Abstract
Background:
Recent advances in EUS techniques (real-time EUS elastography and contrast-enhanced EUS) have allowed a better characterization of focal pancreatic masses. Mean strain histograms (SHs) are considered a good parameter for the semi-quantitative evaluation of focal pancreatic masses, alongside complementary contrast-enhanced EUS parameters which can be quantified during both the early arterial and late venous phase.
Materials and Methods:
The study design was prospective, blinded, and multicentric, assessing real-time EUS elastography and contrast-enhanced EUS results for the characterization of focal pancreatic masses using parametric measurements, in comparison with pathology which is the gold standard. SHs were performed based on the embedded software of the ultrasound system, with the values being reversed as opposed to our initially published data on hue histograms. Consequently, a cutoff of 80 was derived from previous multicentric trials. Contrast-enhanced EUS also allowed the focal masses to be classified as hyper-, iso-, or hypoenhanced in comparison with the normal pancreatic parenchyma. EUS-FNA was then performed for all patients, with a positive cytological diagnosis taken as a final proof of malignancy for the pancreatic masses. The diagnoses obtained by EUS-FNA were verified further either by surgery or during a clinical follow-up of at least 6 months.
Results:
A total number of 97 consecutive patients with focal pancreatic masses were included in the study. Based on previously defined cutoffs of 80, the values of sensitivity, specificity, positive predictive value, negative predictive value, and accuracy of the mean SHs for the diagnosis of pancreatic cancer were 100%, 29.63%, 78.65%, 100%, and 80.41%, respectively. Corresponding values for contrast-enhanced EUS (taking into consideration hypoenhencement as a predictive factor of malignancy) were 98.57%, 77.78%, 92%, 95.45%, and 92.78%, respectively. Combining contrast enhancement-EUS (hypoenhencement) and semi-quantitative EUS elastography (SH cutoffs <80), the resulting values corresponding for sensitivity, specificity, and accuracy were 98.57%, 81.48%, and 93.81%, respectively.
Conclusion:
The current study using objective parametric tools for both EUS elastography and contrast-enhanced EUS confirmed the results of previous studies and meta-analyses that indicated a complementary role for the differential diagnosis of focal pancreatic masses. Moreover, the best values for the receiver operating curves were obtained using a sequential clinical algorithm based on the initial use of elastography, followed by contrast enhancement.
Keywords: Chronic pseudotumoral pancreatitis, contrast-enhanced harmonic imaging, EUS, pancreatic adenocarcinoma, pancreatic neuroendocrine tumors, strain histogram elastography
INTRODUCTION
Due to the fact that pancreatic cancer has a survival rate of 5% over a span of 5 years, it represents one of the most aggressive types of cancer, and the improvement of diagnostic methods is still a challenge. Imaging is essential for the diagnosis of pancreatic cancer, and among all imaging methods, EUS has proven to be the most effective one.[1] Recent advances in EUS-FNA techniques, as well as the development of real-time EUS elastography and contrast-enhanced EUS, have allowed a better characterization of focal pancreatic masses, with possible implications in the management of patients with negative EUS-FNA and a strong suspicion of malignancy. Mean strain histograms (SHs) are considered a good parameter for the semi-quantitative evaluation of focal pancreatic masses,[2,3] alongside complementary contrast-enhanced EUS parameters which can be quantified during both the early arterial and late venous phase.[4,5] The aim of this study was to assess the resulting value of SH EUS elastography combined with contrast-enhanced EUS for the differential diagnosis of focal pancreatic masses.
MATERIALS AND METHODS
The study design was prospective, blinded, and multicentric, assessing real-time EUS elastography and contrast-enhanced EUS results for the characterization of focal pancreatic masses using parametric measurements, in comparison with pathology which is the gold standard. A total number of 97 patients were included, 70 patients with pancreatic carcinoma and 27 patients with benign pancreatic lesions. Patients were consecutively included in six tertiary centers (Germany, Italy, France, Spain, China, and Romania) during routine EUS examinations, with two loops of elastography and one loop of contrast enhancement recorded on the embedded hard disk drive of the ultrasound system. All centers used linear EUS echoendoscopes (Pentax EG 3870 UTK, Pentax Europe GmbH, Hamburg, Germany) combined with the corresponding ultrasound system (Hitachi Avius, Preirus or Ascendus, Hitachi Medical Systems Europe, Zug, Switzerland). SHs were performed based on the embedded software of the ultrasound system, with the values being reversed as opposed to our initially published data on hue histograms. Consequently, a cutoff of 80 was derived from previous multicentric trials. Contrast-enhanced EUS also allowed the focal masses to be classified as hyper-, iso-, or hypoenhanced in comparison with the normal pancreatic parenchyma. EUS-FNA was then performed to all patients, with a positive cytological diagnosis taken as a final proof of malignancy for the pancreatic masses. The diagnoses obtained by EUS-FNA were further verified either by surgery or during a clinical follow-up of at least 6 months.
Strain histogram method
During EUS elastography, one trapezoidal region of interest (ROI) containing at least 50% of the lesion as well as surrounding tissues is manually selected. To calculate SH, a smaller round ROI is selected at the level of the focal lesion without the need to include a normal surrounding tissue (reference area). The elastography images of elemental areas inside a ROI are converted into a graph.[6] The mean SH value corresponds to the global hardness of the lesion expressed on the color scale from hardest (0) to softest (255). A cutoff of 80 was derived from previous multicentric trials. Thus, values <80 were considered predictive for malignancy and values >80 were considered predictive for benign lesions.
Time intensity curve analysis
A movie of 60 s has been recorded in Digital Imaging and Communications in Medicine (DICOM) format based on the low mechanical index (harmonic imaging) software of the ultrasound system. Briefly, 4.8 mL of SonoVue has been injected into a peripheral antecubital vein. Both phases arterial and the venous were recorded on the embedded hard disk drive of the ultrasound system. The movies were then subjected to time intensity curve (TIC) analysis and the tumor was consequently categorized as hypoenhancing, isoenhancing, or hyperenhancing in the early arterial and late venous phases, in comparison with the surrounding pancreatic parenchyma.
The results of the examinations were stored into a structured database, based on Microsoft Excel (software package: Microsoft Office 2010 Professional). Data were subsequently processed statistically using Microsoft Excel (Microsoft Corp., Redmond, WA, USA). Descriptive statistics were initially performed: frequencies, lowest and highest values, mean, and standard deviation. To determine the diagnostic performance of the quantitative real-time EUS elastography and contrast-enhanced EUS, a number of parameters have been calculated such as sensitivity, specificity, accuracy, positive predictive value, and negative predictive value.
RESULTS
A total number of 97 consecutive patients with focal pancreatic masses were included in the study. The final diagnosis of these patients included malignant tumors (70 patients, 72.2%) and benign lesions (27 patients, 27.8%). Patients with pancreatic carcinoma included in the present study had an average age of 68.64 years (standard deviation ± 10.04), ranging between 37 and 91 years, at the time of diagnosis, while for the group of patients with benign lesions the average age was 59.29 years (standard deviation ± 18.38 years), ranging between 18 and 87 years. Most patients with pancreatic carcinomas were men (68.57%–48/70), while patients with benign lesions were in approximately equal numbers men and women (55.55% [15/27] women and 44.44% [12/27] men).
Based on the EUS examination, pancreatic malignant tumors ranged between 14 and 60 mm with an average size of 33.58 mm (standard deviation ± 11.12 mm) in diameter. Almost 90% of the malignant tumors were located in the head (57.62%) with remaining in the body (30.5%).
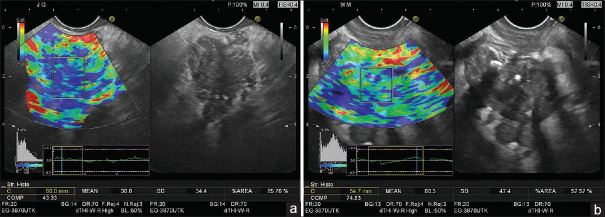
Real-time EUS elastography [Figure 1a and b] was been performed in patients with pancreatic carcinoma, resulting in mean SHs ranging between 3.3 and 70.6 with an average of 22.7 (standard deviation ± 11.2). Thus, all patients with a final diagnosis of pancreatic carcinoma had the mean SH <80, which is considered as a reference for malignant tumors according to the previous studies. For the patients with a final diagnosis of benign lesions, the average of mean SHs was 59.3 (standard deviation ± 45.7), ranging between 9.8 and 152.8. In only 29.62% of the cases (8/27), the mean SH was >80, which is considered as a reference of benign tumors for this group of patients. For the entire group of patients, the average of mean SHs was 32.9 (standard deviation ± 30.5), ranging between 3.3 and 152.8, with a median value of 22.
Figure 1.
Quantitative real-time elastography (strain histogram) in pancreatic ductal adenocarcinoma (a) and chronic pancreatitis (b)
It was further calculated that the values of sensitivity, specificity, positive predictive value, negative predictive value, and accuracy of the mean SHs, based on previously defined cut-offs (< 80), were 100%, 29.63%, 78.65%, 100%, and 80.41%, respectively.
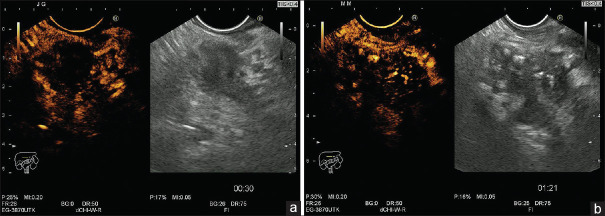
Corresponding values for contrast-enhanced EUS [Figure 2a and b] in the arterial phase (taking into consideration hypoenhancement as a predictive factor of malignancy) were 98.57%, 77.78%, 92%, 95.45%, and 92.78%. For contrast enhancement (CE)-EUS in the venous phase (taking into consideration washout as compared to the surrounding pancreatic parenchyma), the values of sensitivity, specificity, positive predictive value, negative predictive value, and accuracy were 98.57%, 66.67%, 88.46%, 94.74%, and 89.69%, respectively [Table 1].
Figure 2.
Contrast-enhanced EUS with a low mechanical index (0.2) showing an hypoechoic appearance in malignant pancreatic mass (a) and an heterogeneous appearance in chronic pancreatitis (b)
Table 1.
Diagnostic value of semi-quantitative real-time EUS-Elastography and contrast-enhanced-EUS for pancreatic carcinoma
| Strain histogram (cut-off:80) | Strain histogram (cut-off:60) | Strain histogram (cut-off:40) | Strain histogram (cut-off:33) | CE-EUS (arterial phase) | CE-EUS (venous phase) | Combined CE-EUS (hypovascular) and SH (cut-off:80) | |
|---|---|---|---|---|---|---|---|
| Sensitivity (%) | 100 | 98.57 | 92.86 | 85.71 | 98.57 | 98.57 | 98.57 |
| Specificity (%) | 29.63 | 37.04 | 55.56 | 62.96 | 77.78 | 66.67 | 81.48 |
| Accuracy (%) | 80.41 | 81.44 | 82.47 | 79.38 | 92.78 | 89.69 | 93.81 |
| PPV (%) | 78.65 | 80.23 | 84.42 | 85.71 | 92 | 88.46 | 93.24 |
| NPV (%) | 100 | 90.91 | 75 | 62.96 | 95.45 | 94.74 | 95.65 |
NPV: Negative predictive values, PPV: Positive predictive values, CE: Contrast enhanced, SH: Strain histograms
Observation
For all patients, SH values were low (tissue stiffness values were high). Was the cutoff of 80 (derived from previous multicentric studies) inappropriate for this study? So, what happens if we choose the cutoff value of 60, as a reference? Based on cutoffs of 60, it was observed that the values of sensitivity, specificity, and accuracy of the mean SHs were 98.57%, 37.04%, and 81.44%, respectively.
For a cutoff of 40, the mean SHs have sensitivity, specificity, and accuracy of 92.86%, 55.56%, and 82.47%, respectively. For the entire group of study, the average of the mean SHs was 32.9. Using a cutoff of 33, the mean SHs have sensitivity, specificity, and accuracy of 85.71%, 62.96%, and 79.38%, respectively.
Thus, it has been observed that decreasing the cutoff value from 80 to 33, the specificity of the SH for the diagnosis of pancreatic carcinoma increases from 29.63% to 62.96%, but sensitivity decreases only from 100% to 85.71%. The diagnostic accuracy remains approximately unchanged [Table 1].
Nevertheless, the best results were observed for the combination of CE-EUS (taking into consideration hypoenhancement as a predictive factor of malignancy) and semi-quantitative EUS elastography (SH cut-offs <80): sensitivity, specificity, and accuracy were 98.57%, 81.48% and 93.81%, respectively.
DISCUSSION
Pancreatic cancer represents the fourth leading cause of cancer death in the United States, after lung, breast/prostate, and colorectal cancer,[7] with a global annual incidence rate of approximately 8/100,000 persons.[8] Early detection is crucial for the management of the disease because most patients have locally advanced or metastatic disease at the time of diagnosis, thus contraindicating surgical resection. Moreover, the differential diagnosis of focal pancreatic masses can be difficult based on cross-sectional imaging alone.
EUS represents the most sensitive and specific imaging procedures currently available for the management of pancreatic cancer, while EUS-guided FNA allows confirmation of the diagnosis based on cytopatological examination of the aspirated samples.[9,10] There has been remarkable progress in the field of the EUS, especially concerning EUS elastography and contrast-enhanced EUS, which improved considerably the clinical impact of this procedure in focal pancreatic masses.[11,12]
The use of CE-EUS, based on second-generation microbubble ultrasound contrast agents, has been used to improve the characterization of the lesion vascularization, being necessary for the differential diagnosis between benign and malignant pancreatic masses.[13] Pancreatic adenocarcinomas are in general hypoenhanced in both the arterial and late venous phases, while pseudotumoral chronic pancreatitis or neuroendocrine tumors are either isoenhanced or hyperenhanced in the arterial phase as compared to the surrounding parenchyma.[1,14,15,16,17,18] Taking into consideration hypoenhancement as a predictive factor for pancreatic carcinoma, our study demonstrated that CE-EUS (arterial phase) has sensitivity, specificity, and accuracy of 98.57%, 77.78%, and 92.78%, respectively. For CE-EUS in the venous phase, the values of sensitivity, specificity, and accuracy were 98.57%, 66.67%, and 89.69%, respectively.
Elastography is an emerging set of imaging modalities used to reproduce tissue elasticity. Described for the first time in 2005, EUS elastography is a very important technique for the assessment of the pancreatic stiffness,[19] being a useful tool for the differential diagnosis of pancreatic masses.[20] Currently, there are two types of EUS elastography: qualitative and semi-quantitative. In the qualitative method, the tissue stiffness is assessed using a combination of the predominant color pattern inside the lesion and the homogeneity of the color map.[6,20,21] The normal pancreas appears as a homogeneous area with low stiffness, while a markedly stiff heterogeneous area is observed in pancreatic ductal adenocarcinoma and a mixture of variable stiffness is observed in chronic pancreatitis.[22] Most studies demonstrated that qualitative EUS elastography represents a useful tool for differential diagnosis of solid pancreatic masses with very high sensitivity (95%–98%) and relatively low specificity (42%–76%).[20,23,24]
There are two semi-quantitative elastography methods: SH and strain ratio. With regard to the SH method (used in this study), the mean histogram value corresponds to the global hardness of the lesion expressed on the color scale from hardest (0) to softest (255). Most published studies have showed a sensitivity between 85% and 96% and specificity between 64% and 76%.[20,23,25] In the present study, using a cutoff value of 80, derived from the previous multicentric trials, we demonstrated that SH method has a sensitivity, specificity, positive predictive value, negative predictive value, and accuracy of 100%, 29.63%, 78.65%, 100%, and 80.41%, respectively, for the diagnosis of pancreatic carcinoma. Nevertheless, because we observed very low SH values for the entire group of patients, we have tried to gradually decrease the cutoff to observe if there are improvements in the diagnostic performance of SH method in pancreatic cancer. Thus, by decreasing the cutoff from 80 to 33, the specificity of the SH for the diagnosis of pancreatic cancer increases from 29.63% to 62.96% but with a decrease in sensitivity from 100% to only 85.71%.
Our study had several limitations, highlighted also in previous publications.[2] Thus, we have only used SH as the semi-quantitative method of choice, although published data do not consistently show the advantages over strain ratios. Moreover, published meta-analyses did not show clear differences between qualitative and semi-quantitative elastography measurements.[20,24,25,26] Although all efforts were assumed to standardize elastography and contrast-enhanced examinations, there were inherent bias sources caused by manual selection of the ROI for quantitative evaluations.
For the combination between CE-EUS and semi-quantitative EUS elastography, the diagnostic specificity increased considerably to 81.48% with a diagnostic accuracy of 93.81% for pancreatic cancer. Thus, the technique can be used as a complementary EUS method for the differential diagnosis between malignant and benign pancreatic masses.[19] Furthermore, both semi-quantitative EUS elastography and contrast-enhanced EUS might be used for the follow-up of patients with malignant focal pancreatic masses during neoadjuvant chemoradiotherapy and/or antiangiogenic treatments.
CONCLUSION
The current study using objective parametric tools for both semi-quantitative real-time EUS elastography and contrast-enhanced EUS confirmed the results of previous studies and meta-analyses that indicated a complementary role for the differential diagnosis of focal pancreatic masses. Moreover, the best values for the receiver operating curves were obtained using a sequential clinical algorithm based on the initial use of elastography followed by contrast enhancement (sensitivity, specificity, and accuracy of 98.57%, 81.48%, and 93.81%, respectively).
Clinical trial registration
Clinical trials registration number: https://clinicaltrials. gov/ct2/show/NCT02459041.
Partial publication
Partial results of the ADVEUS study protocol (clinical trials registration number: https://clinicaltrials.gov/ct2/show/NCT02459041) have been already published in abstract form during DDW 2017 in Chicago, May 06-09, 2017, GASTROINTESTINAL ENDOSCOPY 2017:85(5), Suppl: AB338-AB338. Meeting Abstract: Su1333.
Financial support and sponsorship
This work was supported by a grant of Ministry of Research and Innovation, CNCS-UEFISCDI, project number PN-III-P4-ID-PCE-2016-0561, within PNCDI III.
Irina M. Cazacu was supported by the 2017 International Travel Grant offered by the American College of Gastroenterology.
Conflicts of interest
Erwan Bories has received speaking and travel support from Pentax Medical Europe
Christoph Dietrich has received speaker honoraria from Bracco, Hitachi, GE, Mindray, Supersonic, Pentax, Olympus, Fuji, Boston Scientific, AbbVie, Falk Foundation, Novartis, Roche; he is also an advisory board member for Hitachi, Mindray, Siemens; Research grant, GE, Mindray, SuperSonic
Adrian Săftoiu has received speaking and travel support from Pentax Medical Singapore Pte. Ltd. He is also an advisory board member for Mediglobe Corporation Gmbh
The other authors declare that they have no competing interests.
Acknowledgement
Dr. Manoop S. Bhutani is Walter H Wriston Distinguished Professor for Pancreatic Cancer Research.
REFERENCES
- 1.Hewitt MJ, McPhail MJ, Possamai L, et al. EUS-guided FNA for diagnosis of solid pancreatic neoplasms: A meta-analysis. Gastrointest Endosc. 2012;75:319–31. doi: 10.1016/j.gie.2011.08.049. [DOI] [PubMed] [Google Scholar]
- 2.Săftoiu A, Vilmann P. Differential diagnosis of focal pancreatic masses by semiquantitative EUS elastography: Between strain ratios and strain histograms. Gastrointest Endosc. 2013;78:188–9. doi: 10.1016/j.gie.2013.01.024. [DOI] [PubMed] [Google Scholar]
- 3.Dietrich CF, Bibby E, Jenssen C, et al. EUS elastography: How to do it? Endosc Ultrasound. 2018;7:20–8. doi: 10.4103/eus.eus_49_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dietrich CF, Averkiou MA, Correas JM, et al. An EFSUMB introduction into dynamic contrast-enhanced ultrasound (DCE-US) for quantification of tumour perfusion. Ultraschall Med. 2012;33:344–51. doi: 10.1055/s-0032-1313026. [DOI] [PubMed] [Google Scholar]
- 5.Dietrich CF, Dong Y, Froehlich E, et al. Dynamic contrast-enhanced endoscopic ultrasound: A quantification method. Endosc Ultrasound. 2017;6:12–20. doi: 10.4103/2303-9027.193595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cui XW, Chang JM, Kan QC, et al. Endoscopic ultrasound elastography: Current status and future perspectives. World J Gastroenterol. 2015;21:13212–24. doi: 10.3748/wjg.v21.i47.13212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 8.Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, et al. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries in 2012. Eur J Cancer. 2013;49:1374–403. doi: 10.1016/j.ejca.2012.12.027. [DOI] [PubMed] [Google Scholar]
- 9.Costache MI, Iordache S, Karstensen JG, et al. Endoscopic ultrasound-guided fine needle aspiration: From the past to the future. Endosc Ultrasound. 2013;2:77–85. doi: 10.4103/2303-9027.117691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cazacu IM, Luzuriaga Chavez AA, Saftoiu A, et al. A quarter century of EUS-FNA: Progress, milestones, and future directions. Endosc Ultrasound. 2018;7:141–60. doi: 10.4103/eus.eus_19_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ignee A, Jenssen C, Arcidiacono PG, et al. Endoscopic ultrasound elastography of small solid pancreatic lesions: A multicenter study. Endoscopy. 2018;50:1071–9. doi: 10.1055/a-0588-4941. [DOI] [PubMed] [Google Scholar]
- 12.Dietrich CF, Sahai AV, D'Onofrio M, et al. Differential diagnosis of small solid pancreatic lesions. Gastrointest Endosc. 2016;84:933–40. doi: 10.1016/j.gie.2016.04.034. [DOI] [PubMed] [Google Scholar]
- 13.Reddy NK, Ioncică AM, Săftoiu A, et al. Contrast-enhanced endoscopic ultrasonography. World J Gastroenterol. 2011;17:42–8. doi: 10.3748/wjg.v17.i1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iordache S, Angelescu R, Filip MM, et al. Power Doppler endoscopic ultrasound for the assessment of pancreatic neuroendocrine tumors. Endosc Ultrasound. 2012;1:150–5. doi: 10.7178/eus.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hocke M, Braden B, Jenssen C, et al. Present status and perspectives of endosonography 2017 in gastroenterology. Korean J Intern Med. 2018;33:36–63. doi: 10.3904/kjim.2017.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dong Y, Jürgensen C, Puri R, et al. Ultrasound imaging features of isolated pancreatic tuberculosis. Endosc Ultrasound. 2018;7:119–27. doi: 10.4103/2303-9027.210901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dong Y, D'Onofrio M, Hocke M, et al. Autoimmune pancreatitis: Imaging features. Endosc Ultrasound. 2018;7:196–203. doi: 10.4103/eus.eus_23_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dietrich CF, Dong Y, Jenssen C, et al. Serous pancreatic neoplasia, data and review. World J Gastroenterol. 2017;23:5567–78. doi: 10.3748/wjg.v23.i30.5567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Costache MI, Dumitrescu D, Săftoiu A. Technique of qualitative and semiquantitative EUS elastography in pancreatic examination. Endosc Ultrasound. 2017;6:S111–4. doi: 10.4103/eus.eus_75_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mei M, Ni J, Liu D, et al. EUS elastography for diagnosis of solid pancreatic masses: A meta-analysis. Gastrointest Endosc. 2013;77:578–89. doi: 10.1016/j.gie.2012.09.035. [DOI] [PubMed] [Google Scholar]
- 21.Dietrich CF, Barr RG, Farrokh A, et al. Strain elastography – How to do it? Ultrasound Int Open. 2017;3:E137–49. doi: 10.1055/s-0043-119412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iglesias-Garcia J, Larino-Noia J, Abdulkader I, et al. EUS elastography for the characterization of solid pancreatic masses. Gastrointest Endosc. 2009;70:1101–8. doi: 10.1016/j.gie.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 23.Ying L, Lin X, Xie ZL, et al. Clinical utility of endoscopic ultrasound elastography for identification of malignant pancreatic masses: A meta-analysis. J Gastroenterol Hepatol. 2013;28:1434–43. doi: 10.1111/jgh.12292. [DOI] [PubMed] [Google Scholar]
- 24.Hu DM, Gong TT, Zhu Q. Endoscopic ultrasound elastography for differential diagnosis of pancreatic masses: A meta-analysis. Dig Dis Sci. 2013;58:1125–31. doi: 10.1007/s10620-012-2428-5. [DOI] [PubMed] [Google Scholar]
- 25.Lu Y, Chen L, Li C, et al. Diagnostic utility of endoscopic ultrasonography-elastography in the evaluation of solid pancreatic masses: A meta-analysis and systematic review. Med Ultrason. 2017;19:150–8. doi: 10.11152/mu-987. [DOI] [PubMed] [Google Scholar]
- 26.Zhang B, Zhu F, Li P, et al. Endoscopic ultrasound elastography in the diagnosis of pancreatic masses: A meta-analysis. Pancreatology. 2018;18:833–40. doi: 10.1016/j.pan.2018.07.008. [DOI] [PubMed] [Google Scholar]