Abstract
Background and Objective:
EUS-FNA sensitivity for malignancy in parenchymal masses of patients with concurrent chronic pancreatitis (CP) has been reported to be unsatisfactory. The aim of the present study was to directly compare the diagnostic accuracy of EUS-FNA and EUS-fine-needle biopsy (FNB) in differentiating between inflammatory masses and malignancies in the setting of CP.
Methods:
We performed a retrospective analysis of prospective, multicentric databases of all patients with pancreatic masses and clinico-radiological-endosonographic features of CP who underwent EUS-FNA or FNB.
Results:
Among 1124 patients with CP, 210 patients (60% males, mean age: 62.7 years) with CP and pancreatic masses met the inclusion criteria and were enrolled. In the FNA group (110 patients), a correct diagnosis was obtained in all but 18 cases (diagnostic accuracy 83.6%, sensitivity 69.5%, specificity 100%, positive predictive value [PPV] 100%, and negative predictive value [NPV] 73.9%); by contrast, among 100 patients undergoing FNB, a correct diagnosis was obtained in all but seven cases (diagnostic accuracy 93%, sensitivity 86.8%, specificity 100%, PPV 100%, and NPV 87%) (P = 0.03, 0.03, 1, 1, and 0.07, respectively). At binary logistic regression, focal pancreatitis (odds of event occurrence [OR]: 4.9; P < 0.001), higher Ca19-9 (OR: 2.3; P = 0.02), and FNB (OR: 2.5; P < 0.01) were the only independent factors associated with a correct diagnosis.
Conclusion:
EUS-FNB is effective in the differential diagnosis between pseudotumoral masses and solid neoplasms in CP, showing higher diagnostic accuracy and sensitivity than EUS-FNA. EUS-FNB should be considered the preferred diagnostic technique for diagnosing cancer in the setting of CP.
Keywords: EUS, fine-needle biopsy, FNA, pancreatic cancer
INTRODUCTION
EUS-FNA has had a dramatic impact on pancreatic tissue acquisition and is considered the current standard of care for sampling pancreatic mass lesions, with reported sensitivity of 64%–95%, specificity of 75%–100%,[1,2] and diagnostic accuracy of 78%–95%.[2,3] However, EUS-FNA presents some limitations. The diagnostic accuracy is influenced by the availability of a cytopathologist to render a rapid on-site evaluation (ROSE),[4,5,6] and its sensitivity for diagnosing malignancies is low in the setting of associated chronic pancreatitis (CP),[7,8,9,10] ranging from 62% to 73.9%.
Up to 35% of patients undergoing EUS-FNA for a suspicion of pancreatic mass have features of underlying CP,[9] which is considered a risk factor for pancreatic cancer. EUS-FNA in the setting of CP is always challenging for physicians due to difficulties in detecting and differentiating malignant from inflammatory lesions at EUS imaging, especially in case of focal pancreatitis[7] and due to significant overlap in the cytological features associated with reactive cellular atypia resulting from CP and those resulting from a well-differentiated pancreatic adenocarcinoma.[5]
In order to overcome some of the EUS-FNA limitations and to improve diagnostic accuracy, several studies have explored the possibility of obtaining tissue samples for histology by performing an EUS-guided fine-needle biopsy (FNB).
However, current data do not demonstrate a significant difference between EUS-FNB and standard EUS-FNA for sample adequacy, diagnostic accuracy, or acquisition of a core specimen, although EUS-FNB establishes the diagnosis with fewer passes and permits to avoid ROSE.[11,12,13,14,15,16]
Conversely, the role of EUS-FNB in discriminating pseudotumoral masses and pancreatic cancer in the setting of CP has not been previously explored.
Starting from this assumption, the aim of the present study was to directly compare the diagnostic accuracy of EUS-FNA and EUS-FNB in differentiating between inflammatory masses and malignancies in the setting of CP.
METHODS
Study population and study design
We performed a retrospective case–control analysis of prospective, multicentric databases in five tertiary Italian endoscopic centers, including all consecutive adult patients with pancreatic masses and clinical, radiological, or endosonographic features consistent with CP, who underwent EUS-FNA or EUS-FNB between January 2015 and October 2018.
The diagnosis of CP was made in accordance with the current international guidelines.[17,18,19,20,21] In particular, the definitive and probable diagnosis of CP was made by imaging (computed tomography [CT], magnetic resonance imaging [MRI], EUS) and histology. Of the cases in which findings for probable diagnosis were present, those that satisfied two or more items among repeated attacks of upper abdominal pain, abnormalities in blood/urine pancreatic enzymes, and exocrine pancreatic dysfunction were definitively diagnosed as CP.[17,19,21]
Endosonographic features of CP were described in accordance with the Rosemont criteria;[22] however, to avoid selection biases, all patients with “indeterminate” or “normal” EUS imaging were excluded from the study.
EUS-FNA and FNB were performed by using a linear array echoendoscope (Pentax EG-3870UTK; Pentax, Tokyo, Japan or Olympus UCT-180; Olympus, Tokyo, Japan), whereas tissue acquisition was done with the 22G or 25G needles (EchoTip Ultra® and EchoTip® ProCore™, Cook Medical Inc., Limerick, Ireland).
Features of pancreatic mass at EUS evaluation (sizes, location, and behavior after intravenous contrast agent administration), number of passes, and laboratory findings (total bilirubin and CA19-9) were recorded for each patient.
The number of passes was not predetermined. However, in accordance with the ESGE recommendations,[23] all endoscopists generally performed three passes with macroscopic on-site evaluation, for every case of FNB and for FNA when cyto-included was performed.
There was no pathologist present in the room, and FNA or FNB samples were recovered and stored for further processing by the endoscopists. The following sampling methods were used: fanning, slow-pull technique, or syringe.
All samples were processed at the pathology departments of each unit for histological analysis. FNA or FNB samples were evaluated by the dedicated pathologist of respective hospitals with particular interest and expertise in evaluating tissue materials obtained via EUS.
In the case of FNA, due to the absence of ROSE, the samples were mainly processed as cyto-included which reduces the difficulty of preparing slides in the absence of the pathologist or cyto-technician. In <30% of the samples for FNA, the sample was processed by the endoscopist (previously trained by pathologists in the preparation of slides) as air-dried and alcohol-stained smears. Air-dried smears were stained with Diff-Quick stain and then sent to a cytopathologist to establish sample adequacy and diagnosis. Alcohol-stained smears were prepared by using Papanicolaou stain.
The samples obtained through FNB were embedded in paraffin. Tissue sections of 3–4 μm were stained by the hematoxylin and eosin technique for morphological evaluation and/or different immunohistochemical analysis. The adequacy of the specimen for diagnosis was defined as clear presence of target organ cells to guarantee an accurate diagnosis. The interpretation of each specimen was judged as “easy” or “not easy,” taking into consideration the percentage of pathological tissue on each slide.[24,25]
However, the reference standards for a final diagnosis of benign or malignant lesion were definite benign or malignant histological diagnosis based on surgical resection specimens from operated patients or histology findings with definite proof of malignancy in patients with unresectable tumors according to EUS and/or other radiological techniques (CT scan and MRI) and compatible clinical follow-up or histology findings without proof of malignancy and a minimum clinical/radiological follow-up time of at least 6 months.
The rate of inadequate samples has been reported in the study; however, patients with inadequate samples were excluded from the final analysis in order to provide the “pure” diagnostic accuracy.
Written informed consent was obtained from each participant.
Statistical analysis
Data were analyzed using the Statistical Package for Social Sciences (SPSS software version 15.0, IBM Corp., Armonk, NY, USA) for Windows. The descriptive statistics used included calculation of mean values and standard deviation of the continuous variables and the percentages and proportions of the categorical variables. Statistical analysis was performed using Chi-square test, Student's t-test, and Mann–Whitney U-test, when appropriate. Sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and overall accuracy of EUS-FNA and FNB with 95% confidence interval (CI) were calculated.
Then, a binary logistic regression was used to examine the possible predictors for a correct diagnosis. Our regression model used a backward step-wise selection (Wald) method. All the continuous variables were dichotomized as being normal or abnormal (yes/not). The coefficients obtained from the logistic regression analysis were also expressed in terms of odds of event occurrence (OR). P < 0.05 was considered statistically significant.
On the basis of previous studies, which demonstrated that the FNA sensitivity in the setting of CP was 65%–75%,[7,8,9] while the FNB sensitivity was 90%, we estimated a sample size of 100 patients per group (alpha error 0.05, beta error 0.2, and power 80%).
RESULTS
By retrieving the databases, a total of 1124 followed-up patients with a diagnosis of CP were found. Among them, 231 were found to have a pancreatic mass and were enrolled in the study.
After the exclusion of 21 patients (12 in the FNA group and 9 in the FNB group, P = NS) due to inadequate samples (9%), 110 patients with CP and pancreatic masses (67% males, mean age: 63.1 ± 8.9) underwent EUS-FNA, whereas 100 patients (59% males, mean age: 62.4 ± 9.4 years) underwent EUS-FNB and met the inclusion criteria to be enrolled. Table 1 reassumes the demographic and clinical features of the study population.
Table 1.
Characteristics of the study population (n = 210)
| FNA (110) | FNB (100) | P | |
|---|---|---|---|
| Males, n (%) | 67 (60.9) | 59 (59) | 0.8 |
| Age (years), mean±SD | 63.1±8.9 | 62.4±9.4 | 0.7 |
| Symptoms, n (%) | |||
| Abdominal pain | 79 (71.8) | 82 (82) | 0.08 |
| Diarrhea | 50 (45.4) | 39 (39) | 0.4 |
| Weight loss | 68 (61.8) | 69 (69) | 0.3 |
| Jaundice | 33 (30) | 35 (35) | 0.5 |
| Total bilirubin (mg/dL), mean±SD | 2.97±5.21 | 3.26±4.45 | 0.2 |
| Ca19-9 (U/mL), mean±SD | 327.3±311.2 | 356.1±347.4 | 0.1 |
| Type of pancreatitis, n (%) | |||
| Focal | 52 (47.3) | 51 (51) | 0.6 |
| Diffuse | 58 (52.7) | 49 (49) | 0.6 |
| Rosemont criteria, n (%) | |||
| Consistent | 47 (42.7) | 41 (41) | 0.9 |
| Suggestive | 63 (57.3) | 59 (59) | 0.9 |
| Site of pancreatic mass, n (%) | |||
| Head | 39 (35.5) | 32 (32) | 0.7 |
| Neck | 24 (21.8) | 26 (26) | 0.5 |
| Body | 26 (23.6) | 20 (20) | 0.6 |
| Tail | 21 (19.1) | 22 (22) | 0.7 |
| Mass dimensions (mm), mean±SD | 25.21±7.83 | 26.13±7 | 0.08 |
| Type of needle, n (%) | |||
| 22G | 49 (44.5) | 43 (43) | 0.9 |
| 25G | 61 (55.5) | 57 (57) | 0.9 |
| Number of passes, mean±SD | 3.5±0.8 | 3±0.6 | 1 |
| Follow-up (months), mean±SD | 14.5±7.1 | 16±6.4 | 0.007 |
SD: Standard deviation, FNB: Fine-needle biopsy
Most of the patients presented abdominal pain (76.6%), weight loss (65.2%), diarrhea (42.4%), and jaundice (32.3%), without differences between the two groups. Pancreatic lesions (mean lesion size: 25.21 ± 7.83 in FNA group vs. 26.1 ± 7 in FNB group, P = 0.08) were more frequently localized at the head (33.8%), neck (23.8%), body (21.9%), or tail (20.5%) (P = NS). On EUS, 42.7% of the patients in the FNA group and 41% of those in the FNB group had an imaging evaluation consistent with CP (P = 0.9), whereas 57.3% and 59% were suggestive of CP (P = 0.9). On the other hand, an EUS diagnosis of focal pancreatitis was made in 47.3% and 51% of cases in FNA and FNB groups, respectively (P = 0.6), whereas the remaining ones configured the picture of diffuse pancreatitis (P = NS).
An intravenous contrast agent (SonoVue®) was administered in 78% of patients, showing ipoenhancement in 61.5% of cases and isoenhancement in 38.5% of cases (P < 0.01).
EUS-FNA was performed by using a 22G or 25G needle in 44.5% and 55.5% f patients, respectively (mean number of passes: 3.5 ± 0.8), while the same needles were used in 43% and 57% of patients undergoing EUS-FNB (mean number of passes: 3 ± 0.6). The following sampling methods were used: fanning (100%) plus slow-pull technique (72%) or syringe (28%).
Based on EUS-FNA, 69 lesions were considered benign and 41 were considered as malignant. However, in accordance with reference standards, 59 lesions (53.6%) were finally considered as malignant and 46.3% as benign. Overall, a correct diagnosis was obtained in all but 18 cases, reporting a diagnostic accuracy for EUS-FNA of 83.64% (95% CI: 75.6%–89.4%), sensitivity of 69.5% (95% CI: 56.85%–79.75%), specificity of 100% (95% CI: 93%–100%), PPV of 100%, and NPV of 73.91% (95% CI: 62.49%–82.81%).
In the case of EUS-FNB, 46 lesions were considered benign and 54 were considered as malignant. Due to reference standards, 53 lesions (53%) were finally considered as malignant and 47% as benign [Figures 1 and 2 show EUS features of two similar pancreatic masses that revealed two different histologic outcomes]. Overall, a correct diagnosis was obtained in all but seven cases, reporting a diagnostic accuracy for EUS-FNB of 93% (95% CI: 86.1%–97.1%), sensitivity of 86.8% (95% CI: 74.6%–94.5%), specificity of 100% (95% CI: 92.5%–100%), PPV of 100%, and NPV of 87% (95% CI: 77.1%–93.1%). Table 2 compares the performance characteristics between FNA and FNB.
Figure 1.
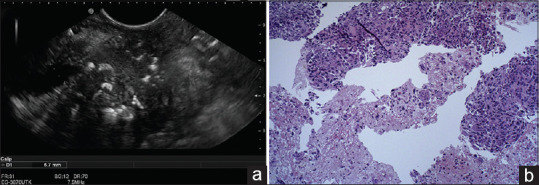
(a) EUS imaging of pancreatic head mass. (b) H and E histological section obtained from EUS-fine-needle biopsy showing atypical cells with prominent nucleoli and variation in nuclear size, desmoplastic fibrosis, and numerous mitoses, revealing a pancreatic adenocarcinoma
Figure 2.
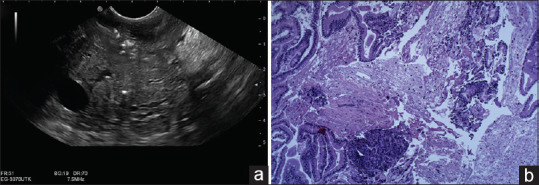
(a) EUS imaging of pancreatic head mass. (b) H and E histological section obtained from EUS-fine-needle biopsy showing the destruction of acinar tissue with replacement by extensive fibrosis and chronic inflammatory cells, revealing a chronic pancreatitis
Table 2.
Comparison of performance characteristics between FNA and fine-needle biopsy
| 95% CI | P | ||
|---|---|---|---|
| FNA | FNB | ||
| Sensitivity (%) | 69.5 (56.85–79.75) | 86.8 (74.6–94.5) | 0.03 |
| Specificity (%) | 100 (93–100) | 100 (92.5–100) | 1 |
| PPV (%) | 100 | 100 | 1 |
| NPV (%) | 73.91 (62.49–82.81) | 87 (77.1–93.1) | 0.07 |
| Accuracy (%) | 83.64 (75.6–89.4) | 93 (86.1–97.1) | 0.03 |
Statistically significant results have been expressed in bold. FNB: Fine-needle biopsy, PPV: Positive predictive value, NPV: Negative predictive value, CI: Confidence interval
When a binary logistic regression was performed in order to evaluate the possible predictors for a correct diagnosis, only focal pancreatitis (OR: 4.9; P < 0.001), higher Ca19-9 (OR: 2.3; P = 0.02), and FNB (OR: 2.5; P < 0.01) were the only independent factors associated with a correct diagnosis [Table 3]. On the other hand, gender, age, Rosemont criteria, mass size, type of needle, number of passes, and features at intravenous contrast agent were not predictive of a correct diagnosis, although ipoenhancement after intravenous contrast agent administration was frequently associated with a diagnosis of pancreatic tumor (OR: 3.2; P < 0.001).
Table 3.
Factors associated with a correct diagnosis
| Variables | Univariate analysis | Binary logistic regression | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | |
| Male sex | 0.9 | 0.8–1 | 0.3 | |||
| Age >60 years | 0.9 | 0.8–1 | 0.4 | |||
| Focal vs. diffuse pancreatitis | 1.2 | 1–1.3 | 0.04 | 4.9 | 2.1–6.3 | <0.001 |
| Rosemont criteria | ||||||
| Consistent | 1 | 0.9–1.1 | 0.7 | |||
| Suggestive | 1 | 0.9–1.2 | 0.3 | |||
| Ca19-9 >60 (U/mL) | 1.6 | 1.4–1.9 | 0.07 | 2.3 | 1.5–4.5 | 0.02 |
| Total bilirubin >2.5 (mg/dL) | 1 | 0.9–1.1 | 0.7 | |||
| Mass size >25 (mm) | 0.9 | 0.8–1.1 | 0.6 | |||
| Isoenhancement at SonoVue | 1 | 0.9–1.1 | 0.9 | |||
| FNB vs. FNA | 1.9 | 1.4–3.5 | 0.02 | 2.5 | 1.4–4.7 | <0.01 |
| Mass site | ||||||
| Head | 1.1 | 0.9–1.2 | 0.2 | |||
| Neck | 1 | 0.9–1.1 | 0.6 | |||
| Body | 1 | 0.9–1.1 | 0.7 | |||
| Tail | 0.8 | 0.6–1 | 0.02 | 0.9 | 0.7–1.6 | 0.3 |
| Needle | ||||||
| 22G | 0.9 | 0.8–1.1 | 0.7 | |||
| 25G | 1 | 0.9–1.1 | 0.4 | |||
| Needle passes >3 versus 1–2 | 1 | 0.8–1.1 | 1 | |||
Significant values have been highlighted in bold. OR: Odds of event occurrence, CI: Confidence interval, FNB: Fine-needle biopsy
DISCUSSION
EUS-guided tissue acquisition has become the gold standard for diagnosing numerous malignant and nonmalignant pancreatic lesions. In effect, several studies reported an EUS-FNA sensitivity up to 95%, specificity of 75%–100%, and diagnostic accuracy ranging from 78% to 95%, especially when an on-site pathologist is available.[1,2,3] By contrast, the absence of ROSE[25] and the presence of CP[7,8,9,10] have been widely considered factors deeply affecting the sensitivity of FNA. Thus, several attempts have been made in order to overcome the limitations of FNA by obtaining tissue for histologic evaluation through FNB. While some studies have focused on the diagnostic accuracy of FNB versus FNA in diagnosing pancreatic cancer,[4,11,12,13,14,15,16] to date, no studies have explored the diagnostic accuracy of FNB in discriminating pseudotumoral masses from pancreatic cancer in the setting of CP, a frequent condition able to simulate a cancer.
In the present study involving 210 pancreatic masses directly confronting EUS-FNA and EUS-FNB, we were able to show that EUS-guided FNB presented a very high diagnostic accuracy (93%), with a sensitivity of 86.8%, a specificity of 100%, a PPV of 100%, and a NPV of 87%. These accuracy and sensitivity were found to be statistically significantly higher than those reported for EUS-FNA (diagnostic accuracy of 83.64% and sensitivity of 69.5%, P = 0.03 for both, specificity of 100%, PPV of 100%, and NPV of 73.91%).
Our results were in accordance with those previously found by Iglesias-Garcia et al.,[26] who performed EUS-FNB with a 19G needle and reported sensitivity, specificity, PPV, NPV, and overall accuracy for the diagnosis of malignancy of 90.2%, 100%, 100%, 78.9%, and 92.9%, respectively.
Recent studies have explored the diagnostic accuracy of EUS-FNA in the detection of malignancy in the setting of CP, reporting cases of false-negative results in patients with underlying CP and highlighting the limitations of FNA in the setting of such a pancreatic disorder.[7,8,9,27]
Fritscher-Ravens et al.[9] analyzed the diagnostic accuracy of EUS-FNA in 207 patients with pancreatic lesions, 74 of them with CP, and found that sensitivity of EUS-FNA was 89% in the absence of CP, but it was only 54% in the presence of CP. Varadarajulu et al.[7] found that EUS-FNA had lower sensitivity for pancreatic mass lesions in patients with CP than in those without CP (73.9% vs. 91.3%, P = 0.02) despite the presence of an on-site pathologist. This decreased sensitivity could be overcome by performing more number of passes at FNA, which improved diagnostic accuracy. More recently, Krishna et al.[8] found that the performance characteristics of EUS-FNA for diagnosing pancreatic cancer were significantly influenced by the presence of CP, which lowered the sensitivity of EUS-FNA for diagnosing pancreatic malignancy (65%) without significantly affecting the NPV (91.7%) or accuracy (91.8%).
To the best of our knowledge, this is the first study confirming, by a direct comparison between FNB and FNA, the higher sensitivity of FNB (86.8%) than that of FNA (69.5%) (P = 0.03). Our results were strengthened by the binary logistic regression, reporting that FNB was a factor associated with a correct diagnosis when compared to FNA (OR: 2.5; P < 0.01). Moreover, our diagnostic accuracy for FNB was very high (93%) also without ROSE, demonstrating the superiority of FNB on FNA in diagnosing cancer in the setting of CP.
However, the current knowledge is not able to demonstrate the superiority of FNB on FNA.
Several randomized and nonrandomized studies have compared the sensitivity and diagnostic accuracy of EUS-FNA and EUS-guided biopsy in patients without CP. Although biopsy had no clear advantage over EUS-FNA in terms of overall sensitivity and diagnostic accuracy, it provided a more specific diagnosis in selected cases, requiring fewer needle passes.[6,14,23,28,29,30,31] Instead, a trial by Chen et al.[12] found a higher histologic diagnostic yield with FNB compared to FNA (91.4% vs. 80%, P = 0.0015) but with no difference in cytologic diagnostic yield (85.5% vs. 78.9%, P = 0.93).
A recent meta-analysis[11] comparing ProCore and standard FNA needles concluded that there was no statistically significant difference in diagnostic adequacy (75.2% vs. 89.0%, OR: 0.39, P = 0.23), diagnostic accuracy (85.8% vs. 86.2%, OR: 0.88, P = 0.53), or rate of histological core specimen acquisition (77.7% vs. 76.5%, OR: 0.94, P = 0.85) between the ProCore and standard FNA needles. The mean number of passes required for diagnosis, however, was significantly lower when using the ProCore needle. Nonetheless, these data did not contemplate the effects of the underlying CP on EUS-FNA or FNB sensitivity.
The recent meta-analysis of seven randomized controlled trials (RCTs) by Facciorusso et al.[31] comparing 25G and 22G needles did not demonstrate significant differences between the needles in terms of sensitivity or specificity for malignancy in patients with solid pancreatic masses, indirectly confirming our results.
Data about 25G ProCore are debated. A recent multicentric RCT[32] comparing standard 25G versus 25G ProCore demonstrated that 25G ProCore provided a better quality in histological samples and a better sample cellularity than the standard 25G, although in the absence of differences for the diagnosis of malignancy between the needles.
Even though two meta-analyses failed to demonstrate a superiority of 22G ProCore on 22G standard needle in terms of diagnostic rates,[11,16] a more recent RCT[12] demonstrated a better diagnostic yield of 22G ProCore versus 22G standard, especially in patients with pancreatic masses (92.68% vs. 81.75%, P = 0.0099). In accordance with the abovementioned studies, we were able to confirm the role of ProCore needles in the histological diagnosis of pancreatic masses.
It is interesting to note that binary logistic regression identified only focal pancreatitis (OR: 4.9; P < 0.001), higher Ca19-9 (OR: 2.3; P = 0.02), and FNB (OR: 2.5; P < 0.01) as the independent factors associated with a correct diagnosis.
Focal pancreatitis is a well-known factor able to simulate a cancer because of similar EUS appearance between the two entities.[7] In effect, also when FNA is used in conjunction with EUS, cytological evaluation of pancreatic tissue in the setting of chronic inflammation is very challenging because the inflammatory infiltrate may obscure or simulate a pancreatic malignancy.[7,8] About this issue, Brand et al.[27] found that the specificity of EUS to diagnose malignancy based on morphological criteria was found to be as low as 53% in the setting of CP. Fritsher-Ravens et al.[9] concluded that sensitivity of EUS-FNA to diagnose malignancy in the setting of CP was much lower when compared with patients with focal pancreatic lesions and a normal pancreas (54% vs. 89%).
Instead, gender, age, Rosemont criteria, mass size, number of passes, and features at intravenous contrast agent were not predictive of a correct diagnosis, although ipoenhancement after intravenous contrast agent administration was frequently associated with a diagnosis of pancreatic tumor (OR: 3.2; P < 0.001), in agreement with recent evidences.[33]
Our study has some limitations, of which we are well aware. First, this study has a retrospective design. However, this is an analysis of prospective, multicentric databases with an active patient follow-up and with a vast sample of patients with CP and pancreatic masses. Further randomized studies comparing FNA and FNB are needed in order to confirm our results and establish the gold standard to discriminate pseudotumoral masses from cancer in the setting of CP.
Another critical point in the present study is the use and definition of the criterion-standard reference method. Ideally, surgical specimens would be the criterion standard also in case of EUS-FNA/FNB-negative results, but it cannot be obtained for ethical reasons in patients in whom surgery is not indicated. Therefore, in agreement with previous studies,[4,8,26] clinical follow-up for at least 6 months with repeated imaging procedures (EUS and CT) was used in our study. Although not ideal, this method is a well-accepted reference standard. Due to the retrospective nature of the study and despite the revision of pathological reports where pathologists specified only the diagnosis, we were not able to provide the rate of histology procurement of each kind of needle used as the current definition of core histology appears quite stringent and not retrievable:[34,35] we need other prospective studies also evaluating this outcome. However, we can speculate that the increased diagnostic accuracy of FNB on FNA could be the result of a more accurate diagnosis thanks to higher tissue amount provided by FNB.
CONCLUSION
This study demonstrated that EUS-FNB was effective in the differential diagnosis between pseudotumoral masses and solid neoplasms in CP, showing a high diagnostic accuracy (93%) especially if compared to EUS-FNA results. EUS-FNB should be considered the preferred diagnostic technique for diagnosing pancreatic cancer in the setting of CP.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Hewitt MJ, McPhail MJ, Possamai L, et al. EUS-guided FNA for diagnosis of solid pancreatic neoplasms: A meta-analysis. Gastrointest Endosc. 2012;75:319–31. doi: 10.1016/j.gie.2011.08.049. [DOI] [PubMed] [Google Scholar]
- 2.Itoi T, Sofuni A, Itokawa F, et al. Current status of diagnostic endoscopic ultrasonography in the evaluation of pancreatic mass lesions. Dig Endosc. 2011;23(Suppl 1):17–21. doi: 10.1111/j.1443-1661.2011.01132.x. [DOI] [PubMed] [Google Scholar]
- 3.Chen G, Liu S, Zhao Y, et al. Diagnostic accuracy of endoscopic ultrasound-guided fine-needle aspiration for pancreatic cancer: A meta-analysis. Pancreatology. 2013;13:298–304. doi: 10.1016/j.pan.2013.01.013. [DOI] [PubMed] [Google Scholar]
- 4.Bang JY, Hebert-Magee S, Trevino J, et al. Randomized trial comparing the 22-gauge aspiration and 22-gauge biopsy needles for EUS-guided sampling of solid pancreatic mass lesions. Gastrointest Endosc. 2012;76:321–7. doi: 10.1016/j.gie.2012.03.1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Erickson RA, Sayage-Rabie L, Beissner RS. Factors predicting the number of EUS-guided fine-needle passes for diagnosis of pancreatic malignancies. Gastrointest Endosc. 2000;51:184–90. doi: 10.1016/s0016-5107(00)70416-0. [DOI] [PubMed] [Google Scholar]
- 6.Song TJ, Kim JH, Lee SS, et al. The prospective randomized, controlled trial of endoscopic ultrasound-guided fine-needle aspiration using 22G and 19G aspiration needles for solid pancreatic or peripancreatic masses. Am J Gastroenterol. 2010;105:1739–45. doi: 10.1038/ajg.2010.108. [DOI] [PubMed] [Google Scholar]
- 7.Varadarajulu S, Tamhane A, Eloubeidi MA. Yield of EUS-guided FNA of pancreatic masses in the presence or the absence of chronic pancreatitis. Gastrointest Endosc. 2005;62:728–36. doi: 10.1016/j.gie.2005.06.051. [DOI] [PubMed] [Google Scholar]
- 8.Krishna NB, Mehra M, Reddy AV, et al. EUS/EUS-FNA for suspected pancreatic cancer: Influence of chronic pancreatitis and clinical presentation with or without obstructive jaundice on performance characteristics. Gastrointest Endosc. 2009;70:70–9. doi: 10.1016/j.gie.2008.10.030. [DOI] [PubMed] [Google Scholar]
- 9.Fritscher-Ravens A, Brand L, Knöfel WT, et al. Comparison of endoscopic ultrasound-guided fine needle aspiration for focal pancreatic lesions in patients with normal parenchyma and chronic pancreatitis. Am J Gastroenterol. 2002;97:2768–75. doi: 10.1111/j.1572-0241.2002.07020.x. [DOI] [PubMed] [Google Scholar]
- 10.Bhutani MS, Gress FG, Giovannini M, et al. The no endosonographic detection of tumor (NEST) study: A case series of pancreatic cancers missed on endoscopic ultrasonography. Endoscopy. 2004;36:385–9. doi: 10.1055/s-2004-814320. [DOI] [PubMed] [Google Scholar]
- 11.Bang JY, Hawes R, Varadarajulu S. A meta-analysis comparing ProCore and standard fine-needle aspiration needles for endoscopic ultrasound-guided tissue acquisition. Endoscopy. 2016;48:339–49. doi: 10.1055/s-0034-1393354. [DOI] [PubMed] [Google Scholar]
- 12.Cheng B, Zhang Y, Chen Q, et al. Analysis of fine-needle biopsy vs.fine-needle aspiration in diagnosis of pancreatic and abdominal masses: A prospective, multicenter, randomized controlled trial. Clin Gastroenterol Hepatol. 2018;16:1314–21. doi: 10.1016/j.cgh.2017.07.010. [DOI] [PubMed] [Google Scholar]
- 13.Nagula S, Pourmand K, Aslanian H, et al. Comparison of endoscopic ultrasound-fine-needle aspiration and endoscopic ultrasound-fine-needle biopsy for solid lesions in a multicenter, randomized trial. Clin Gastroenterol Hepatol. 2018;16:1307–130. doi: 10.1016/j.cgh.2017.06.013. [DOI] [PubMed] [Google Scholar]
- 14.Lee YN, Moon JH, Kim HK, et al. Core biopsy needle versus standard aspiration needle for endoscopic ultrasound-guided sampling of solid pancreatic masses: A randomized parallel-group study. Endoscopy. 2014;46:1056–62. doi: 10.1055/s-0034-1377558. [DOI] [PubMed] [Google Scholar]
- 15.Yang MJ, Yim H, Hwang JC, et al. Endoscopic ultrasound-guided sampling of solid pancreatic masses: 22-gauge aspiration versus 25-gauge biopsy needles. BMC Gastroenterol. 2015;15:122. doi: 10.1186/s12876-015-0352-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khan MA, Grimm IS, Ali B, et al. A meta-analysis of endoscopic ultrasound-fine-needle aspiration compared to endoscopic ultrasound-fine-needle biopsy: Diagnostic yield and the value of onsite cytopathological assessment. Endosc Int Open. 2017;5:E363–75. doi: 10.1055/s-0043-101693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shimosegawa T, Kataoka K, Kamisawa T, et al. The revised Japanese clinical diagnostic criteria for chronic pancreatitis. J Gastroenterol. 2010;45:584–91. doi: 10.1007/s00535-010-0242-4. [DOI] [PubMed] [Google Scholar]
- 18.Ito T, Ishiguro H, Ohara H, et al. Evidence-based clinical practice guidelines for chronic pancreatitis 2015. J Gastroenterol. 2016;51:85–92. doi: 10.1007/s00535-015-1149-x. [DOI] [PubMed] [Google Scholar]
- 19.Conwell DL, Lee LS, Yadav D, et al. American Pancreatic Association Practice guidelines in chronic pancreatitis: Evidence-based report on diagnostic guidelines. Pancreas. 2014;43:1143–62. doi: 10.1097/MPA.0000000000000237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Majumder S, Chari ST. Chronic pancreatitis. Lancet. 2016;387:1957–66. doi: 10.1016/S0140-6736(16)00097-0. [DOI] [PubMed] [Google Scholar]
- 21.Löhr JM, Dominguez-Munoz E, Rosendahl J, et al. United European Gastroenterology evidence-based guidelines for the diagnosis and therapy of chronic pancreatitis (HaPanEU) United European Gastroenterol J. 2017;5:153–99. doi: 10.1177/2050640616684695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Catalano MF, Sahai A, Levy M, et al. EUS-based criteria for the diagnosis of chronic pancreatitis: The Rosemont classification. Gastrointest Endosc. 2009;69:1251–61. doi: 10.1016/j.gie.2008.07.043. [DOI] [PubMed] [Google Scholar]
- 23.Polkowski M, Jenssen C, Kaye P, et al. Technical aspects of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) Technical Guideline – March 2017. Endoscopy. 2017;49:989–1006. doi: 10.1055/s-0043-119219. [DOI] [PubMed] [Google Scholar]
- 24.Petrone MC, Poley JW, Bonzini M, et al. Comparison of pancreatic histology specimens obtained by EUS 19G versus 22G core biopsy needles: A prospective multicentre study among experienced pathologists. United European Gastroenterol J. 2017;5:854–8. doi: 10.1177/2050640616687231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hébert-Magee S, Bae S, Varadarajulu S, et al. The presence of a cytopathologist increases the diagnostic accuracy of endoscopic ultrasound-guided fine needle aspiration cytology for pancreatic adenocarcinoma: A meta-analysis. Cytopathology. 2013;24:159–71. doi: 10.1111/cyt.12071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iglesias-Garcia J, Poley JW, Larghi A, et al. Feasibility and yield of a new EUS histology needle: Results from a multicenter, pooled, cohort study. Gastrointest Endosc. 2011;73:1189–96. doi: 10.1016/j.gie.2011.01.053. [DOI] [PubMed] [Google Scholar]
- 27.Brand B, Pfaff T, Binmoeller KF, et al. Endoscopic ultrasound for differential diagnosis of focal pancreatic lesions, confirmed by surgery. Scand J Gastroenterol. 2000;35:1221–8. doi: 10.1080/003655200750056736. [DOI] [PubMed] [Google Scholar]
- 28.Levy MJ, Jondal ML, Clain J, et al. Preliminary experience with an EUS-guided trucut biopsy needle compared with EUS-guided FNA. Gastrointest Endosc. 2003;57:101–6. doi: 10.1067/mge.2003.49. [DOI] [PubMed] [Google Scholar]
- 29.Wittmann J, Kocjan G, Sgouros SN, et al. Endoscopic ultrasound-guided tissue sampling by combined fine needle aspiration and trucut needle biopsy: A prospective study. Cytopathology. 2006;17:27–33. doi: 10.1111/j.1365-2303.2006.00313.x. [DOI] [PubMed] [Google Scholar]
- 30.Aithal GP, Anagnostopoulos GK, Tam W, et al. EUS-guided tissue sampling: Comparison of “dual sampling” (Trucut biopsy plus FNA) with “sequential sampling” (Trucut biopsy and then FNA as required) Endoscopy. 2007;39:725–30. doi: 10.1055/s-2007-966400. [DOI] [PubMed] [Google Scholar]
- 31.Facciorusso A, Stasi E, Di Maso M, et al. Endoscopic ultrasound-guided fine needle aspiration of pancreatic lesions with 22 versus 25 Gauge needles: A meta-analysis. United European Gastroenterol J. 2017;5:846–53. doi: 10.1177/2050640616680972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kamata K, Kitano M, Yasukawa S, et al. Histologic diagnosis of pancreatic masses using 25-gauge endoscopic ultrasound needles with and without a core trap: A multicenter randomized trial. Endoscopy. 2016;48:632–8. doi: 10.1055/s-0042-106294. [DOI] [PubMed] [Google Scholar]
- 33.Săftoiu A, Vilmann P, Dietrich CF, et al. Quantitative contrast-enhanced harmonic EUS in differential diagnosis of focal pancreatic masses (with videos) Gastrointest Endosc. 2015;82:59–69. doi: 10.1016/j.gie.2014.11.040. [DOI] [PubMed] [Google Scholar]
- 34.Di Leo M, Crinò SF, Bernardoni L, et al. EUS-guided core biopsies of pancreatic solid masses using a new fork-tip needle: A multicenter prospective study. Dig Liver Dis. 2019;51:1275–80. doi: 10.1016/j.dld.2019.03.025. [DOI] [PubMed] [Google Scholar]
- 35.Asokkumar R, Yung Ka C, Loh T, et al. Comparison of tissue and molecular yield between fine-needle biopsy (FNB) and fine-needle aspiration (FNA): A randomized study. Endosc Int Open. 2019;7:E955–63. doi: 10.1055/a-0903-2565. [DOI] [PMC free article] [PubMed] [Google Scholar]


