Abstract
Flaviviruses constitute a public health concern because of their global burden and the lack of specific antiviral treatment. Here we investigated the antiviral activity of the alkaloid anisomycin against dengue (DENV) and Zika (ZIKV) viruses. At non-cytotoxic concentrations, anisomycin strongly inhibited the replication of reference strains and clinical isolates of all DENV serotypes and Asian and African strains of ZIKV in Vero cells. Anisomycin also prevented DENV and ZIKV multiplication in human cell lines. While initial steps of DENV and ZIKV replicative cycle were unaffected, a high inhibition of viral protein expression was demonstrated after treatment with anisomycin. DENV RNA synthesis was strongly reduced in anisomycin treated cultures, but the compound did not exert a direct inhibitory effect on 2′ O-methyltransferase or RNA polymerase activities of DENV NS5 protein. Furthermore, anisomycin-mediated activation of p38 signaling was not related to the antiviral action of the compound. The evaluation of anisomycin efficacy in a mouse model of ZIKV morbidity and mortality revealed that animals treated with a low dose of anisomycin exhibited a significant reduction in viremia levels and died significantly later than the control group. This protective effect was lost at higher doses, though. In conclusion, anisomycin is a potent and selective in vitro inhibitor of DENV and ZIKV that impairs a post-entry step of viral replication; and a low-dose anisomycin treatment may provide some minimal benefit in a mouse model.
Keywords: flavivirus, dengue, Zika, anisomycin, antiviral, p38
1. Introduction
Dengue virus (DENV) and Zika virus (ZIKV) are emerging mosquito-borne viruses that belong to the Flaviviridae family. There are four DENV serotypes (DENV-1, −2, −3 and −4), whereas a single ZIKV serotype, containing the African and Asian lineages, has been identified. Both DENV and ZIKV are transmitted by Aedes aegypti and Aedes albopictus mosquitoes in tropical and subtropical regions of the world. The clinical manifestations of dengue vary from an asymptomatic infection or a mild fever to a life-threatening severe disease (Guzman et al., 2016). In contrast to DENV, sexual and vertical ZIKV transmission has been demonstrated. Although the symptoms of acute ZIKV infection are usually mild an increased risk of serious clinical manifestations, such as Guillain–Barré syndrome in adults and congenital malformations, have been associated with ZIKV infection (Baud et al., 2017).
No specific antiviral treatment or worldwide licensed vaccine against DENV and ZIKV are available and the lack of vector control, unplanned urbanization and increased air travels led to the worldwide expansion of those viral infections (Guarner and Hale, 2019). In the development of antiviral strategies, viral targets provide more specificity and less toxicity whereas cellular targets may offer a wide viral spectrum of action and lower probability of antiviral resistance (García et al., 2017). One of the most studied viral targets is the non-structural protein NS5, the most conserved non-structural protein among flaviviruses. NS5 carries essential enzymatic activities hosted in two domains: the N-terminal methyltransferase domain (NS5-MTase) and the C-terminal RNA dependent RNA polymerase domain (NS5-RdRp). A few compounds with a cellular target have been evaluated in clinical trials for DENV with no promising results (Kaptein et al., 2016; Sung et al., 2016). Recently, the first clinical trial against ZIKV is being performed (Zou and Shi, 2019).
The natural alkaloid anisomycin was originally identified as an antibiotic active against certain protozoa and fungi (Hall et al., 1983; Lynch et al., 1954). Anisomycin inhibits protein synthesis, but at low sub-inhibitory concentrations, anisomycin induces the activation of mitogen-activated protein kinases (MAPKs) such as p38 and the c-Jun N-terminal kinases 1, 2 and 3 (Liu et al., 2014; Stadheim et al., 2002). In addition, anisomycin was reported to suppress in vitro replication of the flavivirus Japanese encephalitis virus (Chang et al., 2005).
In the present study, we evaluated the in vitro antiviral effect of anisomycin against DENV and ZIKV. We also explored the mechanism of anisomycin antiviral action against both viruses and investigated the antiviral activity of the compound in a mouse model for ZIKV infection.
2. Materials and Methods
2.1. Cells and viruses
Vero (ATCC CCL 81), HepG2 (ATCC CRL-10741) and A549 (ATCC CL-185) cells were grown in Eagle’s minimum essential medium (GIBCO, USA) supplemented with 10%, 20% and 5% of newborn calf serum, respectively. For maintenance medium (MM) serum concentration was reduced to 1.5%. U937 cells (ATCC CRL-1593.2) were grown in RPMI-1640 medium (Sigma-Aldrich, USA) supplemented with 10% fetal bovine serum (GIBCO, USA). DENV-2 strain New Guinea C (NGC), DENV-1 strain Hawaii, DENV-3 strain H87, DENV-4 strain 8124 and ZIKV strains DAK-AR-41524, PRVABC59 and P6–740 were used. DENV-2 clinical isolates 67655 and 67702 and ZIKV clinical isolate INEVH11614 were provided by Dr. D. Enria and Dr. S. Levis (Instituto Nacional de Enfermedades Virales Humanas, Argentina). Viral stocks were prepared in C6/36 mosquito cells and titrated by plaque forming units (PFU) in Vero cells.
2.2. Animals
Male and female AG129 mice, lacking interferon α/β and γ receptors, were bred at the Utah State University (USU) Laboratory Animal Research Center (LARC) under germ-free conditions in an in-house breeding colony. Mice between 8 and 10 weeks with an average weight of 22 g were used.
2.3. Compounds
Stock solutions of anisomycin, ribavirin, sinefungin and 3’dATP (Sigma-Aldrich, USA), and SB202190 (Cell Signaling, USA) were prepared in dimethyl sulfoxide (DMSO). Ammonium chloride (Sigma-Aldrich, USA) was dissolved in phosphate buffer saline (PBS).
2.4. Cytotoxicity assay
Cells were exposed to various concentrations of anisomycin in MM for 48 h. Cell viability was examined by the MTS colorimetric assay (Promega, USA). The 50% cytotoxic concentration (CC50) was defined as the compound concentration that reduced cell viability by 50%.
2.5. Virus yield reduction assay
Cells were infected with DENV or ZIKV at a multiplicity of infection (MOI) of 0.1, 1, 10 or 50. After 1 h adsorption cells were covered with MM containing different concentrations of anisomycin. At 48 h post-infection (p.i.), extracellular virus yields were quantified by plaque assay in Vero cells. The 50% effective concentration (EC50), compound concentration that reduced virus yield by 50%, was calculated.
Other DENV-2 (strain NGC) or ZIKV (strain PRVABC59) infected Vero cell cultures (MOI=1) were treated with anisomycin (200 nM) during different time intervals prior or after infection. At 24 h p.i. extracellular virus titer was determined and intracellular infectivity was assessed after two freeze-thaw cycles of cell monolayers.
2.6. Virus internalization assay
Vero cells were exposed to 100 PFU/well of DENV-2 (strain NGC) or ZIKV (strain PRVABC59) and after 1 h adsorption at 4°C, cells were washed with cold PBS and cultures were further incubated at 37°C with or without anisomycin (200 nM). At different times, adsorbed non-internalized virus was inactivated with low pH treatment (Brunetti et al., 2018) and cells were covered with MM containing 1% methylcellulose. Plaques were counted on 6 days p.i. (dpi) for DENV and 4 dpi for ZIKV.
2.7. Immunofluorescence assay
Vero cells grown on coverslips were infected with DENV-2 (strain NGC) or ZIKV (strain PRVABC59) (MOI = 1) and after 1 h adsorption, MM containing anisomycin (200 nM) was added. At different time points, cells were fixed with methanol and further stained using mouse monoclonal antibody anti-DENV-2 E glycoprotein (Abcam, UK) or anti-flavivirus E protein (4G2, Millipore, USA), for ZIKV E protein detection, as primary antibodies and anti-mouse immunoglobulins conjugated to isothiocyanate of fluorescein (Sigma-Aldrich, USA) as secondary antibody. Cell nuclei were stained with Hoechst 33258 reagent (1 μg/ml) and samples were visualized in a fluorescence microscope (Olympus Corporation, Japan). The number of cells expressing E glycoprotein was obtained by counting ten fields chosen at random.
2.8. Real time RT-PCR (qRT-PCR)
Vero cells were infected with DENV-2 (strain NGC) (MOI = 1) and after 1 h adsorption cultures were incubated with anisomycin (200 nM) or ribavirin (80 mM). At different time points, cells were washed with PBS. Total RNA extraction, cDNA synthesis and real time PCR, which allow the amplification of nucleotides 10,419 to 10,493 within the viral 3’ UTR, was performed as previously described (Quintana et al., 2016). Average viral RNA Ct values were normalized to the average Ct values of actin (ΔCt), ΔΔCt based fold-change calculations were set relative to untreated-virus infected cells and QR (2ΔΔCt) was calculated using Bio-Rad iQ5 2.1 software.
2.9. Western blot analysis
DENV-2 (strain NGC) infected Vero cells (MOI=1) were treated with anisomycin (200 nM) and at different time points cells were lysed and viral protein expression was detected by Western blot assay using mouse monoclonal antibody anti-DENV-2 E glycoprotein (Abcam, UK). Mouse anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (Abcam, UK) was also used as primary antibody. Peroxidase conjugated anti-mouse immunoglobulins (Sigma-Aldrich, USA) were employed as secondary antibody.
In order to analyze p38 activation, uninfected or DENV-2 (strain NGC) infected Vero cells (MOI = 1) were treated with different concentrations of anisomycin and incubated at 37°C. In another experiment, Vero cells pretreated 1 h with SB202190 (16 μM) were infected with DENV-2 (MOI=1) in presence of the compound. After 1 h adsorption, cells not treated with SB202190 were incubated with anisomycin (200 nM) and cells previously treated with SB202190 were incubated with SB202190 (16 μM) or a combination of anisomycin (200 nM) and SB202190 (16 μM). At 24 h p.i., cells were lysed and Western blot assay was performed as previously described (Quintana et al., 2016). Rabbit antibody (Cell Signaling, USA) anti phospho- p38 (P-p38) or rabbit antibody anti p38 (Santa Cruz Biotechnology, USA) were used as primary antibodies and peroxidase-conjugated anti-rabbit IgG (Promega, USA) was employed as secondary antibody. The intensity of protein bands, detected by chemiluminescence, was quantified using Image J software.
2.10. DENV NS5 2′O-MTase and RdRp enzyme activity assays
2′O-MTase activity was tested as previously described (Coutard et al., 2014) by measuring the transfer of a [3H]-methyl group from 3H- S-adenosyl methionine to the short GpppAC4 substrate yielding GpppA2′OMeC4 using functionally active recombinant DENV-3 NS5 MTase domain (Egloff et al., 2002).
NS5 RdRp activity was assessed by an in vitro nucleotide incorporation assay using functionally active recombinant DENV-2 NS5 RdRp domain (Selisko et al., 2006) with a homopolymeric RNA template poly(rU) and PicoGreen (Molecular Probes, USA), a fluorescent intercalant agent for detecting double stranded RNA (Benmansour et al., 2016).
2.11. Efficacy study of anisomycin against ZIKV in AG129 mouse model
AG129 mice were randomly assigned to groups of 10 animals each. A challenge dose of ~200 CCID50 (50 % cell culture infectious dose) of ZIKV (strain P6–740) was administered via subcutaneous (s.c.) injection of 0.1 ml. Anisomycin was diluted in sterile saline (NaCl 0.9%). Compound was prepared less than 18 h prior to initial administration and stored at 4°C. Anisomycin was administered s.c., once daily (qd) at 4, 20 or 100 mg/kg/d. Treatment began 4 h after virus challenge and continued for 10 days. A placebo-treated infection control group (n = 10 mice) was included. Uninfected mice (n = 4) treated with 100 mg/kg/d anisomycin qd for 10 days were also included as toxicity controls. Corporal weight was recorded on day 0, 8 and then every day from 9 to 21 dpi. Mice were observed at least twice daily for 28 days for mortality and disease signs, including excitability, lying prone, paralysis, hunching, and conjunctivitis. The first day any of these disease signs was observed was recorded. Mice were humanely euthanized if they could no longer right themselves or were unresponsive to stimuli.
2.12. Statistical analysis
Parametric data were analyzed using one-way ANOVA followed by Dunnett’s multiple comparison tests (Prism 7, GraphPad Software, Inc.). For survival data the Wilcoxon log-rank survival analysis was used. Live/total analyses were performed using a contingency table analysis followed by Fisher’s exact tests when making pairwise comparisons if appropriate.
2.13. Ethics regulation of Laboratory animals
This study was conducted in accordance with the approval of the Institutional Animal Care and Use Committee of USU. The work was done in the AAALAC-accredited LARC of USU. The U. S. Government (National Institutes of Health, NIH) approval was renewed on 9 March 2018 (PHS Assurance no. D16–00468) in accordance with the NIH Guide for the Care and Use of Laboratory Animals (Revision; 2015).
3. Results
3.1. Anisomycin is a dose-dependent inhibitor of DENV and ZIKV multiplication in cell cultures
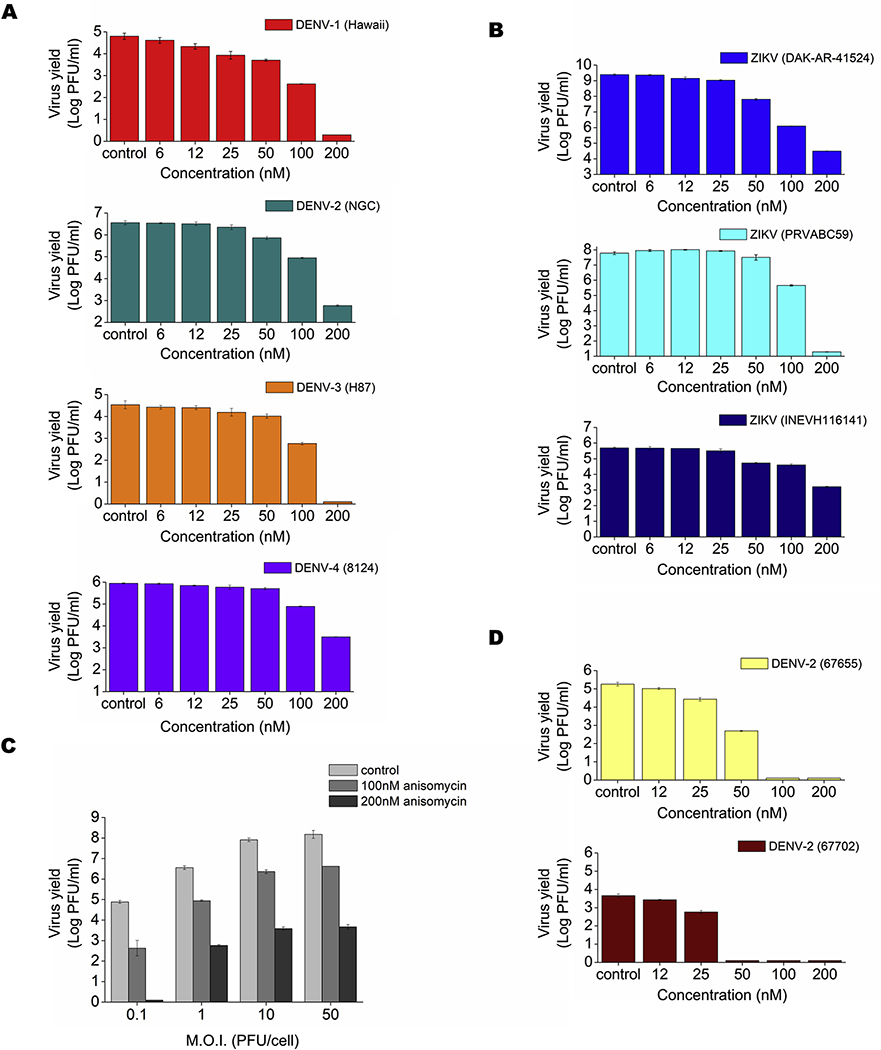
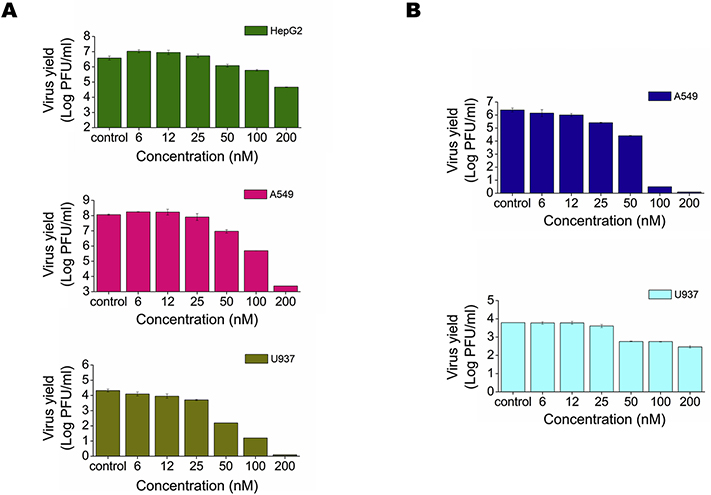
We first carried out an MTS assay to evaluate cell viability in Vero, A549, U937 and HepG2 cells treated for 48 h with different concentrations of anisomycin. Low toxicity was observed after anisomycin treatment in A549 and HepG2 human cell lines in comparison with Vero cells. By contrast, the compound exerted a high cytotoxic effect at high doses on human U937 cells (Table 1). Anisomycin displayed a dose-dependent antiviral activity against all DENV serotypes and ZIKV strains assayed in Vero cells, the cell line more routinely used for flavivirus antiviral testing (Figs. 1A and 1B). Potent inhibition of DENV-2 (NGC) production was achieved even in cultures infected at MOI=50 (Fig. 1C). Anisomycin also exhibited antiviral activity against clinical isolates of ZIKV and DENV-2 (Figs. 1B and 1D) and was effective against DENV-2 (NGC) and ZIKV (PRVABC59) in human cell lines (Figs. 2A and 2B). However, low selectivity was achieved in U937 cells due to the more adverse effect of anisomycin on the viability of this cell line (Table 1).
Table 1:
Cytotoxic and antiviral effects of anisomycin.
| Cell line | CC50 (nM) | Virus | EC50(nM) | SI |
|---|---|---|---|---|
| Vero | 5400± 100 | DENV-1 (Hawaii) | 23.2± 1.5 | 232± 20 |
| DENV-2 (NGC) | 31.3± 1.2 | 172± 10 | ||
| DENV-3 (H87) | 24.8± 1.3 | 217± 16 | ||
| DENV-4 (8124) | 61.6± 1.1 | 87± 5 | ||
| DENV-2 (67655) | 7.6± 1,1 | 710± 64 | ||
| DENV-2 (67702) | 15.0± 1.0 | 360± 31 | ||
| ZIKV (DAK-AR-41524) | 15.9± 1.1 | 340± 30 | ||
| ZIKV (PRVABC59) | 50.7± 1.0 | 107± 9 | ||
| ZIKV (INEVH116141) | 33.0± 1.1 | 163± 9 | ||
| A549 | > 94000 | DENV-2 (NGC) | 32.2± 1.1 | >2919 |
| ZIKV (PRVABC59) | 7.9± 1.2 | >11900 | ||
| U937 | 420± 50 | DENV-2 (NGC) | 13.2± 1.2 | 32± 1 |
| ZIKV (PRVABC59) | 30.9± 1.0 | 14± 2 | ||
| HepG2 | > 94000 | DENV-2 (NGC) | 40.7± 1.1 | >2310 |
Cell viability was assayed by the MTS method and CC50 (compound concentration that reduced cell viability by 50% respect to untreated cultures) was calculated. Antiviral activity was evaluated by a virus yield inhibition assay and EC50 (compound concentration that reduced extracellular virus yield by 50% respect to untreated infected cultures) was determined. Data are mean values from three experiments ± SD. SI (selectivity index): ratio CC50/EC50.
Figure 1: Inhibitory effect of anisomycin against DENV and ZIKV multiplication in Vero cells.
Vero cells were infected with (A) DENV-1 (Hawaii), DENV-2 (NGC), DENV-3 (H87) or DENV-4 (8124) (MOI = 1), (B) ZIKV (strains DAK-AR-41524 or PRVABC59) or ZIKV clinical isolate (INEVH116141) (MOI = 1), (C) DENV-2 (NGC) at different MOIs and (D) DENV-2 clinical isolates (MOI=1). After 1h adsorption at 37°C, cells were incubated in MM containing different concentrations of anisomycin. At 48 h p.i. extracellular virus yield was quantified by plaque assay. Data are mean values from three independent experiments ± standard deviation (SD).
Figure 2: Inhibitory effect of anisomycin against DENV and ZIKV multiplication in human cell lines.
(A) HepG2, A549 or U937 cells were infected with DENV-2 (MOI = 1) and (B) A549 or U937 cells were infected with ZIKV (PRVABC59) (MOI = 1). After 1 h adsorption at 37°C, cells were covered with MM containing different concentrations of anisomycin. At 48 h p.i. extracellular virus yield was quantified by plaque assay. Data are mean values from three independent experiments ± SD.
3.2. Anisomycin inhibits the synthesis of viral RNA and proteins
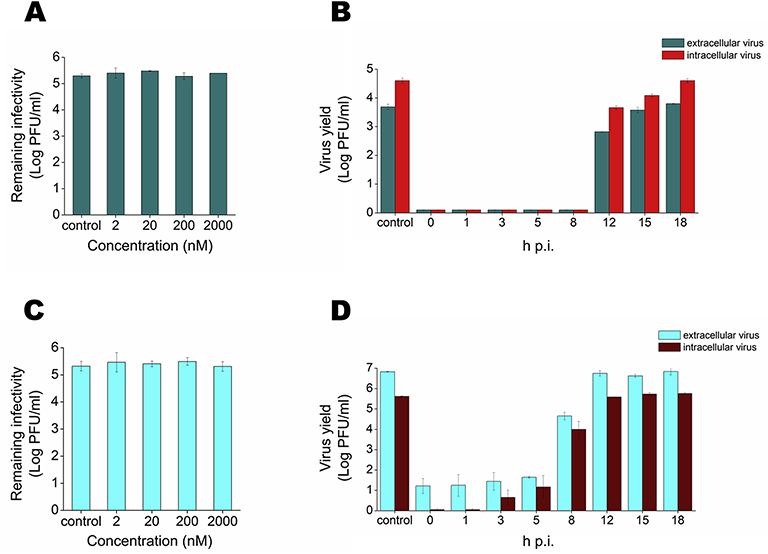
The wide spectrum and selective efficacy of anisomycin against DENV and ZIKV replication encouraged us to further characterize its mode of antiviral action. No direct inactivating effect of anisomycin on DENV-2 or ZIKV particles was observed (Figs. 3A and 3C). Additionally, pretreatment of Vero cell cultures with the compound did not affect DENV-2 multiplication (Fig. S1). A time course study showed that the addition of anisomycin up to 8 h p.i. or 5 h p.i. for DENV or ZIKV, respectively, caused a 99.99% inhibition of viral production. The antiviral effect started to decline when the compound was added later, and was absent when treatment was started at 18 h p.i. for DENV-2 or 12 h p.i for ZIKV (Figs. 3B and 3D) suggesting that anisomycin exerts a high inhibitory action when it is present during the initial or intermediate steps of viral cycle.
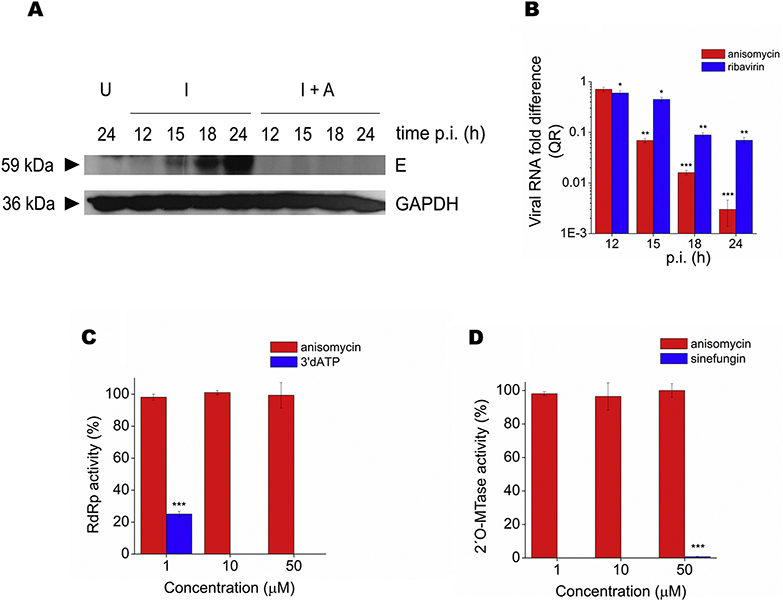
Figure 3: Characterization of anisomycin antiviral effect.
(A) DENV-2 or (C) ZIKV were incubated with different concentrations of anisomycin for 2 h at 37°C and remaining infectivity was determined by plaque assay. Vero cells were infected with (B) DENV-2 or (D) ZIKV (MOI= 1) and anisomycin (200 nM) was added at different times p.i. At 24 h p.i. intracellular and extracellular virus yields were determined by plaque assay. Data are mean values from three independent experiments ± SD.
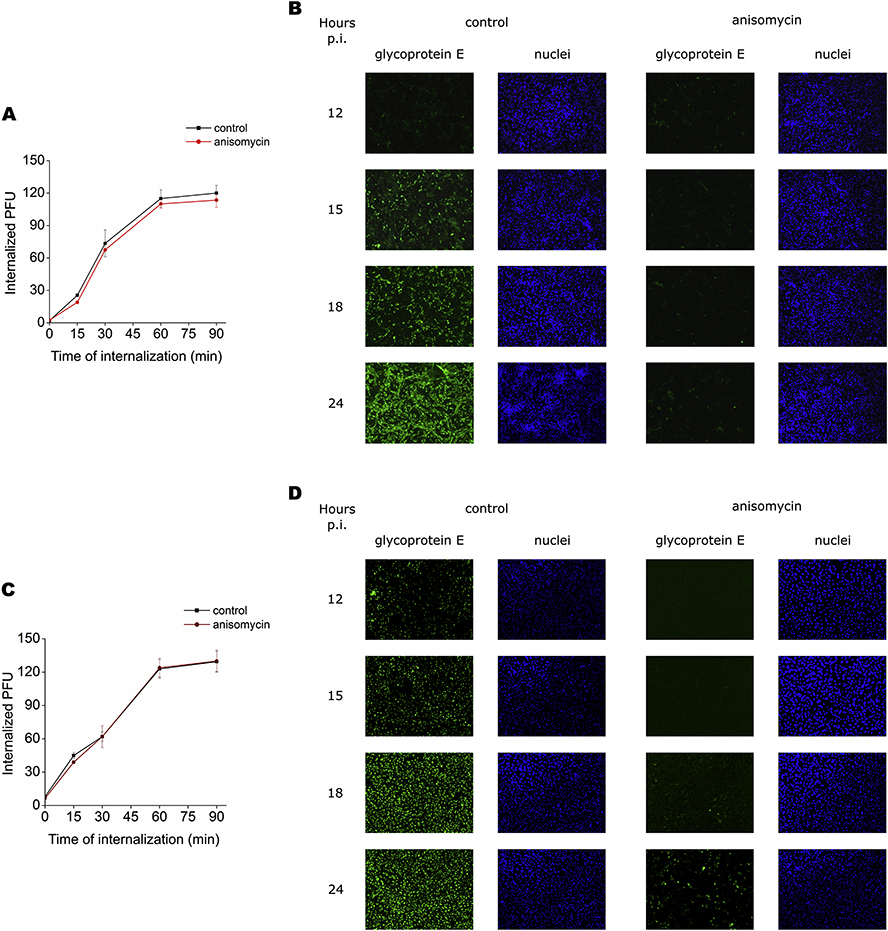
To reveal the target for anisomycin in DENV and ZIKV infective cycle, the effect of anisomycin on initial steps of viral infection was first explored. As shown in Figs. 4A and 4C the kinetics of viral internalization was unaffected by anisomycin treatment, indicating that anisomycin might affect a post-penetration step of viral multiplication. In fact, anisomycin caused a potent inhibition of DENV-2 and ZIKV protein synthesis, detected by an immunofluorescence assay. Treatment with anisomycin completely reduced the number of cells expressing E glycoprotein in DENV-2 infected cells whereas a 99.1± 0.5% and 95.0 ± 3.0% reduction in the number of fluorescent cells was observed in ZIKV infected cultures at 18 and 24 h p.i, respectively (Figs. 4B and 4D). Inhibition of DENV-2 glycoprotein E expression in anisomycin treated cultures was corroborated by Western blot (Fig. 5A). Furthermore, the compound also displayed a strong inhibitory effect on DENV-2 RNA synthesis, quantified by qRT-PCR (Fig. 5B). Overall, these results suggest that anisomycin exerts its inhibitory action mainly affecting the synthesis of viral components. On that basis, we tested the effect of the compound on the activities of DENV NS5 protein directly involved in viral RNA capping and replication processes. Anisomycin did not exhibit inhibitory action against NS5-RdRp activity or NS5–2′O-MTase activity in comparison with the reference inhibitors 3′dATP (1 μM), which inhibited RdRp activity by 75%, and sinefungin (50 μM), which caused a 99% inhibition of 2’O MTase activity (Figs. 5C and 5D).
Figure 4: Mode of anisomycin antiviral action.
Vero cells were exposed to 100 PFU of (A) DENV-2 or (C) ZIKV during 1 h at 4°C, then covered with MM in presence or absence of anisomycin (200 nM) and transferred to 37°C. At the indicated times, non-internalized virus was inactivated and internalized particles were determined by plaque assay. Data are mean values from three independent experiments ± SD. (B) DENV-2 or (D) ZIKV infected Vero cells (MOI= 1) were treated with anisomycin (200 nM) and at different times p.i., cells expressing viral E glycoprotein were detected by an immunofluorescence assay. Cell nuclei were stained with Hoechst. Magnification 100x.
Figure 5: Effect of anisomycin on DENV-2 macromolecular synthesis.
(A) DENV-2 infected Vero cells (MOI= 1) were treated with anisomycin (200 nM) and at different times p.i. E glycoprotein expression was analyzed by Western blot. U: uninfected cells; I: infected cells; I+A: infected cells treated with anisomycin. GAPDH was revealed as loading control. A representative experiment is shown. (B) DENV-2 infected Vero cells (MOI= 1) were treated with anisomycin (200 nM) or ribavirin (80 μM) and at different times p.i. total RNA was extracted and qRT-PCR was performed to determine the relative amount of viral RNA with respect to untreated infected cultures, using actin for normalization. Data are mean values from three independent experiments ± SD (***p <0.001, **p < 0.01, *p < 0.05). (C) Quantification of RdRp and (D) 2′O-MTase activities of DENV NS5 in presence of different concentrations of anisomycin. Compounds 3’dATP and sinefungin were used as reference inhibitors of RdRp and 2′O-MTase activities, respectively. Data, expressed as percentage of enzyme activity with respect to untreated control, are mean values from triplicates ± SD (***p <0.001).
3.3. Anisomycin effect on p38 pathway is not related to its antiviral activity
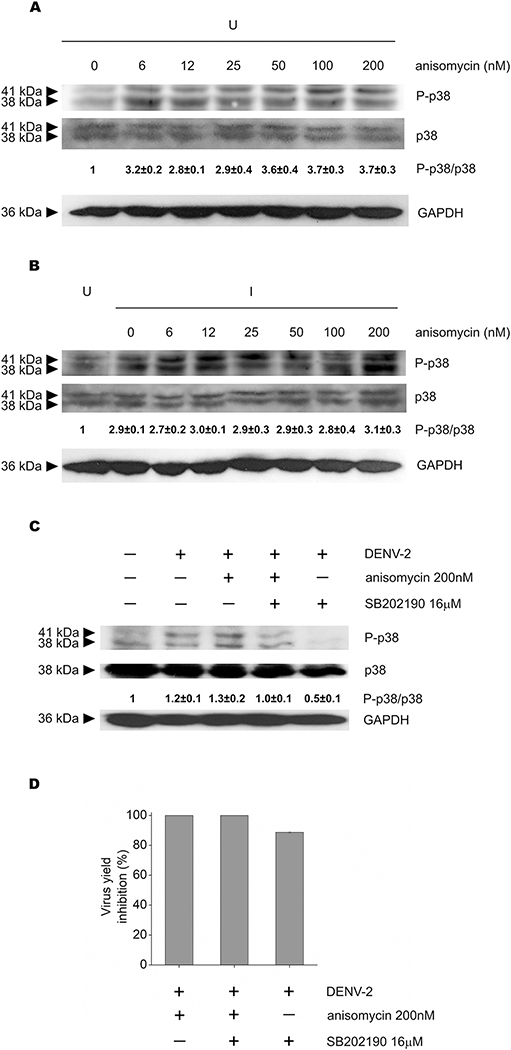
Given that anisomycin is a well-established activator of p38 signaling, we evaluated the effect of viral inhibitory and sub-inhibitory concentrations of anisomycin on p38 pathway activation. In uninfected Vero cells, all concentrations of anisomycin tested promoted p38 phosphorylation, although at high concentrations (50 to 200 nM) a slight increment in the activation of p38 was detected in comparison with low anisomycin doses of the compound (Fig. 6A). On the other hand, DENV-2 infection induced the activation of p38 with respect to uninfected cells (Fig. 6B). However, Fig. 6B shows that in the context of infection no changes in P-p38 levels were detected between cells untreated (0 nM) or treated with either low non-inhibitory concentrations of anisomycin (6 to 25 nM) or high anisomycin concentrations (50 to 200 nM), which display anti DENV activity (see Fig. 1A). These results suggest that p38 activation is not involved in the mechanism of antiviral action of the compound. We also analyzed the effect of anisomycin on p38 phosphorylation and DENV-2 multiplication in cells treated or not with the compound SB202190, a p38 inhibitor. The inhibitory effect of SB202190 on p38 phosphorylation was corroborated by Western blot (Fig. 6C). Combined treatment with anisomycin and SB202190 significantly reduced the level of p38 phosphorylation (p<0.05) in comparison to P-p38 levels in cells incubated with anisomycin as the only treatment (Fig. 6C) but antiviral activity of anisomycin was unaffected by the presence of SB202190 (Fig. 6D). Altogether these results corroborate that the antiviral activity of anisomycin against DENV-2 is not related to the level of p38 activation. It should be noted that P-p38 level in cells treated with anisomycin (200 nM) and SB202190 (16 μM) was still higher than in cells treated with SB202190 (16 μM) alone, so it is likely that higher concentrations of SB202190 would be required to completely inhibit anisomycin-induced p38 activation.
Figure 6: Involvement of p38 activation on anisomycin antiviral activity.
(A) Uninfected or (B) DENV-2 infected Vero cells were treated with different concentrations of anisomycin and p38 phosphorylation was analyzed by Western blot. The relative intensity of P-p38/p38 bands of each sample with respect to uninfected untreated cells is shown. GAPDH was revealed as loading control. U: uninfected cells, I: infected cells. (C and D) DENV-2 infected Vero cells untreated or treated with anisomycin (200 nM), SB202190 (16 μM) or a combination of both compounds were incubated for 24 h at 37°C. (C) p38 activation was analyzed by Western blot and the relative intensity of P-p38/p38 bands of each sample with respect to uninfected untreated cells is shown. GAPDH was used as loading control. (D) Viral yields were determined by plaque assay and results are expressed as percentage of inhibition in treated cells with respect to untreated infected cells. In (A), (B) (C) and (D) each value represents the mean ± SD of three independent experiments.
However, we could not explore this possibility because concentrations of SB202190 greater than 16 μM in combination with anisomycin exhibited cytotoxic effect, as determined by the MTS method (data not shown). In addition, an important reduction of viral titer was detected in cells treated with the p38 inhibitor only (Fig. 6D).
3.4. Anisomycin presented a reverse dose effect against ZIKV in vivo
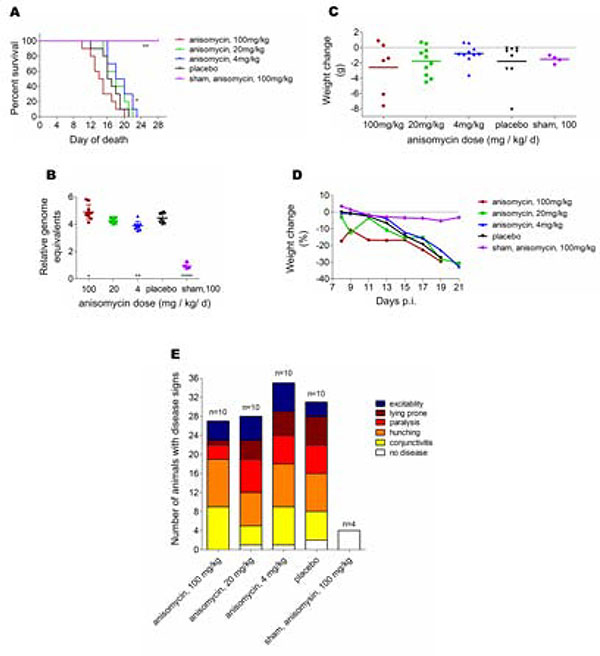
The in vivo efficacy of anisomycin was evaluated using an AG129 mouse model of ZIKV infection and disease (Julander et al., 2017). First, we determined the maximum tolerated dose of anisomycin in AG129 mice (data not shown). In order to test the antiviral activity, three doses of the compound (100, 20 or 4 mg/kg/d) were administered for 10 days starting at 4 h p.i. Infected mice treated with 100 mg/kg/d of anisomycin presented disease signs earlier than infected control mice and a more rapid mortality rate. Despite the earlier onset of disease in mice treated with the highest dose, a longer interval between beginning of disease and mortality was observed as compared with infection controls. By contrast, in mice treated with 4 mg/kg/d of anisomycin the appearance of disease signs was delayed and animals died significantly (p<0.05) later than the control group (Fig. 7A).
Figure 7: In vivo antiviral activity of anisomycin against ZIKV.
(A) Survival of AG129 mice after treatment with various doses of anisomycin administered for 10 days beginning 4 h after challenge with ZIKV (**p<0.01, *p<0.05). (B) Viral RNA obtained at 5dpi from serum of AG129 mice treated with various doses of anisomycin administered for 10 days beginning 4 h after challenge with ZIKV (**** p<0.0001, ** p<0.01, * p<0.05). (C) Weight change between 8 and 13 dpi, (D) time course weight change between 8 and 21 dpi and (E) disease signs of AG129 mice treated with various doses of anisomycin administered for 10 days beginning 4 h after challenge with ZIKV.
Survival data correlated with viremia data, with significantly (p<0.05) higher viral RNA titers found in mice treated with 100 mg/kg/d of anisomycin and a significant (p<0.01) reduction in the amount of viral RNA in samples obtained from animals treated with 4 mg/kg/d of the compound (Fig. 7B). The corporal weight change between 8 and 13 dpi did not significantly differ in treated mice with respect to untreated infected animals (Fig. 7C). Time-course of weight change along the study demonstrated a rapid weight loss in mice treated with the highest dose, while overall weight change was similar in treated animals to that of animals treated with placebo (Fig. 7D). Otherwise, no difference in the number of disease signs was observed in animals treated with anisomycin compared to placebo treated mice (Fig. 7E).
4. Discussion
Since different flaviviruses cocirculate in tropical and subtropical areas, the development of broad-spectrum anti-flavivirus compounds is particularly desirable. In this study, we demonstrate that anisomycin has an effective and selective dose-dependent antiviral activity against the four DENV serotypes and ZIKV strains from the Asian and African lineages, including also clinical isolates, in Vero cells. In addition, the antiviral effect of anisomycin was unaffected by the MOI employed. This advantageous property has also been described for other types of DENV inhibitors (Talarico and Damonte, 2007; Whitby et al., 2005).
Monocytes, macrophages and dendritic cells, which are primary target cells for DENV and ZIKV replication, disseminate the infection via the lymphatic and vascular system to different organs (Foo et al., 2017; Zhang et al., 2019). Anisomycin inhibited DENV-2 and ZIKV production in human cell types that are related to viral replication in vivo, such as the myeloid cell line U937 and the lung carcinoma cell line A549 and also displayed inhibitory action against DENV-2 in HepG2 cells derived from liver carcinoma. Even though the EC50 value against DENV-2 was similar in different cell types, anisomycin caused high cytotoxicity in U937 cells. This fact is in accordance with previously published data, which proved that anisomycin treatment induced apoptosis in U937 cells (Hori et al., 2008) and rabbit macrophages, probably associated with activation of p38 signaling (Croons et al., 2009).
Anisomycin neither presented virucidal effect nor induced a refractory antiviral state in the host cell. Mechanistic studies revealed that DENV and ZIKV internalization step is unaffected by anisomycin whereas viral macromolecular synthesis appears to be the main target of the compound. Neither MTase nor RdRp DENV NS5 in vitro enzyme activity assays were affected by anisomycin. However, it is possible that anisomycin treatment interferes with NS5 interactions with other viral components, such as the NS3 protein (Teramoto et al., 2017), or cellular proteins (Carpp et al., 2014) involved in DENV macromolecular synthesis.
Anisomycin treatment within the range of concentrations 6 to 200 nM rendered similar levels of p38 phosphorylation in DENV-2 infected cells, but only anisomycin concentrations higher than 25 nM displayed anti-DENV-2 activity. Furthermore, the presence of the p38 inhibitor SB202190 did not affect anti-DENV activity of anisomycin. Therefore, the antiviral effect of anisomycin cannot be attributed to its ability to up-regulate p38 signaling. On the other hand, SB202190 reduced both p38 phosphorylation and DENV-2 multiplication, in accordance with previous studies performed with DENV and ZIKV (Ceballos-Olvera et al., 2010; Cheng et al., 2018; Roth et al., 2017). Since it has been reported that anisomycin would also induce the activation of JNK and ERK1/2 cell signaling pathways (Nikaido et al., 2019; Torres et al., 2012), further studies would be necessary to determine whether these MAPKs are involved in anisomycin antiviral action.
Based on previous reports (Tang et al., 2012; Wanisch et al., 2008) we chose s.c. route for anisomycin administration to ZIKV infected AG129 mice. Whereas doses up to 100 mg/kg/d were well-tolerated in uninfected mice, a more rapid mortality rate was observed in infected ones in comparison to untreated infected mice. By contrast, animals treated with a low dose of anisomycin (4 mg/kg/d) exhibited a significant reduction in viremia levels and died significantly later than the control group. These results indicate a potential toxicity of the compound in infected mice at higher doses, despite the tolerability of higher doses in uninfected mice. Interestingly, antiviral studies against influenza virus showed that low doses of a p38 inhibitor, the compound BCT197, improved mouse survival, whereas at higher concentrations of BCT197 this protective effect was reduced (Growcott et al., 2018). p38 MAPK signaling pathway is involved in the inflammatory response, thus the use of p38 modulators as antiviral agents might require an in-depth analysis of dose dependent effects on the proper balance between the regulation of inflammation processes and the prevention of infection. For this reason, we do not rule out that more frequent administration of low doses of anisomycin, combination with a direct-acting antiviral or alternative routes of drug injection might exert a protective effect against in vivo ZIKV infection.
In conclusion, we found that anisomycin potently inhibits DENV and ZIKV replication in cell cultures, mainly affecting viral macromolecular synthesis. The antiviral effect of anisomycin is not mediated by direct inhibition of MTase and RdRp activities of NS5 nor by the activation of p38 signaling. Further studies are required to investigate in more detail anisomycin efficacy, as well as its seemingly complex dose-related effect, in the context of an in vivo flavivirus infection.
Supplementary Material
Figure S1: Effect of pretreatment of cells with anisomycin on DENV-2 production. Vero cells were pretreated with anisomycin (200 nM) at different time points before infection and then cells were infected with DENV-2 (MO I= 1). At 24 h p.i. intracellular and extracellular virus yields were determined by plaque assay. Data are mean values from three independent experiments ± SD.
Highlights.
Anisomycin exerted a selective and potent antiviral activity against dengue (DENV) and Zika (ZIKV) viruses in cell cultures.
A strong inhibition of viral protein expression and RNA synthesis was demonstrated after treatment with anisomycin.
The antiviral effect of anisomycin was not mediated by inhibition of NS5 functions nor by the activation of p38 signaling.
A low dose of anisomycin exhibited a significant reduction in viremia levels in ZIKV infected AG129 mice
Acknowledgments
This work was supported by grants from UBA (20020170100363BA), ANPCyT (PICT 2015 3080), CONICET (PIP 0338), National Institute of Allergy and Infectious Diseases, NIH (contract HHSN272201700041I/HHSN27200004, Task A11). VMQ received Fulbright -Bunge&Born Foundation and The Company of Biologists fellowships. VMQ and JEB are fellows and EBD is researcher from CONICET.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baud D, Gubler DJ, Schaub B, Lanteri MC, Musso D. An update on Zika virus infection. Lancet. 2017; 390(10107):2099–2109. [DOI] [PubMed] [Google Scholar]
- Benmansour F, Eydoux C, Querat G, de Lamballerie X, Canard B, Alvarez K, Guillemot JC, Novel Barral K. 2-phenyl-5-[(E)-2-(thiophen-2-yl)ethenyl]-1,3,4-oxadiazole and 3-phenyl-5-[(E)-2-(thiophen-2-yl)ethenyl]-1,2,4-oxadiazole derivatives as dengue virus inhibitors targeting NS5 polymerase. Eur J Med Chem. 2016; 109:146–56. [DOI] [PubMed] [Google Scholar]
- Brunetti JE, Foscaldi S, Quintana VM, Scolaro LA, López N, Castilla V. Role of the ERK1/2 Signaling Pathway in the Replication of Junín and Tacaribe Viruses. Viruses. 2018. April 17; 10(4). pii: E199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpp LN, Rogers RS, Moritz RL, Aitchison JD. Quantitative proteomic analysis of host-virus interactions reveals a role for Golgi brefeldin A resistance factor 1 (GBF1) in dengue infection. Mol Cell Proteomics. 2014; 13(11):2836–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceballos-Olvera I, Chávez-Salinas S, Medina F, Ludert JE, del Angel RM. JNK phosphorylation, induced during dengue virus infection, is important for viral infection and requires the presence of cholesterol. Virology. 2010; 396(1):30–6. [DOI] [PubMed] [Google Scholar]
- Chang CC, Ou YC, Raung SL, Chen CJ. Antiviral effect of dehydroepiandrosterone on Japanese encephalitis virus infection. J Gen Virol. 2005; 86 (Pt 9):2513–23. [DOI] [PubMed] [Google Scholar]
- Cheng F, Ramos da Silva S, Huang IC, Jung JU, Gao SJ. Suppression of Zika Virus Infection and Replication in Endothelial Cells and Astrocytes by PKA Inhibitor PKI 14–22. J Virol. 2018; 92(4): e02019–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutard B, Decroly E, Li C, Sharff A, Lescar J, Bricogne G, Barral K. Assessment of Dengue virus helicase and methyltransferase as targets for fragment-based drug discovery. Antiviral Res. 2014; 106:61–70. [DOI] [PubMed] [Google Scholar]
- Croons V, Martinet W, Herman AG, Timmermans JP, De Meyer GR. The protein synthesis inhibitor anisomycin induces macrophage apoptosis in rabbit atherosclerotic plaques through p38 mitogen-activated protein kinase. J Pharmacol Exp Ther. 2009; 329(3):856–64. [DOI] [PubMed] [Google Scholar]
- Egloff MP, Benarroch D, Selisko B, Romette JL, Canard B. An RNA cap (nucleoside-2′-O-)-methyltransferase in the flavivirus RNA polymerase NS5: crystal structure and functional characterization. EMBO J. 2002; 21(11):2757–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foo SS, Chen W, Chan Y, Bowman JW, Chang LC, Choi Y, Yoo JS, Ge J, Cheng G, Bonnin A, Nielsen-Saines K, Brasil P, Jung JU. Asian Zika virus strains target CD14+ blood monocytes and induce M2-skewed immunosuppression during pregnancy. Nat Microbiol. 2017; 2(11):1558–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García CG, Quintana VM, Castilla V, Damonte EB. Towards host-cell-targeting therapies to treat dengue virus infections. Frontiers in anti-infective drug discovery. 2017; 7: 45–87. [Google Scholar]
- Growcott EJ, Bamba D, Galarneau JR, Leonard VHJ, Schul W, Stein D, Osborne CS. The effect of P38 MAP kinase inhibition in a mouse model of influenza. J Med Microbiol. 2018; 67(3):452–462. [DOI] [PubMed] [Google Scholar]
- Guarner J and Hale GL. Four human diseases with significant public health impact caused by mosquito-borne flaviviruses: West Nile, Zika, dengue and yellow fever. Semin Diagn Pathol. 2019. pii: S0740–2570(19)30041–3. [DOI] [PubMed] [Google Scholar]
- Guzman MG, Gubler DJ, Izquierdo A, Martinez E, Halstead SB. Dengue infection.Nat Rev Dis Primers. 2016; 2:16055. [DOI] [PubMed] [Google Scholar]
- Hall SS, Loebenberg D, Schumacher DP. Structure-activity relationships of synthetic antibiotic analogues of anisomycin. J Med Chem. 1983; 26(4):469–75. [DOI] [PubMed] [Google Scholar]
- Julander JG, Siddharthan V, Evans J, Taylor R, Tolbert K, Apuli C, Stewart J, Collins P, Gebre M, Neilson S, Van Wettere A, Lee YM, Sheridan WP, Morrey JD, Babu YS. Efficacy of the broad-spectrum antiviral compound BCX4430 against Zika virus in cell culture and in a mouse model. Antiviral Res. 2017; 137:14–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori T, Kondo T, Tabuchi Y, Takasaki I, Zhao QL, Kanamori M, Yasuda T, Kimura T. Molecular mechanism of apoptosis and gene expressions in human lymphoma U937 cells treated with anisomycin. Chem Biol Interact. 2008; 172(2):125–40. [DOI] [PubMed] [Google Scholar]
- Kaptein SJ, Neyts J. Towards antiviral therapies for treating dengue virus infections. Curr Opin Pharmacol. 2016; 30:1–7. [DOI] [PubMed] [Google Scholar]
- Liu Y, Ge J, Li Q, Guo X, Gu L, Ma ZG, Li XH, Zhu YP. Low-dose anisomycin sensitizes glucocorticoid-resistant T-acute lymphoblastic leukemia CEM-C1 cells to dexamethasone-induced apoptosis through activation of glucocorticoid receptor and p38-MAPK/JNK. Leuk Lymphoma. 2014; 55(9):2179–88. [DOI] [PubMed] [Google Scholar]
- Je Lynch, Ar English, Morrison J, Maven I. Protective action of anisomycin in mice infected with Trichomonas foetus. Antibiot Chemother (Northfield). 1954; 4(8):899–904. [PubMed] [Google Scholar]
- Nikaido M, Otani T, Kitagawa N, Ogata K, Iida H, Anan H, Inai T. Anisomycin, a JNK and p38 activator, suppresses cell-cell junction formation in 2D cultures of K38 mouse keratinocyte cells and reduces claudin-7 expression, with an increase of paracellular permeability in 3D cultures. Histochem Cell Biol. 2019; 151(5):369–384. [DOI] [PubMed] [Google Scholar]
- Quintana VM, Piccini LE, Panozzo Zénere JD, Damonte EB, Ponce MA, Castilla V. Antiviral activity of natural and synthetic β-carbolines against dengue virus. Antiviral Res. 2016; 134:26–33. [DOI] [PubMed] [Google Scholar]
- Roth H, Magg V, Uch F, Mutz P, Klein P, Haneke K, Lohmann V, Bartenschlager R, Fackler OT, Locker N, Stoecklin G, Ruggieri A. Flavivirus Infection Uncouples Translation Suppression from Cellular Stress Responses. MBio. 2017; 8(1). pii: e02150–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selisko B, Dutartre H, Guillemot JC, Debarnot C, Benarroch D, Khromykh A, Desprès P, Egloff MP, Canard B. Comparative mechanistic studies of de novo RNA synthesis by flavivirus RNA-dependent RNA polymerases. Virology. 2006; 351(1):145–58. [DOI] [PubMed] [Google Scholar]
- Stadheim TA, Kucera GL. c-Jun N-terminal kinase/stress-activated protein kinase (JNK/SAPK) is required for mitoxantrone- and anisomycin-induced apoptosis in HL-60 cells. Leuk Res. 2002; 26(1):55–65. [DOI] [PubMed] [Google Scholar]
- Sung C, Wei Y, Watanabe S, Lee HS, Khoo YM, Fan L, Rathore AP, Chan KW, Choy MM, Kamaraj US, Sessions OM, Aw P, de Sessions PF, Lee B, Connolly JE, Hibberd ML, Vijaykrishna D, Wijaya L, Ooi EE, Low JG, Vasudevan SG. Extended Evaluation of Virological, Immunological and Pharmacokinetic Endpoints of CELADEN: A Randomized, Placebo-Controlled Trial of Celgosivir in Dengue Fever Patients. PLoS Negl Trop Dis. 2016; 10(8):e0004851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talarico LB, Damonte EB. Interference in dengue virus adsorption and uncoating by carrageenans. Virology. 2007; 363(2):473–85. [DOI] [PubMed] [Google Scholar]
- Tang Z, Xing F, Chen D, Yu Y, Yu C, Di J, Liu J. In vivo toxicological evaluation of anisomycin. Toxicol Lett. 2012; 208(1):1–11. [DOI] [PubMed] [Google Scholar]
- Teramoto T, Balasubramanian A, Choi KH, Padmanabhan R. Serotype-specific interactions among functional domains of dengue virus 2 nonstructural proteins (NS) 5 and NS3 are crucial for viral RNA replication. J Biol Chem. 2017; 292(23):9465–9479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres NI, Castilla V, Bruttomesso AC, Eiras J, Galagovsky LR, Wachsman MB. In vitro antiviral activity of dehydroepiandrosterone, 17 synthetic analogs and ERK modulators against herpes simplex virus type 1. Antiviral Res. 2012; 95(1):37–48. [DOI] [PubMed] [Google Scholar]
- Wanisch K, Wotjak CT. Time course and efficiency of protein synthesis inhibition following intracerebral and systemic anisomycin treatment. Neurobiol Learn Mem. 2008; 90(3):485–94. [DOI] [PubMed] [Google Scholar]
- Whitby K, Pierson TC, Geiss B, Lane K, Engle M, Zhou Y, Doms RW, Diamond MS. Castanospermine, a potent inhibitor of dengue virus infection in vitro and in vivo. J Virol. 2005; 79: 8698–8706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Shen ZL, Feng Y, Li DQ, Zhang NN, Deng YQ, Qi XP, Sun XM, Dai JJ, Yang CG, Yang ZF, Qin CF, Xia XS. Infectivity of Zika virus on primary cells support tree shrew as animal model. Emerg Microbes Infect. 2019; 8(1):232–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou J and Shi PY. Strategies for Zika drug discovery. Curr Opin Virol. 2019; 35:19–26. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Effect of pretreatment of cells with anisomycin on DENV-2 production. Vero cells were pretreated with anisomycin (200 nM) at different time points before infection and then cells were infected with DENV-2 (MO I= 1). At 24 h p.i. intracellular and extracellular virus yields were determined by plaque assay. Data are mean values from three independent experiments ± SD.