Abstract
Background/Aim:
Irritable Bowel Syndrome (IBS) is a common chronic functional bowel disorder and the evidence shows most drug therapies in the treatment of IBS are weak. Recently, some studies showed probiotics may have a positive effect in IBS and they are widely used to improve the symptom of IBS, which indicate probiotics may play an important role in the treatment of IBS. However, the exact effectiveness and safety of probiotics are largely unknown. This systematic review focuses on identifying the efficacy and safety of probiotics in the treatment of IBS.
Materials and Methods:
Data sources were searched up to February 2019. Databases included MEDLINE, CENTRAL, CINAHL, and Embase. Randomized controlled trials (RCTs) comparing probiotics including complex or individual probiotics with placebo or no therapy were screened, extracted, and appraised by two independent reviewers. The data were pooled using a random-effects model. The methodological quality of all RCTs was assessed using the Cochrane risk of bias and Jadad scale. Outcomes included symptom-relevant and patient-relevant characteristics, such as symptom relief, abdominal pain, bloating, flatulence, quality of life, and adverse event.
Results:
This review includes 28 studies with a total of 3606 participants. Particular combinations of probiotics, or specific species and strains, showed probiotics have beneficial effect on overall IBS symptoms (22 studies, n = 3144, RR of improvement in overall IBS symptoms = 1.5, CI 1.23 to 1.83) or overall IBS symptom and abdominal pain scores (18 studies, n = 2766, SMD = -0.31, CI -0.45 to -0.17). In addition, adverse events were not significantly higher with probiotics (8 studies, n = 923, RR = 1.05; 95% CI 0.85-1.31). However, there was no significant benefit on individual IBS symptom scores and quality of life.
Conclusion:
Current evidence shows particular combinations, species or strains of probiotics are effective for overall IBS symptoms. However, it is hard to derive a definite conclusion due to high heterogeneity and unclear risk of bias of some trials. Large well-designed and rigorous trials are warranted.
Keywords: Irritable bowel syndrome, meta-analysis, probiotics, systematic review
INTRODUCTION
Irritable bowel syndrome (IBS) is a chronic and sometimes disabling functional bowel disorder of the gastrointestinal system,[1] characterized by abdominal pain and altered bowel habit, with either predominantly diarrhea, constipation or both.[2] On the basis of Rome IV criteria, IBS is divided into four subtypes based on symptoms, including IBS with constipation (IBS-C), IBS with diarrhea (IBS-D), IBS with a mixed pattern (IBS-M) of constipation and diarrhea, and unclassified IBS (IBS-U), without any of the previous symptoms.[3,4]
The global prevalence of IBS is about 11%,[5] with a range of 9–23%,[6] and it negatively affects quality of life and work productivity. Traditionally, IBS has been conceptualized as a brain-gut disorder because of its high association with coexisting psychiatric and psychological conditions, especially anxiety and depression.[7] However, the exact pathophysiology of IBS remains unclear, and the evidence for the efficacy of most drug therapies in the treatment of IBS is weak.[8] This leads to unsatisfactory control of symptoms for many patients and it has been estimated that patients would give up 10 to 15 years of life expectancy for an instant cure of the disease,[9] therefore, alternative approaches are needed.
Studies showing alterations in gut microbiota structure and composition have been implicated in various gastrointestinal tract disorders including IBS,[10,11] as symptoms of IBS often developed after an infection, known as post-infectious IBS (PI-IBS).[12] Furthermore, a recent study has shown that symptom severity in IBS is negatively associated with microbial richness and a distinct microbial signature,[13] and data suggest that the colonic microbiome is altered in patients with IBS when compared with healthy controls.[14,15,16,17] Probiotics are live microorganisms that have been demonstrated to exhibit potential effects on human health.[18] Probiotics may influence the IBS symptoms including abdominal pain, bloating, distension, flatulence, altered bowel movements, and gut microbiota.[19] Nowadays, although probiotics are used widely in clinical medicine, their efficacy and safety is not entirely clear. In the current study, we aimed to assess the efficacy and safety of probiotics in patients with IBS.
MATERIALS AND METHODS
This systematic review and meta-analysis is registered at PROSPERO, number CRD42019127391.
Inclusion criteria for study selection
Types of studies
We included randomized controlled trials (RCTs) comparing any strain of probiotics with placebo to treat IBS and excluded case reports, case series, commentaries, quasi-RCTs, and non-randomized controlled studies.
Inclusion criteria
(1) Subjects were adult patients (age >16 years); (2) diagnosis of IBS based on either a clinician's opinion or meeting specific diagnostic criteria (Rome I, II, III, IV); (3) duration of treatment and follow-up at least 1 week.
Types of interventions
As experimental intervention, any strain of probiotics or combination of probiotics with any dose was included, while the control group included patients with the same treatment besides comparative interventions.
Types of outcome measures
Primary outcomes
The primary outcomes assessed were the effect on symptom relief, including relief of probiotics compared with placebo on overall IBS symptoms or abdominal pain after cessation of therapy.
Secondary outcomes
The secondary outcomes were as follows:
Effect on overall IBS or abdominal pain symptom scores.
Effect on individual IBS symptom scores including bloating, flatulence.
Quality of life and adverse events.
Search methods for identification
Electronic searches
To cover as much of the relevant literature as possible, we comprehensively searched MEDLINE, CENTRAL, CINAHL, and EMBASE from inception until 1st February 2019, to avoid. To avoid omitting relevant trials, without language restriction. The MEDLINE (Pubmed) search strategy was provided in Table 1, which will also be used in other electronic databases.
Table 1.
Search strategy used in Medline (via PubMed)
| No. | Search items |
|---|---|
| 1 | irritable bowel syndrome* |
| 2 | irritable bowel syndrome [mesh] |
| 3 | 1 OR 2 |
| 4 | probiotics* |
| 5 | probiotics [mesh] |
| 6 | 4 OR 5 |
| 7 | randomized controlled trial [pt] |
| 8 | controlled clinical trial [pt] |
| 9 | randomized controlled trials as topic [mesh] |
| 10 | animals [mesh] NOT humans [mesh] |
| 11 | 7 OR 8 OR 9 NOT 10 |
| 12 | 3 AND 6 AND 11 |
Search of other resources
The references of retrievable studies were manually searched. In addition, the original and references of other relevant literature, including conference proceedings, academic dissertations, reviews, systematic reviews, and meta-analysis, were also searched.
Data collection and extraction
Selection of studies
Two reviewers (JR.S and CF.K) independently screened the titles, abstracts, and references from all identified reports. Potentially eligible studies were confirmed by evaluating the full text. Any disagreements were arbitrated by a third investigator (XK.Q).
Data extraction
In order to ensure the homogeneity of the extracted data, two authors (JR.S and CF.K) independently extracted the original data in the literature onto a standardized form: the first author, year of publication, country, sample size, probiotics used (included strain and species where applicable), duration of therapy, total number of adverse events reported, criteria used to define IBS, primary or secondary outcome measure, proportion of female patients and proportion of patients according to predominant stool pattern (IBS with constipation [IBS-C], diarrhea [IBS-D], or mixed stool pattern [IBS-M]) were recorded. Where necessary, the author of the study was contacted to obtain the study data. Conflicts in data abstraction were resolved by a consensus, and by referring to the original article.
Risk of bias assessment
Two review authors (JR.S and CF.K) independently assessed the quality of the literature following the Cochrane Collaboration Handbook.[20] The scoring system included the following criteria: random sequence generation, allocation concealment, blinding of participants and personnel, blinding the result assessment, incomplete data of the results, selective reporting, and other sources of bias.
Study quality was also assessed with the Jadad scale of randomized controlled trials (RCTs).[21] Two review authors (JR.S and CF.K) independently assessed the quality of the included studies and discrepancies were arbitrated by a third investigator (XK.Q).
Data synthesis and statistical analysis
All included clinical studies were analyzed descriptively and summarized, Dichotomous data were expressed as a relative risk (RR), while continuous data were expressed as standardized mean difference (SMD), with 95% confidence interval (CI).
A fixed model was applied if I2 <50%. Otherwise, data were pooled using a random effects model,[22] to give a more conservative estimate of the range of effects of probiotics, if there was heterogeneity between studies. The heterogeneity among studies was evaluated by χ2 test. Where possible, the potential cause of heterogeneity was interpreted by subgroup analyses and sensitivity analyses. If there were more than 10 studies eligible, the Egger test and funnel plots were performed to detect publication bias,[23] and P< 0.10 was used to define the presence of possible publication bias. All statistical analyses were carried out using the Review Manager version 5.3 software and R software version 3.6.0.
Subgroup analysis
Subgroup analysis was performed to determine the potential cause of heterogeneity and the effectiveness of different probiotics. Therefore, the different strains and species of probiotics were divided into subgroups for analysis.
Sensitivity analysis
To explore the possible sources of heterogeneity, we removed each study by turn, and re-analyzed the remaining studies. The results before and after removing were compared to determine the stability of the integrative results.
RESULTS
Results of the search
Based on our search criteria, we identified 805 papers from electronic databases and other sources, of which 150 duplicate articles were excluded. The remaining 655 studies were screened through titles, abstracts, and full texts, 28 trial reports were identified for the final meta-analysis. A detailed flowchart of the selection process is shown in Figure 1.
Figure 1.
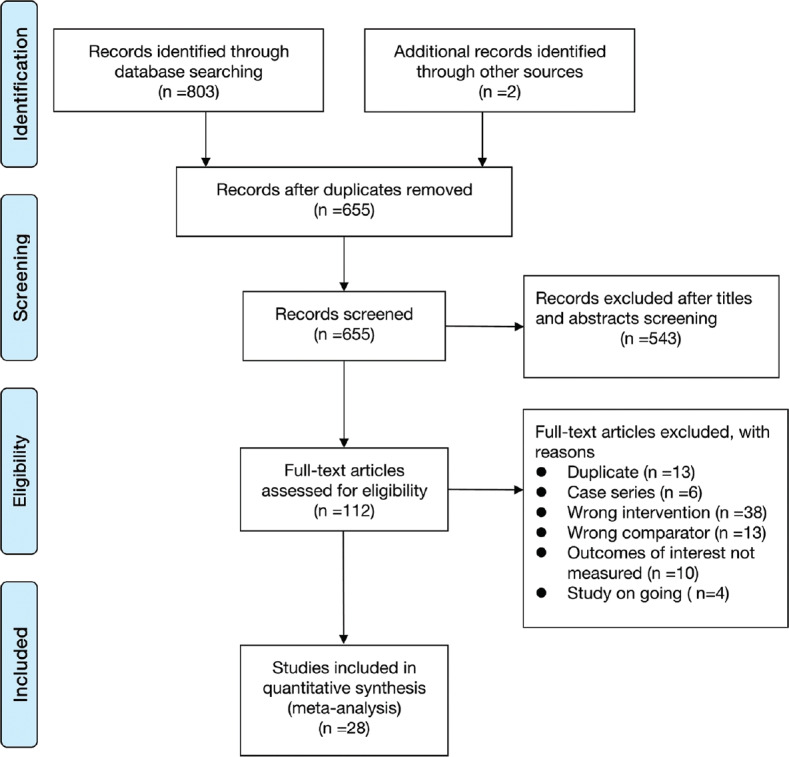
PRISMA diagram
Study characteristics
Twenty-eight RCTs, with a total of 3606 participants, met the inclusion criteria and were included in this review. Sample sizes ranged from 25 to 391, and the proportion of women in trials ranged between 20% and 100%. The time of treatment ranged from 4 to 24 weeks. Eighteen trials used a combination of probiotics, six Lactobacillus, two Bifidobacterium, two E. coli, one Saccharomyces. Detailed characteristics of included RCTs are provided in Table 2.
Table 2.
Characteristics of randomized controlled trials of probiotics vs. placebo in irritable bowel syndrome
| Authors (year) | Region | Sample size (% female) age range | Diagnostic criteria for IBS | Probiotic used and duration of therapy | Measured outcomes included | Jadad score |
|---|---|---|---|---|---|---|
| Kim et al.[40] (2003) | USA | 25 (72) 18-75 |
RomeII,100% IBS-D | Combination One packet containing VSL#3 (225 billion bacteria/packet) b.i.d. for 8 weeks |
Relief of overall IBS symptoms Abdominal pain scores Bloating scores |
5 |
| O’Mahony et al.[50] (2005) | Ireland | 75 (64) 18-75 |
Rome II, subtype not reported | Combination Malted drink containing L. salivarius UCC4331 (1×1010 live bacteria/drink) or B. infantis 35624(live bacteria/drink) q.d. for 8 weeks |
Abdominal pain scores Bloating scores |
5 |
| Kajander et al.[39] (2005) | Finland | 103 (76.7) 20-65 |
Rome I, 47.6% IBS-D, 23.3% IBS-C, 29.1% IBS-M | Combination One capsule containing L. rhamnosus GG,L. rhamnosus Lc705, Propionibacterium freu- denreichii, and Bifidobacterium breve Bb99 (8-9×109 c.f.u./capsule) o.d. for 6 months |
Relief of overall IBS symptoms Overall IBS symptom scores Bloating scores |
3 |
| Whorwell et al.[32] (2006) | UK | 362 (100) 18-65 |
RomeII, subtype not reported | One capsule containing B. infantis 35624 (1×106 live bacteria/capsule, 1×108 live bacteria/capsule, or 1×1010 live bacteria/capsule) o.d. for 4 weeks | Relief of overall IBS symptoms,Abdominal pain scores Bloating scores |
5 |
| Guyonnet et al.[48] (2007) | France | 267 (74.5) 18-65 |
RomeII, subtype not reported | Combination Fermented milk (125 g) containing B. animalis DN173 010 (1.25×1010 c.f.u./125 g) S. thermophilus (1.2×109 c.f.u./125 g) and L. bulgaricus (1.2×109 c.f.u./125 g) b.i.d. for 6 weeks |
Abdominal pain scores Bloating scores Adverse events |
4 |
| Drouault-Holowacz et al.[35] (2008) | France | 106 (76) Unclear |
Rome II, 29% IBS-D, 29% IBSC, 41% IBS-A, 1% Unclassified | One sachet containing B. longum LA 101, L. acidophilus LA 102, L. lactis LA 103, and S. thermophilus LA 104 (1×1010 c.f.u./sachet) o.d. for 4 weeks | Relief of overall IBS symptoms | 5 |
| Sinn et al.[31] (2008) | Korea | 40 (65) 18-70 |
RomeIII, 10% IBS-D, 27.5% IBS-C, 62.5% | One capsule containing L. acidophilus SDC 2012 and 2013 (2×109 c.f.u./ml) b.i.d. for 4 weeks | Relief of overall IBS symptoms | 5 |
| Zeng et al.[46] (2008) | China | 29 (65.5) Unclear |
RomeII, 100% IBS-D | Combination Fermented milk (200 ml) containing S. thermophilus (1×108 c.f.u./ml), L. bulgaricus (1×107 c.f.u./ml), L. acidophilus (1×107 c.f.u./ml), and B. longum (1×107 c.f.u./ml) b.i.d. for 4 weeks |
Relief of overall IBS symptoms Abdominal pain scores Bloating scores |
4 |
| Enck et al.[45] (2009) | Germany | 298 (49.3) 18-70 |
clinical criteria, subtype not reported | E. coli DSM17252 (1.5-4.5×107 c.f.u./ml) 0.75 ml drops t.i.d. for 1 week, then 1.5 ml t.i.d. for weeks 2-8 | Relief of overall IBS symptoms Adverse events |
4 |
| Williams et al.[51] (2009) | UK | 52 (86.5) Unclear |
RomeII, subtype not reported | Combination One capsule containing L. acidophilus CUL- 60 NCIMB 30157 and CUL-21 NCIMB 30156, B. bifidum CUL-20 NCIMB 30153, and B. lactis CUL-34 NCIMB 30172 (2.5×1010 c.f.u./capsule) o.d. for 8 weeks |
Overall IBS symptom scores Bloating scores Flatulence scores Quality of life scores |
4 |
| Hong et al.[38] (2009) | Korea | 70 (32.9) 19-75 |
RomeIII, 45.7% IBS-D, 20% IBS-C, 8.5% IBS-M, 25.8% Unclassified | Combination One sachet containing B. bifidum BGN4, B. lactis AD011, L. acidophilus AD031, and L. casei IBS041 (20 billion bacteria/sachet) b.i.d. for 8 weeks |
Relief of overall IBS symptoms Adverse events |
5 |
| Simren et al.[42] (2010) | Sweden | 74 (70.3) 18-70 |
RomeII, 35.1% IBS-D, 14.9% IBS-C, 50% IBS-M | Combination Fermented milk (200 ml) containing L. paracasei ssp paracasei F19, L. acidophilus La5, and B. lactis Bb12 (5×107 c.f.u./ml) b.i.d. for 8 weeks |
Relief of overall IBS symptoms Abdominal pain scores Bloating scores Flatulence scores |
5 |
| Guglielmetti et al.[36] (2011) | Germany | 122 (67.2) 18-68 |
RomeIII, 21.3% IBS-D, 19.7% IBS-C, 59% IBS-M | Bifidobacterium | Relief of overall IBS symptoms, Abdominal pain scores, Bloating scores, Flatulence scores, adverse events | 5 |
| Ducrotte et al.[44] (2012) | France | 214 (29.4) 18-70 |
RomeIII, subtype not reported | One capsule containing L. plantarum LP299V DSM 9843 (10 billion c.f.u./capsule) o.d. for 4 weeks | Relief of overall IBS symptoms Overall IBS symptom scores |
5 |
| Cui et al.[34] (2012) | China | 60 (70) Unclear |
RomeIII, 48.3% IBS-D, 30% IBS-C, 11.7% IBS-M, 10% Unclassified | Combination Two capsules containing B. longum and L. acidophilus t.i.d. for 4 weeks |
Relief of abdominal pain | 3 |
| Kruis et al.[24] (2012) | Germany | 120 (76.7) 18-65 |
RomeII, subtype not reported | One capsule containing E. coli Nissle 1917 (2.5-25×109 c.f.u./capsule) o.d. for 4 days then b.i.d. for 12 weeks | Relief of overall IBS symptoms Adverse events |
5 |
| Roberts et al.[41] (2013) | UK | 179 (83.2) 18-65 |
RomeIII, 100% IBS-C or IBS-M | Combination One pot containing B. lactis I-2494 (previously known as DN173 010) (1.25×1010 c.f.u./pot), S. thermophilus I-1630 (1.2×109 c.f.u./pot), and L. bulgaricus I-1632 and I-1519 (1.2×109 c.f.u./pot) b.i.d. for 12 weeks |
Relief of overall IBS symptoms | 4 |
| Begtrup et al.[33] (2013) | Denmark | 131 (30) 18-50 |
RomeIII, 40.5% IBS-D, 19.1% IBS-C, 38.2% IBS-M, 2.2% Unclassified | Combination Four capsules containing L. paracasei ssp paracasei F19, L. acidophilus La5, and B. lactis Bb12 (1.3×1010 c.f.u./capsule) o.d. for 6 months |
Relief of overall IBS symptoms, Abdominal pain scores, bloating scores, flatulence scores, quality of life scores | 5 |
| Sisson et al.[43] (2014) | UK | 286 (45.1) 18-65 |
RomeIII, 37.6% IBS-D,21.5% IBS-C, 35.5% IBS-M.5.4% Unclassified | Combinationsuspension of barley extract (1 ml/kg) containing Lactobacillus rhamnosus NCIMB 30174, Lactobacillus plantarum NCIMB 30173, Lactobacillus acidophilus NCIMB 30175 and Enterococcus faecium NCIMB 30176 (1×1010 c.f.u./50 ml) o.d. for 12 weeks | Relief of overall IBS symptoms, Abdominal pain scores, bloating scores, flatulence scores, quality of life scores, adverse events | 5 |
| Jafari et al.[25] (2014) | Iran | 108 (60.2) 20-70 |
RomeIII, subtype not reported | Combinationone capsule containing Bifidobacterium animalis subsp. lactisBB-12®, Lactobacillus acidophilus LA-5®, Lactobacillus delbrueckii subsp. bulgaricus LBY-27, Streptococcus thermophilus STY-31 (4×108 c.f.u./capsule) b.i.d. for 4 weeks | Relief of overall IBS symptoms | 5 |
| Ludidi et al.[26] (2014) | Netherlands | 40 (67.5) 18-65 |
RomeIII, 42.5% IBS-D, 10% IBS-C, 30% IBS-M, 17.5% Unclassified |
Combinationone sachet containing Bifidobacterium lactis W52, Lactobacillus casei W56, Lactobacillus salivarius W57, Lactococcus lactis W58, Lactobacillus acidophilus NCFM, and Lactobacillus rhamnosus W71 (5×109 c.f.u./sachet) o.d. for 6 weeks | Relief of overall IBS symptoms Abdominal pain scores Bloating scores |
4 |
| Wong et al.[47] (2015) | Singapore | 42 (45.2) 20-76 |
RomeIII, subtype not reported | Combinationfour capsules containing VSL#3 (225 billion bacteria/capsule) b.i.d. for 6 weeks | Overall IBS symptom scores Bloating scores |
4 |
| Pineton de Chambrun et al.[29] (2015) | France | 200 (20) 18-75 |
RomeIII, 28.5% IBS-D, 46.9% IBS-C, 24.6% IBS-M | one capsule containing S. cerevisiae CNCM I-3856 (4×109 c.f.u./capsule) o.d. for 8 weeks | Relief of overall IBS symptoms Overall IBS symptom scores Adverse events |
3 |
| Mezzasalma et al.[28] (2016) | Italy | 157 (not reported) 18-65 |
RomeIII, 100% IBS: C | Combination one capsule containing L. acidophilus, L. reuteri, L. plantarum, L. rhamnosus, and B. animalis subsp. lactis (1.5×1010 c.f.u./capsule) o.d. for 2 months | Relief of overall IBS symptoms Flatulence scores Quality of life scores |
3 |
| Lyra et al.[27] (2016) | Finland | 391 (74.7) 18-65 |
RomeIII, 38.9% IBS-D, 16.6% IBS-C, 44% IBS-M, 0.5% Unclassified | one capsule containing L. acidophilus NCFM (ATCC 700396) (low dose: 1×109 c.f.u./capsule, high dose: 1×1010 c.f.u./capsule) o.d. for 12 weeks | Relief of overall IBS symptoms, abdominal pain scores, flatulence scores, quality of life scores, adverse events | 3 |
| Hod et al.[37] (2017) | Isreal | 107 (not reported) 18-70 |
RomeIII, 100% IBS-D | Combinationone capsule c Lactobacillus rhamnosus LR5 (3×109 CFU/capsule), L. casei LC5 (2×109 CFU/capsule), L. paracasei LPC5 (1×109 CFU/capsule), L. plantarum LP3 (1×109 CFU/capsule), L. acidophilus LA1 (5×1099 CFU/capsule), Bifidobacterium bifi- dum BF3 (4×109 CFU/capsule), B. longum BG7 (1×109 CFU/capsule), B. breve BR3 (2×109 CFU/capsule), B. infantis BT1 (1×109 CFU/capsule), Streptococcus thermophilus ST3 (2×109 CFU/capsule), L. bulgaricus LG1, and Lactococcus lactis SL6 (3×109 CFU/capsule) b.i.d. for 8 weeks |
Relief of overall IBS symptoms | 4 |
| Ishaque, S. M et al.[49] (2018) | Bangladesh | 360 (21.9) 18-55 |
RomeIII, 100% IBS-D | Combination two capsules containing Bacillus subtilis PXN 21, Bifidobacterium spp. (B. bifidum PXN 23, B. breve PXN 25, B. infantis PXN 27, B. longum PXN 30), Lactobacillus spp. (L. acidophilus PXN 35, L. delbrueckii spp. Bulgaricus PXN39, L. casei PXN 37, L. plantarum PXN 47, L. rhamnosus PXN 54, L.helveticus PXN 45, L. salivarius PXN 57), Lactococcus lactis PXN 63, and Streptococcus thermophilus PXN 66 (2×109 c.f.u./capsule) b.i.d. for 16 weeks | Abdominal pain scores Bloating scores Flatulence scores Quality of life scores |
3 |
| Shin et al.[30] (2018) | Korea | 51 (56.9) 20-55 |
RomeIII, 100% IBS:D | two capsules containing L. gasseri BNR17 (1×1010 c.f.u./day) b.i.d. for 8 weeks | Relief of overall IBS symptoms | 4 |
Combination: denotes a mixture of probiotics; c.f.u=Colony-forming units; IBS=Irritable bowel syndrome; o.d.=Once daly.
Risk of bias
We judged the risk of bias in the included trials [Figure 2]. Sixteen trials described the method of randomization used. Sixteen trials assessed whether adequate concealment of allocation procedure was used, and 24 trials reported methods for blinding participants. Twenty trials described intention-to-treat analyses (ITT) and reported follow-up data. Selective reporting was not found. Therefore, all of the included trials were determined to have a moderate risk of bias. The quality of studies was generally good, with 22 (75.9%) studies scoring at least 4 out of 5 on the Jadad scale [Table 2].
Figure 2.
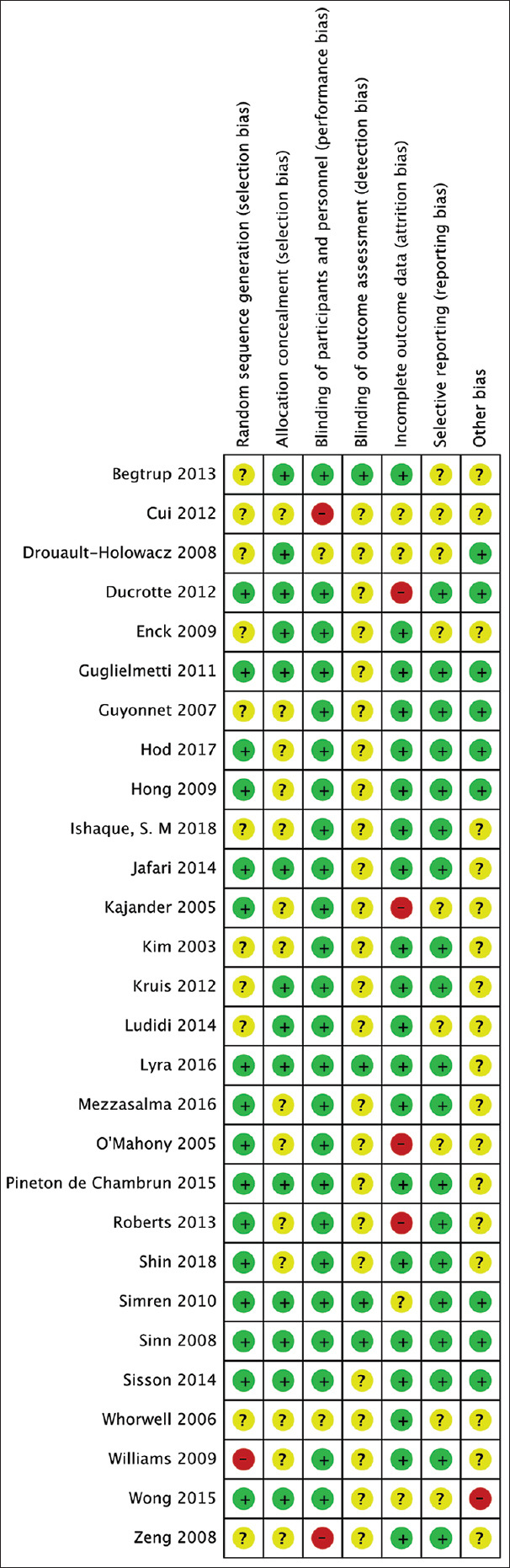
Risk of bias of the included studies. Green, low risk; yellow, unclear; red, high risk
Sensitivity analysis
The influence of a single study on the overall meta-analysis estimate was investigated by removing one study at a time, and the result showed no significant difference, which indicated our results were statistically reliable.
Main Outcomes and Measures
Efficacy of probiotics in the treatment of IBS: effect on symptom relief
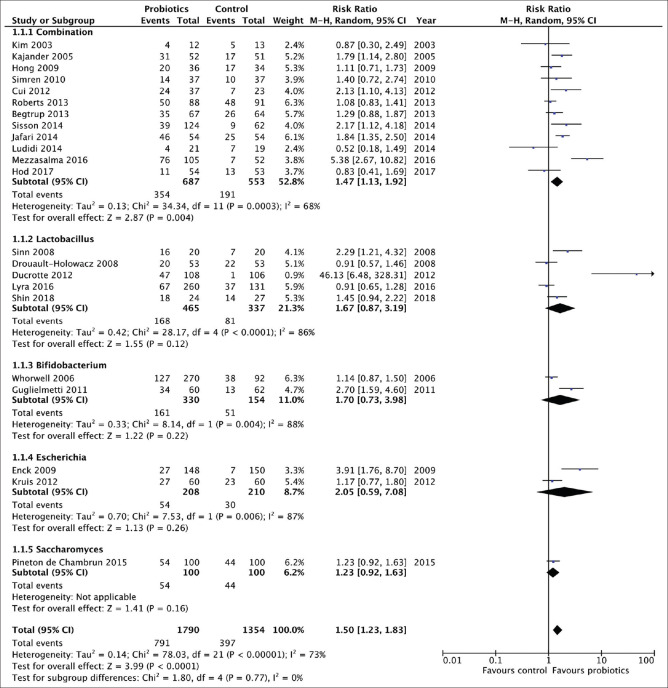
Subgroup analysis was performed by different species and strains. Finally, there were 22 RCTs comparing probiotics with placebo for the treatment of IBS,[24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45] evaluating 3144 patients, which gave outcomes as a dichotomous variable [Figure 3]. The RR of IBS symptoms improving after treatment with probiotics vs. placebo was 1.50 (95% CI 1.23–1.83), with statistically significant heterogeneity detected between studies (I2 = 68%, P < 0.01; Figure 3), There was statistically significant asymmetry detected in the funnel plot (Egger test, P = 0.04), to suggest publication bias or other small study effects. The NNT with probiotics was 5 (95% CI 3–8.7).
Figure 3.
Forest plot of randomized controlled trials of probiotics vs placebo in irritable bowel syndrome: effect on symptom relief
Combination probiotics were assessed in 12 RCTs,[25,26,28,33,34,37,38,39,40,41,42,43] containing 1240 patients, with a significant effect on symptoms (RR = 1.47; 95% CI 1.13–1.92; Figure 3), but with significant heterogeneity between studies (I2 = 68%, P< 0.01), and asymmetry was not detected in the funnel plot (Egger test, P= 0.78). The NNT with combination probiotics was 6 (95% CI 4.5–8.7).
Lactobacillus was used in five trials (802 patients),[27,30,31,35,44] with no clear benefit detected over placebo (RR = 1.67; 95% CI 0.87-3.19; Figure 3), again with significant heterogeneity between studies (I2 = 86%, P< 0.01). Bifidobacterium was studied in two RCTs (484 patients),[32,36] with no benefit over placebo (RR = 1.70; 95% CI 0.73-3.98; Figure 3). E. coli was assessed in two trials (418 patients),[24,45] with little or no benefit detected compared with placebo (RR = 2.05; 95% CI 0.59-7.08; Figure 3). Finally, Saccharomyces was used in one trial recruiting 100 patients,[29] and had little or no beneficial effect on IBS symptoms compared with placebo (RR = 1.23; 95% CI 0.92–1.63; Figure 3).
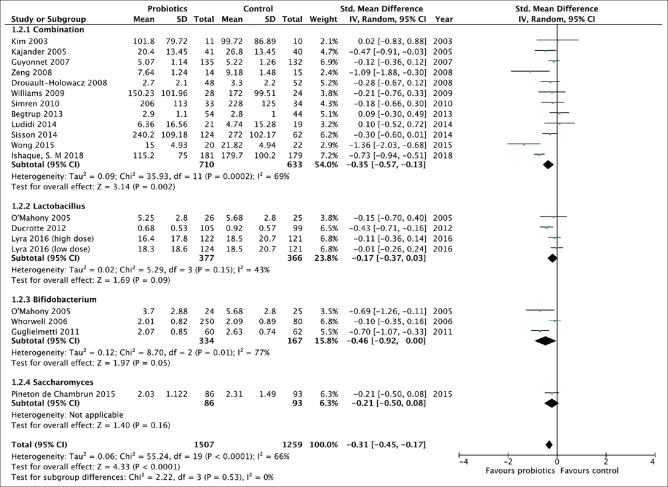
Efficacy of probiotics in the treatment of IBS: effect on overall IBS and abdominal pain symptom scores
There were 18 separate trials,[26,27,29,32,33,35,36,39,40,42,43,44,46,47,48,49,50,51] making 20 comparisons, containing 2766 patients that reported effect of probiotics on overall IBS or abdominal pain scores [Figure 4]. There was a statistically significant effect of probiotics in reducing overall IBS symptoms or abdominal pain (SMD = -0.31; 95% -0.45 to -0.17) with significant heterogeneity (I2 = 66%, P< 0.01; Figure 4). There was no significant asymmetry detected in the funnel plot (Egger test, P= 0.84), to suggest no publication bias or other small study effects. There were three trials (743 patients) that evaluated Lactobacillus,[27,44,50] and three trials (511 patients) that investigated Bifidobacterium,[32,36,50] and neither were statistically significantly more efficacious than placebo [Figure 4], although there was a trend towards a benefit for the latter (SMD = -0.46; 95% CI -0.92 to 0, P= 0.05).
Figure 4.
Forest plot of randomized controlled trials of probiotics vs placebo in irritable bowel syndrome: effect on overall IBS or abdominal pain scores
There were 12 trials,[26,33,35,39,40,42,43,46,47,48,49,51] evaluating 1343 patients, using combinations of probiotics that did suggest a significant improvement in overall IBS symptoms score with active treatment (SMD = -0.35; 95% CI -0.57 to -0.13; Figure 4), with significant heterogeneity between study results (I2 = 68%, P< 0.01). Asymmetry was also not detected in the funnel plot (Egger test, P= 0.82).
Efficacy of probiotics in the treatment of IBS: effect on individual symptom scores
There were 15 separate trials,[26,27,32,33,36,39,40,42,43,46,47,48,49,50,51] making 17 comparisons, and containing 2283 patients, which reported the effect of probiotics on bloating symptom scores. Overall, there was little reduction in bloating scores with probiotics (SMD = -0.20; 95% CI -0.38 to -0.01), with significant heterogeneity between individual study results (I2 = 76%, P< 0.01)
Three trials reported continuous data for the effect of probiotics on flatulence symptom scores in 407 patients,[32,40,46] with a significant benefit over placebo (SMD = -0.27; 95% CI -0.65 to 0.11).
Finally, five RCTs reported the effect of probiotics on quality of life (Qol) in 856 patients.[28,33,43,49,51] There was no apparent benefit detected for probiotics (SMD = -0.07; 95% CI -0.74 to 0.6).
Adverse events with probiotics
Total adverse events were reported by 8 RCTs, containing 1654 patients.[24,27,29,36,38,43,45,48] Overall, 323 (35%) of 923 patients allocated to probiotics experienced any adverse event, compared with 245 (33.5%) of 731 assigned to placebo. The RR of experiencing any adverse event was not significantly higher with probiotics (1.05; 95% CI 0.85–1.31), but there was significant heterogeneity between studies (I2 = 56%, P= 0.03).
DISCUSSION
Probiotics have been used to prevent, mitigate or treat specific diseases for years. A multitude of clinical trials have investigated the use of probiotics for diseases ranging from necrotizing colitis in premature infants to hypertension in adults.[52,53] Although probiotics have also been used clinically to improve the symptoms of IBS for a long time, the precise efficacy of probiotics in IBS is largely unknown. As probiotics have different strains and species, there is no definite conclusion as to which strain and species are more effective. Probiotics have been used safely in foods and dairy products for over a hundred years. However, safety outcomes are inconsistently reported in published clinical trials.[54] In the current study, we assessed the efficacy and safety of probiotics in IBS, and we detected the effect of different strains and species of probiotics by subgroup analysis.
The key finding of our review is that particular combinations of probiotics, appear to have beneficial effects in IBS in terms of effect on overall IBS symptoms and abdominal pain. However, for specific species and strains of probiotics, we could not find sufficient evidence to support their effectiveness. In addition, on account of the existence of significant heterogeneity between studies, and evidence of publication bias in some analyses, it is difficult to draw definitive conclusions about the efficacy and safety of probiotics. In this study, Lactobacillus, E. coli and Saccharomyces showed little or no beneficial effect and there was a trend towards a beneficial effect of Bifidobacterium, in terms of improvement of overall IBS symptoms and abdominal pain scores. Probiotics may have little beneficial effect on bloating scores, and if at all, then the particular strain or species remains unclear.
A previous study showed better beneficial effects of combination probiotics than individual probiotics in IBS, which is a similar conclusion to ours after incorporating two new studies.[55] However, after pooling one new study, our analysis showed that probiotics have no beneficial effect on flatulence, a conclusion different from the previous study. The possible reason for this different outcome that studies included in our current analysis are not quite sufficient, and the two new studies may have an effect on the pooled result. However, there is adequate evidence that combination probiotics provide efficacy in IBS.
In the current systematic review and meta-analysis, eight studies reported adverse events including abdominal pain, diarrhea, constipation, nausea, and skin reactions, which were generally tolerated. Besides, the appearance of adverse events was not significantly different for disparate interventions, which indicated that probiotics were secure for IBS patients.
The strength of this systematic review and meta-analysis is that we performed a subgroup analysis to detect the effectiveness of various strains and species of probiotics and assessed the safety of probiotics in IBS. There are limitations to this analysis, which arise from the nature of the studies available for synthesis. The risk of bias of many of the trials that we identified was unclear, and there was evidence of heterogeneity between RCTs in some of our analyses, although there was no evidence of publication bias among trials of probiotics in IBS. In addition, although we attempted to uncover the species and strains of probiotics which were effective, there were a limited number of trials in some of these subgroup analyses, meaning that we may have had insufficient power to detect any meaningful difference in effect. Therefore, it remains unclear whether a particular combination of probiotics is more likely to be effective, or whether there is a particular IBS subtype that is more likely to benefit. Finally, individual strains of probiotics may have different effects, and pooling all studies from a given species may obscure the beneficial effects of individual strains within that species, although if there were more evaluable studies examining each of these individual strains then we would perhaps be able to make judgments about their efficacy, and compare the efficacy between strains.
In summary, this meta-analysis shows that probiotics can significantly improve the overall symptoms of IBS and abdominal pain scores. Combinations of probiotics appears to show a significant improvement in overall IBS scores. However, as there were various combinations of probiotics, we could not ascertain which combination of probiotics is more effective. Different doses may affect the efficacy of probiotics, therefore, further research should focus on combination probiotics with a fixed dose.
CONCLUSION
The current review has demonstrated that specific combinations of probiotics seem to have beneficial effects on overall IBS symptoms and abdominal pain. However, due to the limited combinations of probiotics and large heterogeneity in the included studies, it is unclear how precisely probiotics can facilitate the relief of IBS symptoms and which particular combination can be the best. Therefore, future research should pay more attention to detect the effect of different probiotic combinations on IBS.
Contributors
LQ.J proposed the concept of the study, JR.S is the drafter of the manuscript. CF.K, LQ.J contributed to revising the manuscript. LQ.J made the search strategy. JR.S and CF.K independently screened eligible studies, extracted data, assessed the quality of included studies and entered data into Review Manager for data synthesis. All authors participated in the design of the study and approved the final manuscript.
Financial support and sponsorship
National Natural Science Foundation of China (NSFC) (NO.81573779).
Conflicts of interest
We declare that we do not have any commercial or associative interest that represents a conflict of interest in connection with the work submitted.
REFERENCES
- 1.Mearin F, Lacy BE, Chang L, Chey WD, Lembo AJ, Simren M, et al. Bowel disorders. Gastroenterology. 2016;150:1393–407. doi: 10.1053/j.gastro.2016.02.031. [DOI] [PubMed] [Google Scholar]
- 2.Canavan C, West J, Card T. The epidemiology of irritable bowel syndrome. Clin Epidemiol. 2014;6:71–80. doi: 10.2147/CLEP.S40245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Longstreth GF, Thompson WG, Chey WD, Houghton La, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006;130:1480–91. doi: 10.1053/j.gastro.2005.11.061. [DOI] [PubMed] [Google Scholar]
- 4.Astegiano M, Pellicano R, Sguazzini C, Berrutti M, Simondi D, Reggiani S, et al. 2008 clinical approach to irritable bowel syndrome. Minerva Gastroenterol Dietol. 2008;54:251–7. [PubMed] [Google Scholar]
- 5.Lovell RM, Ford AC. Global prevalence of and risk factors for irritable bowel syndrome: A meta-analysis. Clin Gastroenterol Hepatol. 2012;10:712–21.e4. doi: 10.1016/j.cgh.2012.02.029. [DOI] [PubMed] [Google Scholar]
- 6.Quigley EM, Fried M, Gwee KA, Khalif I, Hungin AP, Lindberg G, et al. World Gastroenterology Organisation Global Guidelines Irritable Bowel Syndrome: A Global Perspective Update September 2015. J Clin Gastroenterol. 2016;50:704–13. doi: 10.1097/MCG.0000000000000653. [DOI] [PubMed] [Google Scholar]
- 7.Quartero AO, Meineche-Schmidt V, Muris J, Rubin G, de Wit N. Bulking agents, antispasmodic and antidepressant medication for the treatment of irritable bowel syndrome. Cochrane Database Syst Rev. 2005:CD003460. doi: 10.1002/14651858.CD003460.pub2. [DOI] [PubMed] [Google Scholar]
- 8.Holtmann GJ, Ford AC, Talley NJ. Pathophysiology of irritable bowel syndrome. Lancet Gastroenterol Hepatol. 2016;1:133–46. doi: 10.1016/S2468-1253(16)30023-1. [DOI] [PubMed] [Google Scholar]
- 9.Simren M, Brazier J, Coremans G, Dapoigny M, Müller-Lissner SA, Pace F, et al. Quality of life and illness costs in irritable bowel syndrome. Digestion. 2004;69:254–61. doi: 10.1159/000079846. [DOI] [PubMed] [Google Scholar]
- 10.Jeffery IB, O'Toole PW, Öhman L, Claesson MJ, Deane J, Quigley EM, et al. An irritable bowel syndrome subtype defined by species-specific alterations in faecal microbiota. Gut. 2012;61:997–1006. doi: 10.1136/gutjnl-2011-301501. [DOI] [PubMed] [Google Scholar]
- 11.Rajilić-Stojanović M, Biagi E, Heilig HG, Kajander K, Kekkonen RA, Tims S, et al. Global and deep molecular analysis of microbiota signatures in fecal samples from patients with irritable bowel syndrome. Gastroenterology. 2011;141:1792–801. doi: 10.1053/j.gastro.2011.07.043. [DOI] [PubMed] [Google Scholar]
- 12.Jalanka J, Salonen A, Fuentes S, de Vos WM. Microbial signatures in post-infectious irritable bowel syndrome-toward patient stratification for improved diagnostics and treatment. Gut Microbes. 2015;6:364–9. doi: 10.1080/19490976.2015.1096486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tap J, Derrien M, Törnblom H, Brazeilles R, Cools-Portier S, Doré J, et al. Identification of an intestinal microbiota signature associated with severity of irritable bowel syndrome. Gastroenterology. 2016;152:111–23. doi: 10.1053/j.gastro.2016.09.049. [DOI] [PubMed] [Google Scholar]
- 14.Durban A, Abellán JJ, Jiménez-Hernández N, Artacho A, Garrigues V, Ortiz V, et al. Instability of the faecal microbiota in diarrhoea-predominant irritable bowel syndrome. FEMS Microbiol Ecol. 2013;86:581–9. doi: 10.1111/1574-6941.12184. [DOI] [PubMed] [Google Scholar]
- 15.Carroll IM, Ringel-Kulka T, Siddle JP, Ringel Y. Alterations in composition and diversity of the intestinal microbiota in patients with diarrhoea-predominant irritable bowel syndrome. Neurogastroenterol Motil. 2012;24:521–30; e248. doi: 10.1111/j.1365-2982.2012.01891.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jalanka-Tuovinen J, Salojärvi J, Salonen A, Immonen O, Garsed K, Kelly FM, et al. Faecal microbiota composition and host-microbe cross-talk following gastroenteritis and in postinfectious irritable bowel syndrome. Gut. 2014;63:1737–45. doi: 10.1136/gutjnl-2013-305994. [DOI] [PubMed] [Google Scholar]
- 17.Kassinen A, Krogius-Kurikka L, Mäkivuokko H, Rinttilä T, Paulin L, Corander J, et al. The fecal microbiota of irritable bowel syndrome patients differs significantly from that of healthy subjects. Gastroenterology. 2007;133:24–33. doi: 10.1053/j.gastro.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 18.Jones ML, Martoni CJ, Tamber S, Parent M, Prakash S. Evaluation of safety and tolerance of microencapsulated Lactobacillus reuteri NCIMB 30242 in a yogurt formulation: A randomized, placebo-controlled, double-blind study. Food Chem Toxicol. 2012;50:2216–23. doi: 10.1016/j.fct.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 19.Clarke G, Cryan JF, Dinan TG, Quigley EM. Review article: Probiotics for the treatment of irritable bowel syndrome-focus on lactic acid bacteria. Aliment Pharmacol Ther. 2012;35:403–13. doi: 10.1111/j.1365-2036.2011.04965.x. [DOI] [PubMed] [Google Scholar]
- 20.Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomized trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 22.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 23.Egger M, Davey-Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kruis W, Chrubasik S, Boehm S, Stange C, Schulze J. A double-blind placebo-controlled trial to study therapeutic effects of probiotic Escherichia coli Nissle 1917 in subgroups of patients with irritable bowel syndrome. Int J Colorectal Dis. 2012;27:467–74. doi: 10.1007/s00384-011-1363-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jafari E, Vahedi H, Merat S, Momtahen S, Riahi A. Therapeutic effects, tolerability and safety of a multi-strain probiotic in Iranian adults with irritable bowel syndrome and bloating. Arch Iran Med. 2014;17:466–70. [PubMed] [Google Scholar]
- 26.Ludidi S, Jonkers DM, Koning CJ, Kruimel JW, Mulder L, van der Vaart IB, et al. Randomized clinical trial on the effect of a multispecies probiotic on visceroperception in hypersensitive IBS patients. Neurogastroenterol Motil. 2014;26:705–14. doi: 10.1111/nmo.12320. [DOI] [PubMed] [Google Scholar]
- 27.Lyra A, Hillila M, Huttunen T, Mannikko S, Taalikka M, Tennila J, et al. Irritable bowel syndrome symptom severity improves equally with probiotic and placebo. World J Gastroenterol. 2016;22:10631–42. doi: 10.3748/wjg.v22.i48.10631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mezzasalma V, Manfrini E, Ferri E, Sandionigi A, La Ferla B, Schiano I, et al. A randomized, double-blind, placebo-controlled trial: The efficacy of multispecies probiotic supplementation in alleviating symptoms of irritable bowel syndrome associated with constipation. Biomed Res Int. 2016;2016:4740907. doi: 10.1155/2016/4740907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pineton de Chambrun G, Neut C, Chau A, Cazaubiel M, Pelerin F, Justen P, et al. A randomized clinical trial of Saccharomyces cerevisiae versus placebo in the irritable bowel syndrome. Dig Liver Dis. 2015;47:119–24. doi: 10.1016/j.dld.2014.11.007. [DOI] [PubMed] [Google Scholar]
- 30.Shin SP, Choi YM, Kim WH, Hong SP, Park JM, Kim J, et al. A double blind, placebo-controlled, randomized clinical trial that breast milk derived-Lactobacillus gasseri BNR17 mitigated diarrhea-dominant irritable bowel syndrome. J Clin Biochem Nutr. 2018;62:179–86. doi: 10.3164/jcbn.17-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sinn DH, Song JH, Kim HJ, Lee JH, Son HJ, Chang DK, et al. Therapeutic effect of Lactobacillus acidophilus-SDC 2012, 2013 in patients with irritable bowel syndrome. Dig Dis Sci. 2008;53:2714–8. doi: 10.1007/s10620-007-0196-4. [DOI] [PubMed] [Google Scholar]
- 32.Whorwell PJ, Altringer L, Morel J, Bond Y, Charbonneau D, O'Mahony L, et al. Efficacy of an encapsulated probiotic Bifidobacterium infantis 35624 in women with irritable bowel syndrome. Am J Gastroenterol. 2006;101:1581–90. doi: 10.1111/j.1572-0241.2006.00734.x. [DOI] [PubMed] [Google Scholar]
- 33.Begtrup LM, de Muckadell OB, Kjeldsen J, Christensen RD, Jarbol DE. Long-term treatment with probiotics in primary care patients with irritable bowel syndrome--a randomised, double-blind, placebo controlled trial. Scand J Gastroenterol. 2013;48:1127–35. doi: 10.3109/00365521.2013.825314. [DOI] [PubMed] [Google Scholar]
- 34.Cui S, Hu Y. Multistrain probiotic preparation significantly reduces symptoms of irritable bowel syndrome in a double-blind placebo-controlled study. Int J Clin Exp Med. 2012;5:238–44. [PMC free article] [PubMed] [Google Scholar]
- 35.Drouault-Holowacz S, Bieuvelet S, Burckel A, Cazaubiel M, Dray X, Marteau P. A double blind randomized controlled trial of a probiotic combination in 100 patients with irritable bowel syndrome. Gastroenterol Clin Biol. 2008;32:147–52. doi: 10.1016/j.gcb.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 36.Guglielmetti S, Mora D, Gschwender M, Popp K. Randomised clinical trial: Bifidobacterium bifidum MIMBb75 significantly alleviates irritable bowel syndrome and improves quality of life-a double-blind, placebo-controlled study. Aliment Pharmacol Ther. 2011;33:1123–32. doi: 10.1111/j.1365-2036.2011.04633.x. [DOI] [PubMed] [Google Scholar]
- 37.Hod K, Sperber AD, Ron Y, Boaz M, Dickman R, Berliner S, et al. A double-blind, placebo-controlled study to assess the effect of a probiotic mixture on symptoms and inflammatory markers in women with diarrhea-predominant IBS. Neurogastroenterol Motil. 2017:29. doi: 10.1111/nmo.13037. doi: 10.1111/nmo. 13037. [DOI] [PubMed] [Google Scholar]
- 38.Hong KS, Kang HW, Im JP, Ji GE, Kim SG, Jung HC. Effect of probiotics on symptoms in korean adults with irritable bowel syndome. Gut Liver. 2009;3:101–7. doi: 10.5009/gnl.2009.3.2.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kajander K, Hatakka K, Poussa T, Farkkila M, Korpela R. A probiotic mixture alleviates symptoms in irritable bowel syndrome patients: A controlled 6-month intervention. Aliment Pharmacol Ther. 2005;22:387–94. doi: 10.1111/j.1365-2036.2005.02579.x. [DOI] [PubMed] [Google Scholar]
- 40.Kim HJ, Camilleri M, McKinzie S, Lempke MB, Burton DD, Thomforde GM, et al. A randomized controlled trial of a probiotic, VSL#3, on gut transit and symptoms in diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther. 2003;17:895–904. doi: 10.1046/j.1365-2036.2003.01543.x. [DOI] [PubMed] [Google Scholar]
- 41.Roberts LM, McCahon D, Holder R, Wilson S, Hobbs FD. A randomised controlled trial of a probiotic 'functional food' in the management of irritable bowel syndrome. BMC Gastroenterol. 2013;13:45. doi: 10.1186/1471-230X-13-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Simren M, Ohman L, Olsson J, Svensson U, Ohlson K, Posserud I, et al. Clinical trial: The effects of a fermented milk containing three probiotic bacteria in patients with irritable bowel syndrome-a randomized, double-blind, controlled study. Aliment Pharmacol Ther. 2010;31:218–27. doi: 10.1111/j.1365-2036.2009.04183.x. [DOI] [PubMed] [Google Scholar]
- 43.Sisson G, Ayis S, Sherwood RA, Bjarnason I. Randomised clinical trial: A liquid multi-strain probiotic vs. placebo in the irritable bowel syndrome-a 12 week double-blind study. Aliment Pharmacol Ther. 2014;40:51–62. doi: 10.1111/apt.12787. [DOI] [PubMed] [Google Scholar]
- 44.Ducrotte P, Sawant P, Jayanthi V. Clinical trial: Lactobacillus plantarum 299v (DSM 9843) improves symptoms of irritable bowel syndrome. World J Gastroenterol. 2012;18:4012–8. doi: 10.3748/wjg.v18.i30.4012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Enck P, Zimmermann K, Menke G, Klosterhalfen S. Randomized controlled treatment trial of irritable bowel syndrome with a probiotic E.-coli preparation (DSM17252) compared to placebo. Z Gastroenterol. 2009;47:209–14. doi: 10.1055/s-2008-1027702. [DOI] [PubMed] [Google Scholar]
- 46.Zeng J, Li YQ, Zuo XL, Zhen YB, Yang J, Liu CH. Clinical trial: Effect of active lactic acid bacteria on mucosal barrier function in patients with diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther. 2008;28:994–1002. doi: 10.1111/j.1365-2036.2008.03818.x. [DOI] [PubMed] [Google Scholar]
- 47.Wong RK, Yang C, Song GH, Wong J, Ho KY. Melatonin regulation as a possible mechanism for probiotic (VSL#3) in irritable bowel syndrome: A randomized double-blinded placebo study. Dig Dis Sci. 2015;60:186–94. doi: 10.1007/s10620-014-3299-8. [DOI] [PubMed] [Google Scholar]
- 48.Guyonnet D, Chassany O, Ducrotte P, Picard C, Mouret M, Mercier CH, et al. Effect of a fermented milk containing Bifidobacterium animalis DN-173 010 on the health-related quality of life and symptoms in irritable bowel syndrome in adults in primary care: A multicentre, randomized, double-blind, controlled trial. Aliment Pharmacol Ther. 2007;26:475–86. doi: 10.1111/j.1365-2036.2007.03362.x. [DOI] [PubMed] [Google Scholar]
- 49.Ishaque SM, Khosruzzaman SM, Ahmed DS, Sah MP. A randomized placebo-controlled clinical trial of a multi-strain probiotic formulation (Bio-Kult(R)) in the management of diarrhea-predominant irritable bowel syndrome. BMC Gastroenterol. 2018;18:71. doi: 10.1186/s12876-018-0788-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.O'Mahony L, McCarthy J, Kelly P, Hurley G, Luo F, Chen K, et al. Lactobacillus and bifidobacterium in irritable bowel syndrome: Symptom responses and relationship to cytokine profiles. Gastroenterology. 2005;128:541–51. doi: 10.1053/j.gastro.2004.11.050. [DOI] [PubMed] [Google Scholar]
- 51.Williams EA, Stimpson J, Wang D, Plummer S, Garaiova I, Barker ME, et al. Clinical trial: A multistrain probiotic preparation significantly reduces symptoms of irritable bowel syndrome in a double-blind placebo-controlled study. Aliment Pharmacol Ther. 2009;29:97–103. doi: 10.1111/j.1365-2036.2008.03848.x. [DOI] [PubMed] [Google Scholar]
- 52.Bernardo W, Aires FT, Carneiro RM, Sá FP, Rullo VE, Burns DA. Effectiveness of probiotics in the prophylaxis of necrotizing enterocolitis in preterm neonates: A systematic review and meta-analysis. J Pediatr. 2013;89:18–24. doi: 10.1016/j.jped.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 53.Khalesi S, Sun J, Buys N, Jayasinghe R. Effect of probiotics on blood pressure: A systematic review and meta-analysis of randomized, controlled trials. Hypertension. 2014;64:897–903. doi: 10.1161/HYPERTENSIONAHA.114.03469. [DOI] [PubMed] [Google Scholar]
- 54.Doron S, Snydman DR. Risk and safety of probiotics. Clin Infect Dis. 2015;60:S129–34. doi: 10.1093/cid/civ085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ford AC, Harris LA, Lacy BE, Quigley EMM, Paul M. Systematic review with meta-analysis: The efficacy of prebiotics, probiotics, synbiotics and antibiotics in irritable bowel syndrome. Aliment Pharmacol Ther. 2018;48:1044–60. doi: 10.1111/apt.15001. [DOI] [PubMed] [Google Scholar]