Abstract
INTRODUCTION
The Seneca Valley Virus (NTX-010) is an oncolytic picornavirus with tropism for small cell lung cancer (SCLC). This phase II double-blind, placebo controlled trial evaluated NTX-010 in patients with extensive stage (ES) SCLC after completion of first line chemotherapy.
METHODS
ES-SCLC patients who did not progress after ≥4 cycles of platinum-based chemotherapy were randomized 1:1 to a single dose of NTX-010 or placebo within 12 weeks of chemotherapy. Primary end point was progression free survival (PFS). A prespecified interim analysis for futility was performed after 40 events. Viral clearance and the development of neutralizing antibodies were followed.
RESULTS
From January 15, 2010 to January 10, 2013, 50 patients were randomized and received therapy on study (26 NTX-010, 24 placebo). At the specified interim analysis, median PFS was 1.7 months (95% confidence interval (CI) 1.4–3.1 months) for the NTX-010 group versus 1.7 months (95% CI 1.4–4.3 months) for placebo (hazard ratio (HR): 1.03, p = 0.92), and the trial was terminated due to futility. In the NTX-010 group, PFS was shorter in patients with detectable virus at days 7 and 14 versus not detected after treatment (1.0 month (95% CI 0.4–1.5 months) vs 1.8 months (95% CI 1.3–5.5 months; p=0.008); and 0.9 months (95% CI 0.4–2.6 months) vs 1.3 months (95% CI 1.0–5.3 months), p=0.04) respectively.
CONCLUSIONS
Patients with ES-SCLC did not benefit from NTX-010 treatment after chemotherapy with a platinum doublet. Persistence of NTX-010 in the blood 1 or 2 weeks after treatment was associated with a shorter PFS.
Keywords: Small cell lung cancer, virotherapy, NTX-010
INTRODUCTION
Small cell lung cancer (SCLC) is an aggressive malignancy and the majority of patients present with disseminated disease[1]. Patients often experience a significant tumor response to first line therapy but invariably the disease recurs. Additional or prolonged exposure to chemotherapy does not surmount this near uniform recurrence as trials utilizing maintenance or consolidation chemotherapy have not shown improved survival[2]. Recent advances in the standard of care for patients with newly diagnosed ES-SCLC now includes atezolizumab in combination with chemotherapy and as maintenance therapy[3]. While this represents the first change to front-line therapy in decades, the addition of atezolizumab modestly improves the median PFS by 1 month and OS by 2 months compared to chemotherapy alone. Consequently, new approaches are urgently needed to improve the depth and duration of disease response to therapy.
The Seneca valley virus (NTX-010) is an oncolytic, non-enveloped RNA virus within the picornavirus family[4]. Initial studies demonstrated the affinity of NTX-010 to replicate in and lyse tumors with neuroendocrine characteristics, including SCLC[4]. In animal models of SCLC, antitumor effect was observed with a single intravenous dose of NTX-010[4]. Early safety signals supported NTX-010 use in patients. In preclinical testing, NTX-010 did not infect normal human tissue including endothelial cells, myocytes, and leukocytes[4]. Importantly, NTX-010 is not tumorigenic. During replication, NTX-010 does not have a DNA phase and cannot be incorporated into the host genome[5]. The genome of NTX-010 is stable and no viral oncogenes are present[4, 5]. Finally, humans have minimal or no endemic exposure to NTX-010 as only 1 of 120 patient serum samples contained a low-level of neutralizing antibody[4].
On the basis of these preclinical data, a first-in-human phase I trial with NTX-010 enrolled patients with advanced solid tumors with neuroendocrine features, including 6 patients with SCLC[6]. Treatment with NTX-010 was well tolerated and associated with transient flu-like symptoms[6]. In half of SCLC the patients, the virus replicated after administration suggesting intratumoral viral replication. NTX-010 was found only in sites of metastases and not in normal surrounding tissue or uninvolved organs[6]. Notably, the virus persisted in the sites of metastases up to 4 weeks after administration[6]. No objective responses were observed but one SCLC patient experienced disease stabilization for 10 months[6].
Based on the safety profile and signals of potential efficacy from the phase I trial including virus localization to and persistence in tumor tissue, we prospectively evaluated the efficacy of NTX-010 in patients with ES-SCLC who experienced disease control after first line platinum-based chemotherapy in a double-blinded randomized phase II trial. (North Central Cancer Treatment Group N0923). NCCTG is now part of the Alliance for Clinical Trials in Oncology.
METHODS
Patient Population and Study Design
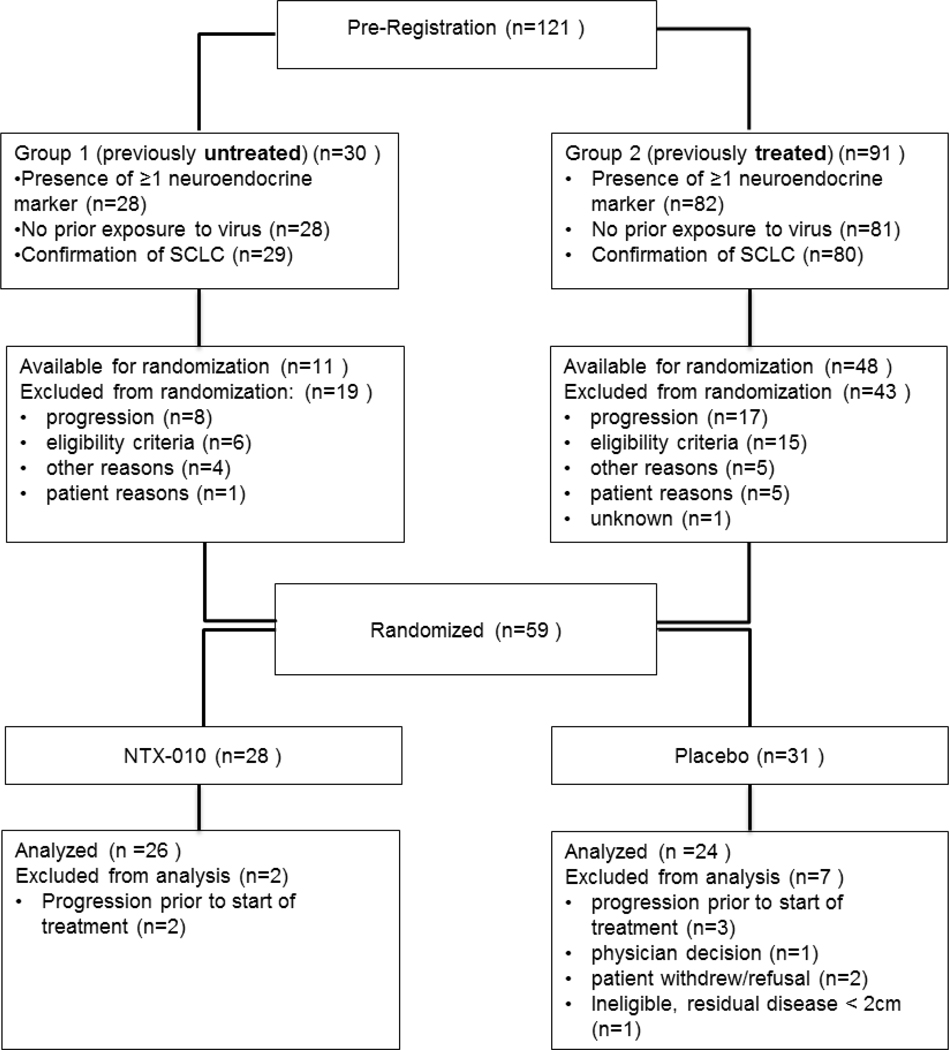
Patients able to give informed consent aged 18 or older with a new diagnosis of ES-SCLC were eligible for this trial after completing 4 to 6 cycles of platinum-based chemotherapy with either stable disease (SD), partial response (PR), or a complete response (CR) ≤12 weeks after completion of chemotherapy (Figure 1). Pathology was centrally reviewed to confirm the diagnosis of SCLC and the presence of neuroendocrine markers synaptophysin, chromogranin, and/or CD56. Patients with residual disease were required to have at least one site of non-nodal measurable disease ≥1.0 cm but <10 cm by CT scan. Lymph nodes were required to be ≥1.5 cm in the short axis to be considered measurable disease. Patients were required to have an Eastern Cooperative Oncology Group performance status of 0 or 1, and adequate bone marrow and organ function[7].
Figure 1.
CONSORT Diagram
Patients were excluded if they had mixed histology, a second invasive primary malignancy unless definitively treated ≥5 years previously with no evidence of recurrence, oxygen dependence, life expectancy < 8 weeks, any clinically significant infection, uncontrolled major illness, or were unwilling to employ adequate contraception. Patients who received consolidation radiation therapy to the chest or palliative radiation to other sites of disease (excluding brain metastasis) ≤4 weeks or bony disease ≤2 weeks before enrollment were not eligible. Patients on combination anti-retroviral therapy for HIV or with active hepatitis B or C virus were excluded. Patients were excluded if they had prior exposure to the Seneca Valley Virus (NTX-010) as determined by positive serum antibodies.
Patients who had not received whole brain radiation therapy prior to randomization were required to receive prophylactic cranial irradiation (25 Gy in 10 fractions) either ≥2 weeks before starting NTX-010/placebo or between day 29 and day 50 after administration of NTX-010/placebo. Imaging was required 4 weeks after completion of radiation therapy to document stable brain metastasis. Patients were randomly assigned in a 1:1 ratio to receive NTX-010 or placebo intravenously as a 1-hour infusion in 100 mL normal saline as a single dose. Patients in the NTX-010 arm received 1011 viral particles (vp)/kg as established by the previous phase I study[6]. Contact isolation precautions were required during and following the NTX-010/placebo infusion. Study personnel administering the therapy and assessing toxicities and responses were blinded to the assigned treatment group, as were patients.
Study blood draws were performed prior to, 48 hours, 7 days, and 14 days after NTX-010/placebo therapy. Viral clearance was assessed by viral load in titer at 48 hours, 7 days and 14 days after NTX-010/placebo administration as described previously[6]. Anti-viral antibody level was evaluated at study day 14[6].
The study was approved by the institutional review board at each participating center. Each participant signed the IRB-approved, protocol-specific informed consent document in accordance with federal and institutional guidelines
Safety Assessments
Patients were evaluated every 3 weeks for vital signs, history, physical exam, CBC, basic chemistry panel and for adverse events. Adverse events were graded as per NCI Common Terminology Criteria for Adverse Events (CTCAE) version 4.0.
This phase randomized phase II therapeutic trial was monitored at least twice annually by the Data and Safety Monitoring Board, a standing committee composed of individuals from within and outside of the Alliance.
Statistical Considerations
The primary end point of this study was PFS. Secondary objectives included overall survival (OS), tumor response rate, and the relationship of neutralizing antibodies, and viral clearance, on any measures of response.
A median PFS of 3 months was expected in the control arm based on historical data [8, 9]. A sample size of 45 evaluable patients per arm (90 evaluable in total) provided 85% power to detect an improvement in median PFS from 3 to 5 months (a hazard ratio of 1.7, control vs. experimental arm) using a 1-sided log-rank test at a significance level of 0.11. The maximum number of events was expected to be 79 in total. Accounting for a 10% non-evaluable rate (drop-out, ineligible, lost to follow-up etc.), the trial was designed to accrue a total sample size was 99 patients.
An interim analysis with a futility rule after one-half of the required number of events for the primary analysis, or specifically, after 40 events (progression or deaths) was planned. At the interim analysis, distribution of PFS in the NTX-010 was compared to the distribution of PFS in the placebo arm. If the observed hazard ratio of NTX-010 vs Placebo ≥1, NTX-010 treatment will be considered inactive in this disease population, the trial was to be terminated early for futility and the results will be reported. Termination of the trial for futility at the interim analysis using this rule was determined to result in minimal loss of power (<2%).
Patients were randomized using a dynamic allocation procedure to balance the marginal distributions of the stratification factors: performance status (0 vs 1), tumor response to first line chemotherapy (SD vs PR vs CR), and time between completion of chemotherapy to randomization (1 vs 2 vs 3 months)[10].
OS was defined as time from randomization to the time of death from any cause. PFS was defined as the time from randomization to documentation of disease progression or death from any cause. Patients were imaged every 6 weeks until documented disease progression. Patients who were lost to follow-up were censored at the time of last evaluation for both endpoints. For patients who died without clear documentation of disease progression, progression was assumed at the time of death. The distribution of PFS and OS were estimated using the Kaplan-Meier method, and differences in treatment arms evaluated using the log rank test[11]. A Cox proportional hazards model was to model the outcomes within defined patient subsets[12].
A confirmed tumor response was defined as a complete or partial response per RECIST 1.1 noted on 2 consecutive evaluations at least 6 weeks apart. Fisher’s exact tests and chi-square tests were used to compare the baseline characteristics, response rates and adverse event patterns (regardless of relationship to study treatment) between the groups.
Data collection and statistical analyses were conducted by the Alliance Statistics and Data Center. Data quality was ensured by review of data by the Alliance Statistics and Data Center and by the study chairperson following Alliance policies.
RESULTS
Patient Characteristics
Between January 2010 and January 2013, 59 patients were randomized to receive NTX-010 or placebo. Eight patients did not receive the study treatment and were excluded from further analyses (2 patients withdrew/refusal, 1 physician decision and 5 experienced disease progression prior to treatment). Upon study audit, 1 randomized patient did not have a site of measurable residual disease and was excluded from further analysis. Fifty patients received NTX-010 (n=26) or placebo (n=24) on trial and baseline patient characteristics are shown in Table 1. Patient characteristics were well balanced between the two treatment groups.
Table 1.
Patient characteristics
| Characteristic | NTX-010 n = 26 | Placebo n = 24 | Overall n = 50 | p-value |
|---|---|---|---|---|
| Age, Median (Range) | 67 (44–81) | 60 (50–82) | 63 (44–82) | 0.18 |
| Sex, n (%) | 0.41 | |||
| Female | 12 (46.2) | 14 (58.3) | 26 (52.0) | |
| Male | 14 (53.9) | 10 (41.7) | 24 (48.0) | |
| Ethnicity, n (%) | 1.00 | |||
| Non-Hispanic | 24 (92.3) | 24 (100) | 48 (96.0) | |
| Not Reported | 1 (3.9) | 0 (0.0) | 1 (2.0) | |
| Unknown | 1 (3.9) | 0 (0.0) | 1 (2.0) | |
| Performance Status, n (%) | 1.00 | |||
| 0 | 8 (30.8) | 7 (29.2) | 15 (30.0) | |
| 1 | 18 (69.2) | 17 (70.8) | 35 (70.0) | |
| Smoking Status, n (%) | 0.34 | |||
| Never smoked | 0 (0.0) | 2 (8.3) | 2 (4.0) | |
| Current smoker | 7 (26.9) | 4 (16.7) | 11 (22.0) | |
| Former smoker | 19 (73.1) | 18 (75.0) | 37 (74.0) | |
| Prior Response to Chemotherapy, n (%) | 0.80 | |||
| Stable Disease | 4 (15.4) | 5 (20.8) | 9 (18.0) | |
| Partial Response | 15 (57.7) | 12 (50.0) | 27 (54.0) | |
| Complete Response | 7 (26.9) | 7 (29.2) | 14 (28.0) | |
| Time Between Completion of Chemotherapy to Randomization, n (%) | 0.93 | |||
| 1 month | 10 (38.5) | 9 (37.5) | 19 (38.0) | |
| 2 months | 10 (38.5) | 10 (41.7) | 20 (40.0) | |
| 3 months | 6 (23.1) | 4 (16.7) | 10 (20.0) | |
| Unknown | 0 (0.0) | 1 (4.2) | 1 (2.0) | |
| Prior CNS Radiation Therapy, n (%) | 0.77 | |||
| Yes | 8 (30.8) | 9 (37.5) | 17 (34.0) | |
| No | 18 (69.2) | 15 (62.5) | 33 (66.0) | |
Efficacy
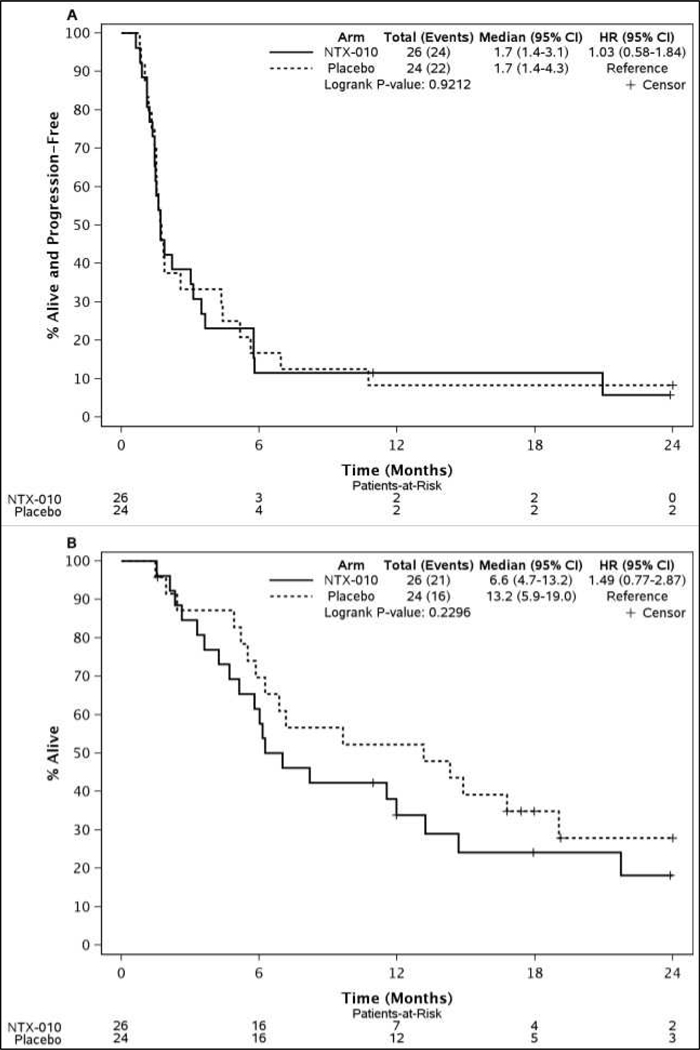
As pre-specified in the trial protocol, after 40 events (progression or death) were observed an interim futility analysis was performed for PFS, the primary end point. The interim analysis did not demonstrate a PFS difference between patients who received NTX-010 or placebo and the trial was terminated early for futility (Figure 2A). The median PFS was 1.7 months (95% CI 1.4 to 3.1) for NTX-010 and 1.7 months (95% CI 1.4 to 4.3) for placebo (p = 0.92) (Figure 2A). No significant OS difference was observed between patients who received NTX-010 or placebo (p = 0.23) (Figure 2B). As of final analysis on May 29, 2014, 5 patients were alive in the NTX-010 group and 8 in the placebo group. Only 2 patients from each group were alive without disease progression.
Figure 2.
Kaplan-Meier curves of (A) progression free survival and (B) overall survival by arm
Five patients treated with NTX-010 and six patients treated with placebo experienced disease control (CR + PR + SD) as a confirmed response (Table 2). No patient who received NTX-010 experienced an improved disease response compared to baseline. No difference was observed between best response rate and confirmed response rate for the two groups (p =1.0, p = 0.18, respectively, Fisher’s Exact Test).
Table 2.
Tumor Response. Best tumor response is defined to be the best overall objective status determined by combining the patient’s status on target lesions, non-target lesions, and new disease. A confirmed tumor response is defined to be a CR or PR noted as the objective status on 2 consecutive evaluations at least 6 weeks apart. NE is defined as not evaluable either due to no measurement available post-baseline or there were not 2 consecutive evaluations at least 6 weeks apart.
| Response Type | NTX-010 (n = 26) | Placebo (n = 24) | Overall (n = 50) |
|---|---|---|---|
| Best Response, n (%) | |||
| CR | 1 (3.8) | 2 (8.3) | 3 (6.0) |
| PR | 3 (11.5) | 2 (8.3) | 5 (10.0) |
| SD | 9 (34.6) | 4 (16.7) | 13 (26.0) |
| PD | 12 (46.2) | 15 (62.5) | 27 (54.0) |
| Non-CR/Non-PD | 1 ( 3.8) | 0 ( 0.0) | 1 ( 2.0) |
| NE* | 0 (0.0) | 1 (4.2) | 1 (2.0) |
| Confirmed Response, n (%) | |||
| CR | 0 (0.0) | 2 (8.3) | 2 (4.0) |
| PR | 1 (3.8) | 2 (8.3) | 3 (6.0) |
| SD | 4 (15.4) | 2 (8.3) | 6 (12.0) |
| PD | 19 (73.1) | 18 (75.0) | 37 (74.0) |
| Non-CR/Non-PD | 1 ( 3.8) | 0 ( 0.0) | 1 ( 2.0) |
| NE* | 1 (3.8) | 0 (0.0) | 1 (2.0) |
Viral Clearance
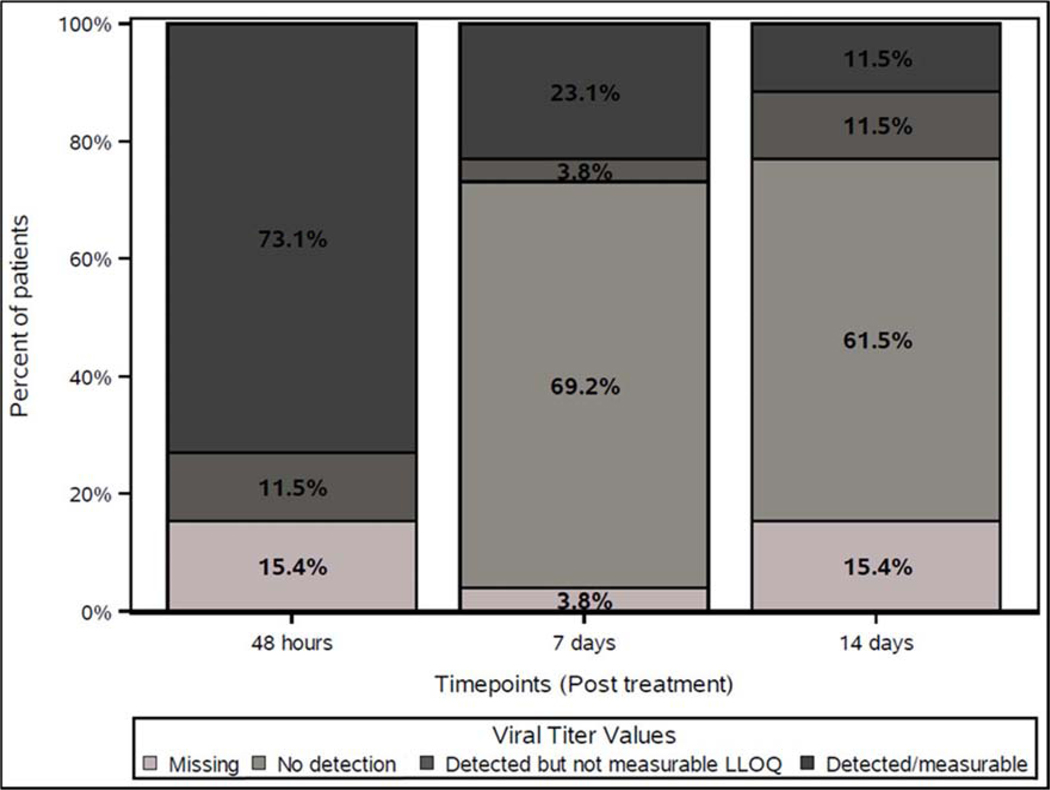
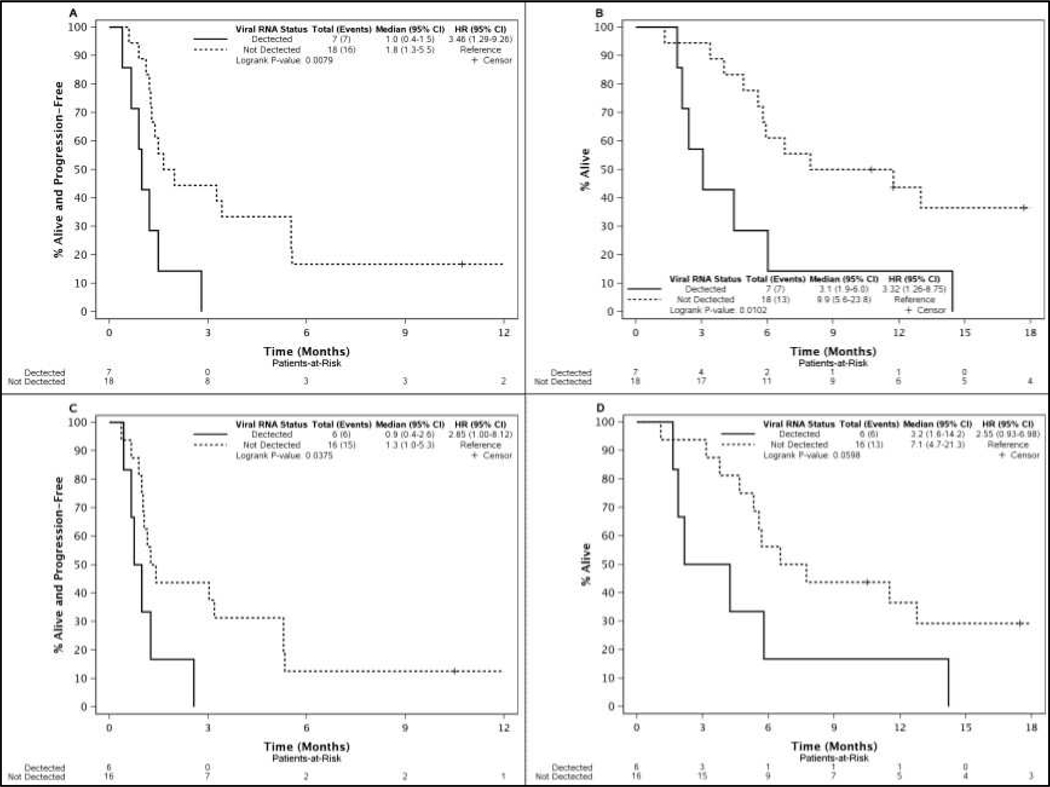
Measures of NTX-010 clearance including neutralizing anti-viral antibodies and viral load titer were followed to explore whether longer viral exposure associates with improved clinical outcomes. Neutralizing anti-viral antibodies were detected 14 days after treatment in all 23 patients who received NTX-010 and underwent testing. Viral clearance by viral titer was observed in a majority of the 26 patients who received NTX-010 either 7 or 14 days after treatment, but several patients had persistent virus similar to findings in the previous phase I trial (Figure 3)[6]. Based on the observations from the phase I trial, detectable levels of virus were thought to be secondary to intratumoral replication and a potential marker of efficacy. Exploratory analyses of PFS and OS stratified by viral clearance status in the NTX-010 cohort were performed. Delayed clearance of virus was associated with shorter PFS and OS. Patients with detectable levels of virus on day 7 or day 14 experienced a significantly shorter PFS compared to patients who had undetectable levels of virus (Figure 4A, B). Patients with detectable levels of virus on day 7 (n=7) experienced an OS of 3.1 months versus 9.9 months for those who cleared the virus (n=18) (p = 0.01) (Figure 4C). A similar trend was observed for patients with detectable virus day 14 post treatment with NTX-010 (n=6) compared to those who cleared the virus (n=16) (p = 0.06) (Figure 4D). Baseline average tumor burden was not associated with persistence of virus at day 7 or 14 (p = 0.24 and p = 0.26, respectively, Rank-sum test).
Figure 3.
Viral titer values for patients in NTX-010 arm (N=26).
Figure 4.
Kaplan-Meier curves of PFS and OS for viral RNA detection at day 7 (A, B) and day 14 (C, D).
Safety
Overall, the rate of grade 3 or higher adverse events, regardless of attribution, was 34.6% vs. 20.8% (p = 0.28); and the rate of grade 4 adverse events, regardless of attribution, was 11.5% vs. 4.2% (p = 0.34) in NTX-010 vs. placebo, respectively. No grade 5 events occurred.
Patients who received NTX-010 were more likely to experience flu-like symptoms (n = 16 vs 6), diarrhea (n = 8 vs 1), and fatigue (n = 8 vs 1). Grade 3 adverse events reported regardless of attribution to NTX-010 therapy include flu-like symptoms, dyspnea, fatigue, neutropenia, confusion, dehydration, hypokalemia, nausea, abdominal pain, arthralgia, insomnia, musculoskeletal deformity, and vomiting (all n = 1). Two patients experienced grade 4 hyponatremia felt to be not related to NTX-010 therapy. One patient with hyponatremia had persistent virus on day 7 and was not tested on day 14. One patient had grade 4 thrombocytopenia possibly related to NTX-010. No persistence of virus was detected in this patient on day 7 or 14.
DISCUSSION
In this randomized phase II trial, treatment with NTX-010 did not improve outcomes in patients with ES SCLC after front line platinum-based chemotherapy. At the pre-specified interim analysis, no significant difference in PFS was observed between patients who received NTX-010 compared to placebo and the trial was closed for futility. Disease response rate and OS were not improved with NTX-010. As observed in the phase I trial, NTX-010 was predominantly associated with flu-like symptoms.
Previously we observed that SCLC patients had persistent, detectable levels of virus in the peripheral blood after treatment with NTX-010[6]. We hypothesized this represented intratumor viral replication and possible additional oncolytic effect[6]. Unexpectedly, in this study detectable virus at day 7 or day 14 post NTX-010 infusion was associated with shorter PFS and OS. An association between viremia and poor patient outcomes has not been reported in other clinical trials with systemically delivered oncolytic viruses. A phase I trial of an oncolytic reovirus in patients with advanced solid malignancies reported detectable viral RNA in 6 of 18 patients[13]. Of these six patients, 1 experienced a partial response over the course of 7 cycles of therapy, 3 had stable disease, and 2 had progressive disease[13]. A single intravenous dose of an oncolytic measles virus resulted in a disease response in 5 of 32 patients with multiple myeloma[14]. Viral persistence was found in 3 of the 5 responders, including the patient who experienced a major hematologic response[14]. In our previous phase I trial, one patient with rapidly progressive SCLC experienced disease stabilization after a single NTX-010 dose of 107 vp/kg and had detectable NTX-010 one week after treatment[6].
In this phase II trial, SCLC patients received 1011 vp/kg NTX-010. The association between detectable virus and patient outcomes may be a previously unknown feature of NTX-010, adverse events related to the dose level, or the underlying disease state. Our findings warrant viral clearance monitoring in future trials as persistent viremia may be an early biomarker of treatment failure.
New potential biomarkers for SCLC permissiveness to NTX-010 have emerged that may better inform patient selection for future trials incorporating NTX-010. Anthrax toxin receptor 1 (ANTXR1) was recently identified as the receptor required for SCLC cell line infection with NTX-010[15]. Optimal viral replication within an ANTXR1 expressing cell lines occurs when interferon signaling genes, an important mediator of host anti-viral immunity, are downregulated[15]. Notably, receptor identification occurred in H446, a human SCLC cell line, and in vivo preclinical studies with H446 demonstrated near complete tumor eradicated by a single dose of NTX-010[16]. Other human SCLC were also tested with NTX-010 for cytotoxicity in vitro and exhibited a range of susceptibility which may more closely mirror the SCLC patient population and ANTXR1 expression[15, 16]. Together these data suggest further work is needed to investigate ANTXR1 and an interferon gene signature to potentially enrich for a cohort of SCLC patients who are likely to benefit from NTX-010.
With the notable exception of atezolizumab in the first line setting, recent randomized trials for subsequent lines of therapy in ES-SCLC have not yielded significant improvements for patient outcomes. Atezolizumab in a noncomparative randomized phase II study showed numerically inferior PFS and similar OS compared to standard chemotherapy[17, 18]. Nivolumab while having received FDA approval as a single agent based on the durable, anti-tumor activity in a subset of patients with recurrent or resistant SCLC did not lead to superior OS compared to standard second-line chemotherapy in the CheckMate 331 study[19, 20]. A phase III trial evaluating rovalpituzumab tesirine, an antibody-drug conjugate that targets DLL3, versus second line topotecan in recurrent SCLC after first line chemotherapy was halted early due to inferior OS compared to control[21]. Radiotherapy has also been studied extensively in ES-SCLC patients. In phase III trials, select ES-SCLC patients benefited from thoracic radiotherapy in terms of survival[22, 23]. The use of PCI has been found to improve survival in meta-analyses and one phase III trial but not another and is controversial[9, 24–26]. Efforts are underway combining multiple therapeutic approaches to improve the number of responders to therapy and those who experience meaningful clinical benefit. Even with these potential new therapy approaches, eradicating all underlying disease in SCLC, with its propensity to recur, remains a significant challenge. Given its unique mechanism of action and selectivity for SCLC, in the appropriately selected patient population NTX-010 in combination with other therapy may improve the depth of tumor elimination.
In conclusion, treatment with 1011 vp/kg NTX-010 after first line therapy for ES SCLC did not improve PFS compared to placebo. NTX-010 was well tolerated and no new safety signals were noted. In patients who received NTX-010, PFS and OS were reduced if viral persistence was detected after therapy. Viral persistence may represent an important biomarker in patients undergoing virotherapy in future trials.
Acknowledgments
Support: Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under Award Numbers U10CA180821 and U10CA180882 (to the Alliance for Clinical Trials in Oncology), U10CA180866, U10CA180790, U10CA180791, U10CA233196, U10CA233290, U10CA180820 and UG1CA189952 (ECOG-ACRIN), and U10CA180888 (SWOG). Also supported in part by funds from Neotropix, Inc. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The following institutional networks participated in this study:
MedStar Georgetown University Hospital, Washington, DC, Filipa Lynce and The Ohio State University Comprehensive Cancer Center LAPS, Columbus, OH, Claire Verschraegen, U10CA180850.
Footnotes
Disclaimers: None
ClinicalTrials.gov Identifier: NCT01017601
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Siegel RL, Miller KD, and Ahmedin J, Cancer statistics, 2019. CA: A Cancer Journal for Clinicians, 2018. 69(1): p. 7–34. [DOI] [PubMed] [Google Scholar]
- 2.Zhou H, et al. , Duration of Chemotherapy for Small Cell Lung Cancer: A Meta-Analysis. PLOS ONE, 2013. 8(8): p. e73805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Horn L, et al. , First-Line Atezolizumab plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer. N Engl J Med, 2018. 379(23): p. 2220–2229. [DOI] [PubMed] [Google Scholar]
- 4.Reddy PS, et al. , Seneca Valley Virus, a Systemically Deliverable Oncolytic Picornavirus, and the Treatment of Neuroendocrine Cancers. JNCI: Journal of the National Cancer Institute, 2007. 99(21): p. 1623–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hales LM, et al. , Complete genome sequence analysis of Seneca Valley virus-001, a novel oncolytic picornavirus. Journal of General Virology, 2008. 89(5): p. 1265–1275. [DOI] [PubMed] [Google Scholar]
- 6.Rudin CM, et al. , Phase I Clinical Study of Seneca Valley Virus (SVV-001), a Replication-Competent Picornavirus, in Advanced Solid Tumors with Neuroendocrine Features. Clinical Cancer Research, 2011. 17(4): p. 888–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oken MM, et al. , Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol, 1982. 5(6): p. 649–55. [PubMed] [Google Scholar]
- 8.Schiller JH, et al. , Topotecan versus observation after cisplatin plus etoposide in extensive-stage small-cell lung cancer: E7593--a phase III trial of the Eastern Cooperative Oncology Group. J Clin Oncol, 2001. 19(8): p. 2114–22. [DOI] [PubMed] [Google Scholar]
- 9.Slotman B, et al. , Prophylactic cranial irradiation in extensive small-cell lung cancer. N Engl J Med, 2007. 357(7): p. 664–72. [DOI] [PubMed] [Google Scholar]
- 10.Pocock SJ and Simon R, Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics, 1975. 31(1): p. 103–15. [PubMed] [Google Scholar]
- 11.Kaplan EL and Meier P, Nonparametric Estimation from Incomplete Observations. Journal of the American Statistical Association, 1958. 53(282): p. 457–481. [Google Scholar]
- 12.Cox DR, Regression Models and Life-Tables. Journal of the Royal Statistical Society. Series B (Methodological), 1972. 34(2): p. 187–220. [Google Scholar]
- 13.Gollamudi R, et al. , Intravenous administration of Reolysin®, a live replication competent RNA virus is safe in patients with advanced solid tumors. Investigational New Drugs, 2010. 28(5): p. 641–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dispenzieri A, et al. , Phase I trial of systemic administration of Edmonston strain of measles virus genetically engineered to express the sodium iodide symporter in patients with recurrent or refractory multiple myeloma. Leukemia, 2017. 31: p. 2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miles LA, et al. , Anthrax toxin receptor 1 is the cellular receptor for Seneca Valley virus. J Clin Invest, 2017. 127(8): p. 2957–2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reddy PS, et al. , Seneca Valley virus, a systemically deliverable oncolytic picornavirus, and the treatment of neuroendocrine cancers. J Natl Cancer Inst, 2007. 99(21): p. 1623–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pujol JL, et al. , 1664OA randomized non-comparative phase II study of anti–PD-L1 ATEZOLIZUMAB or chemotherapy as second-line therapy in patients with small cell lung cancer: Results from the IFCT-1603 trial. Annals of Oncology, 2018. 29(suppl_8): p. mdy298-mdy298. [DOI] [PubMed] [Google Scholar]
- 18.Pujol J-L, et al. , A Randomized Non-Comparative Phase II Study of Anti-Programmed Cell Death-Ligand 1 Atezolizumab or Chemotherapy as Second-Line Therapy in Patients With Small Cell Lung Cancer: Results From the IFCT-1603 Trial. Journal of Thoracic Oncology, 2019. 14(5): p. 903–913. [DOI] [PubMed] [Google Scholar]
- 19.Antonia SJ, et al. , Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): a multicentre, open-label, phase 1/2 trial. The Lancet Oncology, 2016. 17(7): p. 883–895. [DOI] [PubMed] [Google Scholar]
- 20.Reck M, et al. , LBA5Efficacy and safety of nivolumab (nivo) monotherapy versus chemotherapy (chemo) in recurrent small cell lung cancer (SCLC): Results from CheckMate 331. Annals of Oncology, 2018. 29(suppl_10). [Google Scholar]
- 21.Rudin CM, et al. , Rovalpituzumab tesirine, a DLL3-targeted antibody-drug conjugate, in recurrent small-cell lung cancer: a first-in-human, first-in-class, open-label, phase 1 study. The Lancet Oncology, 2017. 18(1): p. 42–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Slotman BJ, et al. , Radiotherapy for extensive stage small-cell lung cancer - Authors’ reply. Lancet, 2015. 385(9975): p. 1292–3. [DOI] [PubMed] [Google Scholar]
- 23.Jeremic B, et al. , Role of radiation therapy in the combined-modality treatment of patients with extensive disease small-cell lung cancer: A randomized study. J Clin Oncol, 1999. 17(7): p. 2092–9. [DOI] [PubMed] [Google Scholar]
- 24.Auperin A, et al. , Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. Prophylactic Cranial Irradiation Overview Collaborative Group. N Engl J Med, 1999. 341(7): p. 476–84. [DOI] [PubMed] [Google Scholar]
- 25.Meert AP, et al. , Prophylactic cranial irradiation in small cell lung cancer: a systematic review of the literature with meta-analysis. BMC Cancer, 2001. 1: p. 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takahashi T, et al. , Prophylactic cranial irradiation versus observation in patients with extensive-disease small-cell lung cancer: a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol, 2017. 18(5): p. 663–671. [DOI] [PubMed] [Google Scholar]