INTRODUCTION
In the presence of a solid pancreatic mass suspicious for malignancy and without major vascular involvement, biopsy proof is not mandatory before proceeding with upfront resection.[1] However, the steady global uptake of neoadjuvant chemotherapy for borderline resectable and even anatomically resectable pancreatic ductal adenocarcinoma (PDAC) has led to an increasing number of EUS-guided biopsies being performed. Novel end-cutting forward-acquiring EUS needles, including the SharkCore™ (Medtronic Parkway, Minneapolis, MN, USA), provide better core biopsy specimens and allow for molecular and genetic studies that will possibly aid in guiding therapy.[2] When tumors are located in the pancreatic body and tail, tissue acquisition is performed through a transgastric route.[3,4] Although exceedingly uncommon, needle tract seeding at the gastric wall level has been described.[3,4]
We herein report two cases of intraoperatively found gastric wall infiltration following EUS-fine needle biopsy (FNB) of resectable PDAC, that required associated partial gastric resection but was demonstrated to be nonneoplastic on final pathological analysis.
CASE 1
The first patient was a 72-year-old female, affected by an incidentally discovered 13 mm pancreatic solid lesion in the body of the pancreas, with upstream main pancreatic duct (MPD) dilation and parenchymal atrophy and without distant metastases on cross-sectional imaging. Ca 19.9 was normal. The lesion was hypoechoic on EUS and hypovascular after contrast injection (Sonovue™, Bracco S. p. a., Milan, Italy) [Figure 1a]. No ultrasonographic signs of neoplastic infiltration of the stomach by the pancreatic lesion were observed. To confirm the diagnosis, transgastric FNB was performed with three passes of a 22G SharkCore™ needle. Ten–twenty to-and-fro movements using the slow-pull and the fanning techniques were performed. Histological diagnosis of PDAC was obtained.
Figure 1.
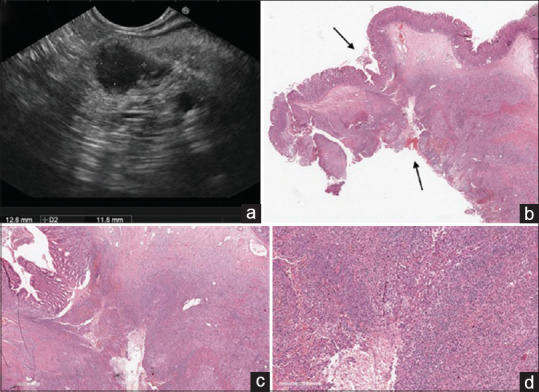
EUS examination revealing a small (13 mm) hypoechoic solid lesion in the body of the pancreas. No signs of suspected gastric infiltration were observed (a). Histological view of the portion of stomach firmly attached to the pancreas (black arrows) and resected during surgical operation (b, low magnification, H and E). A whole-thickness tumor-free gastric wall involved by inflammatory infiltrate with the histological hallmarks of granulation tissue that reach the gastric mucosa from the outer layers of the stomach (c, H and E, ×10 and d, H and E ×40). These are the pathological key features of a “fistula” and witness a secondary gastric involvement from an external underlying disease
CASE 2
The second patient was a 69-year-old male. A small (9 mm) solid lesion in the body of the pancreas was incidentally discovered. Laboratory tests (including Ca 19.9) were normal and features on cross-sectional imaging (upstream MPD dilatation with parenchymal atrophy, no distant metastases) and on EUS (hypoechoic/hypovascular lesion without signs of gastric wall infiltration) were similar to those observed in the first case. Transgastric FNB was performed with two passes of a 25G SharkCore™ needle using the same technique described for Case 1. Histological diagnosis of PDAC was obtained.
SURGICAL AND PATHOLOGICAL FINDINGS
During surgical exploration, in the both cases, unexpected infiltration of the posterior gastric wall was found, and a distal pancreatectomy with en bloc partial gastric resection was performed to achieve complete tumor clearance. The surgical specimens consisted of a distal splenopancreasectomy with an adherent portion of the gastric wall. Although a needle tract seeding was suspected, histological examination revealed no malignant cells implantation, but an inflammatory infiltrate with granulation tissue through the gastric wall, from the outer to the inner layers [Figure 1b-d]. These pathologic hallmarks suggested the formation of a pancreatico-gastric fistula along the needle tract.[5] It is likely that, given the small size of the lesions, the MPD was inadvertently injured during the back and forth movements of the needle, with subsequent leakage of pancreatic juice into the gastric cavity. To the best of our knowledge, this is the first report of EUS-FNB-related nonneoplastic fibroinflammatory reactions mimicking PDAC infiltration of the posterior gastric wall. Surgeons should be aware of this sampling-related adverse event to avoid intraoperative overestimation of tumor stage, especially when no evidence of pancreatico-gastric adherence is observed at preoperative imaging. Future larger prospective EUS-FNB studies employing end-cutting needles and including small resectable PDAC could assess the incidence of this adverse event.
Informed consent statement
The patients involved in this study gave their written informed consent authorizing use and disclosure of their protected health information.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient has given her consent for her images and other clinical information to be reported in the journal. The patient understands that name and initials will not be published and due efforts will be made to conceal identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Asbun HJ, Conlon K, Fernandez-Cruz L, et al. When to perform a pancreatoduodenectomy in the absence of positive histology? A consensus statement by the international study group of pancreatic surgery. Surgery. 2014;155:887–92. doi: 10.1016/j.surg.2013.12.032. [DOI] [PubMed] [Google Scholar]
- 2.Gleeson FC, Levy MJ, Roden AC, et al. EUS fine-needle pancreatic core biopsy can determine eligibility for tumor-agnostic immunotherapy. Endosc Int Open. 2018;6:E1278–E1282. doi: 10.1055/a-0650-4447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Minaga K, Takenaka M, Katanuma A, et al. Needle tract seeding: An overlooked rare complication of endoscopic ultrasound-guided fine-needle aspiration. Oncology. 2017;93(Suppl 1):107–12. doi: 10.1159/000481235. [DOI] [PubMed] [Google Scholar]
- 4.Minaga K, Kitano M, Enoki E, et al. Needle-tract seeding on the proximal gastric wall after EUS-guided fine-needle aspiration of a pancreatic mass. Am J Gastroenterol. 2016;111:1515. doi: 10.1038/ajg.2016.307. [DOI] [PubMed] [Google Scholar]
- 5.Lack EE. Pathology of the Pancreas, Gallbladder, Extrahepatic Biliary Tract, and Ampullary Region. New York: Oxford University Press; 2003. p. 93. [Google Scholar]


