Abstract
Calmodulin is a ubiquitous signalling protein that controls many biological processes due to its capacity to interact and/or regulate a large number of cellular proteins and pathways, mostly in a Ca2+-dependent manner. This complex interactome of calmodulin can have pleiotropic molecular consequences, which over the years has made it often difficult to clearly define the contribution of calmodulin in the signal output of specific pathways and overall biological response. Most relevant for this review, the ability of calmodulin to influence the spatiotemporal signalling of several small GTPases, in particular KRas and Rac1, can modulate fundamental biological outcomes such as proliferation and migration. First, direct interaction of calmodulin with these GTPases can alter their subcellular localization and activation state, induce post-translational modifications as well as their ability to interact with effectors. Second, through interaction with a set of calmodulin binding proteins (CaMBPs), calmodulin can control the capacity of several guanine nucleotide exchange factors (GEFs) to promote the switch of inactive KRas and Rac1 to an active conformation. Moreover, Rac1 is also an effector of KRas and both proteins are interconnected as highlighted by the requirement for Rac1 activation in KRas-driven tumourigenesis. In this review, we attempt to summarize the multiple layers how calmodulin can regulate KRas and Rac1 GTPases in a variety of cellular events, with biological consequences and potential for therapeutic opportunities in disease settings, such as cancer.
Keywords: Calmodulin, KRas, Rac1, calmodulin-binding proteins, signalling
1. Introduction
Calmodulin is a ubiquitously expressed small protein (148 amino acids) and considered the most important Ca2+ sensor in non-muscular cells [1,2]. This Ca2+-sensing function allows calmodulin to act as a signalling molecule, translating transient fluctuations of Ca2+ levels inside cells into rapid and appropriate alterations of a vast number of cellular activities. Calmodulin-mediated cellular responses commonly occur through its interaction with a plethora of effectors named calmodulin-binding proteins (CaMBPs), most of them in a Ca2+-dependent manner [1,3,4]. Multiple calmodulin interaction motifs within CaMBPs exist, comprising basic amphiphilic helices, a cluster of polybasic amino acids, attached prenyl groups, all of which are found in small GTPases (see below), as well as the widely distributed ilimaquinone (IQ) motif [3,5,6,7,8]. Up to date, more than 450 calmodulin interacting proteins in human and mice have been described (BioGRID: https//thebiogrid.og; [9]), suggesting multiple cellular pathways that are triggered or regulated by calmodulin. This large and diverse group of CaMBPs includes enzymes such as kinases and phosphatases, ion channels, and cell surface receptors. Most relevant for this review, calmodulin also binds to members of the Ras superfamily of small GTPases that control cellular processes like endo- and exocytosis, protein and vesicle transport, cytoskeleton organization and dynamics, proliferation, and migration [10,11]. Ras GTPases are divided into five large subfamilies: Ras, Rho, Ran, Arf, and Rab [12]. A substantial number of these small GTPases are calmodulin-binding proteins, pointing at their central role to communicate changes in the local microenvironment and facilitate a rapid cellular response upon transient Ca2+ elevation.
In the following section, we will give a brief overview on calmodulin-binding GTPases (Table 1). Calmodulin acts on these GTPases either via direct binding or indirectly through interaction with CaMBPs that represent regulators of GTPases. Consequently, these interactions control the spatiotemporal localization, activity, and biological outcome of GTPase signalling. Small GTPases are molecular switches that are inactive when bound to GDP and active when bound to GTP. This cycle of GTPase activation and inactivation is controlled by guanine nucleotide exchange factors (GEFs) and GTPase activating proteins (GAPs), respectively. GEF proteins stimulate GDP dissociation, enabling the GTPase to incorporate GTP, which is 10-fold more concentrated than GDP in the cytosol. This turns the GTPase into an active state, capable of interacting with effectors to activate pathways that determine multiple aspects of cellular behavior. GTPase signalling is then terminated by GAP proteins, which enhance GTP hydrolysis through the activation of their intrinsic GTPase activity [13,14,15]. Calmodulin can positively or negatively modulate a variety of GEFs and GAPs, leading to GTPase activation or inhibition depending on the subset of GTPases analyzed. These multiple interactions of calmodulin with GTPases, GEFs and GAPs have made it difficult to dissect and then define calmodulin functions in regard to specific signalling pathways or biological outcomes. However, the use of more sophisticated in vitro protein–protein interaction techniques, pharmacological inhibitors, including small molecules or inhibitory peptides, and state-of-the-art imaging techniques, now provides a clearer picture of how calmodulin regulates different proteins of the Ras GTPase superfamily.
Table 1.
Small GTPases interacting with calmodulin. The various GTPases, their functions, the methodologies to assess their interaction with calmodulin, the cell lines analyzed, the calmodulin function as well as their interacting domains or protein sequences are listed. The relevant references are also given. Abbreviations: AC, affinity chromatography; BRET, bioluminescence resonance energy transfer; CBR, calmodulin binding region (basic amino acids are in red); CoIP, coimmunoprecipitation; CompMod, computational modelling; FS, fluorescence spectrometry; PXL, peptide cross-linking; RC, reconstituted complex; SF-TAP, Strep/Flag tandem affinity purification; YTH, yeast two hybrid.
| GTPase | Function | Interaction method | Species | Calmodulin (CaM) action | Refs |
|---|---|---|---|---|---|
| Cdc42 | Filopodia formation and cell-cycle progression | CoIP, AC, RC | Human (platelets) | Cdc42 inhibition CBR:151AVKYVECSALTQK164 (hypothetical amphipathic α-helix) |
[23] |
| AC | Human (MCF-7) | Regulation of Cdc42 signalling through IQGAP1 | [48] | ||
| Kir/Gem | Cell proliferation, Neurite extension and flattening (Gem); invasion and metastasis (Kir). Inhibition of Ca2+ channels (Kir/Gem) |
FS, RC | Synthetic peptides, in vitro | Kir/Gem inactivation (inhibits GTP binding), regulates intracellular localization CBR:264/265KARRFWGKIVAKNNKNMAFKLKSKS288/289 (hypothetical amphipathic α-helix) |
[31] |
| Xenopus oocytes | Inhibition of high voltage-activated calcium channels and regulation of intracellular distribution | [63] | |||
| Monkey (COS-1) | Regulate intracellular localization between cytoplasm and nucleus, in a complex with 14-3-3 | [61] | |||
| CoIP, RC | Monkey (COS-1) | Inhibits association with importin α5 and nuclear translocation | [66] | ||
| KRas-4B | Cell proliferation, differentiation, survival and apoptosis | CoIP, AC, RC | Mouse (NIH3T3) | KRas inactivation | [19] |
| RC, YTH. | Human (platelets) | Down-regulation of KRas signalling and KRas membrane transport | [20] | ||
| AC | Mouse (NIH3T3) | CBR:151GVDDAFYTLVREIRKH166 (amphipathic α-helix5) CBR: C-terminal prenyl group |
[16] | ||
| RC | CBR:170KMSKDGKKKKKSKTKC185+ prenyl CBR:180KSKTKC185+prenyl group |
[67,68] | |||
| AC | Mouse (NIH3T3) | Inhibits KRas Ser181 phosphorylation | [29] | ||
| Rab3A | Synaptic and axonal transport. Regulated exocytosis in neuronal and endocrine cells |
PXL | Rat brain synaptosomes |
Dissociation of Rab3A from synaptic membranes CBR:62KTIYRNDKRIKLQIWDTA GQERYR85 |
[36,69] |
| Rat (PC12) | Inhibition of exocytosis | [30] | |||
| CoIP, PXL | Rat (pancreatic islets) | Inhibits insulin secretion | [42] | ||
| Rat brain synaptosomes | Promotes GTP binding | [70] | |||
| Human (spermatozoa from sperm donors) | Acrosomal exocytosis | [71] | |||
| Rab3B | Calcium-dependent exocytosis | AC | Mouse brain | [10] | |
| RC | Human (platelets) | Ca2+-dependent secretion in platelets | [33] | ||
| Rab3D | Maintenance of osteoclastic border membrane | AC | Mouse brain | [10] | |
| YTH, BRET, RC | Monkey (COS-1, reconstituted complex) | Promotes osteoclastic bone resorption | [46] | ||
| Rabs: 6B, 8B,10, 15, 33B, 37. | AC | Mouse brain | [10] | ||
| Rac1 | Cytoskeletal organization, migration, adhesion, proliferation, endocytosis, vesicular trafficking | CoIP, AC, RC | Human (platelets) | Rac1 activation CBR:151AVKYLECSALTQRG164 (hypothetical amphipathic α-helix) |
[23] |
| AC, RC, CompMod | Human (HeLa) | Rac1 activation | [21] | ||
| AC | Monkey (COS-1) | Rac1 activation Impairs binding to PIP5K CBR:171EAIRAVLCPPPVKKRKRK188 and the C-terminal prenyl group |
[22] | ||
| SF-TAP | Human (HEK293T) | [72] | |||
| Rad | Skeletal muscle motor function, cytoskeletal organization and glucose transport | RC | GST-Rad incubated with CaM-sepharose in vitro | Regulates intracellular localization CBR:278AKRFLGRIVARNSRKMA FRA297 (hypothetical amphipathic α-helix) |
[31] |
| [65] | |||||
| RalA | Cell proliferation, migration, filopodia formation, differentiation, cytoskeletal organization, vesicular transport, exocytosis and receptor endocytosis. Synaptic and axonal transport |
AC, RC, YTH | Human (platelets) | RalA activation | [38] |
| RC | Human (HeLa) | RalA activation CBR: C-terminal prenyl group |
[39] | ||
| Rat brain synaptosomes | Dissociation of RalA from synaptic membranes | [69] | |||
| Purified RalA, in vitro | Regulates GTP binding CBR:183SKEKNGKKKRKSLAKRIR200 (hypothetical amphipathic α-helix) |
[34,40] | |||
| Rat brain synaptosomes | Regulates GTP binding | [73] | |||
| RalB | Cell proliferation, oncogenic transformation | CoIP, AC, RC, YTH | Human (platelets) | RalB activation CBR: C-terminal prenyl group |
[38,39] |
| Rat brain synaptosomes | Regulates GTP binding | [73] | |||
| Rem | Ca2+channel regulation | CoIP, AC | Human (HEK293, TSA20) | No Ca2+ channel inhibition CBR:165QRARRFLARLTARSARRR282 |
[74] |
| Ric | Dot-blot, AC, CoIP | Drosophila | Regulation of Ca2+-mediated neuronal signal transduction CBR:242RRSRWWRIRSIFALVFRR RR261 |
[35] | |
| Drosophila (cross of Ric mutants with CaM mutants) | Ric inhibition | [56] | |||
| Rin | Ca2+ signalling in neurons | Dot-blot | Mouse |
Regulation of calcium-mediated neuronal signal transduction CBR:194RKLKRKDSLWKKIKASLKKKRENML218 |
[32] |
| CoIP | Monkey (COS-7) | Regulation of Rin activation | [54] | ||
| Rat (PC12) | Induce neurite outgrowth | [55] |
The present review endeavors to compile the current understanding, obtained over more than two decades, summarizing the role of calmodulin in the regulation of small GTPases. We will specifically focus on K-Ras4B (thereafter KRas) and Rac1, which both bind calmodulin in a Ca2+-dependent manner [16,17,18,19,20,21,22,23]. Adding complexity, calmodulin regulates signalling outputs from these GTPases indirectly by binding and influencing the localization and activity of other CaMBPs that act as GTPase regulators or effectors. Calmodulin-dependent GTPase signalling is coupled to pathological settings, as KRas and Rac1 are often drivers of signalling pathways that critically stimulate tumour formation and progression [24,25,26]. The latter has been recently reviewed in more detail in a previous issue of this journal [27,28].
2. Interaction of Calmodulin with GTPases
Up to date, several GTPases have been identified as Ca2+-dependent CaMBPs, including KRas, RalA, RalB, Rin (RIC in Drosophila), Kir/Gem, Rab3A, Rab3B, Cdc42, and Rac1 [16,22,23,29,30,31,32,33,34,35,36] (Table 1). Most of the abovementioned GTPases interact with Ca2+/calmodulin through a C-terminal polybasic region (PBR) and a post-translationally incorporated prenyl group within this region that provides the lipid anchor to direct those GTPases to cell membranes.
2.1. RalA and RalB
RalA and RalB isoforms are both Ras effectors [37] that have a second motif located in the N-terminal domain that interacts with calmodulin [34,38,39]. The interaction of calmodulin with these two Ral GTPases appears to be independent of the Ral-GTP or -GDP status, yet necessary for Ral activation by thrombin in human platelets [38]. Although the biological outcome and significance of these interactions is not completely understood, calmodulin binding to RalA increases its GTP loading and blocks its phosphorylation by protein kinase A (PKA), protein kinase G (PKG) and protein kinase C (PKC) [40,41]. Hence, a role for calmodulin as a GEF for RalA and Rac1 GTPases has been hypothesized [21,40]. Along these lines, calmodulin can also inhibit PKC-mediated serine 181 (Ser181) phosphorylation of KRas, which allows the modulation of KRas function and will be discussed in more detail below (Section 3) [16].
2.2. Rab3A and Rab3D
Rab3A is found on secretory vesicles of neuronal and endocrine cells that regulate neurotransmitter and hormone release [42,43,44,45]. Overexpression of a constitutively active Rab3A mutant (Q81L) inhibits Ca2+-induced exocytosis in pancreatic β-cells. In these cells, calmodulin preferentially binds GTP-bound Rab3A in a Ca2+-dependent manner, and calmodulin dissociation from Rab3A can be triggered by glucose-induced insulin secretion [42]. Accordingly, calmodulin/Rab3A interaction may ensure proper physiological response and prevent secretory events in pancreatic β-cells linked to insulin release in the absence of elevated plasma glucose levels [30]. Upon enhanced glucose levels and the subsequent rise in Ca2+ levels in pancreatic β-cells, Ca2+/calmodulin may dissociate from Rab3A. This could be due to competing CaMBPs with a higher calmodulin affinity, for example Ca2+/calmodulin activated protein kinase II (CaMKII). The preference for calmodulin to bind CaMKII during granule secretion could be the underlying cause for increased insulin release [42]. Additionally, the Rab3D isoform involved in exocytosis in mast cells, osteoclasts and pancreatic β-cells has recently been identified as a Ca2+-dependent CaMBP. Inhibiting calmodulin/Rab3D interaction with calmidazolium chloride diminished osteoclastic bone resorption [46].
2.3. Rac1 and Cdc42
Rac1 and Cdc42 also represent GTPases of the Ras superfamily that bind calmodulin [22,23] (Table 1). Similar to the calmodulin/KRas interaction modules [16], several domains facilitate these protein interactions. This includes a region located between amino acids 151–164 that can adopt an alpha helix conformation, as well as the polybasic domain together with the adjacent prenyl group at the C-terminus. Rac1/calmodulin interaction and its regulatory circuits and downstream effectors will be explained in more detail in Section 4. Inhibition of calmodulin has opposite consequences for Rac1 and Cdc42 activity, leading to decreased Rac1-GTP, but elevated Cdc42-GTP levels [23]. Calmodulin can control Cdc42 activity and Cdc42-mediated regulation of the actin cytoskeleton through binding of the IQ Motif Containing GTPase Activating Protein 1 (IQGAP1), a well-known Cdc42 regulator [47]. IQGAP1 binds Cdc42 and keeps the GTPase bound to GTP (active), which concomitantly enhances F-actin crosslinking activity of IQGAP1 [48,49]. Ca2+/calmodulin, through interaction with IQGAP1, dissociates IQGAP1 from Cdc42 and negatively regulates this GTPase (see section 3.2.2 for further details) [50,51].
IQGAP also binds the ubiquitous GTPase Rap1, which contributes to cell adhesion, synaptic plasticity, and mitogen-activated protein kinase (MAPK) pathway activation. Although Rap1 has yet to be identified as a CaMBP, IQGAP1 binding to Rap1, in contrast to other GTPases interacting with IQGAP1, attenuates its activation triggered by cAMP, fibronectin, or collagen I. Therefore, calmodulin may trigger dissociation of IQGAP1 from Rap1, possibly due to calmodulin and Rap1 competing for IQGAP1 binding, leading to increased Rap1 activity [52]. Accordingly, pharmacological inhibition of calmodulin blocks Rap1 (and Ras) activation after depolarization in cortical neurons. Consequently, activation of the MAPK pathway downstream of Ras and Rap1 is inhibited, making calmodulin a crucial and common regulator of signalling events driven by these two GTPases in neurons [53].
2.4. Ric and Rin
There are members of the Ras superfamily that lack the prenylation motif (also termed CAAX box), which commonly enables membrane association of most GTPases, as is the case for the RGK and Rit subfamilies [32,35,54] (Table 1). These two GTPase subfamilies rely on long stretches of basic amino acids for plasma membrane localization by electrostatic interaction with acidic membrane phospholipids. Rin is a GTPase of the Rit subfamily that is expressed in neurons and necessary for neurite growth [55]. This protein has a polybasic cluster and several hydrophobic residues within a C-terminal 25 amino acid region, allowing interaction with calmodulin in a Ca2+-dependent manner [32,54]. Although the molecular consequences of this interaction are still unclear, blocking calmodulin inhibits the ability of Rin to promote neurite growth [55] and attenuates its activation by growth factor receptors [54]. RIC, the ortholog of Rin in Drosophila, also interacts with calmodulin through a sequence located at position 242–261 [35]. The expression of an active RIC mutant altered the development of adult wing vein structures. This phenotype was intensified when calmodulin levels were reduced. Thus, interaction of calmodulin with RIC seems to negatively regulate this GTPase [56].
2.5. Rad, Kir/Gem, and Rem
Within the RGK GTPase subfamily, Rad, Kir/Gem, and Rem are engaged in the cell surface delivery and inhibition of Ca2+-channels, as well as the control of cell morphology and actin cytoskeleton remodeling by interfering with the Rho/Rho-kinase pathway [57,58,59] (Table 1). Calmodulin interacts with these GTPases in a Ca2+-dependent manner through their C-terminal domains. These C-termini are extended by 31 amino acids compared to Ras, containing polybasic and hydrophobic residues that are typical hallmarks for calmodulin binding [31,60]. Complex formation of calmodulin with Kir and Gem GTPases significantly inhibits their GTP binding [31]. Likewise, calmodulin associates preferentially with Rad-GDP [60]. In this GTPase, the C-terminal region is required for plasma membrane location [58]. Calmodulin, in a cooperative and competitive fashion together with 14-3-3 proteins, regulates the subcellular location of Kir/Gem and Rem2 [61,62]. Mutant versions of these GTPases that are defective in calmodulin binding have been preferentially found inside the nucleus. This change of location may explain the inhibition of voltage-dependent Ca2+-channels and determine cell-shape remodelling [61,63,64]. Beguin et al. demonstrated that binding of Ca2+/calmodulin to Kir/Gem maintains their cytoplasmic localization, which is necessary for their inhibitory effect on surface transport of Ca2+ channels. In such cytoplasmic locations, activated Kir/Gem (GTP-bound), by interacting with beta-subunits of the channel, impairs the physical association with alpha1-subunits and its traffic to the plasma membrane to function as a Ca2+ channel [63]. In addition, calmodulin prevents importins to bind nuclear localization signals within the C-terminal region of these GTPases and interferes with their nuclear translocation [64]. Finally, putative serine-phosphorylated residues neighboring nuclear translocation signals are consensus sites for PKC, PKA and protein kinase B (Akt) kinases. This could affect importin and possibly calmodulin binding to Kir/Gem and cytoplasmic and nuclear shuttling. Interestingly, phosphorylation of the Rad member of the RGK subfamily by PKC or casein kinase II abolishes the interaction of Rad with calmodulin [65].
3. Ras-GTPases and their Post-Translational Modifications Fine-Tune Cellular Signalling
The family of Ras GTPases includes three members: Harvey Ras (HRas), Neuroblastoma Ras (NRas), and Kirsten Ras (KRas), which has two splice variants, KRas-4A and the more prominent KRas-4B isoform (named here KRas). Ras proteins have the vital task to decode a variety of extracellular stimuli. At the cell surface, these stimuli are commonly derived from growth factor-activated tyrosine kinase receptors, surface receptors that participate in cell-extracellular matrix communications or receptors that modulate Ca2+-channels. Ras GTPases are ubiquitously expressed and regulate fundamental cellular endeavors like proliferation, differentiation, survival, apoptosis and cell mobility [75,76,77,78,79] that must be tightly regulated in a timely manner. Hence, constitutive or hyperactive Ras activity due to specific point mutations or overexpression is often associated with cancer initiation and progression or developmental defects.
3.1. The Prominant Role of KRas in Human Cancers
Ras proteins are mutated in 30% of human cancers, with 98% of these mutations being missense mutations in one of three hotspot residues at position G12, G13, and Q61. Amino acid substitutions at these positions prevent efficient Ras-GTP hydrolysis, maintaining Ras proteins in a constitutively active GTP-bound state. Mutations within the Ras family are not evently distributed, with KRas mutations found in 85% of these cancers, followed by NRas (11%) and HRas (4%) [76,80,81,82,83,84].
This indicates prominant roles for KRas mutations in cancer, which are most frequent in pancreatic (<60%), colon (<30%), and lung (<15%) cancers. NRas mutations are prominant in skin (<25%) and hematological (<15%) cancers [82] and HRas mutations often exist in head and neck cancers. This distribution of oncogenic Ras mutations is not well understood and may reflect tissue-specific expression patterns or different potentials to activate shared downstream pathways such as the MAPK and phosphoinositide 3-kinase (PI3K) signalling cascades. Based on the very high (~90%) homology among all Ras isoforms, these findings were initially difficult to interpret. However, over the last two decades, the hypervariable region (HVR), which consists of 24–25 residues at the C-terminus of Ras proteins and shares less than 20% homology within Ras isoforms, is considered to be most relevant. Whereas the N-terminal region (G domain, residues 1–165) is almost identical in Ras isoforms and contains the GTP/GDP-binding site (switch I and II domains), the HVR region is responsible for the anchoring of Ras proteins at the plasma membrane. Biochemical studies and high resolution imaging revealed that the diversity of HVR regions enabled the targeting of Ras isoforms to different microdomains at the plasma membrane. This differential Ras partitioning and nanoclustering within the plasma membrane is now well believed to strongly influence the strength and duration of signals generated, with consequences for signal transmission and output [85,86,87,88,89,90]. Altogether these studies identify HVRs of each Ras isoform to contain unique functions in normal physiological processes as well as in pathogenesis [91,92,93,94].
3.2. The Membrane Association of Ras Proteins
Many research efforts have aimed to dissect the molecular events that ensure HVR-mediated membrane association of Ras proteins and the various steps in Ras membrane insertion are now quite well understood. After Ras synthesis in the cytosol as a hydrophilic protein, several posttranslational modifications at the HVR domain provide the acquisition of signals for membrane insertion and the control for Ras isoform localization. In the cytosol, Ras proteins are firstly farnesylated at a cysteine residue in the C-terminal CAAX motif (C, cysteine; A, aliphatic residue; X, any residue) by a cytosolic farnesyl transferase. This acquisition of a farnesyl group (15-carbon isoprenyl) allows Ras insertion into the endoplasmic reticulum (ER) membrane, where AAX is subsequently subjected to proteolysis and the remaining cysteine is methylated by Ras-converting enzyme 1 (Rce1) and Isoprenylcysteine carboxyl methyltransferase (Icmt), respectively [95]. HRas, NRas, but also the less well-studied KRas-4A isoform, are further modified by palmitoylation, which allows their transport through the Golgi apparatus and subsequent exocytocic vesicular transport routes to isoform-specific plasma membrane localizations. In contrast, KRas is not palmitoylated, but requires a different second signal for membrane localization adjacent to the farnesylated cysteine, that consists of a cluster of polybasic amino acids, the PBR domain. This PBR region exerts a strong electrostatic interaction with acidic phospholipids of the cytosolic membrane leaflet [96,97,98,99]. The molecular mechanism responsible for KRas transport from the ER to the plasma membrane are still not fully understood, and the function of a chaperone protein proposed to hide the farnesyl group after synthesis in the ER have yet to be clarified [93,94,96].
The variety of posttranslational modifications seem to provide the basis for the different microdomain localizations of Ras isoforms. KRas differs substantially from H- and NRas in regard to their trafficking through cellular compartments, in particular in their dynamic association with membranes of the endocytic compartment [100]. During membrane turnover and/or growth factor-induced internalization, all Ras isoforms at the plasma membrane are transported through early endosomes. However, only KRas trafficks through the entire endocytic pathway, including late endosomes/multivesicular bodies, on its path to lysosomes for degradation [101]. Internalized Ras proteins can remain active or get activated and then signal from endosomes, creating additional signal diversity. This aspect has been reviewed in detail previously by our group and others [93,94,95,102,103,104,105,106].
KRas is the only Ras isoform that binds calmodulin [16,17,18,19,20,68,107,108,109,110], confering KRas-specific functions and biological outcomes. The association of calmodulin with KRas and its functionality will be discussed in more detail in the next two sections.
3.3. Domains Responsible for the Interaction of Calmodulin with KRas
Pioneering work by Villalonga and coworkers demonstrated, using affinity chromatography, that calmodulin was able to bind KRas, but not H- or NRas isoforms, from lysates of NIH3T3 fibroblasts. This direct protein–protein interaction occurred in a Ca2+-dependent manner and only when KRas was GTP-bound (active) [19]. In this initial study, it was proposed that binding of calmodulin to KRas in vivo inhibited downstream activation of the Raf and MAPK signalling cascade. Given the high incidence of KRas mutations in human cancers (see above), several groups aimed to dissect the molecular elements required for KRas/calmodulin interaction. Utilizing a wide range of methodologies and techniques such as affinity chromatography, immunoprecipitation, confocal microscopy, fluorescence spectroscopy and isothermal titration calorimetry (ITC), yeast two-hybrid systems, nuclear magnetic resonance (NMR), surface plasmon resonance spectroscopy, artificial liposomes and nanodiscs biomembranes, as well as computational simulations, the KRas/calmodulin interaction was thoroughly established over the years in numerous in vitro and in vivo models and cell types [16,17,18,19,20,68,107,108,109,110,111].
Some discrepancies and variations regarding the requirement of the GTP- or GDP-bound form of KRas, as well as the KRas domains mandatory for the interaction with calmodulin, have been reported. However, it is now well believed that the C-terminal HVR domain of KRas is essential to bind Ca2+/calmodulin. Indeed, the distinctive features of the KRas HVR region, lacking a posttranslational C-terminal palmitoylation found in the other Ras isoforms, but having the polybasic PBR domain, makes KRas the sole Ras isoform capable to bind calmodulin. Vice versa, in H- and NRas, the absence of basic amino acids in the HVR domains and the presence of the palmitoyl group sterically disturbs an interaction with calmodulin. Depalmitoylated KRas-4A containing a polybasic HVR region smaller than KRas may also bind Ca2+/calmodulin [112].
Several studies demonstrated that both the PBR domain and the farnesyl group within the HVR domain of KRas are essential to bind Ca2+/calmodulin [111,113,114,115,116]. López-Alcalá and coworkers further showed that the α-helix between residues 151–166 and the Switch II region, both located upstream the C-terminal HVR domain, also participates in the association with Ca2+/calmodulin [16]. A computational model, with support from experimental data, also supported the catalytic domain of KRas interacting with the N-terminal domain of calmodulin and the PBR wrapping around the calmodulin linker domain. In this model, the KRas farnesyl group docked into the C-terminal pocket of calmodulin [108,110]. NMR and ITC analysis confirmed the PBR domain of KRas as the primary binding site for the calmodulin C-terminal and linker domains. In addition, the catalytic GTP-bound domain of KRas aids the interaction via binding to the N-terminal domain of Ca2+/calmodulin [17]. In this study, it was hypothesized that the farnesyl group of the HVR domain may further enhance the binding affinity of KRas for Ca2+/calmodulin, which indeed was later demonstrated by others [18,117]. Analyzing different fragments and mutants of KRas bound to nanodisc biomembranes mapped the last six C-terminal residues of KRas together with the farnesyl group (KSKTKC-prenyl) as the minimal Ca2+/calmodulin-binding motif [68]. The identification of KRas forming a 2:1 stoichiometric complex with calmodulin suggested that KRas could first interact with the C-terminal domain of calmodulin. This would lead to a conformational change that would then allow the binding of a second KRas protein to the N-terminal domain of calmodulin [68]. Together with findings from several other studies, this suggested that calmodulin could participate as a molecular carrier to transport KRas, independent of its GTP- or GDP-bound form, from the ER to the plasma membrane. Alternatively, this function of calmodulin could translocate KRas from the plasma membrane to intracellular membrane compartments in a Ca2+-dependent manner. It has yet to be determined to what extend an abilty of calmodulin to sequester the polybasic-prenyl motifs of KRas could contribute to the overall membrane-associated trafficking of the entire pool of KRas proteins in each cell. Besides vesicular KRas trafficking, cytosolic KRas transport mechanisms also exist [100,118,119]. This transport route requires shielding the hydrophobic farnesyl group attached to the KRas C-terminus from the hydrophilic cytosol. This could be facilitated by calmodulin, but also others, such as prenylated rab acceptor 1 and phosphodiesterase 6 δ [67,118,119,120]. Together with fluctuations of cellular GTP/GDP ratios, and the reduced affinity of calmodulin towards inactive KRas (KRas-GDP), it remains to be clarified how these different transport routes partition in the cellular movement of KRas proteins [20,67,68,83,109] (Figure 1).
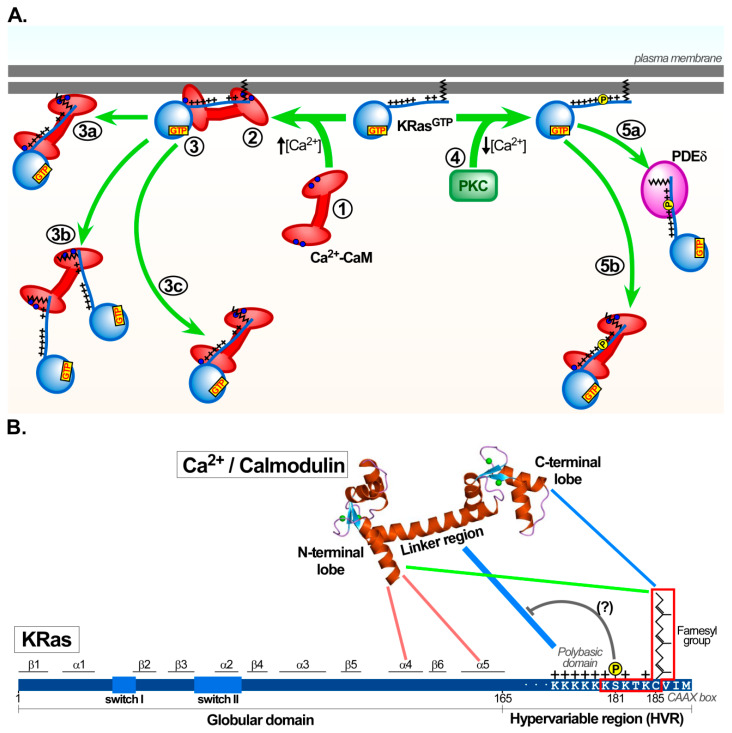
Figure 1.
Calmodulin/KRas interaction motifs and their role in KRas trafficking. The different possibilities how calmodulin may affect KRas trafficking (A) and the protein domains facilitating interaction of calmodulin and KRas are shown (B). (A) Role of calmodulin in KRas trafficking routes. (1) Upon elevation of intracellular [Ca2+] levels, Ca2+ binds and activates calmodulin (Ca2+-CaM), which preferentially interacts with active KRas (KRas-GTP) at the plasma membrane. (2) Initially, the C-terminal lobe and the linker region of Ca2+-CaM interact with the polybasic domain of KRas. The negatively charged Ca2+-CaM linker region is attracted to the polybasic KRas domain through electrostatic coupling. This may be followed by a conformational change in both proteins that permit a secondary interaction between the N-terminal lobe of Ca2+-CaM with the globular domain of KRas, in particular with helices α4 and α5. (3) The majority of Ca2+-CaM/KRas complexes remain associated with the plasma membrane. However, to some extent, the hydrophobic C-terminal lobe of Ca2+-CaM interacts and extracts the KRas farnesyl group from the lipid bilayer. (3a) KRas could remain at the plasma membrane through the interaction of Ca2+-CaM with the acidic membrane leaflet or through interaction with other proteins that are recruited to the plasma membrane, such as phosphoinositide 3-kinase (PI3K). (3b) Alternatively, after binding of the C-terminal lobe of Ca2+-CaM to KRas, followed by a conformational change in Ca2+-CaM, a second KRas protein may bind to the N-terminal lobe of Ca2+-CaM. This would result in a 2:1 Ca2+-CaM/KRas stoichiometry. These KRas proteins could then be removed from the plasma membrane after interaction with Ca2+-CaM. (3c) Ca2+-CaM/KRas complexes with 1:1 stoichiometry may also detach from the plasma membrane. (4) Reduced cytosolic Ca2+ levels lead to the dissociation of the CaM/KRas complex, enabling protein kinase C (PKC) to phosphorylate active KRas at the serine 181 residue (Ser181). (5a) PDEδ or (5b) under certain conditions Ca2+-CaM could cause KRas dissociation from the plasma membrane. The pool of KRas proteins trafficking along the various routes is likely to vary in different cell types and be influenced by changes in the microenvironment and physiological conditions. See text for further details (Section 3.3). (B) Regions involved in KRas and calmodulin interaction. The polybasic domain and the farnesyl group within the hypervariable region (HVR) of KRas are both essential to interact with the linker region and the C-terminal lobe of Ca2+/Calmodulin (blue lines), respectively. The KRas polybasic domain is the initial and primary binding region that interacts with Ca2+/calmodulin (thicker blue line). The minimal Ca2+/calmodulin binding motif of KRas, KSKTKC-farnesyl, is indicated (red square). As part of the KRas/calmodulin interaction, helices α4 and α5 of the globular domain of KRas could also participate interacting with the N-terminal lobe of calmodulin (stoichiometry 1:1; red lines). Alternatively, KRas may first interact with the C-terminal lobe of Ca2+/calmodulin. This could trigger a conformational change of calmodulin to allow interaction of a second KRas protein to the calmodulin N-terminal lobe (stoichiometry 2:1; green line). The position of α-helices, switch I and II within the globular domain (1–165 aas) and the HVR (166–188 aa) of KRas are indicated. The calmodulin N- and the C-terminal lobes, the linker region and the two bound Ca2+ ions in each lobe are also depicted.
3.3.1. Outcomes of KRas/calmodulin Interactions in Cell-Based Studies
In support of Ca2+/calmodulin triggering changes in KRas localization, Ca2+/calmodulin binds to KRas in platelets and MCF-7 cells and dissociates KRas from membranes [20]. In hippocampal neuronal cultures, glutamate activation of N-methyl-D-aspartate (NMDA) receptor induces an elevation of Ca2+ levels. This is associated with the translocation of active (KRas-GTP) and inactive KRas (KRas-GDP) from the plasma membrane to the Golgi apparatus and early endosomal membranes [67]. In both cases, KRas translocation is inhibited by the calmodulin inhibitor W7, strongly implicating Ca2+/calmodulin in this process [20,67]. In HeLa cells, W7 also reduces rapamycin-induced dissociation of the constitutively active KRas G12V mutant from the plasma membrane. In contrast, this W7-sensitive dissociation does not occur with the inactive KRas S17N mutant [118], which is consistent with the concept that calmodulin selectively recognizes and interacts with the activated form of KRas [19].
The binding of calmodulin to KRas may not always contribute to KRas translocation from the plasma membrane to endocytic compartments or participate in KRas transport from the ER to the plasma membrane [16]. In these studies, KRas mutants defective in calmodulin interaction, but with proper plasma membrane location as well as GTP-binding capacity, translocated to endosomal membranes or the Golgi apparatus after Ca2+ elevation in striatal neurons. Hence, in this setting, Ca2+-dependent KRas transport occured independent of calmodulin binding. This raised the possibility that the loss of KRas translocation observed by others [20,67], using the calmodulin inhibitor W7, may be related to an indirect effect of calmodulin on KRas trafficking [16]. Ca2+-insensitive factors, scaffold and carrier proteins influencing KRas localization also need to be considered. For example, in NIH3T3 cells, the binding of calmodulin and KRas in the presence of a Ca2+-ionophore was prominent at the plasma membrane, with only minor amounts of KRas/calmodulin complexes present in intracellular compartments. Along these lines, a KRas mutant defective in calmodulin binding was found predominantly at the plasma membrane [16]. This suggests that calmodulin may not always be essential for KRas transport from the ER to the plasma membrane, implicating other proteins to facilitate KRas transport independent of KRas/calmodulin complex formation. These observations raised the hypothesis that calmodulin could be a key element for selected KRas protein pools to generate discrete microdomains at the plasma membrane. This was indeed supported by Barceló and coworkers [121], providing an exciting model that would allow calmodulin to selectively regulate KRas signalling output.
3.3.2. Additional Insights on the Nucleotide Dependence of KRas/calmodulin Interaction
A notable issue that remains controversial within the field is the nucleotide dependence of the KRas/calmodulin interaction. The majority of earlier reports support Ca2+/calmodulin specifically and preferentially binding active KRas (KRas-GTP) [16,17,18,19,29,117]. The globular domain of KRas bound to GDP resembles an autoinhibited conformation, as this sequesters the HVR region [122], thereby preventing interaction of KRas with calmodulin. Yet, several more recent studies favor nucleotide-independent interaction of these two proteins, irrespective of the GDP- or GTP-bound state of KRas [20,67,68,83,109]. To explain these different experimental outcomes remains difficult, but one plausible scenario could be the loss of the autoinhibited KRas-GDP conformation. Conformational changes may lead to differential sensitivity towards the GTP/GDP ratio in the different models and experimental settings analyzed, possibly allowing the HVR domain to interact with Ca2+/calmodulin, even when GDP is bound to KRas. Nevertheless, further experiments are required to validate the preferential interaction of calmodulin with active KRas in live cells.
Despite these still unresolved matters and based on the currently available literature, the HVR domain of KRas is critical for the interaction with Ca2+/calmodulin. This interaction can prevent PKC-mediated Ser181 phosphorylation in the PBR domain of KRas. Vice versa, phosphorylation of KRas by PKC at this Ser181 residue blocks Ca2+/calmodulin to interact with KRas [16,29,107]. This interplay between PKC-mediated KRas phosphorylation and KRas/calmodulin interaction is an imperative mechanism to modulate KRas activity and downstream signalling [123] and will be discussed in the next section.
3.4. Calmodulin-Dependent KRas Activity—Diverse Outcomes for Cellular Signalling in Normal and Oncogenic Settings
Ras activation at the plasma membrane requires the recruitment of GEFs to promote guanine nucleotide exchange and subsequent association with effectors, in particular Raf1 and PI3K signalling cascades. The serine/threonine kinase Raf1 is vital for MAPK activation that is in command of cell proliferation and differentiation [75,124,125,126,127], while PI3K is upstream of Akt signalling cascades and the Rac1 GTPase, both dictating cell survival, proliferation, and mobility [128,129,130].
To examine this further, the small-molecule calmodulin inhibitors N-(4-aminobutyl)-5-chloro-2-naphthalenesulfonamide, also termed W13, its analog W7, and other related compounds have been utilized widely. This identified Ca2+/calmodulin to modulate Ras activity and downstream effectors by a complex interconnected network of signalling routes. Overall, Ca2+/calmodulin can act at multiple levels to influence Ras activity. This includes regulating upstream receptor tyrosine kinase activity at the cell surface, to controlling recruitment and activity of various GEF and GAP proteins or as discussed in Section 3.1, by a direct interaction with the KRas isoform (Figure 2).
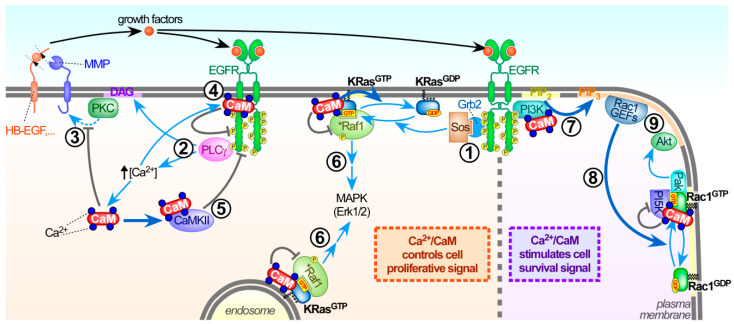
Figure 2.
Model of key signalling events modulated by Ca2+/calmodulin and decisive for cell proliferation and survival. In this scheme, key signalling events regulated by calmodulin (CaM) and critical for cell proliferation (left) and cell survival (right) are highlighted. Left: Several protein–protein interactions of calmodulin enable a negative feedback regulation of the mitogen-activated protein kinase (MAPK) extracellular signal-regulated kinase 1/2 (Erk1/2). This signalling cascade is initially activated by epidermal growth factor receptor (EGFR), and other receptor tyrosine kinases not shown here, at the cell surface and prolonged on endosomes. The overall outcome of these interactions prevents a strong and sustained signal that promotes cell proliferation. Right: Calmodulin can activate phosphoinositide-3 kinase (PI3K) and through protein kinase B (Akt) and Rac1, ensure the generation of signals that stimulate cell survival. Key interactions and signalling events are numbered and underscore the following: (1) Growth factor (e.g., EGF) ¡-induced EGFR activation and autophosphorylation enables the recruitment of adaptors (Grb2) and guanine nucleotide exchange factors (Sos). This is followed by activation of the Ras/Raf1/MAPK signalling pathway. (2) Simultaneously, phospholipase C γ (PLCγ) is recruited to activated EGFR to produce the second messenger diacylglycerol (DAG) and elevate cytosolic Ca2+ levels. (3) The latter binds and activates calmodulin, which disables DAG-dependent and protein kinase C (PKC)-mediated activation of matrix metalloproteases (MMPs). This prevents the shedding and release of membrane-bound growth factor precursors, such as heparin-binding EGF (HB-EGF). This regulatory circuit effectively prevents prolonged EGFR activation in an autocrine manner. (4) In addition, Ca2+/calmodulin binds EGFR and inhibits its tyrosine-kinase activity. (5) Moreover, Ca2+/calmodulin binds Ca2+/calmodulin-dependent kinase II (CaMKII), which has the ability to phosphorylate and inactivate EGFR. These multiple interactions suggest a negative feedback mechanism that allows Ca2+-induced calmodulin activation to trigger downregulation of EGFR signalling. (6) Furthermore, Ca2+/calmodulin may preferentially interact with active KRas (KRas-GTP), which is particularly relevant in settings where KRas is the most prominent Ras family member contributing to MAPK activation, such as in NIH3T3 fibroblasts. In these cells, KRas/calmodulin interaction blocks downstream Raf1 activation. This could possibly be accompanied by the recruitment of GTPase activating proteins (GAPs) that further reduce KRas-GTP levels, thereby advancing to downregulate MAPK signalling and proliferation in these cells. (7) On the other hand, Ca2+/calmodulin together with activated EGFR can stimulate PI3K activity. (8) This increases the recruitment and activity of Rac1 guanine nucleotide exchange factors (Rac1 GEFs) that elevate Rac1-GTP (active) levels. (9) Together with Akt activation, these signalling events are linked to an anti-apoptotic response that triggers cell survival. The GTP/GDP cyles of KRas and Rac1, as well as the membrane location of phosphatidyl-4,5-biphosphate (PIP2) and phosphatidylinositol-3,4,5-triphosphate (PIP3) are indicated. In summary, Ca2+/calmodulin modulates the MAPK pathway driving proliferation and on the other hand, stimulates PI3K activity to induce cell survival. The overall biological outcome probably depends on the signal diversity derived from the extracellular milieu as well as the cell-specific and differential repertoire of calmodulin-responsive players in each cell.
3.4.1. Calmodulin Regulates Several Guanine Nucleotide Exchange Factors (GEFs) and GTPase Activating Proteins (GAPs) in Neuronal Cells
With regard to the impact of calmodulin on GEF and GAP proteins, a lot of current knowledge is based on studies from cells derived from the central nervous system. Adult neurons express substantial amounts of RasGRF, a dual GEF for Ras and Rac1. The Ras GTPase-activating protein SynGAP is highly enriched in postsynaptic density fractions. Both proteins are involved in Ca2+/calmodulin-dependent activation of the Ras/MAPK pathway [131,132,133,134,135,136]. RasGRFs (RasGRF1 and RasGRF2) are activated by Ca2+/calmodulin binding to their N-terminal IQ motif and together with RasGRF phosphorylation, glutamate-induced Ca2+ increase can trigger MAPK activation [132,134,135,136,137]. In contrast, CaMKII-mediated phosphorylation of SynGAP increases its GAP activity thereby inhibits Ras and downstream effectors [133,138,139]. Calmodulin can activate both RasGRF1 and RasGRF2 in the hippocampus, although both GRFs mediate opposite forms of synaptic plasticity. RasGRF2 is believed to mediate the ability of subpopulations of the heterodimeric NMDA receptors to promote long-term potentiation. On the other hand, CaMKII-mediated phosphorylation and activation of SynGAP is associated with other pools of NMDA receptors that allow switching the preference of RasGRF1 from Ras to Rac1 signalling, which thereby promotes long-term depression [137].
3.4.2. Calmodulin Regulates Mitogen-Activated Protein Kinase (MAPK) and Phosphoinositide-3 Kinase (PI3K) Signalling in Non-neuronal Cells
In non-neuronal cells, the modes of calmodulin-dependent Ras inactivation are very diverse and often differ depending on the cell line analyzed, with COS1, CHO, NIH3T3, Swiss3T3, HeLa, and NRK cells being the best-studied cell types to date [19,107,140,141,142,143,144,145,146].
At the outer leaflet of the plasma membrane and upstream of Ras GTPases, Ca2+/calmodulin can inhibit shedding of membrane-anchored growth factors via matrix metalloproteases [147,148]. Consequently, the calmodulin inhibitors W13 and W7 allow increased release of soluble growth factors that potently activate tyrosine kinase receptors like the epidermal growth factor receptor (EGFR) in an autocrine-paracrine manner [140,141,146,149]. EGFR activation by calmodulin inhibitors can be further enhanced as interaction of Ca2+/calmodulin with EGFR inhibits its tyrosine kinase activity [150,151,152]. In addition, CaMKII-mediated EGFR phosphorylation inhibits EGFR tyrosine kinase activity [153]. Blocking these multiple modes of calmodulin-mediated EGFR downregulation effectively permits Ras activation, followed by the recruitment and activation of Raf-1 and the MAPK pathway. This is potentiated further, as Ca2+/calmodulin can bind and not only prevent PKC-mediated KRas Ser181 phosphorylation, but also cause KRas release from the plasma membrane to the cytosol. Both events downregulate KRas-dependent activation of downstream effectors, such as the MAPK pathway (see Section 3.1) [20,67,68,83,107,121,123,141,154]. Hence, calmodulin inhibition leads to KRas activation, followed by Raf1/MAPK activation. Blocking calmodulin can promote Raf/MAPK signalling, although in serum-starved NIH3T3, Swiss3T3, NRK, A431, and HeLa cells, this occured most likely irrespective of tyrosine kinase receptors stimulation [19,144]. Specific expression pattern or preferred signalling routes seem to exist, as inhibition of calmodulin in COS1, CHO, NR6, HEK293, hepatocytes, and PC12 exerted an inhibitory effect on MAPK signalling [140,142,143,155,156]. The different outcomes of calmodulin inhibition on MAPK signalling have been summarized in Table 2.
Table 2.
Effect of calmodulin on MAPK and Rac1 activation in different cell lines. The impact of calmodulin on the activity of the MAPK pathway in different cell lines using calmodulin-specific inhibitors is shown. Some of the cell lines listed here, often in separate studies, have also been analyzed for Rac1 signalling and wherever possible, the outcome of Ca2+/calmodulin mediated activation of Rac1 is also listed. See text for further details. (-, inhibition; +, activation; ND, not determined).
| Cell Type (Species) | Ca2+/Calmodulin Effects | |
|---|---|---|
| MAPK Pathway (Refs) | Rac1 Activity (Refs) | |
| NIH3T3 fibroblasts (mouse) |
- [144] |
+ [157] |
| Swiss3T3 fibroblasts (mouse) |
- [19] |
+ [158] |
| HeLa (cervix carcinoma, human) |
- [145] |
+ [21] |
| NRK (kidney epithelial, rat) |
- [144] |
ND |
| A431 (epidermoid carcinoma, human) |
- [142] |
ND |
| COS1 fibroblasts (kidney, monkey) |
+ [143] |
+ [22] |
| CHO (epithelial ovary, hamster) |
+ [143] |
ND |
| HEK293 (embryonic kidney, human) |
+ [142] |
ND |
| PC12 (adrenal phaeochromocytoma, rat) |
+ [156] |
ND |
| Primary hepatocytes (rat) |
+ [155] |
ND |
This differential reliance on dissimilar signalling routes is reflected in studies that compared calmodulin-mediated PI3K signalling in COS1 and NIH-wt8 cells. Despite calmodulin being considered necessary for PI3K activation [142,143,159,160,161], this is only critical in COS1 cells to ensure Raf1 and MAPK activation [142]. These diverse outcomes may reflect the differential involvement and contribution of other Ras isoforms besides KRas in overall MAPK signal output in the various cell lines. In fact, H- and KRas expression levels, GTP loading, and Raf1 interaction in COS1 and NIH3T3-wt8 cells suggests that the overall role of calmodulin in MAPK signal output is determined by the ratio of activated H- and KRas and the cell-specific contribution of each isoform in Raf-1 activation [142].
The magnitude of interaction between KRas-GTP and calmodulin may be a key element in the modulation of Ras/Raf1/MAPK signalling in a PI3K-independent manner. In fibroblasts, calmodulin prevents activation of KRas and inhibition of GAPs targeting KRas by PKC [29,107,123], which is crucial to elicit Raf1/MAPK activation when calmodulin was inhibited [29,107]. Therefore, balancing interaction of KRas with either PKC or calmodulin is pivotal to regulate Ras effectors that control proliferation, survival and migration [123]. Based on initial studies proposing PKC-mediated Ser181 KRas phosphorylation [162], this was then confirmed in vitro and in vivo to establish Ca2+/calmodulin directly binding to KRas to modulate its signalling capacity [16,29,107].
3.4.3. Calmodulin and Ser181 KRas Phosphorylation
PKC or calmodulin interacting with KRas have consequences for the cellular location of KRas, as Ser181 phosphorylation alleviates the electrostatic interaction with the plasma membrane. This causes partial relocation of active KRas to endocytic compartments, inducing specific KRas signalling outcomes from intracellular sites [119,154,163,164]. Work from our laboratories revealed that KRas can elicit Raf1/MAPK activation from the endocytic compartment. Although these signalling events seem to occur irrespective of KRas phosphorylation, it leads to prolonged MAPK signalling that initiates at the plasma membrane [101].
Given the profound effects of Ser181 phosphorylation on KRas signalling, phosphomimetic, and non-phosphorylatable oncogenic KRas mutants (KRasG12V-S181D, KRasG12V-S181A) were studied extensively. The phosphomimetic KRasG12V-S181D mutant displayed increased MAPK and Akt activity at low growth factor concentration that correlated with increased foci formation, cell mobility and resistance to apoptosis [29]. Although further experimental validation is still required, it was hypothesized that calmodulin interaction and KRas phosphorylation could modulate the localization of KRas in different discrete plasma membrane microdomains. In this model, phosphorylated KRas would possibly be enriched in microdomains containing specific effectors. Our follow-up studies indeed demonstrated by density–gradient fractionation and immunoelectron microscopy that Ser181-phosphorylated oncogenic KRas (KRasG12V) localized to nanoclusters at the plasma membrane that were distinct from the non-phosphorylated KRasG12V-S181A mutant [121]. Both PKC-activated oncogenic KRasG12V as well as the phosphomimetic KRas mutant (KRasG12V-S181D) co-clustered in the same plasma membrane nanodomains that also contained activated PI3K and Raf1. In PKC-inhibited cells, non-phosphorylated oncogenic K-Ras co-clustered with the KRasG12V-S181A mutant into nanodomains where PI3K was absent [121]. Therefore, PKC-mediated and calmodulin-sensitive Ser181 phophorylation permits segregation of KRas into specific plasma membrane nanodomains that favor an increased PI3K and Raf1 activation (Figure 3).
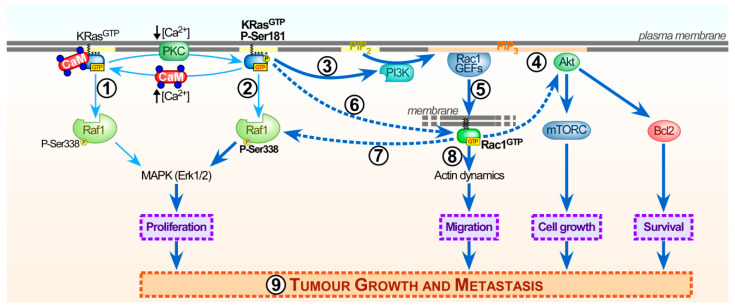
Figure 3.
Model of calmodulin and protein kinase C (PKC) modulating critical features of KRas-driven tumourigenesis. This hypothetical model is based on studies comparing the impact of pharmacological compounds modulating PKC or calmodulin activity in a variety of cell models expressing wild-type or oncogenic and constitutively active KRas (KRasG12V) containing Ser181-phosphomimetic or non-phosphorylatable mutants (see Section 3.4.3 for further details) [16,29,105,121,169,170]. (1) Ca2+/calmodulin binding to the polybasic region (PBR) within the hypervariable region (HVR) of KRas inhibits PKC-mediated Ser181 phosphorylation of KRas (P-Ser181) by sterical hindrance. Complex formation of calmodulin with (active) KRas-GTP may segregate KRas to membrane microdomains where KRas-GTP could be more susceptible to GTPase activating protein (GAP)-mediated KRas inactivation. This ensures KRas inactivation and termination of KRas-GTP-mediated activation of phosphoinositide 3-kinase (PI3K) effector pathways. (2) Low Ca2+ levels disrupt KRas/calmodulin interaction and favor PKC-mediated Ser181 phosphorylation of KRas. (3) This allows a conformational change in P-Ser181 KRas and is followed by its segregation to distinct plasma membrane microdomains or endosomal membranes (omitted in this scheme), where interaction and activation of effectors like PI3K can occur. (4) Ser181 phosphorylation of oncogenic KRas (KRasG12V) triggers PI3K and Akt-dependent anti-apoptotic signals driven by B-cell lymphoma 2 (bcl2) and mammalian target of rapamycin complex (mTORC) that promote survival and cell growth, respectively. (5) PI3K also activates Rac1 guanine nucleotide exchange factors (Rac1 GEFs) that promote activation of Rac1 (Rac1-GTP) on plasma (or endosomal) membranes (dashed lines). The membrane location of phosphatidyl-4,5-biphosphate (PIP2) and phospharidylinositol-3,4,5-triphosphate (PIP3) is indicated. (6) Alternatively, active KRas (KRas-GTP) can directly associate with a Rac1-GEF to activate Rac1. (7) Vice versa, active Rac1-GTP and its effector Pak1 have been suggested to facilitate Ser338 Raf1 phosphorylation and activation, which affects proliferation along the Raf1/mitogen-activated protein kinase (MAPK) pathway. (8) Rac1-GTP drives actin dynamics linked to cell migration. (9) These complex regulatory networks are highlighted by the requirement of Rac1 activity in KRas-driven cancers (see Section 5 for further details). Overall, Ser181 phosphorylation of oncogenic KRas is at the forefront of multiple signalling pathways that are fundamental to cellular events that drive tumour growth and metastasis. This can be counteracted by KRas/calmodulin complex formation, providing a potential tool to reduce signal output of oncogenic KRas.
3.4.4. Alternative Models for Calmodulin-Dependent KRas Signalling
Based on the work from Liao and coworkers [165], another model has been proposed [114,166]. This model favors the assembly of a ternary complex consisting of KRas, calmodulin, and PI3K at the plasma membrane to promote Akt signalling. Together with elevated Ca2+ levels observed clinically, and upregulated calmodulin levels found in many adenocarcinomas, this could support a key role for calmodulin in cancer initiation and progression. Although a ternary KRas/calmodulin/PI3K complex is plausible, experimental evidence for such a complex is still lacking. Additionally, the ability of the KRasG12V-S181A mutant to bind calmodulin but not PI3K, and the KRasG12V-S181D mutant to be defective in calmodulin, but not PI3K interaction (see above), cannot be easily reconciled. It should be noted that recent in vitro studies, using a phosphomimetic KRas S181E mutant, did not support a model of KRas S181 phosphorylation preventing calmodulin binding [83]. More studies, ideally in live cells, are needed to clarify this regulatory aspect of KRas/calmodulin interaction.
Additional support for Ca2+/calmodulin contributing to KRas-driven tumorigenicity has been provided. Wang and coworkers showed that binding of oncogenic KRas to calmodulin sequestered the number of calmodulin molecules available to activate CaMKII. This lack of CaMKII activation caused the suppression of non-canonical Wnt/Ca2+ signalling and triggered β-catenin activation that contributes strongly to tumourigenic properties of oncogenic KRas [167]. Moreover, inhibiting KRas/calmodulin interaction by stimulating PKC-mediated Ser181 phosphorylation of KRas, using the atypical PKC activator prostatin, suppressed tumourigenesis in KRas mutant pancreatic cancer cells as a result of increased CaMKII activity [167].
These results appear somewhat contradictory to those listed above that suggest PKC-mediated KRas phosphorylation to promote oncogenic potential. Different experimental conditions probably need to be considered to explain these discrepancies. For instance, the PKC activator prostratin [167] does not act as a tumour-promoting agent like more commonly used phorbol esters and indirect effects of this agent cannot be excluded. PKC activation stimulates cell survival and proliferation in cells expressing KRasG12V [168]. This is in line with PKC to function as a critical anti-apoptotic signal transducer in cells expressing activated KRas, promoting cell survival through the PI3K/AKT pathway [169,170]. Moreover, PKC inhibition reduced KRasG12V-induced tumour growth in vivo [168].
This highlights the difficulty to compare results obtained from the variety of tools available to modify PKC activity. Yet, the relevance of Ser181 phosphorylation of KRas in tumourigenesis was further demonstrated in pancreatic ductal adenocarcinoma (PDAC) in vivo. In KRas-induced tumours from PDAC cells, Ser181 phosphorylation was critical for KRas to interact with the heterogeneous nuclear ribonucleoproteins A2 and B1, which was required for PI3K/AKT activation, cell survival and tumour formation [171]. Likewise, Ser181 phosphorylation of oncogenic KRas was involved in tumour growth after subcutaneous injection of NIH3T3 fibroblasts or the colorectal cancer cell line DLD1 stably expressing phosphomimetic and non-phosphorylatable KRas mutants in nude mice [172]. Furthermore, treatment with pharmacological PKC inhibitors impaired tumour growth and correlated with KRas dephosphorylation and subsequent apoptosis [172]. Therefore, a substantial number of studies implicate that inhibition of PKC-mediated phosphorylation of KRas at the Ser181 residue, possibly in combination with agents that block calmodulin-mediated PI3K/Akt activation, could be a potential therapeutic target for KRAS-driven tumours (Figure 3).
4. The Multiple Modes of Calmodulin to Influence Rac1 GTPase Activity
4.1. Overview
Rac1 (Ras-related C3 botulinum toxin substrate 1) is a member of the Rho subfamily (Rho, Cdc42, Rac) [173,174] that controls cytoskeleton dynamics. Rho is responsible for stress fibers, while Cdc42 and Rac1 coordinate filopodia and lamellipodia formation, respectively. By controlling rapid actin re-arrangements, Rho-GTPases govern adhesion, migration, endocytosis, vesicular trafficking and proliferation [175,176,177,178,179]. Rac1 is a ubiquitous isoform of the Rac family that contains two other members. This includes Rac2, which is mainly expressed in the hematopoietic lineage, and Rac3, which is only found in neurons of the central nervous system [174,180]. Rac1 is the best studied member and its activity is critical for the proper functioning of various cellular processes such as pinocytosis, phagocytosis, exocytosis, cell–cell contacts, migration, axonal growth, differentiation, but also gene expression [94,176,178,181,182,183,184,185]. Most of these cellular events are facilitated via active Rac1 interacting with effectors that control actin dynamics. p21-activated kinase (Pak) is the central Rac1 effector that influences actin polymerization at the plasma membrane. This initially requires Pak-mediated phosphorylation of LIM kinase and cortactin, and entails actin-related protein 2/3 (Arp2/3) complex, neuronal Wiskott–Aldrich Syndrome protein (N-WASP)/WASP-family verprolin-homologous protein (WAVE) proteins, among others [186,187,188,189,190,191]. A large list of additional Rac1-GTP effectors also participate in actin cytoskeleton dynamics and other cellular responses. Alike all other GTPases, Rho-GTPases cycle between an active (GTP-bound) and inactive (GDP-bound) state, which is regulated by GEFs and GAPs [173,185,192,193,194]. Similar to Rab proteins, Rho guanine nucleotide dissociation inhibitors (RhoGDIs) provide an additional layer of regulation and complexity for Rho GTPases including Rac1. RhoGDIs preferentially bind inactive Rac1-GDP, working as a chaperone to maintain Rac1 as a soluble and inactive complex in the cytosol. This interaction makes Rac1-GDP inaccessible for GEFs located at the plasma membrane or endomembranes, and prevents nucleotide replacement in the Rac1 GDP/GTP cycle [179,195,196,197,198,199]. Highlighting the critical contribution of Ca2+ homeostasis in this regulatory circuit, elevated cellular Ca2+ levels following growth factor stimulation can activate PKC, which then phosphorylates RhoGDI. This promotes Rac1/RhoGDI dissociation, enabling the activation of Rac1 by GEFs associated with membranes [200].
4.1.1. GEF-Mediated Rac1 Activation
Several GEFs specifically activate Rac1 and include Son of Sevenless 1/2 (Sos1/2), PAK-interacting exchange factor α/β (α/β-PIX), kalirin, T-lymphoma invasion and metastasis-inducing protein 1 (Tiam1) and Ras guanine nucleotide releasing factor (RasGRF). Other GEFs also activate Cdc42 or Rho such as Rho guanine nucleotide exchange factor 4 (ARHGEF4/Asef), Active breakpoint cluster region-related protein (Abr), Vav2/3, and Trio. Only few of those GEFs mentioned above have dual activity for Rac1 and Ras GTPases (Sos1 and RasGRF), and Tiam1 can act as a downstream effector of Ras [201]. Typical Rac1-specific GEFs contain tandem of diffuse B-cell lymphoma (DH) [202] and pleckstrin homology (PH) domains. The DH domain entails the GEF activity and the PH domain the phosphoinositode binding capacity with the highest affinity for phosphatidylinositol-3,4,5-triphosphate (PI(3,4,5)P3) [201,203]. Atypical GEFs, called dedicator of cytokinesis protein superfamily proteins [204], lacking DH or PH domains, have also been described [205].
Although many GEFs that activate Rac1 have been reported, each one differentially modulates Rac1-regulated events. This may suggest that spatiotemporal regulation of compartmentalized GEF activity and tissue specificity ensure the final outcomes for Rac1 activity in different cell types. All these GEF proteins are activated by signalling cascades, usually initiated by kinases activated by plasma membrane receptors. GEF activation may occur through different or combinatory modes: exchanging their subcellular localization, releasing their auto-inhibitory conformation and/or by posttranslational modifications, such as serine/threonine (Ser/Thr) or tyrosine (Tyr) phosphorylation [201]. For instance, Rac1 can traffic from the plasma membrane to early and late endosomes by vesicular transport, to be activated after membrane-associated delivery into the vicinity of endosome-associated GEFs Tiam1 or Vav2 [94,106,206,207,208]. Overall, a substantial number of reports demonstrate that calmodulin and CaMBPs modulate many Rac1-related GEF activities. More detailed information supporting prominent functions for calmodulin in GEF-mediated regulation of Rac1 signalling and effector interactions is discussed in the following section (Section 4.3).
4.1.2. Rac1 Membrane Association
Strikingly different from the mode of action of calmodulin on KRas (see Section 2), calmodulin/Rac1 complex formation does not affect dynamics of Rac1 membrane association. Based on results from our group examining Rac1 and KRas endosomal membrane association, the higher membrane affinity of Rac1 compared to KRas might substantially contribute to different Rac1 and KRas membrane dynamics [100]. Rac1 is inserted into membranes by a geranyl-geranyl (20-carbons isoprenyl), and possibly by an additional palmitoyl (16-carbons) group [209], whereas membrane anchoring of KRas only requires a farnesyl (15-carbons) group. In the case of Rac1, after its synthesis in the cytosol as a hydrophilic protein, the leucine residues in the C-terminal CAAX box (CLLL) allow complex formation with geranyl-geranyl transferase type I to link the geranyl-geranyl group to the cysteine residue. This modification enables Rac1 recruitment to ER membranes, where Rce1 and Icmt enzymes (see Section 3) further modify Rac1. After these posttranslational modifications, Rac1 is extracted and solubilized from ER membranes to the cytosol by RhoGDIs, which confers an additional level of regulation compared to KRas [179,196]. In the following section, the domains within calmodulin that interact and influence Rac1 activity will be discussed.
4.2. The Consequences of Direct Rac1/Calmodulin Interactions
Over the years, it has become clear that calmodulin not only regulates Rac1 function via direct protein–protein interactions, but also indirectly through the diverse action of other CaMBPs. These studies reveal a complex molecular crosstalk and interplay of calmodulin with Rac1 and its effectors and regulators. This seems to enable calmodulin to decode signals derived from changes in Ca2+ homeostasis into cytoskeleton re-arrangements that control cell adhesion, morphology, and migration [210].
4.2.1. Calmodulin Interacts with Active Rac1 in a Ca2+-Dependent Manner
Pulldown and co-immunoprecipitation assays using endogenous or ectopically expressed wild-type and mutant versions of Rac1 enabled several groups to thoroughly characterize the interaction of Rac1, but also Cdc42, with calmodulin and its consequences for signal outcome [21,22,23,211,212]. Overall, evidence points at a Ca2+-dependent and direct interaction of endogenous and GTP-bound (active) Rac1 and Cdc42 with calmodulin [22,23]. Alike KRas, Rac1 contains several motifs that participate in calmodulin binding but only some of them are essential (Figure 4).
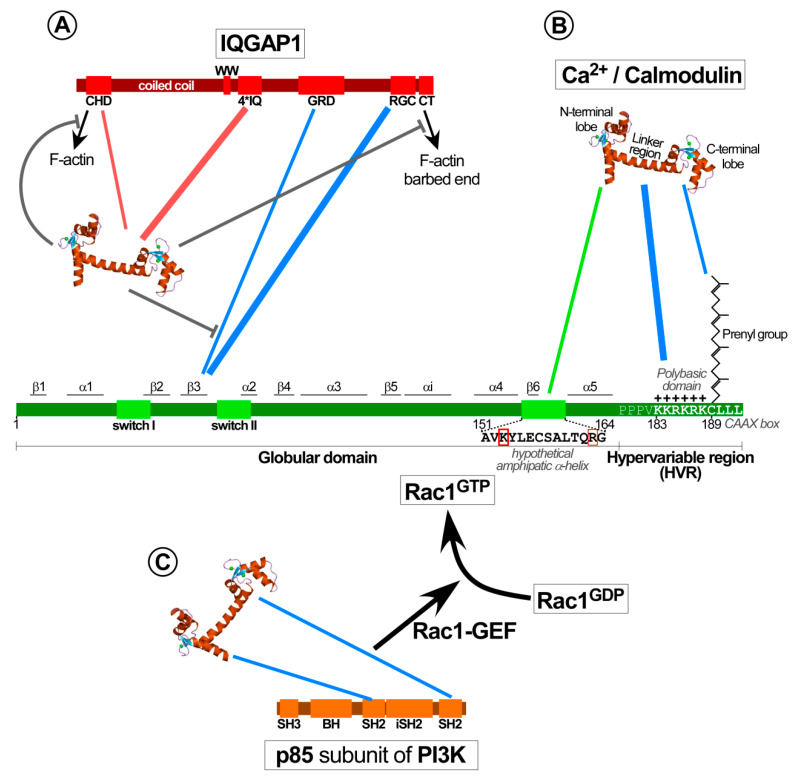
Figure 4.
Calmodulin regulates Rac1 signalling through direct and indirect protein–protein interactions. The scheme summarizes the multiple interactions between calmodulin, Rac1, isoleucine–glutamine (IQ) Motif Containing GTPase Activating Protein 1 (IQGAP1) and phosphoinositide 3-kinase (PI3K). Ca2+/calmodulin affects Rac1 signalling outcome by directly interacting with Rac1 (A), modulating IQGAP1/Rac1 interaction (B) or activating PI3K (C). (A) Multiple Rac1 domains directly interact with Ca2+/calmodulin. Similar to the KRas/calmodulin interaction (see Section 3.3 and Figure 1), the polybasic domain (PBR) and the geranylgeranyl group (prenyl) within the hypervariable region (HVR) of Rac1 are both essential for Ca2+/calmodulin binding (blue lines). Based on the well-characterized KRas/calmodulin interaction, one can speculate that the linker domain and C-terminal lobe of calmodulin interact with the PBR and prenyl group of Rac1, respectively. In addition, amino acids 151-164 in Rac1 may adopt an amphipathic α-helix that contributes to calmodulin interaction (green line) and Rac1 activation. Within this region, the basic amino acid K153 (thick red square), and to a lesser extend R163 (red square) are critical for the interaction with the N-terminal lobe of calmodulin. The position of the switch I and II domains, the PBR (aa 183-188) and the prenyl group attached to C189 of Rac1 are indicated. The N- and the C-terminal lobes and the linker region of calmodulin as well as two bound Ca2+ ions in each lobe are also shown. (B) IQGAP1 interaction with active Rac1 (Rac1-GTP) is inhibited by Ca2+/calmodulin. The Ras GAP-related domain (GRD), RASGAP C-terminal (RGCT) and C-terminal (CT) regions of IQGAP1 bind to the switch I and switch II domains of Rac1-GTP (blue lines) to maintain Rac1 in its active state. Ca2+/calmodulin binds to the four isoleucine/glutamine-containing (IQ) motifs (thick red line) within IQGAP1, which abrogates IQGAP1/Rac1 interaction. In addition, Ca2+/calmodulin binds to the N-terminal calponin homology domain (CHD) (red line) of IQGAP1. This impairs IQGAP1 interaction with F-actin, blocking IQGAP1 from stimulating F-actin crosslinking, bundling, and capping. The CHD, coiled-coil repeat (CC), tryptophan-containing proline-rich motif (WW), IQ, GRD, RGCT and CT domains of IQGAP1 are indicated (see Section 4.2.2 for details) (C) Ca2+/calmodulin interacts and activates PI3K. The N- and C-terminal lobes and the flexible central linker of Ca2+/calmodulin bind to the N-terminal (nSH2) and C-terminal (cSH2) domains of the p85 subunit of PI3K (blue lines). This interaction releases the p85-mediated autoinhibition of the catalytic p110 subunit of PI3K. Activated PI3K then phosphorylates phosphatidylinositol-4,5-biphosphate (PI(4,5)P2) and generates phosphatidylinositol-3,4,5-triphosphate (PI(3,4,5)P3), which can bind and activate several Rac1 guanine nucleotide exchange factors (Rac1 GEFs) to increase Rac1-GTP levels (see Section 4.3 for further details).
Based on the screening of various databases for potential calmodulin-binding domains [7] (http://calcium.uhnres.utoronto.ca/ctdb) and in vitro peptide competition assays, Bhullar and coworkers identified a C-terminal 14 amino acid region (residues 151-164) of Rac1 important for calmodulin binding [23]. Within that region, the positively charged lysine 153 (Lys153) and arginine 163 (Arg163) residues are critical for calmodulin interaction [21]. Replacement of these two residues with alanine in a double mutant (K153A/R163A) strongly diminishes EGF-induced Rac1 activation [21]. Thus, based on these interaction studies, direct binding of calmodulin positively regulates Rac1 activity (Figure 4).
The Rac1 C-terminus (amino acids 170-192) containing the PBR and geranyl-geranyl lipid anchor binds calmodulin albeit with a lower affinity than full length Rac1. In fact, calmodulin binding is abrogated in a Rac1 mutant lacking the PBR or the prenyl group. This indicates that both regions are essential for interaction with calmodulin [22] (Figure 4).
Several Rac1 effectors also interact with the C-terminal Rac1 domain, including phosphatidylinositol-4-phosphate 5-kinase (PIP5K) [213,214,215]. Studies from our group identified that calmodulin competes with PIP5K for Rac1 binding, which has important implications for the efficiency of Rac1 to promote PIP5K-mediated production of phosphatidylinositol-4,5-biphosphate (PI(4,5)P2) [22]. At the plasma membrane, generation of PI(4,5)P2 can regulate actin dynamics to control migration and endocytosis [216,217]. Accordingly, inhibition of calmodulin with W13 increases Rac1 binding to PIP5K. This subsequently elevates PI(4,5)P2 levels, leading to plasma membrane reorganizations that affect the clathrin-independent endocytic pathway. Hence, these W13-induced changes alter clathrin-independent internalization of cargos like major histocompatibility complex 1, cholera toxin and β1-integrins. Ligands that follow the clathrin-dependent internalization pathway, such as transferrin or polymeric immunoglobulin A, are not affected [22,212,218].
Inhibition of calmodulin using W13, or its analog W7, which rapidly diffuse into cells, have been proven powerful tools to address how calmodulin influences the spatiotemporal regulation of particular pathways [219]. Analysis of these small molecule inhibitors not only allowed validation of calmodulin stimulating growth factor-induced Rac1 activation, but also revealed calmodulin contributing to control basal Rac1 activity. W13 and analogs also greatly contributed to better characterize Rac1 activation by different stimuli in various cell types and conditions [21,22,23,157,158,211,220,221,222,223]. However, the underlying molecular mechanism how complex formation between calmodulin and Rac1 alters Rac1 activity and signal output is still not fully understood. As mentioned above, calmodulin might function as a GEF for Rac1 [21]. Alternatively, calmodulin may inhibit the access or activity of one or multiple Rac1-specific GAPs, although experimental evidence for this is lacking. Other more plausible possibilities include the calmodulin-dependent indirect regulation of Rac1 activity via IQGAPs or several Rac1-GEFs, which will be discussed in Section 4.2.2 and Section 4.3.
4.2.2. Calmodulin Modulates the Interaction of IQGAP with Rac1 to Facilitate Cytoskeleton Rearrangements
IQGAP is a multidomain scaffolding protein that directly interacts with Rac1 or Cdc42 and F-actin to modulate actin dynamics at the cell surface to control cell morphology, cell–cell adhesion and motility [224,225,226]. These interactions occur via the C-terminal region of IQGAP that contains a RasGAP-related domain (GRD) and an adjacent Ras-GAP domain that also has high affinity for both GTPases [224,227] (Figure 4). These features probably contribute to specificity, as IQGAPs only interact with Rac1 and Cdc42, but not RhoA or Ras proteins [228,229]. Biochemical assays demonstrate exclusive binding of IQGAP1 to active Rac1-GTP and Cdc42-GTP, but not inactive Rac1/Cdc42-GDP [228]. This correlates with the presence of IQGAP1 at membrane ruffling areas and cell–cell contacts of epithelial cells [228]. The IQGAP2 isoform, which is predominantly expressed in the liver, also interacts with Rac1 and Cdc42 [229]. In contrast to IQGAP1, this interaction appears independent of the activation status (GDP or GTP) of these GTPases. Mechanistically, both IQGAPs contain a GRD domain that does not exert GAP activity towards Rac1 or Cdc42, but rather inhibits GTP hydrolysis, thereby potentiating and stabilizing their active conformation. Hence, complex formation of active Rac1 and Cdc42 with IQGAPs significantly increases and prolongs actin polymerization at the leading edge of migrating cells [51,210,230,231,232,233,234]. This activity critically drives cell motility, has been linked to migration and invasion in cancer metastasis, and has been reviewed in detail elsewhere [232].
Calmodulin binds IQGAP, yet this interaction is complex. The N-terminal calponin homology domain (CHD) in IQGAP can bind Ca2+/calmodulin and F-actin in a competitive, but mutually exclusive manner [48,49,235,236,237]. In addition, IQGAP contains four IQ motifs (IQ1-4), which are commonly considered to bind calmodulin even in the absence of Ca2+ (apocalmodulin) [3]. Interestingly, although all four IQ motifs in IQGAP1 can bind Ca2+/calmodulin, only two of those IQ motifs (IQ3-4) bind apocalmodulin [238,239]. The potential function of the latter interaction remains to be clarified, as apocalmodulin differs greatly from calmodulin, does not require Ca2+ and binds a plethora of other proteins [238].
As described above, calmodulin competes with filamentous actin (F-actin) for the binding to IQGAP1 in a mutually exclusive manner, as evidenced by the purification of two pools of adrenal IQGAP that either contained F-actin or calmodulin [237]. IQGAP1 may stimulate cell migration through two interactions with F-actin. While the N-terminal CHD domain bundles F-actin, the C-terminal domain of IQGAP caps barbed ends of F-actin. Both of these interactions are inhibited by calmodulin in the presence or absence of Ca2+, possibly keeping IQGAP1 in a closed conformation [234]. Similar competitive interactions between IQGAP1 and Ca2+/calmodulin for a variety of others IQGAP1 effectors and signalling proteins have been identified. The underlying mechanism are not fully resolved and some results suggest that Ca2+ binding to calmodulin promotes its dissociation from IQGAP1. This could enhance interactions of IQGAP with effectors responsible for cytoskeleton organization [240]. On the other hand, it appears more likely that Ca2+/calmodulin may dissociate active Rac1 or Cdc42, beta-catenin and E-cadherin from IQGAP1 to affect actin organization and cell–cell adhesion in epithelial cells [224,232,241]. Taking into account that IQGAP1 promotes cell migration by interacting and stabilizing active Rac1 and that Ca2+/calmodulin dissociates Rac1 from IQGAP1, expression of a IQGAP1 mutant defective in Ca2+/calmodulin binding enhances migration in MCF-7 cells [231]. Therefore, inhibition of calmodulin may upregulate the multiple functions related to cell motility that depend on IQGAP1-Rac1/Cdc42 interactions [49,224,226,241]. This role of calmodulin, inhibiting the ability of IQGAP1 to interact with Rac1 and/or Cdc42, has also been shown critical for enteropathogenic Escherichia coli (EPEC) infection in HeLa cells [242]. In these studies, EPEC-induced IQGAP1/calmodulin interaction leads to the dissociation of Rac1 and Cdc42 from IQGAP1. This then triggers an actin reorganization at the plasma membrane to promote an actin pedestal formation necessary for EPEC infection.
Another hypothetical scenario proposes calmodulin to participate as an accessory scaffolding protein to assemble Rac1 with IQGAP, but not Cdc42 [224]. However, this model still remains to be validated experimentally in cell culture models and to date the published data rather supports Ca2+/calmodulin binding to IQGAP1 to favor the dissociation of several partners including Rac1 and Cdc42.
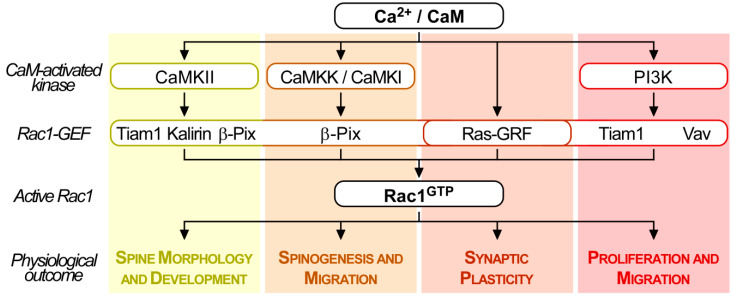
4.3. Calmodulin Influences the Activation of Several Rac1-specific GEFs
Besides the direct interaction of calmodulin with Rac1 (see Section 4.2.1) and its control over IQGAP-dependent Rac1 activation (see Section 4.2.2), calmodulin, and calmodulin-binding effectors can activate Rac1 through several Rac1-GEFs, which are summarized above (Figure 4).
Rac1-GEFs commonly contain a PH domain (see Section 4.1) that allows interaction with phosphoinositides, facilitating Rac1-GEF recruitment to the plasma membrane or endomembranes, and their subsequent activation. Several Rac1-GEFs (Tiam1, Vav, and others) are modulated by PI3K, since its product, PI(3,4,5)P3, can bind to their PH domains [243,244,245]. This regulatory circuit is relevant for a large number of Rac1-GEFs and has been reviewed in great detail by others [205]. Ca2+/calmodulin has the capacity to bind and activate PI3K [143,159,160,161]. Hence, Ca2+/calmodulin may activate Rac1-GEFs and consequently increase Rac1-GTP loading through upregulation of PI3K activity (Figure 4). Indeed, in activated neutrophils, calmodulin, and PI3K inhibitors cooperate to downregulate Rac1 activity [220].
Several CaMBPs, in particular kinases, also modulate Rac1-GEF activity and membrane translocation via phosphorylation events. Several Rac1-GEFs are phosphorylated by different members of the diverse CaMK family, including CaMKI, CaMKII, CaMKIV, and CaMK kinase (CaMKK), all of which being activated upon Ca2+/calmodulin binding that relieves their auto-inhibited conformation [246,247].
A number of studies established that in neurons and other cell types, cytosolic Ca2+ elevation stimulates the Rac1-GEFs, Tiam1, Kalirin, and β-Pix, in a CaMKII-dependent manner [222,248,249,250,251,252,253]. Using NIH3T3 and Swiss 3T3 fibroblasts, Exton and coworkers identified that platelet-derived growth factor activated and translocated Tiam1 to membrane fractions by CaMKII-mediated threonine phosphorylation that was coupled to phospholipase C (PLC)-driven cytosolic Ca2+ increase [157,158,223].
In post-synaptic neurons, Ca2+ entry pulses induced by glutamate binding to the N-methyl-D-aspartate (NMDA) receptor, produce a reciprocal and synergistic activation of CaMKII and Tiam1 to generate persistent Rac1 activation. This then ensures stable actin polymerization to maintain spinal structure during long-term potentiation [249]. This is in line with earlier studies implicating Tiam1 to be essential for Rac1-dependent actin remodeling during NMDA receptor-dependent regulation of spine development [253]. In addition, Ca2+/calmodulin-dependent CaMKII phosphorylated and inactivated RhoGAP is involved in beta-catenin/N-cadherin and NMDA receptor signalling, thereby increasing Rac1-GTP levels required for dendritic spine morphology [254].
The NMDA receptor also stimulates a signalosome complex containing CaMKK, CaMKI, β-PIX and G-protein-coupled receptor (GPCR)-kinase-interacting proteins (GIT) to promote spinogenesis and synaptogenesis in cultured neurons and hippocampal slices. In these models mimicking spine development, Ca2+/calmodulin triggers CaMKI-mediated phosphorylation of Ser516 in β-PIX, which stimulates its GEF activity towards Rac1, driving PAK-dependent spinogenesis [250]. These complex mechanisms are not only relevant for ion channel receptors in neuronal cells, but the same CaMKK/CaMKI/β-PIX/GIT axis also contribute to Rac1 activation required for estrogen-inducible medulloblastoma cell migration [251].
On the other hand, neurotransmitters such as glutamate can activate Ca2+-permeable receptors, including the NMDA receptor. This ultimately stimulates Ca2+/calmodulin binding to RasGRF through IQ motifs, which leads to elevated Rac1-GTP levels to then trigger MAPK signal cascade activation and control neuronal synaptic plasticity [137].
Yet, besides the multiple modes of Ca2+/calmodulin influencing Rac1 activity, it should be noted that calmodulin can also act downstream of Rac1 to control actin dynamics through the regulation of myosin light chain kinase (MLCK) [255]. In this study, phorbol ester-activated PKCδ, through the up-regulation of the Tiam1/Rac1/calmodulin/MLC2/MLCK cascade, elicited actomyosin formation in the protruding leading edge of migratory human peripheral blood T-lymphocytes [255,256].
Finally, transamidation is a post-translational modification that only recently was identified to affect Rac1 activity. In the A1A1v rat embryonic cortical cell line, stimulation of serotonin 2A receptor increases Rac1 transamidation and activity [257], which is coupled to PLC-dependent Ca2+/calmodulin signalling [258]. Ca2+/calmodulin activates transglutaminase, which participates in the addition of amines (e.g., serotonylation), crosslinking of protein-bound glutamine (Gln) or deamination of Gln residues of small GTPases. The fact that stimulation of serotonin 2A receptors in primary cortical neurons, as well as muscarinic acetylcholine receptors or NMDA receptors in neuronal cell lines, results in trans- or deamidation of Rac1 at Gln61, could suggest that Ca2+/calmodulin-induced transamidation activates Rac1 by inhibiting its GTP hydrolysis.
In summary, based on currently available literature summarized in the previous sections, it appears that depending on the microenvironmental changes, cells have an enormous variety of mechanisms at their disposal to allow Ca2+/calmodulin-dependent changes in Rac1 activity to alter a diverse set of cellular activities (Figure 5).
Figure 5.
Calmodulin-mediated activation of Rac1-specific GEFs controls multiple Rac1-dependent cellular functions. This scheme highlights the ability of Ca2+/calmodulin (Ca2+/CaM) to bind and activate several kinases, including calcium/calmodulin-dependent protein kinase I and II (CaMKI, CaMKII), CaMK kinase (CaMKK) and phosphoinositide 3-kinase (PI3K). This leads to the activation of Rac1-specific guanine nucleotide exchange factors (Rac1-GEFs), including T-lymphoma invasion and metastasis-inducing protein 1 (Tiam1), PAK-interacting exchange factor β (β-PIX), kalirin, Ras guanine nucleotide releasing factor (RasGRF), and Vav. These Rac1-GEFs then promote GTP loading of Rac1, promoting Rac1 activation, which is associated with fundamental cellular activities, including spine morphology and development, spinogenesis, synaptic plasticity, proliferation, and migration (see text for further details).
5. Adding Another Layer of Complexity: Rac1 is a KRas Effector
In the previous sections we summarized the extremely complex regulatory network that enables calmodulin to modulate the location, kinetics and effector pathways driven by two small GTPases, KRas and Rac1. It is important to note that current methodologies are still limited to identify a spatiotemporal hierarchy amongst calmodulin-activated signalling cascades. This has made it difficult to determine primary, secondary, and less prominent targets regulated by Ca2+/calmodulin in a ligand- and cell-type specific manner. It is tempting to speculate that a substantial number of effector signals promoted by KRas and Rac1 result from their direct interaction with calmodulin. In addition, Ca2+/calmodulin interacts with a plethora of Rac1 regulators and other CaMBPs to indirectly control Rac1 signal outcome (see Section 4.2, Section 4.2.1, Section 4.2.2 and Section 4.3).
Further complexity is added by Rac1 being an effector of Ras signalling via PI3K-dependent and -independent pathways that activate Rac1-GEFs [205,244,245,253,259,260,261,262,263,264,265]. The most prominent of these routes, with remarkable significance for tumour growth and progression, is represented by Ras-GTP interacting with PI3K, which can then activate Rac1. Firstly, disruption of Ras/PI3K interaction leads to inhibition of Rac1 activation and tumour regression in a mouse model for EGFR mutant lung adenocarcinoma [266]. Secondly, Rac1 activity is required in HRas-induced tumourigenesis [24,266,267,268,269]. Thirdly, loss of Rac1 causes a substantial reduction in a mouse model of KRas mutant-driven pancreatic, epidermal papilloma, and lung tumour growth [25,270,271].
In addition to Rac1-dependent stimulation of proliferation and cell migration (see Section 4), Rac1-mediated anti-apoptotic signals also favor Ras-dependent oncogenicity [272]. Pak1 is considered the main Rac1 effector that influences cortical actin cytoskeleton rearrangements most relevant for cell motility (see Section 4.1). Rac1-mediated Pak1 activation also appears fundamental in KRas-driven skin tumour progression through its decisive role in MAPK and Akt activation [273]. In this setting, Pak1 stimulates Raf1 kinase Ser338 phosphorylation, which increases MAPK activity but also upregulates the PI3K/Akt pathway. The latter signalling cascade is further promoted through a cooperative action of Rac1 with others Rho-GTPases, leading to PI3K activation [274,275].
The events triggered by Ras oncogenes and facilitated by Rac1 and its effectors point at the therapeutic potential of targeting Ca2+/calmodulin in tumour growth and metastasis. The precise roles of calmodulin in Ras-driven tumourigenesis are not fully understood, but Ca2+/calmodulin-induced Rac1 activation may strengthen tumour growth as well as metastasis. As proof-of-principle, calmodulin inhibitors abrogate PI3K and Rac1 activation [22,23,143,159,220]. This concept may indicate some promise for therapeutic anti-cancer approaches, yet aiming to block calmodulin function may only be suitable for some cancers. The interaction of Ca2+/calmodulin with active KRas impairs PKC-mediated KRas Ser181 phosphorylation, which is considered to increase oncogenic potential of KRas [29,172] (for further details see also Section 3) (Figure 3). However, evidence for an involvement of calmodulin in KRas-driven tumourigenesis is still lacking and further studies are needed to clarify its contribution in de-regulated and KRas-dependent oncogenic events.
Finally, one should also consider that calmodulin could couple Ras and Rac1 signalling pathways by regulating GEFs with affinity towards both GTPases [205,276]. For example (see also Section 4.1.1 and Section 4.3), Ca2+/calmodulin binding to RasGRF activates Ras, Rac1, and effectors, including MAPK, in response to signals from a variety of neurotransmitters [137]. On the other hand, earlier studies [245,262] suggest that Ca2+/calmodulin, most likely via PI3K, could enable Sos1 to simultaneously activate Ras and Rac1, hence synergizing signal outcome of both GTPases.
Taken together, besides the direct and indirect modes of action that allow Ca2+/calmodulin to influence KRas and Rac1 activity, Rac1 can also act as an effector of Ras signalling cascades. This connectivity between Ras and Rac1 signalling is often observed in oncogenic settings, which are characterized by continuously active mutant forms of growth factor receptors or Ras isoforms. However, Ca2+/calmodulin-mediated changes in KRas and Rac1 signalling in normal cells are based on the transient nature of environmental signals that trigger fluctuations in Ca2+ levels. Future research therefore needs to clarify how the de-regulation of Ca2+ homeostasis in tumour cells alters the availability of ‘active’ calmodulin and how this might influence the performance of small molecules targeting Ca2+/calmodulin.
6. Perspectives
Over the years, taking advantage of the rapidly improving technologies available, the field has made substantial progress to dissect the highly versatile and complex regulatory circuits that allow calmodulin to influence signalling pathways. This has provided the field with a range of therapeutic targets, including several calmodulin-binding proteins, such as CaMKs. To summarize the enormous variety of Ca2+/calmodulin-dependent mechanisms that cells have at their disposal, some of those with therapeutic potential, would have gone beyond the scope of this review. We therefore refer the reader to recent excellent reviews that cover other aspects of calmodulin in cellular pathophysiology. In this article, we focused to provide an overview on the complex network of calmodulin interactions that influence the spatiotemporal signalling of KRas and Rac1 GTPases, with consequences for biological outcomes in health and disease.
Calmodulin not only directly interacts with KRas and Rac1 to influence their localization and activity, but through other CaMBPs, also modifies the capacity of several GEFs and GAPs to control the GTP/GDP cycle of both GTPases. These multifaceted communications with KRas and Rac1 GTPases triggered by calmodulin have manifold consequences for effector pathways that shape the ability of cells to proliferate, migrate, differentiate or respond to death signals.
Some of these diverse mechanisms for calmodulin to regulate KRas and Rac1 GTPases could provide therapeutic opportunity in cancer pathophysiology, in particular where aberrant KRas signalling drives Rac1-dependent activities that contribute to tumour progression. Given the high incidence of oncogenic KRas mutations in human cancers, and the ability of calmodulin to modulate oncogenic KRas activity, targeting calmodulin in these settings may unravel novel treatment options. Future studies aiming to substantiate a role for calmodulin in KRas-driven tumourigenesis will be required to clarify its therapeutic potential. Despite this concept having therapeutic anti-cancer promise, one also needs to consider that calmodulin is ubiquitously expressed. Hence, avoiding unspecific side-effects and circumventing de-regulation of Ca2+ homeostasis and Ca2+/calmodulin-responsive signalling cascades in neighbouring healthy cells and tissues will be critical. Thus, besides the efforts to advance calmodulin-based anti-cancer techniques, future research will also have to establish drug carriers or platforms that can ensure localized delivery of pharmaceutical agents to specifically block calmodulin-dependent oncogenic signalling in localized tumours.
Acknowledgments
We would like to thank all members of our laboratories, past and present, for their invaluable contributions and apologize to all those researchers whose work could not be discussed owing to space limitations.
Author Contributions
All authors contributed to the conceptualization, writing (original draft preparation, review and editing) of this manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grants BFU2015-66785-P and Consolider-Ingenio (CSD2009-00016 and BFU2016-81912-REDC) from the Ministerio de Economía y Competitividad (Spain) to CE and FT. NA is supported by grant SAF2016- 76239-R from the Ministerio de Economia y Competitividad (Spain). AC is supported by a FPU fellowship from Ministerio de Educación, Cultura y Deporte (Spain). CR is supported by the Serra Húnter Programme (Generalitat de Catalunya). TG is supported by the University of Sydney (RY253, U3367), Sydney, Australia.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Chin D., Means A.R. Calmodulin: A prototypical calcium sensor. Trends Cell Biol. 2000;10:322–328. doi: 10.1016/S0962-8924(00)01800-6. [DOI] [PubMed] [Google Scholar]
- 2.Potter J.D., Strang-Brown P., Walker P.L., Iida S. Ca2+ binding to calmodulin. Methods Enzymol. 1983;102:135–143. doi: 10.1016/s0076-6879(83)02014-5. [DOI] [PubMed] [Google Scholar]
- 3.Rhoads A.R., Friedberg F. Sequence motifs for calmodulin recognition. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 1997;11:331–340. doi: 10.1096/fasebj.11.5.9141499. [DOI] [PubMed] [Google Scholar]
- 4.Sharma R.K., Parameswaran S. Calmodulin-binding proteins: A journey of 40 years. Cell Calcium. 2018;75:89–100. doi: 10.1016/j.ceca.2018.09.002. [DOI] [PubMed] [Google Scholar]
- 5.James P., Vorherr T., Carafoli E. Calmodulin-binding domains: Just two faced or multi-faceted? Trends Biochem. Sci. 1995;20:38–42. doi: 10.1016/S0968-0004(00)88949-5. [DOI] [PubMed] [Google Scholar]
- 6.Cheney R.E., Mooseker M.S. Unconventional myosins. Curr. Opin. Cell Biol. 1992;4:27–35. doi: 10.1016/0955-0674(92)90055-H. [DOI] [PubMed] [Google Scholar]
- 7.Yap K.L., Kim J., Truong K., Sherman M., Yuan T., Ikura M. Calmodulin target database. J. Struct. Funct. Genom. 2000;1:8–14. doi: 10.1023/A:1011320027914. [DOI] [PubMed] [Google Scholar]
- 8.McLaughlin S., Murray D. Plasma membrane phosphoinositide organization by protein electrostatics. Nature. 2005;438:605–611. doi: 10.1038/nature04398. [DOI] [PubMed] [Google Scholar]
- 9.Oughtred R., Stark C., Breitkreutz B.J., Rust J., Boucher L., Chang C., Kolas N., O‘Donnell L., Leung G., McAdam R., et al. The BioGRID interaction database: 2019 update. Nucleic Acids Res. 2019;47:D529–D541. doi: 10.1093/nar/gky1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berggard T., Arrigoni G., Olsson O., Fex M., Linse S., James P. 140 mouse brain proteins identified by Ca2+-calmodulin affinity chromatography and tandem mass spectrometry. J. Proteome Res. 2006;5:669–687. doi: 10.1021/pr050421l. [DOI] [PubMed] [Google Scholar]
- 11.O‘Day D.H. CaMBOT: Profiling and characterizing calmodulin-binding proteins. Cell Signal. 2003;15:347–354. doi: 10.1016/S0898-6568(02)00116-X. [DOI] [PubMed] [Google Scholar]
- 12.Wennerberg K., Rossman K.L., Der C.J. The Ras superfamily at a glance. J. Cell Sci. 2005;118:843–846. doi: 10.1242/jcs.01660. [DOI] [PubMed] [Google Scholar]
- 13.Rajalingam K., Schreck R., Rapp U.R., Albert S. Ras oncogenes and their downstream targets. Biochim. Biophys. Acta. 2007;1773:1177–1195. doi: 10.1016/j.bbamcr.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 14.Downward J. Control of ras activation. Cancer Surv. 1996;27:87–100. [PubMed] [Google Scholar]
- 15.Marshall C.J. Ras effectors. Curr. Opin. Cell Biol. 1996;8:197–204. doi: 10.1016/S0955-0674(96)80066-4. [DOI] [PubMed] [Google Scholar]
- 16.Lopez-Alcala C., Alvarez-Moya B., Villalonga P., Calvo M., Bachs O., Agell N. Identification of essential interacting elements in K-Ras/calmodulin binding and its role in K-Ras localization. J. Biol. Chem. 2008;283:10621–10631. doi: 10.1074/jbc.M706238200. [DOI] [PubMed] [Google Scholar]
- 17.Abraham S.J., Nolet R.P., Calvert R.J., Anderson L.M., Gaponenko V. The hypervariable region of K-Ras4B is responsible for its specific interactions with calmodulin. Biochemistry. 2009;48:7575–7583. doi: 10.1021/bi900769j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu L.J., Xu L.R., Liao J.M., Chen J., Liang Y. Both the C-terminal polylysine region and the farnesylation of K-RasB are important for its specific interaction with calmodulin. PLoS ONE. 2011;6:e21929. doi: 10.1371/journal.pone.0021929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Villalonga P., Lopez-Alcala C., Bosch M., Chiloeches A., Rocamora N., Gil J., Marais R., Marshall C.J., Bachs O., Agell N. Calmodulin binds to K-Ras, but not to H- or N-Ras, and modulates its downstream signaling. Mol. Cell. Biol. 2001;21:7345–7354. doi: 10.1128/MCB.21.21.7345-7354.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sidhu R.S., Clough R.R., Bhullar R.P. Ca2+/calmodulin binds and dissociates K-RasB from membrane. Biochem. Biophys. Res. Commun. 2003;304:655–660. doi: 10.1016/S0006-291X(03)00635-1. [DOI] [PubMed] [Google Scholar]
- 21.Xu B., Chelikani P., Bhullar R.P. Characterization and functional analysis of the calmodulin-binding domain of Rac1 GTPase. PLoS ONE. 2012;7:e42975. doi: 10.1371/journal.pone.0042975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vidal-Quadras M., Gelabert-Baldrich M., Soriano-Castell D., Llado A., Rentero C., Calvo M., Pol A., Enrich C., Tebar F. Rac1 and calmodulin interactions modulate dynamics of ARF6-dependent endocytosis. Traffic. 2011;12:1879–1896. doi: 10.1111/j.1600-0854.2011.01274.x. [DOI] [PubMed] [Google Scholar]
- 23.Elsaraj S.M., Bhullar R.P. Regulation of platelet Rac1 and Cdc42 activation through interaction with calmodulin. Biochim. Biophys. Acta. 2008;1783:770–778. doi: 10.1016/j.bbamcr.2008.01.022. [DOI] [PubMed] [Google Scholar]
- 24.Qiu R.G., Chen J., Kirn D., McCormick F., Symons M. An essential role for Rac in Ras transformation. Nature. 1995;374:457–459. doi: 10.1038/374457a0. [DOI] [PubMed] [Google Scholar]
- 25.Samuel M.S., Lourenco F.C., Olson M.F. K-Ras mediated murine epidermal tumorigenesis is dependent upon and associated with elevated Rac1 activity. PLoS ONE. 2011;6:e17143. doi: 10.1371/journal.pone.0017143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Joneson T., White M.A., Wigler M.H., Bar-Sagi D. Stimulation of membrane ruffling and MAP kinase activation by distinct effectors of RAS. Science. 1996;271:810–812. doi: 10.1126/science.271.5250.810. [DOI] [PubMed] [Google Scholar]
- 27.Villalobo A., Berchtold M.W. The role of calmodulin in tumor cell migration, invasiveness, and metastasis. Int. J. Mol. Sci. 2020;21:765. doi: 10.3390/ijms21030765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berchtold M.W., Villalobo A. The many faces of calmodulin in cell proliferation, programmed cell death, autophagy, and cancer. Biochim. Biophys. Acta. 2014;1843:398–435. doi: 10.1016/j.bbamcr.2013.10.021. [DOI] [PubMed] [Google Scholar]
- 29.Alvarez-Moya B., Lopez-Alcala C., Drosten M., Bachs O., Agell N. K-Ras4B phosphorylation at Ser181 is inhibited by calmodulin and modulates K-Ras activity and function. Oncogene. 2010;29:5911–5922. doi: 10.1038/onc.2010.298. [DOI] [PubMed] [Google Scholar]
- 30.Coppola T., Perret-Menoud V., Luthi S., Farnsworth C.C., Glomset J.A., Regazzi R. Disruption of Rab3-calmodulin interaction, but not other effector interactions, prevents Rab3 inhibition of exocytosis. EMBO J. 1999;18:5885–5891. doi: 10.1093/emboj/18.21.5885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fischer R., Wei Y., Anagli J., Berchtold M.W. Calmodulin binds to and inhibits GTP binding of the ras-like GTPase Kir/Gem. J. Biol. Chem. 1996;271:25067–25070. doi: 10.1074/jbc.271.41.25067. [DOI] [PubMed] [Google Scholar]
- 32.Lee C.H., Della N.G., Chew C.E., Zack D.J. Rin, a neuron-specific and calmodulin-binding small G-protein, and Rit define a novel subfamily of ras proteins. J. Neurosci. Off. J. Soc. Neurosci. 1996;16:6784–6794. doi: 10.1523/JNEUROSCI.16-21-06784.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sidhu R.S., Bhullar R.P. Rab3B in human platelet is membrane bound and interacts with Ca(2+)/calmodulin. Biochem. Biophys. Res. Commun. 2001;289:1039–1043. doi: 10.1006/bbrc.2001.6113. [DOI] [PubMed] [Google Scholar]
- 34.Wang K.L., Khan M.T., Roufogalis B.D. Identification and characterization of a calmodulin-binding domain in Ral-A, a Ras-related GTP-binding protein purified from human erythrocyte membrane. J. Biol. Chem. 1997;272:16002–16009. doi: 10.1074/jbc.272.25.16002. [DOI] [PubMed] [Google Scholar]
- 35.Wes P.D., Yu M., Montell C. RIC, a calmodulin-binding Ras-like GTPase. EMBO J. 1996;15:5839–5848. doi: 10.1002/j.1460-2075.1996.tb00971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park J.B., Farnsworth C.C., Glomset J.A. Ca2+/calmodulin causes Rab3A to dissociate from synaptic membranes. J. Biol. Chem. 1997;272:20857–20865. doi: 10.1074/jbc.272.33.20857. [DOI] [PubMed] [Google Scholar]
- 37.Urano T., Emkey R., Feig L.A. Ral-GTPases mediate a distinct downstream signaling pathway from Ras that facilitates cellular transformation. EMBO J. 1996;15:810–816. doi: 10.1002/j.1460-2075.1996.tb00416.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Clough R.R., Sidhu R.S., Bhullar R.P. Calmodulin binds RalA and RalB and is required for the thrombin-induced activation of Ral in human platelets. J. Biol. Chem. 2002;277:28972–28980. doi: 10.1074/jbc.M201504200. [DOI] [PubMed] [Google Scholar]
- 39.Sidhu R.S., Elsaraj S.M., Grujic O., Bhullar R.P. Calmodulin binding to the small GTPase Ral requires isoprenylated Ral. Biochem. Biophys. Res. Commun. 2005;336:105–109. doi: 10.1016/j.bbrc.2005.08.053. [DOI] [PubMed] [Google Scholar]
- 40.Wang K.L., Roufogalis B.D. Ca2+/calmodulin stimulates GTP binding to the ras-related protein ral-A. J. Biol. Chem. 1999;274:14525–14528. doi: 10.1074/jbc.274.21.14525. [DOI] [PubMed] [Google Scholar]
- 41.van Dam E.M., Robinson P.J. Ral: Mediator of membrane trafficking. Int. J. Biochem. Cell Biol. 2006;38:1841–1847. doi: 10.1016/j.biocel.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 42.Kajio H., Olszewski S., Rosner P.J., Donelan M.J., Geoghegan K.F., Rhodes C.J. A low-affinity Ca2+-dependent association of calmodulin with the Rab3A effector domain inversely correlates with insulin exocytosis. Diabetes. 2001;50:2029–2039. doi: 10.2337/diabetes.50.9.2029. [DOI] [PubMed] [Google Scholar]
- 43.Schluter O.M., Khvotchev M., Jahn R., Sudhof T.C. Localization versus function of Rab3 proteins. Evidence for a common regulatory role in controlling fusion. J. Biol. Chem. 2002;277:40919–40929. doi: 10.1074/jbc.M203704200. [DOI] [PubMed] [Google Scholar]
- 44.Geppert M., Sudhof T.C. RAB3 and synaptotagmin: The yin and yang of synaptic membrane fusion. Annu. Rev. Neurosci. 1998;21:75–95. doi: 10.1146/annurev.neuro.21.1.75. [DOI] [PubMed] [Google Scholar]
- 45.Johannes L., Lledo P.M., Roa M., Vincent J.D., Henry J.P., Darchen F. The GTPase Rab3a negatively controls calcium-dependent exocytosis in neuroendocrine cells. EMBO J. 1994;13:2029–2037. doi: 10.1002/j.1460-2075.1994.tb06476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhu S., Chim S.M., Cheng T., Ang E., Ng B., Lim B., Chen K., Qiu H., Tickner J., Xu H., et al. Calmodulin interacts with Rab3D and modulates osteoclastic bone resorption. Sci. Rep. 2016;6:37963. doi: 10.1038/srep37963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Swart-Mataraza J.M., Li Z., Sacks D.B. IQGAP1 is a component of Cdc42 signaling to the cytoskeleton. J. Biol. Chem. 2002;277:24753–24763. doi: 10.1074/jbc.M111165200. [DOI] [PubMed] [Google Scholar]
- 48.Ho Y.D., Joyal J.L., Li Z., Sacks D.B. IQGAP1 integrates Ca2+/calmodulin and Cdc42 signaling. J. Biol. Chem. 1999;274:464–470. doi: 10.1074/jbc.274.1.464. [DOI] [PubMed] [Google Scholar]
- 49.Fukata M., Kuroda S., Fujii K., Nakamura T., Shoji I., Matsuura Y., Okawa K., Iwamatsu A., Kikuchi A., Kaibuchi K. Regulation of cross-linking of actin filament by IQGAP1, a target for Cdc42. J. Biol. Chem. 1997;272:29579–29583. doi: 10.1074/jbc.272.47.29579. [DOI] [PubMed] [Google Scholar]
- 50.Joyal J.L., Annan R.S., Ho Y.D., Huddleston M.E., Carr S.A., Hart M.J., Sacks D.B. Calmodulin modulates the interaction between IQGAP1 and Cdc42. Identification of IQGAP1 by nanoelectrospray tandem mass spectrometry. J. Biol. Chem. 1997;272:15419–15425. doi: 10.1074/jbc.272.24.15419. [DOI] [PubMed] [Google Scholar]
- 51.Briggs M.W., Sacks D.B. IQGAP1 as signal integrator: Ca2+, calmodulin, Cdc42 and the cytoskeleton. FEBS Lett. 2003;542:7–11. doi: 10.1016/S0014-5793(03)00333-8. [DOI] [PubMed] [Google Scholar]
- 52.Jeong H.W., Li Z., Brown M.D., Sacks D.B. IQGAP1 binds Rap1 and modulates its activity. J. Biol. Chem. 2007;282:20752–20762. doi: 10.1074/jbc.M700487200. [DOI] [PubMed] [Google Scholar]
- 53.Baldassa S., Zippel R., Sturani E. Depolarization-induced signaling to Ras, Rap1 and MAPKs in cortical neurons. Brain Res. Mol. Brain Res. 2003;119:111–122. doi: 10.1016/j.molbrainres.2003.08.020. [DOI] [PubMed] [Google Scholar]
- 54.Hoshino M., Nakamura S. The Ras-like small GTP-binding protein Rin is activated by growth factor stimulation. Biochem. Biophys. Res. Commun. 2002;295:651–656. doi: 10.1016/S0006-291X(02)00731-3. [DOI] [PubMed] [Google Scholar]
- 55.Hoshino M., Nakamura S. Small GTPase Rin induces neurite outgrowth through Rac/Cdc42 and calmodulin in PC12 cells. J. Cell Biol. 2003;163:1067–1076. doi: 10.1083/jcb.200308070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Harrison S.M., Rudolph J.L., Spencer M.L., Wes P.D., Montell C., Andres D.A., Harrison D.A. Activated RIC, a small GTPase, genetically interacts with the Ras pathway and calmodulin during Drosophila development. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2005;232:817–826. doi: 10.1002/dvdy.20346. [DOI] [PubMed] [Google Scholar]
- 57.Kelly K. The RGK family: A regulatory tail of small GTP-binding proteins. Trends Cell Biol. 2005;15:640–643. doi: 10.1016/j.tcb.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 58.Correll R.N., Pang C., Niedowicz D.M., Finlin B.S., Andres D.A. The RGK family of GTP-binding proteins: Regulators of voltage-dependent calcium channels and cytoskeleton remodeling. Cell. Signal. 2008;20:292–300. doi: 10.1016/j.cellsig.2007.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Flynn R., Zamponi G.W. Regulation of calcium channels by RGK proteins. Channels (Austin) 2010;4:434–439. doi: 10.4161/chan.4.6.12865. [DOI] [PubMed] [Google Scholar]
- 60.Moyers J.S., Bilan P.J., Zhu J., Kahn C.R. Rad and Rad-related GTPases interact with calmodulin and calmodulin-dependent protein kinase II. J. Biol. Chem. 1997;272:11832–11839. doi: 10.1074/jbc.272.18.11832. [DOI] [PubMed] [Google Scholar]
- 61.Beguin P., Mahalakshmi R.N., Nagashima K., Cher D.H., Takahashi A., Yamada Y., Seino Y., Hunziker W. 14-3-3 and calmodulin control subcellular distribution of Kir/Gem and its regulation of cell shape and calcium channel activity. J. Cell Sci. 2005;118:1923–1934. doi: 10.1242/jcs.02321. [DOI] [PubMed] [Google Scholar]
- 62.Beguin P., Mahalakshmi R.N., Nagashima K., Cher D.H., Kuwamura N., Yamada Y., Seino Y., Hunziker W. Roles of 14-3-3 and calmodulin binding in subcellular localization and function of the small G-protein Rem2. Biochem. J. 2005;390:67–75. doi: 10.1042/BJ20050414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Beguin P., Nagashima K., Gonoi T., Shibasaki T., Takahashi K., Kashima Y., Ozaki N., Geering K., Iwanaga T., Seino S. Regulation of Ca2+ channel expression at the cell surface by the small G-protein kir/Gem. Nature. 2001;411:701–706. doi: 10.1038/35079621. [DOI] [PubMed] [Google Scholar]
- 64.Mahalakshmi R.N., Ng M.Y., Guo K., Qi Z., Hunziker W., Beguin P. Nuclear localization of endogenous RGK proteins and modulation of cell shape remodeling by regulated nuclear transport. Traffic. 2007;8:1164–1178. doi: 10.1111/j.1600-0854.2007.00599.x. [DOI] [PubMed] [Google Scholar]
- 65.Moyers J.S., Zhu J., Kahn C.R. Effects of phosphorylation on function of the Rad GTPase. Biochem. J. 1998;333:609–614. doi: 10.1042/bj3330609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mahalakshmi R.N., Nagashima K., Ng M.Y., Inagaki N., Hunziker W., Beguin P. Nuclear transport of Kir/Gem requires specific signals and importin alpha5 and is regulated by calmodulin and predicted serine phosphorylations. Traffic. 2007;8:1150–1163. doi: 10.1111/j.1600-0854.2007.00598.x. [DOI] [PubMed] [Google Scholar]
- 67.Fivaz M., Meyer T. Reversible intracellular translocation of KRas but not HRas in hippocampal neurons regulated by Ca2+/calmodulin. J. Cell Biol. 2005;170:429–441. doi: 10.1083/jcb.200409157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Agamasu C., Ghirlando R., Taylor T., Messing S., Tran T.H., Bindu L., Tonelli M., Nissley D.V., McCormick F., Stephen A.G. KRAS prenylation is required for bivalent binding with calmodulin in a nucleotide-independent manner. Biophys. J. 2019;116:1049–1063. doi: 10.1016/j.bpj.2019.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Park J.B., Lee J.Y., Kim J.W. Dissociation of RalA from synaptic membranes by Ca2+/calmodulin. Biochem. Biophys. Res. Commun. 1999;263:765–769. doi: 10.1006/bbrc.1999.1463. [DOI] [PubMed] [Google Scholar]
- 70.Park J.B., Kim J.S., Lee J.Y., Kim J., Seo J.Y., Kim A.R. GTP binds to Rab3A in a complex with Ca2+/calmodulin. Biochem. J. 2002;362:651–657. doi: 10.1042/bj3620651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yunes R., Tomes C., Michaut M., De Blas G., Rodriguez F., Regazzi R., Mayorga L.S. Rab3A and calmodulin regulate acrosomal exocytosis by mechanisms that do not require a direct interaction. FEBS Lett. 2002;525:126–130. doi: 10.1016/S0014-5793(02)03102-2. [DOI] [PubMed] [Google Scholar]
- 72.Boldt K., van Reeuwijk J., Lu Q., Koutroumpas K., Nguyen T.M., Texier Y., van Beersum S.E., Horn N., Willer J.R., Mans D.A., et al. An organelle-specific protein landscape identifies novel diseases and molecular mechanisms. Nat. Commun. 2016;7:11491. doi: 10.1038/ncomms11491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Park J.B. Regulation of GTP-binding state in RalA through Ca2+ and calmodulin. Exp. Mol. Med. 2001;33:54–58. doi: 10.1038/emm.2001.10. [DOI] [PubMed] [Google Scholar]
- 74.Correll R.N., Pang C., Niedowicz D.M., Satin J., Andres D.A. Calmodulin binding is dispensable for Rem-mediated Ca2+ channel inhibition. Mol. Cell. Biochem. 2008;310:103–110. doi: 10.1007/s11010-007-9670-8. [DOI] [PubMed] [Google Scholar]
- 75.Marshall C. How do small GTPase signal transduction pathways regulate cell cycle entry? Curr. Opin. Cell Biol. 1999;11:732–736. doi: 10.1016/S0955-0674(99)00044-7. [DOI] [PubMed] [Google Scholar]
- 76.Malumbres M., Barbacid M. RAS oncogenes: The first 30 years. Nat. Rev. Cancer. 2003;3:459–465. doi: 10.1038/nrc1097. [DOI] [PubMed] [Google Scholar]
- 77.Downward J. Ras signalling and apoptosis. Curr. Opin. Genet. Dev. 1998;8:49–54. doi: 10.1016/S0959-437X(98)80061-0. [DOI] [PubMed] [Google Scholar]
- 78.Khosravi-Far R., Campbell S., Rossman K.L., Der C.J. Increasing complexity of Ras signal transduction: Involvement of Rho family proteins. Adv. Cancer Res. 1998;72:57–107. doi: 10.1016/s0065-230x(08)60700-9. [DOI] [PubMed] [Google Scholar]
- 79.Rebollo A., Martinez A.C. Ras proteins: Recent advances and new functions. Blood. 1999;94:2971–2980. doi: 10.1182/blood.V94.9.2971. [DOI] [PubMed] [Google Scholar]
- 80.Omerovic J., Hammond D.E., Clague M.J., Prior I.A. Ras isoform abundance and signalling in human cancer cell lines. Oncogene. 2008;27:2754–2762. doi: 10.1038/sj.onc.1210925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bos J.L. Ras oncogenes in human cancer: A review. Cancer Res. 1989;49:4682–4689. [PubMed] [Google Scholar]
- 82.Prior I.A., Lewis P.D., Mattos C. A comprehensive survey of Ras mutations in cancer. Cancer Res. 2012;72:2457–2467. doi: 10.1158/0008-5472.CAN-11-2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lakshman B., Messing S., Schmid E.M., Clogston J.D., Gillette W.K., Esposito D., Kessing B., Fletcher D.A., Nissley D.V., McCormick F., et al. Quantitative biophysical analysis defines key components modulating recruitment of the GTPase KRAS to the plasma membrane. J. Biol. Chem. 2019;294:2193–2207. doi: 10.1074/jbc.RA118.005669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Newlaczyl A.U., Hood F.E., Coulson J.M., Prior I.A. Decoding RAS isoform and codon-specific signalling. Biochem. Soc. Trans. 2014;42:742–746. doi: 10.1042/BST20140057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hancock J.F. Ras proteins: Different signals from different locations. Nat. Rev. Mol. Cell Biol. 2003;4:373–384. doi: 10.1038/nrm1105. [DOI] [PubMed] [Google Scholar]
- 86.Prior I.A., Muncke C., Parton R.G., Hancock J.F. Direct visualization of Ras proteins in spatially distinct cell surface microdomains. J. Cell Biol. 2003;160:165–170. doi: 10.1083/jcb.200209091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tian T., Harding A., Inder K., Plowman S., Parton R.G., Hancock J.F. Plasma membrane nanoswitches generate high-fidelity Ras signal transduction. Nat. Cell Biol. 2007;9:905–914. doi: 10.1038/ncb1615. [DOI] [PubMed] [Google Scholar]
- 88.Matallanas D., Sanz-Moreno V., Arozarena I., Calvo F., Agudo-Ibanez L., Santos E., Berciano M.T., Crespo P. Distinct utilization of effectors and biological outcomes resulting from site-specific Ras activation: Ras functions in lipid rafts and Golgi complex are dispensable for proliferation and transformation. Mol. Cell. Biol. 2006;26:100–116. doi: 10.1128/MCB.26.1.100-116.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Omerovic J., Laude A.J., Prior I.A. Ras proteins: Paradigms for compartmentalised and isoform-specific signalling. Cell. Mol. Life Sci. 2007;64:2575–2589. doi: 10.1007/s00018-007-7133-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Henis Y.I., Hancock J.F., Prior I.A. Ras acylation, compartmentalization and signaling nanoclusters (Review) Mol. Membr. Biol. 2009;26:80–92. doi: 10.1080/09687680802649582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Eisenberg S., Henis Y.I. Interactions of Ras proteins with the plasma membrane and their roles in signaling. Cell Signal. 2008;20:31–39. doi: 10.1016/j.cellsig.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 92.Calvo F., Agudo-Ibanez L., Crespo P. The Ras-ERK pathway: Understanding site-specific signaling provides hope of new anti-tumor therapies. Bioessays News Rev. Mol. Cell. Dev. Biol. 2010;32:412–421. doi: 10.1002/bies.200900155. [DOI] [PubMed] [Google Scholar]
- 93.Prior I.A., Hancock J.F. Ras trafficking, localization and compartmentalized signalling. Semin. Cell Dev. Biol. 2012;23:145–153. doi: 10.1016/j.semcdb.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tebar F., Enrich C., Rentero C., Grewal T. GTPases Rac1 and Ras signaling from endosomes. Prog. Mol. Subcell. Biol. 2018;57:65–105. doi: 10.1007/978-3-319-96704-2_3. [DOI] [PubMed] [Google Scholar]
- 95.Fehrenbacher N., Bar-Sagi D., Philips M. Ras/MAPK signaling from endomembranes. Mol. Oncol. 2009;3:297–307. doi: 10.1016/j.molonc.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Heo W.D., Inoue T., Park W.S., Kim M.L., Park B.O., Wandless T.J., Meyer T. PI(3,4,5)P3 and PI(4,5)P2 lipids target proteins with polybasic clusters to the plasma membrane. Science. 2006;314:1458–1461. doi: 10.1126/science.1134389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cho K.J., Park J.H., Piggott A.M., Salim A.A., Gorfe A.A., Parton R.G., Capon R.J., Lacey E., Hancock J.F. Staurosporines disrupt phosphatidylserine trafficking and mislocalize Ras proteins. J. Biol. Chem. 2012;287:43573–43584. doi: 10.1074/jbc.M112.424457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cho K.J., van der Hoeven D., Zhou Y., Maekawa M., Ma X., Chen W., Fairn G.D., Hancock J.F. Inhibition of acid sphingomyelinase depletes cellular phosphatidylserine and mislocalizes K-Ras from the plasma membrane. Mol. Cell. Biol. 2016;36:363–374. doi: 10.1128/MCB.00719-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gulyas G., Radvanszki G., Matuska R., Balla A., Hunyady L., Balla T., Varnai P. Plasma membrane phosphatidylinositol 4-phosphate and 4,5-bisphosphate determine the distribution and function of K-Ras4B but not H-Ras proteins. J. Biol. Chem. 2017;292:18862–18877. doi: 10.1074/jbc.M117.806679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gelabert-Baldrich M., Soriano-Castell D., Calvo M., Lu A., Vina-Vilaseca A., Rentero C., Pol A., Grinstein S., Enrich C., Tebar F. Dynamics of KRas on endosomes: Involvement of acidic phospholipids in its association. Faseb J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2014;28:3023–3037. doi: 10.1096/fj.13-241158. [DOI] [PubMed] [Google Scholar]
- 101.Lu A., Tebar F., Alvarez-Moya B., Lopez-Alcala C., Calvo M., Enrich C., Agell N., Nakamura T., Matsuda M., Bachs O. A clathrin-dependent pathway leads to KRas signaling on late endosomes en route to lysosomes. J. Cell Biol. 2009;184:863–879. doi: 10.1083/jcb.200807186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Miaczynska M., Bar-Sagi D. Signaling endosomes: Seeing is believing. Curr. Opin. Cell Biol. 2010;22:535–540. doi: 10.1016/j.ceb.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.von Zastrow M., Sorkin A. Signaling on the endocytic pathway. Curr. Opin. Cell Biol. 2007;19:436–445. doi: 10.1016/j.ceb.2007.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Omerovic J., Prior I.A. Compartmentalized signalling: Ras proteins and signalling nanoclusters. FEBS J. 2009;276:1817–1825. doi: 10.1111/j.1742-4658.2009.06928.x. [DOI] [PubMed] [Google Scholar]
- 105.Bivona T.G., Philips M.R. Ras pathway signaling on endomembranes. Curr. Opin. Cell Biol. 2003;15:136–142. doi: 10.1016/S0955-0674(03)00016-4. [DOI] [PubMed] [Google Scholar]
- 106.Stasyk T., Huber L.A. Spatio-temporal parameters of endosomal signaling in cancer: Implications for new treatment options. J. Cell. Biochem. 2016;117:836–843. doi: 10.1002/jcb.25418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Villalonga P., Lopez-Alcala C., Chiloeches A., Gil J., Marais R., Bachs O., Agell N. Calmodulin prevents activation of Ras by PKC in 3T3 fibroblasts. J. Biol. Chem. 2002;277:37929–37935. doi: 10.1074/jbc.M202245200. [DOI] [PubMed] [Google Scholar]
- 108.Garrido E., Lazaro J., Jaumot M., Agell N., Rubio-Martinez J. Modeling and subtleties of K-Ras and Calmodulin interaction. PLoS Comput. Biol. 2018;14:e1006552. doi: 10.1371/journal.pcbi.1006552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sperlich B., Kapoor S., Waldmann H., Winter R., Weise K. Regulation of K-Ras4B membrane binding by calmodulin. Biophys. J. 2016;111:113–122. doi: 10.1016/j.bpj.2016.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Jang H., Banerjee A., Marcus K., Makowski L., Mattos C., Gaponenko V., Nussinov R. The Structural basis of the farnesylated and methylated KRas4B interaction with calmodulin. Structure. 2019;27:1647–1659. doi: 10.1016/j.str.2019.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Grant B.M.M., Enomoto M., Back S.I., Lee K.Y., Gebregiworgis T., Ishiyama N., Ikura M., Marshall C.B. Calmodulin disrupts plasma membrane localization of farnesylated KRAS4b by sequestering its lipid moiety. Sci. Signal. 2020;13 doi: 10.1126/scisignal.aaz0344. [DOI] [PubMed] [Google Scholar]
- 112.Nussinov R., Tsai C.J., Chakrabarti M., Jang H. A new view of Ras isoforms in cancers. Cancer Res. 2016;76:18–23. doi: 10.1158/0008-5472.CAN-15-1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ashery U., Yizhar O., Rotblat B., Kloog Y. Nonconventional trafficking of Ras associated with Ras signal organization. Traffic. 2006;7:119–126. doi: 10.1111/j.1600-0854.2006.00459.x. [DOI] [PubMed] [Google Scholar]
- 114.Nussinov R., Muratcioglu S., Tsai C.J., Jang H., Gursoy A., Keskin O. The key role of calmodulin in KRAS-Driven adenocarcinomas. Mol. Cancer Res. 2015;13:1265–1273. doi: 10.1158/1541-7786.MCR-15-0165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lu S., Jang H., Gu S., Zhang J., Nussinov R. Drugging Ras GTPase: A comprehensive mechanistic and signaling structural view. Chem. Soc. Rev. 2016;45:4929–4952. doi: 10.1039/C5CS00911A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cook S.J., Lockyer P.J. Recent advances in Ca(2+)-dependent Ras regulation and cell proliferation. Cell Calcium. 2006;39:101–112. doi: 10.1016/j.ceca.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 117.Chavan T.S., Abraham S., Gaponenko V. Application of reductive (1)(3)C-methylation of lysines to enhance the sensitivity of conventional NMR methods. Molecules. 2013;18:7103–7119. doi: 10.3390/molecules18067103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bhagatji P., Leventis R., Rich R., Lin C.J., Silvius J.R. Multiple cellular proteins modulate the dynamics of K-ras association with the plasma membrane. Biophys. J. 2010;99:3327–3335. doi: 10.1016/j.bpj.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chandra A., Grecco H.E., Pisupati V., Perera D., Cassidy L., Skoulidis F., Ismail S.A., Hedberg C., Hanzal-Bayer M., Venkitaraman A.R., et al. The GDI-like solubilizing factor PDEdelta sustains the spatial organization and signalling of Ras family proteins. Nat. Cell Biol. 2011;14:148–158. doi: 10.1038/ncb2394. [DOI] [PubMed] [Google Scholar]
- 120.Figueroa C., Taylor J., Vojtek A.B. Prenylated Rab acceptor protein is a receptor for prenylated small GTPases. J. Biol. Chem. 2001;276:28219–28225. doi: 10.1074/jbc.M101763200. [DOI] [PubMed] [Google Scholar]
- 121.Barcelo C., Paco N., Beckett A.J., Alvarez-Moya B., Garrido E., Gelabert M., Tebar F., Jaumot M., Prior I., Agell N. Oncogenic K-ras segregates at spatially distinct plasma membrane signaling platforms according to its phosphorylation status. J. Cell Sci. 2013;126:4553–4559. doi: 10.1242/jcs.123737. [DOI] [PubMed] [Google Scholar]
- 122.Chavan T.S., Jang H., Khavrutskii L., Abraham S.J., Banerjee A., Freed B.C., Johannessen L., Tarasov S.G., Gaponenko V., Nussinov R., et al. High-affinity interaction of the K-Ras4B hypervariable region with the Ras active site. Biophys. J. 2015;109:2602–2613. doi: 10.1016/j.bpj.2015.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Alvarez-Moya B., Barcelo C., Tebar F., Jaumot M., Agell N. CaM interaction and Ser181 phosphorylation as new K-Ras signaling modulators. Small Gtpases. 2011;2:99–103. doi: 10.4161/sgtp.2.2.15555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Moodie S.A., Willumsen B.M., Weber M.J., Wolfman A. Complexes of Ras.GTP with Raf-1 and mitogen-activated protein kinase kinase. Science. 1993;260:1658–1661. doi: 10.1126/science.8503013. [DOI] [PubMed] [Google Scholar]
- 125.Koide H., Satoh T., Nakafuku M., Kaziro Y. GTP-dependent association of Raf-1 with Ha-Ras: Identification of Raf as a target downstream of Ras in mammalian cells. Proc. Natl. Acad. Sci. USA. 1993;90:8683–8686. doi: 10.1073/pnas.90.18.8683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lewis T.S., Shapiro P.S., Ahn N.G. Signal transduction through MAP kinase cascades. Adv. Cancer Res. 1998;74:49–139. doi: 10.1016/s0065-230x(08)60765-4. [DOI] [PubMed] [Google Scholar]
- 127.Robinson M.J., Cobb M.H. Mitogen-activated protein kinase pathways. Curr. Opin. Cell Biol. 1997;9:180–186. doi: 10.1016/S0955-0674(97)80061-0. [DOI] [PubMed] [Google Scholar]
- 128.Alessi D.R., Cohen P. Mechanism of activation and function of protein kinase B. Curr. Opin. Genet. Dev. 1998;8:55–62. doi: 10.1016/S0959-437X(98)80062-2. [DOI] [PubMed] [Google Scholar]
- 129.Coffer P.J., Jin J., Woodgett J.R. Protein kinase B (c-Akt): A multifunctional mediator of phosphatidylinositol 3-kinase activation. Biochem. J. 1998;335:1–13. doi: 10.1042/bj3350001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Rodriguez-Viciana P., Warne P.H., Dhand R., Vanhaesebroeck B., Gout I., Fry M.J., Waterfield M.D., Downward J. Phosphatidylinositol-3-OH kinase as a direct target of Ras. Nature. 1994;370:527–532. doi: 10.1038/370527a0. [DOI] [PubMed] [Google Scholar]
- 131.Zippel R., Balestrini M., Lomazzi M., Sturani E. Calcium and calmodulin are essential for Ras-GRF1-mediated activation of the Ras pathway by lysophosphatidic acid. Exp. Cell Res. 2000;258:403–408. doi: 10.1006/excr.2000.4937. [DOI] [PubMed] [Google Scholar]
- 132.Gotoh T., Tian X., Feig L.A. Prenylation of target GTPases contributes to signaling specificity of Ras-guanine nucleotide exchange factors. J. Biol. Chem. 2001;276:38029–38035. doi: 10.1074/jbc.M104658200. [DOI] [PubMed] [Google Scholar]
- 133.Oh J.S., Manzerra P., Kennedy M.B. Regulation of the neuron-specific Ras GTPase-activating protein, synGAP, by Ca2+/calmodulin-dependent protein kinase II. J. Biol. Chem. 2004;279:17980–17988. doi: 10.1074/jbc.M314109200. [DOI] [PubMed] [Google Scholar]
- 134.Norum J.H., Methi T., Mattingly R.R., Levy F.O. Endogenous expression and protein kinase A-dependent phosphorylation of the guanine nucleotide exchange factor Ras-GRF1 in human embryonic kidney 293 cells. FEBS J. 2005;272:2304–2316. doi: 10.1111/j.1742-4658.2005.04658.x. [DOI] [PubMed] [Google Scholar]
- 135.Tian X., Feig L.A. Age-dependent participation of Ras-GRF proteins in coupling calcium-permeable AMPA glutamate receptors to Ras/Erk signaling in cortical neurons. J. Biol. Chem. 2006;281:7578–7582. doi: 10.1074/jbc.M512060200. [DOI] [PubMed] [Google Scholar]
- 136.Farnsworth C.L., Freshney N.W., Rosen L.B., Ghosh A., Greenberg M.E., Feig L.A. Calcium activation of Ras mediated by neuronal exchange factor Ras-GRF. Nature. 1995;376:524–527. doi: 10.1038/376524a0. [DOI] [PubMed] [Google Scholar]
- 137.Feig L.A. Regulation of neuronal function by Ras-GRF exchange factors. Genes Cancer. 2011;2:306–319. doi: 10.1177/1947601911408077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Walkup W.G.T., Washburn L., Sweredoski M.J., Carlisle H.J., Graham R.L., Hess S., Kennedy M.B. Phosphorylation of synaptic GTPase-activating protein (synGAP) by Ca2+/calmodulin-dependent protein kinase II (CaMKII) and cyclin-dependent kinase 5 (CDK5) alters the ratio of its GAP activity toward Ras and Rap GTPases. J. Biol. Chem. 2015;290:4908–4927. doi: 10.1074/jbc.M114.614420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Rockliffe N., Gawler D. Differential mechanisms of glutamate receptor regulation of SynGAP in cortical neurones. FEBS Lett. 2006;580:831–838. doi: 10.1016/j.febslet.2005.12.100. [DOI] [PubMed] [Google Scholar]
- 140.Tebar F., Villalonga P., Sorkina T., Agell N., Sorkin A., Enrich C. Calmodulin regulates intracellular trafficking of epidermal growth factor receptor and the MAPK signaling pathway. Mol. Biol. Cell. 2002;13:2057–2068. doi: 10.1091/mbc.01-12-0571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Tebar F., Llado A., Enrich C. Role of calmodulin in the modulation of the MAPK signalling pathway and the transactivation of epidermal growth factor receptor mediated by PKC. FEBS Lett. 2002;517:206–210. doi: 10.1016/S0014-5793(02)02624-8. [DOI] [PubMed] [Google Scholar]
- 142.Moreto J., Vidal-Quadras M., Pol A., Santos E., Grewal T., Enrich C., Tebar F. Differential involvement of H- and K-Ras in Raf-1 activation determines the role of calmodulin in MAPK signaling. Cell. Signal. 2009;21:1827–1836. doi: 10.1016/j.cellsig.2009.07.018. [DOI] [PubMed] [Google Scholar]
- 143.Moreto J., Llado A., Vidal-Quadras M., Calvo M., Pol A., Enrich C., Tebar F. Calmodulin modulates H-Ras mediated Raf-1 activation. Cell Signal. 2008;20:1092–1103. doi: 10.1016/j.cellsig.2008.01.022. [DOI] [PubMed] [Google Scholar]
- 144.Bosch M., Gil J., Bachs O., Agell N. Calmodulin inhibitor W13 induces sustained activation of ERK2 and expression of p21(cip1) J. Biol. Chem. 1998;273:22145–22150. doi: 10.1074/jbc.273.34.22145. [DOI] [PubMed] [Google Scholar]
- 145.Yoshiki S., Matsunaga-Udagawa R., Aoki K., Kamioka Y., Kiyokawa E., Matsuda M. Ras and calcium signaling pathways converge at Raf1 via the Shoc2 scaffold protein. Mol. Biol. Cell. 2010;21:1088–1096. doi: 10.1091/mbc.e09-06-0455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Belcheva M.M., Szucs M., Wang D., Sadee W., Coscia C.J. Mu-Opioid receptor-mediated ERK activation involves calmodulin-dependent epidermal growth factor receptor transactivation. J. Biol. Chem. 2001;276:33847–33853. doi: 10.1074/jbc.M101535200. [DOI] [PubMed] [Google Scholar]
- 147.Diaz-Rodriguez E., Esparis-Ogando A., Montero J.C., Yuste L., Pandiella A. Stimulation of cleavage of membrane proteins by calmodulin inhibitors. Biochem. J. 2000;346:359–367. doi: 10.1042/bj3460359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Dong J., Wiley H.S. Trafficking and proteolytic release of epidermal growth factor receptor ligands are modulated by their membrane-anchoring domains. J. Biol. Chem. 2000;275:557–564. doi: 10.1074/jbc.275.1.557. [DOI] [PubMed] [Google Scholar]
- 149.Prenzel N., Zwick E., Daub H., Leserer M., Abraham R., Wallasch C., Ullrich A. EGF receptor transactivation by G-protein-coupled receptors requires metalloproteinase cleavage of proHB-EGF. Nature. 1999;402:884–888. doi: 10.1038/47260. [DOI] [PubMed] [Google Scholar]
- 150.Li H., Ruano M.J., Villalobo A. Endogenous calmodulin interacts with the epidermal growth factor receptor in living cells. FEBS Lett. 2004;559:175–180. doi: 10.1016/S0014-5793(04)00067-5. [DOI] [PubMed] [Google Scholar]
- 151.San Jose E., Benguria A., Geller P., Villalobo A. Calmodulin inhibits the epidermal growth factor receptor tyrosine kinase. J. Biol. Chem. 1992;267:15237–15245. [PubMed] [Google Scholar]
- 152.Martin-Nieto J., Villalobo A. The human epidermal growth factor receptor contains a juxtamembrane calmodulin-binding site. Biochemistry. 1998;37:227–236. doi: 10.1021/bi971765v. [DOI] [PubMed] [Google Scholar]
- 153.Feinmesser R.L., Wicks S.J., Taverner C.J., Chantry A. Ca2+/calmodulin-dependent kinase II phosphorylates the epidermal growth factor receptor on multiple sites in the cytoplasmic tail and serine 744 within the kinase domain to regulate signal generation. J. Biol. Chem. 1999;274:16168–16173. doi: 10.1074/jbc.274.23.16168. [DOI] [PubMed] [Google Scholar]
- 154.Bivona T.G., Quatela S.E., Bodemann B.O., Ahearn I.M., Soskis M.J., Mor A., Miura J., Wiener H.H., Wright L., Saba S.G., et al. PKC regulates a farnesyl-electrostatic switch on K-Ras that promotes its association with Bcl-XL on mitochondria and induces apoptosis. Mol. Cell. 2006;21:481–493. doi: 10.1016/j.molcel.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 155.Melien O., Christoffersen T., Sioud M. Evidence for the involvement of Gi2 in activation of extracellular signal-regulated kinases in hepatocytes. BMC Cell Biol. 2001;2:13. doi: 10.1186/1471-2121-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Egea J., Espinet C., Soler R.M., Peiro S., Rocamora N., Comella J.X. Nerve growth factor activation of the extracellular signal-regulated kinase pathway is modulated by Ca(2+) and calmodulin. Mol. Cell. Biol. 2000;20:1931–1946. doi: 10.1128/MCB.20.6.1931-1946.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Buchanan F.G., Elliot C.M., Gibbs M., Exton J.H. Translocation of the Rac1 guanine nucleotide exchange factor Tiam1 induced by platelet-derived growth factor and lysophosphatidic acid. J. Biol. Chem. 2000;275:9742–9748. doi: 10.1074/jbc.275.13.9742. [DOI] [PubMed] [Google Scholar]
- 158.Fleming I.N., Elliott C.M., Buchanan F.G., Downes C.P., Exton J.H. Ca2+/calmodulin-dependent protein kinase II regulates Tiam1 by reversible protein phosphorylation. J. Biol. Chem. 1999;274:12753–12758. doi: 10.1074/jbc.274.18.12753. [DOI] [PubMed] [Google Scholar]
- 159.Perez-Garcia M.J., Cena V., de Pablo Y., Llovera M., Comella J.X., Soler R.M. Glial cell line-derived neurotrophic factor increases intracellular calcium concentration. Role of calcium/calmodulin in the activation of the phosphatidylinositol 3-kinase pathway. J. Biol. Chem. 2004;279:6132–6142. doi: 10.1074/jbc.M308367200. [DOI] [PubMed] [Google Scholar]
- 160.Joyal J.L., Burks D.J., Pons S., Matter W.F., Vlahos C.J., White M.F., Sacks D.B. Calmodulin activates phosphatidylinositol 3-kinase. J. Biol. Chem. 1997;272:28183–28186. doi: 10.1074/jbc.272.45.28183. [DOI] [PubMed] [Google Scholar]
- 161.Fischer R., Julsgart J., Berchtold M.W. High affinity calmodulin target sequence in the signalling molecule PI 3-kinase. FEBS Lett. 1998;425:175–177. doi: 10.1016/S0014-5793(98)00225-7. [DOI] [PubMed] [Google Scholar]
- 162.Ballester R., Furth M.E., Rosen O.M. Phorbol ester- and protein kinase C-mediated phosphorylation of the cellular Kirsten ras gene product. J. Biol. Chem. 1987;262:2688–2695. [PubMed] [Google Scholar]
- 163.Eisenberg S., Giehl K., Henis Y.I., Ehrlich M. Differential interference of chlorpromazine with the membrane interactions of oncogenic K-Ras and its effects on cell growth. J. Biol. Chem. 2008;283:27279–27288. doi: 10.1074/jbc.M804589200. [DOI] [PubMed] [Google Scholar]
- 164.Philips M.R. Ras hitchhikes on PDE6delta. Nat. Cell Biol. 2012;14:128–129. doi: 10.1038/ncb2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Liao J., Planchon S.M., Wolfman J.C., Wolfman A. Growth factor-dependent AKT activation and cell migration requires the function of c-K(B)-Ras versus other cellular ras isoforms. J. Biol. Chem. 2006;281:29730–29738. doi: 10.1074/jbc.M600668200. [DOI] [PubMed] [Google Scholar]
- 166.Nussinov R., Wang G., Tsai C.J., Jang H., Lu S., Banerjee A., Zhang J., Gaponenko V. Calmodulin and PI3K Signaling in KRAS Cancers. Trends Cancer. 2017;3:214–224. doi: 10.1016/j.trecan.2017.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Wang M.T., Holderfield M., Galeas J., Delrosario R., To M.D., Balmain A., McCormick F. K-Ras promotes tumorigenicity through suppression of non-canonical Wnt signaling. Cell. 2015;163:1237–1251. doi: 10.1016/j.cell.2015.10.041. [DOI] [PubMed] [Google Scholar]
- 168.Liou J.S., Chen J.S., Faller D.V. Characterization of p21Ras-mediated apoptosis induced by protein kinase C inhibition and application to human tumor cell lines. J. Cell. Physiol. 2004;198:277–294. doi: 10.1002/jcp.10409. [DOI] [PubMed] [Google Scholar]
- 169.Xia S., Chen Z., Forman L.W., Faller D.V. PKCdelta survival signaling in cells containing an activated p21Ras protein requires PDK1. Cell Signal. 2009;21:502–508. doi: 10.1016/j.cellsig.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Xia S., Forman L.W., Faller D.V. Protein kinase C delta is required for survival of cells expressing activated p21RAS. J. Biol. Chem. 2007;282:13199–13210. doi: 10.1074/jbc.M610225200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Barcelo C., Etchin J., Mansour M.R., Sanda T., Ginesta M.M., Sanchez-Arevalo Lobo V.J., Real F.X., Capella G., Estanyol J.M., Jaumot M., et al. Ribonucleoprotein HNRNPA2B1 interacts with and regulates oncogenic KRAS in pancreatic ductal adenocarcinoma cells. Gastroenterology. 2014;147:882–892.e888. doi: 10.1053/j.gastro.2014.06.041. [DOI] [PubMed] [Google Scholar]
- 172.Barcelo C., Paco N., Morell M., Alvarez-Moya B., Bota-Rabassedas N., Jaumot M., Vilardell F., Capella G., Agell N. Phosphorylation at Ser-181 of oncogenic KRAS is required for tumor growth. Cancer Res. 2014;74:1190–1199. doi: 10.1158/0008-5472.CAN-13-1750. [DOI] [PubMed] [Google Scholar]
- 173.Bishop A.L., Hall A. Rho GTPases and their effector proteins. Biochem. J. 2000;348:241–255. doi: 10.1042/bj3480241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Didsbury J., Weber R.F., Bokoch G.M., Evans T., Snyderman R. Rac, a novel ras-related family of proteins that are botulinum toxin substrates. J. Biol. Chem. 1989;264:16378–16382. [PubMed] [Google Scholar]
- 175.Parri M., Chiarugi P. Rac and Rho GTPases in cancer cell motility control. Cell Commun. Signal. 2010;8:23. doi: 10.1186/1478-811X-8-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Bosco E.E., Mulloy J.C., Zheng Y. Rac1 GTPase: A “Rac“ of all trades. Cell. Mol. Life Sci. 2009;66:370–374. doi: 10.1007/s00018-008-8552-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Ridley A.J. Rho GTPases and actin dynamics in membrane protrusions and vesicle trafficking. Trends Cell Biol. 2006;16:522–529. doi: 10.1016/j.tcb.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 178.Heasman S.J., Ridley A.J. Mammalian Rho GTPases: New insights into their functions from in vivo studies. Nat. Rev. Mol. Cell Biol. 2008;9:690–701. doi: 10.1038/nrm2476. [DOI] [PubMed] [Google Scholar]
- 179.Bustelo X.R., Sauzeau V., Berenjeno I.M. GTP-binding proteins of the Rho/Rac family: Regulation, effectors and functions in vivo. Bioessays News Rev. Mol. Cell. Dev. Biol. 2007;29:356–370. doi: 10.1002/bies.20558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Bolis A., Corbetta S., Cioce A., de Curtis I. Differential distribution of Rac1 and Rac3 GTPases in the developing mouse brain: Implications for a role of Rac3 in Purkinje cell differentiation. Eur. J. Neurosci. 2003;18:2417–2424. doi: 10.1046/j.1460-9568.2003.02938.x. [DOI] [PubMed] [Google Scholar]
- 181.Li Q., Ho C.S., Marinescu V., Bhatti H., Bokoch G.M., Ernst S.A., Holz R.W., Stuenkel E.L. Facilitation of Ca(2+)-dependent exocytosis by Rac1-GTPase in bovine chromaffin cells. J. Physiol. 2003;550:431–445. doi: 10.1113/jphysiol.2003.039073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Hong-Geller E., Cerione R.A. Cdc42 and Rac stimulate exocytosis of secretory granules by activating the IP(3)/calcium pathway in RBL-2H3 mast cells. J. Cell Biol. 2000;148:481–494. doi: 10.1083/jcb.148.3.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Marei H., Malliri A. Rac1 in human diseases: The therapeutic potential of targeting Rac1 signaling regulatory mechanisms. Small Gtpases. 2017;8:139–163. doi: 10.1080/21541248.2016.1211398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Hordijk P.L., ten Klooster J.P., van der Kammen R.A., Michiels F., Oomen L.C., Collard J.G. Inhibition of invasion of epithelial cells by Tiam1-Rac signaling. Science. 1997;278:1464–1466. doi: 10.1126/science.278.5342.1464. [DOI] [PubMed] [Google Scholar]
- 185.Sahai E., Marshall C.J. RHO-GTPases and cancer. Nat. Rev. Cancer. 2002;2:133–142. doi: 10.1038/nrc725. [DOI] [PubMed] [Google Scholar]
- 186.Frost J.A., Khokhlatchev A., Stippec S., White M.A., Cobb M.H. Differential effects of PAK1-activating mutations reveal activity-dependent and -independent effects on cytoskeletal regulation. J. Biol. Chem. 1998;273:28191–28198. doi: 10.1074/jbc.273.43.28191. [DOI] [PubMed] [Google Scholar]
- 187.Yang N., Higuchi O., Ohashi K., Nagata K., Wada A., Kangawa K., Nishida E., Mizuno K. Cofilin phosphorylation by LIM-kinase 1 and its role in Rac-mediated actin reorganization. Nature. 1998;393:809–812. doi: 10.1038/31735. [DOI] [PubMed] [Google Scholar]
- 188.Vidal C., Geny B., Melle J., Jandrot-Perrus M., Fontenay-Roupie M. Cdc42/Rac1-dependent activation of the p21-activated kinase (PAK) regulates human platelet lamellipodia spreading: Implication of the cortical-actin binding protein cortactin. Blood. 2002;100:4462–4469. doi: 10.1182/blood.V100.13.4462. [DOI] [PubMed] [Google Scholar]
- 189.Webb B.A., Zhou S., Eves R., Shen L., Jia L., Mak A.S. Phosphorylation of cortactin by p21-activated kinase. Arch. Biochem. Biophys. 2006;456:183–193. doi: 10.1016/j.abb.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 190.Sauvonnet N., Dujeancourt A., Dautry-Varsat A. Cortactin and dynamin are required for the clathrin-independent endocytosis of gammac cytokine receptor. J. Cell Biol. 2005;168:155–163. doi: 10.1083/jcb.200406174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Grassart A., Meas-Yedid V., Dufour A., Olivo-Marin J.C., Dautry-Varsat A., Sauvonnet N. Pak1 phosphorylation enhances cortactin-N-WASP interaction in clathrin-caveolin-independent endocytosis. Traffic. 2010;11:1079–1091. doi: 10.1111/j.1600-0854.2010.01075.x. [DOI] [PubMed] [Google Scholar]
- 192.Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- 193.Rossman K.L., Der C.J., Sondek J. GEF means go: Turning on RHO GTPases with guanine nucleotide-exchange factors. Nat. Rev. Mol. Cell Biol. 2005;6:167–180. doi: 10.1038/nrm1587. [DOI] [PubMed] [Google Scholar]
- 194.Vetter I.R., Wittinghofer A. The guanine nucleotide-binding switch in three dimensions. Science. 2001;294:1299–1304. doi: 10.1126/science.1062023. [DOI] [PubMed] [Google Scholar]
- 195.Hoffman G.R., Nassar N., Cerione R.A. Structure of the Rho family GTP-binding protein Cdc42 in complex with the multifunctional regulator RhoGDI. Cell. 2000;100:345–356. doi: 10.1016/S0092-8674(00)80670-4. [DOI] [PubMed] [Google Scholar]
- 196.Marei H., Malliri A. GEFs: Dual regulation of Rac1 signaling. Small Gtpases. 2017;8:90–99. doi: 10.1080/21541248.2016.1202635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Olofsson B. Rho guanine dissociation inhibitors: Pivotal molecules in cellular signalling. Cell. Signal. 1999;11:545–554. doi: 10.1016/S0898-6568(98)00063-1. [DOI] [PubMed] [Google Scholar]
- 198.DerMardirossian C., Bokoch G.M. GDIs: Central regulatory molecules in Rho GTPase activation. Trends Cell Biol. 2005;15:356–363. doi: 10.1016/j.tcb.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 199.Grizot S., Faure J., Fieschi F., Vignais P.V., Dagher M.C., Pebay-Peyroula E. Crystal structure of the Rac1-RhoGDI complex involved in nadph oxidase activation. Biochemistry. 2001;40:10007–10013. doi: 10.1021/bi010288k. [DOI] [PubMed] [Google Scholar]
- 200.Price L.S., Langeslag M., ten Klooster J.P., Hordijk P.L., Jalink K., Collard J.G. Calcium signaling regulates translocation and activation of Rac. J. Biol. Chem. 2003;278:39413–39421. doi: 10.1074/jbc.M302083200. [DOI] [PubMed] [Google Scholar]
- 201.Schmidt A., Hall A. Guanine nucleotide exchange factors for Rho GTPases: Turning on the switch. Genes Dev. 2002;16:1587–1609. doi: 10.1101/gad.1003302. [DOI] [PubMed] [Google Scholar]
- 202.Eva A., Aaronson S.A. Isolation of a new human oncogene from a diffuse B-cell lymphoma. Nature. 1985;316:273–275. doi: 10.1038/316273a0. [DOI] [PubMed] [Google Scholar]
- 203.Zheng Y. Dbl family guanine nucleotide exchange factors. Trends Biochem. Sci. 2001;26:724–732. doi: 10.1016/S0968-0004(01)01973-9. [DOI] [PubMed] [Google Scholar]
- 204.Cote J.F., Vuori K. Identification of an evolutionarily conserved superfamily of DOCK180-related proteins with guanine nucleotide exchange activity. J. Cell Sci. 2002;115:4901–4913. doi: 10.1242/jcs.00219. [DOI] [PubMed] [Google Scholar]
- 205.Welch H.C., Coadwell W.J., Stephens L.R., Hawkins P.T. Phosphoinositide 3-kinase-dependent activation of Rac. FEBS Lett. 2003;546:93–97. doi: 10.1016/S0014-5793(03)00454-X. [DOI] [PubMed] [Google Scholar]
- 206.Palamidessi A., Frittoli E., Garre M., Faretta M., Mione M., Testa I., Diaspro A., Lanzetti L., Scita G., Di Fiore P.P. Endocytic trafficking of Rac is required for the spatial restriction of signaling in cell migration. Cell. 2008;134:135–147. doi: 10.1016/j.cell.2008.05.034. [DOI] [PubMed] [Google Scholar]
- 207.Scita G., Di Fiore P.P. The endocytic matrix. Nature. 2010;463:464–473. doi: 10.1038/nature08910. [DOI] [PubMed] [Google Scholar]
- 208.Menard L., Parker P.J., Kermorgant S. Receptor tyrosine kinase c-Met controls the cytoskeleton from different endosomes via different pathways. Nat. Commun. 2014;5:3907. doi: 10.1038/ncomms4907. [DOI] [PubMed] [Google Scholar]
- 209.Navarro-Lerida I., Sanchez-Perales S., Calvo M., Rentero C., Zheng Y., Enrich C., Del Pozo M.A. A palmitoylation switch mechanism regulates Rac1 function and membrane organization. EMBO J. 2012;31:534–551. doi: 10.1038/emboj.2011.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Briggs M.W., Sacks D.B. IQGAP proteins are integral components of cytoskeletal regulation. EMBO Rep. 2003;4:571–574. doi: 10.1038/sj.embor.embor867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Xu B., Bhullar R.P. Regulation of Rac1 and Cdc42 activation in thrombin- and collagen-stimulated CHRF-288-11 cells. Mol. Cell. Biochem. 2011;353:73–79. doi: 10.1007/s11010-011-0776-7. [DOI] [PubMed] [Google Scholar]
- 212.Soriano-Castell D., Chavero A., Rentero C., Bosch M., Vidal-Quadras M., Pol A., Enrich C., Tebar F. ROCK1 is a novel Rac1 effector to regulate tubular endocytic membrane formation during clathrin-independent endocytosis. Sci. Rep. 2017;7:6866. doi: 10.1038/s41598-017-07130-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Tolias K.F., Hartwig J.H., Ishihara H., Shibasaki Y., Cantley L.C., Carpenter C.L. Type Ialpha phosphatidylinositol-4-phosphate 5-kinase mediates Rac-dependent actin assembly. Curr. Biol. 2000;10:153–156. doi: 10.1016/S0960-9822(00)00315-8. [DOI] [PubMed] [Google Scholar]
- 214.Halstead J.R., Savaskan N.E., van den Bout I., Van Horck F., Hajdo-Milasinovic A., Snell M., Keune W.J., Ten Klooster J.P., Hordijk P.L., Divecha N. Rac controls PIP5K localisation and PtdIns(4,5)P(2) synthesis, which modulates vinculin localisation and neurite dynamics. J. Cell Sci. 2010;123:3535–3546. doi: 10.1242/jcs.062679. [DOI] [PubMed] [Google Scholar]
- 215.van Hennik P.B., ten Klooster J.P., Halstead J.R., Voermans C., Anthony E.C., Divecha N., Hordijk P.L. The C-terminal domain of Rac1 contains two motifs that control targeting and signaling specificity. J. Biol. Chem. 2003;278:39166–39175. doi: 10.1074/jbc.M307001200. [DOI] [PubMed] [Google Scholar]
- 216.van den Bout I., Divecha N. PIP5K-driven PtdIns(4,5)P2 synthesis: Regulation and cellular functions. J. Cell Sci. 2009;122:3837–3850. doi: 10.1242/jcs.056127. [DOI] [PubMed] [Google Scholar]
- 217.Kwiatkowska K. One lipid, multiple functions: How various pools of PI(4,5)P(2) are created in the plasma membrane. Cell. Mol. Life Sci. 2010;67:3927–3946. doi: 10.1007/s00018-010-0432-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Apodaca G., Enrich C., Mostov K.E. The calmodulin antagonist, W-13, alters transcytosis, recycling, and the morphology of the endocytic pathway in Madin-Darby canine kidney cells. J. Biol. Chem. 1994;269:19005–19013. [PubMed] [Google Scholar]
- 219.Mostov K.E., Altschuler Y., Chapin S.J., Enrich C., Low S.H., Luton F., Richman-Eisenstat J., Singer K.L., Tang K., Weimbs T. Regulation of protein traffic in polarized epithelial cells: The polymeric immunoglobulin receptor model. Cold Spring Harb. Symp. Quant. Biol. 1995;60:775–781. doi: 10.1101/SQB.1995.060.01.083. [DOI] [PubMed] [Google Scholar]
- 220.Lian J.P., Crossley L., Zhan Q., Huang R., Coffer P., Toker A., Robinson D., Badwey J.A. Antagonists of calcium fluxes and calmodulin block activation of the p21-activated protein kinases in neutrophils. J. Immunol. 2001;166:2643–2650. doi: 10.4049/jimmunol.166.4.2643. [DOI] [PubMed] [Google Scholar]
- 221.Filippi B.M., Mariggio S., Pulvirenti T., Corda D. SRC-dependent signalling regulates actin ruffle formation induced by glycerophosphoinositol 4-phosphate. Biochim. Biophys. Acta. 2008;1783:2311–2322. doi: 10.1016/j.bbamcr.2008.07.021. [DOI] [PubMed] [Google Scholar]
- 222.Mertens A.E., Roovers R.C., Collard J.G. Regulation of Tiam1-Rac signalling. FEBS Lett. 2003;546:11–16. doi: 10.1016/S0014-5793(03)00435-6. [DOI] [PubMed] [Google Scholar]
- 223.Fleming I.N., Elliott C.M., Exton J.H. Phospholipase C-gamma, protein kinase C and Ca2+/calmodulin-dependent protein kinase II are involved in platelet-derived growth factor-induced phosphorylation of Tiam1. FEBS Lett. 1998;429:229–233. doi: 10.1016/S0014-5793(98)00566-3. [DOI] [PubMed] [Google Scholar]
- 224.Nouri K., Timson D.J., Ahmadian M.R. New model for the interaction of IQGAP1 with CDC42 and RAC1. Small Gtpases. 2020;11:16–22. doi: 10.1080/21541248.2017.1321169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Brown M.D., Bry L., Li Z., Sacks D.B. IQGAP1 regulates Salmonella invasion through interactions with actin, Rac1, and Cdc42. J. Biol. Chem. 2007;282:30265–30272. doi: 10.1074/jbc.M702537200. [DOI] [PubMed] [Google Scholar]
- 226.Kuroda S., Fukata M., Nakagawa M., Kaibuchi K. Cdc42, Rac1, and their effector IQGAP1 as molecular switches for cadherin-mediated cell-cell adhesion. Biochem. Biophys. Res. Commun. 1999;262:1–6. doi: 10.1006/bbrc.1999.1122. [DOI] [PubMed] [Google Scholar]
- 227.Nouri K., Fansa E.K., Amin E., Dvorsky R., Gremer L., Willbold D., Schmitt L., Timson D.J., Ahmadian M.R. IQGAP1 Interaction with RHO Family Proteins Revisited: Kinetic and equilibrium evidence for multiple distinct binding sites. J. Biol. Chem. 2016;291:26364–26376. doi: 10.1074/jbc.M116.752121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Kuroda S., Fukata M., Kobayashi K., Nakafuku M., Nomura N., Iwamatsu A., Kaibuchi K. Identification of IQGAP as a putative target for the small GTPases, Cdc42 and Rac1. J. Biol. Chem. 1996;271:23363–23367. doi: 10.1074/jbc.271.38.23363. [DOI] [PubMed] [Google Scholar]
- 229.Brill S., Li S., Lyman C.W., Church D.M., Wasmuth J.J., Weissbach L., Bernards A., Snijders A.J. The Ras GTPase-activating-protein-related human protein IQGAP2 harbors a potential actin binding domain and interacts with calmodulin and Rho family GTPases. Mol. Cell. Biol. 1996;16:4869–4878. doi: 10.1128/MCB.16.9.4869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Mataraza J.M., Briggs M.W., Li Z., Entwistle A., Ridley A.J., Sacks D.B. IQGAP1 promotes cell motility and invasion. J. Biol. Chem. 2003;278:41237–41245. doi: 10.1074/jbc.M304838200. [DOI] [PubMed] [Google Scholar]
- 231.Mataraza J.M., Li Z., Jeong H.W., Brown M.D., Sacks D.B. Multiple proteins mediate IQGAP1-stimulated cell migration. Cell Signal. 2007;19:1857–1865. doi: 10.1016/j.cellsig.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.White C.D., Brown M.D., Sacks D.B. IQGAPs in cancer: A family of scaffold proteins underlying tumorigenesis. FEBS Lett. 2009;583:1817–1824. doi: 10.1016/j.febslet.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Watanabe T., Wang S., Noritake J., Sato K., Fukata M., Takefuji M., Nakagawa M., Izumi N., Akiyama T., Kaibuchi K. Interaction with IQGAP1 links APC to Rac1, Cdc42, and actin filaments during cell polarization and migration. Dev. Cell. 2004;7:871–883. doi: 10.1016/j.devcel.2004.10.017. [DOI] [PubMed] [Google Scholar]
- 234.Pelikan-Conchaudron A., Le Clainche C., Didry D., Carlier M.F. The IQGAP1 protein is a calmodulin-regulated barbed end capper of actin filaments: Possible implications in its function in cell migration. J. Biol. Chem. 2011;286:35119–35128. doi: 10.1074/jbc.M111.258772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Andrews W.J., Bradley C.A., Hamilton E., Daly C., Mallon T., Timson D.J. A calcium-dependent interaction between calmodulin and the calponin homology domain of human IQGAP1. Mol. Cell. Biochem. 2012;371:217–223. doi: 10.1007/s11010-012-1438-0. [DOI] [PubMed] [Google Scholar]
- 236.Liu J., Kurella V.B., LeCour L., Jr., Vanagunas T., Worthylake D.K. The IQGAP1 N-Terminus Forms Dimers, and the Dimer Interface Is Required for Binding F-Actin and Calcium-Bound Calmodulin. Biochemistry. 2016;55:6433–6444. doi: 10.1021/acs.biochem.6b00745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Mateer S.C., McDaniel A.E., Nicolas V., Habermacher G.M., Lin M.J., Cromer D.A., King M.E., Bloom G.S. The mechanism for regulation of the F-actin binding activity of IQGAP1 by calcium/calmodulin. J. Biol. Chem. 2002;277:12324–12333. doi: 10.1074/jbc.M109535200. [DOI] [PubMed] [Google Scholar]
- 238.Li Z., Sacks D.B. Elucidation of the interaction of calmodulin with the IQ motifs of IQGAP1. J. Biol. Chem. 2003;278:4347–4352. doi: 10.1074/jbc.M208579200. [DOI] [PubMed] [Google Scholar]
- 239.Zhang M., Li Z., Jang H., Hedman A.C., Sacks D.B., Nussinov R. Ca(2+)-Dependent switch of calmodulin interaction mode with tandem IQ motifs in the scaffolding protein IQGAP1. Biochemistry. 2019;58:4903–4911. doi: 10.1021/acs.biochem.9b00854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Li Q., Stuenkel E.L. Calcium negatively modulates calmodulin interaction with IQGAP1. Biochem. Biophys. Res. Commun. 2004;317:787–795. doi: 10.1016/j.bbrc.2004.03.119. [DOI] [PubMed] [Google Scholar]
- 241.Brown M.D., Sacks D.B. IQGAP1 in cellular signaling: Bridging the GAP. Trends Cell Biol. 2006;16:242–249. doi: 10.1016/j.tcb.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 242.Brown M.D., Bry L., Li Z., Sacks D.B. Actin pedestal formation by enteropathogenic Escherichia coli is regulated by IQGAP1, calcium, and calmodulin. J. Biol. Chem. 2008;283:35212–35222. doi: 10.1074/jbc.M803477200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Fleming I.N., Batty I.H., Prescott A.R., Gray A., Kular G.S., Stewart H., Downes C.P. Inositol phospholipids regulate the guanine-nucleotide-exchange factor Tiam1 by facilitating its binding to the plasma membrane and regulating GDP/GTP exchange on Rac1. Biochem. J. 2004;382:857–865. doi: 10.1042/BJ20040916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Sander E.E., van Delft S., ten Klooster J.P., Reid T., van der Kammen R.A., Michiels F., Collard J.G. Matrix-dependent Tiam1/Rac signaling in epithelial cells promotes either cell-cell adhesion or cell migration and is regulated by phosphatidylinositol 3-kinase. J. Cell Biol. 1998;143:1385–1398. doi: 10.1083/jcb.143.5.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Han J., Luby-Phelps K., Das B., Shu X., Xia Y., Mosteller R.D., Krishna U.M., Falck J.R., White M.A., Broek D. Role of substrates and products of PI 3-kinase in regulating activation of Rac-related guanosine triphosphatases by Vav. Science. 1998;279:558–560. doi: 10.1126/science.279.5350.558. [DOI] [PubMed] [Google Scholar]
- 246.Swulius M.T., Waxham M.N. Ca(2+)/calmodulin-dependent protein kinases. Cell. Mol. Life Sci. 2008;65:2637–2657. doi: 10.1007/s00018-008-8086-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Wayman G.A., Tokumitsu H., Davare M.A., Soderling T.R. Analysis of CaM-kinase signaling in cells. Cell Calcium. 2011;50:1–8. doi: 10.1016/j.ceca.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Herring B.E., Nicoll R.A. Kalirin and Trio proteins serve critical roles in excitatory synaptic transmission and LTP. Proc. Natl. Acad. Sci. USA. 2016;113:2264–2269. doi: 10.1073/pnas.1600179113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Saneyoshi T., Matsuno H., Suzuki A., Murakoshi H., Hedrick N.G., Agnello E., O‘Connell R., Stratton M.M., Yasuda R., Hayashi Y. Reciprocal activation within a kinase-effector complex underlying persistence of structural LTP. Neuron. 2019;102:1199–1210. doi: 10.1016/j.neuron.2019.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Saneyoshi T., Wayman G., Fortin D., Davare M., Hoshi N., Nozaki N., Natsume T., Soderling T.R. Activity-dependent synaptogenesis: Regulation by a CaM-kinase kinase/CaM-kinase I/betaPIX signaling complex. Neuron. 2008;57:94–107. doi: 10.1016/j.neuron.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Davare M.A., Saneyoshi T., Soderling T.R. Calmodulin-kinases regulate basal and estrogen stimulated medulloblastoma migration via Rac1. J. Neuro-Oncol. 2011;104:65–82. doi: 10.1007/s11060-010-0472-6. [DOI] [PubMed] [Google Scholar]
- 252.Yuan K., Chung L.W., Siegal G.P., Zayzafoon M. Alpha-CaMKII controls the growth of human osteosarcoma by regulating cell cycle progression. Lab. Investig. 2007;87:938–950. doi: 10.1038/labinvest.3700658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Tolias K.F., Bikoff J.B., Burette A., Paradis S., Harrar D., Tavazoie S., Weinberg R.J., Greenberg M.E. The Rac1-GEF Tiam1 couples the NMDA receptor to the activity-dependent development of dendritic arbors and spines. Neuron. 2005;45:525–538. doi: 10.1016/j.neuron.2005.01.024. [DOI] [PubMed] [Google Scholar]
- 254.Okabe T., Nakamura T., Nishimura Y.N., Kohu K., Ohwada S., Morishita Y., Akiyama T. RICS, a novel GTPase-activating protein for Cdc42 and Rac1, is involved in the beta-catenin-N-cadherin and N-methyl-D-aspartate receptor signaling. J. Biol. Chem. 2003;278:9920–9927. doi: 10.1074/jbc.M208872200. [DOI] [PubMed] [Google Scholar]
- 255.Wei S.Y., Lin T.E., Wang W.L., Lee P.L., Tsai M.C., Chiu J.J. Protein kinase C-delta and -beta coordinate flow-induced directionality and deformation of migratory human blood T-lymphocytes. J. Mol. Cell Biol. 2014;6:458–472. doi: 10.1093/jmcb/mju050. [DOI] [PubMed] [Google Scholar]
- 256.Smith A., Bracke M., Leitinger B., Porter J.C., Hogg N. LFA-1-induced T cell migration on ICAM-1 involves regulation of MLCK-mediated attachment and ROCK-dependent detachment. J. Cell Sci. 2003;116:3123–3133. doi: 10.1242/jcs.00606. [DOI] [PubMed] [Google Scholar]
- 257.Dai Y., Dudek N.L., Patel T.B., Muma N.A. Transglutaminase-catalyzed transamidation: A novel mechanism for Rac1 activation by 5-hydroxytryptamine2A receptor stimulation. J. Pharmacol. Exp. Ther. 2008;326:153–162. doi: 10.1124/jpet.107.135046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Dai Y., Dudek N.L., Li Q., Muma N.A. Phospholipase C, Ca2+, and calmodulin signaling are required for 5-HT2A receptor-mediated transamidation of Rac1 by transglutaminase. Psychopharmacology. 2011;213:403–412. doi: 10.1007/s00213-010-1984-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Fleming I.N., Gray A., Downes C.P. Regulation of the Rac1-specific exchange factor Tiam1 involves both phosphoinositide 3-kinase-dependent and -independent components. Biochem. J. 2000;351:173–182. doi: 10.1042/bj3510173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Walsh A.B., Bar-Sagi D. Differential activation of the Rac pathway by Ha-Ras and K-Ras. J. Biol. Chem. 2001;276:15609–15615. doi: 10.1074/jbc.M010573200. [DOI] [PubMed] [Google Scholar]
- 261.Lambert J.M., Lambert Q.T., Reuther G.W., Malliri A., Siderovski D.P., Sondek J., Collard J.G., Der C.J. Tiam1 mediates Ras activation of Rac by a PI(3)K-independent mechanism. Nat. Cell Biol. 2002;4:621–625. doi: 10.1038/ncb833. [DOI] [PubMed] [Google Scholar]
- 262.Nimnual A.S., Yatsula B.A., Bar-Sagi D. Coupling of Ras and Rac guanosine triphosphatases through the Ras exchanger Sos. Science. 1998;279:560–563. doi: 10.1126/science.279.5350.560. [DOI] [PubMed] [Google Scholar]
- 263.Sun H., King A.J., Diaz H.B., Marshall M.S. Regulation of the protein kinase Raf-1 by oncogenic Ras through phosphatidylinositol 3-kinase, Cdc42/Rac and Pak. Curr. Biol. 2000;10:281–284. doi: 10.1016/S0960-9822(00)00359-6. [DOI] [PubMed] [Google Scholar]
- 264.Yamauchi J., Miyamoto Y., Tanoue A., Shooter E.M., Chan J.R. Ras activation of a Rac1 exchange factor, Tiam1, mediates neurotrophin-3-induced Schwann cell migration. Proc. Natl. Acad. Sci. USA. 2005;102:14889–14894. doi: 10.1073/pnas.0507125102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Itoh R.E., Kiyokawa E., Aoki K., Nishioka T., Akiyama T., Matsuda M. Phosphorylation and activation of the Rac1 and Cdc42 GEF Asef in A431 cells stimulated by EGF. J. Cell Sci. 2008;121:2635–2642. doi: 10.1242/jcs.028647. [DOI] [PubMed] [Google Scholar]
- 266.Murillo M.M., Rana S., Spencer-Dene B., Nye E., Stamp G., Downward J. Disruption of the interaction of RAS with PI 3-kinase induces regression of EGFR-mutant-driven lung cancer. Cell Rep. 2018;25:3545–3553. doi: 10.1016/j.celrep.2018.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Khosravi-Far R., Solski P.A., Clark G.J., Kinch M.S., Der C.J. Activation of Rac1, RhoA, and mitogen-activated protein kinases is required for Ras transformation. Mol. Cell. Biol. 1995;15:6443–6453. doi: 10.1128/MCB.15.11.6443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Malliri A., van der Kammen R.A., Clark K., van der Valk M., Michiels F., Collard J.G. Mice deficient in the Rac activator Tiam1 are resistant to Ras-induced skin tumours. Nature. 2002;417:867–871. doi: 10.1038/nature00848. [DOI] [PubMed] [Google Scholar]
- 269.Wang Z., Pedersen E., Basse A., Lefever T., Peyrollier K., Kapoor S., Mei Q., Karlsson R., Chrostek-Grashoff A., Brakebusch C. Rac1 is crucial for Ras-dependent skin tumor formation by controlling Pak1-Mek-Erk hyperactivation and hyperproliferation in vivo. Oncogene. 2010;29:3362–3373. doi: 10.1038/onc.2010.95. [DOI] [PubMed] [Google Scholar]
- 270.Kissil J.L., Walmsley M.J., Hanlon L., Haigis K.M., Bender Kim C.F., Sweet-Cordero A., Eckman M.S., Tuveson D.A., Capobianco A.J., Tybulewicz V.L., et al. Requirement for Rac1 in a K-ras induced lung cancer in the mouse. Cancer Res. 2007;67:8089–8094. doi: 10.1158/0008-5472.CAN-07-2300. [DOI] [PubMed] [Google Scholar]
- 271.Heid I., Lubeseder-Martellato C., Sipos B., Mazur P.K., Lesina M., Schmid R.M., Siveke J.T. Early requirement of Rac1 in a mouse model of pancreatic cancer. Gastroenterology. 2011;141:719–730. doi: 10.1053/j.gastro.2011.04.043. [DOI] [PubMed] [Google Scholar]
- 272.Joneson T., Bar-Sagi D. Suppression of Ras-induced apoptosis by the Rac GTPase. Mol. Cell. Biol. 1999;19:5892–5901. doi: 10.1128/MCB.19.9.5892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Chow H.Y., Jubb A.M., Koch J.N., Jaffer Z.M., Stepanova D., Campbell D.A., Duron S.G., O‘Farrell M., Cai K.Q., Klein-Szanto A.J., et al. P21-Activated kinase 1 is required for efficient tumor formation and progression in a Ras-mediated skin cancer model. Cancer Res. 2012;72:5966–5975. doi: 10.1158/0008-5472.CAN-12-2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Baker N.M., Yee Chow H., Chernoff J., Der C.J. Molecular pathways: Targeting RAC-p21-activated serine-threonine kinase signaling in RAS-driven cancers. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2014;20:4740–4746. doi: 10.1158/1078-0432.CCR-13-1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Yang H.W., Shin M.G., Lee S., Kim J.R., Park W.S., Cho K.H., Meyer T., Heo W.D. Cooperative activation of PI3K by Ras and Rho family small GTPases. Mol. Cell. 2012;47:281–290. doi: 10.1016/j.molcel.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Nimnual A., Bar-Sagi D. The two hats of SOS. Sci. Stke Signal Transduct. Knowl. Environ. 2002;2002:36. doi: 10.1126/stke.2002.145.pe36. [DOI] [PubMed] [Google Scholar]