailability of dexmedetomidine after extravascular doses in healthy subjects. British Journal of Clinical Pharmacology. 2003;56(6):691–693. doi: 10.1046/j.1365-2125.2003.01944.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barends et al. (2017).Barends CR, Absalom A, Minnen Bvan, Vissink A, Visser A. Dexmedetomidine versus midazolam in procedural sedation. A systematic review of efficacy and safety. PLOS ONE. 2017;12(1):e0169525. doi: 10.1371/journal.pone.0169525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulut, Hirshman & Brown (1996).Bulut Y, Hirshman CA, Brown RH. Prevention of lidocaine aerosol-induced bronchoconstriction with intravenous lidocaine. Anesthesiology. 1996;85(4):853–859. doi: 10.1097/00000542-199610000-00021. [DOI] [PubMed] [Google Scholar]
- Chen & Shen (2014).Chen K, Shen X. Dexmedetomidine and Propofol total intravenous anesthesia for airway foreign body removal. Irish Journal of Medical Science. 2014;183(3):481–484. doi: 10.1007/s11845-014-1105-4. [DOI] [PubMed] [Google Scholar]
- Cheng et al. (2017).Cheng HY, Croft QPP, Frise MC, Talbot NP, Petousi N, Robbins PA, Dorrington KL. Human hypoxic pulmonary vasoconstriction is unaltered by 8 h of preceding isocapnic hyperoxia. Physiological Reports. 2017;5(17):e13396. doi: 10.14814/phy2.13396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenfeld et al. (2010).Ehrenfeld JM, Funk LM, Schalkwyk JVan, Merry AF, Sandberg WS, Gawande A. The incidence of hypoxemia during surgery: evidence from two institutions. Canadian Journal of Anaesthesia. 2010;57:888–897. doi: 10.1007/s12630-010-9366-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez et al. (1994).Gonzalez RM, Bjerke RJ, Drobycki T, Stapelfeldt WT, Green JM, Janowitz MJ, Clark M. Prevention of endotracheal tube-induced coughing during emergence from general anesthesia. Anesthesia and Analgesia. 1994;79(4):792–795. doi: 10.1213/00000539-199410000-00030. [DOI] [PubMed] [Google Scholar]
- Groeben et al. (2000).Groeben H, Großwendt T, Silvanus MT, Beste M, Peters J. Lidocaine inhalation for local anesthesia and attenuation of bronchial hyperreactivity with least airway irritation: effect of three different dose regimens. European Journal of Anaesthesiology. 2000;17(11):672–679. doi: 10.1046/j.1365-2346.2000.00745.x. [DOI] [PubMed] [Google Scholar]
- Groeben, Mitzner & Brown (2004).Groeben H, Mitzner W, Brown RH. Effects of the a2-adrenoceptor agonist dexmedetomidine on bronchoconstriction in dogs. Anesthesiology. 2004;100(2):359–363. doi: 10.1097/00000542-200402000-00026. [DOI] [PubMed] [Google Scholar]
- Hasanin et al. (2018).Hasanin A, Taha K, Abdelhamid B, Abougabal A, Elsayad M, Refaie A, Amin S, Wahba S, Omar H, Kamel MM, Abdelwahab Y, Amin SM. Evaluation of the effects of dexmedetomidine infusion on oxygenation and lung mechanics in morbidly obese patients with restrictive lung disease. BMC Anesthesiology. 2018;18(1):104. doi: 10.1186/s12871-018-0572-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang et al. (2017).Huang SQ, Zhang J, Zhang XX, Liu L, Yu Y, Kang XH, Wu XM, Zhu SM. Can dexmedetomidine improve arterial oxygenation and intrapulmonary shunt during one-lung ventilation in adults undergoing thoracic surgery? a meta-analysis of randomized, placebo-controlled trials. Chinese Medical Journal. 2017;30(14):1707–1714. doi: 10.4103/0019-5049.165850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karzai & Schwarzkopf (2009).Karzai W, Schwarzkopf K. Hypoxemia during one-lung ventilation: prediction, prevention, and treatment. Anesthesiology. 2009;110:1402–1411. doi: 10.1097/ALN.0b013e31819fb15d. [DOI] [PubMed] [Google Scholar]
- Kernan et al. (2011).Kernan S, Rehman S, Meyer T, Bourbeau J, Caron N, Tobias JD. Effects of dexmedetomidine on oxygenation during one-lung ventilation for thoracic surgery in adults. Journal of Minimal Access Surgery. 2011;7(4):227–231. doi: 10.4103/0972-9941.85645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee et al. (2016).Lee SH, Kim N, Lee CY, Ban MG, Oh YJ. Effects of dexmedetomidine on oxygenation and lung mechanics in patients with moderate chronic obstructive pulmonary disease undergoing lung cancer surgery. European Journal of Anaesthesiology. 2016;33(4):275–282. doi: 10.1097/EJA.0000000000000405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumb & Slinger (2015).Lumb AB, Slinger xx. Hypoxic pulmonary vasoconstriction: physiology and anesthetic implications. Anesthesiology. 2015;122(4):932–946. doi: 10.1097/ALN.0000000000000569. [DOI] [PubMed] [Google Scholar]
- McCormick et al. (2008).McCormick ASM, Thomas VL, Berry D, Thomas PW. Plasma concentrations and sedation scores after nebulized and intranasal midazolam in healthy volunteers. British Journal of Anaesthesia. 2008;100(5):631–636. doi: 10.1093/bja/aen072. [DOI] [PubMed] [Google Scholar]
- Meng et al. (2018).Meng L, Li L, Lu S, Li K, Su Z, Wang Y, Fan X, Li X, Zhao G. The protective effect of dexmedetomidine on LPS induced acute lung injury through the HMGB1-mediated TLR4/NF-κB and PI3K/Akt/mTOR pathways. Molecular Immunology. 2018;94:7–17. doi: 10.1016/j.molimm.2017.12.008. [DOI] [PubMed] [Google Scholar]
- Nguyen et al. (2017).Nguyen V, Tiemann D, Park E, Salehi A. Alpha-2 Agonists. Anesthesiology Clinics. 2017;35(2):233–245. doi: 10.1016/j.anclin.2017.01.009. [DOI] [PubMed] [Google Scholar]
- Ozcan et al. (2007).Ozcan PE, Sentürk M, Sungur Ulke Z, Toker A, Dilege S, Ozden E, Camci E. Effects of thoracic epidural anaesthesia on pulmonary venous admixture and oxygenation during one-lung ventilation. Acta Anaesthesiologica Scandinavica. 2007;51(8):1117–1122. doi: 10.1111/j.1399-6576.2007.01374.x. [DOI] [PubMed] [Google Scholar]
- Saraswat (2015).Saraswat V. Effects of anaesthesia techniques and drugs on pulmonary function. Indian Journal of Anaesthesia. 2015;59(9):557–564. doi: 10.4103/0019-5049.165850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen et al. (2013).Sen S, Chakraborty J, Santra S, Mukherjee P, Das B. The effect of dexmedetomidine infusion on propofol requirement for maintenance of optimum depth of anaesthesia during elective spine surgery. Indian Journal of Anaesthesia. 2013;57(4):358–363. doi: 10.4103/0019-5049.118558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman & Barrick (2019).Sherman JD, Barrick B. Total intravenous anesthetic versus inhaled anesthetic: pick your poison. Anesthesia and Analgesia. 2019;128(1):13–15. doi: 10.1213/ANE.0000000000003898. [DOI] [PubMed] [Google Scholar]
- Turnaoglu et al. (2001).Turnaoglu S, Tugrul M, Camci E, Cakar N, Akinci O, Ergin xx. Clinical applicability of the substitution of mixed venous oxygen saturation with central venous oxygen saturation. Journal of Cardiothoracic and Vascular Anesthesia. 2001;15(5):574–579. doi: 10.1053/jcan.2001.26534. [DOI] [PubMed] [Google Scholar]
- Wolfe & Braude (2010).Wolfe TR, Braude DA. Intranasal medication delivery for children: a brief review and update. Pediatrics. 2010;126(3):532–537. doi: 10.1542/peds.2010-0616. [DOI] [PubMed] [Google Scholar]
- Xia et al. (2013).Xia R, Yin H, Xia ZY, Mao QJ, Chen GD, Xu W. Effect of intravenous infusion of dexmedetomidine combined with inhalation of isoflurane on arterial oxygenation and intrapulmonary shunt during single-lung ventilation. Cell Biochemistry and Biophysics. 2013;67(3):1547–1550. doi: 10.1007/s12013-013-9659-8. [DOI] [PubMed] [Google Scholar]
- Zanaty & El Metainy (2015).Zanaty OM, El Metainy SA. A comparative evaluation of nebulized dexmedetomidine, nebulized ketamine, and their combination as premedication for outpatient pediatric dental surgery. Anesthesia and Analgesia. 2015;121(1):167–171. doi: 10.1213/ANE.0000000000000728. [DOI] [PubMed] [Google Scholar]
- Zhang et al. (2018).Zhang W, Zhang S, Li B, Sun M, Zhang J. Paravertebral dexmedetomidine as an adjuvant to ropivacaine protects against independent lung injury during one-lung ventilation: a preliminary randomized clinical trial. BMC Anesthesiology. 2018;18(1):67. doi: 10.1186/s12871-018-0532-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Demographic and perioperative data; changes of hemodynamics and respiratory mechanics; amount of anesthetic and hemodynamic agents administrated during OLV.
Data Availability Statement
The following information was supplied regarding data availability:
The raw data are available in Dataset S1.