Abstract
Penile skin (PSG) and the buccal mucosa (BMGs) are the most commonly used grafts for substitution urethroplasty. The aim of this study was to compare the success rates of substitution urethroplasty using either of these grafts. We systematically searched PubMed/Medline, EMBASE, Scopus and Web of science to identify studies comparing the two types of graft urethroplasties. Search strategy was based on Patient, Intervention, Control and Outcome guidelines. Studies reporting data on success of PSG versus BMG within the same manuscript were included. Standard Preferred reporting Items for Systematic reviews and Metaanalysis guidelines were followed while conducting this review and study protocol was registered with PROSPERO in priori (CRD42018114258). Sixteen studies, including 5 prospective and 11 retrospective studies, with a total of 1406 (896 BMG and 510 PSG) patients were included in the final analysis. In the overall analysis, BMG had significantly higher success rate (83.7% vs. 76.1%, P ≤ 0.0001). Duration of followup was heterogeneous across the studies, ranging from 15.9 to 201 months. Comparing the five studies where the data on duration of follow up was available, BMG showed a significantly higher success rate compared to PSG (90% vs. 80.4%; P = 0.02). In the subgroup of patients with bulbar urethral strictures, BMG urethroplasty had significantly higher success rate (87.4% vs. 78.0%; P = 0.0001). From the results of this study, buccal mucosa may appear to be a better choice, however, the data is still immature and a properly conducted randomized controlled trial with an adequate duration of followup is required.
INTRODUCTION
Urethral stricture disease is a common problem faced by urologists worldwide and acquiring the skills of urethral reconstruction is an important part of their training. Along with the knowledge of various available techniques, an evidence base is required for a surgeon to decide upon the best possible approach for a given patient. The techniques of urethral reconstruction are abound. Excision and primary repair is the gold standard for strictures up to 2 cm in the bulbar urethra, whereas for strictures more than 2 cm substitution, urethroplasty is the preferred technique.[1]
Substitution urethroplasty entails harvesting of a graft with suitable characteristics and its placement at the recipient site to reconstruct the strictured urethra. These grafts may be harvested from the genital skin including the preputial skin, the penile shaft skin, and scrotal skin or might be harvested from distant epithelial surfaces such as the inguinal skin, the oral mucosa, lingual mucosa, bladder, and colonic mucosa.[2] With the advent of tissue engineering, artificially produced grafts might also find a place in the arsenal of the reconstructive surgeon.[2]
The ideal graft for urethral reconstruction should be resilient so as to withstand the wet environment, be easily accessible, hairless, and inclined to enable neovascularization.[1] The two most commonly used graft materials used for substitution urethroplasty in today's practice are the penile skin and the buccal mucosa. A penile skin graft (PSG) might be harvested from the prepuce or the penile shaft. The advantage is its easy access within the same operative field. The buccal mucosal graft (BMG) on the other hand requires preparation of two operative fields and thus results in longer surgical time. Despite the relatively tedious access, the structure of the buccal mucosa with a thick epidermis and thin lamina propria makes it a robust graft for urethral reconstruction.[1]
Both, PSG and BMG have their merits and demerits in given situations and till date the use of either is dictated by the surgeons preference and skill. There is a paucity of well conducted trials stating one method is better than the other and the advantages of one over the other haven't been defined objectively. The perception of one being better than the other at this point is purely subjective. Therefore, this study was designed with the aim of evaluating the superiority of one over the other and putting forth an evidence based solution to the above problem.
METHODS
Study design
In the present study, we conducted a systematic review of existing literature to identify all the relevant publications comparing the outcomes of buccal mucosa and penile skin urethroplasties. A prespecified study protocol has been previously registered with PROPSERO (CRD42018114258). Preferred reporting Items for Systematic reviews and Meta-analysis guidelines were followed while conducting this review.[3]
Search strategy
A systematic literature search for relevant papers was performed in various electronic databases PubMed/Medline, EMBASE, Scopus and Web of science by two authors (GS and SS) indenpendently. Keywords used for the search were according to Patient, intervention, control and outcome guidelines, i.e., “Stricture urethra” OR “Urethral stricture” OR “Urethral stenosis” (Patient) AND “Penile skin” OR “Preputial skin;” (Intervention) OR “Urethroplasty” AND “Buccal mucosa” OR “oral” (Control). Following filters for search were applied: date of publication (01.01.2000 and onward), sex (males), species (human), and language (English). Last systematic search was performed on June 2, 2019. Titles and abstracts of these articles were then reviewed for inclusion and exclusion criteria. We also searched the references of the articles selected for full review. Data pertaining to stricture etiology, follow-up duration, duration of procedure, stricture length, stricture location, type of urethroplasty, and complications were also extracted and reviewed. Full details of PubMed search are available in the Supplementary S1.
Supplementary S1.
Search methodlogy for PubMed
| Keyword | Results |
|---|---|
| ALL (“urethral stricture”) | 5936 |
| ALL (“urethral stenosis”) | 584 |
| ALL (“stricture urethra”) | 40 |
| ALL (“penile skin”) | 715 |
| ALL (“preputial skin”) | 175 |
| ALL (“Buccal mucosa”) | 5105 |
| ALL (‘urethroplasty’) | 2870 |
| ALL (‘oral’) | 589,516 |
| ((“buccal mucosa“[All Fields] OR (“mouth”[MeSH Terms] OR “mouth”[All Fields] OR “oral”[All Fields])) AND ((“penile skin”[All Fields] OR “preputial skin”[All Fields]) OR “urethroplasty”[All Fields])) AND ((“urethral stricture”[All Fields] OR “urethral stenosis”[All Fields]) OR “stricture urethra”[All Fields]) AND (“humans”[MeSH Terms] AND English[lang] AND “male”[MeSH Terms]) | 233 |
Selection criteria
GS and SS independently reviewed the titles and abstracts of the relevant articles obtained from the literature search. Based on the inclusion and exclusion criteria, studies were selected for full text review. Studies reporting individualized data on success of PSG versus BMG within the same manuscript were included. Whereas, studies reporting data on success of either PSG or BMG, studies on penile skin flaps or a combined PSG with penile skin flaps versus BMG, studies including patients with primary hypospadias repair, reviews, and case reports were excluded. Any discrepancy on inclusion or exclusion of a study was sort out by arbitration among the three authors (GS, SS, and KP).
Outcomes
No recurrence or need of further endoscopic intervention was considered as success. For successful urethroplasty, apart from the type of the graft used, there are multiple other confounding factors such as duration of follow-up, length of stricture, stricture site, etiology and surgical procedure. In this study, we initially planned to perform a subgroup analysis for each of these factors. However, the data regarding the etiology and the surgical procedure performed was not uniformly recorded in all the studies; hence a subgroup analysis in these two categories could not performed.
Data extraction
Using a predefined template [Table 1] including stricture etiology, follow-up duration, duration of procedure, stricture length, stricture location, type of urethroplasty and complications, the data was extracted by two reviewers (GS and SS) from the studies included in the final analysis. In case of any discrepancy, help of third author (KP) was sought for arbitration.
Table 1.
Summary of data of studies included
| Author, year | Type of study | Country of origin | Etiology of stricture | Duration of surgery (min) | Follow-up (months) | Stricture length (cm) | Stricture Location | Surgical technique | Local Complication rate (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BMG | PSG | BMG | PSG | BMG | PSG | BMG | PSG | BMG | PSG | BMG | PSG | ||||
| Guralnick, 2001 | R | USA | Various | NA | NA | NA | Bulbar | AAR | NA | ||||||
| Wessells, 2002 | R | USA | Various | NA | NA | NA | Bulbar | VO | 5.8 | 0 | |||||
| Alsikafi, 2005 | R | USA | Various | NA | 48.3 | 201 | 5.7 | 4.7 | Anterior urethra | Not specified | NA | ||||
| Raber, 2005 | P | Italy | Various | NA | NA | 3.2 | 4.3 | Bulbar | DO | 30.7 | 11.7 | ||||
| Barbagli, 2006 | R | Italy | Various excluding LS, FHR | NA | 41.6 | 71 | NA | Bulbar | VO, DO, LO, AAR | DO | NA | ||||
| Lumen, 2007 | R | Belgium | Various | NA | 36 | 5.2 | Bulbar | VO | NA | ||||||
| Barbagli BJU Int, 2008 | R | Italy | Various excluding LS, FHR | NA | 57 | 37.8 | NA | Penile | Asopa | NA | |||||
| Barbagli, Eur Urol. 2008 | R | Italy | Various excluding LS, FHR | NA | 38.1 | 109 | NA | Bulbar | AAR (9), DO (38) | AAR (31), OSU, not specified 132) | NA | ||||
| Casey, 2008 | P | USA | NGB | NA | NA | NA | Anterior urethra | OSU TSU | OSU | NA | |||||
| Meeks, 2008 | P | USA | Catheter induced, pancreatic enzyme induced, Unknown In posttransplant patients | NA | 31.5 | 3.5 | Penile | Anterior urethra | Not specified | NA | |||||
| Lumen, 2010 | P | Belgium | Various | NA | 36 | 44.1 | 6.4 | 7.3 | Anterior urethra | Not specified | NA | ||||
| Mathur, 2011 | R | India | Various | NA | NA | NA | Pan-urethral | DO | Not specified | 22.5 | 32 | ||||
| Barbagli, 2014 | R | Italy, India | Various Excluding LS, FHR | NA | 118 months (median) | NA | Penile, Bulbar | Not specified | NA | ||||||
| Granieri, 2014 | R | USA | Various | NA | 15.9 | 17.5 | 2.8 | 2.1 | Bulbar | AAR, DO | AAR, DO | NA | |||
| Lozano, 2015 | R | Spain | Various | NA | 108 | NA | Penile, Bulbar | Not specified | NA | ||||||
| Hussein, 2016 | P | Egypt | Various | 256 | 136 | 55 | 60 | 6.8 | 8.9 | Penile, Peno-bulbar | DO | 6 | 13 | ||
NA: Not available, BMG: Buccal mucosa grafts, PSG: Penile skin graft, LS: Lichen sclerosus, FHR: Failed hypospadias repair, NGB: Neurogenic bladder, TSU: Two-stage urethroplasty, OSU: One-stage urethroplasty, AAR: Augmented anastomotic repair, DO: Dorsal onlay, VO: Ventral onlay, LO: Lateral onlay
Risk of bias assessment
Risk of bias assessment could not be performed as all the studies were either single or multicenter prospective or retrospective case series and we could not find an appropriate tool for the same.
Statistical analysis
All the relevant data pertaining to the study was entered into a Microsoft excel sheet. While performing the pooled analysis, weighted average of the individual summary statistics was calculated for the duration of follow-up and stricture length in the two groups. Mean was estimated from the median and range using the formula reported by Hozo et al.[4]
Statistical heterogeneity was tested using Chi-square and I2. Random effects model was used as it provided a more conservative approach. P = <0.05 indicated statistical significance. Statistical analysis was performed using the Cochrane Collaboration review manager software RevMan 5.2™ (the Cochrane collaboration, Copenhagen, Denmark).
RESULTS
Search strategy and selection
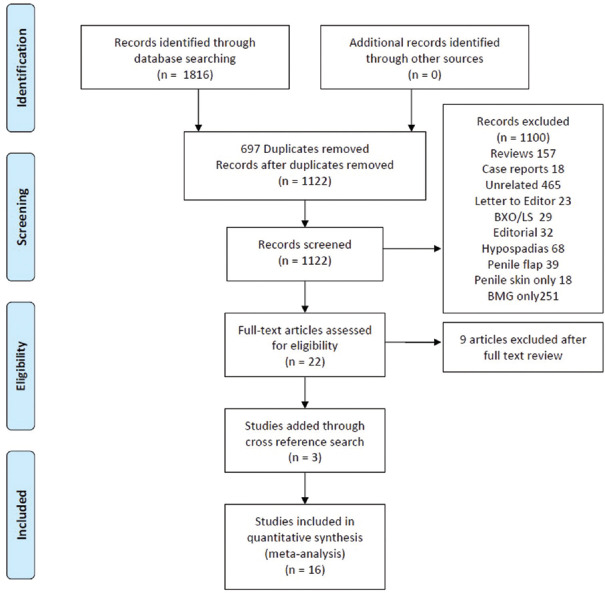
An initial search using keywords and filters described above yielded 1819 articles (233 articles from PubMed, 277 from Web of science, 877 from SCOPUS, and 420 from EMBASE). Of these 1819 articles, 697 duplicates were removed. Further, 1122 nonduplicate citations, abstracts and titles were screened for relevance. After screening of titles and abstracts, 1100 articles were excluded for various reasons [Figure 1]. Full text review of 22 articles was performed out of which 9 were excluded due to lack of individualized data on comparison of BMG and PSG (Supplementary S2). Three additional articles were identified through cross reference search and were included in the final analysis. For the final analysis, 16 studies were included. Data from all these 16 studies[5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20] were extracted and tabulated in a predefined format.
Figure 1.
Preferred reporting Items for Systematic reviews and meta-analysis flow chart depicting search strategy used for this study
Supplementary S2.
Studies excluded after reading full text articles with reasons
| Study | Reason |
|---|---|
| Asopa, 2001 | Lack of individualized data on PSG and BMG |
| Berger, 2005 | Clubbed preputial skin with inguinal skin |
| Dubey, 2003 | Lack of individualized data on PSG and BMG |
| Ekeke, 2017 | Lack of individualized data on PSG and BMG |
| Fossati, 2016 | Lack of individualized data on PSG and BMG |
| Marchal, 2010 | Used different definition for success |
| Reyad, 2018 | Lack of individualized data on PSG and BMG |
| Sawant, 2018 | Lack of individualized data on PSG and BMG |
| Wood, 2006 | Lack of individualized data on PSG and BMG |
BMG: Buccal mucosa grafts, PSG: Penile skin graft
Study characteristics
For the final analysis, 16 studies with 1406 patients were included satisfying the predefined conditions. Out of these 16 studies, 11 were retrospective and 5 were prospective. Out of 1406 patients included in the final analysis, 896 underwent BMG and 510 underwent PSG urethroplasty. Causes of stricture varied across the studies. Duration of surgery was reported only in one study,[20] in which the mean reported duration for BMG was longer as compared to PSG (256 vs. 136 min). Rest of the details regarding site of stricture and type of surgery have been provided in Table 1.
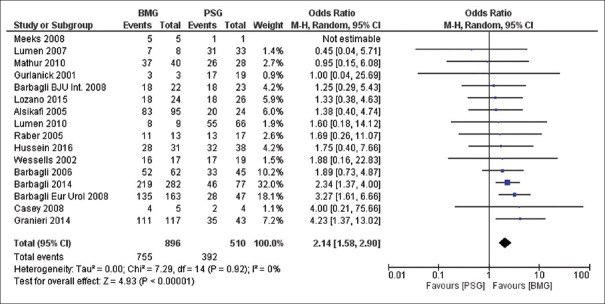
Overall success rate
Overall analysis of data was performed using random effect model. BMG was successful in 83.7% of the patients whereas PSG was successful in 76.1% of the patients (Odds ratio [OR] 2.14, Confidence interval [CI) [1.58, 2.9], P ≤ 0.0001) [Figure 2].
Figure 2.
Forest plot depicting overall success rate of buccal mucosa graft versus penile skin graft
Subgroup analysis
Duration of follow-up
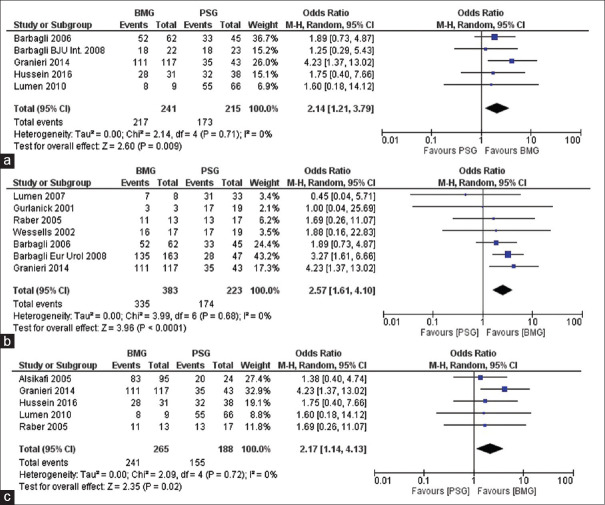
Duration of follow-up was heterogeneous across the studies, ranging from 15.9 to 201 months. Individual data pertaining to the length of follow-up in the two groups was provided by seven studies [Supplementary S3]. Weighted mean duration of follow-up was 35.2 and 95.4 months in the BMG and the PSG groups, respectively. Mean duration of follow-up after excluding studies by Alsikafi et al.[7] and Barbagli et al.[12] was 32 and 46.5 months (as these studies had much longer follow-up for PSG). Comparison of these five studies showed a significantly higher success rate of BMG compared to PSG urethroplasty (90% vs. 80.4%; P = 0.02) [Figure 3a].
Supplementary S3.
Pooled analysis of data for duration of follow-up
| Study | Mean duration (months) | |
|---|---|---|
| BMG (n) | PSG (n) | |
| Alsikafi, 2005 | 48.3 (24) | 201 (95) |
| Barbagli, 2006 | 41.6 (62) | 71 (45) |
| Barbagli, BJU Int, 2008 | 57 (22) | 37.8 (23) |
| Barbagli, Eur Urol, 2008 | 38.1 (163) | 109.3 (47) |
| Lumen, 2010 | 36 (9) | 44.1 (66) |
| Granieri, 2014 | 15.9 (117) | 17.5 (43) |
| Hussein, 2016 | 55 (31) | 60 (38) |
| Weighted mean | 35.2 | 95.4 |
BMG: Buccal mucosa grafts, PSG: Penile skin graft
Figure 3.
(a) Forest plot depicting overall success rate of buccal mucosa grafts versus Penile skin graft for studies with available duration of follow-up, (b) forest plot depicting overall success rate of buccal mucosa grafts versus penile skin graft in bulbar urethra, (c) forest plot depicting overall success rate of buccal mucosa grafts versus Penile skin graft for studies with available length of stricture
Buccal mucosal grafts versus penile skin graft in bulbar urethroplasty
Seven studies reported their data for strictures located in the bulbar urethra [Figure 3b]. For quantitative analysis, we used the random model and the data showed BMG urethroplasty had significantly higher success rates for strictures located in the bulbar region (87.4% vs. 78.0%; P = 0.0001).
Mean length of stricture
Of the 16 studies included in the meta-analysis, mean stricture length was reported only in five studies with range from 2.1 to 8.9 cm. Weighted mean stricture lengths were 4.1 cm and 5.9 cm in the BMG and PSG groups, respectively [Supplementary S4]. Comparing the two groups, i.e., BMG and PSG across these studies again revealed BMG to be significantly better than PSG (RR = 1.1, CI 1.01–1.20, P = 0.003) [Figure 3c].
Supplementary S4.
Pooled analysis of data for stricture length
| Study | Stricture length (cm) | |
|---|---|---|
| BMG (n) | PSG (n) | |
| Alsikafi, 2005 | 4.7 (95) | 5.7 (24) |
| Raber, 2005 | 3.2 (13) | 4.3 (17) |
| Lumen, 2010 | 6.4 (9) | 7.3 (66) |
| Granieri, 2014 | 2.8 (117) | 2.1 (43) |
| Hussein, 2016 | 6.8 (31) | 8.9 (38) |
| Weighted mean | 4.1 | 5.9 |
BMG: Buccal mucosa grafts, PSG: Penile skin graft
Complication rates
Local site complications were reported in four studies. Wessells[6] and Raber et al.[8] reported higher complication with BMG as compared to PSG [Table 1]. Whereas, Mathur et al.[16] and Hussein et al.[20] reported higher complications with PSG.
Publication bias
Funnel plots for the primary outcomes were made. Funnel plots are available in Supplementary S5 (215.4KB, tif) . Test for funnel plot asymmetry for primary outcome i.e., overall success rate was performed using Egger's regression test with P = 0.587, i.e., There was no publication bias.
DISCUSSION
Data from previous retrospective studies suggest an advantage of buccal mucosa over the preputial skin.[21] Purported advantages of BMG are easy and readily availability, concealed donor site, good amount of elastic tissue and thin yet vascular lamina propria which helps in the early phase of graft uptake.[22] Also, the buccal muscosa may be the only available option in certain patients where local penile tissues are unavailable (circumcised) or unsuitable for use (balanitis xerotica obliterans). Use of PSG has been in practice for a long time and the studies reporting on the outcomes of PSG have a longer duration of follow-up as compared to BMG, the use of which has been popularized recently at the turn of century. Lumen et al.[23] had acknowledged this limitation in their meta-analysis. Furthermore, there are numerous other confounding factors such as the length of stricture, the site of stricture, and the surgical technique used which must be taken into account. Alsikafi et al.[7] reported no significant difference in the two groups of urethroplasties despite the fact that duration of follow-up was longer in the PSG arm. As the experience with BMG urethroplasty has grown in the present century, this review aims to overcome the shortcomings highlighted in the previous studies and provide best quality evidence.
In the present meta-analysis, of the 16 studies included, 5 were prospective and 11 were retrospective (Level of evidence IIIA). An improvement in the quality of studies and the resulting level of evidence can be brought by conducting randomized control trials in this field. Saying this, it should also be kept in mind that conducting a randomized control trial in urethroplasty is a daunting task due to the heterogeneity in the site of stricture, surgical techniques and outcome assessment parameters. Level of evidence obtained from the present study is low (IIIA); in the paucity of well-conducted randomized controlled trials (RCTs), however, it is the best available evidence in the literature.
Length of stricture
Effect of length of stricture on the outcome is controversial. Hussein et al.[20] reported similar success rates (87% vs. 87%, P = 0.9) and functional outcomes in patients with long segment anterior urethral strictures (<8 cm vs. >8 cm) postdorsal onlay urethroplasty. On the contrary, Breyer et al. have reported a stricture length of >4 cm to be an independent predictor of failure posturethroplasty.[24] Longer strictures have been associated with longer operative time, higher amount of blood loss and postvoid dribbling.[20] In the subgroup analysis of studies with data on the length of stricture, weighted mean stricture length was longer in PSG group (5.9 cm vs. 4.1 cm). Subgroup analysis of these 5 studies for primary outcome favored the BMG group (90.9 vs. 82.4, P = 0.03).
Duration of follow-up
In this meta-analysis, seven studies have reported individual follow-up data [Supplementary S3]. Weighted mean duration of follow-up was longer for PSG urethroplasty (95.4 vs. 35.2 months). Andrich et al.[25] have reported an increase in the re-stricture rates after substitution urethroplasty as the duration of follow-up increases, probably related to the tendency of these grafts to shrink in size over time. In order reduce this disparity in the follow-up duration between two arms of our analysis, the studies by Alsikafi et al.[7] and Barbagli et al.[12] were excluded and the revised weighted mean duration of follow-up was 32 months in BMG and 46.5 months in PSG. Subgroup analysis of these five studies also favored the BMG group (90% vs. 80.4%; P = 0.02). This ten percent difference between the two groups seems to be quite significant, even if we consider the mean difference of 14.5 months in the duration of follow-up.
Site
Apart from the surgical technique, the stricture and the graft length, the site of the stricture can also influence outcomes. Some studies have reported better outcomes in bulbar[26,27] as compared to penile urethral strictures whereas others have not reported a significant difference[17]. From the studies included in this review, a comparison was not possible, however we performed a subgroup analysis of the seven studies that reported their outcome specifically for the bulbar urethral strictures and the results again favored BMG over PSG (87.4% vs. 78.0%, P = 0.01) although there might have been other confounding factors.
Etiology
The causes of urethral strictures are varied and it is logical to think that the pathology leading to the disease might also have some bearing on the success of the methods used for reconstruction. This question becomes more important when the etiology is related to a local infective or inflammatory cause such as lichen sclerosis. The study by Mathur et al.[16] endeavors to answer this very question and their results state that the outcomes for postinflammatory strictures are the poorest.
The studies included in this meta-analysis tackle various etiologies and many have excluded strictures due to lichen sclerosis and failed hypospadias repair. The etiologies are diverse and their distribution within the two groups of interest is unknown. In cases of inflammatory diseases, local tissue is usually not available for reconstruction, which makes it possible that more inflammatory pathology might be included in the BMG group. However, despite this assumption, our results show an improved outcome in the BMG group.
Surgical technique
The surgical techniques available for graft placement for urethral reconstruction are as diverse as the etiologies involved. Consequently the studies included in the meta-analysis use a wide variety of techniques with the dorsal onlay and the ventral onlay techniques being the most frequently used. Only few of the studies have maintained a uniformity in the position of graft placement such as the one by Wessells[6] which describes the experience of the author with the ventral onlay technique.
The proponents for dorsal onlay technique state decreased blood loss as the advantage while those for ventral onlay technique cite the fact that lesser and easier dissection is required to reach the desired location of graft placement. However, the most important determinant of graft take remains the condition of the graft bed. Surgeon experience and preference play an equally important role in the outcomes of the various techniques. There might be equal chances of hemorrhage in the dorsal approach and equal propensity for disturbing the vasculature of the corpus spongiosa in the ventral approach in hands of surgeons who are not used to the respective procedures. No significant difference has been found in the take of the graft pertaining to its location.[1] Thus, it is unlikely that the results of this meta-analysis would be influenced by the distribution of the technique of surgery within the cohorts of interest.
As described above, the main limitation of this study is the poor quality of studies included in this review. Studies included in this review are case series conducted in a single or multicentre setting. Two groups, i.e., BMG and PSG are quite heterogeneous with multiple confounding factors influencing the primary outcome, i.e., duration of follow-up, etiology of stricture, surgical technique, surgeon experience, length of stricture and the site of stricture. Although we have tried to eliminate some of them by performing a subgroup analysis, many other confounding factors could not be controlled.
CONCLUSION
This meta-analysis shows that BMG may be superior to PSG urethroplasty. We attempted to tackle each of the confounding factors, one at a time and still found BMG to be superior to PSG. However, controlling for one factor at a time is not enough when multiple confounding factors are involved (duration of follow-up, stricture length, type of surgical technique, and site of stricture). In any given situation, other factors such as surgeon preference, local tissue inflammation, poor oral hygiene might influence the choice of graft. Buccal mucosa, due to its various properties, appear to be better than PSG; but, the data are still immature, and a RCT with an adequate duration of follow up is required to better answer the question.
Funnel plot for primary outcome
Footnotes
Financial support and sponsorship: Nil.
Conflicts of Interest: There are no conflicts of interest.
REFERENCES
- 1.Levy ME, Elliott SP. Graft use in bulbar urethroplasty. Urol Clin North Am. 2017;44:39–47. doi: 10.1016/j.ucl.2016.08.009. [DOI] [PubMed] [Google Scholar]
- 2.Mangera A, Chapple C. Management of anterior urethral stricture: An evidence-based approach. Curr Opin Urol. 2010;20:453–8. doi: 10.1097/MOU.0b013e32833ee8d5. [DOI] [PubMed] [Google Scholar]
- 3.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guralnick ML, Webster GD. The augmented anastomotic urethroplasty: Indications and outcome in 29 patients. J Urol. 2001;165:1496–501. [PubMed] [Google Scholar]
- 6.Wessells H. Ventral onlay graft techniques for urethroplasty. Urol Clin North Am. 2002;29:381–7, vii. doi: 10.1016/s0094-0143(02)00029-0. [DOI] [PubMed] [Google Scholar]
- 7.Alsikafi NF, Eisenberg M, McAninch JW. Long-term outcomes of penile skin graft versus buccal mucosal graft for substitution urethroplasty of the anterior urethra. J Urol. 2005;73:87. [Google Scholar]
- 8.Raber M, Naspro R, Scapaticci E, Salonia A, Scattoni V, Mazzoccoli B, et al. Dorsal onlay graft urethroplasty using penile skin or buccal mucosa for repair of bulbar urethral stricture: Results of a prospective single center study. Eur Urol. 2005;48:1013–7. doi: 10.1016/j.eururo.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 9.Barbagli G, Lazzeri M. Urethral reconstruction. Curr Opin Urol. 2006;16:391–5. doi: 10.1097/01.mou.0000250277.44990.ab. [DOI] [PubMed] [Google Scholar]
- 10.Lumen N, Browaeys H, Hoebeke P, Oosterlinck W. Ventral onlay graft urethroplasty using genital skin or buccal mucosa in the treatment of bulbar strictures: A retrospective analysis of 41 cases. Current Urol. 2008;2:10–4. [Google Scholar]
- 11.Barbagli G, Morgia G, Lazzeri M. Retrospective outcome analysis of one-stage penile urethroplasty using a flap or graft in a homogeneous series of patients. BJU Int. 2008;102:853–60. doi: 10.1111/j.1464-410X.2008.07741.x. [DOI] [PubMed] [Google Scholar]
- 12.Barbagli G, Guazzoni G, Lazzeri M. One-stage bulbar urethroplasty: Retrospective analysis of the results in 375 patients. Eur Urol. 2008;53:828–33. doi: 10.1016/j.eururo.2008.01.041. [DOI] [PubMed] [Google Scholar]
- 13.Casey JT, Erickson BA, Navai N, Zhao LC, Meeks JJ, Gonzalez CM. Urethral reconstruction in patients with neurogenic bladder dysfunction. J Urol. 2008;180:197–200. doi: 10.1016/j.juro.2008.03.056. [DOI] [PubMed] [Google Scholar]
- 14.Meeks JJ, Gonzalez CM. Urethroplasty in patients with kidney and pancreas transplants. J Urol. 2008;180:1417–20. doi: 10.1016/j.juro.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 15.Lumen N, Hoebeke P, Oosterlinck W. Urethroplasty for urethral strictures: Quality assessment of an in-home algorithm: Original article: Clinical investigation. Int J Urol. 2010;17:167–74. doi: 10.1111/j.1442-2042.2009.02435.x. [DOI] [PubMed] [Google Scholar]
- 16.Mathur R, Aggarwal G, Satsangi B. A retrospective analysis of delayed complications of urethroplasty at a tertiary care centre. Updates Surg. 2011;63:185–90. doi: 10.1007/s13304-011-0093-4. [DOI] [PubMed] [Google Scholar]
- 17.Barbagli G, Kulkarni SB, Fossati N, Larcher A, Sansalone S, Guazzoni G, et al. Long-term followup and deterioration rate of anterior substitution urethroplasty. J Urol. 2014;192:808–13. doi: 10.1016/j.juro.2014.02.038. [DOI] [PubMed] [Google Scholar]
- 18.Granieri MA, Webster GD, Fraser MO, Peterson AC. The evolution of urethroplasty for bulbar urethral stricture disease; more options, better outcomes. Eur Urol Suppl. 2014;13:e87. doi: 10.1016/j.juro.2014.05.085. [DOI] [PubMed] [Google Scholar]
- 19.Lozano JL, Arruza A. Substitution urethroplasty. Long term follow up results in a group of 50 patients. Arch Esp Urol. 2015;68:424–8. [PubMed] [Google Scholar]
- 20.Hussein MM, Almogazy H, Mamdouh A, Farag F, Rashed E, Gamal W, et al. Urethroplasty for treatment of long anterior urethral stricture: Buccal mucosa graft versus penile skin graft-does the stricture length matter? Int Urol Nephrol. 2016;48:1831–5. doi: 10.1007/s11255-016-1366-0. [DOI] [PubMed] [Google Scholar]
- 21.Barbagli G. Interview with Dr Guido Barbagli? Substitution urethroplasty: Which tissues and techniques are optimal for urethral replacement. Eur Urol. 2007;52:602–4. [PubMed] [Google Scholar]
- 22.Wood DN, Allen SE, Andrich DE, Greenwell TJ, Mundy AR. The morbidity of buccal mucosal graft harvest for urethroplasty and the effect of nonclosure of the graft harvest site on postoperative pain. J Urol. 2004;172:580–3. doi: 10.1097/01.ju.0000132846.01144.9f. [DOI] [PubMed] [Google Scholar]
- 23.Lumen N, Oosterlinck W, Hoebeke P. Urethral reconstruction using buccal mucosa or penile skin grafts: Systematic review and meta-analysis. Urol Int. 2012;89:387–94. doi: 10.1159/000341138. [DOI] [PubMed] [Google Scholar]
- 24.Breyer BN, McAninch JW, Whitson JM, Eisenberg ML, Mehdizadeh JF, Myers JB, et al. Multivariate analysis of risk factors for long-term urethroplasty outcome. J Urol. 2010;183:613–7. doi: 10.1016/j.juro.2009.10.018. [DOI] [PubMed] [Google Scholar]
- 25.Andrich DE, Dunglison N, Greenwell TJ, Mundy AR. The long-term results of urethroplasty. J Urol. 2003;170:90–2. doi: 10.1097/01.ju.0000069820.81726.00. [DOI] [PubMed] [Google Scholar]
- 26.Wang K, Miao X, Wang L, Li H. Dorsal onlay versus ventral onlay urethroplasty for anterior urethral stricture: A meta-analysis. Urol Int. 2009;83:342–8. doi: 10.1159/000241680. [DOI] [PubMed] [Google Scholar]
- 27.Spilotros M, Sihra N, Malde S, Pakzad MH, Hamid R, Ockrim JL, et al. Buccal mucosal graft urethroplasty in men-risk factors for recurrence and complications: A third referral centre experience in anterior urethroplasty using buccal mucosal graft. Transl Androl Urol. 2017;6:510–6. doi: 10.21037/tau.2017.03.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Funnel plot for primary outcome