Abstract
The purpose of this article was to review current literature on peri-operative pain management in hip arthroscopy. A systematic review of the literature on pain control in hip arthroscopy published January 2008 to December 2018 was performed. Inclusion criteria consisted of English language or articles with English translations, subjects undergoing hip arthroscopy with documented peri-operative pain control protocols in studies reporting Level I to IV evidence. Exclusion criteria were non-English articles, animal studies, prior systematic review or meta-analyses, studies not reporting peri-operative pain control protocols, studies documenting only pediatric (<18 years of age) patients, studies with Level V evidence and studies including less than five subjects. Statistical analysis was performed to assess pain protocols on narcotic consumption in PACU, VAS score on discharge, time to discharge from PACU and incidence of complications. Seventeen studies were included, comprising 1674 patients. Nerve blocks were administered in 50% of patients (n = 838 of 1674), of which 88% (n = 740 of 838) received a pre-operative block while 12% (n = 98 of 838) post-operative block. Sixty-eight complications were recorded: falls (54%, n = 37), peripheral neuritis (41%, n = 28), seizure (1.5%, n = 1), oxygen desaturation and nausea (1.5%, n = 1) and epidural spread resulting in urinary retention (1.5%, n = 1). No significant differences in narcotic consumption, VAS score at discharge, time until discharge or incidence of complication was found based on pain control modality utilized. No statistically significant difference in PACU narcotic utilization, VAS pain scores at discharge, time to discharge or incidence of complications was found between peri-operative pain regimens in hip arthroscopy.
INTRODUCTION
The use of hip arthroscopy for the treatment of patients with hip pathology has increased during the last two decades, with majority performed as outpatient procedures [1, 2]. While minimally invasive in nature, many patients report post-operative pain despite steady narcotic consumption [3–5]. Higher post-operative pain scores are associated with prolonged discharge times [6, 7] and unexpected hospital admission [8], resulting in increasing costs incurred by the patient and hospital [9]. Post-operative pain in the peri-operative period is of particularly concern as orthopedic procedures have the highest rate of unexpected hospital admissions and incidence of postoperative pain among surgical subspecialties in the setting of ambulatory surgery [5]. To date, the ideal peri-operative pain management protocol to effectively decrease pain scores, increase patient satisfaction, allow early mobilization and discharge following arthroscopic hip surgery remains largely unknown [1].
In the setting of the current opioid crisis, the use of multimodal pain control regimens has become an increasing focus in orthopedic procedures, including hip arthroscopy, to decrease post-operative narcotic consumption [10]. The causes of pain following hip arthroscopy are multifactorial and related to a combination of traction on the operative leg, capsulotomy, labral repair and osteochondroplasty [2, 6, 11, 12]. Moreover, the complex innervation of the hip joint and surrounding musculature make effective pain control difficult [13]. Current studies have examined the efficacy of peripheral nerve block administration, local injection analgesia, and use of adjunct medications in decreasing post-operative pain and narcotic consumption, while facilitating early discharge following hip arthroscopy [1, 2, 8, 12, 14–18]. However, no study has evaluated reported post-operative pain outcomes based on pain control regimen. The purpose of this investigation is to provide a comprehensive systematic review of the current literature on peri-operative pain management protocols in patients undergoing arthroscopic hip surgery. The authors hypothesized no significant differences in narcotic consumption, Visual Analog Scale (VAS) score at discharge or incidence of complication depending on pain regimen utilized.
MATERIALS AND METHODS
A systematic review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines using a PRISMA checklist [19]. All literature pertaining to studies reporting on patients undergoing arthroscopic hip surgery with documented peri-operative pain management regimens published between January 2008 and December 2018 was identified. Two reviewers (J.G.K., D.M.K.) independently conducted a literature search in December 2018 using the following database: Biosis Previews, SPORTdiscus, PEDRO and EMBASE. Each search included the following terms: hip AND arthroscopy AND pain AND management AND nerve block AND analgesia AND outcomes.
Inclusion criteria consisted of English language or articles with English translations, subjects undergoing arthroscopic hip surgery with documented peri-operative pain control protocols in studies reporting Level I to IV evidence. Exclusion criteria were non-English articles, animal studies, prior systematic review or meta-analyses, studies not reporting peri-operative pain control protocols, studies documenting only pediatric (<18 years of age) patients, studies with Level V evidence and studies with less than five subject participants.
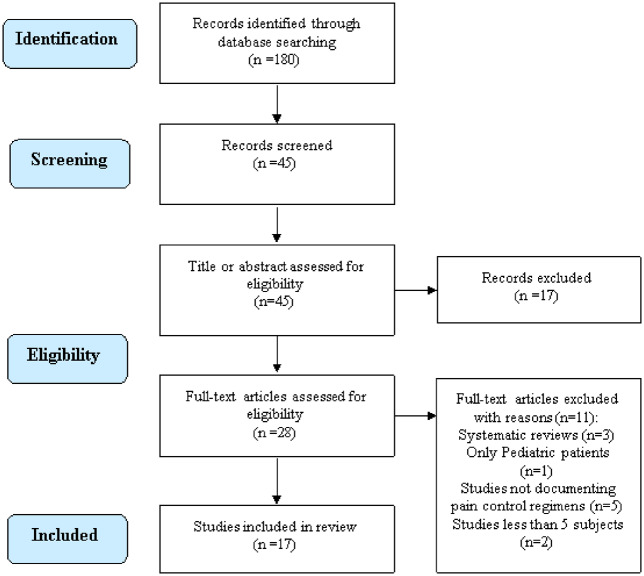
Following the 2 independent authors search of the literature a total of 180 citations were identified. The search process is shown in the flow diagram (Fig. 1). Following title and abstract evaluation, a total of 28 articles were selected for further evaluation. Of these studies, 11 studies were excluded due to systematic reviews (n = 3 studies), reporting on only pediatric patients (n = 1 study), studies not documenting pain control regimens (n = 5 studies), studies with <5 participants (n = 2). Following application of the inclusion/exclusion criteria, a total of 17 studies were identified for further analysis. To ensure that all available studies were identified, references cited in the included articles were cross-referenced for inclusion if they were overlooked during the initial search.
Fig. 1.
Preferred reporting items for systematic reviews and meta-analyses (PRISMA) flowchart.
Patient sex and age at the time of surgery, pain management regimens, time from arrival to discharge from post-anesthesia care unit (PACU), VAS pain score at the time of discharge from PACU, and any complications occurring secondary to pain control interventions or medication were recorded. Narcotic consumption in the PACU was calculated based on morphine equivalents [20]. Unexpected hospital admissions due to poorly controlled pain in the immediate post-operative period were also documented. The impact on time to discharge from PACU, VAS score on discharge, PACU narcotic consumption and incidence of complications related to pain regimen was analysed based on: (i) performance of nerve block versus no nerve block, (ii) performance of pre-operative versus post-operative nerve block, (iii) site of nerve block (femoral nerve versus lumbar plexus versus fascia iliac compartment), (iv) performance of isolated nerve block versus nerve block + local analgesic injection versus isolated local injection versus use of only oral or intravenous narcotics. Due to the small size of included studies, Fischer’s exact test was used to determine differences between two variables while a one-way analysis of variance was performed when three or more variables were analysed. A P values of <0.05 was used to determine statistical significance. All statistical analyses were performed using SPSS (Version 25.0, IBM, Armonk, NY, USA) software.
QUALITY ASSESSMENT OF INCLUDED EVIDENCE
The JADAD quality evaluation scale [21] was used to assess the quality of included randomized controlled trials. Studies were assigned a score based on the presence of certain domains (0 = not present, 1 = present and adequate, −1 = present but inadequate). Studies were graded as low quality if they scored 0–1 points, fair quality for 2–3 points or high-quality if they scored 4–5 points. The methodological index for non-randomized studies (MINORS) score [22] was used for non-randomized control trial studies which consists of 12 categories (non-comparative studies use only the first 8). Each category is scored out of two points for reported domains (0 = not reported, 1 = reported but inadequate, 2 = reported and adequate). The max score therefore is 16 for non-comparative studies and 24 for comparative studies. An inter-class correlation coefficient was calculated between two authors (J.G.K., D.M.K.) to evaluate agreement between authors using quality appraisal scores. If a score discrepancy was greater than one point between the two authors, the study was evaluated and scored by the senior author (M.J.S.).
RESULTS
The current study includes a combination of blinded randomized control trials and retrospective/prospective cohort studies. In total, 17 articles were included in the present study: 8 randomized controlled trials [2, 11, 23, 24, 25, 27, 29, 30], 7 retrospective [8–10, 12, 14, 26, 28] and 2 prospective cohort studies [4, 16]. Level 1 evidence made up 47% (n = 8 of 17) of the included studies, Level 2 evidence comprised 6% (n = 1 of 17) of studies, Level 3 41% (n = 7 of 17) and Level 4 6% (n = 1 of 17). The mean JADAD score was 4.1 ± 0.6.and the mean MINORS Score was 17.6 ± 3.2 (Table I) The ICC between the two authors was 0.96, indicating excellent inter-observer agreement, while no study required consultation with the senior author due to score discrepancy of greater than one point.
Table I.
Methodology assessment scores of articles
| Study | Assessment score #1 | Assessment score #2 |
|---|---|---|
| Philippi et al. [26] | MINORS score: 13/24 | MINORS score: 13/24 |
| Purcell et al. [9] | MINORS score: 18/24 | MINORS score: 18/24 |
| Wolff et al. [28] | MINORS score: 16/24 | MINORS score: 16/24 |
| Baker et al. [23] | JADAD score: 4/5 | JADAD score: 4/5 |
| Childs et al. [10] | MINORS score: 20/24 | MINORS score: 20/24 |
| Dold et al. [8] | MINORS score: 17/24 | MINORS score: 17/24 |
| Kahlenberg et al. [25] | JADAD score: 4/5 | JADAD score: 4/5 |
| Schroeder et al. [12] | MINORS score: 18/24 | MINORS score: 18/24 |
| Jaffe et al. [14] | MINORS score: 18/24 | MINORS score: 18/24 |
| Krych et al. [16] | MINORS score: 12/16 | MINORS score: 12/16 |
| Shlaifer et al. [27] | JADAD score: 4/5 | JADAD score: 4/5 |
| Xing et al. [2] | JADAD score: 5/5 | JADAD score: 5/5 |
| Behrends et al. [24] | JADAD score: 4/5 | JADAD score: 4/5 |
| Zhang et al. [30] | JADAD score: 3/5 | JADAD score: 3/5 |
| Potter et al. [4] | MINORS score: 22/24 | MINORS score: 22/24 |
| Garner et al. [11] | JADAD score: 4.5/5 | JADAD score: 4.5/5 |
| YaDeua et al. [29] | JADAD score: 4/5 | JADAD score: 4/5 |
The 17 studies meeting inclusion/exclusion criteria consisted of 1674 patients undergoing arthroscopic hip surgery (Table II). Mean patient age at the time of surgery was 34.7 ± 3.9 years. Males comprised 37% (n = 617) of patients, while patient sex was not reported in one study [12]. Mean PACU VAS pain scores at the time of discharge was 4.79 ± 2.04, while VAS scores were not reported in 14 studies [2, 8, 9, 11, 12, 14, 16, 23–29]. Mean time from arrival to discharge from the PACU was 143.6 ± 57.6 min and not reported in four studies [10, 11, 14, 16]. Mean reported post-operative narcotic utilization following surgery while in PACU was 10.9 ± 10.3 mg, while narcotic consumption was not reported in three studies [2, 16, 30].
Table II.
Summary of studies included in review
| Author | Journal | Level of evidence | Number of patients | Age (mean years) | Sex | Pre-operative block | Intra-operative local injection | Post- operative block | PACU morphine equivalents (mg) | Time until discharge (Min) | Post- operative pain at discharge (VAS) | Complications | Pre- versus post-procedure local injection analgesia | Localization for block | Procedures performed |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Philippi et al. [26] | J Hip Preserv Surg (2018) | 3 | 50 | 25.47 |
|
NR | Extracapsular 20 ml 0.25% bupivacaine-epinephrine (1:200 000) |
|
14.51 | 151.2 | NR | NR | Post-procedure | NR | NR |
| 50 | 25.09 |
|
NR | None |
|
14.08 | 139.8 | NR | NR | ||||||
| Purcell et al. [9] | Knee Surg Sports Traumatol Arthrosc (2018) | 3 | 34 | 34.7 |
|
|
NR | NR | 32.53 | 110.6 | NR | None | NA | US | NR |
| 34 | 32.4 |
|
|
NR | NR | 29.46 | 108.6 | NR | None | ||||||
| Wolff et al. [28] | J Hip Preserv Surg (2016) | 3 | 55 | 34.51 |
|
None | NR | NR | 7.02 | 108.6 | NR | None | NA | US (FNB, LPB)+nerve stimulator for (LBP) | NR |
| 30 | 30.57 |
|
|
NR | NR | 8.8 | 112.2 | NR | None | ||||||
| 60 | 35.45 |
|
|
NR | NR | 7.16 | 111.5 | NR | Seizure (n=1) | ||||||
| Baker et al. [23] | Hip Int (2011) | 1 | 40 | 37.8 |
|
NR |
|
NR | 0.57 | 48 | NR | NR | Post-procedure | NA | Labral/chondral tissue debridement; MFX/osteoplasty |
| 33 | 38.8 |
|
NR |
|
NR | 2.33 | 48 | NR | NR | ||||||
| Childs et al. [10] | Arthroscopy (2017) | 3 | 105 | 33.4 |
|
|
None | NR | 10.425 | NR | 3.55 |
|
Post-procedure | US | Labral debridement/repair/reconstruction; synovectomy; femoral osteochondroplasty |
| 88 | 31.3 |
|
None |
|
NR | 12.525 | NR | 4.28 |
|
||||||
| Dold et al. [8] | Am J Sports Med (2014) | 3 | 40 | 34.2 |
|
None | NR | NR | 4 | 81.53 | NR | Admission for pain (n=2) | NA | US | Femoral osteochondroplastyloose body removal; labral debridement |
| 56 | 33.1 |
|
|
NR | NR | 2.04 | 85.96 | NR | None | ||||||
| Kahlenberg et al. [25] | Arthroscopy (2017) | 1 | 50 | 34.2 |
|
None | NR | NR | 15.326 | 152.96 | NR | None | NA | NA | Labral repair/debridement/reconstruction alone; revision labral repair; Labral repair with acetabular osteoplasty/chondroplasty; labral repair with femoral head chondroplasty |
| 48 | 35.8 |
|
None | NR | NR | 15.419 | 172.96 | NR | None | ||||||
| Schroeder et al. [12] | Hip Int (2013) | 3 | 118 | 41.1 | NR |
|
Intracapsular 4 mg of morphine | NR | 5 | 240 | NR | NR | Post-procedure | Nerve stimulator | NR |
| 118 | 38.7 | NR | None |
|
NR | 6.7 | 217.5 | NR | NR | ||||||
| Jaffe et al. [14] | J Pain Palliat Care Pharmacother (2017) | 3 | 141 | 32.4 |
|
LPB+sciatic block | NR | NR | 0 | NR | NR | NR | NA | NR | NR |
| 36 | 38.2 |
|
None | NR | NR | 40.05 | NR | NR | NR | ||||||
| Krych et al. [16] | Knee Surg Sports Traumatol Arthrosc (2014) | 2 | 30 | 34 |
|
|
NR | NR | NR | NR | NR | None | NA | US | Labral repair; femoral osteochondroplasty; pincer resection |
| Shlaifer et al. [27] | Arthroscopy (2017) | 1 | 21 | 39.6 |
|
None |
|
NR | 3.15 | 123 | NR | None | Pre- and post-procedure | NA | Labral repair/reconstruction; femoral/acetabular osteoplasty |
| 21 | 36 |
|
None | Intracapsular 20 ml of bupivacaine 0.5% | NR | 7.23 | 213 | NR | None | ||||||
| Xing et al. [2] | Am J Sports Med (2015) | 1 | 27 | 32 |
|
|
NR | None | NR | 246 | NR | Falls (n=6) | NA | US | Labral repair; osteochondroplasty |
| 23 | 31 |
|
None | NR | None | NR | 252.5 | NR | None | ||||||
| Behrends et al. [24] | Anesthesiology (2018) | 1 | 38 | 35 |
|
|
|
NR | 15 | 123 | NR | Falls (n=4) | Post-procedure | US | Osteochondroplast; labral repair; loose body removal; screw removal; MFX; debridement |
| 40 | 32 |
|
None |
|
NR | 16 | 128 | NR | Fall (n=1) | ||||||
| Zhang et al. [30] | Eur J Orthop Surg Traumatol (2013) | 1 | 27 | 41 |
|
None | NR | NR | NR | 147 | 7.23 | NR | NA | NA | NR |
| 26 | 43.5 |
|
None | NR | NR | NR | 152 | 7.46 | NR | ||||||
| Potter et al. [4] | Arthroscopy (2014) | 4 | 53 | 37.1 |
|
NR | NR |
|
4.4 | 102 | 2.8 | Admission for pain (n=3) | NA | US | Femoral osteoplasty; labral repair; capsular repair; unlisted additional repair |
| 54 | 34.9 |
|
NR | NR | None | 4.4 | 97 | 3.4 | None | ||||||
| Garner et al. [11] | Arthroscopy (2017) | 1 | 20 | 33.6 |
|
None |
|
NR | 2.4 | NR | NR | None | Post-procedure | US | Excision of CAM lesion; labral debridement/repair; removal of loose bodies; chondroplasty; MFX |
| 26 | 31.9 |
|
|
None | NR | 5.5 | NR | NR | None | ||||||
| YaDeua et al. [29] | Anesth Analg (2012) | 1 | 41 | 37 |
|
None | None | NR | 29 | 187 | NR | Oxygen desaturation (n=1) Admission for pain (n=1) | NA | Nerve stimulator | Labral debridement; labral/capsular repair; synovectomy; cam decompression; osteochondroplasty; pincer debridement; subspine decompression; rim decompression; psoas release; loose body removal |
| 41 | 33 |
|
|
None | NR | 2 | 216 | NR |
|
GA, general anesthesia; FICB, fascia iliac compartment block; LPB, lumbar plexus block; FNB, femoral nerve block; LIA, local injection analgesia; CSE, continuous spinal epidural; PA, periacetabular; IA, intra-articular; NR, not reported; PACU, post-anesthesia care unit; VAS, visual analog score; NRS, numeric rating scale; SDCU, surgical day care unit; Min, minutes; mg, milligrams; MFX, microfracture; US, Ultrasound; NR, Not reported; NA, Not applicable.
Nerve blocks were performed in 50% of patients (n = 838 of 1674), of which 88% (n = 740 of 838) received a pre-operative block while 12% (n = 98 of 838) were administered following surgery. Blocks were administered to the: fascia iliaca compartment (29%, n = 245 of 838) [4, 9, 11, 16, 24, 28], femoral nerve (28%, n = 233 of 838) [2, 8, 10, 26], lumbar plexus (26%, n = 219 of 838) [12, 28, 29] and lumbar plexus + sciatic nerve (17%, n = 141 of 838) [14]. Blocks were performed either under ultrasound guidance or with the use of a nerve stimulator, with block localization not reported in two studies [14, 26]. Seven studies reported use of local injection analgesia intra-operatively, with injections provided to the intracapsular space (n = 5 studies) [10, 12, 23, 24, 27], extracapsular space (n = 3 studies) [11, 23, 26], or both intra-and extracapsular spaces (n = 1 study) [27]. Statistical analysis found no significant difference in consumption of narcotic pain medication in PACU, time until PACU discharge, VAS scores on discharge, or the incidence of complications based on pain control regimen (Table III). Time from PACU arrival to discharge was not significantly different in patients provided with femoral nerve blocks (mean, 166 min) compared with those receiving fascia iliac compartment (mean, 111 min) or lumbar plexus blocks (mean 189 min) (P = 0.21).
Table III.
Fischer’s exact test and analysis of variables based on pain control modality
|
P-value |
||||
|---|---|---|---|---|
| Pain control modality | Time admission to PACU discharge (min) | PACU narcotic utilization (Meq) | VAS at PACU discharge | Complications |
| Nerve block only versus no nerve block | 0.89 | 0.75 | 0.1 | 0.3 |
| Pre-operative nerve block versus post-operative nerve block | – | – | – | – |
| Nerve block only versus nerve block + LIA | 0.58 | 0.85 | – | – |
| LIA pre and post versus LIA post-procedure | 0.62 | 0.28 | – | 0.24 |
| LIA only versus LIA + nerve block | 0.53 | 0.6 | – | – |
| FNB versus LPB versus FICB block | 0.21 | 0.16 | – | 0.17 |
| Nerve block only versus nerve block + LIA versus LIA only | 0.64 | 0.53 | – | 0.86 |
| Nerve block only versus nerve block +LIA versus LIA only versus narcotics only | 0.74 | 0.35 | – | 0.75 |
LIA, local injection analgesia; FNB, femoral nerve block; LPB, lumbar plexus nerve block; FICB, fascia iliac compartment block; Meq, narcotic use based on calculated morphine equivalents; VAS, visual analog score; PACU, post-anesthesia care unit.
Statistical significance.
A total of 68 complications related to post-operative pain management interventions were reported in 5 studies. Reported complications included: falls (54%, n = 37 patients) [2, 10, 24, 29], peripheral neuritis (41%, n = 28 patients) [10], seizure (1.5%, n = 1 patient) [28], oxygen desaturation and nausea (1.5%, n = 1 patient) [29], and epidural spread resulting in urinary retention (1.5%, n = 1 patient) [29]. Eighty-four percent (n = 31 of 37) of falls occurred in patients receiving a peripheral nerve block, of which 81% (n = 25 of 31) of fall patients received a femoral nerve block [2, 10]. No reported falls required further operative intervention. A total of six patients were admitted following surgery due to poorly controlled post-operative pain [4, 8, 29]. No significant difference was appreciated between patients admitted for poorly controlled pain compared with patients discharged on the day of surgery based on patient age (P = 0.60), narcotic consumption in PACU (P = 0.86) or time from PACU arrival to discharge (P = 0.57).
DISCUSSION
A total of 50% of patients undergoing arthroscopic hip surgery received a peripheral nerve block, with the majority of patients undergoing pre-operative nerve blocks. No significant difference in narcotic consumption in PACU, time until PACU discharge, VAS score on discharge or incidence of complications related to peri-operative pain management was appreciated based on pain control regimen. Patients treated with femoral nerve blocks had higher rate of falls post-operatively compared with fascia iliaca compartment blocks or lumbar plexus blocks. To our knowledge, this investigation represents the largest collection of studies evaluating the impact of peri-operative pain control regimens on outcomes following arthroscopic hip surgery (Table IV).
Table IV.
Mean values based on pain control modality utilized
| Pain control modality | Mean time admission to PACU discharge (min) | Mean PACU narcotic utilization (Meq) | Mean VAS at PACU discharge | Mean number of complications |
|---|---|---|---|---|
| Nerve block only | 137 | 11.7 | 3.18 | 5 |
| No nerve block | 142 | 11.6 | 5.59 | 0.75 |
| Nerve block (FNB, LBP or FICB) | 146 | 10.2 | 3.18 | 4.92 |
| Pre-operative nerve block | 150 | 10.7 | 3.55 | 5.34 |
| Post-operative nerve block+ | 102 | 4.4 | 2.80 | 0 |
| Nerve block + LIA | 181.5 | 10 | – | 4 |
| LIA post-procedure | 137 | 8.75 | 3.92 | 9.5 |
| LIA pre- and post-procedure | 168 | 5.19 | – | 0 |
| LIA only | 130 | 6.36 | 4.28 | 1.60 |
| FNB | 166 | 6.23 | 3.55 | 17 |
| LBP | 189 | 3.54 | – | 2 |
| FICB | 111 | 15.9 | 2.8 | 0.57 |
| Narcotics only | 150 | 16.5 | 6.03 | 0.14 |
LIA, local injection analgesia; FNB, femoral nerve block; LPB, lumbar plexus nerve block; FICB, fascia iliaca compartment block; Meq, narcotic use based on calculated morphine equivalents; VAS, visual analog score; PACU, post-anesthesia care unit; +, denotes single study.
Statistical significance.
Nerve blocks were utilized as part of the pain control regimen in 50% of patients undergoing hip arthroscopy. The use of selective nerve blocks have demonstrated good efficacy in controlling pain following hip fracture fixation, as well as total knee arthroplasty [31, 32]. One retrospective review found that patients who underwent pre-operative femoral nerve blocks prior to hip arthroscopy reported significantly lower pain scores 1 h following PACU arrival, required lower PACU morphine equivalent doses, and experienced no unexpected admissions due to poorly controlled pain compared with control patients not receiving femoral nerve blocks [8]. Another retrospective review found that patients who received a pre-operative lumbar plexus block in addition to standard intra-articular injection of morphine reported significantly reduced immediate and peak postoperative recovery pain scores, as well as decreased narcotic consumption post-operatively when compared with control patients [12]. As such, the utilization of selective nerve blocks has become increasingly common for the control of post-operative pain following hip arthroscopy. The current investigation found that patients treated with FICB (mean 111 min) experienced shorter times to discharge when compared with FNB (mean 166 min) and LPB (mean 189 min). While not statistically significant, the shorter PACU stay times for FICB blocks could result in decrease hospital costs. A recent analysis on operating room and PACU cost found that PACU operations were ∼$12.14 per minute for services offered [33]. Thus, hospital costs could be theoretically decreased with shorter PACU stays based on the type of nerve block provided. However, in order to delineate these cost savings and benefits, future studies examining different outcome variables based on types of nerve blocks performed for hip arthroscopy are warranted.
Local analgesia alone or when administered with nerve blocks was not found to significantly change post-operative pain outcomes. Local injections are generally provided to effectively control localized pain and supplement the anatomical areas missed by peripheral nerve blocks, specifically the hip capsule. Difficulty in controlling pain from the hip capsule occurs as a result of its complex innervation from the network of surrounding nerves: the anterior and medial capsule being innervated by the femoral and obturator nerves while the posterior and lateral capsule are innervated by the sciatic, superior gluteal and nerve to quadratus femoris [13]. One randomized control trial reported that patients treated with local analgesia to the hip capsule versus FICB nerve blocks had significantly lower PACU pain scores, hypothesizing that the innervation provided by the sciatic, superior gluteal and nerve to quadratus femoris to the hip capsule were not treated using an FICB block [11]. The same study also proposed that local analgesia injections diffuse outside the portal tracts, thereby more completely anesthetizing the hip capsule/joint [11]. Moreover, the concentration of diffused local anesthetic effectively block the fibers responsible for pain, lacking the potency to block motor fibers, decreasing fall risks [11]. Other investigations have similarly reported that FICB as well as FNB do not affect nerves from the sacral plexus innervating the hip capsule, leading to continued hip pain with the potential for admission for pain control [4, 9, 28]. When compared with extra-capsular injection, one study reported that intra-capsular injections have been shown to result in significantly lower morphine requirements in the immediate post-operative period [23]. The authors further hypothesized that a combination of intra-articular and portal local injection analgesia to be optimal for post-operative pain control [23].
While no differences in the incidence of complications were reported based on pain regimen, peripheral nerve blocks and local anesthesia injections each carry the potential for complications. Falls remains a significant area of concern following peripheral nerve blocks in hip surgery due to muscle weakness and neuraxial spread [10, 24, 29]. One retrospective review reported that patients receiving femoral nerve blocks had over 3× the fall risk and 14× the risk for post-operative neuropathy when compared with local injection anesthesia [10]. Meanwhile, intra-articular injections possess the potential for chondrotoxic effects, with one study reporting that a single intra articular injection of bupivacaine into the knees of rat subjects resulted in a 50% decrease in chondrocyte density compared with saline-injected rat knees after 6 months [34]. As such, post-operative precautions must be taken into consideration in patients undergoing utilization of nerve blocks for pain control following hip arthroscopy.
The current study is not without limitations. Due to the heterogeneity and variability of data reporting post-operative pain regimen, the authors utilized surrogate metrics to standardize data to allow for pooled analysis. Moreover, there remains a lack of a standardized reporting measures to evaluate post-operative pain outcomes following arthroscopic hip surgery. Due to limited reported data, the authors were unable to determine differences in outcomes based on the performance of pre-operative versus post-operative nerve blocks or outcomes based on pre-procedural versus post-procedural intra-operative local injection analgesia. As the arthroscopic procedures performed within the hip were not regularly reported nor were outcomes based on procedures performed explicitly reported, the authors were unable to conduct any sub-analysis based on the number and type of arthroscopic procedures performed within the hip. Patients who underwent revision surgeries and those with a history of chronic opiate or narcotic abuse/use were not explicitly excluded in all of the chosen articles, which may alter post-operative outcome scores when compared with primary surgery patients and opiate naive patients. In addition, due to heterogeneity of the reported data, the authors were unable to perform any meaningful statistical analysis on pre-operative non-narcotic medication regimens.
In moving forward, the treatment of peri-operative pain following hip arthroscopy will require further investigation using a multiple modal pain control protocol given the complex innervation of the hip capsule and surrounding tissue. Determining the optimal pain protocol will involve providing appropriate sensory anesthesia for pain control while preserving motor function to prevent the incidence of falls and other associated injuries in the acute postoperative period. The senior author’s institution has implemented a novel opiate sparing pain protocol for peri-operative pain control following arthroscopic hip surgery that includes non-narcotic preoperative pain medications (acetaminophen, celecoxib, neurontin); preoperative ultrasound-guided pure sensory transversalis fascia plane block performed by a fellowship-trained anesthesiologist; the use of both intra capsular and portal site local injection analgesia; muscle relaxer to combat spasms; and the use of anti-inflammatory postoperative pain medication with minimal postoperative narcotics utilization. Further prospective studies utilizing a combination of peripheral nerve block, peri-operative and postoperative pain control with minimal use of narcotics are necessary to help better understand the best methods of treating the complex series of pain generators activated following hip arthroscopy.
In conclusion, no statistically significant difference in PACU narcotic utilization, time to discharge, VAS pain scores at discharge or incidence of complications was appreciated when comparing different peri-operative pain regimens following hip arthroscopy in the present study. Further research and standardization of pain control regimens is required to determine the best protocol for the treatment of postoperative pain during the peri-operative period to optimize patient satisfaction, allow early mobilization and decease number of pain-related complications.
FUNDING
None declared.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1. Cogan CJ, Knesek M, Tjong VK.. Assessment of intraoperative intra-articular morphine and clonidine injection in the acute postoperative period after hip arthroscopy. Orthop J Sports Med 2016; 4: 2325967116631335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Xing JG, Abdallah FW, Brull R.. Preoperative femoral nerve block for hip arthroscopy: a randomized, triple-masked controlled trial. Am J Sports Med 2015; 43: 2680–7. [DOI] [PubMed] [Google Scholar]
- 3. Lee EM, Murphy KP, Ben-David B.. Postoperative analgesia for hip arthroscopy: combined L1 and L2 paravertebral blocks. J Clin Anesth 2008; 20: 462–5. [DOI] [PubMed] [Google Scholar]
- 4. Potter MQ, Sun GS, Fraser JA. et al. Psychological distress in hip arthroscopy patients affects postoperative pain control. Arthroscopy 2014; 30: 195–201. [DOI] [PubMed] [Google Scholar]
- 5. Ward JP, Albert DB, Altman R. et al. Are femoral nerve blocks effective for early postoperative pain management after hip arthroscopy? Arthroscopy 2012; 28: 1064–9. [DOI] [PubMed] [Google Scholar]
- 6. Baker JF, Byrne DP, Hunter K. et al. Post-operative opiate requirements after hip arthroscopy. Knee Surg Sports Traumatol Arthrosc 2011; 19: 1399–402. [DOI] [PubMed] [Google Scholar]
- 7. Pavlin DJ, Chen C, Penaloza DA. et al. Pain as a factor complicating recovery and discharge after ambulatory surgery. Anesth Analg 2002; 95: 627–34. [DOI] [PubMed] [Google Scholar]
- 8. Dold AP, Murnaghan L, Xing J. et al. Preoperative femoral nerve block in hip arthroscopic surgery: a retrospective review of 108 consecutive cases. Am J Sports Med 2014; 42: 144–9. [DOI] [PubMed] [Google Scholar]
- 9. Purcell RL, Nappo KE, Griffin DW. et al. Fascia iliaca blockade with the addition of liposomal bupivacaine vs. plain bupivacaine for perioperative pain management following hip arthroscopy. Knee Surg Sports Traumatol Arthrosc 2018; 26: 2536–41. [DOI] [PubMed] [Google Scholar]
- 10. Childs S, Pyne S, Nandra K. et al. The effect of intra-articular cocktail versus femoral nerve block for patients undergoing hip arthroscopy. Arthroscopy 2017; 33: 2170–6. [DOI] [PubMed] [Google Scholar]
- 11. Garner M, Alsheemeri Z, Sardesai A. et al. A prospective randomized controlled trial comparing the efficacy of fascia iliaca compartment block versus local anesthetic infiltration after hip arthroscopic surgery. Arthroscopy 2017; 33: 125–32. [DOI] [PubMed] [Google Scholar]
- 12. Schroeder KM, Donnelly MJ, Anderson BM. et al. The analgesic impact of preoperative lumbar plexus blocks for hip arthroscopy. A retrospective review. Hip Int 2013; 23: 93–8. [DOI] [PubMed] [Google Scholar]
- 13. Birnbaum K, Prescher A, Heßler S. et al. The sensory innervation of the hip joint–an anatomical study. Surg Radiol Anat 1997; 19: 371–5. [DOI] [PubMed] [Google Scholar]
- 14. Jaffe JD, Morgan TR, Russell GB.. Combined sciatic and lumbar plexus nerve blocks for the analgesic management of hip arthroscopy procedures: a retrospective review. J Pain Palliat Care Pharmacother 2017; 31: 121–5. [DOI] [PubMed] [Google Scholar]
- 15. Kay J, de SA D, Memon M. et al. Examining the role of perioperative nerve blocks in hip arthroscopy: a systematic review. Arthroscopy 2016; 32: 704–15.e1. [DOI] [PubMed] [Google Scholar]
- 16. Krych AJ, Baran S, Kuzma SA. et al. Utility of multimodal analgesia with fascia iliaca blockade for acute pain management following hip arthroscopy. Knee Surg Sports Traumatol Arthrosc 2014; 22: 843–7. [DOI] [PubMed] [Google Scholar]
- 17. Shin JJ, McCrum CL, Mauro CS. et al. Pain management after hip arthroscopy: systematic review of randomized controlled trials and cohort studies. Am J Sports Med 2018; 46: 3288–98. [DOI] [PubMed] [Google Scholar]
- 18. Yu HC, Al-Shehri M, Johnston KD. et al. Anesthesia for hip arthroscopy: a narrative review. Can J Anaesth 2016; 63: 1277–90. [DOI] [PubMed] [Google Scholar]
- 19. Liberati A, Altman DG, Tetzlaff J. et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol 2009; 62: 1–34. [DOI] [PubMed] [Google Scholar]
- 20.Morphine Equivalent Narcotic Calculator. Available at: https://www.ohiopmp.gov/MED_Calculator.aspx. Accessed: 23 April 2019.
- 21. Jadad AR, Moore RA, Carroll D. et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996; 17: 1–12. [DOI] [PubMed] [Google Scholar]
- 22. Slim K, Nini E, Forestier D. et al. Methodological index for non-randomized studies (MINORS): development and validation of a new instrument. ANZ J Surg 2003; 73: 712–6. [DOI] [PubMed] [Google Scholar]
- 23. Baker JF, McGuire CM, Byrne DP. et al. Analgesic control after hip arthroscopy: a randomised, double-blinded trial comparing portal with intra-articular infiltration of bupivacaine. Hip Int 2011; 21: 373–7. [DOI] [PubMed] [Google Scholar]
- 24. Behrends M, Yap EN, Zhang AL. et al. Preoperative fascia iliaca block does not improve analgesia after arthroscopic hip surgery, but causes quadriceps muscles weakness: a randomized, double-blind trial. Anesthesiology 2018; 129: 536–43. [DOI] [PubMed] [Google Scholar]
- 25. Kahlenberg CA, Patel RM, Knesek M. et al. Efficacy of celecoxib for early postoperative pain management in hip arthroscopy: a prospective randomized placebo-controlled study. Arthroscopy 2017; 33: 1180–5. [DOI] [PubMed] [Google Scholar]
- 26. Philippi MT, Kahn TL, Adeyemi TF. et al. Extracapsular local infiltration analgesia in hip arthroscopy: a retrospective study. J Hip Preserv Surg 2018; 5: 60–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shlaifer A, Sharfman ZT, Martin HD. et al. Preemptive analgesia in hip arthroscopy: a randomized controlled trial of preemptive periacetabular or intra-articular bupivacaine in addition to postoperative intra-articular bupivacaine. Arthroscopy 2017; 33: 118–24. [DOI] [PubMed] [Google Scholar]
- 28. Wolff AB, Hogan GW, Capon JM. et al. Pre-operative lumbar plexus block provides superior post-operative analgesia when compared with fascia iliaca block or general anesthesia alone in hip arthroscopy. J Hip Preserv Surg 2016; 3: 338–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. YaDeau JT, Tedore T, Goytizolo EA. et al. Lumbar plexus blockade reduces pain after hip arthroscopy: a prospective randomized controlled trial. Anesth Analg 2012; 115: 968–72. [DOI] [PubMed] [Google Scholar]
- 30. Zhang Z, Zhu W, Zhu L. et al. Efficacy of celecoxib for pain management after arthroscopic surgery of hip: a prospective randomized placebo-controlled study. Eur J Orthop Surg Traumatol 2014; 24: 919–23. [DOI] [PubMed] [Google Scholar]
- 31. Morrison RS, Dickman E, Hwang U. et al. Regional nerve blocks improve pain and functional outcomes in hip fracture: a randomized controlled trial. J Am Geriatr Soc 2016; 64: 2433–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Paul JE, Arya A, Hurlburt L. et al. Femoral nerve block improves analgesia outcomes after total knee arthroplasty: a meta-analysis of randomized controlled trials. Anesthesiology 2010; 113: 1144–62. [DOI] [PubMed] [Google Scholar]
- 33. Raft J, Millet F, Meistelman C.. Example of cost calculations for an operating room and a post-anaesthesia care unit. Anaesth Crit Care Pain Med 2015; 34: 211–5. [DOI] [PubMed] [Google Scholar]
- 34. Chu CR, Coyle CH, Chu CT. et al. In vivo effects of single intra-articular injection of 0.5% bupivacaine on articular cartilage. J Bone Joint Surg Am 2010; 92: 599–608. [DOI] [PMC free article] [PubMed] [Google Scholar]