Abstract
Background
Lyme disease (LD) is emerging in many parts of central and eastern Canada. Serological testing is most commonly used to support laboratory diagnosis of LD. Standard two-tiered testing (STTT) for LD involves detection of Borrelia burgdorferi antibodies using an enzyme immunoassay (EIA) followed by IgM and/or IgG immunoblots. However, improved sensitivity has been demonstrated using a modified two-tiered testing (MTTT) approach, in which a second EIA instead of the traditional immunoblot is used. This article summarises the evidence supporting the MTTT versus STTT for laboratory diagnosis of LD in Canada.
Methods
Peer reviewed literature on the sensitivity and specificity of different EIAs were compared by Canadian experts in LD diagnostic for MTTT vs STTT in patients with clinical history of LD residing in LD endemic areas or in samples from the LD serum repository.
Results
The MTTT approach consistently demonstrated improved sensitivity to detect early infections with B. burgdorferi and also maintained high specificity vs STTT.
Conclusion
Diagnostic improvements in sensitivity of LD testing without significant loss of specificity have been consistently reported when MTTT is compared with STTT in studies conducted in highly LD endemic regions. Our working group agrees with the recommendation by the United States Centers for Disease Control that serological testing for LD using MTTT is an acceptable alternative to STTT. This recommendation is contingent on development and implementation of comprehensive validation studies on the performance of MTTT vs STTT within the Canadian context, including evaluation of the test performance in areas of low endemicity for LD.
Keywords: Borrelia burgdorferi, Lyme disease, serology, standard two-tiered testing, enzyme immunoassays, immunoblots, diagnostics
Introduction
Lyme disease (LD) is an emerging tick-borne infection caused by spirochetes belonging to the Borrelia burgdorferi sensu lato species complex, which are transmitted to humans by infected ticks (1). The principal tick vectors are the blacklegged tick (Ixodes scapularis) and the western blacklegged tick (Ixodes pacificus) in eastern/central Canada and British Columbia, respectively (2). In Canada, infected blacklegged tick populations are endemic in parts of British Columbia, Manitoba, Ontario, Quebec, New Brunswick and Nova Scotia (3). The number of Canadians with LD has risen since it became nationally reportable, from 144 cases in 2009 to 2,025 in 2017, which is likely an under-representation of the true numbers (1,2,4). As the geographic range of blacklegged ticks continues to expand, more Canadians will be at risk for acquiring LD (5). It is estimated that more than 300,000 cases of LD occur in the United States (US) each year (6). The volume of diagnostic tests for LD performed in the US is much greater compared with Canada (7). In part, this has driven efforts to improve testing efficiencies for LD, including the development and approval of the modified two-tiered testing (MTTT) (8). The objective of this document is to summarise the evidence supporting the improved performance of the MTTT approach compared to the currently used diagnostic algorithm for LD.
Intervention
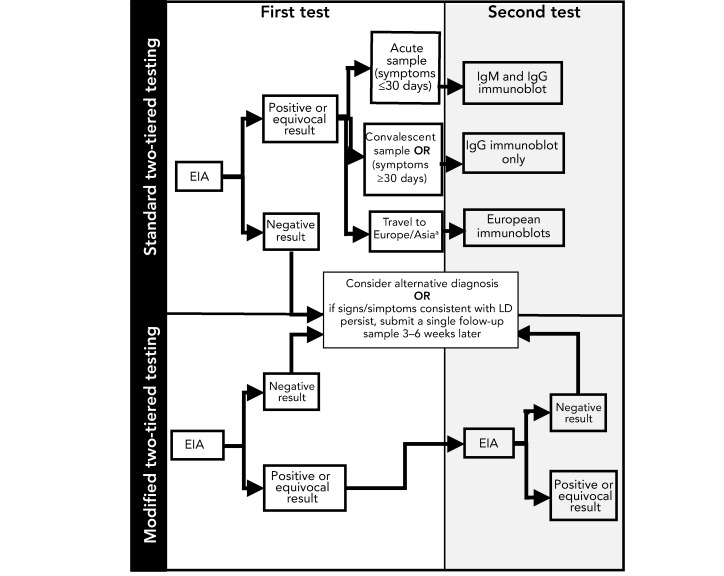
The current reference method most commonly used for laboratory diagnosis of LD is serology, which detects antibodies to B. burgdorferi using standard two-tiered testing (STTT), using an enzyme immunoassay (EIA) as the first tier test followed by IgM and/or IgG immunoblots as a supplemental test (Figure 1). Most provincial public health or hospital laboratories perform the EIA testing locally while immunoblot testing is performed independently at provincial public health labs in British Columbia, Ontario (and shortly in Quebec) or at the National Microbiology Laboratory (NML). NML performs immunoblot testing for all provinces when LD is suspected in patients who travelled outside of North America (Figure 1). Regardless of the type of testing, results are reviewed by laboratory staff and reported to the requesting physician and positive results are also reported to local provincial public health.
Figure 1.
Schematic depicting steps in standard two-tiered testing and modified two-tiered testing for Lyme diseasea
Abbreviations: EIA, enzyme immunoassay; IgG, immunoglobulin G; IgM, Immunoglobulin M; LD, Lyme disease
a Note that suspect LD cases from Europe or Asia would continue to be investigated using the standard two-tiered testing
A number of different EIAs are available for the first tier in the STTT including those composed of whole cell sonicates (WCS) of the laboratory strain of B. burgdorferi B31. More recently, EIAs based on synthetic peptides that contain regions conserved among multiple B. burgdorferi strains, such as the surface lipoprotein variable major protein-like sequence, expressed (VlsE), C6 (the invariable region 6 of VlsE) or C10 peptide (the conserved amino-terminal portion of outer surface protein C), have been developed (8,9). While the specificity of the newer assays is better than WCS, they are still not sufficiently specific to be used as a standalone assay. As a result, supplemental testing with immunoblots is recommended (9–12). The STTT does have a number of technical limitations, including that immunoblots are more laborious to perform than EIAs and the scoring of the immunoblots can be subjective, which may lead to inter and intralaboratory variability (11). In addition, immunoblot testing is performed in relatively few reference diagnostic laboratories in the US (7) and Canada so turnaround times are typically longer than for EIAs alone (8,11).
The performance characteristics of the STTT algorithm also depend on the stage of infection. A recent systematic review has shown that the sensitivity of STTT for LD is poor in early localized infection (less than 50%) but in late stages of infection the sensitivity approaches 100% (13). As such, diagnosis and treatment of early localized LD is based on clinical symptoms alone in patients who have exposure history in blacklegged tick endemic areas (10). However, the diagnosis of early LD can be challenging since some patients with early localized B. burgdorferi infections do not present with an erythema migrans rash and may have symptoms that overlap with those of other diseases (9,14). Thus, improving the sensitivity of testing in early localized infections is important in identifying patients with LD, allowing for early treatment and potentially preventing infection from disseminating and causing severe disease.
Outcomes
Modified two-tiered testing for serologic diagnosis of Lyme disease
There have been a number of studies evaluating the use of a MTTT approach in which a second EIA is performed instead of the traditional immunoblots (Figure 1). A number of different combinations of EIAs have been used in this so-called “two EIA approach” including WCS EIA followed by C6 EIA, VlsE EIA followed by C6 EIA, C6 EIA followed by VlsE and VlsE/C10 followed by WCS (15–20). Samples for these evaluations have been drawn from smaller cohorts of patients with acute LD (15,18) or comparisons were made using well-characterised samples from the Centers for Disease Control (CDC) LD serum repository (16,19,21). Studies were performed on samples from children (17,20) as well as adults (16,19,21). With few exceptions (22), these evaluations have only been performed on patients from the US and the MTTT has not been fully validated for use on patients with exposure in Europe or Asia.
Although different combinations of EIAs were used in the MTTT algorithms, the MTTT was consistently more sensitive in detecting B. burgdorferi infections, particularly in early localized LD compared with STTT. Importantly, these MTTT had equivalent sensitivity for detecting late infections and comparable specificities to STTT regardless of the combinations of EIAs used in the MTTT (see summaries in Table 1 and Table 2). Recently, the US Food and Drug Administration approved a MTTT algorithm for the laboratory confirmation of LD acquired in North America (23). This alternative testing algorithm has been endorsed by the US CDC that states that it is an acceptable alternative to the STTT because “the new Lyme disease assays indicates that test performance has been evaluated and is substantially equivalent to or better than a legally marketed predicate test” (24). It is unknown whether the MTTT approach will be validated in the US for patients who potentially acquired LD outside of North America; however, in Canada the STTT algorithm will be maintained using European-specific assays on Canadians with suspect LD acquired outside of North America (Figure 1).
Table 1. Sensitivity of modified two-tiered testing and standard two-tiered testing for Lyme disease.
| Sample size | Reference | Disease manifestations | EIAs combinations useda | MTTT sensitivity % (CI or range) |
STTT sensitivity % (CI or range)b |
|---|---|---|---|---|---|
| 140 | (15) | EM, ENB, LC | WCS f/b C6 | 61 (CI 53–69) | 48 (CI 40–56) |
| 318 | (11) | EM, ENB | WCS f/b C6 | 60 (CI 55–66) | 41 (CI 36–46) |
| 55 | (18) | Acute EM | WCS f/b C6; WCS f/b VlsE CFLIA; VlsE FLIA f/b C6 | 42.7 (R 38.0–54.0) | 32 (R 25–36) |
| 47 | (18) | Convalescent EM | WCS f/b C6; WCS f/b VlsE CFLIA; VlsE FLIA f/b C6 | 70 (R 66–72) | 57.3 (R 55.0–60.0) |
| 95 | (16) | EM, ENB, LC | Vidas f/b C6 or VlsEc | 66.8 (R 65.2–68.4) | 60.2 (R 56.8–64.2) |
| 114 | (17) | All disease stages combined | WCS f/b C6 | 79.8 (CI 71.1–86.5) | 81.6 (CI 73.0–88.0) |
| 40 | (19) | Acute EM | VlsE f/b C6; WCS f/b C6; WCS f/b VlsE | 54.3 (R 50.0–58.0) | 45.3 (R 43.0–50.0) |
| 38 | (19) | Convalescent EM | VlsE f/b C6; WCS f/b C6; WCS f/b VlsE | 77 (R 76–79) | 61; 61; 63 |
| 124 | (19) | All disease stages combined | VlsE f/b C6; WCS f/b C6; WCS f/b VlsE | 76.7 (R 75.0–78.0) | 66; 67; 71 |
| 30 | (25) | Acute EM | VlsE/pepC10 f/b WCS | 73.3 | 50 |
| 30 | (25) | Convalescent EM | VlsE/pepC10 f/b WCS | 83.3 | 76.7 |
| 56 | (25) | Early disseminated disease-stagea | VlsE/pepC10 f/b WCS | 66.1 | 60.7 |
| 29 | (15) | LA, LNB | WCS f/b C6 | 100 (CI 86–100) | 100 (CI 86–100) |
| 122 | (11) | LA, LNB | WCS f/b C6 | 98 (CI 93–99) | 96 (CI 91–98) |
| 29 | (16) | LA | Vidas f/b C6 or VlsE | 100 | 98.9 (R 97–100) |
| 50 | (25) | Late disseminated disease-stagec | VlsE/pepC10 f/b WCS | 100 | 100 |
Abbreviations: EIA, enzyme immunoassay; EM, erythema migrans; ENB, early neuroborreliosis; LA, Lyme arthritis; LC, Lyme carditis; LNB, late neuroborreliosis; MTTT, modified two-tiered testing; STTT, standard two-tiered testing; VlsE, variable major protein-like sequence, expressed; WCS, whole cell sonicates
a Type of EIA and order that EIAs were performed in; f/b-followed by, see original publications for manufacturer's information
b See original publications for precisely EIA and immunoblots used in STTT algorithms
c Data from these two different EIA combinations pooled because no significant difference between them
Table 2. Specificity of modified two-tiered testing and standard two-tiered testing for Lyme disease.
| Sample size | Reference | Patient cohorta | EIAs combinations usedb | MTTT sensitivity % (CI or range) | STTT sensitivity % (CI or range)c |
|---|---|---|---|---|---|
| Overall controls | |||||
| 1,300 | (15) | Healthy and symptomatic controls | WCS f/b C6 | 99.5 (CI 98.9–99.8) | 99.5 (CI 98.9–99.8) |
| 2,208 | (11) | Healthy controls & patients with other diseases | WCS f/b C6 | 99.5 (CI 99.1–99.8) | 99.5 (CI 99.1–99.7) |
| 347 | (16) | Healthy controls & patients with other diseases | Vidas f/b C6 or VlsEc | 98.3 (CI 96.2–99.3) | 98.3 (CI 96.2–99.3) |
| 931 | (17) | Healthy and symptomatic controls | WCS f/b C6 | 96.6 (R 94.6–97.6) | 98.7 (R 96.6–100.0) |
| 347 | (19) | Healthy controls & patients with other diseases | VlsE f/b C6; WCS f/b C6; WCS f/b VlsE | 98.6 (R 97.7–99.4) | 98.1 (R 95.7–99.7) |
| 190 | (25) | Healthy controls & patients with other diseases | VlsE/pepC10 f/b WCS | 98.9 (R 97.8–100.0) | 100 |
| Unhealthy controls | |||||
| 54 | (15) | Symptomatic controls | WCS f/b C6 | 100 | 100 |
| 50 | (18) | Patients with other diseases | WCS f/b C6; WCS f/b VlsE CLIA; VlsE CLIA f/b C6 | 99.3 (R 98.0–100.0) | 100 |
| 144 | (16) | Patients with other diseases | Vidas f/b C6 or VlsEd | 98.2 (R 96.5–100.0) | 97.1 (R 94.4–99.3) |
| 830 | (17) | Symptomatic controls | WCS f/b C6 | 96.5 (R 94.6–97.6) | 98.7 (R 96.6–100.0) |
| 144 | (19) | Patients with other diseases | VlsE f/b C6; WCS f/b C6; WCS f/b VlsE | 98.1 (R 96.5–100.0) | 97.4 (R 95.7–99.7) |
| 90 | (25) | Patients with other diseases | VlsE /PEPC10 f/b WCS | 97.8 | 100 |
Abbreviations: EIA, enzyme immunoassay; MTTT, modified two-tiered testing; STTT, standard two-tiered testing; VlsE, variable major protein-like sequence, expressed; WCS, whole cell sonicates
a Unlike healthy controls, symptomatic controls were subjects with clinical symptoms compatible with Lyme disease (LD) but who did not meet authors LD case definitions; see original publications for list of other diseases but these are look-alike diseases such as syphilis, fibromyalgia and multiple sclerosis
b Type of EIA and order of EIAs were performed in the MTTT; f/b-followed by; see original publications for manufacturer's information
c See original publications for precisely EIA and immunoblots used in STTT algorithms
d Data from these two different EIA combinations were pooled because no significant difference between them
Benefits and limitations of the modified two-tiered testing
In addition to greater sensitivity for the detection of early B. burgdorferi infections, the interpretation of the results of MTTT is less subjective than immunoblot testing (Table 3). The MTTT has also been shown to be more cost-effective than the STTT (26). The tests are also less labour-intensive and can be performed using automated instruments or platforms (8). As such, the MTTT does not require specialized testing (i.e. immunoblots) in a reference laboratory and can be performed by any laboratory that currently does serologic testing. These differences can lead to faster turnaround time for results (8,9).
Table 3. Advantages and disadvantages of the modified two-tiered testing compared to standard two-tiered testing for Lyme disease.
| Advantages | Disadvantages |
|---|---|
| • Improved sensitivity for the detection of early infection (greater than 25% improvement) • Less costly than the STTT • Less laborious • Less subjective • Enzyme immunoassay testing performed locally rather than referral to a specialized laboratory, reducing turnaround times • Faster turnaround time facilitates acute and convalescent testing for non-erythema migrans early localized LD |
• Patients presenting with erythema migrans will still require empiric treatment with antibiotics as the test algorithm sensitivity is less than 90% • As occurs for the STTT, cannot differentiate between recent and past infections or reinfections • Impacts of MTTT on specificity in areas of low prevalence are unclear • STTT may be necessary/beneficial in patients with Lyme arthritis, given the potential for reduced specificity of some polyvalent enzyme immunoassays |
Abbreviations: LD, Lyme disease; MTTT, modified two-tiered testing; STTT, standard two-tiered testing
The interpretation of the results of the MTTT diagnostic testing is either positive or negative, which is more straightforward than for STTT where IgM and IgG immunoblots can produce different outcomes, which can cause confusion for physicians (11). Although more sensitive than STTT, the sensitivity of the MTTT is still less than 90%, so patients with early localized LD should continue to be treated based on their clinical presentation rather than serologic results. However, the rapid turnaround time for MTTT may be particularly useful in evaluating patients with a clinical suspicion of LD but without an erythema migrans rash, or in those who present with signs that overlap with other infections (e.g. Bell’s palsy or arthritis) where serologic results will help establish the diagnosis (8). The most recent evidence-based guidelines from the United Kingdom suggested that "if LD is still suspected in people with a negative ELISA who were tested within four weeks from symptom onset, repeat the ELISA 4–6 weeks after the first ELISA test” (12). Currently if the convalescent EIA is positive, it would still require further supplemental testing with an immunoblot in the STTT. Given the anticipated faster turnaround time for the MTTT, clinicians may be more inclined to follow the National Institute for Health and Care Excellence recommendation and consider acute and convalescent testing, which increases diagnostic certainty of the testing on patients who do not present with erythema migrans rash. This is a particularly important consideration when the clinical suspicion is not high, such as for patients without known tick exposure in LD risk areas.
Despite the numerous advantages of the MTTT, there are associated limitations. Since antibodies to B. burgdorferi can persist for months to years after initial infection (27), the MTTT algorithm (and the STTT) cannot differentiate between active versus past infections, which further confounds serological diagnosis of reinfection with B. burgdorferi. In addition, it is possible that the MTTT algorithm may generate false positives based on the IgM component of the polyclonal EIAs used, since false positive IgM immunoblots are known to occur in healthy patients or in those with long-standing symptoms (28–31). The excellent performance characteristics of STTT in late stage LD may be difficult to match in the MTTT format, especially when polyvalent EIAs (containing epitopes for IgM) are used and it is likely that immunoblots will still need to be used in evaluating difficult LD cases (8). As such, the use of immunoblots may still have value in patients with manifestations of late stage LD such as Lyme arthritis or in suspect false positive cases where serologic results do not fit with the clinical presentation. In these circumstances, it is reasonable to consider performing an IgG immunoblot as patients with late stage LD have high IgG antibody responses and the immunoblot may allow for the evaluation of the response to specific Borrelial proteins, which some clinicians may find helpful (32,33). Finally, most of the evaluations of the MTTT algorithm have been conducted in areas of high LD endemicity and testing has been restricted to primarily adult patients. Evaluations of the performance of the MTTT in areas of lower risk of LD and in pediatric populations are knowledge gaps that should be filled over time (20).
Discussion
The Canadian Public Health Laboratory Network agrees with the CDC recommendation (24) that serologic assays for LD that utilize a MTTT approach (i.e. substitute a second EIA for the immunoblot in the second tier of testing) are acceptable alternatives to STTT. This recommendation assumes that the MTTT approach has been validated and shown to have comparable performance characteristics to the STTT in regions of Canada where incidence of LD is high, as well as in low incidence jurisdictions. At present, only Nova Scotia has data validating the MTTT approach for LD diagnostics. Based on 447 samples from LD patients in that province, a MTTT consisting of an EIA based on a WCS of B. burgdorferi followed by a C6 EIA, detected 25% more cases of early localized infection compared to the STTT and had a specificity of 99.5% (34). These results are consistent with previously published data from studies conducted in highly LD endemic areas in the US (11,15) and support the use of the MTTT in this province. However, this validation study was conducted in the province with the highest incidence of LD in Canada (35). Further validation studies of the MTTT will need to be conducted in regions of Canada where LD incidence is lower, as it will be critical to document the performance characteristics of the MTTT in populations with a lower pre-test probability of infection (15,36). Small reductions in specificity can reduce the predictive value of the test (Table 4), which has led to the recommendation that LD testing should not be considered when the pre-test probability is less than 20% (37). Given the strain variation within B. burgdorferi populations observed across Canada (38), and the possible impact that this strain variability may have on LD diagnostic assays (39), it seems prudent to verify that the improved sensitivity of MTTT reported in the literature will be maintained when applied within different jurisdictions in Canada that host diverse and varied strains of B. burgdorferi.
Table 4. Estimated predictive values of modified two-tier testing depends on the stage of infection and the estimated prevalence of B. burgdorferi infectiona.
| Estimated prevalence (%) | MTTT positive predictive value (%) | MTTT negative predictive value (%) | ||
|---|---|---|---|---|
| Early localized LDb | Late LDc | Early localized LDa | Late LDb | |
| 5 | 84.8 | 91.3 | 97.6 | 100 |
| 3 | 76.6 | 86.0 | 98.6 | 100 |
| 1 | 51.7 | 66.8 | 99.5 | 100 |
| 0.1 | 9.6 | 16.6 | 100 | 100 |
Abbreviations: LD, Lyme disease; MTTT, modified two-tiered testing
a Positive and negative predictive values calculated using http://vassarstats.net/clin2.html
b Sensitivity was considered 53% based on data from reference 15; specificity was considered 99.5% based on data from Nova Scotia studies (33)
c Sensitivity was considered 99.3% based on data from reference 15; specificity was considered 99.5% based on data from Nova Scotia studies (33)
The Lyme Disease Diagnostic Working Group of the Canadian Public Health Laboratory Network is working with provincial laboratories to develop validation plans for the MTTT. The goals of the validation will be to define the performance characteristics of the MTTT in areas with different incidences of LD (and possibly different strains of B. burgdorferi) and to evaluate which combination of the different EIAs available in Canada provide the data necessary to ensure that the benefits of the new MTTT algorithms are realized and specificity of LD serological testing is maintained. A second report will be publicly available once these validation studies are completed.
Conclusion
The US Food and Drug Administration has recently approved a MTTT diagnostic algorithm for LD serology and the US CDC has recommended this new approach as an acceptable alternative to STTT. There are a growing number of scientific publications, using patients from the US, that report improved sensitivity in detection of early localized LD infection, while maintaining high specificity, when MTTT algorithms are compared to STTT. Recent data from Nova Scotia, generated using MTTT, draws similar conclusions. The improved sensitivity of the MTTT and shorter turnaround times associated with this new approach warrant further validation studies and possible rollout of this new diagnostic algorithm for LD in Canada.
Authors’ statement
Lyme disease Diagnostics Working Group of the Canadian Public Health Laboratory Network comprises T Hatchette (co-chair), LR Lindsay (co-chair), K Bernat, G Desnoyers, A Dibernardo, K Fonseca, G German, A Lang, M Morshed, R Needle, S Patel, K Thivierge and P VanCaeseele.
Conflict of interest
Authors have no conflicts of interest to report.
Acknowledgements
The Canadian Public Health Laboratory Network kindly provided secretariat support that was extremely helpful during the development of this position statement.
Funding
No specific funding was provided for this project.
References
- 1.Ogden NH, Bouchard C, Badcock J, Drebot MA, Elias SP, Hatchette TF, Koffi JK, Leighton PA, Lindsay LR, Lubelczyk CB, Peregrine AS, Smith RP, Webster D. What is the real number of Lyme disease cases in Canada? [PubMed]. BMC Public Health 2019. Jun;19(1):849. 10.1186/s12889-019-7219-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Public Health Agency of Canada. Surveillance of Lyme disease. Ottawa (ON): PHAC; 2018 (Accessed 18-09-2019). https://www.canada.ca/en/public-health/services/diseases/lyme-disease/surveillance-lyme-disease.html
- 3.Ogden NH, Koffi JK, Pelcat Y, Lindsay LR. Environmental risk from Lyme disease in central and eastern Canada: a summary of recent surveillance information [PubMed]. Can Commun Dis Rep 2014. Mar;40(5):74–82. 10.14745/ccdr.v40i05a01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Henry B, Roth D, Reilly R, MacDougall L, Mak S, Li M, Muhamad M. How big is the Lyme problem? Using novel methods to estimate the true number of Lyme disease cases in British Columbia residents from 1997 to 2008 [PubMed]. Vector Borne Zoonotic Dis 2011. Jul;11(7):863–8. 10.1089/vbz.2010.0142 [DOI] [PubMed] [Google Scholar]
- 5.Leighton PA, Koffi JK, Pelcat Y, Lindsay LR, Ogden N. Predicting the speed of tick invasion: an empirical model of range expansion for the Lyme disease vector Ixodes scapularis. J Appl Ecol 2012;49(2):457–64. 10.1111/j.1365-2664.2012.02112.x [DOI] [Google Scholar]
- 6.Kuehn BM. CDC estimates 300,000 US cases of Lyme disease annually [PubMed]. JAMA 2013. Sep;310(11):1110. 10.1001/jama.2013.278331 [DOI] [PubMed] [Google Scholar]
- 7.Hinckley AF, Connally NP, Meek JI, Johnson BJ, Kemperman MM, Feldman KA, White JL, Mead PS. Lyme disease testing by large commercial laboratories in the United States [PubMed]. Clin Infect Dis 2014. Sep;59(5):676–81. 10.1093/cid/ciu397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marques AR. Revisiting the Lyme disease serodiagnostic algorithm: the momentum gathers [PubMed]. J Clin Microbiol 2018. Jul;56(8):e00749–18. 10.1128/JCM.00749-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moore A, Nelson C, Molins C, Mead P, Schriefer M. Current guidelines, common clinical pitfalls, and future directions for laboratory diagnosis of Lyme Disease, United States [PubMed]. Emerg Infect Dis 2016. Jul;22(7):1169–77. 10.3201/eid2207.151694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wormser GP, Dattwyler RJ, Shapiro ED, Halperin JJ, Steere AC, Klempner MS, Krause PJ, Bakken JS, Strle F, Stanek G, Bockenstedt L, Fish D, Dumler JS, Nadelman RB. The clinical assessment, treatment, and prevention of lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America [PubMed]. Clin Infect Dis 2006. Nov;43(9):1089–134. 10.1086/508667 [DOI] [PubMed] [Google Scholar]
- 11.Branda JA, Body BA, Boyle J, Branson BM, Dattwyler RJ, Fikrig E, Gerald NJ, Gomes-Solecki M, Kintrup M, Ledizet M, Levin AE, Lewinski M, Liotta LA, Marques A, Mead PS, Mongodin EF, Pillai S, Rao P, Robinson WH, Roth KM, Schriefer ME, Slezak T, Snyder J, Steere AC, Witkowski J, Wong SJ, Schutzer SE. Advances in serodiagnostic testing for Lyme disease are at hand [PubMed]. Clin Infect Dis 2018. Mar;66(7):1133–9. 10.1093/cid/cix943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Institute for Health and Care Excellence. Guidance: Lyme disease. London (UK): NICE; 2018 (Accessed 30-09-2019). https://www.nice.org.uk/guidance/ng95
- 13.Waddell LA, Greig J, Mascarenhas M, Harding S, Lindsay R, Ogden N. The accuracy of diagnostic tests for Lyme disease in humans, a systematic review and meta-analysis of North American research [PubMed]. PLoS One 2016. Dec;11(12):e0168613. 10.1371/journal.pone.0168613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shapiro ED. Lyme disease [PubMed]. N Engl J Med 2014. Aug;371(7):684. 10.1056/NEJMcp1314325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Branda JA, Linskey K, Kim YA, Steere AC, Ferraro MJ. Two-tiered antibody testing for Lyme disease with use of 2 enzyme immunoassays, a whole-cell sonicate enzyme immunoassay followed by a VlsE C6 peptide enzyme immunoassay [PubMed]. Clin Infect Dis 2011. Sep;53(6):541–7. 10.1093/cid/cir464 [DOI] [PubMed] [Google Scholar]
- 16.Molins CR, Delorey MJ, Sexton C, Schriefer ME. Lyme Borreliosis serology: performance of several commonly used laboratory diagnostic tests and a large resource panel of well-characterized patient samples [PubMed]. J Clin Microbiol 2016. Nov;54(11):2726–34. 10.1128/JCM.00874-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lipsett SC, Branda JA, McAdam AJ, Vernacchio L, Gordon CD, Gordon CR, Nigrovic LE. Evaluation of the C6 Lyme enzyme immunoassay for the diagnosis of Lyme disease in children and adolescents [PubMed]. Clin Infect Dis 2016. Oct;63(7):922–8. 10.1093/cid/ciw427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Branda JA, Strle K, Nigrovic LE, Lantos PM, Lepore TJ, Damle NS, Ferraro MJ, Steere AC. Evaluation of modified 2-tiered serodiagnostic testing algorithms for early Lyme disease [PubMed]. Clin Infect Dis 2017. Apr;64(8):1074–80. 10.1093/cid/cix043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pegalajar-Jurado A, Schriefer ME, Welch RJ, Couturier MR, MacKenzie T, Clark RJ, Ashton LV, Delorey MJ, Molins CR. Evaluation of modified two-tiered testing algorithms for Lyme disease laboratory diagnosis using well-characterized serum samples [PubMed]. J Clin Microbiol 2018. Jul;56(8):e01943–17. 10.1128/JCM.01943-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lipsett SC, Branda JA, Nigrovic LE. Evaluation of the modified two-tiered testing (MTTT) method for the diagnosis of Lyme disease in children [PubMed]. J Clin Microbiol 2019. Sep;57(10):e00547–19. 10.1128/JCM.00547-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Molins CR, Sexton C, Young JW, Ashton LV, Pappert R, Beard CB, Schriefer ME. Collection and characterization of samples for establishment of a serum repository for lyme disease diagnostic test development and evaluation [PubMed]. J Clin Microbiol 2014. Oct;52(10):3755–62. 10.1128/JCM.01409-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Branda JA, Strle F, Strle K, Sikand N, Ferraro MJ, Steere AC. Performance of United States serologic assays in the diagnosis of Lyme borreliosis acquired in Europe [PubMed]. Clin Infect Dis 2013. Aug;57(3):333–40. 10.1093/cid/cit235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.U.S. Food and Drug Administration. FDA News Release: FDA clears new indications for existing Lyme disease tests that may help streamline diagnoses. FDA; 2019 (Accessed 21-09-2019). https://www.fda.gov/news-events/press-announcements/fda-clears-new-indications-existing-lyme-disease-tests-may-help-streamline-diagnoses
- 24.Mead P, Petersen J, Hinckley A. Updated CDC recommendation for serologic diagnosis of Lyme disease [PubMed]. MMWR Morb Mortal Wkly Rep 2019. Aug;68(32):703. 10.15585/mmwr.mm6832a4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zweitzig D, Kopnitsky M. and Zeus Scientific. Validation of a modified two-tiered testing (MTTT) algorithm for the improved diagnosis of Lyme disease. Unpublished Technical report; 2019. 13 pp.
- 26.Wormser GP, Levin A, Soman S, Adenikinju O, Longo MV, Branda JA. Comparative cost-effectiveness of two-tiered testing strategies for serodiagnosis of lyme disease with noncutaneous manifestations [PubMed]. J Clin Microbiol 2013. Dec;51(12):4045–9. 10.1128/JCM.01853-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kalish RA, McHugh G, Granquist J, Shea B, Ruthazer R, Steere AC. Persistence of immunoglobulin M or immunoglobulin G antibody responses to Borrelia burgdorferi 10-20 years after active Lyme disease [PubMed]. Clin Infect Dis 2001. Sep;33(6):780–5. 10.1086/322669 [DOI] [PubMed] [Google Scholar]
- 28.Seriburi V, Ndukwe N, Chang Z, Cox ME, Wormser GP. High frequency of false positive IgM immunoblots for Borrelia burgdorferi in clinical practice [PubMed]. Clin Microbiol Infect 2012. Dec;18(12):1236–40. 10.1111/j.1469-0691.2011.03749.x [DOI] [PubMed] [Google Scholar]
- 29.Fallon BA, Pavlicova M, Coffino SW, Brenner C. A comparison of lyme disease serologic test results from 4 laboratories in patients with persistent symptoms after antibiotic treatment [PubMed]. Clin Infect Dis 2014. Dec;59(12):1705–10. 10.1093/cid/ciu703 [DOI] [PubMed] [Google Scholar]
- 30.Lantos PM, Lipsett SC, Nigrovic LE. False positive Lyme disease IgM immunoblots in children [PubMed]. J Pediatr 2016. Jul;174:267–269.e1. 10.1016/j.jpeds.2016.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Webber BJ, Burganowski RP, Colton L, Escobar JD, Pathak SR, Gambino-Shirley KJ. Lyme disease overdiagnosis in a large healthcare system: a population-based, retrospective study [PubMed]. Clin Microbiol Infect 2019. Oct;25(10):1233–8. 10.1016/j.cmi.2019.02.020 [DOI] [PubMed] [Google Scholar]
- 32.Akin E, McHugh GL, Flavell RA, Fikrig E, Steere AC. The immunoglobulin (IgG) antibody response to OspA and OspB correlates with severe and prolonged Lyme arthritis and the IgG response to P35 correlates with mild and brief arthritis [PubMed]. Infect Immun 1999. Jan;67(1):173–81. 10.1128/IAI.67.1.173-181.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Steere AC. Treatment of Lyme arthritis [PubMed]. J Rheumatol 2019. Aug;46(8):871–3. 10.3899/jrheum.190320 [DOI] [PubMed] [Google Scholar]
- 34.Davis I, McNeil SA, Allen W, MacKinnon-Cameron D, Wilson K, Bernat K, Dibernardo A, Lindsay LR, Hatchette TF. Performance of two EIA algorithm for Lyme disease (LD) in Nova Scotia. JAMMI. 2019;4(S1): poster P57. https://doi.org/ 10.3138/jammi_4.s1.abst-02 [DOI] [PMC free article] [PubMed]
- 35.Gasmi S, Ogden NH, Lindsay LR, Burns S, Fleming S, Badcock J, Hanan S, Gaulin C, Leblanc MA, Russell C, Nelder M, Hobbs L, Graham-Derham S, Lachance L, Scott AN, Galanis E, Koffi JK. Surveillance for Lyme disease in Canada: 2009-2015 [PubMed]. Can Commun Dis Rep 2017. Oct;43(10):194–9. 10.14745/ccdr.v43i10a01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lantos PM, Branda JA, Boggan JC, Chudgar SM, Wilson EA, Ruffin F, Fowler V, Auwaerter PG, Nigrovic LE. Poor Positive predictive value of Lyme disease serologic testing in an area of low disease incidence [PubMed]. Clin Infect Dis 2015. Nov;61(9):1374–80. 10.1093/cid/civ584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tugwell P, Dennis DT, Weinstein A, Wells G, Shea B, Nichol G, Hayward R, Lightfoot R, Baker P, Steere AC. Laboratory evaluation in the diagnosis of Lyme disease [PubMed]. Ann Intern Med 1997. Dec;127(12):1109–23. 10.7326/0003-4819-127-12-199712150-00011 [DOI] [PubMed] [Google Scholar]
- 38.Mechai S, Margos G, Feil EJ, Lindsay LR, Ogden NH. Complex population structure of Borrelia burgdorferi in southeastern and south central Canada as revealed by phylogeographic analysis [PubMed]. Appl Environ Microbiol 2015. Feb;81(4):1309–18. 10.1128/AEM.03730-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ogden NH, Arsenault J, Hatchette TF, Mechai S, Lindsay LR. Antibody responses to Borrelia burgdorferi detected by western blot vary geographically in Canada [PubMed]. PLoS One 2017. Feb;12(2):e0171731. 10.1371/journal.pone.0171731 [DOI] [PMC free article] [PubMed] [Google Scholar]