Abstract
It is important to monitor serotonin neurochemistry in the context of brain disorders. Specifically, a better understanding of biophysical alterations and associated biochemical functionality within sub-regions of the brain will enable better of understanding of diseases such as depression. Fast voltammetric tools at carbon fiber microelectrodes (CFMs) provide an opportunity to make direct evoked and ambient serotonin measurements in vivo in mice. In this study, we characterize novel stimulation and measurement circuitries for serotonin analyses in brain regions relevant to psychiatric disease. Evoked and ambient serotonin in these brain areas, the CA2 region of the hippocampus and the medial prefrontal cortex, are compared to ambient and evoked serotonin in the substantia nigra pars reticulata, an area well established previously for serotonin measurements with fast voltammetry. Stimulation of a common axonal location evoked serotonin in all three brain regions. Differences are observed in the serotonin release and reuptake profiles between these three brain areas which we hypothesize to arise from tissue physiology heterogeneity around the CFM. We validate this hypothesis mathematically and via confocal imaging. We thereby show that fast voltammetric methods can provide accurate information about local physiology and highlight implications for chemical mapping.
Keywords: Hippocampus, prefrontal cortex, substantia nigra, medial forebrain bundle, FSCV, confocal microscopy
Graphical Abstract
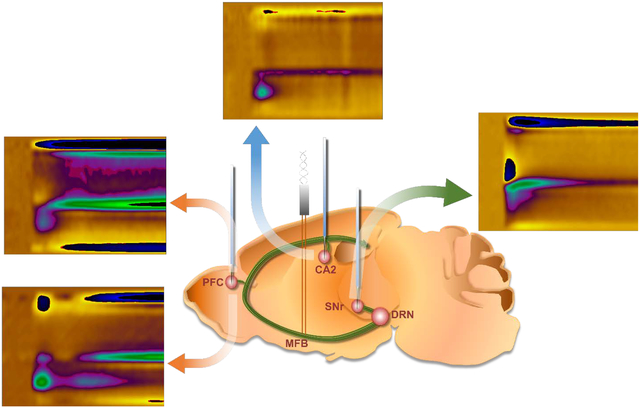
To demonstrate that chemical responses at carbon fiber microelectrodes can give information about local tissue physiology, we characterize novel medial forebrain bundle (MFB) stimulation and measurement circuitries for serotonin in the hippocampus (CA2), the medial prefrontal cortex (mPFC) and the substantia nigra (SNr). Differences are observed in serotonin chemistry which we show experimentally, mathematically and via imaging, to be indicative of tissue heterogeneity. This finding has important implications for chemical mapping.
Introduction
Neuromodulators, such as serotonin, are thought to regulate the brain’s major excitatory and inhibitory processes. As such, subtle alterations in the function of these modulators are thought to underlie the phenotypes of many disorders of the brain (Owens & Nemeroff 1994; Muller et al. 2016; Abi-Dargham et al. 1997; Oades 2008; Volkow & Fowler 2000). To better understand the functional importance of messengers like serotonin in the brain, analytical measurements of neurotransmitters is a thriving and rapidly evolving field (Johnson et al. 2018; Shen et al. 2018; Meunier et al. 2017). To this end, we have thus far focused on pioneering in vivo measurements of evoked and ambient serotonin using fast-scan cyclic voltammetry (FSCV) and fast-scan controlled adsorption voltammetry (FSCAV) at carbon fiber microelectrodes (CFMs) (Hashemi et al. 2009; Abdalla et al. 2017).
Quantitative chemical measurements of brain serotonin in vivo are highly challenging because of very low extracellular concentrations (Parsons & Justice 1993). An additional difficulty for electroanalytical measurements is that serotonin and serotonin metabolites are detrimental to electrode stability due to polymer filming (Jackson et al. 1995). In our initial efforts to develop an FSCV tool for serotonin measurements, we chose to make measurements in the substantia nigra, pars reticulata (SNr) because this region contains the most dense innervation by serotonin axons in the brain (Palkovits et al. 1974). When it comes to interest in serotonin’s actions in the brain however, there is much focus on psychiatric, cognitive and developmental disorders (Owens & Nemeroff 1994; Muller et al. 2016; Abi-Dargham et al. 1997; Oades 2008; Volkow & Fowler 2000) which implicate brain regions other than the SNr. These brain regions include the hippocampus (Gyorfi et al. 2017), amygdala (Schumann et al. 2011), hypothalamus (Nestler et al. 2002) and prefrontal cortex (PFC) (Jin et al. 2016). Our own research interests incline us towards the hippocampus and medial prefrontal cortex (mPFC). Biophysical alterations are seen in these regions during disease. For example, volume changes in the hippocampus and the prefrontal cortex have been associated with depression (Frodl et al. 2006; Bremner et al. 2000; Bremner et al. 2002; Rigucci et al. 2010).
It is very important to address the chemical functionality associated with such biophysical changes. We have performed pilot studies in these and other brain areas (Abdalla et al. 2017; West et al. 2018; Srejic et al. 2016), here we present a formal characterization of the voltammetry stimulation and measurement circuits for serotonin in the CA2 region of the hippocampus and the mPFC, compared with the SNr.
Serotonin is shown to be evoked in the CA2 region of the hippocampus, the mPFC and the SNr via a common electrical stimulation of the medial forebrain bundle (MFB). Comparison of the evoked and ambient serotonin chemistry in these three regions reveals significant differences which we postulate to arise from differences in tissue physiology local to the CFM, specifically in the characteristics of serotonin reuptake via different monoamine transporters. The chemical signals are modeled mathematically according to our previously established models of serotonin transmission which accounts for Uptake 1 (Serotonin transporter (SERT) mediated) and Uptake 2 (Dopamine transporter (DAT), norepinephrine transporter (NET), and organic cation transporter (OCT) mediated) (Wood et al. 2014) and single vs. dual evoked events (West et al. 2018). Our modeling also, independently, hypothesizes differences in how serotonin is reuptaken via Uptake 1 and 2, while bringing forth additional hypotheses about the strength of input to the three locales and autoreceptor contribution. Our models’ predictions are subsequently verified with confocal imaging of axons and stimulation parameter experiments.
We thereby show that FSCV and FSCAV are multi-faceted tools that provide information on the proteins directly around the CFM and tell us about the strength of the input circuit. This combination of techniques presents an innovative approach for chemical brain mapping which has future implications for chemically defining the functionality of local circuitry during health and disease. A better understanding of the circuits within specific brain regions will ultimately allow for improved drug targeting for disease treatment.
Materials and Methods
Study Design and Exclusion Criteria
Previously collected sample data (see Supplementary Material) informed upon values necessary to determine statistical difference between two serotonin signals. This data was input into a power analysis according to literature standards (Charan & Kantharia 2013). The following formula to calculate sample size between two groups with a quantitative endpoint was used:
The pooled standard deviation from the sample data was found to be 1.06, and the effect size 1.96, resulting in a calculated Cohen’s d of 1.85 (Cohen 1988). The Zα term used was 1.96 according to a 95% confidence interval and the Zβ term was 0.842 according to the 80% power assumed. This power analysis resulted in a n=5.25, which was rounded to 5. Finally, the sample size was corrected to account for loss of animals due to death or exclusion criteria (Charan & Kantharia 2013). We began with 141 animals to accommodate this adjustment and still achieve sufficient power. The percent of animals expected to die due to urethane anesthesia, based on empirical observations from our own lab, was about 38% (54 of 141 animals). This number appears high, though research has shown that many studies severely under-report unexpected death from anesthesia (Uhlig et al. 2015). Urethane is a terminal anesthetic and the dose is difficult to adjust. However, the fact that our experiments require long periods of stable anesthesia, combined with the non-survival nature of our experiments makes urethane an ideal choice for our purposes. Of the remaining 87 animals, 34 were excluded for because of exclusion criteria fully detailed below, leaving 53 animals that were included in the data analysis (16 in the SNr, 16 in the CA2 and 21 in the mPFC). No randomization was used to determine which mice would be used for measurement in each region.
For all FSCV experiments, the CV of the evoked signal was compared to well-established serotonin cyclic voltammograms (CVs) in vivo and in vitro and mice in which the CVs did not contain the characteristic serotonin redox peaks or included interference by dopamine were excluded. For FSCAV experiments, a stimulated serotonin response was collected prior to the start of FSCAV and the same aforementioned test was performed. Data which contained a stimulation artifact resulting from the stimulation electrode touching the skull that masked, delayed, or minimized the serotonin response (stimulation glitch) were excluded. Electrodes that displayed drift or instability during file collection, as a result of damage during insertion, were excluded. While stereotaxic technique was utilized for this study, individual variation between mice oftentimes limits the control we have over the exact position of the working electrode. We confirmed placement of the electrode in our target region in two ways, first during surgery, if the cyclic voltammogram is characteristic for serotonin and second, post-surgery histology confirmed if the electrode was in the target region. Signals not collected in the target regions were excluded from the study.
Carbon-Fiber Microelectrodes
CFM’s were constructed through the aspiration of a single T-650 carbon fiber (7 μm, Goodfellow, Coraopolis, PA) through a cylindrical glass capillary (internal diameter: 0.4 mm, external diameter: 0.6 mm, Product # 624500, A-M Systems, Carlsborg, WA). This capillary was then placed in a vertical pipette puller (Narishige Group, Setagaya-Ku, Tokyo, Japan) to make a carbon – glass seal by gravity. The protruding carbon fiber was then cut to 150 μm in length. Subsequently, a solution of Nafion (LQ-1105-US-25, 5% by weight Nafion, Ion Power, DE) was electrodeposited, as previously described, onto the exposed carbon fiber (Hashemi et al. 2009). The CFM was then dried for 10 minutes at 70°C.
Animal Surgery
C57BL/6J (RRID: IMSR_JAX:000664) male mice, 6 to 12 weeks old and between 20–30 g, were procured from Jackson Laboratories (Bar Harbor, ME). The study was originally started with 141 mice. Mice were offered food and water ad libitum and housed in 12 hours light/dark cycles. The Guide for the Care and Use of Laboratory Animals, as accepted by the Institutional Animal Care and Use Committees of the University of South Carolina (Institution Approval # A3049–01), was followed in all animal care and procedures. A urethane solution was produced (25% (w/v)) by dissolving urethane (Product # U2500, Sigma Aldrich Co., St. Louis, MO, USA) in sterile saline (0.9% NaCl solution, NDC 0409–4888-20, Hospira, Lake Forest, IL). The anesthetic urethane, was administered intraperitoneally (i.p.) at a volume of 7 μL per 1 g mouse weight, followed by stereotaxic surgeries (Model 962, David Kopf Instruments, Tujunga, CA) during the light cycle. To maintain the ideal mouse body temperature of 37° C, a heating pad from Braintree Scientific was utilized. For stereotaxic coordinates of MFB [AP: −1.58, ML: +1.0, DV: −4.8 to −5.0], SNr [AP: −3.28, ML: +1.40, DV: −4.21 to −5.0] CA2 [AP: −2.9, ML: +3.35, DV: −2.5 to −3.0], and mPFC [AP: +1.7, ML: +0.2, DV: −2.0 to −3.0], bregma was used as a reference from Franklin and Paxinos (2008). In order to access the MFB, CA2, SNr, and mPFC holes were drilled in line with the above stereotaxic coordinates. No randomization method was used to assign mice for measurements in a specific region. For stimulation, a stainless-steel electrode (diameter 0.2 mm, MS303/2-A/SPC; Plastics One, Roanoke, VA) was inserted into the MFB. For measurements, the Nafion-coated CFM was then inserted into either the CA2, SNr, or the mPFC. The reference electrode is made of a silver wire (diameter: 0.010 in, 787000; A-M Systems, Sequim, WA), which was electroplated with chloride through immersion of in hydrochloric acid for 30 seconds (0.1 M, 4 V vs. tungsten). This is then placed into the contralateral hemisphere of the CFM placement.
Data Collection
FSCV and FSCAV were both performed through instrumentation and software (WCCV 3.05) developed by Knowmad Technologies LLC (Tucson, AZ) (available upon request). FSCV was performed by applying the “Jackson” waveform (Jackson et al. 1995). Measurements were collected every 10 minutes with a 30–60 second file. Five seconds into the file, a stimulation was applied (60 Hz biphasic 360 μA, 120 pulse stimulation, 2 ms per phase) through employing a linear constant current stimulus isolator (NL800A Neurolog; Digitimer Ltd.) to elicit the serotonin response. This stimulation was varied slightly, one parameter at a time, for the stimulation parameter experiments. FSCAV was applied through a CMOS precision analog switch, ADG419 (Analog Devices), which is used to control the application of the computer-generated “Jackson” waveform to the CFM. The logic was software - controlled to either apply a series of ramps (0.2 V to 1.0 V to −0.1 V to 0.2 V, scan rate = 1000 V/s) every 10 ms (100 Hz) or apply a constant potential of 0.2 V to the CFM for a specified controlled adsorption period (10 s) (Abdalla et al. 2017).
Data analysis
Signals collected from FSCV and FSCAV were processed using custom software (vide supra) using LabVIEW 2009 (available upon request). The processing used includes signal deconvolution, filtering, and smoothing. For FSCAV, the cyclic voltammogram (CV) at the 3rd scan (following the controlled adsorption period) was extracted to integrate the serotonin oxidation peak approximately between 0.4 V and 0.85 V. The charge value found, in pC, was plotted versus [serotonin] to generate calibration curves that were then used to calculate in vivo values which were specific to each electrode (Abdalla et al. 2017). No blinding was necessary for the purpose of the experiments presented herein.
Statistical Analysis
Post-Hoc Validation of Sample Size
A sample size of 5 mice for each of the three groups was utilized. This choice of sample size was initially estimated by previous studies (see Study Design) as the data necessary for proper sample size calculation was not yet collected. Following collection of experimental data, this sample size was reevaluated based on power considerations. We assumed that two-sample t-tests applied at 5% level of significance and an 80% power with a Cohen’s d of 2.0 (associated with an effect size of 2 from experimental data), when the common standard deviation is 1.0. Note that based on the obtained sample data, the pooled standard deviation was found to be 1.06, which is close to 1.0. Based on the sample size formula, the appropriate sample size to use is thus n = 3.92, rounded to a sample size of 4 for each group. Taking into consideration that the assumed common standard deviation of 1.0 may have been too liberal (in light of the sample standard deviation of 1.06), a sample size of 5 per group appears acceptable for determining statistical differences.
Basal Level Analysis
In vitro calibrations related charge with the concentration, as explained previously (Abdalla et al. 2017). We compared the basal responses for the three regions (CA2, SNr, mPFC; five animals for each region), where the basal value was measured every minute for 60 minutes. Hence, a repeated measures two-factor model was fitted, with the factors being the region and time. To accommodate the repeated-measures feature, random effects were included in the model, as well as the possible interaction effects between region and time. The model developed is presented here:
Where i = 1, 2, 3 represents the region; j = 1, 2, …, 5, represents the animal number (within the group), and t = 0, 1, 2, …, 60, represents the time. M is a common mean; the A(i)’s are the effects of the region levels; the B(t)’s are the effects of the time levels; the C(I,t)’s are the interaction effects; V(i,j) is a random term (which is normally distributed) to account for the repeated measurements over time; and E(I,j,t)’s are the error terms (which are also normally distributed and independent of the V(I,j)’s).
A test of the null hypotheses that the B(t)’s and C(I,t)’s are all zeros based on the repeated-measures analysis of deviance, implemented through the R Statistical Package, resulted in not being able to reject these null hypotheses, hence both the interaction effect and the time effect were not significant. The p-value for testing for no interaction effects was 0.8539, while the p-value for testing that there is no time effect is 0.8249.
Because the time and region*time effects were not significant, we therefore combined the concentration over the 60 minutes for each of the animals in each group by obtaining their sample means. These sample mean values were then used to compare whether there were differences between the three regions; this analysis was performed using a one-way analysis of variance. By analyzing the sample means of the observations over time, we were able to eliminate dependencies over time among the observations. This analysis of variance showed that at least two of the regions are indeed different statistically with a p-value of 0.00000756 (ANOVA).
To determine which regions are different, we performed pairwise t-tests with the Welch-Sattertwaite’s degrees-of-freedom approximation to allow for unequal variances, and we implemented a multiple testing correction, using the Holm procedure. We concluded that CA2 and mPFC regions are not statistically different (adjusted p = 0.23), while the SNr and CA2 are different (adjusted p = 0.000012), and the SNr and mPFC are also different (adjusted p = 0.000043). A summary of the statistics from these group means are provided in the table below, which includes 95% confidence interval for the group means.
| Region | Sample Size | Mean (mean of the sample means) | SD (standard deviation) | ME (margin of error) | 95% CI for the group mean response |
|---|---|---|---|---|---|
| SNr | 5 | 39.706 | 4.391 | 5.452 | 34.254 – 45.158 |
| CA2 | 5 | 72.822 | 7.170 | 8.903 | 63.919 – 81.725 |
| mPFC | 5 | 67.565 | 7.636 | 9.481 | 58.084 – 77.046 |
Depletion Analysis
Repeated stimulations were employed to determine if serotonin release could be depleted in each of the three regions over time. Maximum serotonin release for each animal in each group was measured in response to stimulations every minute for 20 minutes. The main goal was to compare the maximum amplitude during the first stimulation (t=1) with those at later times to see whether serotonin release was depleted in response to repeated stimulations. Because this is a repeated measures experiment, for any fixed time t (taking values from 2, 3, …, 20), we took the differences in the level at time t and at time 1 for each of the animals within each group. Using these differences, we then performed a one-sample t-test to test the null hypothesis that the population mean difference is zero versus less than zero (indicating a decrease from time 1 to time t). We performed this procedure for each of the time values t, yielding a t-test statistic value and a p-value. Since we performed multiple tests (19), we applied the Benjamini-Hochberg (BH) false discovery rate (FDR) mulitplicity correction. The results are as follows, based on the BH-adjusted p-values:
For the CA2 data set, we concluded that the maximum amplitude at t=20 is significantly lower than at t=1, with BH-adjusted p-value of 0.0325. This is the only time point for which we concluded significance based on the BH-adjusted p-values. It should be noted that some of the unadjusted p-values arising from these t-tests were less than .05, however the necessity of the multiplicity adjustments increased these values. It is possible that with more animals, statistical significance could be achieved. Another way to globally test if the serotonin level was decreasing over time is via an analysis of covariance with the covariate being the serotonin level at t=1. This analysis of covariance yielded an estimate of the regression coefficient associated with time of −0.4542 (p-value = 0.00048) and with estimate of the coefficient of determination of R2 = 59.66%. The sign of the estimated regression coefficient (negative), indicates that the serotonin level decreases over time from t=1.
For the SNr data set, we did not find any significant differences between t=1 and any of the other time points, even before the BH adjustment for multiplicity. An analysis of covariance also did not show a significant decrease.
For the mPFc data set, prior to the BH multiplicity adjustments, there were p-values that were less than 0.05. After employing the BH-adjustments the analysis concluded no significant differences between the t=1 and later time values. An analysis of covariance did not show a significant difference in the serotonin level over time.
Modeling
We use the mathematical model that we previously presented (Wood et al. 2014):
S(t) is the concentration of serotonin in the extracellular space, R(t) is the firing rate of the serotonin neuron that rises briefly after stimulation, and A(t) represents the strength of the autoreceptor effect. The first negative term represents reuptake by SERTs with Vmax1 = 19.25 nM/hr and Km1 = 5 nM. The second term represents Uptake 2 (DATs, NETs, and OCTs) with Vmax2 = 780 nM/hr and Km2 = 170 nM. Uptake 2 operates only above the steady state concentration of serotonin. α and β give the balance between Uptake 1 and Uptake 2. For details and discussion, see Wood et al. 2014. To model the double hump in the mPFC we use the extended model presented in West et al. 2018 that allows diffusion of serotonin from regions with only Uptake 1 to regions that have both Uptake 1 and Uptake 2.
Histology
In order to confirm the spatial placement of the CFM in vivo, a small lesion was created at the end of the FSCV experiment, by applying a constant potential at the CFM (~10 V for 1 min). Subsequently, the mice were euthanized via cervical dislocation followed by decapitation, and the brain was removed from the skull and stored in 4% paraformaldehyde in PBS solution. At least 2 days prior to sectioning, the brain was transferred into a 30% sucrose solution, until it was saturated with the medium. The brain was then flash-frozen, sectioned into 30 μm slices mounted onto frosted glass slides, and stained with 0.2% thionin. The slices were then photographed with an optical microscope.
Immunohistochemistry
Slc6a4-EGFP male mice (RRID: MMRRC_030692-UCD), 6–12 weeks old, were anesthetized with urethane (25% dissolved in 0.9% NaCl solution, Hospira, Lake Forest, IL) administered intraperitoneally (i.p.) at a volume of 7 μL per 1 g mouse weight. Mice were then perfused intracardially with phosphate-buffered saline (PBS) followed by 4% paraformaldehyde in PBS at 4 °C. The entire brain was removed and fixed in 4% paraformaldehyde for 3 hours at room temperature and then cryoprotected in 15% sucrose in PBS overnight at 4 °C, followed by a switch to 30% sucrose on the next day and continuing overnight. Sections of the mouse brain (40 μm thick) were prepared using a microtome and were washed with PBS and then blocked with 5% normal goat serum and 0.3 % Triton X-100 in PBS for 2 hours at room temperature. The sections were incubated in primary antibody diluted in blocking buffer, overnight at 4 °C. The primary antibodies used were chicken anti-GFP (1:5000, Aves Labs #GFP-1010, RRID: AB_2307313), guinea pig anti-SERT (1: 1,000, Frontier Institute #HTT-GP-Af1400), rabbit anti-TH (1: 1,000, Millipore #AB152), and mouse anti-NeuN (1:500, Millipore #MAB377, RRID: AB_2298772). The sections were then washed with PBS and incubated in the secondary antibody in a blocking buffer for 2 hours at room temperature. The secondary antibodies used were Alexa Fluor 488-labeled goat anti-chicken (1:1000, Life Technologies #A11039), Cy3-labeled goat anti-guinea pig (1:800, Jackson ImmunoResearch Laboratories #106–165-003), Cy3-labeled goat anti-rabbit (1:800, Jackson ImmunoResearch Laboratories #111–165-003), and Cy5-labeled goat anti-mouse (1:200, Jackson ImmunoResearch Laboratories #115–175-146). Then, the sections were mounted on slides, and images were acquired using a single-photon confocal microscope (Zeiss). Maximum projection images of 10 z-planes 1 micron apart were analyzed using NIH ImageJ (https://imagej.nih.gov/ij/), and % pixel intensity of EGFP signal above threshold was measured as an index of SERT innervation.
Results
Serotonin is Evoked via a Common MFB Stimulation in Three Distinct Brain Regions
Stimulation of the MFB at one location evokes serotonin in the CA2 region of the hippocampus, the mPFC and the SNr. Figure 2 displays representative examples of color plots and CVs collected in each of these 3 brain areas upon stimulation. In Panel i are illustrations of the positions of the stimulating and working electrode in sagittal sections of the mouse brain. Panel ii shows representative 30 second color plots enveloping the 5 seconds before and 25 seconds after the stimulation. Interpretation of color plots is explained in detail elsewhere (Michael et al. 1999), briefly voltage is on the y-axis, time on the x-axis, and current illustrated in false color. Panel iii shows CVs extracted from the vertical dashed lines on the color plots with the oxidation peak of serotonin at approximately 0.7 V.
Figure 2.

(i) Representation of a sagittal section of a mouse brain. WE shows the position of the working electrode and STIM shows the stimulating electrode. Green track represents the serotonergic axons that originate in the raphe nucleus and traverse the MFB to innervate different brain regions. (ii) Representative FSCV color plots of the CA2 (A) (n=5), the mPFC (B) (n=5) and (C) (n=5), and the SNr (D) (n=5). The red bar below the color plots denote the stimulation period (2 s) iii) Cyclic voltammograms extracted from the vertical dashed lines in A(ii), B(ii), C(ii), and D(ii) with current on the y-axis and voltage vs. Ag / AgCl on the x-axis. Red and yellow stars on C(ii) denote the two successive oxidation events seen in the mPFC. Cyclic voltammograms extracted at both these positions are seen in C(iii), marked with their respective stars. (n=number of animals)
In contrast to the CA2 and SNr where a single serotonin release and uptake event is evoked upon stimulation, in the mPFC we often also observe a dual peak, the origins of which we’ve recently described (West et al. 2018). Two representative examples of mPFC signals are illustrated in Figure 2C.
To study whether there is physiological relevant information in these traces, we carry out a comparison of how serotonin concentration changes over the file collection time. Serotonin concentration [serotonin] vs. time traces are extracted at the position of the peak oxidation current and averaged between 5 animals per region. Figure 3 shows the averaged serotonin time courses per region (n=5, ± standard error of the mean ((SEM)). The responses are confirmed to be in the target region via lesioning the tissue adjacent to the CFM after the experiment. A representative stained slice from each region, along with the location of the CFM, for each experiment performed can be found in Panel B of Figure 3. The decay of these traces indicates the transporters responsible for removing serotonin from the extracellular space. A fast decay is a result of Uptake 2 (DATs, NETs, and OCTs), a slow decay results from Uptake 1 (SERTs), and a trace which has both fast and slow decay is a hybrid response and results from a combination of the two uptakes. Serotonin release in the CA2 appears primarily mediated by Uptake 2. The mPFC serotonin responses, as we previously characterized (West et al. 2018), involve clearance by both Uptake 1 and 2 transporters, which are linked inextricably to the two release domains. Serotonin released in the first domain, observed as a single peak, is reuptaken by Uptake 2 mechanisms, while serotonin in the second domain is solely reuptaken by Uptake 1 processes. This was verified pharmacologically using escitalopram. Domain 2 is only readily observed when measurements occur in layers 5–6 of the mPFC and is more sparsely innervated, resulting in a second peak due to the longer diffusion time to reach the electrode surface. In the SNr, serotonin seems to be reuptaken via a hybrid of Uptake 1 and 2.
Figure 3.
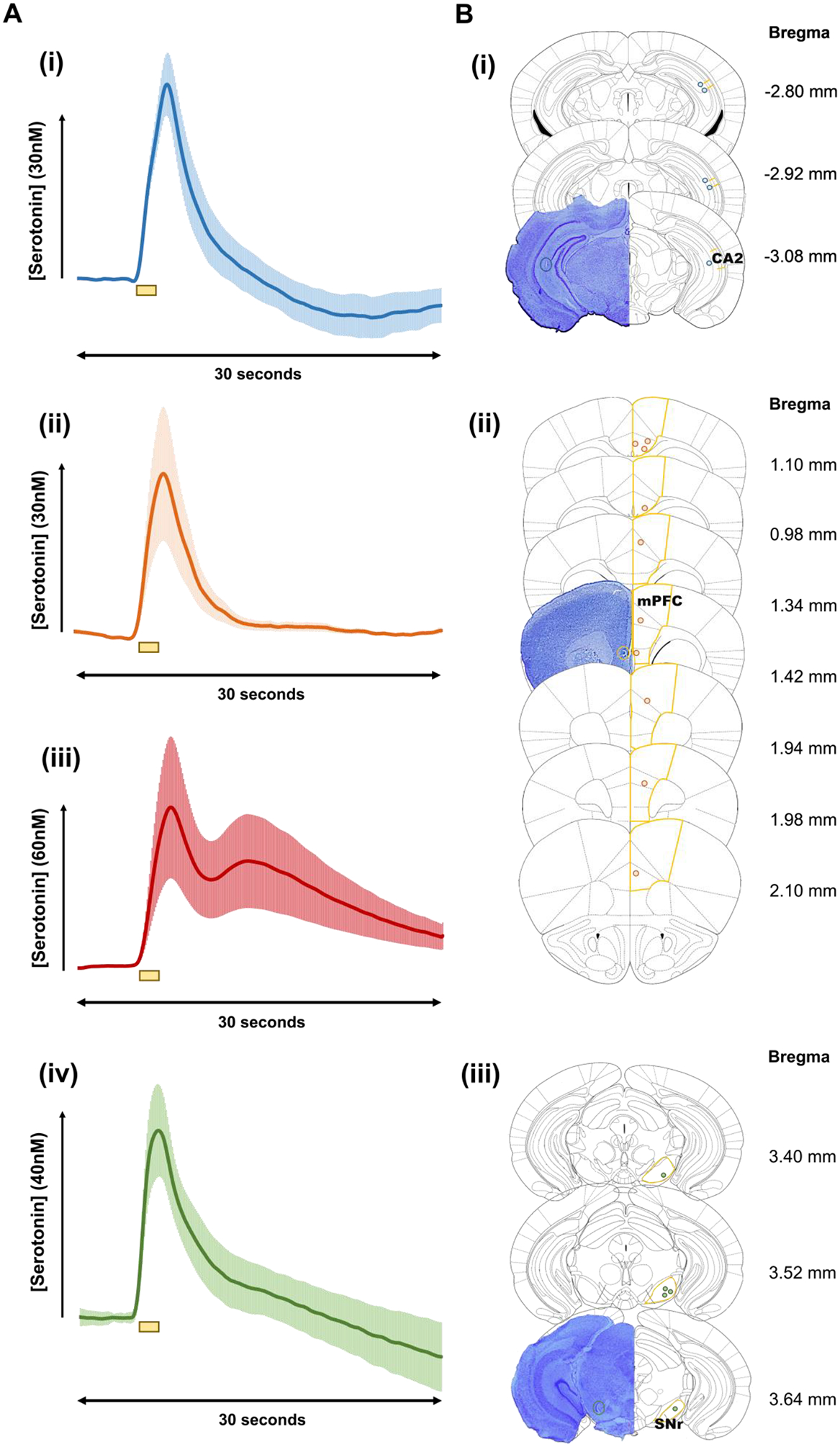
(A) Averaged [Serotonin] – time profiles (n=5 ± SEM) i) CA2, ii) mPFC single peak response, and iii) mPFC double peak response, and iv) SNr. Yellow bars beneath the plot denote the stimulation period (2 s). (B) Thionin stained representative brains displayed on the left with a colored circle denoting the actual placement of the CFM. On the right, yellow lines represent the outlines of the i) CA2 ii) mPFC, and iii) SNr regions. Blue, orange, and green circles denote the placement of the CFM in each individual mouse, for the CA2 (n=5), the SNr (n=5), and the mPFC (n=10), respectively. Coordinates with respect to Bregma are shown to the right of each coronal slice. Region specific coordinates are explained in the methods section. (n=number of animals)
This data lead us to formulate a hypothesis that the chemical decay profile after stimulation offered by the CFM is indicative of the ratio of SERT (Uptake 1) : non-SERT (Uptake 2), specifically that serotonin in the SNr is cleared by a higher Uptake 1 : Uptake 2 ratio when compared to the other two regions. To strengthen this hypothesis, we perform ambient serotonin measurements with FSCAV in the three brain areas, shown in Figure 4.
Figure 4.
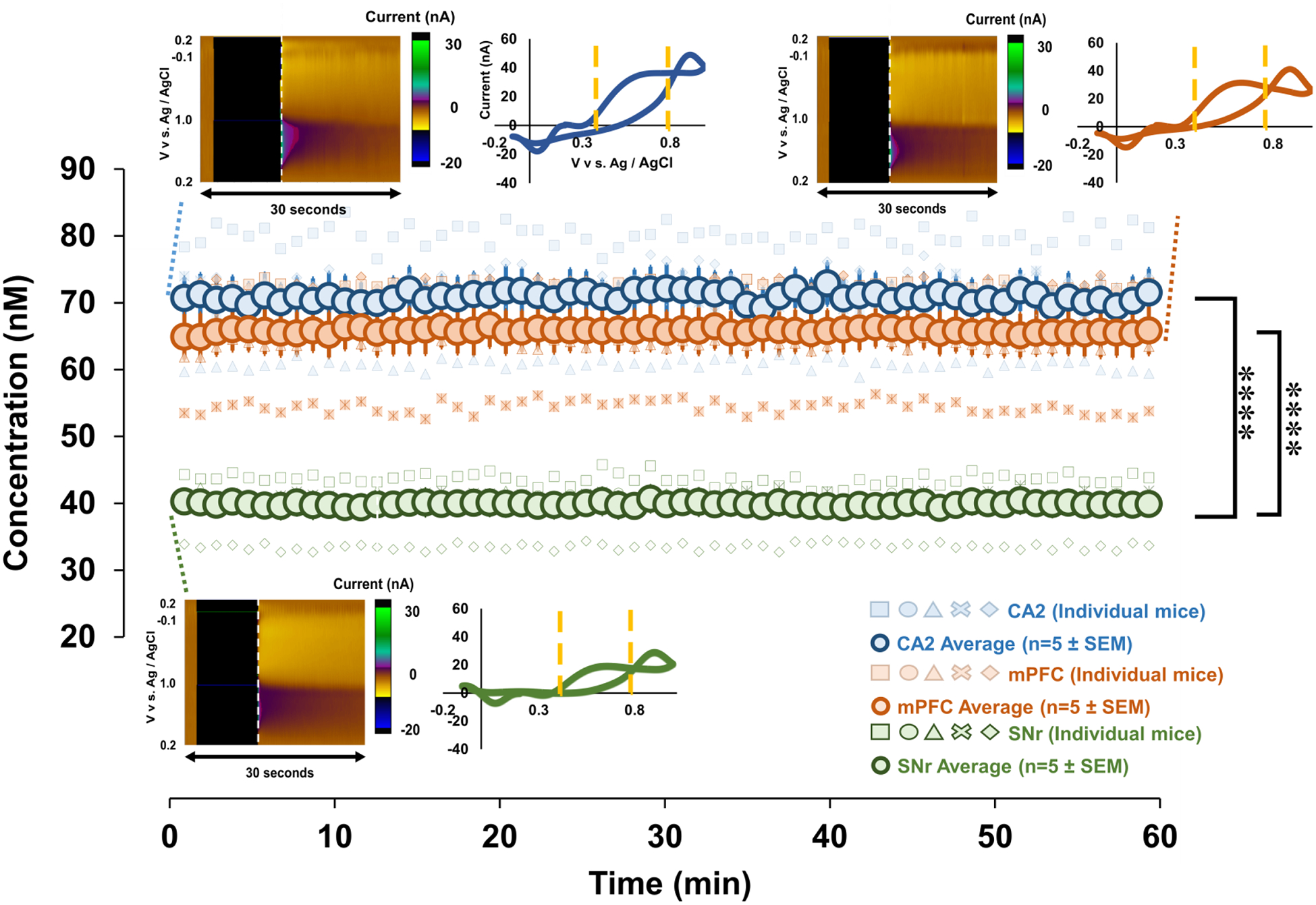
Blue, orange and green circles represent the weighted averaged response (n=5 mice each region ± SEM), and faint blue, orange, and green markers represent individual mice responses. Files were collected for 60 mins to obtain a baseline reading. Representative FSCAV color plots and CVs (extracted from vertical dashed lines) are inset, on top left for the CA2, top right for the mPFC, and at the bottom for SNr. Yellow lines on the CV denote the limits of integration. ****p<0.0001.
Serotonin Basal Levels Differ Based on Brain Region
To measure ambient serotonin levels in each of three brain localities, a new method, FSCAV is applied (Abdalla et al. 2017). First MFB stimulation is employed in mice to verify the presence of serotonin with FSCV. FSCAV is then performed on the same CFM for 60 mins to obtain a baseline reading of ambient serotonin concentrations. The dark blue, green, and orange circles on the central trace of Figure 4 represent the weighted average response (n=5 ± SEM) in the three regions. Individual mice traces are displayed by faint markers, of similar color, on the same plot. Representative in vivo color plots, along with CVs extracted from the 3rd scan (vertical dashed line), post controlled adsorption period, can be seen on Figure 4. All CVs demonstrate the characteristic redox serotonin peaks, thus confirming the identity of the signal measured. To convert this signal to serotonin concentration in the 3 regions, we use a chemometric approach, to take into account the variability between the CFMs used, along with the in vivo variability. This approach was explained in detail previously (Abdalla et al. 2017). Using 5 mice for each region, the average ambient serotonin level in the CA2 is 72.82 ± 3.21 nM (n = 5 mice, weighted average ± standard error), the mPFC is 67.57 ± 3.42 nM (n = 5 mice, weighted average ± standard error), whilst the level in the SNr is 39.71 ± 1.96 nM (n = 5 mice, weighted average ± standard error). A pairwise t-test confirms statistically significant differences in the basal levels between the CA2 and SNr (p= 0.000012), and the mPFC and SNr (p= 0.000043). While the CA2 and mPFC are not statistically different from one another (p= 0.23). See the analysis reported earlier.
Mathematical Modeling of Evoked Release
The ambient measurements support the idea that serotonin release in the SNr is reuptaken with a higher Uptake 1: Uptake 2 ratio than in the CA2 or mPFC. Here we analyze the experimental curves with previously established models and allow the model to generate an independent hypothesis to explain the experimental data. The model utilizes the Uptake 1, Uptake 2, autoreceptor, and two domain theories to determine the best parameters for fitting the experimental data presented here (Wood et al. 2014; West et al. 2018). Figure 5 shows the mean experimental curves for the CA2, mPFC and the SNr (solid are experimental and dotted are models).
Figure 5.

The [Serotonin] vs. time plots display the experimental data (solid) and model curves (dotted) for each region: CA2 (A), mPFC layers 5–6 (B), mPFC layers 1–3 (C), and the SNr (D). The table below outlines the parameters used to generate the model curves for each region.
This analysis gives us an opportunity to compare the three areas by comparing the models’ parameters that were optimized to fit each curve. We allow ourselves to vary the strength of the input (R(t)), the strength of the autoreceptor effect (A(t)), and the strengths of Uptake 1 and Uptake 2 (α and β). And, to obtain the double hump in the mPFC, we allow diffusion from a region with only Uptake 1 to a region with both Uptake 1 and 2 (see West et. al. 2018). The table in Figure 5 shows the qualitative differences in the parameters for different regions.
The results from the modeling are as follows:
(a) Diffusion is needed to explain the double humps seen in the PFC.
(b) There is higher contribution of Uptake 1 in the SNr than in the other regions.
(c) The autoreceptor effect is strong in the SNr and CA2 but weak in the mPFC.
(d) The input strength to regions of the mPFC is more variable than to the SNr and CA2.
The model’s results bring forth 2 additional hypotheses, (b) and (d) that we are able to test below.
Imaging Axonal Density in Three Brain Regions
To test the hypothesis brought forth by the model’s 2nd result (higher contribution of Uptake 1 in SNr) we employ a single photon imaging approach, whereby axons are imaged in brain slices of adult serotonin transporter-EGFP BAC transgenic mice (Gong et al. 2003). The imaging locations are chosen to correspond to the regions of our voltammetric measurements as ascertained with the histological analysis in Figure 3.
Figure 6 shows exemplar confocal images taken from the prefrontal cortex, hippocampus CA2, and substantia nigra pars recticulata (SNr) from serotonin transporter-EGFP BAC transgenic mice.
Figure 6.
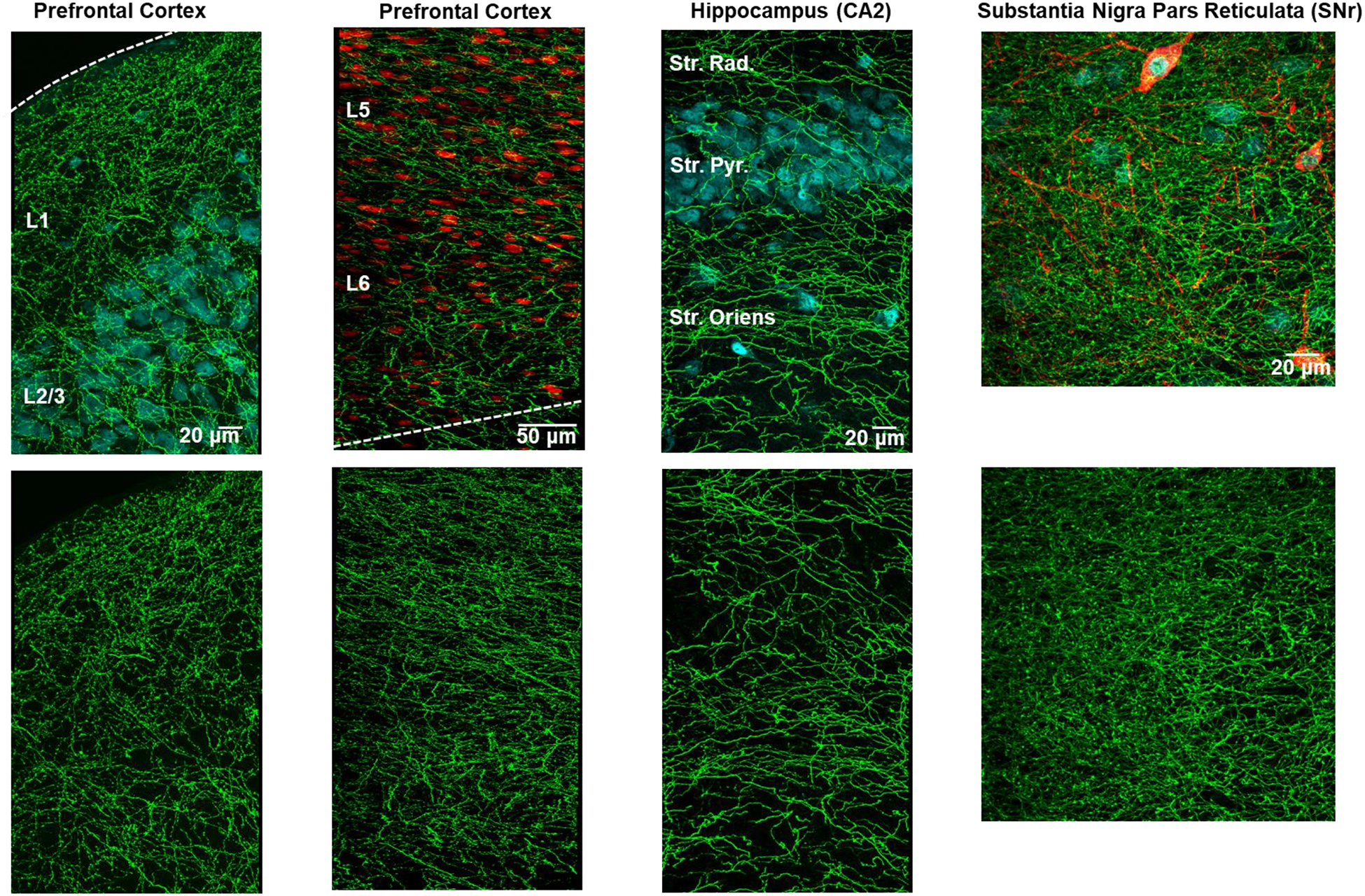
Immunohistochemistry for GFP in serotonin transporter-EGFP BAC transgenic mice reveals serotonin axon density across brain regions (n=1 mouse per region). Top panels: The SERT signal is shown in green with NeuN in cyan in all regions except the prefrontal cortex layer 5 and 6 where NeuN is shown in red. Layers 1–6 are all clearly defined in the prefrontal cortex and the stratum radiatum (Str. Rad.), stratum pyrimidale (Str. Pyr.), and the stratum oriens are labeled in the hippocampus. In the SNr, the red immunofluorescence is signal from an antibody directed against tyrosine hydroxylase, a marker of dopamine neurons. The top panels show single photon confocal micrographs of axonal innervation in the three brain areas. The bottom panels show the green channel alone to allow for the clear comparison of serotonin axon density.
To compare the degree of innervation between the regions, the percent pixels were calculated across the same number of focal planes for each region. The results, seen in Table 1, illustrate a higher degree of serotonin axon innervation in the SNr compared to the two regions (innervation depends on layer and sub-region of CA2 and mPFC).
Table 1.
The degree of serotonin axon innervation was calculated as the percent of GFP-immunopositive pixels above threshold for each layer/ sub-region within the three target regions.
| Region | Sub-Region/ Layer | % Pixels |
|---|---|---|
| CA2 | Oriens | 16.3 |
| Pyramidale | 10.6 | |
| Radiatum | 15.9 | |
| SN | Pars Reticulata | 27.4 |
| mPFC | L 1 | 19.7 |
| L 2/3 | 14.6 | |
| L 5 | 17.4 | |
| L 6 | 14.9 |
Axonal Reaction to Electrical Stimulation
To test the hypothesis brought forth by the model’s 4th result (input strength to mPFC is more variable than other two regions) we perform a stimulation parameter study. Figure 7 (top panel) shows the maximum evoked concentration of serotonin after systematic variation of stimulation conditions frequency, amplitude and pulse width. This data shows that the axons react in a similar manner to stimulation since increased terminal serotonin accompanies increased stimulation intensity, frequency and pulse width. It is only the mPFC response that does not substantially plateau during any of the stimulation paradigms, whereas the CA2 and SNr responses plateau during one or more of the experiments (CA2 and SNr during amplitude and SNr during frequency). The plateau of the curves are confirmed utilizing the first derivative of each point with respect to the previous one. In one or more of the parameter studies, the first derivative of the CA2 and SNr data fall below zero, indicating that the slope is plateauing. A depletion study where serotonin release is stimulated once a minute over a one-hour period is shown in Figure 7 (bottom panel). Here, when accounting for multiplicity, serotonin release in the CA2, but in the SNr or mPFC, shows significant depleted over 20 stimulations (see Statistical Analysis section).
Figure 7.
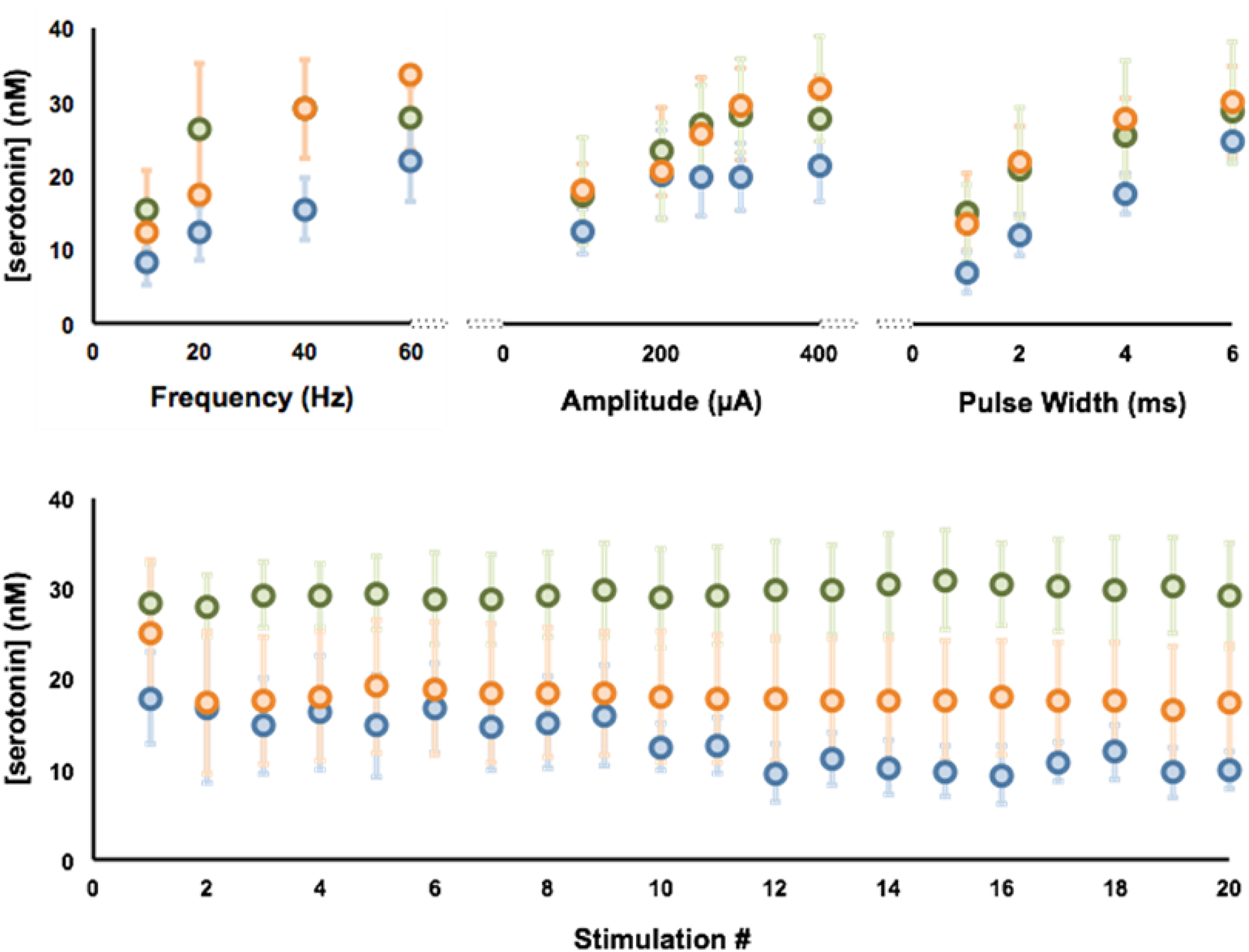
Top Panel– Averaged serotonin responses (n=5, ± SEM), CA2 (blue), mPFC (orange) and in the SNr (green) with variation in the stimulation frequency (top left), stimulation amplitude (middle), and stimulation pulse width (top right). Bottom Panel – Average serotonin response (n=5, ±SEM) to a stimulation train for 20 mins at 60 Hz, 4 ms pulse width, and 360μA in the SNr (green), CA2 (blue), and mPFC (orange). (n=number of animals)
Discussion
Expanding Serotonin Voltammetry Beyond the SNr
In vivo FSCV measurements are largely limited to dopamine. Serotonin is significantly more problematic to electroanalyze in vivo for two reasons. First, during the oxidative scan, serotonin and similarly structured metabolites form unstable intermediary species that rapidly restabilize by forming polymers (Jackson et al. 1995). Unfortunately, as this happens on the electrode surface, serotonin films are created which poison the electrode. Second, extracellular serotonin levels are low, being subject to a thorough level of regulation, likely because of serotonin’s well-established neurotoxic effects (Lane & Baldwin 1997). The former issue was largely resolved ex vivo via a combination of kinetic and physical modifications to the electrode and detection scheme (Hashemi et al. 2009; Jackson et al. 1995). The latter challenge was addressed by targeting an in vivo brain region with the highest density of serotonergic innervation, the SNr (Palkovits et al. 1974), under the rationale that the extracellular serotonin levels in that region should be high. Paradoxically, for reasons described below, we now know that extracellular serotonin levels in the SNr are not as high as in the other regions we studied.
Recent interest in serotonin dynamics is largely for its roles in affective, cognitive and developmental disorders (Owens & Nemeroff 1994; Muller et al. 2016; Abi-Dargham et al. 1997; Oades 2008; Volkow & Fowler 2000). To investigate these diseases, brain regions other than the SNr are thought to play important roles. For example, the hippocampus is heavily implicated in emotion and memory, and as such is often the focus of depression studies (Gyorfi et al. 2017). Similarly, the amygdala, nucleus accumbens, and hypothalamus are investigated for their roles in depression and anxiety (Nestler et al. 2002; Gina L. Forster 2012). The prefrontal cortex plays a role in controlling cognition, motor function, affective and social behaviors (Kolb et al. 2012). The region also has a prolonged development period which means particular susceptibility to influence from exposure to environmental risk factors (Muhammad et al. 2012). As such, the PFC is often associated with and considered in the study of developmental disorders. General and our own interest in these regions shifted our efforts towards serotonin FSCV measurements in the CA2 region of the hippocampus and the mPFC.
It is not trivial to simply apply our current FSCV approach to serotonin measurements in new brain regions. This is primarily because of interference from other transmitters, metabolites and ions, and the heterogeneity of serotonin release sites within each region. Nonetheless, we optimized stereotaxic coordinates that yield serotonin FSCV signals in the CA2 region of the hippocampus, the mPFC and the SNr, in separate animals, through electrical stimulation of a common MFB site. Figure 2 shows 3 representative color plots and corresponding CVs for signals obtained in these regions. It is important to mention that the examples we choose in this figure are highly representative of the signals we obtain in each region. The color plots and CVs are different in these regions, with unique additional features, speak to the different interferences and microenvironment around the CFM. One such unique feature is the ‘switching’ peaks of each region that occur when analytes adsorb to the electrode surface and change the capacitative current that occurs as a function of the electrical bilayer on the electrode surface. This capacitative or charging current is normally subtracted out, however when analytes adsorb and change the current, a signal is manifested at the positive and negative switching potentials. It is not easily possible to identify which analytes are responsible for the switching peaks, but the switching peaks are different in region depending on the microenvironment. The characteristic serotonin oxidation peak is at around 0.7 V and we use this to quantify serotonin. Upon initial inspection of the CV, this peak can occasionally be difficult to observe due to the large switching peaks. However, upon closer inspection, including subtracting out the large switching peaks, the characteristic serotonin oxidation peak is observed.
The mPFC is an intrinsically unique brain region in the context of evoked serotonin responses where two types of signals are routinely observed. In contrast to the CA2 and SNr where a single serotonin release and uptake event is evoked upon stimulation, in the mPFC we often also observe a dual peak. We recently characterized mPFC responses and showed that the dual response was due to stimulation of two distinct axonal bundles that traverse the MFB with diffusion to the electrode surface resulting in the two distinct peaks. Each domain was also found to have a specific reuptake mechanisms (Uptake 1 or Uptake 2) which was verified pharmacologically using escitalopram (West et al. 2018).
These data thus confirm serotonin release in three regions via stimulation of the same MFB coordinate. This common stimulation site is significant because it will enable future, simultaneous measurements of serotonin in multiple brain localities in one animal.
We perceive that the most striking feature of these responses is the difference in reuptake profiles. To better provide a visual comparison, in Figure 8, these responses are normalized such that the maximum amplitude in each case is at the level of the horizontal blue dotted line. In Wood et al. 2012, we modeled the uptake of serotonin in the SNr via two mechanisms, Uptake 1 and 2, first coined by Sol Snyder in the 1970s (Shaskan & Snyder 1970). Uptake 1 is reuptake by the serotonin transporters (SERTs) at high affinity and low efficiency. We observe the Uptake 1 mechanism as a single, slow decay curve (approx. 12–15 seconds). Uptake 2 transpires due to the activity of non-serotonin (non-SERT) transporters, that uptake serotonin with high efficiency, but with low capacity. Uptake 2 generates a decay curve with a single slope that reaches baseline quickly (around 5 seconds). When there is a combination of SERTs and non-SERTs, the result is a hybrid signal, with a curve that decays quickly for a few seconds, followed by a slow decay until it reaches baseline, resulting in decay with two different slopes (Wood et al. 2014).
Figure 8.

The averages from each region have been normalized such that the maximum amplitude of each curve is at the horizontal blue dotted line. This allows for simple visual comparison of the reuptake curves.
While the latter portions of the reuptake curves appear to be the most different between the curves, important information is garnered by comparing the entirety of the reuptake profiles. Serotonin release in the CA2 appears primarily mediated by Uptake 2. The mPFC serotonin responses, as we previously characterized (West et al. 2018), involve clearance by both Uptake 1 and 2 transporters, which are linked inextricably to the two release domains. Serotonin released in the first domain, observed as a single peak, is reuptaken by Uptake 2 mechanisms, while serotonin in the second domain is solely reuptaken by Uptake 1 processes. Domain 2 is only readily observed when measurements occur in layers 5–6 of the mPFC. In the SNr, serotonin seems to be reuptaken via a hybrid of Uptake 1 and 2.
This data lead us to formulate a hypothesis that the chemical decay profile after stimulation offered by the CFM is indicative of the ratio of SERT (Uptake 1) : non-SERT (Uptake 2) mediated serotonin reuptake. It follows that serotonin in the SNr and mPFC is more subject to Uptake 1 than serotonin in the CA2. It is important to note that in the mPFC, we measure as many single evoked events mediated by Uptake 2 (exclusively in layers 1–3) as we do double events (layers 5–6) incorporating both Uptake 1 and 2 (West et al. 2018). For this reason, we narrow down our postulate to serotonin in the SNr being cleared by a higher Uptake 1 : Uptake 2 ratio when compared to the other two regions.
To strengthen this hypothesis, we perform ambient serotonin measurements with FSCAV in the three brain areas, shown in Figure 4. In this study we find that extracellular serotonin levels in the SNr are significantly lower than in the other two regions. This finding is in contrast to our early rationale, that because the SNr is highly innervated by serotonin fibers and this region contains a high tissue content of serotonin, that the SNr should contain a higher extracellular serotonin concentration.
We believe this low SNr basal level can also be explained by a higher ratio of SERT mediated reuptake in the SNr. At rest, ambient serotonin levels are primarily controlled by SERTs that are located, at high density, in the synaptic area (Zhou et al. 1998). However, because serotonin communication is characterized by volume transmission, this modulator can escape the synapse where it is more likely cleared by Uptake 2 mechanisms. Our electrical MFB stimulation is intense, with a high frequency and as such causes an atypically heavy efflux of serotonin, large enough to significantly escape the synapse. Thus, we observe Uptake 2 once the extracellular serotonin concentration is high enough to overcome a specific threshold. The stimulation allows us to test the local physiology of serotonin reuptake, yet the ambient level is more indicative of local SERT density (higher SERT density = lower extracellular serotonin).
This finding further supports our hypothesis that the SNr serotonin is reuptaken by a higher Uptake 1 : Uptake 2 ratio. To corroborate the rationale, we next turned to computational models to analyze the experimental data.
Mathematical Modeling Suggests Physiological Differences Around CFM
We bring forth the idea that serotonin release in the SNr is reuptaken with a higher Uptake 1: Uptake 2 ratio than in the CA2 or mPFC (vide supra). Here we use previously established models to model experiment data and generate independent hypotheses (Figure 5). As a reminder, the conclusions from the modeling are:
(a) Diffusion is needed to explain the double humps seen in the PFC.
(b) There is a higher contribution of Uptake 1 in the SNr than in the other regions.
(c) The autoreceptor effect is strong in the SNr and CA2 but weak in the mPFC.
(d) The input strength to regions of the PFC is more variable than to the SNr and CA2.
Result (a) has already been validated and discussed in detail in prior work (West et al. 2018). Conclusion (b) is in line with hypotheses brought forth from experimental data. The model has additionally suggested two more ideas in conclusions (c) and (d). We next further verify the Uptake 1 : Uptake ratio 2 hypothesis and address these two novel concepts.
Verification of Model Hypotheses
The data and modeling thus far are highly interesting in that they imply that the voltammetric signal can provide information about local tissue physiology and the voltammetric circuitry. According to our model, the SNr evoked serotonin is subject to a higher ratio of Uptake 1 vs. Uptake 2. It is not possible to say for certain, because the model reports a ratio, but this finding implies a higher density of SERTs in the SNr compared to the other regions. To address SERT density in each region, we first turned to the literature but were unable to find a single study that utilized a common technique to compare SERTs in the specific areas of all three regions we are interested in. Thus we employ a single photon imaging approach, whereby axons are imaged in brain slices of adult serotonin transporter-EGFP BAC transgenic mice (Gong et al. 2003). It is well established that axonal innervation corresponds to SERT density (Jin et al. 2016). Images of axons and subsequent quantification of density (Figure 6 and Table 1) illustrate a higher degree of serotonin axon innervation in the SNr compared to the two regions (innervation depends on layer and sub-region of CA2 and mPFC). This is consistent with our model’s hypothesis of higher ratio of Uptake 1 to 2 serotonin reuptake in the SNr. Thus these images provide support for the experimental and model Uptake 1 : Uptake 2 hypothesis.
We next explore an additional postulation offered by the model, that the excitation input to the mPFC is more variable than to the other two regions. While serotonin is elicited in all three regions via the common MFB stimulation, this does not mean that the stimulation location is optimal for evoking maximal serotonin in all regions. If the stimulation location is optimal for eliciting maximal serotonin in all areas, systematically increasing the stimulation intensity, frequency and width should provide three serotonin profiles that plateau with the same characteristic. Thus, we perform experiments in 5 separate mice in each region whereby electrical stimulation frequency, amplitude and pulse width are systematically altered. The standard stimulation utilized in our FSCV experiments is 120 pulses at 60Hz and 360 μA, so the parameters are varied around this range Figure 7 (top panel). The results from the stimulation paradigm study show that while the axons react in a similar manner to stimulation, the mPFC response does not plateau during any of the stimulation trains, whereas the CA2 and SNr responses clearly plateau during one or more of the experiments. This experiment shows that the stimulation location is less optimal for evoking maximal serotonin release in the mPFC, in accord with the model’s postulate that the excitation input to the mPFC is more variable than to the other two regions.
An interesting side note is that a key difference between regions is observed during a depletion study where serotonin release is stimulated once a minute over a one-hour period in Figure 7 (bottom panel). Here, only CA2 serotonin release is significantly depleted over 20 stimulations while the SNr shows no depletion. A simplistic explanation for this difference is the tissue content differences between these regions, of which SNr contains much higher serotonin levels than the other regions (21.0 ± 1.6 ng/mg in the SNr vs. 0.98 ± 0.13 ng/mg in the hippocampus and 0.81 ± 0.065 ng/mg in the frontal cortex) (Shaskan & Snyder 1970; Kim et al. 2014). While it is worth noting that none of these experiments demonstrate the profound depletion that occurs in similar depletion studies with dopamine, this was previously attributed to the high cytosolic serotonin compared to the low amount released during each stimulation (Hashemi et al. 2012).
Thus, independently, two of the model’s hypotheses are verified, again providing context that the chemical signals at the CFMs provide important information about localities and circuits. Another aspect of the model is to hypothesize that serotonin in the CA2 is more strongly autoregulated in comparison to the other two locals. It would be extremely challenging to independently verify this notion due to the large scope of serotonin autoreceptors and autoreceptor sub-types, and these autoreceptors’ ability to synergize. However, the chemical signal and modeling that allude to autoreceptor control provide direction for future interest in autoreceptor mediated serotonin regulation.
In sum, serotonin neurochemistry is important to study in the context of many brain disorders. FSCV and FSCAV are important tools to probe in vivo serotonin chemistry. In this paper, we compared two new voltammetric serotonin stimulation - measurement circuitries, in the CA2 region of the hippocampus and the mPFC, to well established SNr measurements. A common MFB stimulation evoked serotonin in all three brain localities where evoked and ambient serotonin were measured at the same electrode. We found differences in the serotonin chemistry between these three regions that we postulated to arise from differences in tissue physiology local to the CFM. By taking mathematical and imaging approaches, we validated this hypothesis. We therefore highlight the strength of fast voltammetric tools for providing physiological information that has implications for circuit mapping. A map of the circuits within specific brain regions ultimately allows for improved drug targeting for disease treatment.
Supplementary Material
Figure 1.

In each of the three brain regions (CA2, SNr, mPFC) serotonin measurements were taken using either FSCV or FSCAV. The three main methods, outlined here, led to the collection of evoked control (SNr: n=5, CA2: n=5, mPFC: n=10), evoked stimulation parameter experiments (SNr: n=5, CA2: n=5, mPFC: n=5), and basal (SNr: n=5, CA2: n=5, mPFC: n=5) serotonin measurements, and staining of serotonin axons (SNr: n=1, CA2: n=1, mPFC: n=1) in each of the three regions of interest. (n= number of animals)
Acknowledgements and Conflicts of Interest
This study was not pre-registered.
This work was supported by the National Institutes of Health R01MH106563 (PH, JB, MCR and HFN), P30GM103336–01A1 (EP), NS106491 and NS081467 (DJL), and Johns Hopkins Brain Science Institute grant (DJL).
The authors declare no conflicts of interest for this work.
Abbreviations:
- CFM
Carbon Fiber Microelectrode
- FSCV
Fast-Scan Cyclic Voltammetry
- FSCAV
Fast-Scan Controlled Adsorption Voltammetry
- SNr
Substantia Nigra pars Reticulata
- PFC
Prefrontal Cortex
- mPFC
Medial Prefrontal Cortex
- MFB
Medial Forebrain Bundle
- CV
Cyclic Voltammogram
- SEM
Standard Error of the Mean
- RRID
Research Resource Identifiers
References
- Abdalla A, Atcherley CW, Pathirathna P, Samaranayake S, Qiang B, Pena E, Morgan SL, Heien ML and Hashemi P (2017) In Vivo Ambient Serotonin Measurements at Carbon-Fiber Microelectrodes. Anal Chem 89, 9703–9711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abi-Dargham A, Laruelle M, Aghajanian GK, Charney D and Krystal J (1997) The role of serotonin in the pathophysiology and treatment of schizophrenia. J Neuropsychiatry Clin Neurosci 9, 1–17. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Narayan M, Anderson ER, Staib LH, Miller HL and Charney DS (2000) Hippocampal volume reduction in major depression. Am J Psychiatry 157, 115–118. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Vythilingam M, Vermetten E, Nazeer A, Adil J, Khan S, Staib LH and Charney DS (2002) Reduced volume of orbitofrontal cortex in major depression. Biol Psychiatry 51, 273–279. [DOI] [PubMed] [Google Scholar]
- Charan J and Kantharia ND (2013) How to calculate sample size in animal studies? J Pharmacol Pharmacother 4, 303–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J (1988) Statistical Power Analysis for the Behavioral Sciences. Lawrence Erlbaum Associates. [Google Scholar]
- Frodl T, Schaub A, Banac S et al. (2006) Reduced hippocampal volume correlates with executive dysfunctioning in major depression. J Psychiatry Neurosci 31, 316–323. [PMC free article] [PubMed] [Google Scholar]
- Forster Gina L., N. AM, Scholl Jamie L., Watt Michael J. (2012) The Role of the Amygdala in Anxiety Disorders In: The Amygdala, (Ferry B ed.). IntechOpen. [Google Scholar]
- Gong S, Zheng C, Doughty ML et al. (2003) A gene expression atlas of the central nervous system based on bacterial artificial chromosomes. Nature 425, 917–925. [DOI] [PubMed] [Google Scholar]
- Gyorfi O, Nagy H, Bokor M, Moustafa AA, Rosenzweig I, Kelemen O and Keri S (2017) Reduced CA2-CA3 Hippocampal Subfield Volume Is Related to Depression and Normalized by l-DOPA in Newly Diagnosed Parkinson’s Disease. Front Neurol 8, 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashemi P, Dankoski EC, Lama R, Wood KM, Takmakov P and Wightman RM (2012) Brain dopamine and serotonin differ in regulation and its consequences. Proc Natl Acad Sci U S A 109, 11510–11515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashemi P, Dankoski EC, Petrovic J, Keithley RB and Wightman RM (2009) Voltammetric detection of 5-hydroxytryptamine release in the rat brain. Anal Chem 81, 9462–9471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson BP, Dietz SM and Wightman RM (1995) Fast-Scan Cyclic Voltammetry of 5-Hydroxytryptamine. Analytical Chemistry 67, 1115–1120. [DOI] [PubMed] [Google Scholar]
- Jin Y, Dougherty SE, Wood K et al. (2016) Regrowth of Serotonin Axons in the Adult Mouse Brain Following Injury. Neuron 91, 748–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JA, Rodeberg NT and Wightman RM (2018) Measurement of Basal Neurotransmitter Levels Using Convolution-Based Nonfaradaic Current Removal. Anal Chem 90, 7181–7189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TH, Choi J, Kim HG and Kim HR (2014) Quantification of neurotransmitters in mouse brain tissue by using liquid chromatography coupled electrospray tandem mass spectrometry. J Anal Methods Chem 2014, 506870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb B, Mychasiuk R, Muhammad A, Li Y, Frost DO and Gibb R (2012) Experience and the developing prefrontal cortex. Proc Natl Acad Sci U S A 109 Suppl 2, 17186–17193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane R and Baldwin D (1997) Selective serotonin reuptake inhibitor-induced serotonin syndrome: review. J Clin Psychopharmacol 17, 208–221. [DOI] [PubMed] [Google Scholar]
- Meunier CJ, Roberts JG, McCarty GS and Sombers LA (2017) Background Signal as an in Situ Predictor of Dopamine Oxidation Potential: Improving Interpretation of Fast-Scan Cyclic Voltammetry Data. ACS Chem Neurosci 8, 411–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael DJ, Joseph JD, Kilpatrick MR, Travis ER and Wightman RM (1999) Improving data acquisition for fast-scan cyclic voltammetry. Anal Chem 71, 3941–3947. [DOI] [PubMed] [Google Scholar]
- Muhammad A, Carroll C and Kolb B (2012) Stress during development alters dendritic morphology in the nucleus accumbens and prefrontal cortex. Neuroscience 216, 103–109. [DOI] [PubMed] [Google Scholar]
- Muller CL, Anacker AM and Veenstra-VanderWeele J (2016) The serotonin system in autism spectrum disorder: From biomarker to animal models. Neuroscience 321, 24–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ and Monteggia LM (2002) Neurobiology of depression. Neuron 34, 13–25. [DOI] [PubMed] [Google Scholar]
- Oades RD (2008) Dopamine-serotonin interactions in attention-deficit hyperactivity disorder (ADHD). Prog Brain Res 172, 543–565. [DOI] [PubMed] [Google Scholar]
- Owens MJ and Nemeroff CB (1994) Role of serotonin in the pathophysiology of depression: focus on the serotonin transporter. Clin Chem 40, 288–295. [PubMed] [Google Scholar]
- Palkovits M, Brownstein M and Saavedra JM (1974) Serotonin content of the brain stem nuclei in the rat. Brain Res 80, 237–249. [DOI] [PubMed] [Google Scholar]
- Parsons LH and Justice JB Jr. (1993) Serotonin and dopamine sensitization in the nucleus accumbens, ventral tegmental area, and dorsal raphe nucleus following repeated cocaine administration. J Neurochem 61, 1611–1619. [DOI] [PubMed] [Google Scholar]
- Rigucci S, Serafini G, Pompili M, Kotzalidis GD and Tatarelli R (2010) Anatomical and functional correlates in major depressive disorder: the contribution of neuroimaging studies. World J Biol Psychiatry 11, 165–180. [DOI] [PubMed] [Google Scholar]
- Schumann CM, Bauman MD and Amaral DG (2011) Abnormal structure or function of the amygdala is a common component of neurodevelopmental disorders. Neuropsychologia 49, 745–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaskan EG and Snyder SH (1970) Kinetics of serotonin accumulation into slices from rat brain: relationship to catecholamine uptake. J Pharmacol Exp Ther 175, 404–418. [PubMed] [Google Scholar]
- Shen M, Qu Z, DesLaurier J, Welle TM, Sweedler JV and Chen R (2018) Single Synaptic Observation of Cholinergic Neurotransmission on Living Neurons: Concentration and Dynamics. J Am Chem Soc 140, 7764–7768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srejic LR, Wood KM, Zeqja A, Hashemi P and Hutchison WD (2016) Modulation of serotonin dynamics in the dorsal raphe nucleus via high frequency medial prefrontal cortex stimulation. Neurobiol Dis 94, 129–138. [DOI] [PubMed] [Google Scholar]
- Uhlig C, Krause H, Koch T, de Abreu MG and Spieth PM (2015) Anesthesia and Monitoring in Small Laboratory Mammals Used in Anesthesiology, Respiratory and Critical Care Research: A Systematic Review on the Current Reporting in Top-10 Impact Factor Ranked Journals. Plos One 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND and Fowler JS (2000) Addiction, a disease of compulsion and drive: involvement of the orbitofrontal cortex. Cereb Cortex 10, 318–325. [DOI] [PubMed] [Google Scholar]
- West A, Best J, Abdalla A, Nijhout F, Reed M and Hashemi P (2018) Voltammetric evidence for discrete serotonin circuits, linked to specific reuptake domains, in the mouse medial prefrontal cortex. Neurochem Int. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood KM, Zeqja A, Nijhout HF, Reed MC, Best J and Hashemi P (2014) Voltammetric and mathematical evidence for dual transport mediation of serotonin clearance in vivo. J Neurochem 130, 351–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou FC, Tao-Cheng JH, Segu L, Patel T and Wang Y (1998) Serotonin transporters are located on the axons beyond the synaptic junctions: anatomical and functional evidence. Brain Res 805, 241–254. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


