Summary
Introduction
The incidence of chronic kidney disease (CKD) has increased in recent years. CKD is associated with obesity, type 2 diabetes, and cardiovascular disease, although the mechanism remains unclear. Elevated soluble form of the receptor for advanced glycation end products ( RAGE) is related to proinflammatory signaling pathways that may promote diabetic nephropathy and vascular dysfunction. Because lifestyle modification reduces systematic inflammation in adults with obesity and hyperglycaemia, the hypothesis that exercise plus caloric restriction would lower soluble RAGE in adults with CKD was tested in this study.
Methods
Eight adults (n = 6 females; age: 56.3 ± 2.8 y; BMI: 43.7 ± 2.2 kg/m2; 2‐h OGTT glucose: 215 ± 9.8 mg/dL; eGFR: 49.6 ± 3.3 mL/min/1.73 m2) were enrolled in a 12‐week pilot lifestyle intervention (supervised aerobic exercise [5 d/wk, up to 60 min/d at approximately 65%‐85% HRmax] plus low‐fat dietary counseling). Body composition (DXA), aerobic fitness (VO2max), insulin sensitivity (120 min 75 g OGTT; Matsuda Index), plasma levels of soluble RAGE and fetuin‐A were measured before and after the intervention.
Results
Exercise reduced body weight, fasting glucose, and fetuin‐A as well as increased VO2max, glucose tolerance, and insulin sensitivity (all P < .05). Lifestyle intervention decreased plasma soluble RAGE (pre: 1018.1 ± 163 vs post: 810.6 ± 119.6 ng/mL; P = .02), and the decrease was associated with a lower 2‐hour blood glucose (r = 0.76, P = .03) and with increased insulin sensitivity (r = −0.90, P < .01).
Conclusions
Exercise and caloric restriction are effective at lowering soluble RAGE in relation to glucose regulation in patients with CKD.
Keywords: cardiometabolic risk, insulin sensitivity, type 2 diabetes
1. INTRODUCTION
Nearly 14% of the US population has chronic kidney disease (CKD).1 The presence of CKD is clinically concerning since it increases the risk of cardiovascular disease three‐fold over 5 years compared with people with normal kidney function.2 Abdominal obesity in particular has been implicated in not only a two‐fold risk of developing CKD but also the associated obesity co‐morbidities hypertension, glucose intolerance, and insulin resistance.3 Indeed, insulin resistance is tightly associated with inflammation,4 and there is growing evidence that advanced glycation end products (AGEs), and their receptors are increased during conditions of inflammation.5 In fact, receptor for AGEs (RAGE) activates cellular signalling pathways, such as nuclear factor‐κB, thereby resulting in disturbances in oxidative stress–mediated vascular dysfunction.6 As such, RAGE is a novel member of a group of immunoglobins that are implicated in the development of atherosclerosis, metabolic syndrome, and type 2 diabetes.7
Interestingly, mice that genetically overexpress RAGE have increased diabetic nephropathy compared with RAGE null mice who do not develop kidney dysfunction.8 However, the soluble form of RAGE has been suggested to prevent the development and progression of atherosclerosis in diabetic apo‐E‐null mice by acting as a decoy for AGEs.9 To date, controversy exists surrounding soluble RAGE, with several studies reporting that it is elevated in adults with type 2 diabetes10, 11 and impaired renal function12 but lower in adults with essential hypertension and coronary heart disease.12, 13 Therefore, determining the effect of treatments on soluble RAGE is paramount to understanding cardiometabolic risk reduction mechanisms and relevance in people with CKD. Exercise and weight loss interventions reduce cardiometabolic risk by, at least partially, lowering inflammation (eg, fetuin‐A and leptin) related to insulin resistance in adults with obesity.14, 15, 16 As a result, exercise and diet are recommended as a first‐line therapy in weight management and glycemic control. Exercise with weight loss generally improves endothelial function and glucose regulation via reductions in inflammation in adults with CKD,17, 18 although not all studies agree.19 Nevertheless, based on the established health benefits of weight loss, it would seem that soluble RAGE should decrease after interventions aimed at improving insulin sensitivity for glycaemic control in adults with CKD.17 However, data on the effect of exercise and/or weight loss on soluble RAGE are equivocal, with some showing reductions,20 increases,21 or no change.22, 23 These studies are limited though to patients with obesity, who have or do not have type 2 diabetes, and there are no data determining the effect of an exercise plus diet intervention programme on soluble RAGE in adults with CKD. To address this gap in the literature, this study tested the hypothesis that lifestyle‐induced weight loss would decrease plasma soluble RAGE in conjunction with improved glucose regulation.
2. METHODS
2.1. Participants
Eight adults (56.3 ± 2.8 y, BMI: 43.7 ± 2.1 kg/m2, Table 1) with CKD (eGFR: 49.6 ± 3.3 mL/min/1.73 m2) volunteered for this study after being identified using electronic medical records. Although some cardiometabolic data were previously reported,17 the data are provided here for convenience. All participants were nonsmoking, weight stable (<2 kg in the previous 6 months), sedentary (less than <60 min/wk), and had blood pressure < 180/110 mmHg, HbA1c < 8%, and an eGFR < 60 mL/min/1.73 m2. Participants were verbally briefed about the study and signed written informed consent documents approved by the Cleveland Clinic Institutional Review Board.
Table 1.
Cardiometabolic risk factors before and after a 12‐week lifestyle intervention
| Pre | Post | P Value | |
|---|---|---|---|
| Population (n, M/F) | 8 (2 M, 6F) | ‐ | ‐ |
| Race (W/B) | 3/5 | ‐ | ‐ |
| Age (y) | 56.3 ± 2.8 | ‐ | ‐ |
| Body composition | |||
| Weight (kg) | 122.6 ± 7.0 | 117.3 ± 6.3 | .001 |
| Body mass index (kg/m2) | 43.7 ± 2.1 | 41.8 ± 1.9 | <.001 |
| Body fat (%) | 48.5 ± 3.2 | 46.2 ± 3.1 | .001 |
| Fat‐free mass (kg) | 63.2 ± 3.9 | 63.4 ± 4.1 | .74 |
| Waist circumference (cm) | 127.3 ± 4.9 | 123.2 ± 4.7 | .02 |
| Cardiovascular fitness | |||
| Resting heart rate (bpm) | 82.5 ± 1.6 | 79.0 ± 2.9 | .30 |
| HR max (bpm) | 155.6 ± 5.8 | 155.5 ± 6.5 | .60 |
| VO2max (L/min) | 2.4 ± 0.2 | 2.7 ± 0.2 | <.001 |
| VO2max (mL/kg/min) | 20.2 ± 1.6 | 23.5 ± 1.8 | <.001 |
| Systolic blood pressure (mmHg) | 137.6 ± 9.1 | 140.9 ± 7.8 | .92 |
| Diastolic blood pressure (mmHg) | 81.6 ± 2.9 | 82.0 ± 2.6 | .94 |
| Glucose metabolism | |||
| Fasting PG (mg/dL) | 111.2 ± 7.2 | 91.7 ± 2.0 | .03 |
| Fasting PI (μU/mL) | 36.0 ± 9.2 | 41.1 ± 16.9 | .68 |
| 2‐h PG (mg/dL) | 211.9 ± 31.0 | 178.7 ± 7.2 | .06 |
| 2‐h PI (μU/mL) | 150.2 ± 29.2 | 110.8 ± 23.3 | .07 |
| Early‐phase PG AUC (mg/dL* 30 min) | 4275.9 ± 285.7 | 3635.4 ± 188.9 | .10 |
| Early‐phase PI AUC (μU/mL* 30 min) | 2366.2 ± 413.2 | 2382.0 ± 561.1 | .97 |
| Total‐phase PG AUC (mg/dL* 120 min) | 22 725.9 ± 2313.6 | 19 077.9 ± 1584.2 | .05 |
| Total‐phase PI AUC (μU/mL* 120 min) | 14 336.6 ± 2731.2 | 12 481.8 ± 2513.7 | .16 |
| Insulin sensitivity (au) | 1.9 ± 0.5 | 2.5 ± 0.5 | .02 |
Note. Data reported as mean ± SEM. Insulin sensitivity was estimated via the Matsuda Index.
Abbreviations: AUC = total area under the curve; PG = plasma glucose; PI = plasma insulin; WC = waist circumference.
2.2. Exercise training
Participants were screened with a resting ECG and an incremental maximally graded exercise stress test before participation in the intervention to ensure cardiac function and safety. Participants underwent a supervised progressive treadmill‐walking exercise intervention 5 d/wk for 30 to 60 min/d at approximately 65% to 85% of heart rate max (HRmax) for 12 weeks. Participants progressed from 30 to 40 min/d during weeks 1 to 4, 40 to 50 min/d during weeks 5 to 8, and 50 to 60 min/d thereafter, all at 85% HRmax. Exercise was performed in the Exercise Physiology Laboratory located within the CRU, and sessions were supervised by an exercise physiologist and/or nurse. Exercise sessions commenced and ended with an approximate 5‐minute warm‐up and cool‐down on a cycle ergometer. The target exercise intensity was managed using HR monitors (Polar Electro, Inc, Woodbury, New York). Postintervention outcome measures were obtained the following morning approximately 16 to 18 hours after the last exercise session.
2.3. Dietary intervention
At baseline, resting metabolic rate (RMR) was examined after participants rested in the supine position for 30 minutes following an overnight fast. Expired air (ie, VO2 and VCO2) was collected using a ventilated hood and indirect calorimetry (Vmax Encore, Viasys, Yorba Linda, California). RMR was multiplied by an activity factor of 1.2 to estimate total daily energy requirements. The registered dietitian developed individualized 500‐kcal hypocaloric diets with 50% to 60% carbohydrate, 25% to 30% fat, and 15% (ie, 0.8‐1.0 g/kg/day) protein for each patient throughout the intervention. Compliance with the diet was monitored weekly by verification of journaling by the participants during visits to the Clinical Research Unit (CRU) for the exercise programme.
2.4. Body composition and aerobic fitness
After an overnight fast, weight was recorded on a digital platform scale in a hospital gown, and height was measured without shoes using a wall‐mounted stadiometer. Dual X‐ray absorptiometry (DXA; Lunar Prodigy, Madison, Wisconsin) was used to quantify total fat mass, and waist circumference was measured with a plastic tape measure 2 cm above the umbilicus. Maximum oxygen consumption (VO2max) was determined using a continuous incremental treadmill exercise test, (Jaeger Oxygcon Pro; Viasys, Yorba Linda, California). The HRmax obtained during this test was used during exercise training.
2.5. Cardiometabolic risk
Resting HR as well as systolic and diastolic blood pressure was recorded in the seated position after approximately 10 minutes of rest. Fasting glucose, insulin, fetuin‐A, and soluble RAGE were obtained from an antecubital vein. A standard 75‐g oral glucose tolerance test (OGTT) was performed to measure postprandial glucose and insulin as previously described.24 Blood samples were obtained every 30 minutes up to 120 minutes. Total area under the curve (AUC) was calculated using the trapezoidal model. Insulin sensitivity was estimated using the Matsuda Index.25
2.6. Biochemical analysis
Plasma glucose was determined by a glucose oxidase assay (YSI 2300 STAT Plus, Yellow Springs, Ohio). Blood samples were centrifuged at 4°C for 10 minutes at 1000 rpm and then stored at −80°C until subsequent analysis. Plasma soluble RAGE and fetuin‐A were measured using a Human‐specific enzyme‐linked immunosorbent assay (Roche Modular Diagnostics, Indianapolis, Indiana and Quantikine R & D Systems, Minneapolis, Minnesota, respectively). Plasma insulin was measured using a radioimmunoassay (Millipore, Billerica, Massachusetts).
2.7. Statistical analysis
Pre‐group and post‐group means were compared using the statistical programme R (Mavericks build, R Foundation, Vienna, Austria, 2014). Non‐normally distributed data were log transformed for statistical analysis. All outcomes were assessed using a paired, two‐tailed t test. Spearman‐ranked correlation analysis was used to determine associations between variables, and significance was accepted as P < .05. Data are expressed as mean ± standard error of the mean.
3. RESULTS
3.1. Fitness and body composition
Exercise and diet significantly reduced body weight by −4.1 ± 0.6% (P < .001), and this effect was predominately due to a decrease in fat mass (−4.6 ± 0.7%, P < .001) since fat‐free mass was unchanged at the end of the intervention (0.2 ± 1.0%, P = .74; Table 1). Exercise training increased VO2max (mL/kg/min) by approximately 16.9 ± 2.9% (P < .001).
3.2. Cardiometabolic risk factors
The intervention had no significant effect on lowering resting HR or blood pressure (Table 1). However, exercise plus diet significantly lowered fetuin‐A (pre = 0.92 ± 0.15 vs post = 0.77 ± 0.12 ng/mL, P = .02) as well as fasting glucose and improved glucose tolerance as reflected by total phase AUC calculations (Table 1). Exercise training with weight loss increased insulin sensitivity by 29.2 ± 16.0% (P < .02; Table 1).
3.3. Soluble RAGE and correlations
Exercise training with weight loss significantly decreased plasma soluble RAGE (pre = 1018.1 ± 163.0 vs post = 810.6 ± 119.6 ng/mL, P = .02; Figure 1). These lower soluble RAGE levels were associated with reductions in 2‐hour blood glucose (r = 0.76, P = .03, Figure 2A) and improved insulin sensitivity (r = −0.90, P < .01, Figure 2B). The correlation between changes in body weight (r = −0.57, P = .15), VO2max (r = −0.25, P = .59), and iGRF (r = 0.02, P = .97) with reductions in soluble RAGE were not statistically significant.
Figure 1.
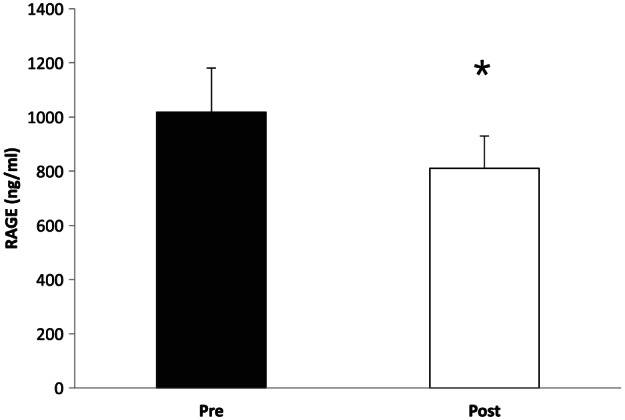
Effect of a 12‐week exercise intervention on plasma soluble RAGE concentrations. *Significant compared with pretest (P = .02)
Figure 2.
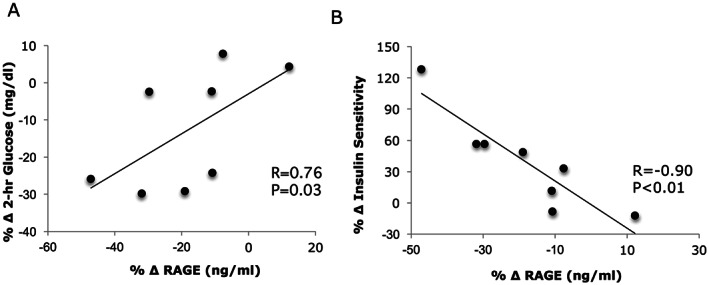
Correlation between the change (Δ) in circulating soluble RAGE and glucose tolerance (A) and insulin sensitivity (B) after a 12‐week lifestyle intervention
4. DISCUSSION
The main finding from this study is that exercise training plus caloric restriction decreased plasma soluble RAGE concentrations (Figure 1). The reduction in soluble RAGE was significantly correlated with lower circulating postprandial glucose and increased insulin sensitivity (Figure 2). These findings are consistent with a recent report that increasing physical activity led to a reduction in soluble RAGE in elderly adults with normoglycemia.20 In contrast, Danzig et al23 reported that a single bout of aerobic exercise performed to maximal HR had no effect on soluble RAGE in adults with coronary artery disease. Further adding to the opposing findings, Choi et al21 demonstrated that 12 weeks of aerobic exercise training raised soluble RAGE in adults with type 2 diabetes, and these findings were directly correlated with reductions in C‐reactive protein. Importantly, unlike previous work,20, 21, 23 the study design herein had participants undergo a rigorous dietary control 3 days before metabolic testing and exercised with full supervision at approximately 65% to 85% of HRmax for up to 60 minutes per session 5 d/wk for 12 weeks. Thus, these data strengthen the current body of literature and show for the first time that moderate to vigorous intensity exercise training reduces soluble RAGE in adults with CKD.
The mechanism by which lifestyle‐induced weight loss reduces soluble RAGE has yet to be fully elucidated. Physically active individuals typically have more favourable cardiometabolic profiles than less active people.26 However, no such correlation of fitness and RAGE was detected in this study, suggesting VO2max is unlikely a primary mechanism underlying the reduction in RAGE. Alternatively, renal clearance may be an important factor regulating the removal of AGEs27 that, in turn, would impact expression of soluble RAGE. Prior work17 though showed that 12 weeks of lifestyle‐induced weight loss had no effect on kidney function as measured by iGFR, and no such association with soluble RAGE was observed with this same approach in the present study. Thus, changes in soluble RAGE after lifestyle treatment appear independent of renal function. Weight loss on the other hand is established to reduce cardiometabolic disease risk,28 and some,20 but not all,21, 29 have proposed that decreased body weight per se is directly correlated with reductions in soluble RAGE. While the results presented here are consistent with lifestyle‐induced weight loss altering soluble RAGE in patients with CKD, further work is warranted. Nonetheless, another possible mechanism by which weight loss could have reduced soluble RAGE relates to circulating glucose. Hyperglycaemia induces nonenzymatic alterations in extracellular and intracellular proteins, thereby promoting AGEs and increasing the expression of RAGE.30, 31 Although the current study did not measure AGEs, it is worth noting that caloric restriction and 12‐weeks of aerobic exercise decrease AGEs.32, 33 Further, a direct correlation between reductions in postprandial plasma glucose and decreased soluble RAGE following lifestyle treatment was observed (Figure 2A). Thus, reductions in glucose may play a role in affecting the expression of RAGE.
Exercise combined with caloric restriction has been show to improve insulin sensitivity34 in part through lower inflammation.15, 35 Herein, while lifestyle‐induced weight loss was shown to decrease circulating fetuin‐A in patients with CKD, no significant correlation with insulin sensitivity was noted. In contrast, the results of the present study suggest that changes in soluble RAGE after lifestyle‐induced therapy were associated with improvements in insulin sensitivity (Figure 2B). These findings oppose pharmacological36 and bariatric surgery interventions29 that have reported improved insulin sensitivity is related to elevated soluble RAGE. The precise mechanism by which lifestyle‐induced insulin sensitivity associates with decreased soluble RAGE is beyond the scope of the present work, but there are two distinct pathways by which soluble RAGEs are generated. These include (a) the ectodomain shedding of the membrane‐associated receptor and (b) the alternative splicing of RAGE pre‐mRNA transcript.5 Thus, future work should consider determining the cellular mechanism by which exercise and/or diet affects RAGE in order to optimize therapies for preventing and/or treating diabetic complications.
This study has limitations that may affect interpretation of results. This is a small pilot study, and interpretation should be made with caution. Moreover, despite the absence of an age‐ and weight‐matched control group, recent work demonstrates that soluble RAGE in at‐risk adults for renal dysfunction do not change over a 3‐month period.21 This provides confidence that the decrease in soluble RAGE observed herein is due to lifestyle‐induced weight loss and not diurnal variation. The intervention selected was a combined exercise plus caloric restriction, thereby limiting ability to isolate exercise versus diet per se. Further work is required to test independent effects of exercise and caloric restriction on soluble RAGE. It is also recognized that this study is specific to patients with stage 2 and 3 CKD, and the data may not be applicable to more or less severe forms of CKD. Further, circulating total soluble RAGE was measured. As a result, results herein may not be generalized or directly compared with studies that have measured endogenous secretory (es)RAGE. This may be of physiologic relevance since esRAGE constitutes only part but not all of the human soluble RAGE in plasma.37 Nevertheless, these findings have clinical relevance for not only understanding how exercise plus weight loss regulates cytokines implicated in glucose regulation but also providing novel insight that may lay the groundwork for elucidating mechanisms involved in renal rehabilitation.
In conclusion, 12 weeks of aerobic exercise training with weight loss reduced soluble RAGE in previously sedentary, adults with CKD. The decrease in soluble RAGE concentrations was associated with reductions in circulating postprandial blood glucose and elevations in insulin sensitivity. Given that hyperglycaemia is implicated in the development of vascular damage, these findings suggest that soluble RAGE may be an important cytokine related to macrovascular and microvascular complications related to end‐stage organ damage in CKD. Further work is required to elucidate the mechanism whereby different RAGE variants relate to glucose metabolism in order to gain insight into how exercise with weight loss contributes to the prevention and/or reversal of type 2 diabetes and cardiovascular disease in patients with CKD.
FUNDING
S.D.N. was supported by a career development award from the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health (grant no. TR000440). S.K.M. was supported by an NIH training grant T32 DK007319 to J.P.K. This work was “Supported in part by UL1TR000439, National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health.” The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
CONFLICT OF INTEREST STATEMENT
No conflict of interest was declared.
AUTHOR CONTRIBUTIONS
S.K.M, S.N., and J.P.K. contributed to the study design. S.K.M was primarily responsible for data analysis and statistical integrity. S.K.M. drafted the manuscript. S.N. A.M., H.H., and J.P.K. contributed to discussion and interpretation and edited the manuscript.
DATA AVAILABILITY STATEMENT
Data were available upon request to the corresponding author with reason.
Malin SK, Navaneethan SD, Fealy CE, et al. Exercise plus caloric restriction lowers soluble RAGE in adults with chronic kidney disease. Obes Sci Pract. 2020;6:307–312. 10.1002/osp4.408
REFERENCES
- 1. Dharmarajan SH, Bragg‐Gresham JL, Morgenstern H, et al. State‐level awareness of chronic kidney disease in the U.S. Am J Prev Med. 2017;53:300‐307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chrysohoou C, Panagiotakos DB, Pitsavos C, et al. Renal function, cardiovascular disease risk factors' prevalence and 5‐year disease incidence; the role of diet, exercise, lipids and inflammation markers: The ATTICA study. QJM. 2010;103:413‐422. [DOI] [PubMed] [Google Scholar]
- 3. Chen J, Muntner P, Hamm LL, et al. The metabolic syndrome and chronic kidney disease in U.S. adults. Ann Intern Med. 2004;140:167‐174. [DOI] [PubMed] [Google Scholar]
- 4. Stump CS. Physical activity in the prevention of chronic kidney disease. Cardiorenal Med. 2011;1:164‐173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vazzana N, Santilli F, Cuccurullo C, Davi G. Soluble forms of RAGE in internal medicine. Intern Emerg Med. 2009;4:389‐401. [DOI] [PubMed] [Google Scholar]
- 6. Schmidt AM, Yan SD, Wautier JL, Stern D. Activation of receptor for advanced glycation end products: a mechanism for chronic vascular dysfunction in diabetic vasculopathy and atherosclerosis. Circ Res. 1999;84:489‐497. [DOI] [PubMed] [Google Scholar]
- 7. Yamagishi S, Nakamura K, Imaizumi T. Advanced glycation end products (AGEs) and diabetic vascular complications. Curr Diabetes Rev. 2005;1:93‐106. [DOI] [PubMed] [Google Scholar]
- 8. Yamamoto Y, Yamagishi S, Yonekura H, et al. Roles of the AGE‐RAGE system in vascular injury in diabetes. Ann N Y Acad Sci. 2000;902:163‐170. discussion 170‐2 [DOI] [PubMed] [Google Scholar]
- 9. Bucciarelli LG, Wendt T, Qu W, et al. RAGE blockade stabilizes established atherosclerosis in diabetic apolipoprotein E‐null mice. Circulation. 2002;106:2827‐2835. [DOI] [PubMed] [Google Scholar]
- 10. Tan KC, Shiu SW, Chow WS, Leng L, Bucala R, Betteridge DJ. Association between serum levels of soluble receptor for advanced glycation end products and circulating advanced glycation end products in type 2 diabetes. Diabetologia. 2006;49:2756‐2762. [DOI] [PubMed] [Google Scholar]
- 11. Nakamura K, Yamagishi S, Adachi H, et al. Serum levels of soluble form of receptor for advanced glycation end products (sRAGE) are positively associated with circulating AGEs and soluble form of VCAM‐1 in patients with type 2 diabetes. Microvasc Res. 2008;76:52‐56. [DOI] [PubMed] [Google Scholar]
- 12. Kalousova M, Hodkova M, Kazderova M, et al. Soluble receptor for advanced glycation end products in patients with decreased renal function. Am J Kidney Dis. 2006. Mar;47:406‐411. [DOI] [PubMed] [Google Scholar]
- 13. Geroldi D, Falcone C, Emanuele E, et al. Decreased plasma levels of soluble receptor for advanced glycation end‐products in patients with essential hypertension. J Hypertens. 2005;23:1725‐1729. [DOI] [PubMed] [Google Scholar]
- 14. Malin SK, Mulya A, Fealy CE, et al. Fetuin‐A is linked to improved glucose tolerance after short‐term exercise training in nonalcoholic fatty liver disease. J Appl Physiol. 2013;115:988‐994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kelly KR, Navaneethan SD, Solomon TP, et al. Lifestyle‐induced decrease in fat mass improves adiponectin secretion in obese adults. Med Sci Sports Exerc. 2014;46:920‐926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. You T, Nicklas B. Effects of exercise on adipokines and the metabolic syndrome. Curr Diab Rep. 2008;8:7‐11. [DOI] [PubMed] [Google Scholar]
- 17. Navaneethan SD, Fealy CE, Scelsi AC, Arrigain S, Malin SK, Kirwan JP. A trial of lifestyle modification on cardiopulmonary, inflammatory, and metabolic effects among obese with chronic kidney disease. Am J Nephrol. 2015;42:274‐281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Martens CR, Kirkman DL, Edwards DG. The vascular endothelium in chronic kidney disease: a novel target for aerobic exercise. Exerc Sport Sci Rev. 2016. Jan;44:12‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Leehey DJ, Collins E, Kramer HJ, et al. Structured exercise in obese diabetic patients with chronic kidney disease: a randomized controlled trial. Am J Nephrol. 2016;44:54‐62. [DOI] [PubMed] [Google Scholar]
- 20. Kotani K, Caccavello R, Sakane N, Yamada T, Taniguchi N, Gugliucci A. Influence of physical activity intervention on circulating soluble receptor for advanced glycation end products in elderly subjects. J Clin Med Res. 2011;3:252‐257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Choi KM, Han KA, Ahn HJ, et al. Effects of exercise on sRAGE levels and cardiometabolic risk factors in patients with type 2 diabetes: a randomized controlled trial. J Clin Endocrinol Metab. 2012;97:3751‐3758. [DOI] [PubMed] [Google Scholar]
- 22. Parikh M, Chung M, Sheth S, et al. Randomized pilot trial of bariatric surgery versus intensive medical weight management on diabetes remission in type 2 diabetic patients who do NOT meet NIH criteria for surgery and the role of soluble RAGE as a novel biomarker of success. Ann Surg. Oct 2014;260:617‐622. discussion 622‐4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Danzig V, Mikova B, Kuchynka P, et al. Levels of circulating biomarkers at rest and after exercise in coronary artery disease patients. Physiol Res. 2010;59:385‐392. [DOI] [PubMed] [Google Scholar]
- 24. Malin SK, Solomon TPJ, Blaszczak A, Finnegan S, Filion J, Kirwan JP. Pancreatic beta cell function increases in a linear dose‐response manner following exercise training in adults with prediabetes. Am J Physiol Endocrinol Metab. 2013;305:E1248‐E1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22:1462‐1470. [DOI] [PubMed] [Google Scholar]
- 26. Church TS, LaMonte MJ, Barlow CE, Blair SN. Cardiorespiratory fitness and body mass index as predictors of cardiovascular disease mortality among men with diabetes. Arch Intern Med. 2005;165:2114‐2120. [DOI] [PubMed] [Google Scholar]
- 27. Dolhofer‐Bliesener R, Lechner B, Gerbitz KD. Possible significance of advanced glycation end products in serum in end‐stage renal disease and in late complications of diabetes. Eur J Clin Chem Clin Biochem. 1996;34:355‐361. [DOI] [PubMed] [Google Scholar]
- 28. Bays H, Blonde L, Rosenson R. Adiposopathy: How do diet, exercise and weight loss drug therapies improve metabolic disease in overweight patients? Expert Rev Cardiovasc Ther. 2006;4:871‐895. [DOI] [PubMed] [Google Scholar]
- 29. Brix JM, Hollerl F, Kopp HP, Schernthaner GH, Schernthaner G. The soluble form of the receptor of advanced glycation endproducts increases after bariatric surgery in morbid obesity. Int J Obes (Lond). 2012;36:1412‐1417. [DOI] [PubMed] [Google Scholar]
- 30. Lee EJ, Park JH. Receptor for advanced glycation endproducts (RAGE), its ligands, and soluble RAGE: potential biomarkers for diagnosis and therapeutic targets for human renal diseases. Genomics Inform. 2013;11:224‐229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Goldin A, Beckman JA, Schmidt AM, Creager MA. Advanced glycation end products: sparking the development of diabetic vascular injury. Circulation. 2006;114:597‐605. [DOI] [PubMed] [Google Scholar]
- 32. Gugliucci A, Kotani K, Taing J, et al. Short‐term low calorie diet intervention reduces serum advanced glycation end products in healthy overweight or obese adults. Ann Nutr Metab. 2009;54:197‐201. [DOI] [PubMed] [Google Scholar]
- 33. Goon JA, Aini AH, Musalmah M, Anum MY, Nazaimoon WM, Ngah WZ. Effect of tai chi exercise on DNA damage, antioxidant enzymes, and oxidative stress in middle‐age adults. J Phys Act Health. 2009;6:43‐54. [DOI] [PubMed] [Google Scholar]
- 34. Yassine H, Marchetti C, Krishnan R, Vrobel T, Gonzalez F, Kirwan JP. Effects of exercise and caloric restriction on insulin resistance and cardiometabolic risk factors in older obese adults—a randomized clinical trial. J Gerontol Biol Med Sci. 2009;64:90‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Malin SK, del Rincon JP, Huang H, Kirwan JP. Exercise‐induced lowering of fetuin‐A may increase hepatic insulin sensitivity. Med Sci Sports Exerc. 2014;46:2085‐2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tan KC, Chow WS, Tso AW, et al. Thiazolidinedione increases serum soluble receptor for advanced glycation end‐products in type 2 diabetes. Diabetologia. 2007;50:1819‐1825. [DOI] [PubMed] [Google Scholar]
- 37. Koyama H, Shoji T, Yokoyama H, et al. Plasma level of endogenous secretory RAGE is associated with components of the metabolic syndrome and atherosclerosis. Arterioscler Thromb Vasc Biol. 2005;25:2587‐2593. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data were available upon request to the corresponding author with reason.


