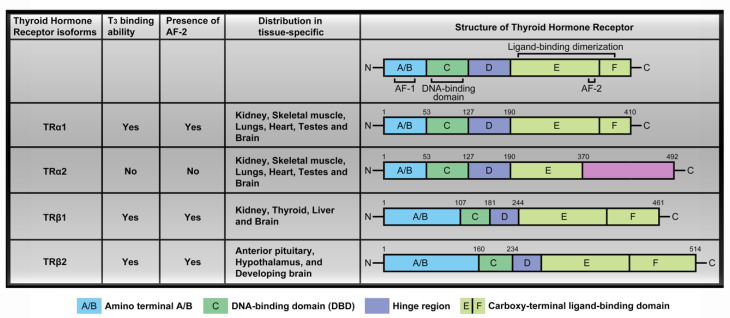
Dear Editor,
In Dr. Magdalena Szaryńska’s recent letter, she expressed one confusing aspect she met in Figure 1 [1]. We had already corrected and published on [2], circulating thyroid hormones (THs) interact with thyroid hormone receptors to promote downstream signaling pathways and activate transcription factors. Thyroid hormone receptors (TR) including TRα and TRβ contain several domains; specifically, these are the amino-terminal A/B that may function as a gene enhancer, the DNA-binding domain (DBD), the hinge region containing the nuclear localization signal and the carboxy-terminal ligand-binding domain (LBD) that binds T3 through AF-2, which is a surface-exposed hydrophobic included residue from H3 and H5 and is completed by T3-dependent packing of C-terminal H12 against the LBD which affects target genes transcription [3,4]. (Figure 1). The four major TR isoforms, TRα1, TRα2, TRβ1, and TRβ2, are produced by c-erbAα and c-erbAβ genes. Their human homologs are designated THRA and THRB. The c-erbAα gene located on chromosome 17 encodes two different TRα isoforms. One is functional TH-binding TRα1 and the other is a dominant-negative splice variant, TRα2, lacking TH binding activity [5]. T3 interacts with thyroid hormone receptors via C-terminal activation function-2 (AF-2) in the ligand-binding domain (LBD), however, only TRα2 has a distinct C-terminal extension and is absent the activation function-2 (AF-2) region, which suggested that TRα2 does not bind T3 [6]. TRα2 is unique in consideration of its lack of binding to THs while interacting with DNA, and its precise function is unclear at present. We have corrected to show that the TRα2 didn’t bind T3 and have marked the presence or absence of the AF-2 domain in Figure 1 as follows:
Figure 1.
Thyroid hormone receptors (TR) isoforms and structure distribution. Thyroid hormone receptors (TR) contain several domains, specifically, amino-terminal A/B that may function as a gene enhancer, DNA-binding domain (DBD), hinge region containing the nuclear localization signal and carboxy-terminal ligand-binding domain that binds T3. The four major TR isoforms, TRα1, TRα2, TRβ1, and TRβ2 undergo TH binding and are widely distributed in a tissue-specific manner, such as TRα1 and TRα2 are expressed in the kidney, skeletal muscle, lungs, heart, and testes, with particularly high levels detected in the brain. TRβ1 expression is significant in the brain, thyroid, liver, and kidney while the TRβ2 isoform is specifically expressed in the anterior pituitary, hypothalamus, and developing brain.
References
- 1.Liu Y.C., Yeh C.T., Lin K.H. Molecular Functions of Thyroid Hormone Signaling in Regulation of Cancer Progression and Anti-Apoptosis. Int. J. Mol. Sci. 2019;20:4986. doi: 10.3390/ijms20204986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu Y.-C., Yeh C.-T., Lin K.-H. Correction: Lin, K.-H., et al. Molecular Functions of Thyroid Hormone Signaling in Regulation of Cancer Progression and Anti-Apoptosis. Int. J. Mol. Sci., 2019, 20, 4986. Int. J. Mol. Sci. 2020;21:3185. doi: 10.3390/ijms21093185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feng W., Ribeiro R.C., Wagner R.L., Nguyen H., Apriletti J.W., Fletterick R.J., Baxter J.D., Kushner P.J., West B.L. Hormone-dependent coactivator binding to a hydrophobic cleft on nuclear receptors. Science. 1998;280:1747–1749. doi: 10.1126/science.280.5370.1747. [DOI] [PubMed] [Google Scholar]
- 4.Nascimento A.S., Dias S.M., Nunes F.M., Aparicio R., Ambrosio A.L., Bleicher L., Figueira A.C., Santos M.A., de Oliveira Neto M., Fischer H., et al. Structural rearrangements in the thyroid hormone receptor hinge domain and their putative role in the receptor function. J. Mol. Biol. 2006;360:586–598. doi: 10.1016/j.jmb.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 5.Mitsuhashi T., Tennyson G.E., Nikodem V.M. Alternative splicing generates messages encoding rat c-erbA proteins that do not bind thyroid hormone. Proc. Natl. Acad. Sci. USA. 1988;85:5804–5808. doi: 10.1073/pnas.85.16.5804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bianco A.C., da Conceicao R.R. The Deiodinase Trio and Thyroid Hormone Signaling. Methods Mol. Biol. 2018;1801:67–83. doi: 10.1007/978-1-4939-7902-8_8. [DOI] [PMC free article] [PubMed] [Google Scholar]