Abstract
The increasing of intracellular calcium concentration is a fundamental process for mediating osteoclastogenesis, which is involved in osteoclastic bone resorption. Cytosolic calcium binds to calmodulin and subsequently activates calcineurin, leading to NFATc1 activation, a master transcription factor required for osteoclast differentiation. Targeting the various activation processes in osteoclastogenesis provides various therapeutic strategies for bone loss. Diverse compounds that modulate calcium signaling have been applied to regulate osteoclast differentiation and, subsequently, attenuate bone loss. Thus, in this review, we summarized the modulation of the NFATc1 pathway through various compounds that regulate calcium signaling and the calcium influx machinery. Furthermore, we addressed the involvement of transient receptor potential channels in osteoclastogenesis.
Keywords: osteoclast, calcium signaling, NFAT, transient receptor potential channels
1. Osteoclastogenesis in Bone Remodeling
Bone remodeling is balanced by the coordinated activities of osteoclastic resorption and osteoblastic formation [1]. Imbalanced bone remodeling leads to bone diseases including osteoporosis, periodontitis and rheumatoid arthritis, which are characterized by enhanced osteoclast activity. In other words, an excessive increase in osteoclast differentiation and bone resorption gives rise to various bone-resorptive diseases [2]. Osteoclasts are the cells responsible for bone resorption. These large multinucleated cells originate from the monocyte/macrophage hematopoietic lineage [3,4]. Osteoclast differentiation depends on two essential cytokines, receptor activator of nuclear factor-κB (NF-κB) ligand (RANKL) and macrophage colony-stimulating factor (M-CSF) [5,6,7]. M-CSF is involved in the proliferation and survival of osteoclast precursors and RANKL induce osteoclast differentiation through binding to its receptor RANK and subsequent activation of nuclear factor of activated T cells (NFATc1), a master transcription factor required for osteoclast differentiation [8]. Osteoclasts are formed by the fusion of osteoclast precursor cells. Cellular fusion is an essential element in osteoclast development that results in the formation of multinucleated giant cells responsible for bone resorption activity. This process is called osteoclastogenesis. To resorb bone, osteoclasts attach to the bone surface, form a “ruffled border” and dissolve bone mineral by massive secretion of acidic elements [3].
2. The Role of Calcium (Ca2+) Signaling in Osteoclastogenesis
Ca2+ signaling in osteoclasts is important for multiple cellular functions, including proliferation, differentiation, gene transcription and bone resorption [9]. Ca2+ is released from intracellular stores, or enters the cell via plasma membrane ion channels [10]. RANKL-mediated signaling in osteoclasts is the initial step of bone resorption initiation. RANK-bound RANKL induces activation of the tumor necrosis factor (TNF) receptor-associated factor 6 (TRAF6) [11], subsequently involved in the activation of mitogen-activated protein kinases (MAPKs), nuclear factor-κB (NF-κB), and a component of activator protein-1 (AP-1) [8,12,13]. Activated NF-κB induces NFATc1 transcription to differentiate osteoclasts [6,14]. RANKL also stimulates phospholipase Cγ (PLCγ) during the early stages of osteoclastogenesis. Activated PLCγ produces inositol 1, 4, 5-triphosphate (IP3) in the cytosol. Son et al. [15] reported that RANKL-mediated activation of PLC induces an increase of cytosolic IP3 levels, which increases intracellular Ca2+ concentration ([Ca2+]i) through inducing its release from the endoplasmic reticulum (ER). Ca2+ influx through store-operated Ca2+ entry (SOCE) and transient receptor potential (TRP) channels causes RANKL-induced [Ca2+]i oscillations during osteoclastogenesis [16,17,18,19]. TRP channels are involved in not only extracellular but also intracellular Ca2+ balance in osteoclasts [20]. Ca2+ release and reuptake into the ER stores is also necessary for [Ca2+]i oscillations. Sarco/endoplasmic reticulum Ca2+-ATPase (SERCA) transports Ca2+ from the cytosol into the ER, and SERCA activity is also essential for [Ca2+]i oscillations. Disruption of SERCA2 expression impairs RANKL-induced [Ca2+]i oscillations [21]. Furthermore, RANKL induces a reactive oxygen species (ROS) pathway and causes long lasting [Ca2+]i oscillations [22]. Cytosolic Ca2+ binds to calmodulin (CaM), which results in the activation of CaM-dependent enzymes such as the phosphatase calcineurin [23]. Activated calcineurin dephosphorylates serine residues in NFATc1, resulting in translocation of NFATc1 into the nucleus [24,25]. A recent study using Homer2 and Homer3 (Homer2/3) double-knockout (DKO) mice showed that Homer proteins regulate NFATc1 function through interaction with calcineurin to regulate RANKL-induced osteoclastogenesis [26]. Thus, increased [Ca2+]i is a fundamental process mediating osteoclastogenesis (Figure 1). In this review, we focused on modulation of Ca2+ signaling through Ca2+ influx via TRP channels and highlighted the diverse compounds, involved in the Ca2+ -mediated signaling pathway, in osteoclastogenesis.
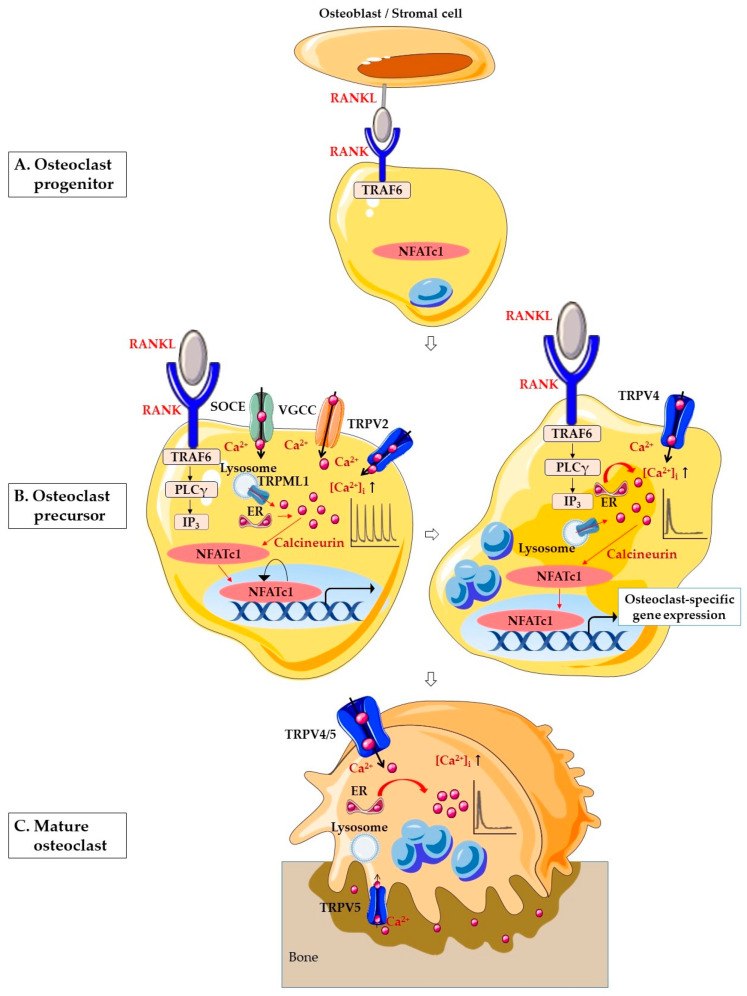
Figure 1.
Schematic illustration of Ca2+ signaling in osteoclastogenesis. (A) RANK on the surface of osteoclast progenitor activates signaling by RANKL on the surface of osteoblasts/stromal cell to promote osteoclastogenesis. (B) Osteoclast precursor stage. In the early stages of osteoclastogenesis, RANK-bound RANKL induces activation of TRAF6 and stimulates PLCγ. PLCγ produces IP3, which evokes Ca2+ release from the ER. In addition, RANK-bound RANKL induces lysosomal Ca2+ release through TRPML1 and generates Ca2+ oscillation. SOCE, VGCC and TRPV2 are also involved in Ca2+ oscillation. The Ca2+ oscillations induce Ca2+-calcineurin-NFATc1 signaling. In the late stages of osteoclastogenesis, the Ca2+ oscillation is sustained by TRPV4-mediated Ca2+ influx. In the nucleus, NFATc1 induces the expression of various osteoclast-specific genes. (C) In mature osteoclasts, TRPV4 and TRPV5 in the basolateral membrane are necessary for the regulation of osteoclastic bone resorption. TRPV5 is predominantly located on the ruffled border of resorbing osteoclasts. Abbreviations: RANKL, receptor activator of nuclear factor-κB (NF-κB) ligand; RANK, receptor activator of nuclear factor-κB (NF-κB); NFATc1, nuclear factor of activated T cells cytoplasmic 1; TRAF6, tumor necrosis factor (TNF) receptor-associated factor 6; PLCγ, phospholipase Cγ; IP3, inositol 1,4,5-triphosphate; ER, endoplasmic reticulum; Ca2+, calcium; [Ca2+]i, intracellular Ca2+ concentration; SOCE, store-operated Ca2+ entry; VGCC, voltage-gated Ca2+ channel; TRPV2, transient receptor potential vanilloid 2; TRPV4, transient receptor potential vanilloid 4; TRPV5, transient receptor potential vanilloid 5; TRPML1, transient receptor potential mucolipin 1.
3. Transient Receptor Potential (TRP) Channels in Osteoclast
Cytosolic Ca2+ modulation is crucial in osteoclastogenesis. TRP channels are widely expressed in several mammalian tissues and involved in diverse physiological processes such as differentiation, proliferation, and apoptosis [27,28]. Several studies have focused on TRP channels as Ca2+-influx channels in RANKL-induced osteoclastogenesis. Generally, TRP channels are non-selective cation channels and are divided into six subfamilies: canonical (TRPCs), vanilloid (TRPVs), melastatin (TRPMs), mucolipin (TRPMLs), polycystins (TRPPs), and ankyrin (TRPA) [29]. Among the TRP channels, TRPV2 [30], TRPV4 [31], and TRPV5 [32] contribute to intracellular Ca2+ signaling in osteoclast differentiation. TRPC1 also regulates osteoclast differentiation through SOCE [33]. This section discusses the roles of TRPC, TRPV, and TRPML channels in osteoclastogenesis.
3.1. TRPC
Mildly enhanced bone mass was observed in TRPC1 null mice and its effect was revealed only in mice lacking inhibitor of MyoD family isoform a (I-mfa) [33]. TRPC1 binds I-mfa [34]. Trpc1 and I-mfa functionally interact to regulate the early differentiation stage of the osteoclast through antagonistic regulation of SOCE. Although there are limited studies on TRPC, the modulation of the Ca2+ release-activated Ca2+ current (ICRAC) by TRPC1, and I-mfa is crucial for NFATc1 activation and subsequent osteoclast differentiation [33].
3.2. TRPV
TRPV family members act as sensory channels for receptor-operated Ca2+ influx and are critically involved in the regulating of osteoclast differentiation [20]. The TRPV family consists of six members, TRPV1–TRPV6, composed of six transmembrane domains that form a cation-permeable pore [35,36,37].
Among the TRPV family members, TRPV1 is a non-selective cation channel activated by various stimuli such as heat, noxious stimuli, low pH, and numerous chemicals [38]. The physiological role of TRPV1 in bone biology was addressed one decade ago. TRPV1 is expressed in osteoclasts and promotes their differentiation [39]. Human osteoclast expresses functional TRPV1, as well as the cannabinoid receptors type 1 and 2 (CB1/CB2). The involvement of both receptors is controversial. Expression levels of TRPV1 are enhanced in osteoclasts derived from osteoporotic subjects, whereas CB2 are reduced [40]. More recently, TRPV1 desensitization and/or CB2 stimulation were found beneficial for reducing osteoclast over-activity [41,42]. There are several reports showing that application of the TRPV1 agonist capsaicin suppresses LPS-induced prostaglandin E2 (PGE2) production in osteoblasts and suppressed LPS-induced osteoclast formation [39,43]. On the other hand, the TRPV1 antagonist capsazepine inhibits bone formation and bone resorption activity of osteoclasts in OVX mice [44]. [6]-Gingerol, a major constituent of ginger, augments osteoclast function via TRPV1 and induces bone loss in adult ovary-intact mice [45]. Zoledronic acid is nitrogen containing bisphosphonate that inhibit bone resorption. Effects of the Zoledronic acid were antagonized by capsazepine supporting the involvement of TRPV1 channel in osteoblastogenesis and mineralization, but this mechanism is not effective in osteoclasts lacking the TRPV1 [46]. Sirtuin 1 (SIRT1), also known as nicotinamide adenine dinucleotide (NAD+)-dependent lysine deacetylase, directly inhibits the osteoclast differentiation by inhibiting ROS generation and TNF-α-mediated TRPV1 channel activation [47]. In addition, TRPV1, as a pain receptor, is expressed in peripheral sensory nerves [48,49]. A pathological role of TRPV1 has been revealed in both osteoporosis and osteoarthritis [41,50].
TRPV2 is closely related to TRPV1 [38,51]. TRPV2 is expressed in RANKL-treated RAW264.7 cells and TRPV2-mediated spontaneous [Ca2+]i oscillations activate NFATc1 and promote osteoclast differentiation [30]. More recently, TRPV2 was found to regulate RANKL-dependent osteoclastic differentiation through the Ca2+-calcineurin-NFATc1 signaling pathway in multiple myeloma (MM) patients [52].
TRPV4 also plays an essential role in osteoclast differentiation [31]. It is known as a mechano- and osmo-sensor [53,54], and localizes to the basolateral membranes of mature osteoclasts [31]. TRPV4-mediated Ca2+ influx and intracellular Ca2+ signaling activate NFATc1 and induce osteoclast differentiation and resorption activity [31,55]. A protein–protein interaction between TRPV4 and myosin IIa regulates Ca2+/CaM signaling, which supports the migration and fusion of osteoclast precursors [55]. In addition, the TRPV4-specific antagonist, RN1734, inhibits osteoclast formation, whereas the TRPV4-specific agonist 4-α-PDD enhances osteoclast formation under mild acidic conditions [56,57]. Stromal interaction molecule 1 (STIM1)-mediated SOCE is involved in fluid shear stress (FSS)-induced [Ca2+]i oscillations at the early differentiation stage of osteoclasts, whereas TRPV4 is highly associated with the Ca2+ response at the late stage of differentiation under FSS simulation [58]. TRPV4 knockdown significantly suppresses osteoclast differentiation and osteoporosis by inhibiting the Ca2+-calcineurin-NFATc1 pathway [59].
TRPV5, a highly selective Ca2+ channel, is activated by low [Ca2+]i [60]. It is predominantly located on the ruffled borders of the membranes of resorbing osteoclasts [32]. TRPV5 knockout mice showed increased osteoclast numbers and reduced trabecular and cortical bone thickness [61]. In contrast, TRPV5 knockout mice had impaired osteoclastic function in vivo [32]. Although controversial, these findings suggest that TRPV5 plays an important role in osteoclastic function, again demonstrating the significance of Ca2+ influx in mature osteoclasts. In addition, small interfering RNA (siRNA) knockdown of TRPV5 completely inhibits RANKL-induced Ca2+ influx at the late differentiation stage of osteoclasts in vitro and enhances bone resorption activity in human osteoclasts [20,62]. The lack of estrogen leads to osteoporosis. Estrogen inhibits osteoclast differentiation and bone resorption activity by increasing TRPV5 expression in postmenopausal osteoporosis [63]. Song et al. also demonstrated that estrogen increases TRPV5 expression through the interaction of the estrogen receptor α (ERα) in RAW 264.7 cells. Furthermore, NF-κB binds to the putative site on the trpv5 promoter, and TRPV5 is regulated by NF-κB [64]. Thus, TRPV5 contributes to the processes of estrogen-mediated osteoclast formation, bone resorption activity, and osteoclast apoptosis. A recent study showed that vitamin D (1,25(OH)2D3) inhibits TRPV5 expression at the early stage of osteoclastogenesis by suppressing osteoclast differentiation [65].
3.3. TRPML
The TRPML family has three members: TRPML1, TRPML2, and TRPML3. Among these, TRPML1 is a non-selective cation channel that permeates Ca2+ [66]. TRPML1 is a Ca2+-permeable channel in lysosomes and plays vital roles in lysosomal trafficking and functions [67]. Erkhembaatar et al. [68] showed that deleting TRPML1 inhibits RANKL-induced [Ca2+]i oscillations, which reduces osteoclastogenesis and bone remodeling.
4. Diverse Compounds Modulating Ca2+ Signaling in Osteoclastogenesis
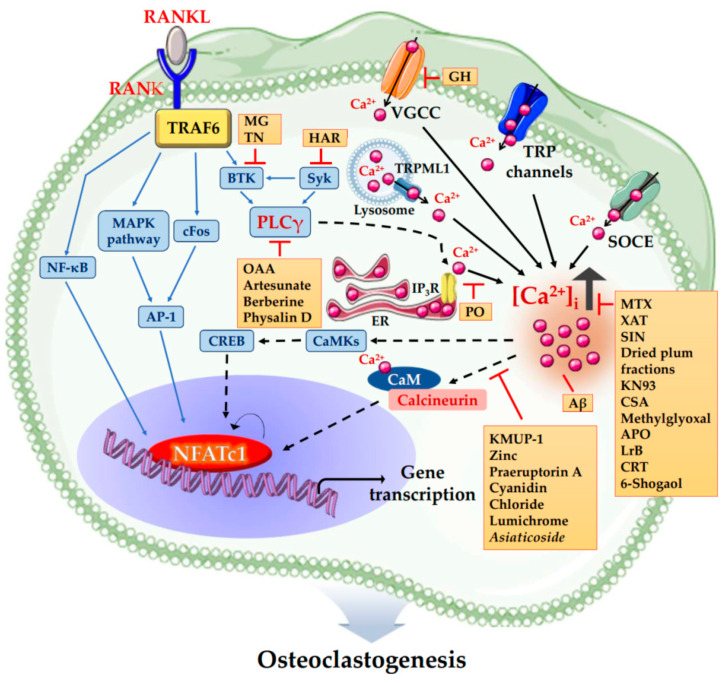
Osteoclasts are responsible for bone resorption and are therefore considered targets of anti-osteoporosis therapies. Novel treatment strategies aimed at preventing excessive bone resorption have been studied [69]. The study of antiresorptive agents derived from diverse compounds has become a recent topic of interest. The aim of this section is to summarize the current knowledge on diverse compounds that regulate osteoclast differentiation by modulating Ca2+ signaling. Thus, in this section, we mentioned by listing diverse compounds depending on their mode of action. Table 1 and Figure 2 summarize diverse compounds that regulate Ca2+ signaling in osteoclastogenesis.
Table 1.
Diverse compounds that regulate Ca2+ signaling in osteoclastogenesis.
| Compound | Mechanism of Inhibition of RIO (1) | Species | Administered Dose | Ref | |
|---|---|---|---|---|---|
| In Vitro | In Vivo | ||||
| Mode of action: Ca2+-Calcineurin-NFATc1(CCN (2)) signaling | |||||
| KMUP-1 | CCN signaling independently of PLCγ | RAW264.7 cell, BALB/c mice |
1–10 μM | 1, 5, 10 mg/kg | [70] |
| Zinc | CCN signaling independently of PLCγ | RAW264.7 cell, BMMs (C57BL/6 mice) |
10–100 μM | N/A (4) | [71] |
| Praeruptorin A | Inhibition of PLCγ-independent [Ca2+]i oscillations | BMMs (ICR mice) | 10 μM | N/A | [72] |
| Cyanidin Chloride | Suppression of NF-κB, ERK and CCN signaling | RAW264.7 cell, BMMs (C57BL/6 mice), C57BL/6 mice |
5–10 μM | 5 mg/kg | [73] |
| Lumichrome | Suppression of NF-κB, MAPK and CCN signaling | RAW264.7 cell, BMMs (C57BL/6 mice), C57BL/6 mice |
7.5–10 μM | 7.5 mg/kg | [74] |
| Asiaticoside | Suppression of NF-κB and CCN signaling | RAW264.7 cell, BMMs (C57BL/6 mice) |
2.5–20 μM | N/A | [75] |
| Mode of action: PLCγ- Ca2+-NFATc1(PCN (3)) signaling | |||||
| OAA | PCN signaling | BMMs (ICR mice), ICR mice |
20 μM | 10 mg/kg | [76] |
| HAR | Syk-Btk-PLCγ- Ca2+ Signaling | BMMs (ICR mice), C57BL/6 mice |
25–100 μM | 10 mg/kg | [77] |
| Artesunate | PCN signaling | RAW264.7 cell, BMMs (C57BL/6 mice), C57BL/6 mice |
3.125–12.5 μM | 5, 30 mg/kg | [78] |
| MG | Akt and Btk-PLCγ- Ca2+ Signaling | BMMs (ICR mice), ICR mice |
1–10 μM | 10 mg/kg | [79] |
| Berberine | * Inhibition of LPS-induced osteoclastogenesis through TRAF6 and PCN signaling | RAW264.7 cell | 5–20 μM | N/A | [80] |
| TN | Suppression of Btk-PLCγ cascade, NF-κB, MAPKs and CCN signaling | BMMs (C57BL/6 mice) | 1.25–5 μM | N/A | [81] |
| Physalin D | Suppression of PLCγ-CaMK-CREB pathway | BMMs (C57BL/6 mice), C57BL/6 mice |
5 μM | 10, 100 mg/kg | [82] |
| Mode of action: Negative regulation of Ca2+ signaling | |||||
| GH | Abrogation of RANKL-induced [Ca2+]i oscillations by inactivating VGCCs independently of Ca2+ release from intracellular Ca2+ stores | BMMs (C57BL/6 mice) | 5–50 μg/mL | N/A | [83] |
| PO | Suppression of RANKL-induced [Ca2+]i oscillations by inhibiting Ca2+ release from intracellular Ca2+ stores | murine BMMs | 50 μg/mL | N/A | [84] |
| MTX | Decrease of RANKL-induced Ca2+ influx | BMMs (C57BL/6 mice) | 1, 5 μM | N/A | [85] |
| XAT | Suppression of RANKL-induced [Ca2+]i oscillations and Ca2+-CaMKK-PYK2 signaling | BMMs (C57BL/6 mice), C57BL/6 mice |
0.1, 1 μM | 0.5, 5 mg/kg | [86] |
| SIN | * Inhibition of LPS-induced osteoclastogenesis by decreasing expression of NF-κB, AP-1 and Ca2+-NFATc1 | RAW264.7 cell, C57BL/6 mice |
0.25–1 mM | 25, 50, 100 mg/kg | [87] |
| Dried plum fractions | Suppression of MAPKs and Ca2+ signaling, resulting in inhibition of NFATc1 | RAW264.7 cell, BMMs (C57BL/6 mice) |
1, 10 μg/mL | N/A | [88] |
| KN93 | Decreasing of [Ca2+]i | RAW264.7 cell | 10 μM | N/A | [89] |
| CSA | Block of ROS activity and [Ca2+]i oscillations | RAW264.7 cell, BMMs (C57BL/6 mice), C57BL/6 mice |
5–10 μM | 10 mg/kg | [90] |
| Methylglyoxal | Suppression of [Ca2+]i, mitochondrial biogenesis, mitochondrial membrane potential, and glyoxalase I | RAW264.7 cell | 10–200 μM | N/A | [91] |
| APO | Decreasing of [Ca2+]i | BMMs (C57BL/6 mice) | 1 μM | N/A | [92] |
| LrB | Suppression of [Ca2+]i oscillations, ROS production, and NFATc1 translocation | RAW264.7 cell, BMMs (C57BL/6 mice), C57BL/6 mice |
5–10 μM | 4 mg/kg | [93] |
| CRT | Suppression of RANKL-induced [Ca2+]i oscillations and expression of NFATc1 and c-Fos, independently of ionomycin-induced Ca2+ influx | RAW264.7 cell, BMMs (C57BL/6 mice), C57BL/6, NOD mice, |
0.5–500 ng/ml | 0.2 mg/kg | [94] |
| 6-Shogaol | Suppression of [Ca2+]i oscillations, ROS production, and NFATc1 activity | BMMs (C57BL/6 mice), C57BL/6 mice |
2.5–10 μM | 10 mg/kg | [95] |
| Mode of action: Increasing [Ca2+]i oscillations | |||||
| Aβ | * Enhancement of osteoclast activation by activating NF-κB, ERK and increasing [Ca2+]i oscillations, resulting in upregulation of NFAT-c1 | BMMs (C57BL/6 mice) | 1–10 μM | N/A | [96] |
* Another mechanism besides RIO, Abbreviations: (1) RIO, RANKL-induced osteoclastogenesis; (2) CCN, Ca2+-Calcineurin-NFATc1; (3) PCN, PLCγ- Ca2+-NFATc1; (4) N/A, not applicable; The other abbreviations are listed in the last paragraph.
Figure 2.
The schematic illustration summarized diverse compounds that regulate Ca2+ signaling in osteoclastogenesis. KMUP-1 (7-[2-[4-(2-chlorophenyl)piperazinyl]ethyl]-1,3-dimethylxanthine), Zinc, Praeruptorin A, Cyanidin Chloride, Lumichrome and Asiaticoside inhibit osteoclastogenesis via inhibiting Ca2+-Calcineurin-NFATc1 signaling independent of PLCγ. Methotrexate (MTX), Xanthotoxin (XAT), Sinomenine (SIN), Dried plum fractions, KN93, Cajaninstilbene acid (CSA), Methylglyoxal, Apocynin (APO), Loureirin B (LrB), Calreticulin (CRT) and 6-Shogaol inhibit osteoclastogenesis via decreasing [Ca2+]i. On the contrary, Amyloid beta peptide (Aβ) enhances osteoclastic bone resorption by increasing [Ca2+]i oscillations, resulting in upregulation of NFATc1. Portulaca oleracea (PO) inhibits osteoclastogenesis by inhibiting Ca2+ release from intracellular Ca2+ stores. Oleanolic acid acetate (OAA), Artesunate, Berberine and Physalin D inhibit osteoclastogenesis via inhibiting PLCγ-Ca2+-NFATc1 signaling. Harpagoside (HAR) inhibits osteoclastogenesis via inhibiting Syk-Btk-PLCγ-Ca2+ Signaling. Methyl gallate (MG) and Tatarinan N (TN) inhibit osteoclastogenesis by suppression of Btk-PLCγ cascade. Glechoma hederacea (GH) inhibits osteoclastogenesis by inactivating VGCCs independent of Ca2+ release from intracellular Ca2+ stores. Abbreviations: RANKL, receptor activator of nuclear factor-κB (NF-κB) ligand; RANK, receptor activator of nuclear factor-κB (NF-κB); NFATc1, nuclear factor of activated T cells cytoplasmic 1; TRAF6, tumor necrosis factor (TNF) receptor-associated factor 6; MAPK, mitogen-activated protein kinases; AP-1, activator protein-1; Btk, Bruton’s tyrosine kinase; Syk, spleen tyrosine kinase; PLCγ, phospholipase Cγ; IP3R, inositol 1,4,5-triphosphate receptor; ER, endoplasmic reticulum; Ca2+, calcium; [Ca2+]i, intracellular Ca2+ concentration; SOCE, store-operated Ca2+ entry; VGCC, voltage-gated Ca2+ channel; TRP channels, transient receptor potential cation channels; CaMKs, Ca2+/calmodulin dependent protein kinases; CREB, cAMP-responsive element-binding protein; CaM, calmodulin.
4.1. Ca2+-Calcineurin-NFATc1 (CCN) Pathway
4.1.1. KMUP-1
KMUP-1 (7-[2-[4-(2-chlorophenyl)piperazinyl]ethyl]-1,3-dimethylxanthine), a chemical synthetic xanthine-based derivative, effectively suppresses RANKL-induced osteoclast differentiation in vitro, and also attenuated ovariectomized (OVX)-induced osteoclast differentiation and prevented bone resorption in vivo [18]. Especially KMUP-1 inhibits RANKL-induced [Ca2+]i oscillations, and subsequently, inhibits calcineurin-NFATc1 signaling [70].
4.1.2. Zinc
It has been shown that zinc, an important trace element, inhibits osteoclast differentiation by suppressing the Ca2+-calcineurin-NFATc1 signaling pathway in vitro and in vivo [19]. Specifically, zinc inhibits calcineurin activity but not expression and RANKL-induced [Ca2+]i oscillations, without decreasing PLCγ phosphorylation. In addition, it was proposed that zinc inhibits calcineurin in the early stage of osteoclast differentiation and [Ca2+]i oscillations in the middle or late stage of osteoclast differentiation [71].
4.1.3. Praeruptorin A
Praeruptorin A is isolated from the dried root of Peucedanum praeruptorum Dunn. It also has anti-osteoclastogenic activity by inhibiting [Ca2+]i oscillations without decreasing PLCγ phosphorylation [72].
4.1.4. Cyanidin
Cyanidin, a particular type of anthocyanidins, is the sugar-free counterpart of anthocyanins. Anthocyanins are reddish pigments widely spread in colored fruits and vegetables [97,98]. Cyanidin chloride inhibits RANKL-induced osteoclast formation and osteoclast resorptive activity in vitro and protects against OVX-induced bone loss in vivo. Furthermore, cyanidin chloride impairs RANKL-induced [Ca2+]i oscillations, which leads to the suppression of the activation of NFATc1 in cultured primary bone marrow-derived macrophages (BMMs) [73]
4.1.5. Lumichrome
Lumichrome is a natural metabolite of riboflavin, a member of the B family of vitamins, and has been shown to have a beneficial effect on bone formation [99,100]. Chuan et al. [74] found that lumichrome inhibits RANKL-induced [Ca2+]i oscillations in BMMs. Furthermore, lumichrome suppresses NFATc1, NF-κB, and MAPK signaling activation and decreases bone loss in OVX-mice by inhibiting osteoclastogenesis.
4.1.6. Asiaticoside
Asiaticoside, a natural compound, is extracted from Centella asiatica and is a member of the triterpenoid family [101]. It significantly inhibits RANKL-induced [Ca2+]i oscillations and NFATc1 expression in BMMs. Therefore, Asiaticoside suppresses the differentiation and function of the osteoclast via inhibiting the NF-κB and NFATc1 pathways [75].
4.2. PLCγ-Ca2+-NFATc1 (PCN) Pathway
4.2.1. Oleanolic Acid Acetate
Oleanolic acid acetate (OAA) is a compound isolated from Vigna angularis (azuki bean). Kim et al. [76] have reported that OAA negatively regulates osteoclast differentiation by RANKL-induced PLCγ2 and [Ca2+]i oscillations, which leads to NFATc1 activation. In vitro, OAA inhibits RANKL-induced osteoclast differentiation through PLCγ2-Ca2+-NFATc1 signaling. OAA administration also suppresses lipopolysaccharide (LPS)-induced bone loss in vivo.
4.2.2. Harpagoside
Harpagoside (HAR), an iridoid glycoside isolated from Harpagophytum procumbens (devil’s claw), inhibits [Ca2+]i oscillations via inactivation of several kinases such as Bruton’s tyrosine kinase (Btk), spleen tyrosine kinase (Syk), and PLCγ2, which leads to the suppression of RANKL-induced osteoclast differentiation [77]. HAR also restored bone density in an LPS-induced, but not in an OVX-induced bone loss mouse model in vivo [77].
4.2.3. Artesunate
Artesunate is one of the effective clinical treatments for falciparum malaria [102]. It suppresses RANKL-induced Ca2+ influx and calcineurin expression. Furthermore, phosphorylation of PLCγ1 is decreased by artesunate treatment in RANKL-stimulated RAW264.7 cells. Therefore, artesunate suppresses RANKL-induced osteoclast differentiation and function by inhibiting the PLCγ1-Ca2+-calcineurin-NFATc1 pathway [78].
4.2.4. Methyl Gallate
Methyl gallate (MG) is a polyphenolic compound that is known to have antioxidant [103], antitumor [104], anti-inflammatory [105], and antimicrobial activities [106]. MG is a dominant inhibitor of sodium and potassium ion channels in skeletal muscle cells [107]. Baek et al. [79] showed that MG attenuates RANKL-induced osteoclast differentiation by inhibiting both Akt (Protein kinase B) phosphorylation and intracellular Ca2+ influx mediated by Btk and PLCγ2.
4.2.5. Berberine Hydrochloride
Berberine hydrochloride, an isoquinoline alkaloid, is found in many plants of the Berberidaceae families [108]. It inhibits the activation of PLCγ1, and thereby, inhibits Ca2+ influx, which reduces intracellular Ca2+ concentration, and subsequently, inhibits osteoclast differentiation and bone destruction through suppression of the TRAF6-Ca2+-calcineurin-NFATc1 signaling pathway in LPS-stimulated RAW264.7 cells [80].
4.2.6. Tatarinan N
Tatarinan N (TN), a lignin-like component, is extracted from Acorus tatarinowii Schott [109]. It attenuates RANKL-induced osteoclast differentiation via reducing NFATc1 and c-Fos expression as well as inhibiting the ERK1/2 or p38 signaling pathway. Besides, TN significantly reduces the elevation of intracellular Ca2+ concentration induced by RANKL and attenuates RANKL-induced phosphorylation of Btk and PLCγ2 in a dose-dependent manner in BMMs [81].
4.2.7. Physalin D
Physalin D is isolated from Physalis alkekengi L., known as “winter cherry”, and grows in western Asia and Europe [110]. Physalin D has been shown to have anti-inflammatory, antimalarial, and antinociceptive effects [110,111,112]. Physalin D attenuates RANKL-induced [Ca2+]i oscillations by inhibiting phosphorylation of PLCγ2 and blocks the downstream activation of Ca2+/calmodulin-dependent protein kinase (CaMK) type IV and cAMP-responsive element-binding protein (CREB) in BMMs. Moreover, physalin D protects RANKL-induced bone loss in vivo [82].
4.3. Negative Regulation on Ca2+ Signaling
4.3.1. Glechoma Hederacea
Glechoma hederacea (GH), known as ‘ground ivy’ or ‘creeping Charlie’, is a perennial hairy herb of the mint family Lamiaceae. Hwang et al. [83] have shown that GH induces a transient and large increase in [Ca2+]i, through the involvement of Ca2+ influx via voltage-gated Ca2+ channels (VGCCs), resulting in the abrogation of RANKL-induced [Ca2+]i oscillations and the inhibition of NFATc1 expression in BMMs. However, GH-induced intracellular [Ca2+]i elevation was independent of Ca2+ release from intracellular Ca2+ stores in BMMs. Taken together, these findings suggest that GH abrogates RANKL-induced [Ca2+]i oscillations, inhibits NFATc1 expression, and reduces osteoclast differentiation by inactivating VGCCs.
4.3.2. Portulaca Oleracea
Portulaca oleracea (PO), also known as verdolaga, red root, or pursley, has been widely used as traditional medicine. PO ethanol extract (POEE) has dual and contrary effects on RANKL-induced osteoclast differentiation. The POEE inhibits RANKL-induced [Ca2+]i oscillations and NFATc1 activation, while it enhances RANKL-induced osteoclast differentiation by reducing RANKL-mediated cytotoxicity. Erkhembaatar et al. [84] proposed that RANKL-mediated cytotoxicity due to Ca2+ release from intracellular Ca2+ stores is attenuated by POEE, which leads to enhanced RANKL-induced osteoclast differentiation.
4.3.3. Methotrexate
Methotrexate (MTX) is used to treat sarcoma, leukemia, and auto-inflammatory diseases such as rheumatoid arthritis [113,114]. MTX inhibits osteoclast differentiation by inhibiting RANKL-induced Ca2+ influx in osteoclast progenitor cells [85].
4.3.4. Xanthotoxin
Xanthotoxin (XAT) is isolated from the seeds of a plant of the carrot family Ammi majus [115]. XAT has been shown to have antitumor activity and antioxidant activity [116,117]. Interestingly, XAT affects the intracellular Ca2+ levels in melanocytes, resulting in reorganization of actin stress fiber cytoskeleton [118]. Dou et al. [86] showed that XAT suppresses RANKL-induced [Ca2+]i oscillations and the activation of downstream targets of Ca2+-CaMKK (Calmodulin-dependent protein kinase kinase)/Pyk2 (Proline-rich tyrosine kinase 2) signaling during osteoclast differentiation, resulting in the inhibition of NFATc1 and c-FOS in BMMs. In addition, an in vivo study showed that XAT treatment prevents bone loss and increases new bone formation in OVX-mice.
4.3.5. Sinomenine
Sinomenine (SIN) is an alkaloid found in the roots and stems of Sinomenium acutum. SIN has been used for the treatment of rheumatoid arthritis (RA) in China [119]. SIN dramatically reduces LPS-induced upregulation of intracellular Ca2+ in matured RAW264.7 cells. In addition, SIN decreases expression of osteoclast-specific genes and tumor necrosis factor-α (TNF-α) production, and inhibits LPS-induced osteolysis and osteoclast differentiation in vitro and in vivo [87].
4.3.6. Dried Plum Fractions
In preclinical trials, bone resorption is decreased by dietary supplementation with dried plum in ovariectomized rat and mouse models [120,121]. Graef et al. [88] showed that polyphenolic compounds in dried plums suppress intracellular Ca2+ signaling and MAPK signaling, resulting in the inhibition of NFATc1 expression, which reduces osteoclast differentiation in BMMs.
4.3.7. KN93
KN93 is an inhibitor of multifunctional Ca2+/CaMKs [122]. It inhibits the formation and activation of the osteoclast. KN93 also downregulates the expression of NFATc1 and AP-1 protein family members in RANKL-stimulated RAW 264.7 cells. Furthermore, KN93 significantly decreases intracellular Ca2+ concentration in differentiated osteoclasts [89].
4.3.8. Cajaninstilbene Acid
Cajaninstilbene acid (CSA) is a bioactive compound derived from pigeon pea leaves [123]. It suppresses osteoclast differentiation and bone resorption via inhibiting RANKL-induced ROS activity and [Ca2+]i oscillations in RAW264.7 cells and BMMs. CSA also protects the bone loss of OVX- induced C57BL/6 mice [90].
4.3.9. Methylglyoxal
Methylglyoxal is derived from organic compounds and is a precursor of advanced glycation end products. Its formation involves several metabolic pathways [124]. The formation of Methylglyoxal is increased in diabetic patients [125]. Diabetes can give rise to a state of low bone turnover osteoporosis [126]. The Methylglyoxal decreases [Ca2+]I, mitochondrial biogenesis, mitochondrial membrane potential, and glyoxalase I, resulting in the inhibition of RANKL-induced osteoclast differentiation and bone resorbing activity in RAW264.7 cells [91].
4.3.10. Apocynin
The catechol apocynin (APO) is used as a NADPH oxidase (NOX) inhibitor [127]. Soares et al. [92] evaluated the effects of APO on osteoclast differentiation. APO reduces [Ca2+]i by blocking Ca2+ channels except two pore segment channel 2 (TPC2) and inositol 1,4,5-triphosphate receptor type 1 (IP3R1). TPC2 is a Ca2+-permeable channel expressed in lysosomes, and IP3R1 is a Ca2+ channel that mediates Ca2+ release from the ER, following IP3 stimulation. APO inhibits osteoclast differentiation by decreasing [Ca2+]i.
4.3.11. Loureirin B
Loureirin B (LrB) is an active component isolated from Sanguis draxonis, which is a Chinese traditional herb also known as Dragon’s Blood [128]. Yuhao et al. [93] investigated the effects of LrB on RANKL-induced osteoclast activity in vitro and in an OVX-induced osteoporosis mouse model in vivo. LrB attenuates RANKL-induced [Ca2+]i oscillations, ROS production, and NFATc1 translocation into the nucleus in BMMs. Therefore, LrB can inhibit osteoclast differentiation and function by suppressing [Ca2+]i oscillations, ROS, and NFATc1 activities. LrB also exerts a protective effect on OVX-induced osteoporosis in a mouse model [93].
4.3.12. Calreticulin
Calreticulin (CRT) is a Ca2+-binding protein that regulates intracellular Ca2+ homeostasis by modulating cytoplasmic and ER Ca2+ levels [129,130,131]. Fischer et al. [94] found that exogenous CRT has an anti-osteoclastogenic effect in vitro and in vivo. Recombinant CRT Inhibits RANKL-induced [Ca2+]i oscillations, but not ionomycin-induced Ca2+ influx in BMMs. Recombinant CRT also blocks expression of NFATc1 and c-Fos, but not CREB and NF-κB in RAW264.7 cells.
4.3.13. 6-Shogaol
Shogaols are significant biomarkers used for the quality control of ginger-containing products and responsible for the pungent flavor in dried ginger. Among them, 6-shogaol is the most common type [132]. The 6-shogaol inhibits RANKL-induced [Ca2+]i oscillations, ROS production, and NFATc1 activities in BMMs. Furthermore, 6-shogaol attenuates osteoclastogenesis and alveolar bone resorption in a ligature-induced periodontitis model in vivo [95].
4.4. Increasing [Ca2+]i Oscillations
Amyloid Beta Peptide
Amyloid beta peptide (Aβ) is the principal component of the accumulations of β-amyloid found in the brains of Alzheimer’s patients [133]. Various studies have addressed the role of Aβ in osteoclasts [134,135,136]. Specifically, a recent study showed that Aβ enhances RANKL-induced osteoclast activation and functions through nuclear factor-κB inhibitor α (IκB-α) degradation, extracellular-signal-regulated kinase (ERK) phosphorylation, and increased [Ca2+]i oscillations in BMMs [96].
5. Closing Remarks and Perspectives
The crucial studies on Ca2+ signaling in osteoclastogenesis have highlighted its role in bone biology. Considering the involvement of Ca2+ signaling in bone biology, the relatively few studies available to date suggest the importance of TRP channels for modulating osteoclastogenesis and bone loss. Therefore, most of the therapeutic potentials remain open. We estimate that pharmacological targeting of this membrane channels may result in the development of therapeutics that facilitate or inhibit Ca2+ influx.
Abbreviations
| RANKL | receptor activator of nuclear factor-κB (NF-κB) ligand |
| M-CSF | macrophage colony-stimulating factor |
| NFATc1 | nuclear factor of activated T cells |
| Ca2+ | calcium |
| TRAF6 | tumor necrosis factor (TNF) receptor-associated factor 6 |
| MAPKs | mitogen-activated protein kinases |
| NF-κB | nuclear factor-κB |
| AP-1 | activator protein-1 |
| PLCγ | phospholipase Cγ |
| IP3 | inositol 1, 4, 5-triphosphate |
| [Ca2+]i | intracellular Ca2+ concentration |
| ER | endoplasmic reticulum |
| SOCE | store-operated Ca2+ entry |
| TRP | transient receptor potential |
| SERCA | Sarco/endoplasmic reticulum Ca2+-ATPase |
| ROS | reactive oxygen species |
| CaM | calmodulin |
| Homer2/3 | Homer2 and Homer3 |
| DKO | double-knockout |
| TRPCs | Transient receptor potential canonical channel |
| TRPVs | Transient receptor potential vanilloid channel |
| TRPMs | Transient receptor potential melastatin channel |
| TRPMLs | Transient receptor potential mucolipin channel |
| TRPPs | Transient receptor potential polycystin channel |
| TRPAs | Transient receptor potential ankyrin channel |
| I-mfa | inhibitor of MyoD family isoform a |
| ICRAC | Ca2+ release-activated Ca2+ current |
| CB1/CB2 | cannabinoid receptors type 1 and 2 |
| PGE2 | prostaglandin E2 |
| SIRT1 | Sirtuin 1 |
| MM | multiple myeloma |
| STIM1 | Stromal interaction molecule 1 |
| FSS | fluid shear stress |
| siRNA | small interfering RNA |
| ERα | estrogen receptor α |
| CCN | Ca2+-Calcineurin-NFATc1 |
| OVX | ovariectomized |
| BMMs | bone marrow–derived macrophages |
| OAA | Oleanolic acid acetate |
| LPS | lipopolysaccharide |
| HAR | Harpagoside |
| Btk | Bruton’s tyrosine kinase |
| Syk | spleen tyrosine kinase |
| MG | Methyl gallate |
| TN | Tatarinan N |
| CaMK | Ca2+/calmodulin dependent protein kinase |
| CREB | cAMP-responsive element-binding protein |
| GH | Glechoma hederacea |
| VGCCs | voltage-gated Ca2+ channels |
| PO | Portulaca oleracea |
| POEE | Portulaca oleracea ethanol extract |
| MTX | Methotrexate |
| XAT | Xanthotoxin |
| CaMKK | Calmodulin-dependent protein kinase kinase |
| Pyk2 | Proline-rich tyrosine kinase 2 |
| SIN | Sinomenine |
| RA | rheumatoid arthritis |
| TNF-α | tumor necrosis factor-α |
| CSA | Cajaninstilbene acid |
| APO | apocynin |
| NOX | NADPH oxidase |
| TPC2 | two pore segment channel 2 |
| IP3R1 | inositol 1,4,5-triphosphate receptor type 1 |
| LrB | Loureirin B |
| CRT | Calreticulin |
| Aβ | Amyloid beta peptide () |
| IκB-α | nuclear factor-κB inhibitor α |
| ERK | extracellular-signal-regulated kinase |
Author Contributions
J.H.H., D.M.S., and J.Y.K. contributed to conception and design of manuscript; J.Y.K. drafted the article and revised it critically for important intellectual contents; N.K. and Y.-M.Y. collected data; J.H.H. and D.M.S. contributed to final approval of the version to be published. All authors are accountable for all aspects of the work. All authors have read and agreed to the published version of the manuscript
Funding
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean Government MSIT (2019R1F1A1046785 (to J.H.H.), 2020R1A2C2003409 (to D.M.S.)).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Harada S., Rodan G.A. Control of osteoblast function and regulation of bone mass. Nature. 2003;423:349–355. doi: 10.1038/nature01660. [DOI] [PubMed] [Google Scholar]
- 2.Lazner F., Gowen M., Pavasovic D., Kola I. Osteopetrosis and osteoporosis: Two sides of the same coin. Hum. Mol. Genet. 1999;8:1839–1846. doi: 10.1093/hmg/8.10.1839. [DOI] [PubMed] [Google Scholar]
- 3.Boyle W.J., Simonet W.S., Lacey D.L. Osteoclast differentiation and activation. Nature. 2003;423:337–342. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- 4.Ash P., Loutit J.F., Townsend K.M. Osteoclasts derived from haematopoietic stem cells. Nature. 1980;283:669–670. doi: 10.1038/283669a0. [DOI] [PubMed] [Google Scholar]
- 5.Fuller K., Owens J.M., Jagger C.J., Wilson A., Moss R., Chambers T.J. Macrophage colony-stimulating factor stimulates survival and chemotactic behavior in isolated osteoclasts. J. Exp. Med. 1993;178:1733–1744. doi: 10.1084/jem.178.5.1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tanaka S., Takahashi N., Udagawa N., Tamura T., Akatsu T., Stanley E.R., Kurokawa T., Suda T. Macrophage colony-stimulating factor is indispensable for both proliferation and differentiation of osteoclast progenitors. J. Clin. Investig. 1993;91:257–263. doi: 10.1172/JCI116179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yasuda H., Shima N., Nakagawa N., Yamaguchi K., Kinosaki M., Mochizuki S., Tomoyasu A., Yano K., Goto M., Murakami A., et al. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to trance/rankl. Proc. Natl. Acad. Sci. USA. 1998;95:3597–3602. doi: 10.1073/pnas.95.7.3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takayanagi H., Kim S., Koga T., Nishina H., Isshiki M., Yoshida H., Saiura A., Isobe M., Yokochi T., Inoue J., et al. Induction and activation of the transcription factor nfatc1 (nfat2) integrate rankl signaling in terminal differentiation of osteoclasts. Dev. Cell. 2002;3:889–901. doi: 10.1016/S1534-5807(02)00369-6. [DOI] [PubMed] [Google Scholar]
- 9.Hogan P.G., Chen L., Nardone J., Rao A. Transcriptional regulation by calcium, calcineurin, and nfat. Genes Dev. 2003;17:2205–2232. doi: 10.1101/gad.1102703. [DOI] [PubMed] [Google Scholar]
- 10.Clapham D.E. Calcium signaling. Cell. 2007;131:1047–1058. doi: 10.1016/j.cell.2007.11.028. [DOI] [PubMed] [Google Scholar]
- 11.Wong B.R., Josien R., Lee S.Y., Vologodskaia M., Steinman R.M., Choi Y. The traf family of signal transducers mediates nf-kappab activation by the trance receptor. J. Biol. Chem. 1998;273:28355–28359. doi: 10.1074/jbc.273.43.28355. [DOI] [PubMed] [Google Scholar]
- 12.David J.P., Rincon M., Neff L., Horne W.C., Baron R. Carbonic anhydrase ii is an ap-1 target gene in osteoclasts. J. Cell. Physiol. 2001;188:89–97. doi: 10.1002/jcp.1099. [DOI] [PubMed] [Google Scholar]
- 13.Kobayashi N., Kadono Y., Naito A., Matsumoto K., Yamamoto T., Tanaka S., Inoue J. Segregation of traf6-mediated signaling pathways clarifies its role in osteoclastogenesis. EMBO J. 2001;20:1271–1280. doi: 10.1093/emboj/20.6.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boyce B.F., Xiu Y., Li J., Xing L., Yao Z. Nf-κb-mediated regulation of osteoclastogenesis. Endocrinol. Metab. (Seoul Korea) 2015;30:35–44. doi: 10.3803/EnM.2015.30.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Son A., Kim M.S., Jo H., Byun H.M., Shin D.M. Effects of inositol 1,4,5-triphosphate on osteoclast differentiation in rankl-induced osteoclastogenesis. Korean J. Physiol. Pharm. 2012;16:31–36. doi: 10.4196/kjpp.2012.16.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mao D., Epple H., Uthgenannt B., Novack D.V., Faccio R. Plcgamma2 regulates osteoclastogenesis via its interaction with itam proteins and gab2. J. Clin. Investig. 2006;116:2869–2879. doi: 10.1172/JCI28775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dolmetsch R.E., Lewis R.S., Goodnow C.C., Healy J.I. Differential activation of transcription factors induced by ca2+ response amplitude and duration. Nature. 1997;386:855–858. doi: 10.1038/386855a0. [DOI] [PubMed] [Google Scholar]
- 18.Parekh A.B., Putney J.W. Store-operated calcium channels. Physiol. Rev. 2005;85:757–810. doi: 10.1152/physrev.00057.2003. [DOI] [PubMed] [Google Scholar]
- 19.Yang Y.M., Jung H.H., Lee S.J., Choi H.J., Kim M.S., Shin D.M. Trpm7 is essential for rankl-induced osteoclastogenesis. Korean J. Physiol. Pharm. 2013;17:65–71. doi: 10.4196/kjpp.2013.17.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lieben L., Carmeliet G. The involvement of trp channels in bone homeostasis. Front. Endocrinol. 2012;3:99. doi: 10.3389/fendo.2012.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang Y.M., Kim M.S., Son A., Hong J.H., Kim K.H., Seo J.T., Lee S.I., Shin D.M. Alteration of rankl-induced osteoclastogenesis in primary cultured osteoclasts from serca2+/− mice. J. Bone Min. Res. 2009;24:1763–1769. doi: 10.1359/jbmr.090420. [DOI] [PubMed] [Google Scholar]
- 22.Kim M.S., Yang Y.M., Son A., Tian Y.S., Lee S.I., Kang S.W., Muallem S., Shin D.M. Rankl-mediated reactive oxygen species pathway that induces long lasting ca2+ oscillations essential for osteoclastogenesis. J. Biol. Chem. 2010;285:6913–6921. doi: 10.1074/jbc.M109.051557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feske S., Giltnane J., Dolmetsch R., Staudt L.M., Rao A. Gene regulation mediated by calcium signals in t lymphocytes. Nat. Immunol. 2001;2:316–324. doi: 10.1038/86318. [DOI] [PubMed] [Google Scholar]
- 24.Kim K., Kim J.H., Lee J., Jin H.M., Lee S.H., Fisher D.E., Kook H., Kim K.K., Choi Y., Kim N. Nuclear factor of activated t cells c1 induces osteoclast-associated receptor gene expression during tumor necrosis factor-related activation-induced cytokine-mediated osteoclastogenesis. J. Biol. Chem. 2005;280:35209–35216. doi: 10.1074/jbc.M505815200. [DOI] [PubMed] [Google Scholar]
- 25.Negishi-Koga T., Takayanagi H. Ca2+-nfatc1 signaling is an essential axis of osteoclast differentiation. Immunol. Rev. 2009;231:241–256. doi: 10.1111/j.1600-065X.2009.00821.x. [DOI] [PubMed] [Google Scholar]
- 26.Son A., Kang N., Oh S.Y., Kim K.W., Muallem S., Yang Y.M., Shin D.M. Homer2 and homer3 modulate rankl-induced nfatc1 signaling in osteoclastogenesis and bone metabolism. J. Endocrinol. 2019;242:241–249. doi: 10.1530/JOE-19-0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gees M., Colsoul B., Nilius B. The role of transient receptor potential cation channels in ca2+ signaling. Cold Spring Harb. Perspect. Biol. 2010;2:a003962. doi: 10.1101/cshperspect.a003962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gees M., Owsianik G., Nilius B., Voets T. Trp channels. Compr. Physiol. 2012;2:563–608. doi: 10.1002/cphy.c110026. [DOI] [PubMed] [Google Scholar]
- 29.Venkatachalam K., Montell C. Trp channels. Annu. Rev. Biochem. 2007;76:387–417. doi: 10.1146/annurev.biochem.75.103004.142819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kajiya H., Okamoto F., Nemoto T., Kimachi K., Toh-Goto K., Nakayana S., Okabe K. Rankl-induced trpv2 expression regulates osteoclastogenesis via calcium oscillations. Cell Calcium. 2010;48:260–269. doi: 10.1016/j.ceca.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 31.Masuyama R., Vriens J., Voets T., Karashima Y., Owsianik G., Vennekens R., Lieben L., Torrekens S., Moermans K., Vanden Bosch A., et al. Trpv4-mediated calcium influx regulates terminal differentiation of osteoclasts. Cell Metab. 2008;8:257–265. doi: 10.1016/j.cmet.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 32.Van der Eerden B.C., Hoenderop J.G., de Vries T.J., Schoenmaker T., Buurman C.J., Uitterlinden A.G., Pols H.A., Bindels R.J., van Leeuwen J.P. The epithelial ca2+ channel trpv5 is essential for proper osteoclastic bone resorption. Proc. Natl. Acad. Sci. USA. 2005;102:17507–17512. doi: 10.1073/pnas.0505789102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ong E.C., Nesin V., Long C.L., Bai C.X., Guz J.L., Ivanov I.P., Abramowitz J., Birnbaumer L., Humphrey M.B., Tsiokas L. A trpc1 protein-dependent pathway regulates osteoclast formation and function. J. Biol. Chem. 2013;288:22219–22232. doi: 10.1074/jbc.M113.459826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ma R., Rundle D., Jacks J., Koch M., Downs T., Tsiokas L. Inhibitor of myogenic family, a novel suppressor of store-operated currents through an interaction with trpc1. J. Biol. Chem. 2003;278:52763–52772. doi: 10.1074/jbc.M309610200. [DOI] [PubMed] [Google Scholar]
- 35.Harteneck C., Plant T.D., Schultz G. From worm to man: Three subfamilies of trp channels. Trends Neurosci. 2000;23:159–166. doi: 10.1016/S0166-2236(99)01532-5. [DOI] [PubMed] [Google Scholar]
- 36.Clapham D.E., Runnels L.W., Strubing C. The trp ion channel family. Nat. Rev. Neurosci. 2001;2:387–396. doi: 10.1038/35077544. [DOI] [PubMed] [Google Scholar]
- 37.Vennekens R., Owsianik G., Nilius B. Vanilloid transient receptor potential cation channels: An overview. Curr. Pharm. Des. 2008;14:18–31. doi: 10.2174/138161208783330763. [DOI] [PubMed] [Google Scholar]
- 38.Clapham D.E. Trp channels as cellular sensors. Nature. 2003;426:517–524. doi: 10.1038/nature02196. [DOI] [PubMed] [Google Scholar]
- 39.Rossi F., Siniscalco D., Luongo L., De Petrocellis L., Bellini G., Petrosino S., Torella M., Santoro C., Nobili B., Perrotta S., et al. The endovanilloid/endocannabinoid system in human osteoclasts: Possible involvement in bone formation and resorption. Bone. 2009;44:476–484. doi: 10.1016/j.bone.2008.10.056. [DOI] [PubMed] [Google Scholar]
- 40.Rossi F., Bellini G., Luongo L., Torella M., Mancusi S., De Petrocellis L., Petrosino S., Siniscalco D., Orlando P., Scafuro M., et al. The endovanilloid/endocannabinoid system: A new potential target for osteoporosis therapy. Bone. 2011;48:997–1007. doi: 10.1016/j.bone.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 41.Rossi F., Bellini G., Torella M., Tortora C., Manzo I., Giordano C., Guida F., Luongo L., Papale F., Rosso F., et al. The genetic ablation or pharmacological inhibition of trpv1 signalling is beneficial for the restoration of quiescent osteoclast activity in ovariectomized mice. Br. J. Pharmacol. 2014;171:2621–2630. doi: 10.1111/bph.12542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bellini G., Torella M., Manzo I., Tortora C., Luongo L., Punzo F., Colacurci N., Nobili B., Maione S., Rossi F. Pkcbetaii-mediated cross-talk of trpv1/cb2 modulates the glucocorticoid-induced osteoclast overactivity. Pharmacol. Res. 2017;115:267–274. doi: 10.1016/j.phrs.2016.11.039. [DOI] [PubMed] [Google Scholar]
- 43.Kobayashi M., Watanabe K., Yokoyama S., Matsumoto C., Hirata M., Tominari T., Inada M., Miyaura C. Capsaicin, a trpv1 ligand, suppresses bone resorption by inhibiting the prostaglandin e production of osteoblasts, and attenuates the inflammatory bone loss induced by lipopolysaccharide. ISRN Pharmacol. 2012;2012:439860. doi: 10.5402/2012/439860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Idris A.I., Landao-Bassonga E., Ralston S.H. The trpv1 ion channel antagonist capsazepine inhibits osteoclast and osteoblast differentiation in vitro and ovariectomy induced bone loss in vivo. Bone. 2010;46:1089–1099. doi: 10.1016/j.bone.2010.01.368. [DOI] [PubMed] [Google Scholar]
- 45.Khan K., Singh A., Mittal M., Sharan K., Singh N., Dixit P., Sanyal S., Maurya R., Chattopadhyay N. [6]-gingerol induces bone loss in ovary intact adult mice and augments osteoclast function via the transient receptor potential vanilloid 1 channel. Mol. Nutr. Food Res. 2012;56:1860–1873. doi: 10.1002/mnfr.201200200. [DOI] [PubMed] [Google Scholar]
- 46.Scala R., Maqoud F., Angelelli M., Latorre R., Perrone M.G., Scilimati A., Tricarico D. Zoledronic acid modulation of trpv1 channel currents in osteoblast cell line and native rat and mouse bone marrow-derived osteoblasts: Cell proliferation and mineralization effect. Cancers (Basel) 2019;11:206. doi: 10.3390/cancers11020206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yan S., Miao L., Lu Y., Wang L. Sirtuin 1 inhibits tnf-alpha-mediated osteoclastogenesis of bone marrow-derived macrophages through both ros generation and trpv1 activation. Mol. Cell. Biochem. 2019;455:135–145. doi: 10.1007/s11010-018-3477-7. [DOI] [PubMed] [Google Scholar]
- 48.Caterina M.J., Schumacher M.A., Tominaga M., Rosen T.A., Levine J.D., Julius D. The capsaicin receptor: A heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 49.Moran M.M., McAlexander M.A., Biro T., Szallasi A. Transient receptor potential channels as therapeutic targets. Nat. Rev. Drug Discov. 2011;10:601–620. doi: 10.1038/nrd3456. [DOI] [PubMed] [Google Scholar]
- 50.Kalff K.M., El Mouedden M., van Egmond J., Veening J., Joosten L., Scheffer G.J., Meert T., Vissers K. Pre-treatment with capsaicin in a rat osteoarthritis model reduces the symptoms of pain and bone damage induced by monosodium iodoacetate. Eur. J. Pharmacol. 2010;641:108–113. doi: 10.1016/j.ejphar.2010.05.022. [DOI] [PubMed] [Google Scholar]
- 51.Kanzaki M., Zhang Y.-Q., Mashima H., Li L., Shibata H., Kojima I. Translocation of a calcium-permeable cation channel induced by insulin-like growth factor-i. Nat. Cell Biol. 1999;1:165–170. doi: 10.1038/11086. [DOI] [PubMed] [Google Scholar]
- 52.Bai H., Zhu H., Yan Q., Shen X., Lu X., Wang J., Li J., Chen L. Trpv2-induced ca2+-calcineurin-nfat signaling regulates differentiation of osteoclast in multiple myeloma. Cell Commun. Signal. 2018;16:68. doi: 10.1186/s12964-018-0280-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mizuno A., Matsumoto N., Imai M., Suzuki M. Impaired osmotic sensation in mice lacking trpv4. Am. J. Physiol. Cell Physiol. 2003;285:C96–C101. doi: 10.1152/ajpcell.00559.2002. [DOI] [PubMed] [Google Scholar]
- 54.Suzuki M., Mizuno A., Kodaira K., Imai M. Impaired pressure sensation in mice lacking trpv4. J. Biol. Chem. 2003;278:22664–22668. doi: 10.1074/jbc.M302561200. [DOI] [PubMed] [Google Scholar]
- 55.Masuyama R., Mizuno A., Komori H., Kajiya H., Uekawa A., Kitaura H., Okabe K., Ohyama K., Komori T. Calcium/calmodulin-signaling supports trpv4 activation in osteoclasts and regulates bone mass. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2012;27:1708–1721. doi: 10.1002/jbmr.1629. [DOI] [PubMed] [Google Scholar]
- 56.Kato K., Morita I. Promotion of osteoclast differentiation and activation in spite of impeded osteoblast-lineage differentiation under acidosis: Effects of acidosis on bone metabolism. Biosci. Trends. 2013;7:33–41. doi: 10.5582/bst.2013.v7.1.33. [DOI] [PubMed] [Google Scholar]
- 57.Kato K., Morita I. Acidosis environment promotes osteoclast formation by acting on the last phase of preosteoclast differentiation: A study to elucidate the action points of acidosis and search for putative target molecules. Eur. J. Pharm. 2011;663:27–39. doi: 10.1016/j.ejphar.2011.04.062. [DOI] [PubMed] [Google Scholar]
- 58.Li P., Bian X., Liu C., Wang S., Guo M., Tao Y., Huo B. Stim1 and trpv4 regulate fluid flow-induced calcium oscillation at early and late stages of osteoclast differentiation. Cell Calcium. 2018;71:45–52. doi: 10.1016/j.ceca.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 59.Cao B., Dai X., Wang W. Knockdown of trpv4 suppresses osteoclast differentiation and osteoporosis by inhibiting autophagy through ca(2+) -calcineurin-nfatc1 pathway. J. Cell. Physiol. 2019;234:6831–6841. doi: 10.1002/jcp.27432. [DOI] [PubMed] [Google Scholar]
- 60.Hoenderop J.G., Nilius B., Bindels R.J. Calcium absorption across epithelia. Physiol. Rev. 2005;85:373–422. doi: 10.1152/physrev.00003.2004. [DOI] [PubMed] [Google Scholar]
- 61.Hoenderop J.G., van Leeuwen J.P., van der Eerden B.C., Kersten F.F., van der Kemp A.W., Mérillat A.M., Waarsing J.H., Rossier B.C., Vallon V., Hummler E., et al. Renal ca2+ wasting, hyperabsorption, and reduced bone thickness in mice lacking trpv5. J. Clin. Investig. 2003;112:1906–1914. doi: 10.1172/JCI200319826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chamoux E., Bisson M., Payet M.D., Roux S. Trpv-5 mediates a receptor activator of nf-kappab (rank) ligand-induced increase in cytosolic ca2+ in human osteoclasts and down-regulates bone resorption. J. Biol. Chem. 2010;285:25354–25362. doi: 10.1074/jbc.M109.075234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen F., Ouyang Y., Ye T., Ni B., Chen A. Estrogen inhibits rankl-induced osteoclastic differentiation by increasing the expression of trpv5 channel. J. Cell. Biochem. 2014;115:651–658. doi: 10.1002/jcb.24700. [DOI] [PubMed] [Google Scholar]
- 64.Song T., Lin T., Ma J., Guo L., Zhang L., Zhou X., Ye T. Regulation of trpv5 transcription and expression by e2/erα signalling contributes to inhibition of osteoclastogenesis. J. Cell. Mol. Med. 2018;22:4738–4750. doi: 10.1111/jcmm.13718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gu J., Tong X., Chen Y., Zhang C., Ma T., Li S., Min W., Yuan Y., Liu X., Bian J., et al. Vitamin d inhibition of trpv5 expression during osteoclast differentiation. Int. J. Endocrinol. Metab. 2019;17:e91583. doi: 10.5812/ijem.91583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dong X.P., Shen D., Wang X., Dawson T., Li X., Zhang Q., Cheng X., Zhang Y., Weisman L.S., Delling M., et al. Pi(3,5)p(2) controls membrane trafficking by direct activation of mucolipin ca(2+) release channels in the endolysosome. Nat. Commun. 2010;1:38. doi: 10.1038/ncomms1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xu H., Ren D. Lysosomal physiology. Annu. Rev. Physiol. 2015;77:57–80. doi: 10.1146/annurev-physiol-021014-071649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Erkhembaatar M., Gu D.R., Lee S.H., Yang Y.M., Park S., Muallem S., Shin D.M., Kim M.S. Lysosomal ca(2+) signaling is essential for osteoclastogenesis and bone remodeling. J. Bone Min. Res. 2017;32:385–396. doi: 10.1002/jbmr.2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rachner T.D., Khosla S., Hofbauer L.C. Osteoporosis: Now and the future. Lancet (Lond. Engl.) 2011;377:1276–1287. doi: 10.1016/S0140-6736(10)62349-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liou S.F., Hsu J.H., Lin I.L., Ho M.L., Hsu P.C., Chen L.W., Chen I.J., Yeh J.L. Kmup-1 suppresses rankl-induced osteoclastogenesis and prevents ovariectomy-induced bone loss: Roles of mapks, akt, nf-kappab and calcium/calcineurin/nfatc1 pathways. PLoS ONE. 2013;8:e69468. doi: 10.1371/journal.pone.0069468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Park K.H., Park B., Yoon D.S., Kwon S.H., Shin D.M., Lee J.W., Lee H.G., Shim J.H., Park J.H., Lee J.M. Zinc inhibits osteoclast differentiation by suppression of ca2+-calcineurin-nfatc1 signaling pathway. Cell Commun. Signal. CCS. 2013;11:74. doi: 10.1186/1478-811X-11-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yeon J.T., Kim K.J., Choi S.W., Moon S.H., Park Y.S., Ryu B.J., Oh J., Kim M.S., Erkhembaatar M., Son Y.J., et al. Anti-osteoclastogenic activity of praeruptorin a via inhibition of p38/akt-c-fos-nfatc1 signaling and plcgamma-independent ca2+ oscillation. PLoS ONE. 2014;9:e88974. doi: 10.1371/journal.pone.0088974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cheng J., Zhou L., Liu Q., Tickner J., Tan Z., Li X., Liu M., Lin X., Wang T., Pavlos N.J., et al. Cyanidin chloride inhibits ovariectomy-induced osteoporosis by suppressing rankl-mediated osteoclastogenesis and associated signaling pathways. J. Cell. Physiol. 2018;233:2502–2512. doi: 10.1002/jcp.26126. [DOI] [PubMed] [Google Scholar]
- 74.Liu C., Cao Z., Zhang W., Tickner J., Qiu H., Wang C., Chen K., Wang Z., Tan R., Dong S., et al. Lumichrome inhibits osteoclastogenesis and bone resorption through suppressing rankl-induced nfat activation and calcium signaling. J. Cell. Physiol. 2018;233:8971–8983. doi: 10.1002/jcp.26841. [DOI] [PubMed] [Google Scholar]
- 75.He L., Hong G., Zhou L., Zhang J., Fang J., He W., Tickner J., Han X., Zhao L., Xu J. Asiaticoside, a component of centella asiatica attenuates rankl-induced osteoclastogenesis via nfatc1 and nf-kappab signaling pathways. J. Cell. Physiol. 2019;234:4267–4276. doi: 10.1002/jcp.27195. [DOI] [PubMed] [Google Scholar]
- 76.Kim J.Y., Cheon Y.H., Oh H.M., Rho M.C., Erkhembaatar M., Kim M.S., Lee C.H., Kim J.J., Choi M.K., Yoon K.H., et al. Oleanolic acid acetate inhibits osteoclast differentiation by downregulating plcgamma2-ca(2+)-nfatc1 signaling, and suppresses bone loss in mice. Bone. 2014;60:104–111. doi: 10.1016/j.bone.2013.12.013. [DOI] [PubMed] [Google Scholar]
- 77.Kim J.Y., Park S.H., Baek J.M., Erkhembaatar M., Kim M.S., Yoon K.H., Oh J., Lee M.S. Harpagoside inhibits rankl-induced osteoclastogenesis via syk-btk-plcgamma2-ca(2+) signaling pathway and prevents inflammation-mediated bone loss. J. Nat. Prod. 2015;78:2167–2174. doi: 10.1021/acs.jnatprod.5b00233. [DOI] [PubMed] [Google Scholar]
- 78.Zeng X., Zhang Y., Wang S., Wang K., Tao L., Zou M., Chen N., Xu J., Liu S., Li X. Artesunate suppresses rankl-induced osteoclastogenesis through inhibition of plcgamma1-ca(2+)-nfatc1 signaling pathway and prevents ovariectomy-induced bone loss. Biochem. Pharm. 2017;124:57–68. doi: 10.1016/j.bcp.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 79.Baek J.M., Kim J.Y., Lee C.H., Yoon K.H., Lee M.S. Methyl gallate inhibits osteoclast formation and function by suppressing akt and btk-plcgamma2-ca(2+) signaling and prevents lipopolysaccharide-induced bone loss. Int. J. Mol. Sci. 2017;18:581. doi: 10.3390/ijms18030581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ye F., Zhou Q., Tian L., Lei F., Feng D. The protective effect of berberine hydrochloride on lpsinduced osteoclastogenesis through inhibiting traf6ca2+calcineurinnfatcl signaling pathway. Mol. Med. Rep. 2017;16:6228–6233. doi: 10.3892/mmr.2017.7338. [DOI] [PubMed] [Google Scholar]
- 81.Zhang Y., Wang Z., Xie X., Wang J., Wang Y., Peng Q.S., Zhang M., Wu D., Liu N., Wang H.B., et al. Tatarinan n inhibits osteoclast differentiation through attenuating nf-kappab, mapks and ca(2+)-dependent signaling. Int. Immunopharmacol. 2018;65:199–211. doi: 10.1016/j.intimp.2018.09.030. [DOI] [PubMed] [Google Scholar]
- 82.Ding N., Lu Y., Cui H., Ma Q., Qiu D., Wei X., Dou C., Cao N. Physalin d inhibits rankl-induced osteoclastogenesis and bone loss via regulating calcium signaling. BMB Rep. 2020;53:154–159. doi: 10.5483/BMBRep.2020.53.3.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hwang J.K., Erkhembaatar M., Gu D.R., Lee S.H., Lee C.H., Shin D.M., Lee Y.R., Kim M.S. Glechoma hederacea suppresses rankl-mediated osteoclastogenesis. J. Dent. Res. 2014;93:685–690. doi: 10.1177/0022034514536579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Erkhembaatar M., Choi E.J., Lee H.Y., Lee C.H., Lee Y.R., Kim M.S. Attenuated rankl-induced cytotoxicity by portulaca oleracea ethanol extract enhances rankl-mediated osteoclastogenesis. BMC Complement. Altern. Med. 2015;15:226. doi: 10.1186/s12906-015-0770-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kanagawa H., Masuyama R., Morita M., Sato Y., Niki Y., Kobayashi T., Katsuyama E., Fujie A., Hao W., Tando T., et al. Methotrexate inhibits osteoclastogenesis by decreasing rankl-induced calcium influx into osteoclast progenitors. J. Bone Min. Metab. 2016;34:526–531. doi: 10.1007/s00774-015-0702-2. [DOI] [PubMed] [Google Scholar]
- 86.Dou C., Chen Y., Ding N., Li N., Jiang H., Zhao C., Kang F., Cao Z., Quan H., Luo F., et al. Xanthotoxin prevents bone loss in ovariectomized mice through the inhibition of rankl-induced osteoclastogenesis. Osteoporos. Int. 2016;27:2335–2344. doi: 10.1007/s00198-016-3496-8. [DOI] [PubMed] [Google Scholar]
- 87.He L., Duan H., Li X., Wang S., Zhang Y., Lei L., Xu J., Liu S., Li X. Sinomenine down-regulates tlr4/traf6 expression and attenuates lipopolysaccharide-induced osteoclastogenesis and osteolysis. Eur. J. Pharmacol. 2016;779:66–79. doi: 10.1016/j.ejphar.2016.03.014. [DOI] [PubMed] [Google Scholar]
- 88.Graef J.L., Rendina-Ruedy E., Crockett E.K., Ouyang P., Wu L., King J.B., Cichewicz R.H., Lin D., Lucas E.A., Smith B.J. Osteoclast differentiation is downregulated by select polyphenolic fractions from dried plum via suppression of mapks and nfatc1 in mouse c57bl/6 primary bone marrow cells. Curr. Dev. Nutr. 2017;1:e000406. doi: 10.3945/cdn.117.000406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fu Y., Niu D., Su W., Yang Q., Wang W., Tang B., Li Z., Zhang D., Mao Y., Li C., et al. Effects of ca2+/calmodulindependent protein kinase pathway inhibitor kn93 on osteoclastogenesis. Int. J. Mol. Med. 2018;42:2294–2302. doi: 10.3892/ijmm.2018.3793. [DOI] [PubMed] [Google Scholar]
- 90.Sun Y., Liu Y., He W., Wang C., Tickner J., Kuek V., Zhou C., Wang H., Zou X., Hong Z., et al. Cajaninstilbene acid inhibits osteoporosis through suppressing osteoclast formation and rankl-induced signaling pathways. J. Cell. Physiol. 2019;234:11792–11804. doi: 10.1002/jcp.27868. [DOI] [PubMed] [Google Scholar]
- 91.Suh K.S., Chon S., Jung W.W., Choi E.M. Effects of methylglyoxal on rankl-induced osteoclast differentiation in raw264.7cells. Chem. Biol. Interact. 2018;296:18–25. doi: 10.1016/j.cbi.2018.09.005. [DOI] [PubMed] [Google Scholar]
- 92.Soares M.P.R., Silva D.P., Uehara I.A., Ramos E.S., Jr., Alabarse P.V.G., Fukada S.Y., da Luz F.C., Vieira L.Q., Oliveira A.P.L., Silva M.J.B. The use of apocynin inhibits osteoclastogenesis. Cell Biol. Int. 2019;43:466–475. doi: 10.1002/cbin.11110. [DOI] [PubMed] [Google Scholar]
- 93.Liu Y., Wang C., Wang G., Sun Y., Deng Z., Chen L., Chen K., Tickner J., Kenny J., Song D., et al. Loureirin b suppresses rankl-induced osteoclastogenesis and ovariectomized osteoporosis via attenuating nfatc1 and ros activities. Theranostics. 2019;9:4648–4662. doi: 10.7150/thno.35414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fischer C.R., Mikami M., Minematsu H., Nizami S., Goo Lee H., Stamer D., Patel N., Yu Soung D., Back J.H., Song L., et al. Calreticulin inhibits inflammation-induced osteoclastogenesis and bone resorption. J. Orthop. Res. 2017;35:2658–2666. doi: 10.1002/jor.23587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kim Y.G., Kim M.O., Kim S.H., Kim H.J., Pokhrel N.K., Lee J.H., Lee H.J., Kim J.Y., Lee Y. 6-shogaol, an active ingredient of ginger, inhibits osteoclastogenesis and alveolar bone resorption in ligature-induced periodontitis in mice. J. Periodontol. 2019 doi: 10.1002/JPER.19-0228. [DOI] [PubMed] [Google Scholar]
- 96.Li S., Yang B., Teguh D., Zhou L., Xu J., Rong L. Amyloid beta peptide enhances rankl-induced osteoclast activation through nf-kappab, erk, and calcium oscillation signaling. Int. J. Mol. Sci. 2016;17:1683. doi: 10.3390/ijms17101683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hou D.X., Ose T., Lin S., Harazoro K., Imamura I., Kubo M., Uto T., Terahara N., Yoshimoto M., Fujii M. Anthocyanidins induce apoptosis in human promyelocytic leukemia cells: Structure-activity relationship and mechanisms involved. Int. J. Oncol. 2003;23:705–712. doi: 10.3892/ijo.23.3.705. [DOI] [PubMed] [Google Scholar]
- 98.Jayaprakasam B., Vareed S.K., Olson L.K., Nair M.G. Insulin secretion by bioactive anthocyanins and anthocyanidins present in fruits. J. Agric. Food Chem. 2005;53:28–31. doi: 10.1021/jf049018+. [DOI] [PubMed] [Google Scholar]
- 99.Bro-Rasmussen F. The riboflavin requirement of animals and man and associated metabolic relations. II. Relation of requirement to the metabolism of protein and energy. Nutr. Abstr. Rev. 1958;28:369–386. [PubMed] [Google Scholar]
- 100.Yazdanpanah N., Uitterlinden A.G., Zillikens M.C., Jhamai M., Rivadeneira F., Hofman A., de Jonge R., Lindemans J., Pols H.A., van Meurs J.B. Low dietary riboflavin but not folate predicts increased fracture risk in postmenopausal women homozygous for the mthfr 677 t allele. J. Bone Min. Res. 2008;23:86–94. doi: 10.1359/jbmr.070812. [DOI] [PubMed] [Google Scholar]
- 101.Prasad A., Mathur A., Singh M., Gupta M.M., Uniyal G.C., Lal R.K., Mathur A.K. Growth and asiaticoside production in multiple shoot cultures of a medicinal herb, Centella asiatica (L.) urban, under the influence of nutrient manipulations. J. Nat. Med. 2012;66:383–387. doi: 10.1007/s11418-011-0588-9. [DOI] [PubMed] [Google Scholar]
- 102.Rosenthal P.J. Artesunate for the treatment of severe falciparum malaria. N. Engl. J. Med. 2008;358:1829–1836. doi: 10.1056/NEJMct0709050. [DOI] [PubMed] [Google Scholar]
- 103.Asnaashari M., Farhoosh R., Sharif A. Antioxidant activity of gallic acid and methyl gallate in triacylglycerols of kilka fish oil and its oil-in-water emulsion. Food Chem. 2014;159:439–444. doi: 10.1016/j.foodchem.2014.03.038. [DOI] [PubMed] [Google Scholar]
- 104.Lee H., Lee H., Kwon Y., Lee J.H., Kim J., Shin M.K., Kim S.H., Bae H. Methyl gallate exhibits potent antitumor activities by inhibiting tumor infiltration of cd4+cd25+ regulatory t cells. J. Immunol. 2010;185:6698–6705. doi: 10.4049/jimmunol.1001373. [DOI] [PubMed] [Google Scholar]
- 105.Chae H.S., Kang O.H., Choi J.G., Oh Y.C., Lee Y.S., Brice O.O., Chong M.S., Lee K.N., Shin D.W., Kwon D.Y. Methyl gallate inhibits the production of interleukin-6 and nitric oxide via down-regulation of extracellular-signal regulated protein kinase in raw 264.7 cells. Am. J. Chin. Med. 2010;38:973–983. doi: 10.1142/S0192415X10008391. [DOI] [PubMed] [Google Scholar]
- 106.Choi J.G., Kang O.H., Lee Y.S., Oh Y.C., Chae H.S., Jang H.J., Kim J.H., Sohn D.H., Shin D.W., Park H., et al. In vitro activity of methyl gallate isolated from galla rhois alone and in combination with ciprofloxacin against clinical isolates of salmonella. J. Microbiol. Biotechnol. 2008;18:1848–1852. doi: 10.4014/jmb.0800.025. [DOI] [PubMed] [Google Scholar]
- 107.Quevedo L., Aguayo L., Concha J., Cid H., Saez J.C. Electrophysiological effects of methyl 3-o-methyl gallate on single muscle fibres. Pharmacology. 1981;23:293–296. doi: 10.1159/000137563. [DOI] [PubMed] [Google Scholar]
- 108.Zhang Q., Cai L., Zhong G., Luo W. Simultaneous determination of jatrorrhizine, palmatine, berberine, and obacunone in phellodendri amurensis cortex by rp-hplc. Zhongguo Zhong Yao Za Zhi. 2010;35:2061–2064. [PubMed] [Google Scholar]
- 109.Tong X.G., Qiu B., Luo G.F., Zhang X.F., Cheng Y.X. Alkaloids and sesquiterpenoids from acorus tatarinowii. J. Asian Nat. Prod. Res. 2010;12:438–442. doi: 10.1080/10286020.2010.490522. [DOI] [PubMed] [Google Scholar]
- 110.Bastos G.N., Silveira A.J., Salgado C.G., Picanco-Diniz D.L., do Nascimento J.L. Physalis angulata extract exerts anti-inflammatory effects in rats by inhibiting different pathways. J. Ethnopharmacol. 2008;118:246–251. doi: 10.1016/j.jep.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 111.Sa M.S., de Menezes M.N., Krettli A.U., Ribeiro I.M., Tomassini T.C., Ribeiro dos Santos R., de Azevedo W.F., Jr., Soares M.B. Antimalarial activity of physalins b, d, f, and g. J. Nat. Prod. 2011;74:2269–2272. doi: 10.1021/np200260f. [DOI] [PubMed] [Google Scholar]
- 112.Yang Y.J., Yi L., Wang Q., Xie B.B., Dong Y., Sha C.W. Anti-inflammatory effects of physalin e from physalis angulata on lipopolysaccharide-stimulated raw 264.7 cells through inhibition of nf-kappab pathway. Immunopharmacol. Immunotoxicol. 2017;39:74–79. doi: 10.1080/08923973.2017.1282514. [DOI] [PubMed] [Google Scholar]
- 113.Bleyer W.A. The clinical pharmacology of methotrexate: New applications of an old drug. Cancer. 1978;41:36–51. doi: 10.1002/1097-0142(197801)41:1<36::AID-CNCR2820410108>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 114.Kremer J.M., Lee J.K. The safety and efficacy of the use of methotrexate in long-term therapy for rheumatoid arthritis. Arthritis Rheum. 1986;29:822–831. doi: 10.1002/art.1780290702. [DOI] [PubMed] [Google Scholar]
- 115.Purohit M., Pande D., Datta A., Srivastava P.S. Enhanced xanthotoxin content in regenerating cultures of ammi majus and micropropagation. Planta Med. 1995;61:481–482. doi: 10.1055/s-2006-958144. [DOI] [PubMed] [Google Scholar]
- 116.Sumiyoshi M., Sakanaka M., Taniguchi M., Baba K., Kimura Y. Anti-tumor effects of various furocoumarins isolated from the roots, seeds and fruits of angelica and cnidium species under ultraviolet a irradiation. J. Nat. Med. 2014;68:83–94. doi: 10.1007/s11418-013-0774-z. [DOI] [PubMed] [Google Scholar]
- 117.Ng T.B., Liu F., Wang Z.T. Antioxidative activity of natural products from plants. Life Sci. 2000;66:709–723. doi: 10.1016/S0024-3205(99)00642-6. [DOI] [PubMed] [Google Scholar]
- 118.Zhang X.Q., Zheng M., Mou K.H., Feng J. Effects of 8-methoxypsoralen on intracellular [ca(2+)]i and cytoskeleton actin organization in human melanocytes in vitro. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2009;38:348–351. [PubMed] [Google Scholar]
- 119.Zhao X.X., Peng C., Zhang H., Qin L.P. Sinomenium acutum: A review of chemistry, pharmacology, pharmacokinetics, and clinical use. Pharm. Biol. 2012;50:1053–1061. doi: 10.3109/13880209.2012.656847. [DOI] [PubMed] [Google Scholar]
- 120.Rendina E., Lim Y.F., Marlow D., Wang Y., Clarke S.L., Kuvibidila S., Lucas E.A., Smith B.J. Dietary supplementation with dried plum prevents ovariectomy-induced bone loss while modulating the immune response in c57bl/6j mice. J. Nutr. Biochem. 2012;23:60–68. doi: 10.1016/j.jnutbio.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 121.Smith B.J., Bu S.Y., Wang Y., Rendina E., Lim Y.F., Marlow D., Clarke S.L., Cullen D.M., Lucas E.A. A comparative study of the bone metabolic response to dried plum supplementation and pth treatment in adult, osteopenic ovariectomized rat. Bone. 2014;58:151–159. doi: 10.1016/j.bone.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 122.Linseman D.A., Bartley C.M., Le S.S., Laessig T.A., Bouchard R.J., Meintzer M.K., Li M., Heidenreich K.A. Inactivation of the myocyte enhancer factor-2 repressor histone deacetylase-5 by endogenous ca(2+) //calmodulin-dependent kinase ii promotes depolarization-mediated cerebellar granule neuron survival. J. Biol. Chem. 2003;278:41472–41481. doi: 10.1074/jbc.M307245200. [DOI] [PubMed] [Google Scholar]
- 123.Kong Y., Fu Y.-J., Zu Y.-G., Chang F.-R., Chen Y.-H., Liu X.-L., Stelten J., Schiebel H.-M. Cajanuslactone, a new coumarin with anti-bacterial activity from pigeon pea [Cajanus cajan (l.) millsp.] leaves. Food Chem. 2010;121:1150–1155. doi: 10.1016/j.foodchem.2010.01.062. [DOI] [Google Scholar]
- 124.Yim H.S., Kang S.O., Hah Y.C., Chock P.B., Yim M.B. Free radicals generated during the glycation reaction of amino acids by methylglyoxal. A model study of protein-cross-linked free radicals. J. Biol. Chem. 1995;270:28228–28233. doi: 10.1074/jbc.270.47.28228. [DOI] [PubMed] [Google Scholar]
- 125.Scheijen J.L., Schalkwijk C.G. Quantification of glyoxal, methylglyoxal and 3-deoxyglucosone in blood and plasma by ultra performance liquid chromatography tandem mass spectrometry: Evaluation of blood specimen. Clin. Chem. Lab. Med. 2014;52:85–91. doi: 10.1515/cclm-2012-0878. [DOI] [PubMed] [Google Scholar]
- 126.Mori K., Kitazawa R., Kondo T., Mori M., Hamada Y., Nishida M., Minami Y., Haraguchi R., Takahashi Y., Kitazawa S. Diabetic osteopenia by decreased beta-catenin signaling is partly induced by epigenetic derepression of sfrp-4 gene. PLoS ONE. 2014;9:e102797. doi: 10.1371/journal.pone.0102797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Stolk J., Hiltermann T.J., Dijkman J.H., Verhoeven A.J. Characteristics of the inhibition of nadph oxidase activation in neutrophils by apocynin, a methoxy-substituted catechol. Am. J. Respir. Cell Mol. Biol. 1994;11:95–102. doi: 10.1165/ajrcmb.11.1.8018341. [DOI] [PubMed] [Google Scholar]
- 128.Hou Z., Zhang Z., Wu H. Effect of sanguis draxonis (a chinese traditional herb) on the formation of insulin resistance in rats. Diabetes Res. Clin. Pract. 2005;68:3–11. doi: 10.1016/j.diabres.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 129.Fliegel L., Burns K., MacLennan D.H., Reithmeier R.A., Michalak M. Molecular cloning of the high affinity calcium-binding protein (calreticulin) of skeletal muscle sarcoplasmic reticulum. J. Biol. Chem. 1989;264:21522–21528. [PubMed] [Google Scholar]
- 130.Krause K.H., Michalak M. Calreticulin. Cell. 1997;88:439–443. doi: 10.1016/S0092-8674(00)81884-X. [DOI] [PubMed] [Google Scholar]
- 131.Park B.J., Lee D.G., Yu J.R., Jung S.K., Choi K., Lee J., Lee J., Kim Y.S., Lee J.I., Kwon J.Y., et al. Calreticulin, a calcium-binding molecular chaperone, is required for stress response and fertility in Caenorhabditis elegans. Mol. Biol. Cell. 2001;12:2835–2845. doi: 10.1091/mbc.12.9.2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Semwal R.B., Semwal D.K., Combrinck S., Viljoen A.M. Gingerols and shogaols: Important nutraceutical principles from ginger. Phytochemistry. 2015;117:554–568. doi: 10.1016/j.phytochem.2015.07.012. [DOI] [PubMed] [Google Scholar]
- 133.Hamley I.W. The amyloid beta peptide: A chemist’s perspective. Role in alzheimer’s and fibrillization. Chem. Rev. 2012;112:5147–5192. doi: 10.1021/cr3000994. [DOI] [PubMed] [Google Scholar]
- 134.Li S., Liu B., Zhang L., Rong L. Amyloid beta peptide is elevated in osteoporotic bone tissues and enhances osteoclast function. Bone. 2014;61:164–175. doi: 10.1016/j.bone.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 135.Zhou Z., Immel D., Xi C.X., Bierhaus A., Feng X., Mei L., Nawroth P., Stern D.M., Xiong W.C. Regulation of osteoclast function and bone mass by rage. J. Exp. Med. 2006;203:1067–1080. doi: 10.1084/jem.20051947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Cui S., Xiong F., Hong Y., Jung J.U., Li X.S., Liu J.Z., Yan R., Mei L., Feng X., Xiong W.C. Appswe/abeta regulation of osteoclast activation and rage expression in an age-dependent manner. J. Bone Min. Res. 2011;26:1084–1098. doi: 10.1002/jbmr.299. [DOI] [PMC free article] [PubMed] [Google Scholar]