Abstract
Wickerhamomyces anomalus LBCM1105 is a yeast isolated from cachaça distillery fermentation vats, notable for exceptional glycerol consumption ability. We report its draft genome with 20.5x in-depth coverage and around 90% extension and completeness. It harbors the sequences of proteins involved in glycerol transport and metabolism.
Keywords: Non-conventional yeast, glycerol, “de novo” assembly, glycerol
Wickerhamomyces anomalus (synonyms Pichia anomala, Hansenula anomala and Candida pelliculosa) are found in several diverse natural habitats, frequently associated with spoilage or processing of food and grain products (Passoth et al., 2006). Different strains of W. anomalus were reported (i) to be able to grow on a wide variety of conditions, including different carbon and nitrogen sources (Conceição et al., 2015; Cunha et al., 2019), at both low and high pH (2.0 to 12.4) and from 3 to 37 °C (Fredlund et al., 2002), (ii) to be highly tolerant to different stress conditions, like osmotic stress (salt), high concentrations of ethanol, and the presence of heavy metals, and (iii) to produce ethanol from glucose, sucrose or xylose. W. anomalus strains have also been reported to display constitutive cyanide-resistant alternative oxidase (Cunha et al., 2019). W. anomalus has been used as a cell factory for the production, among others, of enzymes (Díaz-Rincón et al., 2017), biosurfactants (Teixeira Souza et al., 2018) and fermented-beverages (Aplin et al., 2019). Although W. anomalus strains show a high industrial versatility, only two strains have its genome sequenced to date (Schneider et al., 2012; Riley et al., 2016).
W. anomalus strain LBCM1105 (previously LBCM105) was isolated from sugarcane fermentation vats in a cachaça distillery in Brazil (Conceição et al., 2015), (S22.099694, W41.511090). Extraction of DNA was carried out using the phenol/chloroform method, and purification was performed using the PowerClean DNA Clean-UP kit (MoBio, QIAGEN, Carlsbad, US). The genome size was determined by flow cytometry as previously described (Hare and Johnston, 2011). Cell samples were stained with 2 μM Sytox Green (Thermo Fisher Scientific, MA, US) and the assessment was made in triplicate. The genomic library for sequencing was prepared with the Nextera DNA Library kit (Illumina, San Diego, California, US). Genome sequencing (1.0 million paired-end reads of 151 bp) was performed with an Illumina HiSeq 2500. Quality trimming, and the removal of reads shorter than 90 nucleotides, were carried out using Trimommatic v.0.32 (Bolger et al., 2014). The genome was assembled into contigs (20.5 x in depth coverage, ≥ 1 kb) using SPAdes v.3.11.1, dipSPAdes mode (Bankevich et al., 2012). The completeness was evaluated by BUSCO v.3.0 (Simão et al., 2015), using the Fungi and Saccharomycetales datasets. Genome statistics were computed with QUAST v5.0.2 (Gurevich et al., 2013). A multilocus phylogenetic analysis was performed using RAxML v.8 (Stamatakis, 2014) building a Maximum Likelihood tree based on DNA sequences from the Internal Transcribed Spacers 1 and 2 (ITS1, ITS2), the large and small ribosomal subunits (LSU, SSU), and the Elongation Factor-1α (EF-1α) from species within the genus Barnettozyma, Wickerhamomyces and Candida. The species and the accession numbers of loci LSU, SSU and EF-1α of the related microorganism were previously described (Kobayashi et al., 2017). The accession numbers for ITS are listed in Figure S1 (245KB, pdf) ). Saccharomyces cerevisiae S288c was used as the outgroup. The sequences of the loci SSU, LSU and EF-1α of the LBCM1105 strain were identified via Blast searches using the proper sequences from W. anomalus NRRL Y-366 as baits (SSU- EF550479.1, LSU- EF550341.1 and EF-1α- EF552565.1). ITS1 and ITS2 sequences from W. anomalus LBCM1105 was extracted using ITSx v.1.0.11 (Bengtsson-Palme et al., 2013). The sequences of ITS1, ITS2, LSU and SSU were aligned using MXSCARNA v.2.1 (Tabei et al., 2008), and of EF-1α protein using MAFFT v.7 (Katoh et al., 2017). rtREV was selected using IQ-TREE v1.6 (Nguyen et al., 2015) as the best evolutionary model for the EF-1α phylogenetic analysis. All the alignments were concatenated in a supermatrix using FASconCAT v.1.04 (Kuck and Meusemann, 2010), which was used to conduct a partitioned phylogenetic analysis. A phylogenetic tree based on the alignments and in the evolutionary model (rtREV for EF-1α and GTR for the others – ITS1, ITS2, LSU and SSU), was inferred using RAxML v.8.4 (Stamatakis, 2014), with 1,000 bootstrap replicates. Genome annotation was done using Augustus v3.3.1 (Stanke et al., 2008) and BRAKER2 v2.1.2 (Hoff et al., 2019), using as extrinsic evidence for training the proteins of W. anomalus deposited in GenBank. Proteins related to glycerol transport and metabolism were identified in the LBCM1105 genome using Blastx.
The GC content of the genome was 34.51%. The phylogenetic analysis (Figure S1 (245KB, pdf) ) confirmed that LBCM1105 is, in fact, a strain within W. anomalus, in the same clade with the W. anomalus NRRL Y-366-8, with a bootstrap of 100%. Moreover, according to flow cytometry analyses, the genome of strain LBCM1105 is 13.93 ± 0.11 Mb. The total genome assembly corresponds to 12.72 Mb, i.e., 91.31% of the expected size, and 89.89% in relation to the genome of the W. anomalus strain NRRL Y-366-8 (GCA_001661255.1) which has a genome size of 14.15 Mb. The completeness of the genome assembly, as evaluated on the gene space by BUSCO, was 88.6% for the fungi dataset (290 genes) and 85.5% for the Saccharomycetales dataset (1711 genes). Half of the data is present in 51 scaffolds (L50) larger than 76 kb (N50), the largest being 229 kb. The total number of contigs was 389 with 6,812 predicted protein-coding genes. This number is similar to the 6,421 ORFs previously reported from the genome of W. anomalus NRRL Y-366-8 (Riley et al., 2016), and to the 5,885 ORFs of Saccharomyces cerevisiae (Goffeau et al., 1996). We compared the genome annotation of LBCM1105 (Augustus and BRAKER2) to that of NRRL Y-366-8, S. cerevisiae S288c and W. ciferrii using OrthoFinder (Emms and Kelly, 2015). This comparison clearly showed that most predicted genes in LBCM1105 can be assigned to orthologous groups and are shared with the other genomes in the analysis (Figure S2 (190.8KB, pdf) and Table 1). This Whole Genome Shotgun project has been deposited at DDBJ/ENA/GenBank under the accession SHLV00000000. The version described in this paper is version SHLV01000000.
Table 1. Comparison of groups of orthologous genes between W. anomalus LBCM1105 with two annotation strategies A) Augustus, B) BRAKER2, W. anomalus NRRL Y-366-8, W. ciferrii NRRL Y-1031 and S. cerevisiae S288c.
| Groups of orthologous genes | LBCM1105-A | LBCM1105-B | S288c | NRRL Y-366-8 | NRRL Y-1031 |
|---|---|---|---|---|---|
| Number of genes in strains/species | 6812 | 6159 | 6002 | 6421 | 6702 |
| Number of genes in orthogroups | 5965 | 6106 | 4651 | 6227 | 5936 |
| Number of unassigned genes | 847 | 53 | 1351 | 194 | 766 |
| Percentage of genes in orthogroups | 87,6 | 99,1 | 77,5 | 97,0 | 88,6 |
| Number of species-specific orthogroups | 0 | 0 | 7 | 0 | 7 |
| Number of genes in species-specific orthogroups | 0 | 0 | 17 | 0 | 79 |
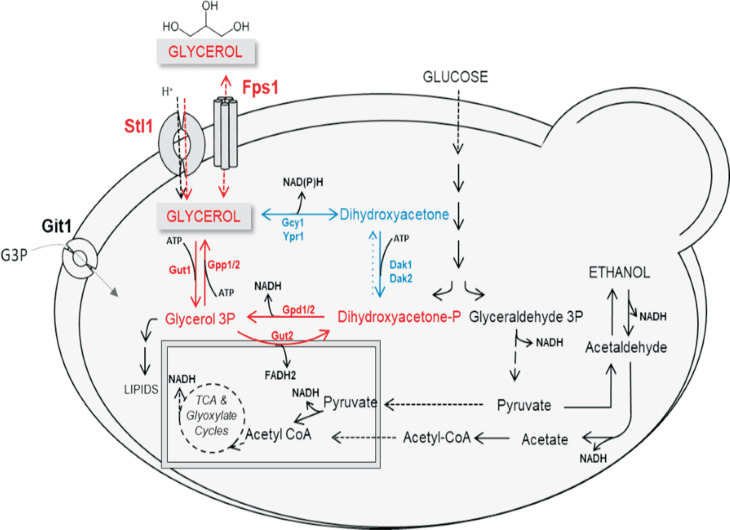
DNA sequences from S. cerevisiae S288c encoding the proteins that perform glycerol transport (the channel Fps1p and the high affinity transporter Stl1p) and metabolism (the consumption Gut1p/Gut2p, the production Gpd1p/Gpd2p and Gpp1p/Gpp2p, as well as the putative pathway Gcy1p, Ypr1p and Dak1p/Dak2p) (Figure 1, and Table 2) were obtained from SGD (https://www.yeastgenome.org) and used to identify the correspondent putative ORFs in the W. anomalus LBCM1105 genome. Homologous sequences to the proteins were found (Table 2), in some cases different S. cerevisiae proteins aligned to the same protein in the W. anomalus LBCM1105 genome, it is not clear which will be the exact function of the LBCM1105's protein, more studies are need to elucidate this. The W. anomalus Stl1p was previously studied in detail, showing very high affinity for glycerol (Cunha et al., 2019). The genome sequence presented here provides evidence for the existence of the genes needed to ensure the two glycerol consumption and production pathways known in S. cerevisiae. Further studies are required to verify how intrinsic characteristics of these proteins and their expression and regulation are the cause underlying the LBCM1105's extraordinary ability to grow on glycerol as single a carbon source (Conceição et al., 2015).
Figure 1. Global yeast metabolism overview focusing on glycerol transport, consumption and production pathways. Red: main metabolic pathway. Blue: alternative pathway with unclear physiological relevance in S. cerevisiae.
Table 2. Similarity between the S. cerevisiae genes encoding the proteins responsible for glycerol transport and metabolism as in Figure 1, and the corresponding sequences identified in the genome of W. anomalus LBCM1105. Protein Sequences are available at https://doi.org/10.6084/m9.figshare.11441061.v1.
| Protein role | S. cerevisiae - SGD database | Gene | Percentage target aligned | Similarity | |||
|---|---|---|---|---|---|---|---|
| Gene | ID | ||||||
| Regular pathway | Transport | Glycerol channel | FPS1 | S000003966 | g1373.t1 | 45.3 | 56% |
| Glycerol active permease/ H+ symporter | STL1 | S000002944 | g4293.t1 | 85.4 | 57% | ||
| Consumption | Glycerol kinase | GUT1 | S000001024 | g1371.t1 | 91.2 | 72% | |
| Glycerol 3P | GUT2 | S000001417 | g5045.t1 | 98.8 | 72% | ||
| dehydrogenase/mitochondria | |||||||
| Production | Glycerol 3P dehydrogenase | GPD1 | S000002180 | g1302.t1 | 100 | 78% | |
| Glycerol 3P dehydrogenase | GPD2 | S000005420 | g1302.t1 | 81.1 | 82% | ||
| Glycerol 3P phosphatase | GPP1 | S000002180 | g4575.t1 | 99.2 | 71% | ||
| Glycerol 3P phosphatase | GPP2 | S000005420 | g4575.t1 | 99.2 | 71% | ||
| Alternative pathway | Consumption/Production | Glycerol dehydrogenase | GCY1 | S000005646 | g1045.t1 | 98.7 | 79% |
| Glycerol dehydrogenase | YPR1 | S000002776 | g1045.t1 | 98.7 | 78% | ||
| Consumption | Dihydroxyacetone kinase | DAK1 | S000004535 | g4297.t1 | 98.5 | 56% | |
| Dihydroxyacetone kinase | DAK2 | S000001841 | g4297.t1 | 97.8 | 52% | ||
Acknowledgments
The authors gratefully acknowledge Laboratório Nacional de Ciência e Tecnologia do Bioetanol (CTBE) and the Centro Nacional de Pesquisa em Energia e Materiais (CNPEM) for support with the sequencing of LBCM1105. This work was supported by CAPES/Brazil (PNPD 2755/2011; PCF-PVE 021/2012), by CNPq (Brazil), processes 304815/2012 (research grant) and 305135/2015-5, and by AUXPE-PVES 1801/2012 (Process 23038.015294/2016-18) from Brazilian Government and by UFOP. C.L. is supported by the strategic program UID/BIA/04050/2013 [POCI-01-0145-FEDER-007569] funded by national funds through the FCT I.P. and by the ERDF through the COMPETE2020 - Programa Operacional de Competitividade e Internacionalização (POCI). DMRP is a fellow from the CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico) – Brazil (310080/2018-5).
Supplementary Material
The following online material is available for this study
Footnotes
Associate editor: Ana Tereza Vasconcelos
References
- Aplin JJ, White KP, Edwards CG. Growth and metabolism of non-Saccharomyces yeasts isolated from Washington state vineyards in media and high sugar grape musts. Food Microbiol. 2019;77:158–165. doi: 10.1016/j.fm.2018.09.004. [DOI] [PubMed] [Google Scholar]
- Bankevich A, Nurk S, Antipov D, Gurevich AA, Dvorkin M, Kulikov AS, Lesin VM, Nikolenko SI, Pham S, Prjibelski AD, et al. SPAdes: A new genome assembly algorithm and its applications to single-Cell sequencing. J Comput Biol. 2012;19:455–477. doi: 10.1089/cmb.2012.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengtsson-Palme J, Ryberg M, Hartmann M, Branco S, Wang Z, Godhe A, De Wit P, Sánchez-García M, Ebersberger I, Sousa F, et al. Improved software detection and extraction of ITS1 and ITS2 from ribosomal ITS sequences of fungi and other eukaryotes for analysis of environmental sequencing data. Methods Ecol Evol. 2013;4:914–919. [Google Scholar]
- Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conceição LE, Saraiva MA, Diniz RH, Oliveira J, Barbosa GD, Alvarez F, Correa LF, Mezadri H, Coutrim MX, Afonso RJ, et al. Biotechnological potential of yeast isolates from cachaça: the Brazilian spirit. J Ind Microbiol Biotechnol. 2015;42:237–246. doi: 10.1007/s10295-014-1528-y. [DOI] [PubMed] [Google Scholar]
- Cunha AC, Gomes LS, Godoy-Santos F, Faria-Oliveira F, Teixeira JA, Sampaio GMS, Trópia MJM, Miranda Castro I, Lucas C, Brandão RL. High-affinity transport, cyanide-resistant respiration, and ethanol production under aerobiosis underlying efficient high glycerol consumption by Wickerhamomyces anomalus . J Ind Microbiol Biotechnol. 2019;46:709–723. doi: 10.1007/s10295-018-02119-5. [DOI] [PubMed] [Google Scholar]
- Díaz-Rincón DJ, Duque I, Osorio E, Rodríguez-López A, Espejo-Mojica A, Parra-Giraldo CM, Poutou-Piñales RA, Alméciga-Díaz CJ, Quevedo-Hidalgo B. Production of recombinant Trichoderma reesei cellobiohydrolase II in a new expression system based on Wickerhamomyces anomalus . Enzyme Res. 2017:6980565–6980565. doi: 10.1155/2017/6980565. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emms DM, Kelly S. OrthoFinder: solving fundamental biases in whole genome comparisons dramatically improves orthogroup inference accuracy. Genome Biol. 2015;16:157–157. doi: 10.1186/s13059-015-0721-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredlund E, Druvefors U, Boysen ME, Lingsten KJ, Schnurer J. Physiological characteristics of the biocontrol yeast Pichia anomala J121. FEMS Yeast Res. 2002;2:395–402. doi: 10.1016/S1567-1356(02)00098-3. [DOI] [PubMed] [Google Scholar]
- Goffeau A, Barrell BG, Bussey H, Davis RW, Dujon B, Feldmann H, Galibert F, Hoheisel JD, Jacq C, Johnston M, et al. Life with 6000 genes. Science. 1996;274:563–547. doi: 10.1126/science.274.5287.546. [DOI] [PubMed] [Google Scholar]
- Gurevich A, Saveliev V, Vyahhi N, Tesler G. QUAST: quality assessment tool for genome assemblies. Bioinformatics. 2013;29:1072–1075. doi: 10.1093/bioinformatics/btt086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare EE, Johnston JS. Genome size determination using flow cytometry of propidium iodide-stained nuclei. Methods Mol Biol. 2011;772:3–12. doi: 10.1007/978-1-61779-228-1_1. [DOI] [PubMed] [Google Scholar]
- Hoff KJ, Lomsadze A, Borodovsky M, Stanke M. Whole-Genome Annotation with BRAKER. Methods Mol Biol. 2019;1962:65–95. doi: 10.1007/978-1-4939-9173-0_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh K, Rozewicki J, Yamada KD. MAFFT online service: multiple sequence alignment, interactive sequence choice and visualization. Brief Bioinform. 2017;20:1160–1166. doi: 10.1093/bib/bbx108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi R, Kanti A, Kawasaki H. Three novel species of d-xylose-assimilating yeasts, Barnettozyma xylosiphila sp. nov., Barnettozyma xylosica sp. nov. and Wickerhamomyces xylosivorus f.a., sp. nov. Int J Syst Evol Microbiol. 2017;67:3971–3976. doi: 10.1099/ijsem.0.002233. [DOI] [PubMed] [Google Scholar]
- Kuck P, Meusemann K. FASconCAT: Convenient handling of data matrices. Mol Phylogenet Evol. 2010;56:1115–1118. doi: 10.1016/j.ympev.2010.04.024. [DOI] [PubMed] [Google Scholar]
- Nguyen LT, Schmidt HA, von Haeseler A, Minh BQ. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol Biol Evol. 2015;32:268–274. doi: 10.1093/molbev/msu300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passoth V, Fredlund E, Druvefors UA, Schnurer J. Biotechnology, physiology and genetics of the yeast Pichia anomala. FEMS Yeast Res. 2006;6:3–13. doi: 10.1111/j.1567-1364.2005.00004.x. [DOI] [PubMed] [Google Scholar]
- Riley R, Haridas S, Wolfe KH, Lopes MR, Hittinger CT, Goker M, Salamov AA, Wisecaver JH, Long TM, Calvey CH, et al. Comparative genomics of biotechnologically important yeasts. Proc Natl Acad Sci U S A. 2016;113:9882–9887. doi: 10.1073/pnas.1603941113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider J, Rupp O, Trost E, Jaenicke S, Passoth V, Goesmann A, Tauch A, Brinkrolf K. Genome sequence of Wickerhamomyces anomalus DSM 6766 reveals genetic basis of biotechnologically important antimicrobial activities. FEMS Yeast Res. 2012;12:382–386. doi: 10.1111/j.1567-1364.2012.00791.x. [DOI] [PubMed] [Google Scholar]
- Simão FA, Waterhouse RM, Ioannidis P, Kriventseva EV, Zdobnov EM. BUSCO: assessing genome assembly and annotation completeness with single-copy orthologs. Bioinformatics. 2015;3:3210–3212. doi: 10.1093/bioinformatics/btv351. [DOI] [PubMed] [Google Scholar]
- Stamatakis A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 2014;30:1312–1313. doi: 10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanke M, Diekhans M, Baertsch R, Haussler D. Using native and syntenically mapped cDNA alignments to improve de novo gene finding. Bioinformatics. 2008;24:637–644. doi: 10.1093/bioinformatics/btn013. [DOI] [PubMed] [Google Scholar]
- Tabei Y, Kiryu H, Kin T, Asai K. A fast structural multiple alignment method for long RNA sequences. BMC Bioinformatics. 2008;9:33–33. doi: 10.1186/1471-2105-9-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira Souza KS, Gudina EJ, Schwan RF, Rodrigues LR, Dias DR, Teixeira JA. Improvement of biosurfactant production by Wickerhamomyces anomalus CCMA 0358 and its potential application in bioremediation. Toxins. 2018;346:152–158. doi: 10.1016/j.jhazmat.2017.12.021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.