Abstract
Objectives
To define the characteristics of post-traumatic headache with cluster headache phenotype (PTH-CH) and to compare these characteristics with primary CH.
Methods
A retrospective study was conducted of patients seen between 2007 and 2017 in a headache centre and diagnosed with PTH-CH that developed within 7 days of head trauma. A control cohort included 553 patients with primary CH without any history of trauma who attended the headache clinic during the same period. Data including demographics, attack characteristics and response to treatments were recorded.
Results
Twenty-six patients with PTH-CH were identified. Multivariate analysis revealed significant associations between PTH-CH and family history of CH (OR 3.32, 95% CI 1.31 to 8.63), chronic form (OR 3.29, 95% CI 1.70 to 6.49), parietal (OR 14.82, 95% CI 6.32 to 37.39) or temporal (OR 2.04, 95% CI 1.10 to 3.84) location of pain, and presence of prominent cranial autonomic features during attacks (miosis OR 11.24, 95% CI 3.21 to 41.34; eyelid oedema OR 5.79, 95% CI 2.57 to 13.82; rhinorrhoea OR 2.65, 95% CI 1.26 to 5.86; facial sweating OR 2.53, 95% CI 1.33 to 4.93). Patients with PTH-CH were at a higher risk of being intractable to acute (OR 12.34, 95% CI 2.51 to 64.73) and preventive (OR 16.98, 95% CI 6.88 to 45.52) treatments and of suffering from associated chronic migraine (OR 10.35, 95% CI 3.96 to 28.82).
Conclusion
This largest series of PTH-CH defines it as a unique entity with specific evolutive profile. Patients with PTH-CH are more likely to suffer from the chronic variant, have marked autonomic features, be intractable to treatment and have associated chronic migraine compared with primary CH.
Introduction
Post-traumatic headache (PTH) is defined as headache that develops within 7 days of head trauma.1 It is difficult to determine the true prevalence of this type of headache; however, it appears to be more frequently associated with mild rather than severe head injury.2 3 Tension-type headache and migraine without aura are the usual associated phenotypes.4 Trigeminal autonomic cephalalgias (TAC) have been reported following head injury5–7; however, only two cases fulfil the International Classification of Headache Disorders (ICHD) criteria for post-traumatic headache and cluster headache (CH), the vast majority of cases being described with a prolonged or unknown latency between CH onset and head trauma.8–10
Described as one of the most painful conditions known to humans, CH is characterised by a strictly unilateral headache with associated ipsilateral cranial autonomic features.1 It is the most frequent TAC, with an estimated lifetime prevalence of 0.12%. There have been several advances in delineating the pathophysiology of CH over the last decade. Recent functional imaging data suggest that ipsilateral hypothalamic activation with subsequent trigeminovascular and cranial autonomic activation underpins the pathogenesis of CH.11 12 However, its relationship with head trauma remains unclear. According to Lambru and Matharu,8 there are three hypotheses explaining the link between head injury and CH. First, CH may occur as a direct result of head trauma; second, head trauma may only increase the risk of developing CH; and finally that the personality traits associated with CH may predispose to head trauma.
Although most PTHs resolve within 12 months of injury, approximately 18%–33% of PTHs persist, leading to loss of work capacity and significant fiscal consequences.13 14 Postconcussion syndrome, with associated depression, cognitive dysfunction and insomnia, also contributes to this economic burden.15 This highlights both the clinical and medicolegal imperative to define post-traumatic headache with cluster headache phenotype (PTH-CH) accurately. Thus, this cohort study attempts to define the characteristics of PTH-CH and compare them with primary CH.
Materials and methods
Patients
This retrospective cohort study was conducted in a tertiary headache centre at the National Hospital for Neurology and Neurosurgery (Queen Square, London, UK) between January 2007 and May 2017. All consecutive patients with prior head trauma and diagnosed with CH according to the current International Classification of Headache Disorders - Third Edition (ICHD-3) diagnostic criteria were assessed.1 All patients were evaluated and examined by a neurologist. After careful review of medical records, we only included patients with a latency period between head trauma and the first CH attack of 7 days at the most, thus meeting the ICHD criteria for both post-traumatic headache and CH.
Data collection
Demographic characteristics, attack frequency, duration, laterality and associated symptoms including autonomic or migrainous features were recorded. Each site of pain and/or referred pain was categorised as orbital, temporal, frontal, parietal, occipital or cheek. Patients were diagnosed with episodic CH (ECH) when attacks occurred in clusters lasting weeks to months, with remissions lasting longer than 3 months, and with chronic CH (CCH) if they had no such attack-free periods. Two independent neurologists established a diagnosis of probable CH in cases where one of the ICHD-3 criteria was not met. We also recorded any other associated headaches using the ICHD-3 diagnostic criteria. The patients underwent the same assessment of diagnosis and specific characteristics of headache over the years by the same neurologist team. The data were prospectively recorded during clinic visits.
In order to evaluate the response to acute and preventive treatment, we used a modified definition of intractable CH based on the European Headache Federation and the work of Goadsby et al (box 1).16 17 A satisfactory response to a trial was defined as a 50% or more reduction in mean attack frequency for preventive medications and as a 50% or more reduction in pain at least 50% of the time for acute medications.18 Patients were classified as indeterminate in case of insufficient data (less than four trials of preventive treatment or lack of either sumatriptan injection or high-flow oxygen as acute treatment). The response to treatment was determined based on the last available clinical letter.
Box 1. Definitions of intractable response to treatment.
Adequate trial performed.
Appropriate dose: decision left to the clinical physician.
-
Appropriate length of time:
At least 1 month for melatonin trial.
At least 3 months for all other preventive therapies.
Failed trial.
-
Unsatisfactory response:
Less than 50% reduction in mean attack frequency for preventive treatment.
Less than 50% reduction in pain at least 50% of the time for acute treatment.
Side effects requiring cessation of treatment.
Contraindications to use.
Intractable to acute treatment.
Failure to respond within 15 min of subcutaneous sumatriptan use.
Failure within 30 min of high-dose and flow rate oxygen use.
Intractable to preventive treatment.
-
Failure of at least four classes among the following:
Verapamil.
Lithium.
Topiramate.
Gabapentin.
Methysergide.
Melatonin
Modified from the European Headache Federation consensus guideline and Goadsby et al 17
For each patient, we evaluated the cause and mechanism of head trauma, initial symptoms, such as post-traumatic amnesia or loss of consciousness, and neuroimaging. In case of headache of greater than 3 months’ duration, the PTH-CH was called ‘persistent’. According to the International Headache Society classification, we defined head trauma as severe/moderate or mild.
A family history of CH was collected according to proxy reports from patients. To confirm a diagnosis of CH, available affected relatives were interviewed by a neurologist either over the phone or in person at the clinic. Relatives who had died or were unavailable for interview were included if they had previously been diagnosed with CH by a neurologist or had a history highly suspicious for CH.
Comparison with a control cohort
In order to ascertain specific characteristics in patients with PTH-CH, a comparison with a control cohort was done. All patients who attended the headache clinic during the same period and who fulfilled the criteria for primary CH were included in our control cohort (n=631). The same evaluation and data collection conducted in the PTH-CH group were performed in the cohort. In addition, any patient with history of head trauma (n=52) or patients diagnosed with CH considered as secondary to another disorder (n=26) were excluded from the final control cohort (n=553), given the possible impact on pain pathways of those events or disorders. No difference between patients with PTH-CH and patients with primary CH was found with regard to demographic data, such as current age, sex, age of onset and follow-up duration. Therefore, this allowed further comparisons regarding clinical characteristics.
Statistics
Descriptive statistics were expressed as mean with SD. The two groups of patients were compared using a Mann-Whitney non-parametric test for continuous data and a Fisher’s exact or χ2 test for categorical data. For the multivariate analysis, we adopted a three-step approach. First, we balanced our two groups of patients with PTH-CH and primary CH. Indeed, when dealing with highly imbalanced classes, classification rules tend to be overwhelmed by the majority class to the detriment of the minority class. A common statistical approach to deal with imbalanced learning is to alter the class distribution to get a more balanced sample. Second, we performed variable selection to select a subset of relevant covariates for our model construction and finally a logistic regression with the previously selected features. Note that we did not split the data into a training and a test set as done usually. Indeed, due to the highly imbalanced data set, adopting the standard holdout strategy would lead to high variance estimates of the accuracy measure. Instead, we adopted a specific holdout version adapted to imbalanced learning. More specifically, we handled missing values by imputation techniques based on random decision forests. Then we used the ROSE (Random Over Sampling Examples) algorithm to balance the data.19 The ROSE function creates an artificial balanced sample according to a smoothed bootstrap approach. Then, on the ROSE sample we performed variable selection to get a subset of important covariates to feed into the logistic regression. We resorted to the powerful LASSO (Least Absolute Shrinkage and Selection Operator) algorithm to select relevant explanatory variables.20 The LASSO performs automatic variable selection and is capable of selecting groups of correlated variables. Eventually, we performed a logistic regression with the variables selected by the LASSO. All the numerical results were performed with the R software. The randomForest, ROSE and glmnet packages were used. The threshold for statistical significance was set to p≤0.05.
Results
Demographics and clinical characteristics
We identified 26 patients diagnosed with PTH-CH within the defined study period, of whom 19 (73.0%) were male (tables 1 and 2). PTH-CH was persistent in all of them. The mean age was 48.4 years (SD 11.2) and the mean age at CH onset was 31.8 years old (SD 13.5). The mean follow-up time in our headache clinic was 6.5 years (SD 13.5). Five (19.2%) patients with PTH-CH were diagnosed with ECH or probable ECH phenotype and 21 (80.7%) with CCH or probable CCH phenotype.
Table 1.
Demographics and clinical characteristics of patients with post-traumatic cluster headache
| Sex | Age of onset | Current age | Follow-up duration (years) | Type of CH | Other associated headache | Characteristics of attacks | Response to treatment | |||||||
| Laterality | Average duration (min) | Average frequency (per day) | Autonomic feature | Restlessness | Migrainous symptoms | Intractable to acute treatment | Intractable to preventive treatment | |||||||
| 1 | M | 15 | 34 | 10 | CCH* | – | Side variable | 50 | 3 | C, L, N, R, E, FS, P | Yes | N, V | No | No |
| 2 | M | 32 | 54 | 10 | CCH | Episodic migraine | L | 60 | 2 | C, L, N, R, E, FF, P | Yes | PT, PN, OSM, N | No | No |
| 3 | M | 18 | 38 | 10 | ECH | – | R | 120 | 3 | C, L, N, E, FS, P | Yes | PT, PN, N, V, OSM | No | No |
| 4 | F | 36 | 54 | 10 | CCH* | Episodic migraine | Side variable | 90 | 3 | N, E, P | Yes | PT, PN, N, V, OSM | No | Yes |
| 5 | M | 33 | 64 | 8 | CCH* | – | R | 90 | 6 | L, N, R, FS, FF, P, AF | Yes | PT, PN, OSM | No | Yes |
| 6 | M | 43 | 57 | 8 | CCH | Episodic migraine | L | 150 | 1 | L, R | Yes | PT, PN | No | No |
| 7 | M | 20 | 34 | 3 | CCH* | – | R | 40 | 5 | C, L, N, R, E, FS, P | Yes | – | No | No |
| 8 | M | 10 | 55 | 8 | PECH | – | R | 180 | 6 | C, L, N, R, FS, FF, P, AF | Yes | PT, PN | No | Yes |
| 9 | M | 22 | 38 | 8 | CCH | Chronic migraine | L | 50 | 5 | C, L, N, R, E, FS, FF, P | Yes | PT, PN, OSM | No | No |
| 10 | M | 26 | 52 | 8 | CCH* | – | R | 120 | 2 | C, L, N, FF, FS, P | Yes | OSM | Yes | Yes |
| 11 | F | 45 | 58 | 8 | CCH | Episodic migraine | L | 120 | 2 | FS, AF, P | Yes | PN, N, MS | No | Yes |
| 12 | F | 29 | 42 | 7 | CCH | – | L | 120 | 6 | C, L, N, R, E, P | Yes | N, OSM | No | Only 3 trials done |
| 13 | F | 11 | 23 | 5.5 | CCH | – | Side variable | 90 | 2 | C, L, R, E, P | Yes | PT, N | NA | NA |
| 14 | M | 54 | 57 | 5 | CCH | – | L | 150 | 1 | C, FF, FS | No | – | Yes | Yes |
| 15 | M | 48 | 59 | 5 | CCH | L | 45 | 2 | C, L, N, R, E, FS, P | Yes | PT, PN | No | Yes | |
| 16 | F | 47 | 61 | 4 | CCH | Chronic migraine | Side variable | 120 | 3 | L, N, FF, FS | Yes | – | No | Yes |
| 17 | M | 24 | 33 | 2.5 | CCH | Chronic migraine | L | 30 | 5 | C, L, N, R, E, FS, FF, P | Yes | PT, PN, N, V, OSM | No | Only 3 trials done |
| 18 | M | 45 | 52 | 5 | PECH | Episodic migraine | L | 210 | 3 | C, R, E, FS | Yes | N | No | No |
| 19 | M | 56 | 62 | 2 | CCH | Chronic migraine | L | 180 | 2 | NA | Yes | PT, PN | No | Yes |
| 20 | F | 51 | 64 | 8 | ECH | – | L | 30 | 3 | C, L, N, R, FS, P | Yes | PT | No | Only 3 trials done |
| 21 | M | 28 | 45 | 1 | CCH* | – | L | 120 | 4 | C, L, N, R, E, FS, FF, P | Yes | PT | No | No |
| 22 | M | 38 | 48 | 4.5 | CCH | – | R | 30 | 6 | C, L, N, R, E, FS, FF, P | Yes | – | No | Yes |
| 23 | M | 26 | 39 | 10.5 | CCH | – | L | 20 | 8 | C, L, N, R, FS, FF | Yes | PT, PN | No | Yes |
| 24 | M | 24 | 36 | 6.5 | CCH | Chronic migraine | R | 60 | 2 | C, L, N, P, AF | No | PT, PN, N | No | No |
| 25 | M | 33 | 48 | 7 | CCH | – | L | 60 | 4 | C, L, N, R, E, P | Yes | PN, N | No | Only 3 trials done |
| 26 | F | 17 | 52 | 5 | ECH | Episodic migraine | L | 45 | 3 | L, N, R | Yes | PT, PN, N, OSM | No | Only 3 trials done |
Autonomic feature: AF, aural fullness; C, conjunctival injection; E, eyelid oedema; FF, facial flushing; FS, facial sweating; L, lacrimation; N, nasal congestion; P, ptosis; R, rhinorrhoea.
Migrainous symptoms: MS, motion sensitivity; N, nausea; OSM, osmophobia; PN, phonophobia; PT, photophobia; V, vomiting.
*Secondary chronic cluster headache phenotype (initially episodic and subsequently transformed to chronic variant).
CCH, chronic cluster headache; CH, cluster headache; ECH, episodic cluster headache; F, female; L, left; M, male; NA, not available; PECH, probable episodic cluster headache; R, right.
Table 2.
Characteristics of head trauma in patients with post-traumatic cluster headache
| Side of injury | Association with headache | Details of head trauma | Associated features | Neuroimaging | ||||
| Ipsilaterality | Time from injury to CH onset | Loss of consciousness | Amnesia | Skin bruise or laceration | ||||
| 1 | Vertex | Indeterminate | 5 days | Head hit by a block of concrete while standing on a riverbank for fishing. | 2–3 min | – | Deep laceration | Normal |
| 2 | Left | Yes | Immediate | Cheek and occiput hit while attacked by a group, then was thrown down flight of stairs. | 20–25 min | Anterograde | Bruising and laceration | Left zygoma fracture |
| 3 | Left | No | 24 hours | Hitting the front left aspect of head while walking into a metal pole. | – | – | – | Normal |
| 4 | Unclear | Indeterminate | 24 hours | Concussion to occiput and neck while playing netball. | – | – | – | Normal |
| 5 | Forehead | Indeterminate | 7 days | Banging the head on the beam of a door at home. | – | – | – | Normal |
| 6 | Left | Yes | 7 days | Accidental fall during water skiing, hitting on the left face. | 2 min | – | – | Normal |
| 7 | Unclear | Indeterminate | 24 hours | Unknown cause of syncope during driving, then hitting a tree with head concussion. | 15 min | – | – | Normal |
| 8 | Unclear | Indeterminate | Immediate | Involved in road traffic accident but no further details available. | – | – | – | Normal |
| 9 | Left | Yes | Few days | Attacked by a group. Left side of head was punched and then kicked twice. | 10 min | Anterograde | Bruising | Normal |
| 10 | Unclear | Indeterminate | 7 days | Head was hit when he was serving in the army. No further details available. | 5 min | – | – | Normal |
| 11 | Left | Yes | Immediate | Fell forward and hit on left forehead while ice skating. | 4 hours | – | Marked bruising | Basal skull fracture |
| 12 | Left | Yes | Few days | Attacked in a pub with the left temple punched by a customer. | – | – | – | Normal |
| 13 | Unclear | Indeterminate | 5 hours | Head was hit when the bus swerved suddenly. | – | – | Mild bruising | NA |
| 14 | Left | Yes | Few days | A piece of glass fell on the left side of the head when dismantling a conservatory. | – | – | – | Incidental aneurysm |
| 15 | Left | Yes | Immediate | Assaulted by a prisoner with a metal mop base on the left cheek. | – | – | Deep laceration | Normal |
| 16 | Left | Yes | Immediate | Left side of head and neck was hit by an object falling from a building. | – | – | – | Normal |
| 17 | Unclear | Indeterminate | Few hours | Involved in road traffic accident; was run over by a car while riding a motorcycle. | 5 min | – | – | Normal |
| 18 | Left | Yes | 3 days | Involved in road traffic accident but no further details available. | Duration unknown | – | – | Normal |
| 19 | Left | Yes | Few hours | Fell off the bed and hit the corner of left eye on table. | – | – | – | Normal |
| 20 | Left | Yes | Immediate | Bashed her head against the corner of a desk. | – | – | Bruising | Normal |
| 21 | Unclear | Indeterminate | 3 days | Involved in road traffic accident: collision with a car while riding a motorcycle. | – | – | – | Normal |
| 22 | Right | Yes | 1 day | Root canal treatment of a right upper tooth followed 7 days after by insertion of crown. | – | – | – | Normal |
| 23 | Left | Yes | 3 days | Dental extraction with dislocation of the jaw. | – | – | – | Normal |
| 24 | Right | Yes | 7 days | Lower right dental extraction. | – | – | – | Normal |
| 25 | Left | Yes | Few days | Upper left dental extraction. | – | – | – | Normal |
| 26 | Bilateral | Indeterminate | 24 hours | Four wisdom teeth extraction. | – | – | – | Normal |
CH, cluster headache; NA, not available.
Of the 21 patients with CCH phenotype, 15 were chronic at onset. Of the remaining patients, six developed the ECH phenotype immediately after the injury. The mean duration of attacks was 87.3 min (SD 55) and the mean frequency was 3.3 daily (SD 1.8). At least one autonomic feature was present during the attacks in the entire case group, with restlessness during an attack reported in all but two patients. At least one migrainous feature was reported in 24 (92.3%) patients. There was a distinct circadian periodicity, with the attacks occurring predictably during the night in 24 (92.3%) patients. Regarding concomitant headaches, six (23.0%) patients suffered from episodic migraine and five (19.2%) patients from chronic migraine. Interestingly, one developed chronic migraine at the same time as PTH-CH, whereas three patients also suffered from post-traumatic headache with a chronic migraine phenotype but related to another head injury. All patients could clearly distinguish CH attacks from migraine pain. Of the 26 patients with PTH-CH, a family history of CH in at least one first-degree relative was confirmed in 15.3% of patients, consisting of 4 individuals from 3 families. Two patients were brother and sister and their mother also suffered from CH, confirmed by us at the clinic. The affected relatives of the two remaining patients with PTH-CH with probable family history had passed away and therefore were not available for interview. Nevertheless, one (the mother of the patient with PTH-CH) had previously been diagnosed with CH and the other (the uncle of the patient with PTH-CH) had a history that was highly suspicious for CH (extremely severe headaches localised behind the eye and occurring during the night).
Regarding the response to medical treatment, 2 (7.6%) patients were considered as intractable to acute treatment and 11 (42.3%) as intractable to preventive treatment. Three of them benefited from invasive neuromodulation, including occipital nerve stimulation, sphenopalatine ganglion stimulation and ventral tegmental region deep brain stimulation.
Head trauma and its relationship to headache
The details on head trauma are summarised in table 2. The vast majority of patients (n=23, 88.4%) sustained mild injury. Three (11.6%) traumas were considered as severe due to the duration of loss of consciousness and/or presence of anterograde amnesia. No intracranial haemorrhage was reported. There were however two skull fractures described in our cohort. The different mechanisms of head injury included road traffic accident (n=5), mechanical fall (n=4), collision with an object (n=7), assault (n=3) or direct penetration of the head by metal or glass (n=2). In addition, dental extractions (n=5) were assessed as the ICHD defines head injury as ‘penetration of the head by a foreign body’ but were not included for comparison with the control cohort, considering that they were not strictly identical to other patients with PTH-CH. Following the trauma, five patients had bruising and three had deep laceration. The first CH attacks occurred a few hours after the trauma in seven patients and immediately after it in five patients. The CH attacks were ipsilateral to the head injury in all patients in whom the trauma was clearly one-sided, except one (patient 2). Due to a wider and often bilateral injury, laterality was indeterminate in 11 patients.
Comparison with control cohort
Univariate analyses comparing the 21 patients with PTH-CH (without dental extractions) with the control cohort are presented in table 3. A family history of CH was identified in 50 (9.0%) patients according to medical records and confirmed over the phone or in person in 33 (5.9%) patients of the total control cohort. Regarding the headache diagnosis, patients with PTH-CH were more likely to be diagnosed with the CCH phenotype (80.9% vs 50.4%; p=0.006) and with associated chronic migraine (19.0% vs 7.6%; p=0.05) than the control cohort. Univariate analysis revealed statistical differences in terms of location, cranial autonomic features and response to preventive treatment. Indeed, a parietal location of referred pain, which was the most common site of injury, was more common in the PTH-CH group (38.0% vs 16.0%; p=0.008). They were at a higher risk of being intractable to preventive treatment than the control cohort (42.8% vs 16.0%; p=0.002). The results remained unchanged after including the five dental extractions cases (data not shown).
Table 3.
Univariate analysis results of comparison between PTH-CH and primary CH (control cohort)
| PTH-CH | Control cohort | P value | |
| (n=21) | (n=553) | ||
| Demographics | |||
| Age (years) | 49.3±11.9 | 48.8±12.4 | 0.56 |
| Gender (male/female) | 15/6 | 379/174 | 0.77 |
| Follow-up duration (years) | 6.47±3.0 | 6.04±2.8 | 0.43 |
| Age at onset (years) | 32.9±14.4 | 31.3±13.6 | 0.56 |
| Family history of CH | 4 (19.0) | 50 (9.0) | 0.12 |
| Headache diagnosis | |||
| CH | |||
| ECH and/or PECH | 4 (19.1) | 274 (49.5) | 0.006 |
| CCH and/or PCCH | 17 (80.9) | 279 (50.4) | |
| Associated migraine | |||
| Episodic migraine | 5 (23.8) | 99 (17.9) | 0.49 |
| Chronic migraine | 4 (19.0) | 42 (7.6) | 0.05 |
| Duration and frequency of attacks | |||
| Usual duration (min) | 97.8±56.0 | 90.8±64.0 | 0.44 |
| Average frequency per day | 3.0±1.5 | 2.8±2.1 | 0.08 |
| Laterality | |||
| Strictly unilateral | 16 (76.1) | 444 (80.2) | 0.64 |
| Side variable | 5 (23.8) | 91 (16.4) | 0.37 |
| Bilateral | 0 | 18 (3.2) | 0.4 |
| Site and referred pain | |||
| Orbital/retro-orbital | 11 | 398 | 0.05 |
| Frontal | 6 | 176 | 0.75 |
| Temple | 11 | 267 | 0.71 |
| Parietal | 8 | 89 | 0.008 |
| Occiput | 5 | 111 | 0.67 |
| Cranial autonomic features and restlessness | |||
| Ptosis | 16 | 304 | 0.05 |
| Eyelid oedema | 12 | 192 | 0.03 |
| Conjunctival injection | 15 | 365 | 0.6 |
| Miosis | 1 | 22 | 0.85 |
| Lacrimation | 16 | 428 | 0.89 |
| Nasal blockage | 14 | 320 | 0.42 |
| Rhinorrhoea | 13 | 330 | 0.83 |
| Facial sweating | 14 | 265 | 0.09 |
| Facial flush | 9 | 208 | 0.63 |
| Aural fullness | 3 | 86 | 0.87 |
| Restlessness | 20 (95.2) | 428 (83.9) | 0.06 |
| Response to medical treatment | |||
| Acute treatment | |||
| Intractable | 2 | 16 | 0.23 |
| Indeterminate | 4 | 130 | |
| Responsive | 16 | 407 | |
| Preventive treatment | |||
| Intractable | 9 | 89 | 0.002 |
| Indeterminate | 8 | 210 | |
| Responsive | 4 | 254 | |
Mean±SD for quantitative data; n (%) for qualitative data.
CCH, chronic cluster headache; CH, cluster headache; ECH, episodic cluster headache; PCCH, probable chronic cluster headache; PECH, probable episodic cluster headache; PTH-CH, post-traumatic headache with cluster headache phenotype.
For multivariate analysis, we first approximately balanced the two classes with the ROSE algorithm and obtained a new sample generated from the first one with 270 patients with PTH-CH and 304 patients with no PTH-CH. On this balanced data set, in a binary logistic framework, the LASSO selected 33 variables over the 37 original ones. Then we fed the 33 selected variables into a logistic regression. For the sake of simplicity, we only represented the items with significant results out of the 33 variables in figure 1 (n=17 items).
Figure 1.
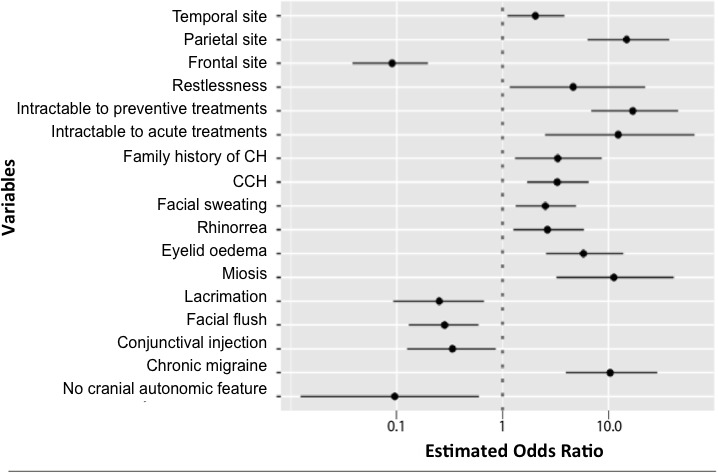
Estimated OR in log-10 scale of each selected item entered into the logistic regression model with significant result (n=17). Eventually, we used the built-in ROSE.eval function in order to estimate our model accuracy. The ROSE.eval function implemented a ROSE version of holdout: the logistic regression was fitted on one sample generated by ROSE and tested on the original data set. The threshold for the logistic regression was set to 0.5. It turned out that the area under the curve was 0.874, which shows the accuracy of the estimated model. CCH, chronic cluster headache; CH, cluster headache.
We found significant positive association between PTH-CH and family history of CH (OR 3.32; 95% CI 1.31 to 8.63), CCH phenotype (OR 3.29; 95% CI 1.70 to 6.49), temporal location (OR 2.04; 95% CI 1.10 to 3.84), parietal location (OR 14.82; 95% CI 6.32 to 37.39), eye oedema during attacks (OR 5.79; 95% CI 2.57 to 13.82), miosis during attacks (OR 11.24; 95% CI 3.21 to 41.34), rhinorrhoea (OR 2.65; 95% CI 1.26 to 5.86), facial sweating (OR 2.53; 95% CI 1.33 to 4.93) and restlessness (OR 4.63; 95% CI 1.16 to 22.19). Multivariate analysis confirmed intractability to acute (OR 12.34; 95% CI 2.51 to 64.73) and preventive (OR 16.98; 95% CI 6.88 to 45.52) treatment as independent characteristics of patients with PTH-CH. Associated chronic migraine had one of the highest ORs (10.35; 95% CI 3.96 to 28.82) (table 4 and figure 1). Conversely, patients with PTH-CH were less likely to present with frontal referred pain (OR 0.09; 95% CI 0.03 to 0.19), absence of cranial autonomic features during attacks (OR 0.09; 95% CI 0.01 to 0.60), conjunctival injection (OR 0.33; 95% CI 0.12 to 0.86), lacrimation (OR 0.25; 95% CI 0.09 to 0.66) or flush (OR 0.28; 95% CI 0.12 to 0.59).
Table 4.
Results of multivariate logistic regression model comparing post-traumatic headache with cluster headache phenotype (n=21) and primary cluster headache (control cohort; n=553)
| Predictive factor | OR | 95% CI | P value |
| Family history of cluster headache | 3.32 | 1.31 to 8.63 | 0.012 |
| Chronic cluster headache phenotype | 3.29 | 1.70 to 6.49 | <0.001 |
| Temporal location | 2.04 | 1.10 to 3.84 | 0.024 |
| Parietal location | 14.82 | 6.32 to 37.39 | <0.001 |
| Presence of eye oedema | 5.79 | 2.57 to 13.82 | <0.001 |
| Presence of miosis | 11.24 | 3.21 to 41.34 | <0.001 |
| Presence of rhinorrhoea | 2.65 | 1.26 to 5.86 | 0.013 |
| Presence of facial sweating | 2.53 | 1.33 to 4.93 | 0.005 |
| Restlessness | 4.63 | 1.16 to 22.19 | 0.039 |
| Associated chronic migraine | 10.35 | 3.96 to 28.82 | <0.001 |
| Intractable to acute treatment | 12.34 | 2.51 to 64.73 | 0.002 |
| Intractable to preventive treatment | 16.98 | 6.88 to 45.52 | <0.001 |
Discussion
To our knowledge, this is the largest series of PTH-CH reported to date. Here, we describe the clinical characteristics of 26 patients with PTH-CH who developed headaches within 7 days of head trauma that strictly fulfil the ICHD-3 criteria for both PTH and CH. We have kept five patients who underwent dental extractions apart, considering that these cases have a postsurgical rather than a typical post-traumatic aetiology. Univariate analysis comparing the remaining 21 patients with PTH-CH with a control cohort of 553 patients with primary CH revealed that patients with PTH-CH were more likely to have the chronic variant of CH, parietal site of pain, prominent cranial autonomic features particularly ptosis and eyelid oedema, intractability to preventive treatments, and have chronic migraine. A multivariate logistic regression model comparing the 21 patients with PTH-CH with the 553 patients with primary CH confirmed that patients with PTH-CH were more likely to have a family history of CH, the chronic variant of CH, temporal and parietal site of pain, prominent cranial autonomic features (particularly eyelid oedema, miosis, rhinorrhoea and facial sweating), restlessness, and intractability to acute and preventive treatments, as well as associated chronic migraine. These findings suggest that PTH-CH has a distinct clinical phenotype with more severe CH phenotype that is less responsive to treatments compared with primary CH. Furthermore, it implies that PTH-CH may have an alternative pathophysiological mechanism with its own evolutive profile.
In primary CH, wide activation of ipsilateral trigeminal nociceptive pathways, involving the trigeminal-autonomic reflex, leads to central activation through the trigeminal nucleus caudalis and the superior salivatory nucleus in the brainstem.8 21 In contrast, it is suggested that PTH results from more localised changes in the trigeminal pathway due to direct damage at the site of trauma.22 23 Factors including axonal injury, reduced cerebral circulation and the inappropriate release of local neurotransmitters are likely to play a role in the initial emergence of PTH-CH.4 8 Consistent with previous reports, there was a propensity for referred pain in the parietal and temporal regions during PTH-CH attacks in our cohort.24 This likely reflects localised changes. Furthermore, the majority of patients with strictly unilateral headache reported pain on the side ipsilateral to the injury. Although the pain distribution may lead to attribution to some event in the past, this is consistent with previous findings and supports the hypothesis of peripheral involvement in PTH-CH pathogenesis via direct damage to local nociceptive structures.25 26
Nevertheless, diffuse changes such as excessive neuronal depolarisation and the release of excitotoxic neurotransmitters may underpin the pathogenesis of PTH-CH.4 The sensitisation of central trigeminal neurons in PTH as a consequence of the initial trauma-related inflammatory process within the cranial meninges and the calvarial periosteum may also be involved.27 28 During primary CH attacks, the ipsilateral cranial autonomic features testify of cranial parasympathetic activation and sympathetic hypofunction, due to a central disinhibition of the trigeminal autonomic reflex.29 The more frequent eyelid oedema, miosis, rhinorrhoea and facial sweating in PTH-CH compared with primary CH in our study may reveal a marked disinhibition of pain pathways after trauma. Such long-term modulation of central pain pathways in CH following trauma would also explain the vast majority of chronic variant found in our study. Indeed, more than 80% of patients with PTH-CH suffered from the chronic variant and 15 of them developed the chronic form at onset, without any intermittent pain-free period. This further supports a continuous sensitivity to common headache triggering factors.28 PTH with a chronic migraine phenotype co-occurred in 19% (4 of 21) of our cohort, exceeding the estimated 3% incidence in the general population and reaching the high OR of 10.35 in multivariate analysis.30 Although PTH can be heterogenous, patients could clearly distinguish their CH attacks from their migraine pain, arguing more for true concomitant headaches than one heterogeneous phenotype of the same type of headache. Similarly, this may result from central sensitisation with dysfunction of brainstem antinociceptive centres (implicated in migraine) as well as the hypothalamus (implicated in CH).31 Thus, head trauma may lead to global alteration in the ‘pain neuromatrix’, with concomitant forms of CH and migraine. The marked intractability of PTH-CH to treatment, both acute and preventive, could be another direct consequence of these modifications.
The rarity of CH following trauma, compared with migraine and tension-type headache, remains unexplained.8 Nevertheless, in a study in military service members with a history of mild traumatic brain injury (n=95), TAC type (n=6) was the second most prevalent, after migraine type.10 The emergence of CH phenotype may be due to a genetic susceptibility. We found that four (15.3%) patients with PTH-CH had family history of CH in first-degree relatives, exceeding the estimated prevalence of CH of 1.2% in the general population. Interestingly, there were three times the odds of having a family history of CH in case of PTH-CH. This implies that genetic risk in combination with an environmental trigger, such as head trauma, may be required to develop the phenotype.
PTH-CH may occur only in cases where there is hypothalamic disruption secondary to trauma and subsequent reorganisation.32 Indeed, the somatosensory system is capable of reorganisation following peripheral denervation and pain.33 34 There is supporting evidence for the involvement of posterior hypothalamus in primary CH attacks.29 35 Head trauma can lead to a loss of hypocretin-producing neurons in the posterolateral hypothalamus, and CH attacks are known to occur when the orexinergic system is downregulated.36 37 Indeed, the circadian periodicity is one of the hallmarks of CH attacks and concerns 70% of patients.38 The unusual high rate of circadian periodicity, documented in 92% of our patients with PTH-CH, may be suggestive of hypothalamic dysfunction related to trauma.
Weaknesses of this study include that our data were collected at a tertiary headache centre. Comparison with a large control cohort, who attended the same clinic during the same period, minimises this referral bias. Retrospective data may involve memory biases, but the rarity of the PTH-CH entity makes a prospective study unlikely to be feasible. Disability from PTH is compounded by coexisting post-traumatic stress disorder.39 Unfortunately, we did not formally perform psychological assessment or comparison with primary CH in terms of quality of life. Post-traumatic sleep and mood disturbances can plausibly influence the development and perpetuation of headache, but also the response to treatment.15 In terms of intractability, a large number of patients were classified as indeterminate due to incomplete trials. Finally, rather broad CIs were found after logistic regression owing to a small number of patients with PTH-CH. In order to take into account those imbalanced groups and give relevant results, we used several powerful statistical strategies such as ROSE and LASSO algorithms.
The strengths of the study include the large sample of patients who strictly fulfil the ICHD criteria for both PTH and CH, allowing us to consider our conclusions as strongly reliable. An association between head injury and CH has already been described in the past, but most injuries were quite remote from the first CH attack.8 The definition of PTH implies a close temporal relationship, established as the occurrence of headache within 7 days after head trauma. This stipulation might be somewhat arbitrary but yields a stronger evidence of causation, leading to a higher specificity of the ICHD criteria.1 Indeed, a high proportion of patients with CH sustained head injury several years prior to CH onset, suggesting an association between trauma and CH, which goes beyond the rare occurrence of PTH-CH cases, thus raising the hypothesis of distinctive lifestyles in patients with CH.40
In conclusion, this series is the first to describe in detail the specific clinical characteristics of PTH-CH. We demonstrated that PTH-CH is more likely to present as chronic form, with marked cranial autonomic features and temporoparietal location of attacks in patients with family history of CH. They have a considerably higher risk of intractability to treatment and associated chronic migraine. This unique evolutive profile possibly reflects sensitisation of the pain neuromatrix and hypothalamus following trauma.
Acknowledgments
We would like to thank our headache specialist nurses for their help with completion of the clinical database and management of patients. We also thank the patients and their families for their help with this project.
Footnotes
Contributors: LG: collection, analysis and interpretation of data, and drafting and revision of the manuscript. EO: analysis and interpretation of data, and drafting and revision of the manuscript. C-KC: collection and analysis of data. LA and TMPN: statistical analysis and interpretation of data, and drafting of the manuscript. MSM: study concept, recruitment of subjects, interpretation of data and manuscript revision.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: MSM serves on the advisory board for Allergan, St Jude Medical, Medtronic and Eli Lilly, and has received payment for the development of educational presentations from Allergan, St Jude Medical, Medtronic and electroCore.
Patient consent for publication: Not required.
Ethics approval: Ethics board approval was obtained from the National Hospital for Neurology and Neurosurgery Research Ethics Committee, London, UK (REC number: 07/Q0512/26).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available upon reasonable request. De-identified database and statistical analysis plan will be shared upon reasonable request for 2 years after publication.
References
- 1. Headache classification Committee of the International headache Society (IHS) the International classification of headache disorders, 3rd edition. Cephalalgia 2018;38:1–211. 10.1177/0333102417738202 [DOI] [PubMed] [Google Scholar]
- 2. Sheftell FD, Tepper SJ, Lay CL, et al. Post-Traumatic headache: emphasis on chronic types following mild closed head injury. Neurol Sci 2007;28:S203–7. 10.1007/s10072-007-0777-1 [DOI] [PubMed] [Google Scholar]
- 3. Nampiaparampil DE. Prevalence of chronic pain after traumatic brain injury: a systematic review. JAMA 2008;300:711–9. 10.1001/jama.300.6.711 [DOI] [PubMed] [Google Scholar]
- 4. Packard RC, Ham LP. Pathogenesis of posttraumatic headache and migraine: a common headache pathway? Headache 1997;37:142–52. 10.1046/j.1526-4610.1997.3703142.x [DOI] [PubMed] [Google Scholar]
- 5. Matharu MJ, Goadsby PJ. Post-Traumatic chronic paroxysmal hemicrania (CPH) with aura. Neurology 2001;56:273–5. 10.1212/WNL.56.2.273 [DOI] [PubMed] [Google Scholar]
- 6. Lambru G, Castellini P, Manzoni GC, et al. Post-Traumatic cluster headache: from the periphery to the central nervous system? Headache 2009;49:1059–61. 10.1111/j.1526-4610.2009.01456.x [DOI] [PubMed] [Google Scholar]
- 7. Putzki N, Nirkko A, Diener HC. Trigeminal autonomic cephalalgias: a case of post-traumatic SUNCT syndrome? Cephalalgia 2005;25:395–7. 10.1111/j.1468-2982.2004.00860.x [DOI] [PubMed] [Google Scholar]
- 8. Lambru G, Matharu M. Traumatic head injury in cluster headache: cause or effect? Curr Pain Headache Rep 2012;16:162–9. 10.1007/s11916-012-0248-0 [DOI] [PubMed] [Google Scholar]
- 9. Manzoni GC, Lambru G, Torelli P. Head trauma and cluster headache. Curr Pain Headache Rep 2006;10:130–6. 10.1007/s11916-006-0024-0 [DOI] [PubMed] [Google Scholar]
- 10. Finkel AG, Yerry JA, Klaric JS, et al. Headache in military service members with a history of mild traumatic brain injury: a cohort study of diagnosis and classification. Cephalalgia 2017;37:548–59. 10.1177/0333102416651285 [DOI] [PubMed] [Google Scholar]
- 11. Matharu M, Lambru G. Trigeminal autonomic cephalalgias: a review of recent diagnostic, therapeutic and pathophysiological developments. Ann Indian Acad Neurol 2012;15:51 10.4103/0972-2327.100007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yang F-C, Chou K-H, Fuh J-L, et al. Altered hypothalamic functional connectivity in cluster headache: a longitudinal resting-state functional MRI study. J Neurol Neurosurg Psychiatry 2015;86:437–45. 10.1136/jnnp-2014-308122 [DOI] [PubMed] [Google Scholar]
- 13. Kjeldgaard D, Forchhammer H, Teasdale T, et al. Chronic post-traumatic headache after mild head injury: a descriptive study. Cephalalgia 2014;34:191–200. 10.1177/0333102413505236 [DOI] [PubMed] [Google Scholar]
- 14. Yilmaz T, Roks G, de Koning M, et al. Risk factors and outcomes associated with post-traumatic headache after mild traumatic brain injury. Emerg Med J EMJ. [DOI] [PubMed] [Google Scholar]
- 15. Minen MT, Boubour A, Walia H, et al. Post-Concussive syndrome: a focus on post-traumatic headache and related cognitive, psychiatric, and sleep issues. Curr Neurol Neurosci Rep 2016;16:100. 10.1007/s11910-016-0697-7 [DOI] [PubMed] [Google Scholar]
- 16. Mitsikostas DD, Edvinsson L, Jensen RH, et al. Refractory chronic cluster headache: a consensus statement on clinical definition from the European headache Federation. J Headache Pain 2014;15:79. 10.1186/1129-2377-15-79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Goadsby PJ, Schoenen J, Ferrari MD, et al. Towards a definition of intractable headache for use in clinical practice and trials. Cephalalgia 2006;26:1168–70. 10.1111/j.1468-2982.2006.01173.x [DOI] [PubMed] [Google Scholar]
- 18. Schürks M, Kurth T, de Jesus J, et al. Cluster headache: clinical presentation, lifestyle features, and medical treatment. Headache 2006;46:1246–54. 10.1111/j.1526-4610.2006.00534.x [DOI] [PubMed] [Google Scholar]
- 19. Lunardon N, Menardi G, Torelli N. Rose: a package for binary imbalanced learning. R J 2014;6:79 10.32614/RJ-2014-008 [DOI] [Google Scholar]
- 20. Tibshirani R. Regression shrinkage and selection via the LASSO. J R Stat Soc Ser B 1996;58:267–88. 10.1111/j.2517-6161.1996.tb02080.x [DOI] [Google Scholar]
- 21. May A, Goadsby PJ. The trigeminovascular system in humans: pathophysiologic implications for primary headache syndromes of the neural influences on the cerebral circulation. J Cereb Blood Flow Metab 1999;19:115–27. 10.1097/00004647-199902000-00001 [DOI] [PubMed] [Google Scholar]
- 22. Turkewitz LJ, Wirth O, Dawson GA, et al. Cluster headache following head injury: a case report and review of the literature. Headache 1992;32:504–6. 10.1111/j.1526-4610.1992.hed3210504.x [DOI] [PubMed] [Google Scholar]
- 23. Benromano T, Defrin R, Ahn AH, et al. Mild closed head injury promotes a selective trigeminal hypernociception: implications for the acute emergence of post-traumatic headache: closed head injury promotes trigeminal hypernociception. Eur J Pain 2015;19:621–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Defrin R. Chronic post-traumatic headache: clinical findings and possible mechanisms. J Man Manip Ther 2014;22:36–43. 10.1179/2042618613Y.0000000053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rogado AZ, Graham JR. Through a glass darkly. Headache 1979;19:58–62. 10.1111/j.1526-4610.1979.hed1902058.x [DOI] [PubMed] [Google Scholar]
- 26. Lance JW, Anthony M. Migrainous neuralgia or cluster headache? J Neurol Sci 1971;13:401–14. 10.1016/0022-510X(71)90003-7 [DOI] [PubMed] [Google Scholar]
- 27. Burstein R. Deconstructing migraine headache into peripheral and central sensitization. Pain 2001;89:107–10. 10.1016/S0304-3959(00)00478-4 [DOI] [PubMed] [Google Scholar]
- 28. Bree D, Levy D. Development of CGRP-dependent pain and headache related behaviours in a rat model of concussion: implications for mechanisms of post-traumatic headache. Cephalalgia 2018;38:246–58. 10.1177/0333102416681571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Leone M, Bussone G. Pathophysiology of trigeminal autonomic cephalalgias. Lancet Neurol 2009;8:755–64. 10.1016/S1474-4422(09)70133-4 [DOI] [PubMed] [Google Scholar]
- 30. May A, Schulte LH. Chronic migraine: risk factors, mechanisms and treatment. Nat Rev Neurol 2016;12:455–64. 10.1038/nrneurol.2016.93 [DOI] [PubMed] [Google Scholar]
- 31. May A, Goadsby PJ. Hypothalamic involvement and activation in cluster headache. Curr Pain Headache Rep 2001;5:60–6. 10.1007/s11916-001-0011-4 [DOI] [PubMed] [Google Scholar]
- 32. Tanriverdi F, Unluhizarci K, Kelestimur F. Pituitary function in subjects with mild traumatic brain injury: a review of literature and proposal of a screening strategy. Pituitary 2010;13:146–53. 10.1007/s11102-009-0215-x [DOI] [PubMed] [Google Scholar]
- 33. Saab CY. Pain-Related changes in the brain: diagnostic and therapeutic potentials. Trends Neurosci 2012;35:629–37. 10.1016/j.tins.2012.06.002 [DOI] [PubMed] [Google Scholar]
- 34. Sörös P, Knecht S, Bantel C, et al. Functional reorganization of the human primary somatosensory cortex after acute pain demonstrated by magnetoencephalography. Neurosci Lett 2001;298:195–8. 10.1016/S0304-3940(00)01752-3 [DOI] [PubMed] [Google Scholar]
- 35. May A, Bahra A, Büchel C, et al. Pet and MRA findings in cluster headache and MRA in experimental pain. Neurology 2000;55:1328–35. 10.1212/WNL.55.9.1328 [DOI] [PubMed] [Google Scholar]
- 36. Baumann CR, Bassetti CL, Valko PO, et al. Loss of hypocretin (orexin) neurons with traumatic brain injury. Ann Neurol 2009;66:555–9. 10.1002/ana.21836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Holland PR, Goadsby PJ. Cluster headache, hypothalamus, and orexin. Curr Pain Headache Rep 2009;13:147–54. 10.1007/s11916-009-0025-x [DOI] [PubMed] [Google Scholar]
- 38. Almoznino G, Benoliel R, Sharav Y, et al. Sleep disorders and chronic craniofacial pain: characteristics and management possibilities. Sleep Med Rev 2017;33:39–50. 10.1016/j.smrv.2016.04.005 [DOI] [PubMed] [Google Scholar]
- 39. Roper LS, Nightingale P, Su Z, et al. Disability from posttraumatic headache is compounded by coexisting posttraumatic stress disorder. J Pain Res 2017;10:1991–6. 10.2147/JPR.S129808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lambru G, Castellini P, Manzoni GC, et al. Mode of occurrence of traumatic head injuries in male patients with cluster headache or migraine: is there a connection with lifestyle? Cephalalgia 2010;30:1502–8. 10.1177/0333102409359710 [DOI] [PubMed] [Google Scholar]


